Relationship of SOD-1 Activity in Metabolic Syndrome and/or Frailty in Elderly Individuals
Abstract
1. Introduction
- Mitochondrial Electron Transport Chain: Within mitochondria, during cellular respiration, the oxidative phosphorylation involves electron transfer that can produce ROS such as superoxide anion (O2•−) and hydrogen peroxide (H2O2). Imperfections in the electron transport system may increase ROS production.
- Reactions Catalyzed by Oxidoreductases: Oxidoreductase enzymes participate in redox reactions during metabolic processes, such as lipid peroxidation, which can inadvertently produce ROS as metabolic byproducts (resulting in the formation of reactive aldehydes), playing a role in regular cellular metabolism.
- Oxidation of Low-Molecular-Weight Compounds (RH2): Various compounds such as amino acids, thiols, and reducing sugars can undergo oxidation, leading to the production of ROS (e.g., protein oxidation), including superoxide anions and free radicals derived from oxidized compounds (•RH).
- Peroxisomes: Organelles that contain enzymes, such as xanthine oxidase, are responsible for purine metabolism and the oxidation of fatty acids, which also generates ROS.
- Phagocyte Activation: Neutrophils and other phagocytic cells produce ROS, such as superoxide anion and hydrogen peroxide, in response to infections via NADPH oxidase. This process is crucial for their bactericidal activity, and the Fenton reaction (production of hydroxyl radical in the presence of Fe2+ ions) can further increase the amount of ROS [19,20].
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.3. Clinical Examination
2.4. Frailty Diagnosis
2.5. Blood Sampling and Biochemical Analysis
2.5.1. Glucose and Lipids Measurements
2.5.2. The Activity of Cu-, Zn-Superoxide Dismutase (SOD-1) in Red Blood Cell
Preparation of Hemolysate
Measurement Procedure
Calculating SOD-1 Activity
2.6. Metabolic Syndrome
2.7. Group Analysed in the Study
2.8. Statistical Analysis
3. Results
4. Discussion
Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vollset, S.E.; Goren, E.; Yuan, C.W.; Cao, J.; Smith, A.E.; Hsiao, T.; Bisignano, C.; Azhar, G.S.; Castro, E.; Chalek, J.; et al. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: A forecasting analysis for the Global Burden of Disease Study. Lancet 2020, 396, 1285–1306. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, A.E.; Aali, A.; Abate, Y.H.; Abbasgholizadeh, R.; Abbasian, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd ElHafeez, S. GBD 2021 Fertility and Forecasting Collaborators. Global fertility in 204 countries and territories, 1950–2021, with forecasts to 2100: A comprehensive demographic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2057–2099. [Google Scholar] [CrossRef]
- Kawai, K.; Oshita, K.; Kusube, T. Model for projecting the generation of used disposable diapers in the era of depopulation and aging in Japan. Waste Manag. Res. 2023, 41, 1089–1101. [Google Scholar] [CrossRef]
- Lucke, J.A.; Mooijaart, S.P.; Heeren, P.; Singler, K.; McNamara, R.; Gilbert, T.; Nickel, C.H.; Castejon, S.; Mitchell, A.; Mezera, V.; et al. Providing care for older adults in the Emergency Department: Expert clinical recommendations from the European Task Force on Geriatric Emergency Medicine. Eur. Geriatr. Med. 2022, 13, 309–317. [Google Scholar] [CrossRef]
- Luengo-Fernandez, R.; Walli-Attaei, M.; Gray, A.; Torbica, A.; Maggioni, A.P.; Huculeci, R.; Bairami, F.; Aboyans, V.; Timmis, A.D.; Vardas, P.; et al. Economic burden of cardiovascular diseases in the European Union: A population-based cost study. Eur. Heart J. 2023, 44, 4752–4767. [Google Scholar] [CrossRef] [PubMed]
- Zdrowie i Ochrona Zdrowia w 2022. Główny Urząd Statystyczny. Available online: https://stat.gov.pl/obszary-tematyczne/zdrowie/zdrowie/zdrowie-i-ochrona-zdrowia-w-2022-roku,1,13.html (accessed on 9 July 2024).
- Zhang, K.; Ma, Y.; Luo, Y.; Song, Y.; Xiong, G.; Ma, Y.; Sun, X.; Kan, C. Metabolic diseases and healthy aging: Identifying environmental and behavioral risk factors and promoting public health. Front. Public Health 2023, 11, 1253506. [Google Scholar] [CrossRef]
- Tomlinson, D.J.; Erskine, R.M.; Morse, C.I.; Onambélé, G.L. Body Fat Percentage, Body Mass Index, Fat Mass Index and the Ageing Bone: Their Singular and Combined Roles Linked to Physical Activity and Diet. Nutrients 2019, 11, 195. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, Z.; Wang, G.; Li, Q.; Xu, Y.; Li, M.; Hu, R.; Chen, G.; Su, Q.; Mu, Y.; et al. Age-related disparities in diabetes risk attributable to modifiable risk factor profiles in Chinese adults: A nationwide, population-based, cohort study. Lancet Healthy Longev. 2021, 2, e618–e628. [Google Scholar] [CrossRef]
- Caprara, G. Mediterranean-Type Dietary Pattern and Physical Activity: The Winning Combination to Counteract the Rising Burden of Non-Communicable Diseases (NCDs). Nutrients 2021, 13, 429. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Jung, Y.J.; Yoon, J.L.; Kim, H.S.; Lee, A.Y.; Kim, M.Y.; Cho, J.J. Atypical Clinical Presentation of Geriatric Syndrome in Elderly Patients With Pneumonia or Coronary Artery Disease. Ann. Geriatr. Med. Res. 2017, 21, 158–163. [Google Scholar] [CrossRef]
- Yang, Y.C.; Lin, M.H.; Wang, C.S.; Lu, F.H.; Wu, J.S.; Cheng, H.P.; Lin, S.I. Geriatric syndromes and quality of life in older adults with diabetes. Geriatr. Gerontol. Int. 2019, 19, 518–524. [Google Scholar] [CrossRef]
- Buchmann, N.; Spira, D.; König, M.; Demuth, I.; Steinhagen-Thiessen, E. Frailty and the Metabolic Syndrome—Results of the Berlin Aging Study II (BASE-II). J. Frailty Aging 2019, 8, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Brivio, P.; Paladini, M.S.; Racagni, G.; Riva, M.A.; Calabrese, F.; Molteni, R. From Healthy Aging to Frailty: In Search of the Underlying Mechanisms. Curr. Med. Chem. 2019, 26, 3685–3701. [Google Scholar] [CrossRef]
- Clegg, A.; Young, J. The frailty syndrome. Clin. Med. 2011, 11, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Wang, Y.; Mak, J.K.L.; Ericsson, M.; Hägg, S.; Jylhävä, J. Is Frailty Different in Younger Adults Compared to Old? Prevalence, Characteristics, and Risk Factors of Early-Life and Late-Life Frailty in Samples from Sweden and UK. Gerontology 2023, 69, 1385–1393. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–48. [Google Scholar] [CrossRef]
- Sinenko, S.A.; Starkova, T.Y.; Kuzmin, A.A.; Tomilin, A.N. Physiological Signaling Functions of Reactive Oxygen Species in Stem Cells: From Flies to Man. Front. Cell Dev. Biol. 2021, 9, 714370. [Google Scholar] [CrossRef]
- de Almeida, A.J.P.O.; de Oliveira, J.C.P.L.; da Silva Pontes, L.V.; de Souza Júnior, J.F.; Gonçalves, T.A.F.; Dantas, S.H.; de Almeida Feitosa, M.S.; Silva, A.O.; de Medeiros, I.A. ROS: Basic Concepts, Sources, Cellular Signaling, and its Implications in Aging Pathways. Oxid. Med. Cell Longev. 2022, 2022, 1225578. [Google Scholar] [CrossRef]
- Bernabeu-Wittel, M.; Gómez-Díaz, R.; González-Molina, Á.; Vidal-Serrano, S.; Díez-Manglano, J.; Salgado, F.; Soto-Martín, M.; Ollero-Baturone, M.; on behalf of the Proteo Researchers. Oxidative Stress, Telomere Shortening, and Apoptosis Associated to Sarcopenia and Frailty in Patients with Multimorbidity. J. Clin. Med. 2020, 9, 2669. [Google Scholar] [CrossRef]
- Dzięgielewska-Gęsiak, S. Metabolic syndrome in an aging society—Role of oxidant-antioxidant imbalance and inflammation markers in disentangling atherosclerosis. Clin. Interv. Aging 2021, 16, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef] [PubMed]
- Trist, B.G.; Hilton, J.B.; Hare, D.J.; Crouch, P.J.; Double, K.L. Superoxide Dismutase 1 in Health and Disease: How a Frontline Antioxidant Becomes Neurotoxic. Angew. Chem. Int. Ed. Engl. 2021, 60, 9215–9246. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide dismutase. An enzymatic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Milani, P.; Gagliardi, S.; Cova, E.; Cereda, C. SOD1 transcriptional and posttranscriptional regulation and its potential implications in ALS. Neurol. Res. Int. 2011, 2011, 458427. [Google Scholar] [CrossRef] [PubMed]
- Kozakiewicz, M.; Kornatowski, M.; Krzywińska, O.; Kędziora-Kornatowska, K. Changes in the blood antioxidant defense of advanced age people. Clin. Interv. Aging 2019, 14, 763–771. [Google Scholar] [CrossRef]
- Parascandolo, A.; Laukkanen, M.O. Carcinogenesis and Reactive Oxygen Species Signaling: Interaction of the NADPH Oxidase NOX1-5 and Superoxide Dismutase 1–3 Signal Transduction Pathways. Antioxid. Redox Signal. 2019, 30, 443–486. [Google Scholar] [CrossRef] [PubMed]
- Rybka, J.; Kędziora-Kornatowska, K.; Banaś-Leżańska, P.; Majsterek, I.; Carvalho, L.A.; Cattaneo, A.; Anacker, C.; Kędziora, J. Interplay between the pro-oxidant and antioxidant systems and proinflammatory cytokine levels, in relation to iron metabolism and the erythron in depression. Free Radic. Biol. Med. 2013, 63, 187–194. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, P.; Dong, C.; Zhang, J.; Wang, X.; Pei, H. Oxidative Stress Signaling Mediated Pathogenesis of Diabetic Cardiomyopathy. Oxid. Med. Cell Longev. 2022, 2022, 5913374. [Google Scholar] [CrossRef]
- Deepa, S.S.; Bhaskaran, S.; Espinoza, S.; Brooks, S.V.; McArdle, A.; Jackson, M.J.; Van Remmen, H.; Richardson, A. A new mouse model of frailty: The Cu/Zn superoxide dismutase knockout mouse. Geroscience 2017, 39, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Lorente, H.; Herrera-Quintana, L.; Molina-López, J.; López-González, B.; Planells, E. Sociodemographic, Anthropometric, Body Composition, Nutritional, and Biochemical Factors Influenced by Age in a Postmenopausal Population: A Cross-Sectional Study. Metabolites 2023, 13, 78. [Google Scholar] [CrossRef]
- Dzięgielewska-Gęsiak, S.; Wyszomirska, K.; Fatyga, E.; Wysocka, E.; Muc-Wierzgoń, M. The role of oxidant-antioxidant markers and resistin in metabolic syndrome elderly individuals. Sci. Prog. 2021, 104, 368504211006510. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 1953–2041. [Google Scholar] [CrossRef]
- Rockwood, K.; Theou, O. Using the Clinical Frailty Scale in allocating scarce health care resources. Can. Geriatr. J. 2020, 23, 254–259. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed]
- Perry, J.J.P.; Shin, D.S.; Getzoff, E.D.; Tainer, J.A. The structural biochemistry of the superoxide dismutases. Biochim. Biophys. Acta. 2010, 1804, 245–262. [Google Scholar] [CrossRef]
- Kozakiewicz, M.; Rowiński, R.; Kornatowski, M.; Dąbrowski, A.; Kędziora-Kornatowska, K.; Strachecka, A. Relation of Moderate Physical Activity to Blood Markers of Oxidative Stress and Antioxidant Defense in the Elderly. Oxid. Med. Cell Longev. 2019, 2019, 5123628. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Kattimani, S.; Sridhar, M.G. Oxidative stress during alcohol withdrawal and its relationship with withdrawal severity. Indian. J. Psychol. Med. 2015, 37, 175–180. [Google Scholar] [CrossRef]
- Moraes, L.; Dries, S.S.; Seibert, B.S.; Linden, R.; Perassolo, M.S. Evaluation of oxidative stress markers in ethanol users. Braz. J. Med. Biol. Res. 2023, 56, e12465. [Google Scholar] [CrossRef]
- Haque, S.; Kodidela, S.; Sinha, N.; Kumar, P.; Cory, T.J.; Kumar, S. Differential packaging of inflammatory cytokines/chemokines and oxidative stress modulators in U937 and U1 macrophages-derived extracellular vesicles upon exposure to tobacco constituents. PLoS ONE 2020, 15, e0233054. [Google Scholar] [CrossRef] [PubMed]
- Oates, N.; Pamphlett, R. An epigenetic analysis of SOD1 and VEGF in ALS. Amyotroph. Lateral Scler. 2007, 8, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Bilski, J.; Mazur-Bialy, A.; Wojcik, D.; Magierowski, M.; Surmiak, M.; Kwiecien, S.; Magierowska, K.; Hubalewska-Mazgaj, M.; Sliwowski, Z.; Brzozowski, T. Effect of Forced Physical Activity on the Severity of Experimental Colitis in Normal Weight and Obese Mice. Involvement of Oxidative Stress and Proinflammatory Biomarkers. Nutrients 2019, 11, 1127. [Google Scholar] [CrossRef]
- Lennicke, C.; Cochemé, H.M. Redox signalling and ageing: Insights from Drosophila. Biochem. Soc. Trans. 2020, 48, 367–377. [Google Scholar] [CrossRef]
- Trist, B.G.; Genoud, S.; Roudeau, S.; Rookyard, A.; Abdeen, A.; Cottam, V.; Hare, D.J.; White, M.; Altvater, J.; Fifita, J.A.; et al. Altered SOD1 maturation and post-translational modification in amyotrophic lateral sclerosis spinal cord. Brain 2022, 145, 3108–3130. [Google Scholar] [CrossRef] [PubMed]
- Franco, C.; Canzoniero, L.M.T. Zinc homeostasis and redox alterations in obesity. Front. Endocrinol. 2024, 14, 1273177. [Google Scholar] [CrossRef]
- Bandeira Sde, M.; Guedes Gda, S.; da Fonseca, L.J.; Pires, A.S.; Gelain, D.P.; Moreira, J.C.; Rabelo, L.A.; Vasconcelos, S.M.; Goulart, M.O. Characterization of blood oxidative stress in type 2 diabetes mellitus patients: Increase in lipid peroxidation and SOD activity. Oxid. Med. Cell Longev. 2012, 2012, 819310. [Google Scholar] [CrossRef]
- Javed, A.; Mehboob, K.; Rashid, A.; Majid, A.; Khan, S.; Baig, Z.A. Oxidative Stress and Lipid Peroxidation in NAFLD with and without Type 2 Diabetes Mellitus. J. Coll. Physicians Surg. Pak. 2023, 33, 1254–1258. [Google Scholar] [CrossRef]
- Kane, A.E.; Gregson, E.; Theou, O.; Rockwood, K.; Howlett, S.E. The association between frailty, the metabolic syndrome, and mortality over the lifespan. Geroscience 2017, 39, 221–229. [Google Scholar] [CrossRef]
- Grygiel-Górniak, B.; Marcinkowska, J.; Szczepanik, A.; Przysławski, J. Nutritional habits and oxidative stress in postmenopausal age. Pol. Arch. Med. Wewn. 2014, 124, 298–305. [Google Scholar] [CrossRef]
- Dzięgielewska-Gęsiak, S.; Bielawska, L.; Zowczak-Drabarczyk, M.; Hoffmann, K.; Cymerys, M.; Muc-Wierzgoń, M.; Wysocka, E.; Bryl, W. The impact of high-density lipoprotein on oxidant-antioxidant balance in healthy elderly people. Pol. Arch. Med. Wewn. 2016, 126, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Luna-Luna, M.; Niesor, E.; Pérez-Méndez, Ó. HDL as Bidirectional Lipid Vectors: Time for New Paradigms. Biomedicines 2022, 10, 1180. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Marcos, M.A.; Blázquez-Medela, A.M.; Gamella-Pozuelo, L.; Recio-Rodriguez, J.I.; García-Ortiz, L.; Martínez-Salgado, C. Serum superoxide dismutase is associated with vascular structure and function in hypertensive and diabetic patients. Oxidative Med. Cell. Longev. 2016, 2016, 9124676. [Google Scholar] [CrossRef] [PubMed]
- Eleutherio, E.C.A.; Silva Magalhães, R.S.; de Araújo Brasil, A.; Monteiro Neto, J.R.; de Holanda Paranhos, L. SOD1, more than just an antioxidant. Arch. Biochem. Biophys. 2021, 697, 108701. [Google Scholar] [CrossRef] [PubMed]
- Adachi, Y.; Nojima, M.; Mori, M.; Yamano, H.O.; Sasaki, Y.; Nakase, H.; Lin, Y.; Wakai, K.; Tamakoshi, A. Association of serum superoxide dismutase activity and the incidence of colorectal cancer in a nested case-control study. Cancer Epidemiol. 2023, 87, 102455. [Google Scholar] [CrossRef]
- Skórska, K.B.; Płaczkowska, S.; Prescha, A.; Porębska, I.; Kosacka, M.; Pawełczyk, K.; Zabłocka-Słowińska, K. Serum Total SOD Activity and SOD1/2 Concentrations in Predicting All-Cause Mortality in Lung Cancer Patients. Pharmaceuticals 2021, 14, 1067. [Google Scholar] [CrossRef]
| Variable | NonMetS-Nonfrail n = 19 | NonMetS-Frail n = 20 | MetS-Nonfrail n = 17 | MetS-Frail n = 10 | p |
|---|---|---|---|---|---|
| Age [years] | 70.0 (66.0–75.0) | 81.0 (73.0–88.5) | 71.0 (68.0–74.0) | 71.5 (68.0–78.0) | 0.004 |
| Waist [cm] | 83.0 (78.0–92.0) | 80.0 (71.0–86.5) | 95.0 (92.0–102.0) | 80.0 (74.0–98.0) | 0.0001 |
| SBP [mmHg] | 130.0 (125.0–140.0) | 122.5 (107.5–140.0) | 140.0 (135.0–150.0) | 142.5 (140.0–145.0) | 0.001 |
| DBP [mmHg] | 80.0 (70.0–90.0) | 77.5 (67.5–80.0) | 85.0(80.0–85.0) | 77.5 (70.0–90.0) | >0.05 |
| G 0’ [mg/dL] | 93.0 (90.4–97.5) | 89.1 (86.0–101.0) | 111.0 (103.2–115.2) | 125.2 (114.8–149.0) | 0.0000 |
| Mean G [mg/dL] | 117.0 (111.0–123.0) | 117.0 (108.0–131.0) | 123.0 (114.0–131.0) | 126.0 (108.0–126.0) | >0.05 |
| HbA1c [%] | 5.7 (5.5–5.9) | 5.7 (5.4–6.2) | 5.9 (5.6–6.2) | 6.0 (5.4–6.0) | >0.05 |
| TC [mg/dL] | 200.0 (185.0–222.0) | 164.5 (125.5–184.0) | 186.0 (160.0–201.0) | 133.5 (116.0–151.0) | 0.0000 |
| TG [mg/dL] | 82.0 (70.0–120.0) | 95.0 (70.0–133.0) | 92.0 (70.0–182.0) | 148.5 (116.0–188.0) | >0.05 |
| HDL-C [mg/dL] | 66.7 (59.2–71.4) | 45.5 (39.0–50.8) | 54.0 (45.0–65.8) | 27.0 (24.1–37.0) | 0.0000 |
| LDL-C [mg/dL] | 116.0 (99.6–134.8) | 78.0 (62.6–113.2) | 107.6 (90.0–118.7) | 74.4 (70.0–88.0) | 0.003 |
| WBC [G/L] | 5.4 (4.9–6.9) | 7.8 (5.9–9.4) | 6.0 (5.3–7.5) | 7.2 (6.5–9.2) | 0.0146 |
| PLT [G/L] | 303 (273–318) | 324 (246–399) | 242 (164–338) | 402 (239–482) | >0.05 |
| RBC [T/L] | 4.56 (4.36–4.80) | 4.29 (4.09–4.61) | 4.77 (4.52–5.38) | 4.04 (3.90–4.69) | 0.0453 |
| HCT [%] | 40.5 (38,8–41,8) | 37.0 (33.4–40.2) | 41.9 (41.0–42.1) | 36.2 (33.0–40.8) | 0.0081 |
| HGB [g/dL] | 12.7 (12.1–13.8) | 11.6 (11.1–12.6) | 12.8 (12.3–14.2) | 12.1 (11.0–13.9) | 0.0197 |
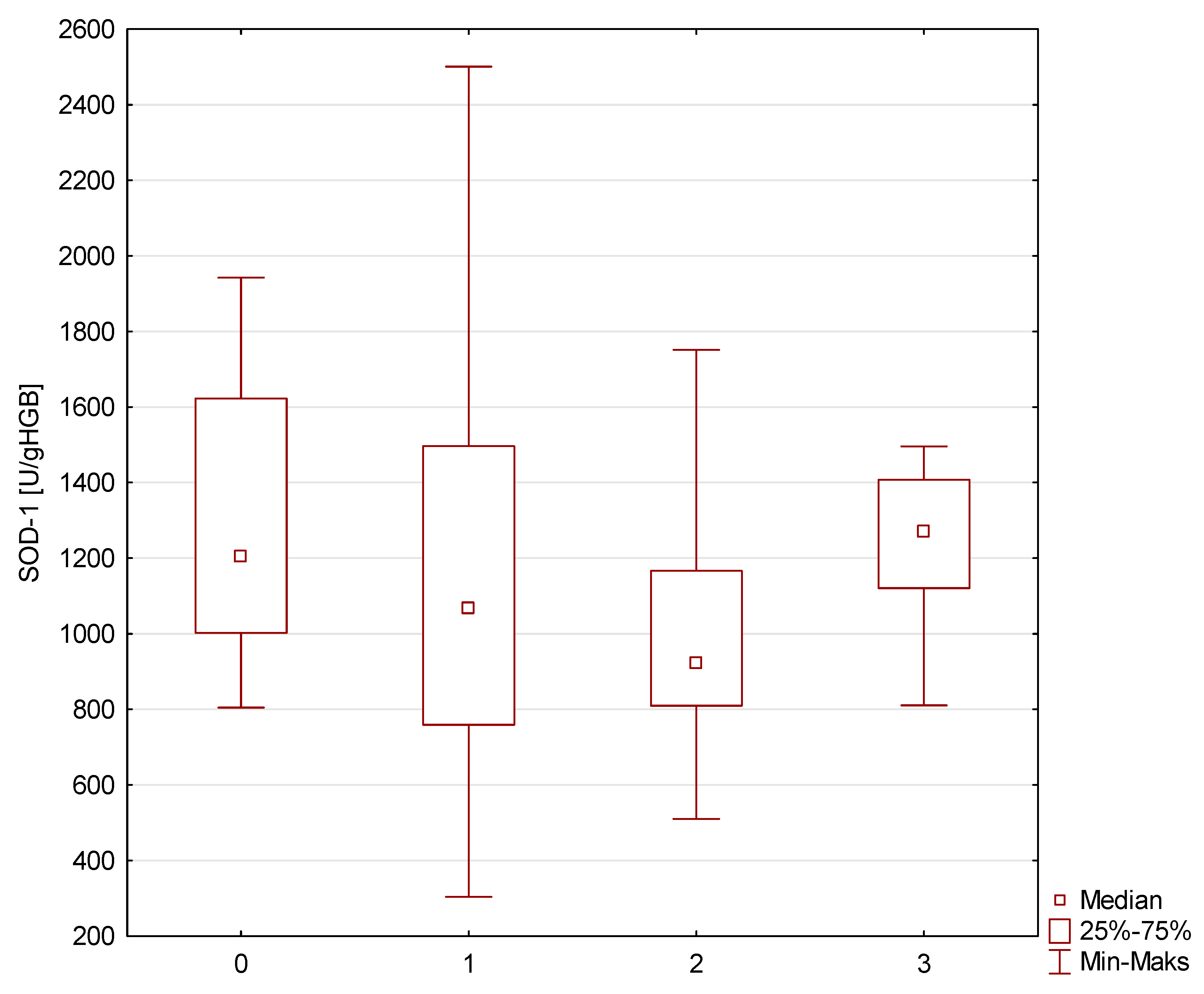
| SOD-1 [U/gHGB] | 1202.8 (1002.6–1622.7) | 1067.2 (759.1–1496.5) | 919.8 (809.7–1166.6) | 1270.3 (1121.0–1407.4) | >0.05 |
| Variable | NonMetS-Nonfrail SOD-1 Activity Spearman’s R Coefficient | NonMetS-Frail SOD-1 Activity Spearman’s R Coefficient | MetS-Nonfrail SOD-1 Activity Spearman’s R Coefficient | MetS-Frail SOD-1 Activity Spearman’s R Coefficient |
|---|---|---|---|---|
| Age | −0.6293 | 0.0023 | 0.322- | 0.6000 |
| Waist | −0.0079 | −0.3003 | 0.0602 | −0.1471 |
| SBP | 0.1348 | −0.1881 | 0.0625 | 0.0294 |
| DBP | 0.3978 | −0.0927 | −0.2555 | 0.1852 |
| G 0’ | −0.2511 | 0.2339 | −0.0686 | −0.8426 |
| HbA1c | 0.2941 | 0.0818 | 0.0012 | 0.6377 |
| TC | 0.4866 | −0.4301 | 0.1669 | −0.6000 |
| TG | 0.1795 | −0.1343 | −0.3514 | −0.2000 |
| HDL-C | 0.0246 | −0.4586 | 0.0245 | 0.3143 |
| LDL-C | 0.5160 | −0.2075 | 0.3211 | −0.6000 |
| WBC | −0.3426 | −0.2250 | 0.2143 | 0.4857 |
| PLT | 0.1366 | −0.4650 | 0.2143 | 0.1428 |
| RBC | −0.5315 | 0.1714 | 0.6071 | −0.2571 |
| HCT | −0.5315 | −0.1023 | 0.8829 | −0.2571 |
| HGB | −0.2827 | −0.0956 | 0.4196 | −0.0857 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzięgielewska-Gęsiak, S.; Wysocka, E.; Fatyga, E.; Muc-Wierzgoń, M. Relationship of SOD-1 Activity in Metabolic Syndrome and/or Frailty in Elderly Individuals. Metabolites 2024, 14, 514. https://doi.org/10.3390/metabo14090514
Dzięgielewska-Gęsiak S, Wysocka E, Fatyga E, Muc-Wierzgoń M. Relationship of SOD-1 Activity in Metabolic Syndrome and/or Frailty in Elderly Individuals. Metabolites. 2024; 14(9):514. https://doi.org/10.3390/metabo14090514
Chicago/Turabian StyleDzięgielewska-Gęsiak, Sylwia, Ewa Wysocka, Edyta Fatyga, and Małgorzata Muc-Wierzgoń. 2024. "Relationship of SOD-1 Activity in Metabolic Syndrome and/or Frailty in Elderly Individuals" Metabolites 14, no. 9: 514. https://doi.org/10.3390/metabo14090514
APA StyleDzięgielewska-Gęsiak, S., Wysocka, E., Fatyga, E., & Muc-Wierzgoń, M. (2024). Relationship of SOD-1 Activity in Metabolic Syndrome and/or Frailty in Elderly Individuals. Metabolites, 14(9), 514. https://doi.org/10.3390/metabo14090514








