Structural Analysis of Cardanol and Its Biological Activities on Human Keratinocyte Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Materials
2.2. NMR Spectroscopy Analysis of Cardanol
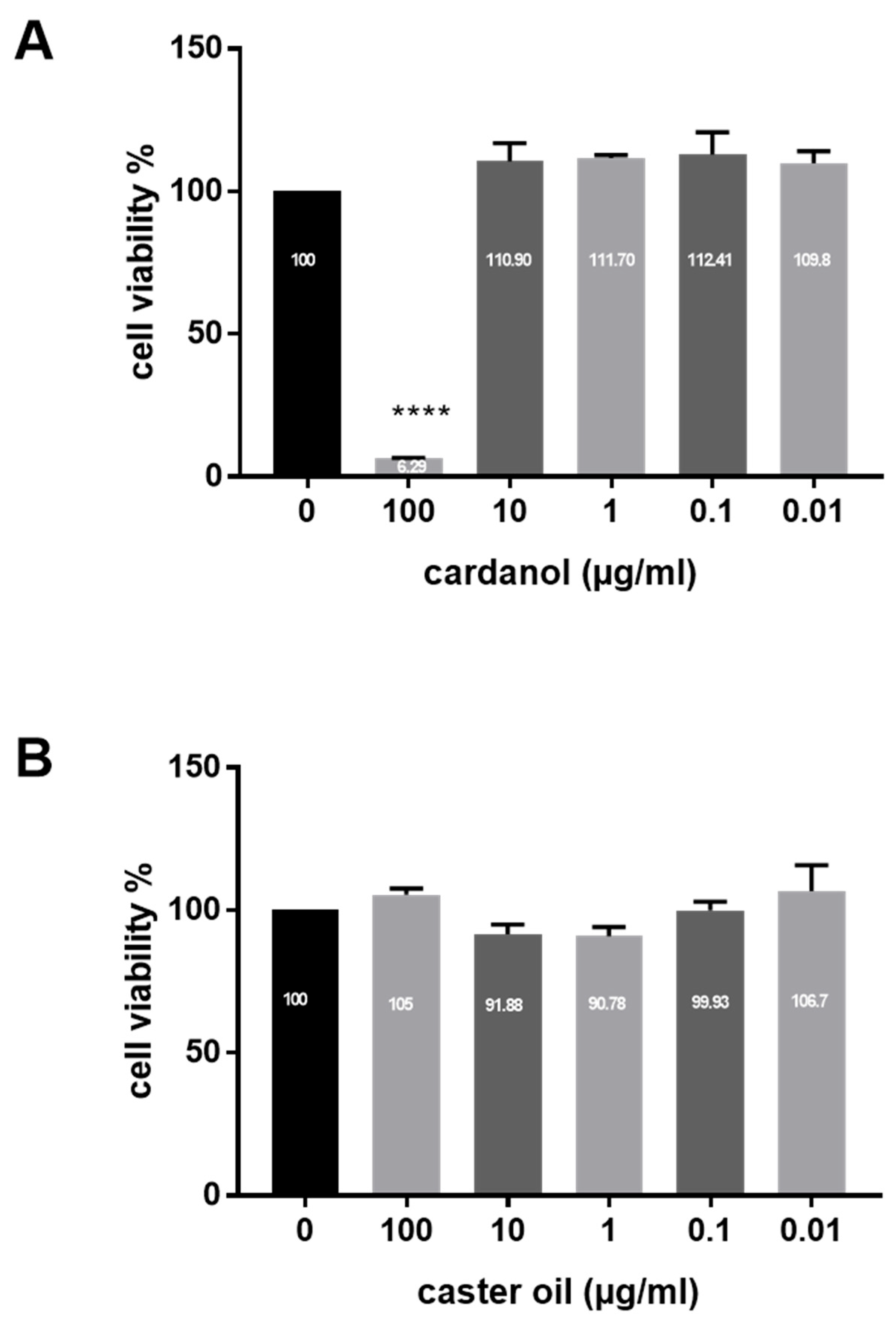
2.3. Cytotoxicity Assay
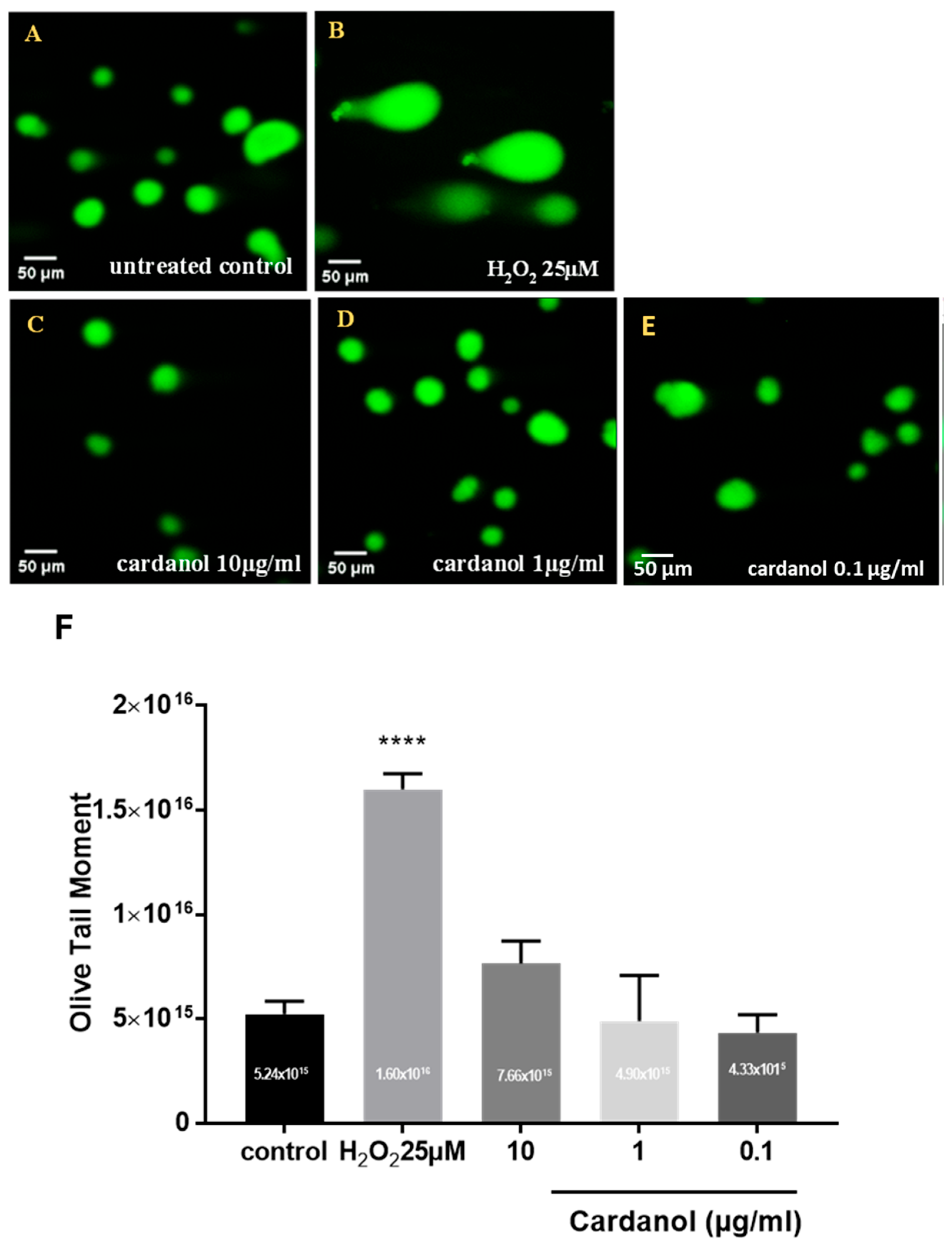
2.4. Genotoxicity Assay
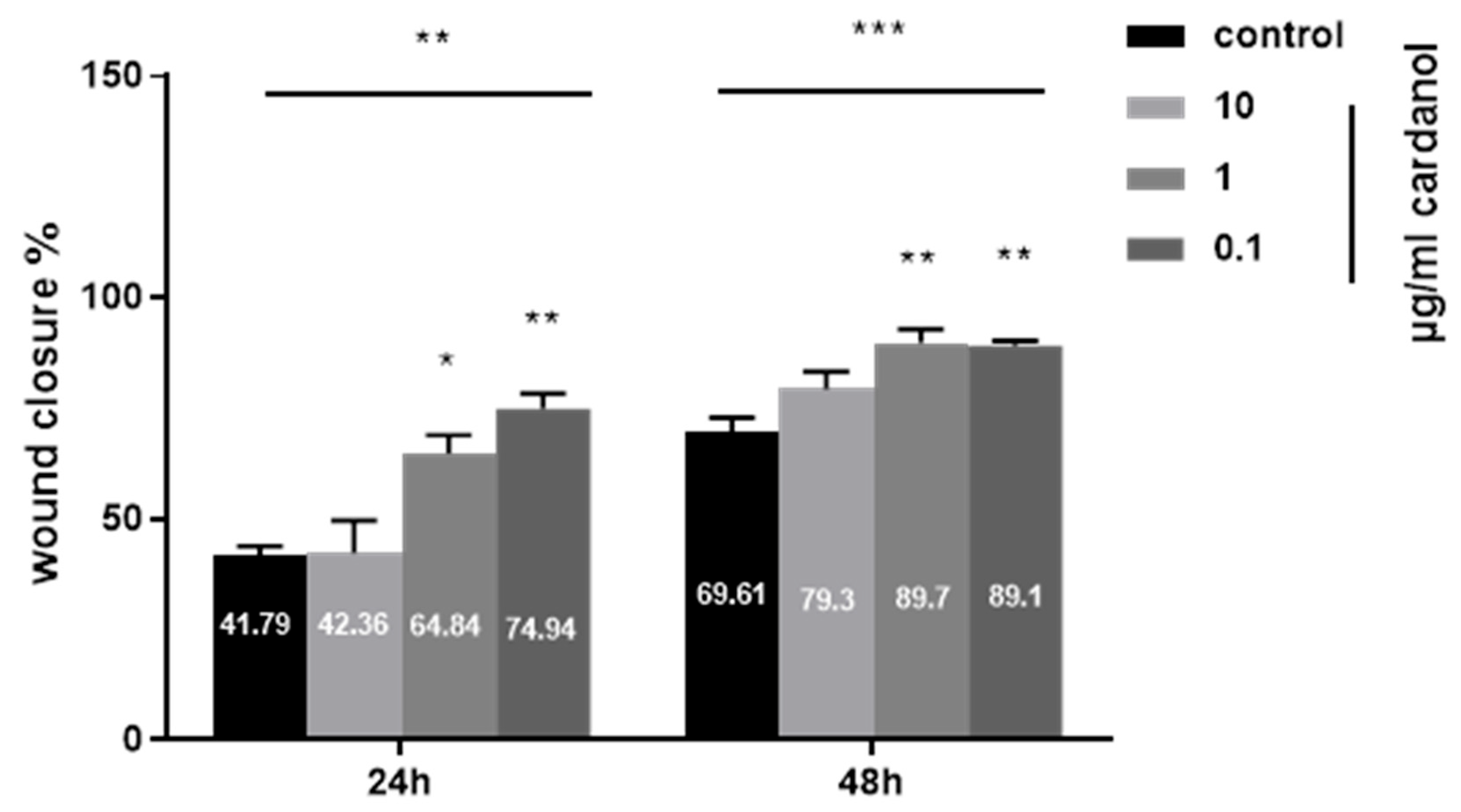
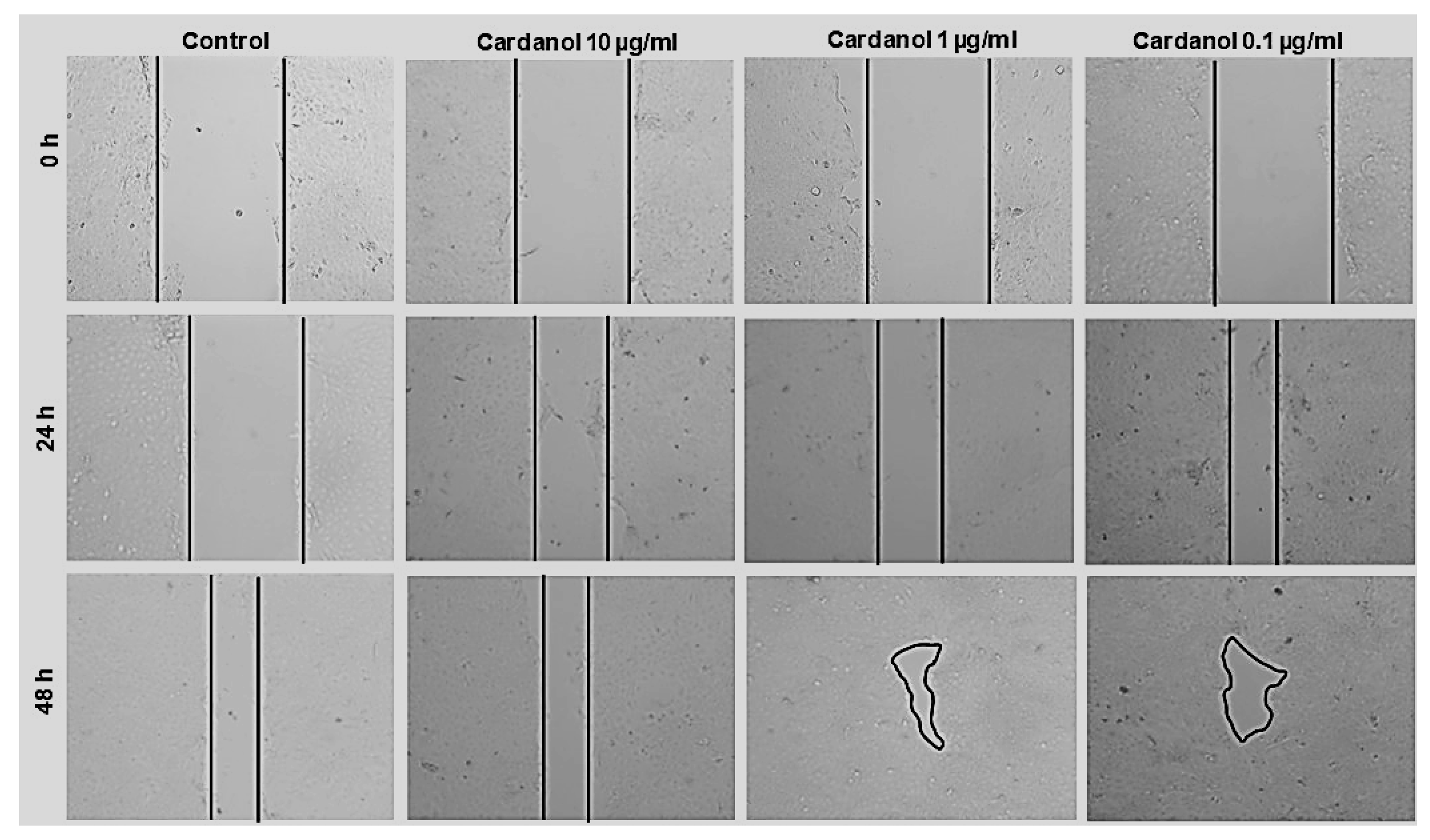
2.5. Wound-Healing Assay
2.6. Proliferation Assay
2.7. Fatty Acid Profiles of HaCaT Cells
2.8. Statistical Analysis
3. Results
3.1. NMR Analysis
3.2. Cytotoxicity of Cardanol on HaCaT Keratinocytes
3.3. Genotoxicity (Comet Assay)
3.4. Wound Healing Properties of Cardanol
3.5. Proliferative Effect of Cardanol on HaCaT Cells
3.6. Modulatory Effect of Cardanol on the Cellular Fatty Acid Profile
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuhn, S.; Colreavy-Donnelly, S.; Santana De Souza, J.; Borges, R.M. An Integrated Approach for Mixture Analysis Using MS and NMR Techniques. Faraday Discuss. 2019, 218, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Z.; Ma, Y.; Liu, Y.; Lin, C.-C.; Li, S.; Zhan, J.; Ho, C.-T. Immunomodulatory Effects of Green Tea Polyphenols. Molecules 2021, 26, 3755. [Google Scholar] [CrossRef] [PubMed]
- Anis, A.; Sharshar, A.; El Hanbally, S.; Shehata, A.A. Histopathological Evaluation of the Healing Process of Standardized Skin Burns in Rabbits: Assessment of a Natural Product with Honey and Essential Oils. J. Clin. Med. 2022, 11, 6417. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-K.; Zhong, L.; Santiago, J.L. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef]
- Tyman, J.H.P. Synthetic and Natural Phenols; Studies in organic chemistry; Elsevier: Amsterdam, The Netherlands, 1996; ISBN 978-0-444-88164-9. [Google Scholar]
- Phani Kumar, P.; Paramashivappa, R.; Vithayathil, P.J.; Subba Rao, P.V.; Srinivasa Rao, A. Process for Isolation of Cardanol from Technical Cashew (Anacardium Occidentale L.) Nut Shell Liquid. J. Agric. Food Chem. 2002, 50, 4705–4708. [Google Scholar] [CrossRef]
- Patel, R.N.; Bandyopadhyay, S.; Ganesh, A. Extraction of Cashew (Anacardium Occidentale) Nut Shell Liquid Using Supercritical Carbon Dioxide. Bioresour. Technol. 2006, 97, 847–853. [Google Scholar] [CrossRef]
- Rodrigues, F.H.A.; França, F.C.F.; Souza, J.R.R.; Ricardo, N.M.P.S.; Feitosa, J.P.A. Comparison between Physico-Chemical Properties of the Technical Cashew Nut Shell Liquid (CNSL) and Those Natural Extracted from Solvent and Pressing. Polímeros 2011, 21, 156–160. [Google Scholar] [CrossRef]
- Senthil Kumar, P.; Arun Kumar, N.; Sivakumar, R.; Kaushik, C. Experimentation on Solvent Extraction of Polyphenols from Natural Waste. J. Mater. Sci. 2009, 44, 5894–5899. [Google Scholar] [CrossRef]
- Acero, L.H. CASHEW (Anacardium Occidentale) Nutshell Ethanol Extract in the Control of Cokroach (Periplaneta Americana). Sci. Int. 2018, 30, 427–429. [Google Scholar]
- Kubo, I.; Muroi, H.; Himejima, M.; Yamagiwa, Y.; Mera, H.; Tokushima, K.; Ohta, S.; Kamikawa, T. Structure-Antibacterial Activity Relationships of Anacardic Acids. J. Agric. Food Chem. 1993, 41, 1016–1019. [Google Scholar] [CrossRef]
- Trevisan, M.T.S.; Pfundstein, B.; Haubner, R.; Würtele, G.; Spiegelhalder, B.; Bartsch, H.; Owen, R.W. Characterization of Alkyl Phenols in Cashew (Anacardium Occidentale) Products and Assay of Their Antioxidant Capacity. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2006, 44, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.S.C.; Morais, S.M.d.; Magalhães, D.V.; Batista, W.P.; Vieira, I.G.P.; Craveiro, A.A.; de Manezes, J.E.S.A.; Carvalho, A.F.U.; de Lima, G.P.G. Antioxidant, Larvicidal and Antiacetylcholinesterase Activities of Cashew Nut Shell Liquid Constituents. Acta Trop. 2011, 117, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Muroi, H.; Kubo, I. Bactericidal Activity of Anacardic Acids against Streptococcus Mutans and Their Potentiation. J. Agric. Food Chem. 1993, 41, 1780–1783. [Google Scholar] [CrossRef]
- Wu, Y.; He, L.; Zhang, L.; Chen, J.; Yi, Z.; Zhang, J.; Liu, M.; Pang, X. Anacardic Acid (6-Pentadecylsalicylic Acid) Inhibits Tumor Angiogenesis by Targeting Src/FAK/Rho GTPases Signaling Pathway. J. Pharmacol. Exp. Ther. 2011, 339, 403–411. [Google Scholar] [CrossRef]
- López, C.A.A.; Lima, K.R.S.; Manno, M.C.; Tavares, F.B.; Fernandes Neto, D.L.; Jesus, M.L.C.; Viana, M.A.O.; Fonseca, L.A.B. Effects of Cashew Nut Shell Liquid (CNSL) on the Performance of Broiler Chickens. Arq. Bras. Med. Veterinária E Zootec. 2012, 64, 1027–1035. [Google Scholar] [CrossRef]
- Watanabe, Y.; Suzuki, R.; Koike, S.; Nagashima, K.; Mochizuki, M.; Forster, R.J.; Kobayashi, Y. In Vitro Evaluation of Cashew Nut Shell Liquid as a Methane-Inhibiting and Propionate-Enhancing Agent for Ruminants. J. Dairy Sci. 2010, 93, 5258–5267. [Google Scholar] [CrossRef]
- Shinkai, T.; Enishi, O.; Mitsumori, M.; Higuchi, K.; Kobayashi, Y.; Takenaka, A.; Nagashima, K.; Mochizuki, M.; Kobayashi, Y. Mitigation of Methane Production from Cattle by Feeding Cashew Nut Shell Liquid. J. Dairy Sci. 2012, 95, 5308–5316. [Google Scholar] [CrossRef]
- Hosokawa, M.; Hikita, C.; Suzuki, Y.; Koike, S.; Kobayashi, Y. Effects of Cashew Nut Shell Liquid on Hindgut Fermentation and Microbiota of Chickens. J. Anim. Sci. Res. 2023, 7. [Google Scholar] [CrossRef]
- Vendrame, S.; Alaba, T.; Marchi, N.; Tsakiroglou, P.; Klimis-Zacas, D. In Vitro and In Vivo Evaluation of Bioactive Compounds from Berries for Wound Healing. Curr. Dev. Nutr. 2024, 2024, 102078. [Google Scholar] [CrossRef]
- Andonaba, J.-B.; Lompo, S.S.; Ouédraogo, V.; Ouédraogo, F.; Ouédraogo, M.S.; Konaté, I.; Diallo, B.; Traoré, A. Skin Damage and Aesthetic Disadvantage Observed in Women in the Hand Craft Shelling Chain of Cashew Nuts in a Factory to Bobo-Dioulasso, Burkina Faso. J. Cosmet. Dermatol. Sci. Appl. 2017, 7, 211–220. [Google Scholar] [CrossRef][Green Version]
- Kumaravel, T.S.; Vilhar, B.; Faux, S.P.; Jha, A.N. Comet Assay Measurements: A Perspective. Cell Biol. Toxicol. 2009, 25, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Buranasukhon, W.; Athikomkulchai, S.; Tadtong, S.; Chittasupho, C. Wound Healing Activity of Pluchea Indica Leaf Extract in Oral Mucosal Cell Line and Oral Spray Formulation Containing Nanoparticles of the Extract. Pharm. Biol. 2017, 55, 1767–1774. [Google Scholar] [CrossRef] [PubMed]
- Iwami, D.; Nonomura, K.; Shirasugi, N.; Niimi, M. Immunomodulatory Effects of Eicosapentaenoic Acid through Induction of Regulatory T Cells. Int. Immunopharmacol. 2011, 11, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Green, I.R.; Tocoli, F.E.; Lee, S.H.; Nihei, K.-I.; Kubo, I. Molecular Design of Anti-MRSA Agents Based on the Anacardic Acid Scaffold. Bioorg. Med. Chem. 2007, 15, 6236–6241. [Google Scholar] [CrossRef]
- Green, I.R.; Tocoli, F.E.; Lee, S.H.; Nihei, K.; Kubo, I. Design and Evaluation of Anacardic Acid Derivatives as Anticavity Agents. Eur. J. Med. Chem. 2008, 43, 1315–1320. [Google Scholar] [CrossRef]
- Swamy, M.T.; Ashok, M.A.; Yathirajan, H.S.; Narayana, B.; Bolte, M. Desipraminium Picrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2007, 63, o4919. [Google Scholar] [CrossRef]
- Suo, M.; Isao, H.; Ishida, Y.; Shimano, Y.; Bi, C.; Kato, H.; Takano, F.; Ohta, T. Phenolic Lipid Ingredients from Cashew Nuts. J. Nat. Med. 2012, 66, 133–139. [Google Scholar] [CrossRef]
- Morais, S.; Silva, K.; Araujo, H.; Vieira, I.; Alves, D.; Fontenelle, R.; Silva, A. Anacardic Acid Constituents from Cashew Nut Shell Liquid: NMR Characterization and the Effect of Unsaturation on Its Biological Activities. Pharmaceuticals 2017, 10, 31. [Google Scholar] [CrossRef]
- Kumar, P.; Gowda, B.; Deepu, V.; Mantelingu, K.; Rangappa, S. Development and Validation of a Normal Phase HPLC Method for Separation of Anacardic Acid Isomers in Cashew Nut Shell Liquid. J. Chem. Pharm. Res. 2013, 5, 369–373. [Google Scholar]
- De Souza, M.Q.; Teotônio, I.M.S.N.; De Almeida, F.C.; Heyn, G.S.; Alves, P.S.; Romeiro, L.A.S.; Pratesi, R.; De Medeiros Nóbrega, Y.K.; Pratesi, C.B. Molecular Evaluation of Anti-Inflammatory Activity of Phenolic Lipid Extracted from Cashew Nut Shell Liquid (CNSL). BMC Complement. Altern. Med. 2018, 18, 181. [Google Scholar] [CrossRef]
- Souza, N.d.O.; Cunha, D.A.; Rodrigues, N.d.S.; Pereira, A.L.; Medeiros, E.J.T.; Pinheiro, A.d.A.; de Vasconcelos, M.A.; do Nascimento Neto, L.G.; Bezerra, T.T.; Mazzetto, S.E.; et al. Cashew Nut Shell Liquids: Antimicrobial Compounds in Prevention and Control of the Oral Biofilms. Arch. Oral Biol. 2022, 133, 105299. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Hua, X.; Liu, N.; Li, X.; Liu, S.; Chen, X.; Zhao, C.; Lan, X.; Yang, C.; Dou, Q.P.; et al. Anacardic Acid Induces Cell Apoptosis Associated with Induction of ATF4-Dependent Endoplasmic Reticulum Stress. Toxicol. Lett. 2014, 228, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Attanasi, O.A.; Favi, G.; Menichetti, S.; Pedulli, G.F.; Viglianisi, C. Amphiphilic Antioxidants from “Cashew Nut Shell Liquid” (CNSL) Waste. Org. Biomol. Chem. 2011, 9, 1352. [Google Scholar] [CrossRef] [PubMed]
- Andrade, T.d.J.A.d.S.; Araújo, B.Q.; Citó, A.M.d.G.L.; da Silva, J.; Saffi, J.; Richter, M.F.; Ferraz, A..d.B.F. Antioxidant Properties and Chemical Composition of Technical Cashew Nut Shell Liquid (tCNSL). Food Chem. 2011, 126, 1044–1048. [Google Scholar] [CrossRef]
- Silva, R.B.D.; Araújo, J.L.; Passos, I.N.G.; Lima, J.R.D.O.; Souza, J.S.N.D.; Caldas, N.M.; Figueiredo, F.C.; Santos Junior, J.R.D. Síntese Antioxidante à Base de Cashel Nut Shell Liquid (CNSL) Através Da Eletrólise de Hidroquinona. Res. Soc. Dev. 2022, 11, e49111528636. [Google Scholar] [CrossRef]
- Hollands, A.; Corriden, R.; Gysler, G.; Dahesh, S.; Olson, J.; Raza Ali, S.; Kunkel, M.T.; Lin, A.E.; Forli, S.; Newton, A.C.; et al. Natural Product Anacardic Acid from Cashew Nut Shells Stimulates Neutrophil Extracellular Trap Production and Bactericidal Activity. J. Biol. Chem. 2016, 291, 13964–13973. [Google Scholar] [CrossRef]
- M Ashraf, S.; Rathinasamy, K. Antibacterial and Anticancer Activity of the Purified Cashew Nut Shell Liquid: Implications in Cancer Chemotherapy and Wound Healing. Nat. Prod. Res. 2018, 32, 2856–2860. [Google Scholar] [CrossRef]
- Xie, H.; Mason, M.M.; Wise, J.P. Genotoxicity of Metal Nanoparticles. Rev. Environ. Health 2011, 26, 251–268. [Google Scholar] [CrossRef]
- Shah, S.U. Importance of Genotoxicity & S2A Guidelines for Genotoxicity Testing for Pharmaceuticals. IOSR J. Pharm. Biol. Sci. 2012, 1, 43–54. [Google Scholar] [CrossRef]
- Jena, G.; Kaul, C.; Poduri, R. Genotoxicity Testing, a Regulatory Requirement for Drug Discovery and Development: Impact of ICH Guidelines. Indian J. Pharmacol. 2002, 34, 86–99. [Google Scholar]
- Snyder, R.D.; Green, J.W. A Review of the Genotoxicity of Marketed Pharmaceuticals. Mutat. Res. 2001, 488, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Brendler-Schwaab, S.; Hartmann, A.; Pfuhler, S.; Speit, G. The in Vivo Comet Assay: Use and Status in Genotoxicity Testing. Mutagenesis 2005, 20, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Speit, G.; Henderson, L. Review of the in Vivo Genotoxicity Tests Performed with Styrene. Mutat. Res. 2005, 589, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Bolt, H.M.; Stewart, J.D.; Hengstler, J.G. A Comprehensive Review about Micronuclei: Mechanisms of Formation and Practical Aspects in Genotoxicity Testing. Arch. Toxicol. 2011, 85, 861–862. [Google Scholar] [CrossRef]
- Mohamed, S.; Sabita, U.; Rajendra, S.; Dang, R. Genotoxicity: Mechanisms, Testing Guidelines and Methods. Glob. J. Pharm. Pharm. Sci. 2017, 1, 1–6. [Google Scholar]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef]
- MacLeod, A.S.; Mansbridge, J.N. The Innate Immune System in Acute and Chronic Wounds. Adv. Wound Care 2016, 5, 65–78. [Google Scholar] [CrossRef]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from Inflammation to Proliferation: A Critical Step during Wound Healing. Cell. Mol. Life Sci. CMLS 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Santoro, M.M.; Gaudino, G. Cellular and Molecular Facets of Keratinocyte Reepithelization during Wound Healing. Exp. Cell Res. 2005, 304, 274–286. [Google Scholar] [CrossRef]
- Mariamenatu, A.H.; Abdu, E.M. Overconsumption of Omega-6 Polyunsaturated Fatty Acids (PUFAs) versus Deficiency of Omega-3 PUFAs in Modern-Day Diets: The Disturbing Factor for Their “Balanced Antagonistic Metabolic Functions” in the Human Body. J. Lipids 2021, 2021, 8848161. [Google Scholar] [CrossRef]
- Simpson, J.M.; McCracken, V.J.; Gaskins, H.R.; Mackie, R.I. Denaturing Gradient Gel Electrophoresis Analysis of 16S Ribosomal DNA Amplicons to Monitor Changes in Fecal Bacterial Populations of Weaning Pigs after Introduction of Lactobacillus Reuteri Strain MM53. Appl. Environ. Microbiol. 2000, 66, 4705–4714. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The Omega-6/Omega-3 Fatty Acid Ratio: Health Implications. Ol. Corps Gras Lipides 2010, 17, 267–275. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Omega-6 Fatty Acids and Inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Bosma, K.J.; Kaiser, C.E.; Kimple, M.E.; Gannon, M. Effects of Arachidonic Acid and Its Metabolites on Functional Beta-Cell Mass. Metabolites 2022, 12, 342. [Google Scholar] [CrossRef]
- Bäck, M. Leukotriene Signaling in Atherosclerosis and Ischemia. Cardiovasc. Drugs Ther. 2009, 23, 41–48. [Google Scholar] [CrossRef]
- Black, P.N.; Sharpe, S. Dietary Fat and Asthma: Is There a Connection? Eur. Respir. J. 1997, 10, 6–12. [Google Scholar] [CrossRef]
- Kelley, D.S.; Taylor, P.C.; Nelson, G.J.; Mackey, B.E. Arachidonic Acid Supplementation Enhances Synthesis of Eicosanoids without Suppressing Immune Functions in Young Healthy Men. Lipids 1998, 33, 125–130. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Importance of the Omega-6/Omega-3 Balance in Health and Disease: Evolutionary Aspects of Diet. World Rev. Nutr. Diet. 2011, 102, 10–21. [Google Scholar] [CrossRef]
- Artmann, A.; Petersen, G.; Hellgren, L.I.; Boberg, J.; Skonberg, C.; Nellemann, C.; Hansen, S.H.; Hansen, H.S. Influence of Dietary Fatty Acids on Endocannabinoid and N-Acylethanolamine Levels in Rat Brain, Liver and Small Intestine. Biochim. Biophys. Acta 2008, 1781, 200–212. [Google Scholar] [CrossRef]
- Calder, P.C. N-3 PUFA and Inflammation: From Membrane to Nucleus and from Bench to Bedside. Proc. Nutr. Soc. 2020, 79, 404–416. [Google Scholar] [CrossRef]
- Massaro, M.; Scoditti, E.; Carluccio, M.A.; De Caterina, R. Nutraceuticals and Prevention of Atherosclerosis: Focus on Ω-3 Polyunsaturated Fatty Acids and Mediterranean Diet Polyphenols. Cardiovasc. Ther. 2010, 28, e13–e19. [Google Scholar] [CrossRef] [PubMed]
- Limbu, R.; Cottrell, G.S.; McNeish, A.J. Characterisation of the Vasodilation Effects of DHA and EPA, n-3 PUFAs (Fish Oils), in Rat Aorta and Mesenteric Resistance Arteries. PLoS ONE 2018, 13, e0192484. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mastana, S.S.; Lindley, M.R. N-3 Fatty Acids and Asthma. Nutr. Res. Rev. 2016, 29, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Balić, A.; Vlašić, D.; Žužul, K.; Marinović, B.; Bukvić Mokos, Z. Omega-3 Versus Omega-6 Polyunsaturated Fatty Acids in the Prevention and Treatment of Inflammatory Skin Diseases. Int. J. Mol. Sci. 2020, 21, 741. [Google Scholar] [CrossRef]
- Sawada, Y.; Saito-Sasaki, N.; Nakamura, M. Omega 3 Fatty Acid and Skin Diseases. Front. Immunol. 2021, 11, 623052. [Google Scholar] [CrossRef]



| Fatty Acid | HaCaT Grown in Basic Medium 1 | HaCaT Grown in Medium with Cardanol 2 | p-Value |
|---|---|---|---|
| Palmitic acid | 194.00 ± 6.37 | 249.75 ± 8.43 ** | p < 0.001 |
| Oleic acid | 323.35 ± 11.06 | 420.44 ± 23.44 * | p < 0.01 |
| Linoleic acid | 19.82 ± 0.24 | 25.92 ± 1.17 ** | p < 0.001 |
| Arachidonic acid | 46.98 ± 0.14 | 60.04 ± 2.15 ** | p < 0.001 |
| Eicosapentaenoic acid | 3.12 ± 0.29 | 4.10 ± 0.70 | p < 0.01 |
| Docosahexaenoic acid | 23.53 ± 0.74 | 32.33 ± 1.92 * | p < 0.01 |
| Docosapenatenoic acid | 23.22 ± 0.67 | 30.24 ± 1.93 * | p < 0.01 |
| Total ω6 fatty acids | 89.57 ± 0.75 | 114.42 ± 5.13 ** | p < 0.001 |
| Total ω3 fatty acids | 51.65 ± 1.52 | 68.56 ± 4.49 * | p < 0.01 |
| Total ω9 fatty acids | 357.25 ± 12.40 | 463.23 ± 72.28 * | p < 0.01 |
| Total saturated fatty acids | 295.19 ± 11.07 | 382.22 ± 14.35 ** | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basiouni, S.; Abel, N.; Eisenreich, W.; May-Simera, H.L.; Shehata, A.A. Structural Analysis of Cardanol and Its Biological Activities on Human Keratinocyte Cells. Metabolites 2025, 15, 83. https://doi.org/10.3390/metabo15020083
Basiouni S, Abel N, Eisenreich W, May-Simera HL, Shehata AA. Structural Analysis of Cardanol and Its Biological Activities on Human Keratinocyte Cells. Metabolites. 2025; 15(2):83. https://doi.org/10.3390/metabo15020083
Chicago/Turabian StyleBasiouni, Shereen, Nina Abel, Wolfgang Eisenreich, Helen L. May-Simera, and Awad A. Shehata. 2025. "Structural Analysis of Cardanol and Its Biological Activities on Human Keratinocyte Cells" Metabolites 15, no. 2: 83. https://doi.org/10.3390/metabo15020083
APA StyleBasiouni, S., Abel, N., Eisenreich, W., May-Simera, H. L., & Shehata, A. A. (2025). Structural Analysis of Cardanol and Its Biological Activities on Human Keratinocyte Cells. Metabolites, 15(2), 83. https://doi.org/10.3390/metabo15020083








