Methimazole-Induced Hypothyroidism Increases the Content of Glycogen and Changes the Expression of LDH, GLUT4, and Aromatase in the Pregnant Uterus of Rabbits
Abstract
:1. Introduction
2. Materials and Methods
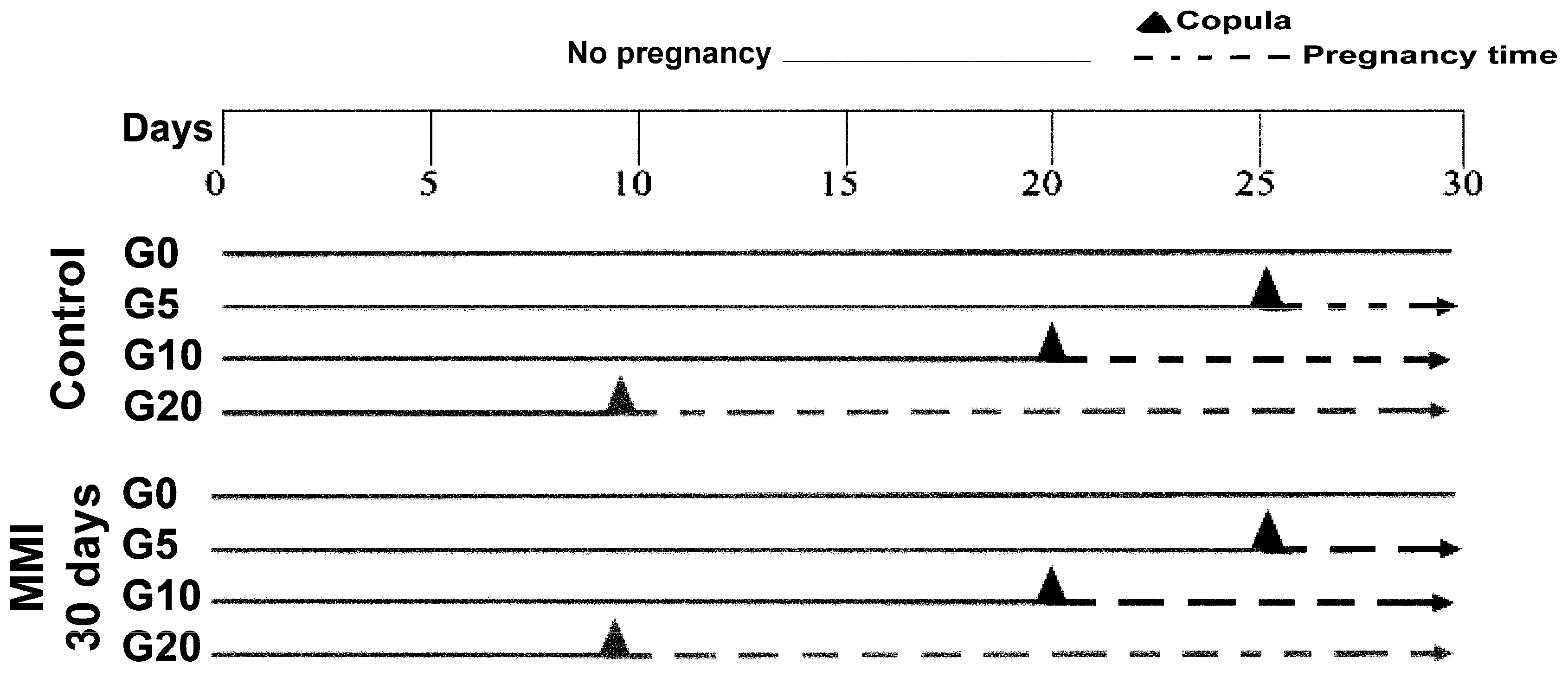
2.1. Animals
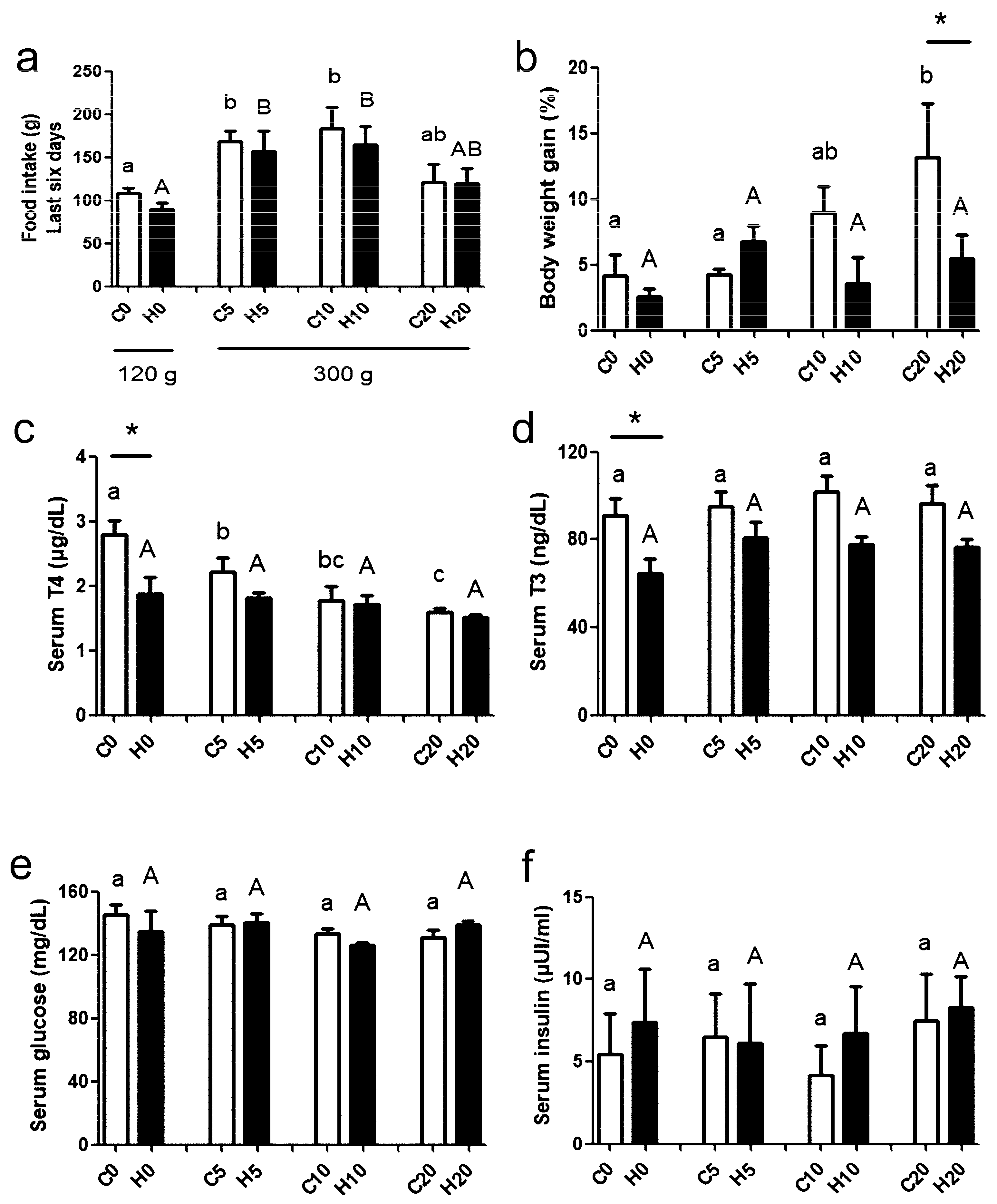
2.2. Hormones, Glucose, and Glycogen Quantification in Dams
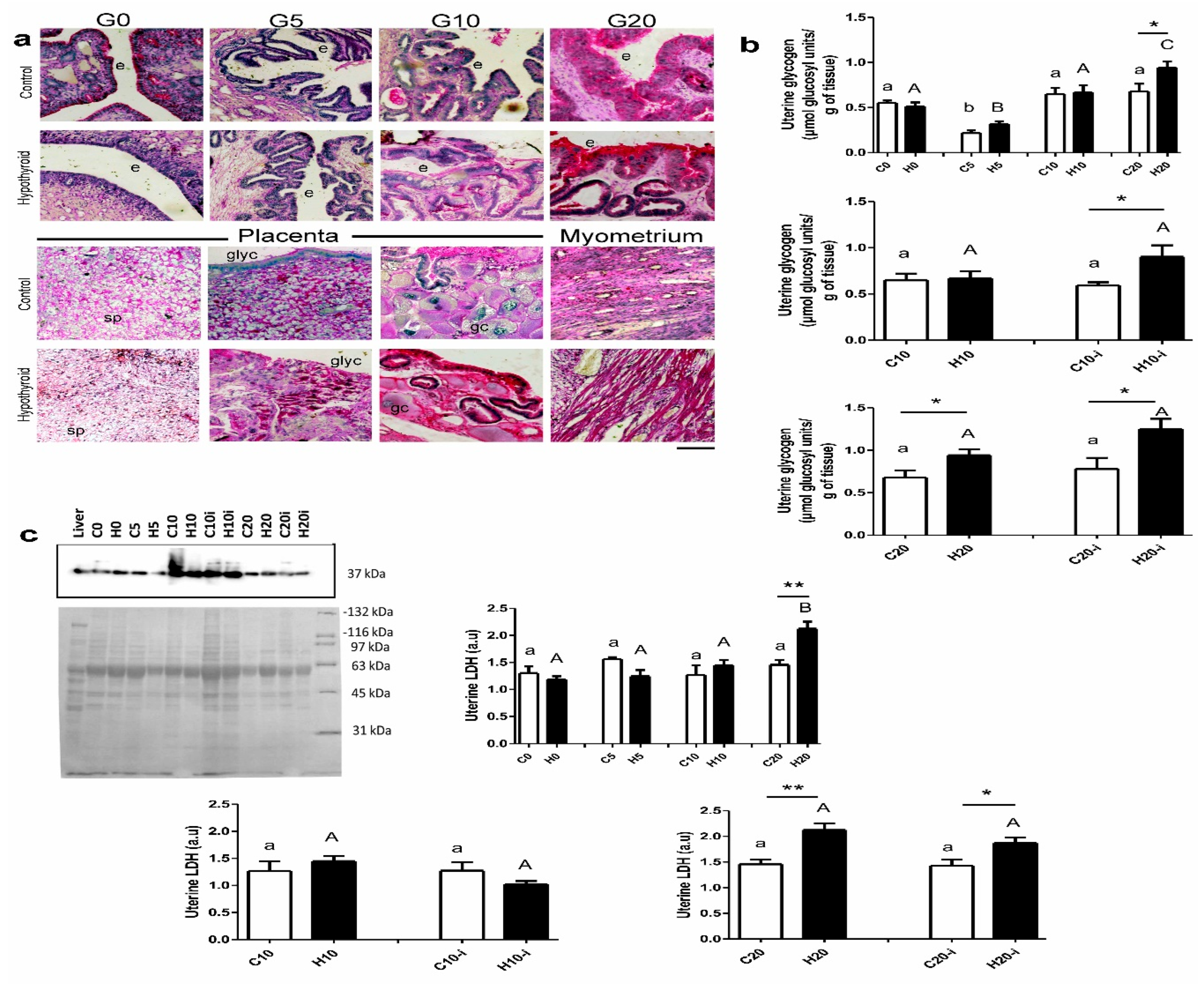
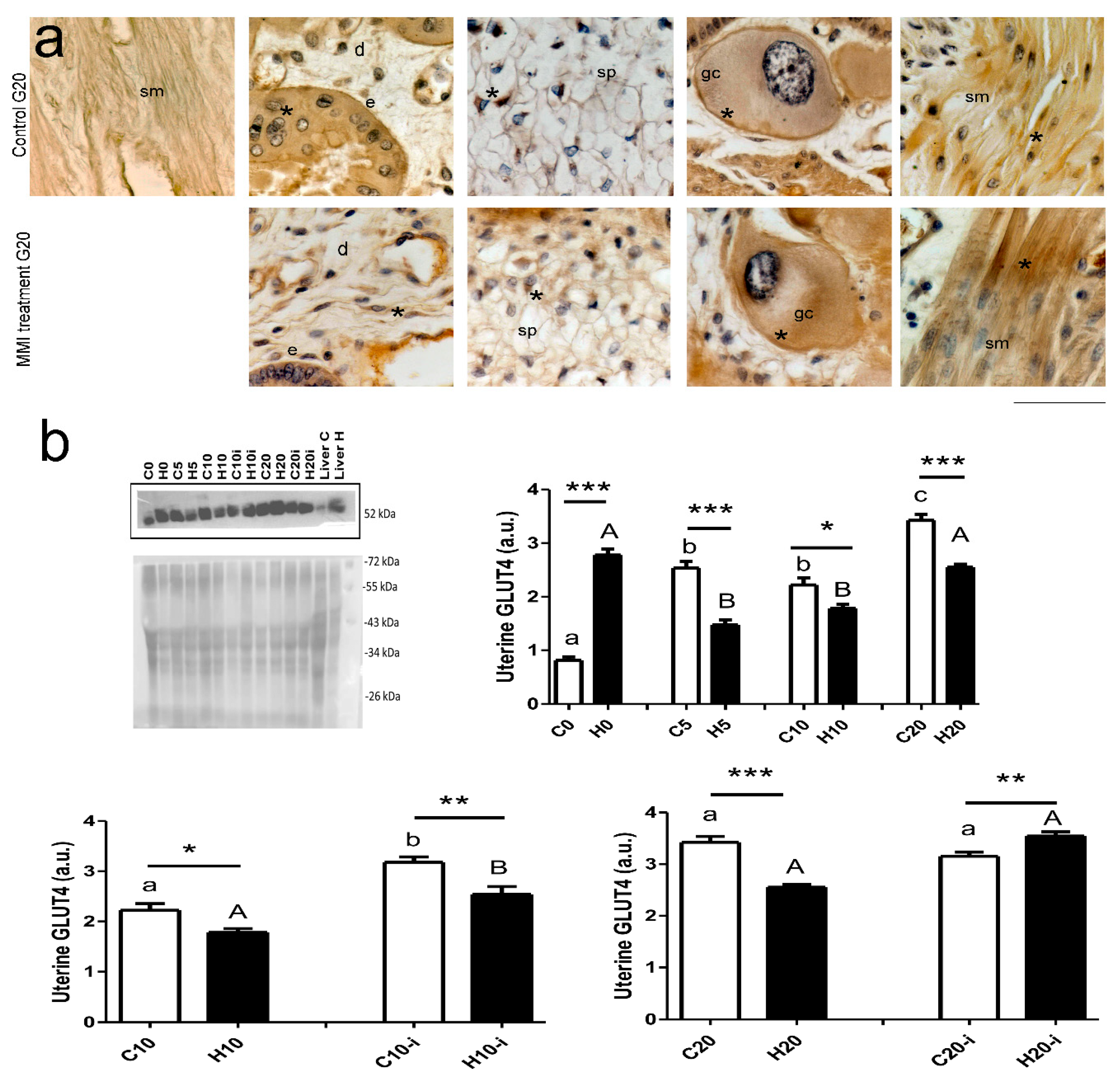
2.3. Expression of GLUT4, LDH, and Aromatase
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodríguez-Castelán, J.; Del Moral-Morales, A.; Piña-Medina, A.G.; Zepeda-Pérez, D.; Castillo-Romano, M.; Méndez-Tepepa, M.; Espindola-Lozano, M.; Camacho-Arroyo, I.; Cuevas-Romero, E. Hypothyroidism Induces Uterine Hyperplasia and Inflammation Related to Sex Hormone Receptors Expression in Virgin Rabbits. Life Sci. 2019, 230, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.D.L.; Obeng-Gyasi, B.; Hall, J.E.; Shekhar, S. The Thyroid Hormone Axis and Female Reproduction. Int. J. Mol. Sci. 2023, 24, 9815. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Castelán, J.; Zepeda-Pérez, D.; Méndez-Tepepa, M.; Castillo-Romano, M.; Espíndola-Lozano, M.; Anaya-Hernández, A.; Berbel, P.; Cuevas-Romero, E. Hypothyroidism Alters the Uterine Lipid Levels in Pregnant Rabbits and Affects the Fetal Size. Endocr. Metab. Immune Disord. Drug Targets 2018, 19, 818–825. [Google Scholar] [CrossRef]
- Andersen, S.L.; Olsen, J.; Wu, C.S.; Laurberg, P. Low Birth Weight in Children Born to Mothers with Hyperthyroidism and High Birth Weight in Hypothyroidism, Whereas Preterm Birth Is Common in Both Conditions: A Danish National Hospital Register Study. Eur. Thyroid J. 2013, 2, 135–144. [Google Scholar] [CrossRef]
- Pande, A.; Anjankar, A. A Narrative Review on the Effect of Maternal Hypothyroidism on Fetal Development. Cureus 2023, 15, e34824. [Google Scholar] [CrossRef]
- Serakides, R.; Silva, J.F.; Vidigal, P.N.; Galvo, D.D.; Boeloni, J.N.; Nunes, P.P.; Ocarino, N.M.; Nascimento, E.F. Fetal Growth Restriction in Hypothyroidism Is Associated with Changes in Proliferative Activity, Apoptosis and Vascularisation of the Placenta. Reprod. Fertil. Dev. 2012, 24, 923–931. [Google Scholar] [CrossRef]
- Colicchia, M.; Campagnolo, L.; Baldini, E.; Ulisse, S.; Valensise, H.; Moretti, C. Molecular Basis of Thyrotropin and Thyroid Hormone Action during Implantation and Early Development. Hum. Reprod. Update 2014, 20, 884–904. [Google Scholar] [CrossRef]
- Brison, D.R.; Leese, H.J. Energy Metabolism in Late Preimplantation Rat Embryos. J. Reprod. Fertil. 1991, 93, 245–251. [Google Scholar] [CrossRef]
- Chen, Z.; Sandoval, K.; Dean, M. Endometrial Glycogen Metabolism during Early Pregnancy in Mice. Mol. Reprod. Dev. 2022, 89, 431–440. [Google Scholar] [CrossRef]
- Vasilenko, P.; Adams, W.C.; Frieden, E.H. Uterine Size and Glycogen Content in Cycling and Pregnant Rats: Influence of Relaxin. Biol. Reprod. 1981, 25, 162–169. [Google Scholar] [CrossRef]
- Zuo, R.J.; Gu, X.W.; Qi, Q.R.; Wang, T.S.; Zhao, X.Y.; Liu, J.L.; Yang, Z.M. Warburg-like Glycolysis and Lactate Shuttle in Mouse Decidua during Early Pregnancy. J. Biol. Chem. 2015, 290, 21280–21291. [Google Scholar] [CrossRef] [PubMed]
- Vrhovac Madunić, I.; Karin-Kujundžić, V.; Madunić, J.; Šola, I.M.; Šerman, L. Endometrial Glucose Transporters in Health and Disease. Front. Cell Dev. Biol. 2021, 9, 703671. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Wang, Y.C.; Yuan, D.Z.; Dai, X.H.; Liao, L.C.; Zhang, X.Q.; Zhang, L.X.; Ma, Y.D.; Lei, Y.; Cui, Z.H.; et al. GLUT4 in Mouse Endometrial Epithelium: Roles in Embryonic Development and Implantation. Front. Physiol. 2021, 12, 674924. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.K. Lactate Production by the Mammalian Blastocyst: Manipulating the Microenvironment for Uterine Implantation and Invasion? Bioessays 2015, 37, 364–371. [Google Scholar] [CrossRef]
- Gurner, K.H.; Evans, J.; Hutchison, J.C.; Harvey, A.J.; Gardner, D.K. A Microenvironment of High Lactate and Low PH Created by the Blastocyst Promotes Endometrial Receptivity and Implantation. Reprod. Biomed. Online 2022, 44, 14–26. [Google Scholar] [CrossRef]
- Madaan, A.; Nadeau-Vallée, M.; Rivera, J.C.; Obari, D.; Hou, X.; Sierra, E.M.; Girard, S.; Olson, D.M.; Chemtob, S. Lactate Produced during Labor Modulates Uterine Inflammation via GPR81 (HCA1). Am. J. Obstet. Gynecol. 2017, 216, 60.e1–60.e17. [Google Scholar] [CrossRef]
- Burton, G.J.; Scioscia, M.; Rademacher, T.W. Endometrial Secretions: Creating a Stimulatory Microenvironment within the Human Early Placenta and Implications for the Aetiopathogenesis of Preeclampsia. J. Reprod. Immunol. 2011, 89, 118–125. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Ali-Hassanzadeh, M.; Nadimi, E.; Karbalay-Doust, S.; Noorafshan, A.; Gharesi-Fard, B. Stereological Study of the Placental Structure in Abortion-Prone Mice Model (CBA/J×DBA/2J). Ann. Anat. 2020, 230, 151508. [Google Scholar] [CrossRef]
- Santos, B.R.; dos Anjos Cordeiro, J.M.; Santos, L.C.; Barbosa, E.M.; Mendonça, L.D.; Santos, E.O.; de Macedo, I.O.; de Lavor, M.S.L.; Szawka, R.E.; Serakides, R.; et al. Kisspeptin Treatment Improves Fetal-Placental Development and Blocks Placental Oxidative Damage Caused by Maternal Hypothyroidism in an Experimental Rat Model. Front. Endocrinol. 2022, 13, 908240. [Google Scholar] [CrossRef]
- Bowman, K.; Rose, J. Estradiol Stimulates Glycogen Synthesis Whereas Progesterone Promotes Glycogen Catabolism in the Uterus of the American Mink (Neovison Vison). Anim. Sci. J. 2017, 88, 45–54. [Google Scholar] [CrossRef]
- Hodonu, A.; Escobar, M.; Beach, L.; Hunt, J.; Rose, J. Glycogen Metabolism in Mink Uterine Epithelial Cells and Its Regulation by Estradiol, Progesterone and Insulin. Theriogenology 2019, 130, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Steinsapir, J.; Rojas, A.M.; Mena, M.; Tchernitchin, A.N. Effects of Thyroid Hormone on some Uterine Responses to Estrogen. Endocrinology 1982, 110, 1773–1779. [Google Scholar] [CrossRef] [PubMed]
- Gardners, R.M.; Kirkland, J.L.; Ireland, J.S.; Stancel, G.M. Regulation of the Uterine Response to Estrogen by Thyroid Hormone. Endocrinology 1978, 103, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk-Zieba, I.; Staszkiewicz-Chodor, J.; Boruszewska, D.; Lukaszuk, K.; Jaworska, J.; Woclawek-Potocka, I. Hypothyroidism Affects Uterine Function via the Modulation of Prostaglandin Signaling. Animals 2021, 11, 2636. [Google Scholar] [CrossRef] [PubMed]
- Erbaş, E.; Gedikli, S. Investigation of the Endometrial Receptivity Status in Experimental Hypothyroid-Induced Female Rats. Iran. J. Basic Med. Sci. 2022, 25, 1077–1083. [Google Scholar] [CrossRef]
- Fischer, B.; Chavatte-Palmer, P.; Viebahn, C.; Santos, A.N.; Duranthon, V. Rabbit as a Reproductive Model for Human Health. Reproduction 2012, 144, 1. [Google Scholar] [CrossRef]
- Krusche, C.A.; Vloet, T.D.; Herrler, A.; Black, S.; Beier, H.M. Functional and Structural Regression of the Rabbit Corpus Luteum Is Associated with Altered Luteal Immune Cell Phenotypes and Cytokine Expression Patterns. Histochem. Cell Biol. 2002, 118, 479–489. [Google Scholar] [CrossRef]
- Chavatte-Palmer, P.; Tarrade, A.; Rousseau-Ralliard, D. Diet before and during Pregnancy and Offspring Health: The Importance of Animal Models and What Can Be Learned from Them. Int. J. Environ. Res. Public Health 2016, 13, E586. [Google Scholar] [CrossRef]
- Anaya-Hernández, A.; Rodríguez-Castelán, J.; Nicolás, L.; Martínez-Gómez, M.; Jiménez-Estrada, I.; Castelán, F.; Cuevas, E. Hypothyroidism Affects Differentially the Cell Size of Epithelial Cells among Oviductal Regions of Rabbits. Reprod. Domest. Anim. 2015, 50, 104–111. [Google Scholar] [CrossRef]
- Yap, Y.W.; Onyekwelu, E.; Alam, U. Thyroid Disease in Pregnancy. Clin. Med. 2023, 23, 125–128. [Google Scholar] [CrossRef]
- Kent, N.L.; Atluri, S.C.; Cuffe, J.S.M. Maternal Hypothyroidism in Rats Reduces Placental Lactogen, Lowers Insulin Levels, and Causes Glucose Intolerance. Endocrinology 2022, 163, bqab231. [Google Scholar] [CrossRef] [PubMed]
- Tsou, M.T. Subclinical Hypothyroidism Represents Visceral Adipose Indices, Especially in Women With Cardiovascular Risk. J. Endocr. Soc. 2021, 5, bvab028. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Castelán, J.; Nicolás, L.; Morimoto, S.; Cuevas, E. The Langerhans Islet Cells of Female Rabbits Are Differentially Affected by Hypothyroidism Depending on the Islet Size. Endocrine 2015, 48, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Pickard, M.R.; Leonard, A.J.; Ogilvie, L.M.; Edwards, P.R.; Evans, I.M.; Sinha, A.K.; Ekins, R.P. Maternal Hypothyroidism in the Rat Influences Placental and Liver Glycogen Stores: Fetal Growth Retardation near Term Is Unrelated to Maternal and Placental Glucose Metabolic Compromise. J. Endocrinol. 2003, 176, 247–255. [Google Scholar] [CrossRef]
- Korgun, E.T.; Demir, R.; Hammer, A.; Dohr, G.; Desoye, G.; Skofitsch, G.; Hahn, T. Glucose Transporter Expression in Rat Embryo and Uterus during Decidualization, Implantation, and Early Postimplantation. Biol. Reprod. 2001, 65, 1364–1370. [Google Scholar] [CrossRef]
- Cui, P.; Li, X.; Wang, X.; Feng, Y.; Lin, J.F.; Billig, H.; Shao, R. Lack of Cyclical Fluctuations of Endometrial GLUT4 Expression in Women with Polycystic Ovary Syndrome: Evidence for Direct Regulation of GLUT4 by Steroid Hormones. BBA Clin. 2015, 4, 85–91. [Google Scholar] [CrossRef]
- Bukulmez, O.; Hardy, D.B.; Carr, B.R.; Word, R.A.; Mendelson, C.R. Inflammatory Status Influences Aromatase and Steroid Receptor Expression in Endometriosis. Endocrinology 2008, 149, 1190–1204. [Google Scholar] [CrossRef]
- Knapp, P.; Chabowski, A.; Błachnio-Zabielska, A.; Walentowicz-Sadłecka, M.; Grabiec, M.; Knapp, P.A. Expression of Estrogen Receptors (α, β), Cyclooxygenase-2 and Aromatase in Normal Endometrium and Endometrioid Cancer of Uterus. Adv. Med. Sci. 2013, 58, 96–103. [Google Scholar] [CrossRef]
- Ha, L.X.; Wu, Y.Y.; Yin, T.; Yuan, Y.Y.; Du, Y.D. Effect of TNF-Alpha on Endometrial Glucose Transporter-4 Expression in Patients with Polycystic Ovary Syndrome through Nuclear Factor-Kappa B Signaling Pathway Activation. J. Physiol. Pharmacol. 2021, 72, 965–973. [Google Scholar] [CrossRef]
- Cuevas, E.; Ausó, E.; Telefont, M.; Morreale De Escobar, G.; Sotelo, C.; Berbel, P. Transient Maternal Hypothyroxinemia at Onset of Corticogenesis Alters Tangential Migration of Medial Ganglionic Eminence-Derived Neurons. Eur. J. Neurosci. 2005, 22, 541–551. [Google Scholar] [CrossRef]

| Control n = 6 | MMI-Treated n = 6 | Statistic | |
|---|---|---|---|
| Both uterine horns | |||
| Range of implants (min–max) | 6–10 | 9–12 | |
| Number of fetuses per dam | 8.2 ± 0.7 | 9.8 ± 0.5 | t = 1.78; p = 0.09 |
| Number of fetal resorptions per dam | 0.3 ± 0.3 | 0.2 ± 0.2 | U = 17.5; p = 1.0 |
| Body weight of fetuses (g) | 4.0 ± 0.3 | 4.4 ± 0.2 | t = 1.02; p = 0.33 |
| Abdominal diameter of fetuses (mm) | 13.7 ± 0.5 | 14.1 ± 0.1 | t = 0.76; p = 0.46 |
| Body length of fetuses (mm) | 39.2 ± 1.9 | 42.0 ± 0.8 | t = 1.31; p = 0.21 |
| 48 fetuses | 59 fetuses | ||
| % of slim fetuses (<3.4 g) | 10.7 ± 7.5 | 5.0 ± 3.6 | U = 17.0; p = 0.92 |
| % of medium fetuses (3.4–4.5 g) | 62.8 ± 14.9 | 57.9 ± 11.6 | t = 0.25; p = 0.80 |
| % of heavy fetuses (>4.6 g) | 26.3 ± 16.7 | 36.9 ± 13.8 | U = 14.0; p = 0.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espindola-Lozano, M.; Méndez-Tepepa, M.; Castillo-Romano, M.; Rojas-Juárez, R.; Nicolás-Toledo, L.; Rodríguez-Antolín, J.; Castelán, F.; Cuevas-Romero, E. Methimazole-Induced Hypothyroidism Increases the Content of Glycogen and Changes the Expression of LDH, GLUT4, and Aromatase in the Pregnant Uterus of Rabbits. Metabolites 2025, 15, 82. https://doi.org/10.3390/metabo15020082
Espindola-Lozano M, Méndez-Tepepa M, Castillo-Romano M, Rojas-Juárez R, Nicolás-Toledo L, Rodríguez-Antolín J, Castelán F, Cuevas-Romero E. Methimazole-Induced Hypothyroidism Increases the Content of Glycogen and Changes the Expression of LDH, GLUT4, and Aromatase in the Pregnant Uterus of Rabbits. Metabolites. 2025; 15(2):82. https://doi.org/10.3390/metabo15020082
Chicago/Turabian StyleEspindola-Lozano, Marlen, Maribel Méndez-Tepepa, Marlenne Castillo-Romano, Rubicela Rojas-Juárez, Leticia Nicolás-Toledo, Jorge Rodríguez-Antolín, Francisco Castelán, and Estela Cuevas-Romero. 2025. "Methimazole-Induced Hypothyroidism Increases the Content of Glycogen and Changes the Expression of LDH, GLUT4, and Aromatase in the Pregnant Uterus of Rabbits" Metabolites 15, no. 2: 82. https://doi.org/10.3390/metabo15020082
APA StyleEspindola-Lozano, M., Méndez-Tepepa, M., Castillo-Romano, M., Rojas-Juárez, R., Nicolás-Toledo, L., Rodríguez-Antolín, J., Castelán, F., & Cuevas-Romero, E. (2025). Methimazole-Induced Hypothyroidism Increases the Content of Glycogen and Changes the Expression of LDH, GLUT4, and Aromatase in the Pregnant Uterus of Rabbits. Metabolites, 15(2), 82. https://doi.org/10.3390/metabo15020082






