Effects of Roasting Process on Sensory Qualities, Color, Physicochemical Components, and Identification of Key Aroma Compounds in Hubei Strip-Shaped Green Tea
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Tea Samples
2.2. Sensory Quality Assessment
2.3. Determination of HSSGT Color Quality
2.4. Physicochemical Quality Determination
2.5. Analysis of the Volatile Compounds by Gas Chromatography–Mass Spectrometry (GC-MS)
2.6. ROAV Calculation
2.7. GC–O Analysis
2.8. Data Analysis
3. Results
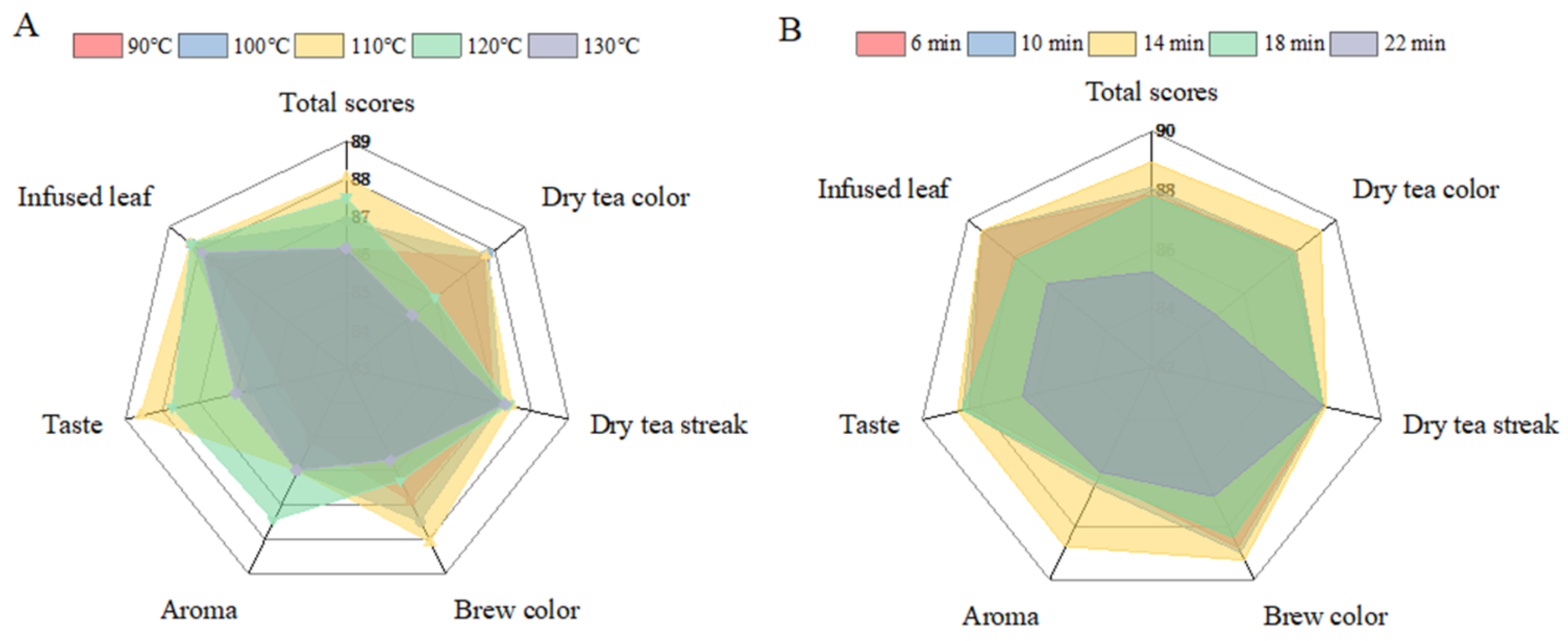
3.1. Effect of Varying Roasting Treatments on HSSGT Sensory Qualities
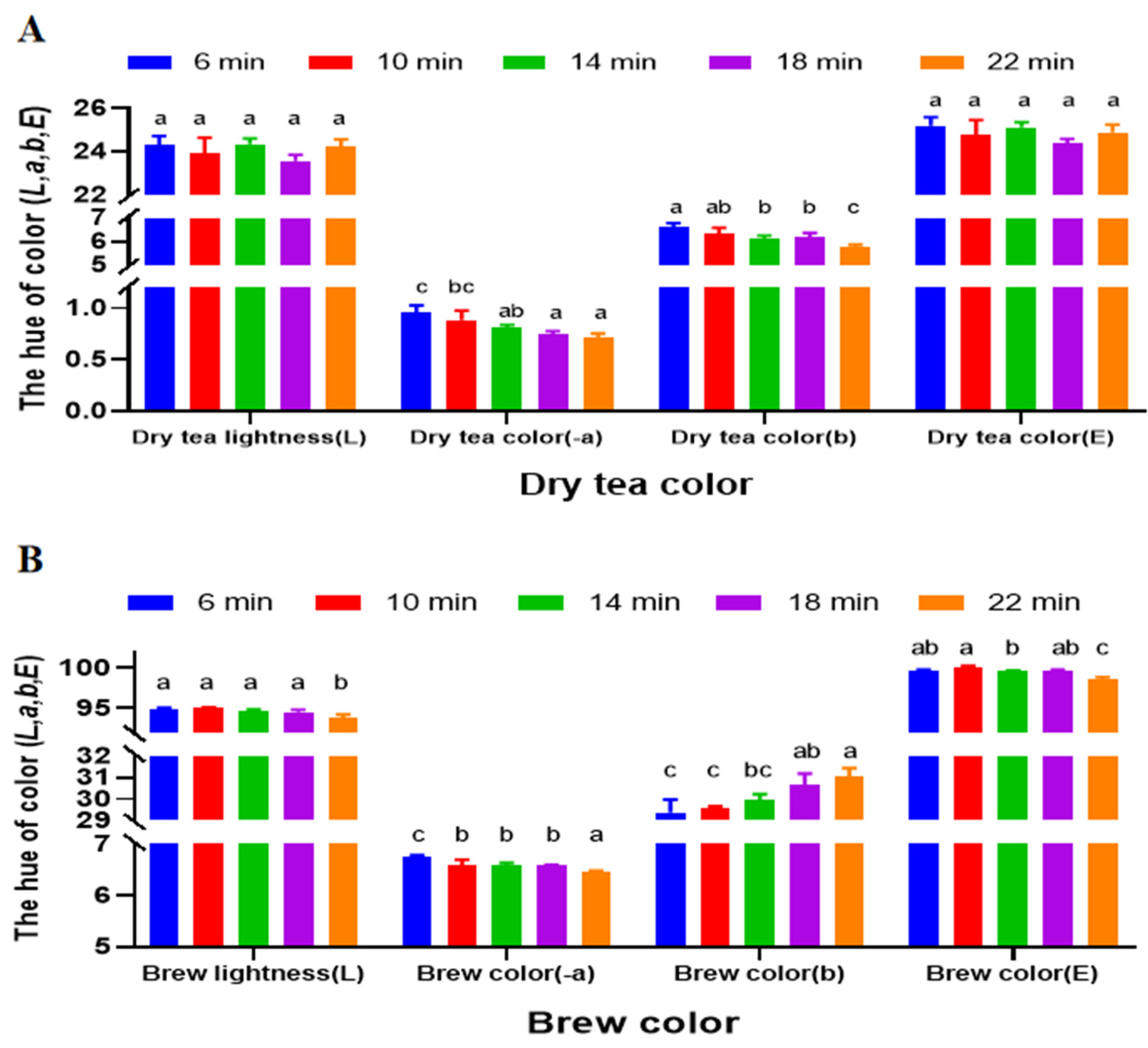
3.2. Impact of Varying Roasting Time on HSSGT Color Quality
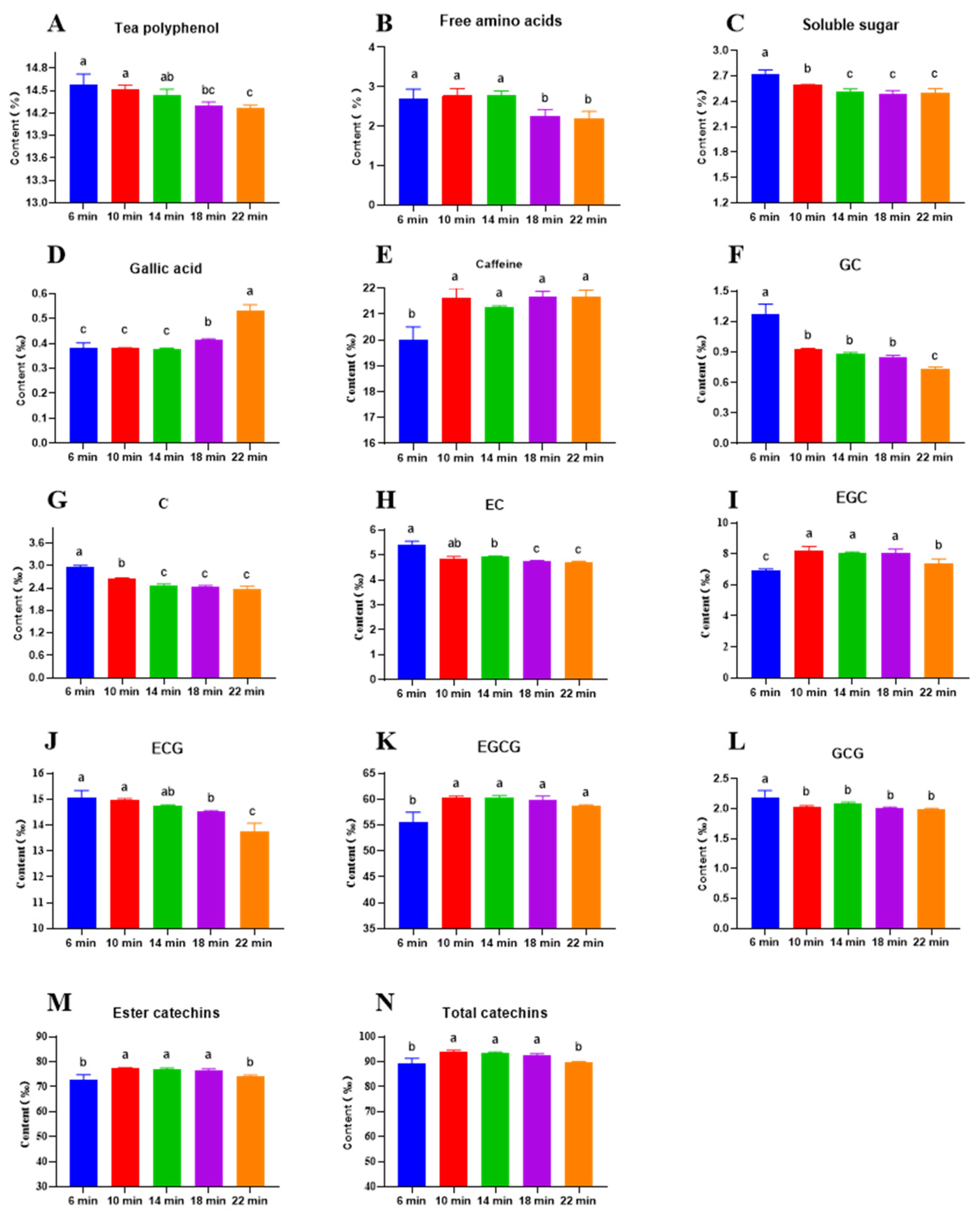
3.3. Impact of Roasting Time on Bioactive Compound Content
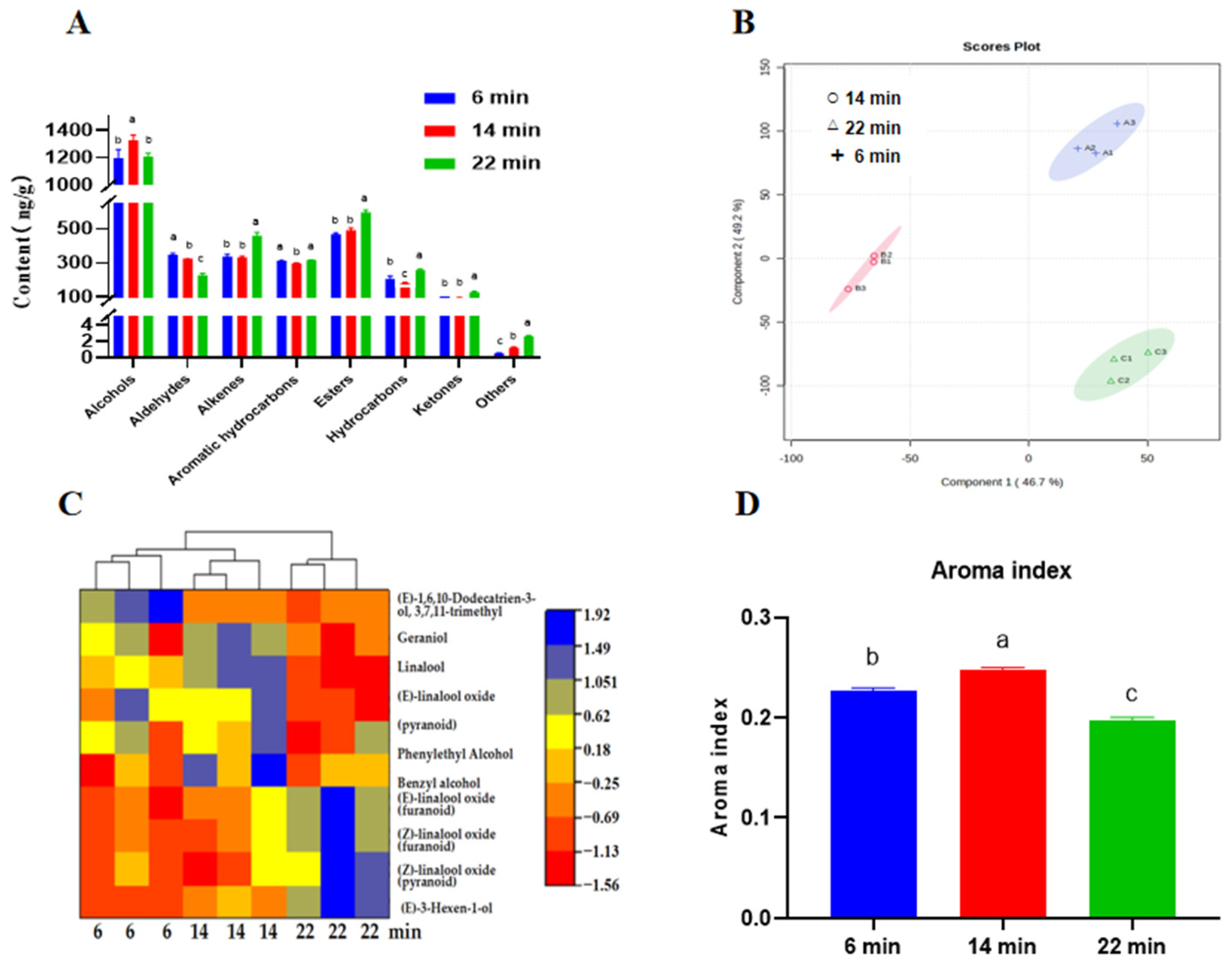
3.4. Profile of HSSGT Volatile Compounds After Roasting for Different Durations
3.5. Comparison of the Relative Odor Activity Values of Key HSSGT Odorants After Roasting
3.6. GC-O Analysis of Aroma Compounds in HSSGT Roasted with Different Times
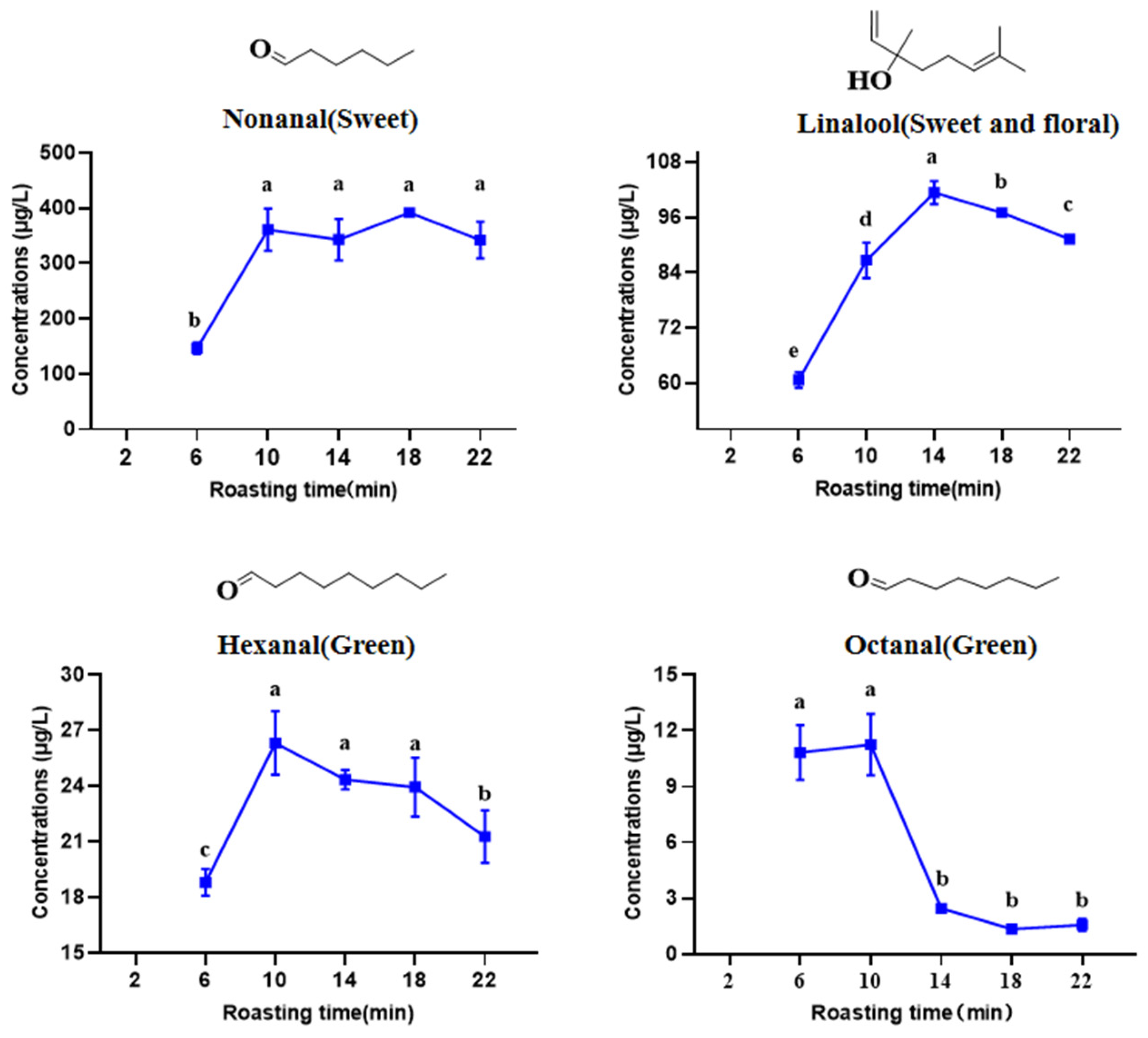
3.7. Absolute Quantification of Key Aroma Compounds in the HSSGT
3.8. The Possible Formation Pathways of Key Odorants in HSSGT During the Roasting Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Gao, L.; Chen, Z.; Zeng, X.; Huang, J.A.; Gong, Y.; Li, Q.; Liu, S.; Lin, Y.; Cai, S. Leading progress on genomics, health benefits and utilization of tea resources in China. Nature 2019, 566, S15–S19. [Google Scholar]
- Zhang, Y.; Yan, K.; Peng, Q.; Zhu, Y.; Dai, W.; Fu, J.; Lv, H.; Lin, Z.; Shi, J.; Baldermann, S. Comprehensive analysis of pigment alterations and associated flavor development in strip and needle green teas. Food Res. Int. 2024, 175, 113713. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ran, W.; He, C.; Zhou, J.; Chen, Y.; Yu, Z.; Ni, D. Effects of different tea tree varieties on the color, aroma, and taste of Chinese Enshi green tea. Food Chem. X 2022, 14, 100289. [Google Scholar] [CrossRef]
- Zou, D.; Yin, X.-L.; Gu, H.-W.; Peng, Z.-X.; Ding, B.; Li, Z.; Hu, X.-C.; Long, W.; Fu, H.; She, Y. Insight into the effect of cultivar and altitude on the identification of EnshiYulu tea grade in untargeted metabolomics analysis. Food Chem. 2024, 436, 137768. [Google Scholar] [CrossRef]
- Fei, Y.; Ziming, G.; Anhui, G.; Jing, T.; Shiwei, G. Physico-chemical characteristics and quality improvement of machine picking green tea by automatic production line. Trans. Chin. Soc. Agric. Eng. Trans. CSAE 2019, 35, 281. [Google Scholar]
- Ouyang, W.; Yu, Y.; Wang, H.; Jiang, Y.; Hua, J.; Ning, J.; Yuan, H. Analysis of volatile metabolite variations in strip green tea during processing and effect of rubbing degree using untargeted and targeted metabolomics. Food Res. Int. 2022, 162, 112099. [Google Scholar] [CrossRef]
- Jeszka-Skowron, M.; Frankowski, R.; Zgoła-Grześkowiak, A. Comparison of methylxantines, trigonelline, nicotinic acid and nicotinamide contents in brews of green and processed Arabica and Robusta coffee beans—Influence of steaming, decaffeination and roasting processes on coffee beans. LWT Food Sci. Technol. 2020, 125, 109344. [Google Scholar] [CrossRef]
- Yang, Y.; Qian, M.C.; Deng, Y.; Yuan, H.; Jiang, Y. Insight into aroma dynamic changes during the whole manufacturing process of chestnut-like aroma green tea by combining GC-E-Nose, GC-IMS, and GC × GC-TOFMS. Food Chem. 2022, 387, 132813. [Google Scholar] [CrossRef]
- Zhu, Y.; Lv, H.P.; Shao, C.Y.; Kang, S.; Zhang, Y.; Guo, L.; Dai, W.D.; Tan, J.F.; Peng, Q.H.; Lin, Z. Identification of key odorants responsible for chestnut-like aroma quality of green teas. Food Res. Int. 2018, 108, 74–82. [Google Scholar] [CrossRef]
- Jiang, Z.; Han, Z.; Wen, M.; Ho, C.T.; Wu, Y.; Wang, Y.; Xu, N.; Xie, Z.; Zhang, J.; Zhang, L.; et al. Comprehensive comparison on the chemical metabolites and taste evaluation of tea after roasting using untargeted and pseudotargeted metabolomics. Food Sci. Hum. Wellness 2022, 11, 12. [Google Scholar] [CrossRef]
- Fu, Y.Q.; Wang, J.Q.; Chen, J.X.; Wang, F.; Xu, Y.Q. Effect of baking on the flavor stability of green tea beverages. Food Chem. 2020, 331, 127258. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Chen, L.; Kun, J.; He, S.; Tong, H.; Chen, Y. The unique aroma of ripened Pu-erh tea, Liupao tea and Tietban tea: Associated post-fermentation condition and dominant microorganism with key aroma-active compound. Food Chem. 2025, 464, 141788. [Google Scholar] [CrossRef] [PubMed]
- GB/T 23376-2018; Methodology for Sensory Evaluation of Tea. Standardization of Administration of the People’s Republic of China: Beijing, China, 2018.
- GB/T14487-2017; Tea Vocabulary for Sensory Evaluation. Standardization of Administration of the People’s Republic of China: Beijing, China, 2017.
- Ye, F.; Qiao, X.; Gui, A.; Liu, P.; Wang, S.; Wang, X.; Teng, J.; Zheng, L.; Feng, L.; Han, H.; et al. Characterization of Roasting Time on Sensory Quality, Color, Taste, and Nonvolatile Compounds of Yuan An Yellow Tea. Molecules 2022, 27, 4119. [Google Scholar] [CrossRef]
- Cao, Q.Q.; Wang, F.; Wang, J.Q.; Chen, J.X.; Yin, J.F.; Li, L.; Meng, F.K.; Cheng, Y.; Xu, Y.Q. Effects of brewing water on the sensory attributes and physicochemical properties of tea infusions. Food Chem. 2021, 364, 130235. [Google Scholar] [CrossRef]
- Ye, F.; Qiao, X.; Gui, A.; Wang, S.; Liu, P.; Wang, X.; Teng, J.; Zheng, L.; Feng, L.; Han, H.; et al. Metabolomics Provides a Novel Interpretation of the Changes in Main Compounds during Black Tea Processing through Different Drying Methods. Molecules 2021, 26, 6739. [Google Scholar] [CrossRef]
- GB/T 8304-2013; Tea-Determination of Moisture Content. Standardization of Administration of the People’s Republic of China: Beijing, China, 2017.
- GB/T 5009.124-2003; Determination of Amino Acids in Foods. Standardization of Administration of the People’s Republic of China: Beijing, China, 2003.
- ISO 14502-1:2005 EN; Determination of Substances Characteristic of Green and Black Tea—Part 1: Content of Total Polyphenols in Tea—Colorimetric Method Using Folin-Ciocalteu Reagent. International Standard ISO: Geneva, Switzerland, 2005.
- Wang, H.; Hua, J.; Jiang, Y.; Yang, Y.; Yuan, H. Influence of fixation methods on the chestnut-like aroma of green tea and dynamics of key aroma substances. Food Res. Int. 2020, 136, 109479. [Google Scholar] [CrossRef]
- Liu, P.; Zheng, P.; Feng, L.; Gong, Z.; Zheng, L.; Gao, S.; Wang, X.; Ye, F.; Huang, J.; Liu, Z. Dynamic changes in the aroma profile of Qingzhuan tea during its manufacture. Food Chem. 2022, 375, 131847. [Google Scholar] [CrossRef]
- Schieberle, P. New Developments in Methods for Analysis of Volatile Flavor Compounds and their Precursors. In Characterization of Food; Elsevier: Amsterdam, The Netherlands, 1995; pp. 403–431. [Google Scholar]
- Wang, M.Q.; Ma, W.J.; Shi, J.; Zhu, Y.; Lin, Z.; Lv, H.P. Characterization of the key aroma compounds in Longjing tea using stir bar sorptive extraction (SBSE) combined with gas chromatography-mass spectrometry (GC-MS), gas chromatography-olfactometry (GC-O), odor activity value (OAV), and aroma recombination. Food Res. Int. 2020, 130, 108908. [Google Scholar] [CrossRef]
- Wang, H.; Ouyang, W.; Yu, Y.; Wang, J.; Yuan, H.; Hua, J.; Jiang, Y. Analysis of non-volatile and volatile metabolites reveals the influence of second-drying heat transfer methods on green tea quality. Food Chem. X 2022, 14, 100354. [Google Scholar] [CrossRef]
- Wang, J.Q.; Fu, Y.Q.; Chen, J.X.; Wang, F.; Feng, Z.H.; Yin, J.F.; Zeng, L.; Xu, Y.Q. Effects of baking treatment on the sensory quality and physicochemical properties of green tea with different processing methods. Food Chem. 2022, 380, 132217. [Google Scholar] [CrossRef]
- Wang, B.; Qu, F.; Wang, P.; Zhao, L.; Wang, Z.; Han, Y.; Zhang, X. Characterization analysis of flavor compounds in green teas at different drying temperature. LWT 2022, 161, 113394. [Google Scholar] [CrossRef]
- Zeng, Y.; Liang, J.; Zhang, J.; Chen, W.; Hu, D.; Xia, H.; Ma, C.; Qiao, X. Improvement of the sensory characteristics of Dancong tea using a dry-heating post-treatment approach. LWT 2023, 185, 115118. [Google Scholar] [CrossRef]
- Wang, H.; Xie, H.; Chen, S.; Fu, Q.; Wang, R.; Zhang, W.; Hu, Z. Effect of different drying methods on drying characteristics and qualities of lemon slices. Trans. Chin. Soc. Agric. Eng. Trans. CSAE 2017, 33, 292. [Google Scholar]
- Luo, L.; Wang, J.; Li, M.; Zhang, Y.; Wang, Y.; Xu, Y.; Chen, H.; Zhu, Y.; Feng, Z.; Yin, J. Characterization of the key odorants and investigation of the effects of drying methods on the aroma, taste, color and volatile profiles of the fruit of Clausena anisum-olens (Blanco) Merr. LWT 2023, 175, 114476. [Google Scholar] [CrossRef]
- Peng, Y.; Du, Z.; Wang, X.; Wu, R.; Zheng, C.; Han, W.; Liu, L.; Gao, F.; Liu, G.; Liu, B.; et al. From heat to flavor: Unlocking new chemical signatures to discriminate Wuyi rock tea under light and moderate roasting. Food Chem. 2024, 431, 137148. [Google Scholar] [CrossRef]
- Kręcisz, M.; Kolniak-Ostek, J.; Łyczko, J.; Stępień, B. Evaluation of bioactive compounds, volatile compounds, drying process kinetics and selected physical properties of vacuum impregnation celery dried by different methods. Food Chem. 2023, 413, 135490. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Zhang, Y.N.; Chen, J.X.; Wang, F.; Du, Q.Z.; Yin, J.F. Quantitative analyses of the bitterness and astringency of catechins from green tea. Food Chem. 2018, 258, 16–24. [Google Scholar] [CrossRef]
- Zhang, J.; Sun-Waterhouse, D.; Su, G.; Zhao, M. New insight into umami receptor, umami/umami-enhancing peptides and their derivatives: A review. Trends Food Sci. Technol. 2019, 88, 429–438. [Google Scholar] [CrossRef]
- Protiva Rani, D.; Youngmok, K.; Seong-Jin, H.; Jong-Bang, E. Profiling of volatile and non-phenolic metabolites—Amino acids, organic acids, and sugars of green tea extracts obtained by different extraction techniques. Food Chem. 2019, 296, 69–77. [Google Scholar]
- Zeng, L.; Fu, Y.Q.; Liu, Y.Y.; Huang, J.S.; Chen, J.X.; Yin, J.F.; Jin, S.; Sun, W.J.; Xu, Y.Q. Comparative analysis of different grades of Tieguanyin oolong tea based on metabolomics and sensory evaluation. LWT 2023, 174, 114423. [Google Scholar] [CrossRef]
- Wang, L.F.; Kim, D.M.; Lee, C.Y. Effects of heat processing and storage on flavanols and sensory qualities of green tea beverage. J. Agric. Food Chem. 2000, 48, 4227–4232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xia, Y.; Peterson, D.G. Identification of bitter modulating maillard-catechin reaction products. J. Agric. Food Chem. 2014, 62, 8470–8477. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ho, C.T.; Schwab, W.; Wan, X. Effect of the roasting degree on flavor quality of large-leaf yellow tea. Food Chem. 2021, 347, 129016. [Google Scholar] [CrossRef]
- Fan, F.Y.; Shi, M.; Nie, Y.; Zhao, Y.; Ye, J.H.; Liang, Y.R. Differential behaviors of tea catechins under thermal processing: Formation of non-enzymatic oligomers. Food Chem. 2016, 196, 347–354. [Google Scholar] [CrossRef]
- Jiang, G.; Xue, R.; Wang, Y.; Liu, B.; Yuan, Y.; Pu, Q.; Fang, X.; Huang, Y.; Xiang, J.; Hu, X. Dynamic changes in the aroma profiles and volatiles of Enshi Yulu tea throughout its industrial processing. Food Chem. 2024, 458, 140145. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Y.; Yang, J.; Wang, H.; Ding, Y.; Lei, P. Comprehensive profiling of volatile components in Taiping Houkui green tea. LWT 2022, 163, 113523. [Google Scholar] [CrossRef]
- Yang, W.Z. Understanding the biosyntheses and stress response mechanisms of aroma compounds in tea (Camellia sinensis) to safely and effectively improve tea aroma. Crit. Rev. Food Sci. Nutr. 2019, 59, 2321–2334. [Google Scholar]
- Ho, C.T.; Zheng, X.; Li, S. Tea aroma formation. Food Sci. Hum. Wellness 2015, 4, 9–27. [Google Scholar] [CrossRef]
- Xu, S.; Zeng, X.; Wu, H.; Shen, S.; Ning, J. Characterizing Volatile Metabolites in Raw Pu’er Tea Stored in Wet-Hot or Dry-Cold Environments by Performing Metabolomic Analysis and using the Molecular Sensory Science Approach. Food Chem. 2021, 350, 129186. [Google Scholar] [CrossRef]
- Wang, H.; Cao, X.; Yuan, Z.; Guo, G. Untargeted metabolomics coupled with chemometrics approach for Xinyang Maojian green tea with cultivar, elevation and processing variations. Food Chem. 2021, 352, 129359. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Dong, J.J.; Jin, J.; Liu, J.H.; Zheng, X.Q.; Lu, J.L.; Liang, Y.R.; Ye, J.H. Roasting process shaping the chemical profile of roasted green tea and the association with aroma features. Food Chem. 2021, 353, 129428. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, J.; Zhu, L. Tea quality evaluation by applying E-nose combined with chemometrics methods. J. Food Sci. Technol. 2021, 58, 1549–1561. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, J.E.; Li, Z.; Liu, Q.; Zhao, X.; Yang, H. Metabolomic analysis of energy regulated germination and sprouting of organic mung bean (Vigna radiata) using NMR spectroscopy. Food Chem. 2019, 286, 87–97. [Google Scholar] [CrossRef]
- Wang, Y.S.; Fang, M.Z.; Zheng, S.D.; Cho, J.G.; Yi, T.H. Identification of Chinese green tea (Camellia sinensis) marker metabolites using GC/MS and UPLC-QTOF/MS. Food Sci. Biotechnol. 2021, 30, 1293–1301. [Google Scholar] [CrossRef]
- Li, H.; Luo, L.; Ma, M.; Zeng, L. Characterization of Volatile Compounds and Sensory Analysis of Jasmine Scented Black Tea Produced by Different Scenting Processes. J. Food Sci. 2018, 83, 2718–2732. [Google Scholar] [CrossRef]
- Yu, X.; Li, Y.; He, C.; Zhou, J.; Chen, Y.; Yu, Z.; Wang, P.; Ni, D. Nonvolatile metabolism in postharvest tea (Camellia sinensis L.) leaves: Effects of different withering treatments on nonvolatile metabolites, gene expression levels, and enzyme activity. Food Chem. 2020, 327, 126992. [Google Scholar] [CrossRef]
- Wu, Y.; Lv, S.; Lian, M.; Wang, C.; Gao, X.; Meng, Q. Study of characteristic aroma components of baked Wujiatai green tea by HS-SPME/GC-MS combined with principal component analysis. CyTA J. Food 2016, 14, 423–432. [Google Scholar] [CrossRef]
- Schuh, C.; Schieberle, P. Characterization of the key aroma compounds in the beverage prepared from Darjeeling black tea: Quantitative differences between tea leaves and infusion. J. Agric. Food Chem. 2006, 54, 916–924. [Google Scholar] [CrossRef]
- Qiao, X.; Zhang, S.; He, S.; Liu, S. Urate oxidase treatment increases the quality of autumn Yellowish Yinghong 9 black tea. LWT 2023, 184, 115092. [Google Scholar] [CrossRef]
- Yin, X.; Xiao, Y.; Wang, K.; Wu, W.; Huang, J.; Liu, S.; Zhang, S. Effect of shaking manners on floral aroma quality and identification of key floral-aroma-active compounds in Hunan black tea. Food Res. Int. 2023, 174, 113515. [Google Scholar] [CrossRef]
- Huang, D.; Li, M.; Wang, H.; Fu, M.; Hu, S.; Wan, X.; Wang, Z.; Chen, Q. Combining gas chromatography-ion mobility spectrometry and olfactory analysis to reveal the effect of filled-N2 anaerobic treatment duration on variation in the volatile profiles of gabaron green tea. LWT 2023, 179, 114630. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, Q.; Jiang, R.; Liu, Y.; Xie, H.; Ou, X.; Li, Q.; Liu, Z.; Huang, J.A. Characteristic volatiles of Fu brick tea formed primarily by extracellular enzymes during Aspergillus cristatus fermentation. Food Res. Int. 2024, 177, 113854. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Y.; Hu, C.; Yu, B.; Wan, C.; Chen, B.; Lu, L.; Yuan, L.; Wu, Z.; Chen, H. The flavor substances changes in Fuliang green tea during storage monitoring by GC–MS and GC-IMS. Food Chem. X 2024, 21, 101047. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Li, S.; Tao, M.; He, W.; Shu, Z.; Wang, S.; Liu, Z. Effect of shaking and piling processing on improving the aroma quality of green tea. Food Res. Int. 2025, 201, 115624. [Google Scholar] [CrossRef]


| No. | Volatile Compounds * | Rt (min) | CAS | RI/NIST RI | Odor Descriptions | Threshold (μg/kg) * | ROVA (Mean ± SD) | ||
|---|---|---|---|---|---|---|---|---|---|
| 6 min | 14 min | 22 min | |||||||
| 1 | β-Ionone | 31.17 | 79-77-6 | 1478 | Violet-like, raspberry, floral | 0.007 | 1695 ± 92 a | 1809 ± 67 a | 1676 ± 166 a |
| 2 | β-Ocimene | 18.60 | 1040397-46-3 | 1045 | Warm, floral, herbal, sweet | 0.02 | 1286 ± 131 b | 1576 ± 137 b | 2348 ± 286 a |
| 3 | trans-Calamenene | 32.34 | 73209-42-4 | 1525 | Herbal, spicy | 0.02 | 748 ± 99 b | 751 ± 50 b | 1163 ± 59 a |
| 4 | δ-cadinene | 31.85 | 13862-36-7 | 1520 | Herbal, woody | 1.5 | 87 ± 2 a | 63 ± 3 b | 58 ± 1 c |
| 5 | Jasmone | 28.92 | 488-10-8 | 1391 | Floral, creamy | 1.9 | 26.7 ± 3.3 b | 28.1 ± 1.3 b | 45.7 ± 1.4 a |
| 6 | Geraniol | 24.835 | 103606-17-3 | 1246 | Rose-like | 7.5 | 21.6 ± 1.6 ab | 23.0 ± 0.3 a | 20.6 ± 0.5 b |
| 7 | (Z)-3-Hexenyl hexanoate | 28.43 | 294177-31-4 | 1373 | Floral, fruity, sweet | 16 | 18.1 ± 0.5 b | 18.7 ± 0.3 b | 22.3 ± 0.5 a |
| 8 | Nonanal | 22.47 | 124-19-6 | 1102 | Fat, citrus, green | 1 | 16.4 ± 3.2 b | 23.1 ± 0.7 a | 17.0 ± 1.1 b |
| 9 | Heptanal | 13.73 | 111-71-7 | 900 | Chestnut-like, sweet, grass | 3 | 10.9 ± 0.2 a | 9.9 ± 0.3 a | 5.1 ± 0.9 b |
| 10 | Octanal | 17.16 | 124-13-0 | 1001 | Fat, soap, lemon, green | 0.7 | 9.2 ± 0.5 a | 6.8 ± 0.2 b | 4.9 ± 0.2 c |
| 11 | Hexanal | 10.4 | 66-25-1 | 799 | Strong green, grassy, fruity | 4.5 | 6.1 ± 0.2 a | 5.9 ± 0.6 b | 4.8 ± 0.4 c |
| 12 | Benzeneacetaldehyde | 18.56 | 122-78-1 | 1044 | Sweet, floral honey, rosy | 4 | 2.8 ± 0.1 a | 3.0 ± 0.9 a | 1.3 ± 0.1 b |
| 13 | trans, trans-2,4-Heptadienal | 17.43 | 5910-85-0 | 1009 | Fatty, green odor | 15.4 | 1.9 ± 0.1 a | 1.8 ± 0.1 ab | 1.6 ± 0.1 b |
| 14 | cis-3-Hexenyl cis-3-hexenoate | 28.53 | 61444-38-0 | 1377 | Tender, fresh, clean | 16 | 1.2 ± 0.2 b | 1.2 ± 0.0 b | 1.6 ± 0.1 a |
| 15 | Linalool | 20.32 | 78-70-6 | 1098 | Pleasant floral | 6 | 1.0 ± 0.1 b | 1.3 ± 0.0 a | 0.97 ± 0.0 c |
| 16 | o-Cymene | 17.97 | 527-84-4 | 1026 | Aromatic | 11.4 | 1.0 ± 0.1 b | 1.82 ± 0.0 a | 1.67 ± 0.1 a |
| No. | Rt (min) | Compounds | Odor Descriptions | Average AI | AI | ||
|---|---|---|---|---|---|---|---|
| 6 min | 14 min | 22 min | |||||
| 1 | 2.06 | Dimethyl sulfide | Sweet, chestnut-like | 1.5 | 1.5 ± 0.2 | 1.5 ± 0.2 | 1.5 ± 0.2 |
| 2 | 4.73 | Hexanal | Green | 1.2 | 1.5 ± 0.3 | 1.0 ± 0.2 | 1.0 ± 0.2 |
| 3 | 8.07 | Heptaldehyde | Green | 1.4 | 1.5 ± 0.2 | 1.8 ± 0.2 | 1.8 ± 0.3 |
| 4 | 11.79 | 1-Octen-3-ol | Sweet and floral | 2.0 | 2.5 ± 0.3 | 2.0 ± 0.3 | 2.0 ± 0.3 |
| 5 | 13.20 | Octanal | Green | 1.6 | 1.5 ± 0.2 | 1.9 ± 0.3 | 1.5 ± 0.2 |
| 6 | 14.43 | 2,2,6-Trimethylcyclohexanone | Like burnt, baked, pungent | 1.0 | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.2 |
| 7 | 17.83 | cis-Linalool oxide | Earthy floral, caramel-like | 1.2 | 1.5 ± 0.3 | 1.0 ± 0.2 | 1.0 ± 0.2 |
| 8 | 18.02 | Linalool | Sweet and floral | 2.7 | 2.5 ± 0.3 | 2.8 ± 0.3 | 3.0 ± 0.3 |
| 9 | 18.18 | Nonanal | Sweet | 2.6 | 2.0 ± 0.3 | 2.8 ± 0.3 | 3.0 ± 0.3 |
| 10 | 22.15 | (E)-3-hexenyl butanoate | Baked, pungent | 0.8 | 1.5 ± 0.2 | 0.8 ± 0.2 | - |
| 11 | 23.13 | β-cyclocitral | Green, fruity | 1.2 | 1.5 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.2 |
| 12 | 28.89 | (-)-α-Cubebene | Tender floral | 1.2 | 1.5 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.2 |
| 13 | 30.88 | (Z)-hex-3-en-1-yl hexanoate | Tender floral, fresh | 1.0 | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.2 |
| 14 | 39.81 | Cedrol | Tender floral | 1.0 | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.2 |
| No. | Compounds | Standard Curve | R2 | Linear Range (μg/L) |
|---|---|---|---|---|
| 1 | Linalool | Y = 0.79 × 10−6X − 2.08 | 0.9841 | 2.07~20,654.90 |
| 2 | Nonanal | Y = 0.23 × 10−3X − 315.75 | 0.9924 | 1.79~17,866.70 |
| 3 | Octanal | Y = 4.74 × 10−8X − 0.033 | 0.9983 | 0.82~82.00 |
| 4 | Hexanal | Y = 6.41 × 10−6X + 1.1548 | 0.9973 | 1.31~13,102.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, F.; Gui, A.; Qiao, X.; Liu, P.; Wang, X.; Wang, S.; Feng, L.; Teng, J.; Xue, J.; Chen, X.; et al. Effects of Roasting Process on Sensory Qualities, Color, Physicochemical Components, and Identification of Key Aroma Compounds in Hubei Strip-Shaped Green Tea. Metabolites 2025, 15, 155. https://doi.org/10.3390/metabo15030155
Ye F, Gui A, Qiao X, Liu P, Wang X, Wang S, Feng L, Teng J, Xue J, Chen X, et al. Effects of Roasting Process on Sensory Qualities, Color, Physicochemical Components, and Identification of Key Aroma Compounds in Hubei Strip-Shaped Green Tea. Metabolites. 2025; 15(3):155. https://doi.org/10.3390/metabo15030155
Chicago/Turabian StyleYe, Fei, Anhui Gui, Xiaoyan Qiao, Panpan Liu, Xueping Wang, Shengpeng Wang, Lin Feng, Jin Teng, Jinjin Xue, Xun Chen, and et al. 2025. "Effects of Roasting Process on Sensory Qualities, Color, Physicochemical Components, and Identification of Key Aroma Compounds in Hubei Strip-Shaped Green Tea" Metabolites 15, no. 3: 155. https://doi.org/10.3390/metabo15030155
APA StyleYe, F., Gui, A., Qiao, X., Liu, P., Wang, X., Wang, S., Feng, L., Teng, J., Xue, J., Chen, X., Mei, Y., Zhang, B., Han, H., Liao, A., Zheng, P., & Gao, S. (2025). Effects of Roasting Process on Sensory Qualities, Color, Physicochemical Components, and Identification of Key Aroma Compounds in Hubei Strip-Shaped Green Tea. Metabolites, 15(3), 155. https://doi.org/10.3390/metabo15030155





