The Functions of Major Gut Microbiota in Obesity and Type 2 Diabetes
Abstract
1. Introduction
2. Introduction of Gut Microbiota and Obesity
3. Environmental and Lifestyle Factors Influencing Gut Microbiota Composition
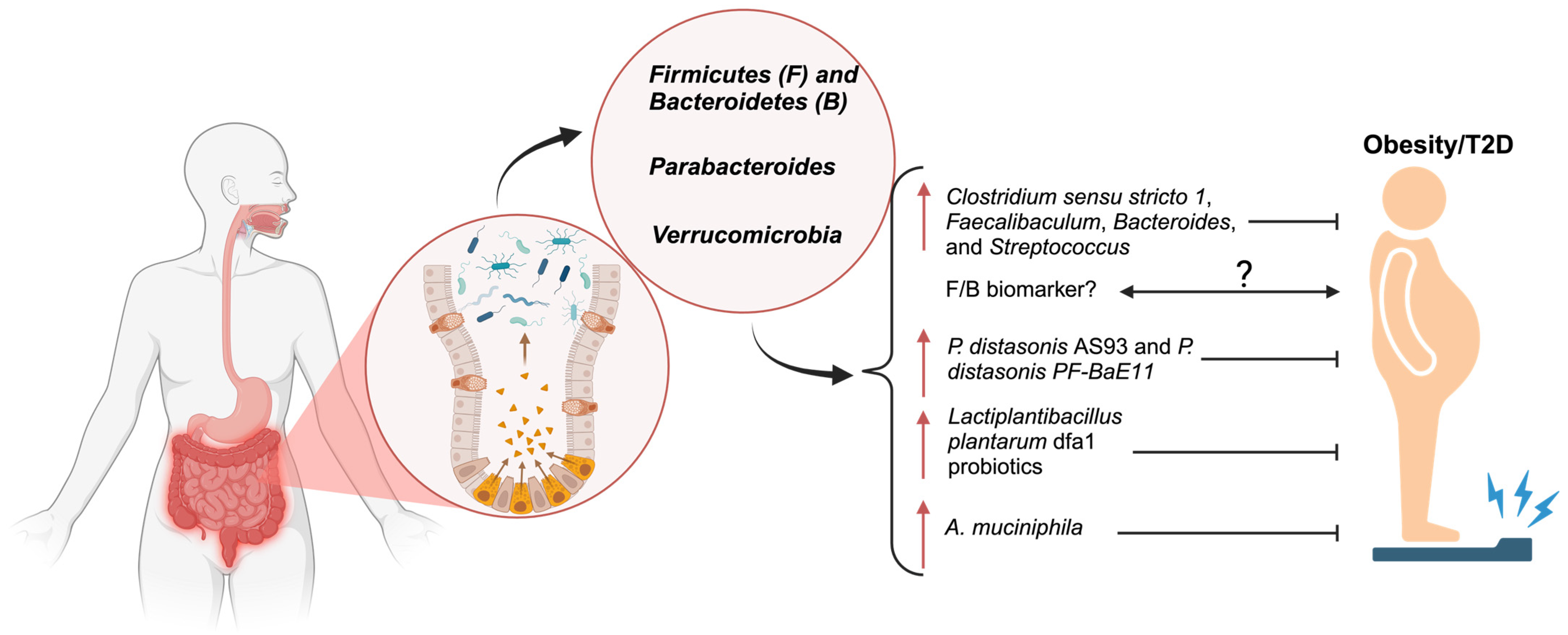
4. Current Updates in Gut Microbiota Research
4.1. Firmicutes (F) and Bacteroidetes (B)
4.2. Parabacteroides
4.3. Verrucomicrobiota
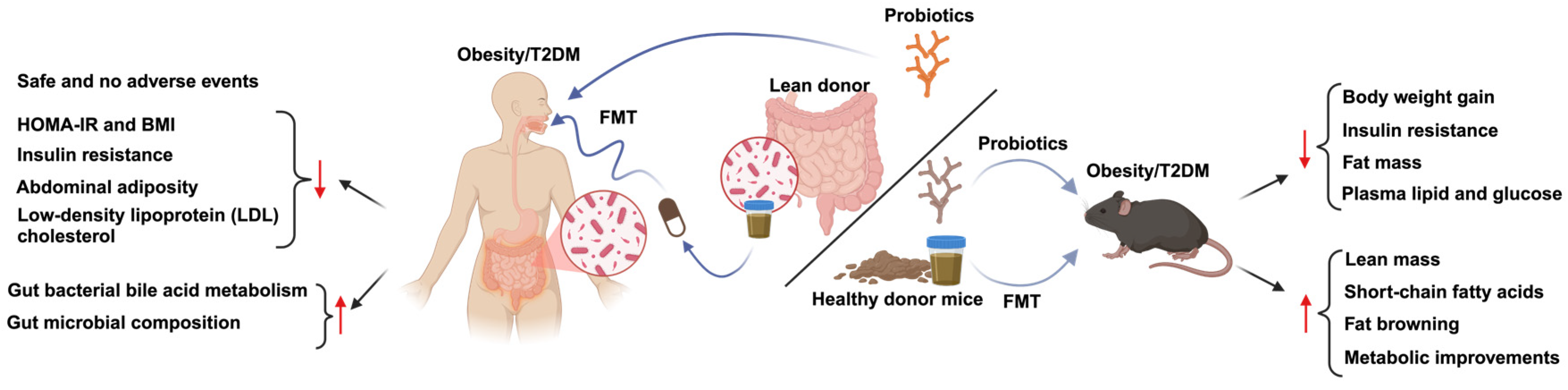
5. Therapeutic Strategies
6. Beyond Gut Microbiota: Gut Microbiota–Brain Axis
7. Challenges and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faith, J.J.; Guruge, J.L.; Charbonneau, M.; Subramanian, S.; Seedorf, H.; Goodman, A.L.; Clemente, J.C.; Knight, R.; Heath, A.C.; Leibel, R.L.; et al. The Long-Term Stability of the Human Gut Microbiota. Science 2013, 341, 1237439. [Google Scholar] [CrossRef] [PubMed]
- Kamada, N.; Seo, S.-U.; Chen, G.Y.; Núñez, G. Role of the Gut Microbiota in Immunity and Inflammatory Disease. Nat. Rev. Immunol. 2013, 13, 321–335. [Google Scholar] [CrossRef]
- Pant, A.; Maiti, T.K.; Mahajan, D.; Das, B. Human Gut Microbiota and Drug Metabolism. Microb. Ecol. 2023, 86, 97–111. [Google Scholar] [CrossRef]
- Walker, A.W.; Ince, J.; Duncan, S.H.; Webster, L.M.; Holtrop, G.; Ze, X.; Brown, D.; Stares, M.D.; Scott, P.; Bergerat, A.; et al. Dominant and Diet-Responsive Groups of Bacteria within the Human Colonic Microbiota. ISME J. 2011, 5, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut Biogeography of the Bacterial Microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Sweatt, K.; Garvey, W.T.; Martins, C. Correction: Strengths and Limitations of BMI in the Diagnosis of Obesity: What Is the Path Forward? Curr. Obes. Rep. 2024, 13, 831. [Google Scholar] [CrossRef]
- Gilbert, M. Role of Skeletal Muscle Lipids in the Pathogenesis of Insulin Resistance of Obesity and Type 2 Diabetes. J. Diabetes Investig. 2021, 12, 1934–1941. [Google Scholar] [CrossRef]
- Hariharan, R.; Odjidja, E.N.; Scott, D.; Shivappa, N.; Hébert, J.R.; Hodge, A.; De Courten, B. The Dietary Inflammatory Index, Obesity, Type 2 Diabetes, and Cardiovascular Risk Factors and Diseases. Obes. Rev. 2022, 23, e13349. [Google Scholar] [CrossRef]
- Schnurr, T.M.; Jakupović, H.; Carrasquilla, G.D.; Ängquist, L.; Grarup, N.; Sørensen, T.I.A.; Tjønneland, A.; Overvad, K.; Pedersen, O.; Hansen, T.; et al. Obesity, Unfavourable Lifestyle and Genetic Risk of Type 2 Diabetes: A Case-Cohort Study. Diabetologia 2020, 63, 1324–1332. [Google Scholar] [CrossRef]
- Wiertsema, S.P.; Van Bergenhenegouwen, J.; Garssen, J.; Knippels, L.M.J. The Interplay between the Gut Microbiome and the Immune System in the Context of Infectious Diseases throughout Life and the Role of Nutrition in Optimizing Treatment Strategies. Nutrients 2021, 13, 886. [Google Scholar] [CrossRef]
- Li, B.; Yan, Y.; Zhang, T.; Xu, H.; Wu, X.; Yao, G.; Li, X.; Yan, C.; Wu, L.-L. Quercetin Reshapes Gut Microbiota Homeostasis and Modulates Brain Metabolic Profile to Regulate Depression-like Behaviors Induced by CUMS in Rats. Front. Pharmacol. 2024, 15, 1362464. [Google Scholar] [CrossRef]
- Park, M.; Choi, J.; Lee, H.-J. Flavonoid-Rich Orange Juice Intake and Altered Gut Microbiome in Young Adults with Depressive Symptom: A Randomized Controlled Study. Nutrients 2020, 12, 1815. [Google Scholar] [CrossRef]
- Xia, C.-X.; Gao, A.X.; Zhu, Y.; Dong, T.T.-X.; Tsim, K.W.-K. Flavonoids from Seabuckthorn (Hippophae Rhamnoides L.) Restore CUMS-Induced Depressive Disorder and Regulate the Gut Microbiota in Mice. Food Funct. 2023, 14, 7426–7438. [Google Scholar] [CrossRef]
- Li, P.; Jiang, J.; Li, Y.; Lan, Y.; Yang, F.; Wang, J.; Xie, Y.; Xiong, F.; Wu, J.; Liu, H.; et al. Metagenomic Analysis Reveals Distinct Changes in the Gut Microbiome of Obese Chinese Children. BMC Genom. 2023, 24, 721. [Google Scholar] [CrossRef]
- Ling, Z.; Liu, X.; Cheng, Y.; Yan, X.; Wu, S. Gut Microbiota and Aging. Crit. Rev. Food Sci. Nutr. 2022, 62, 3509–3534. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, D.Y.; Kang, H.J.; Kang, J.H.; Cho, M.G.; Jang, H.W.; Kim, B.K.; Hur, S.J. Differences in the Gut Microbiota between Young and Elderly Persons in Korea. Nutr. Res. 2021, 87, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, J.L.; Musaad, S.M.; Holscher, H.D. Time of Day and Eating Behaviors Are Associated with the Composition and Function of the Human Gastrointestinal Microbiota. Am. J. Clin. Nutr. 2017, 106, 1220–1231. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Hogenesch, J.B.; Kay, S.A. Circadian Rhythms from Flies to Human. Nature 2002, 417, 329–335. [Google Scholar] [CrossRef]
- Yu, X.; Zuo, T. Editorial: Food Additives, Cooking and Processing: Impact on the Microbiome. Front. Nutr. 2021, 8, 731040. [Google Scholar] [CrossRef]
- Muralidharan, J.; Moreno-Indias, I.; Bulló, M.; Lopez, J.V.; Corella, D.; Castañer, O.; Vidal, J.; Atzeni, A.; Fernandez-García, J.C.; Torres-Collado, L.; et al. Effect on Gut Microbiota of a 1-y Lifestyle Intervention with Mediterranean Diet Compared with Energy-Reduced Mediterranean Diet and Physical Activity Promotion: PREDIMED-Plus Study. Am. J. Clin. Nutr. 2021, 114, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Hamjane, N.; Mechita, M.B.; Nourouti, N.G.; Barakat, A. Gut Microbiota Dysbiosis -Associated Obesity and Its Involvement in Cardiovascular Diseases and Type 2 Diabetes. A Systematic Review. Microvasc. Res. 2024, 151, 104601. [Google Scholar] [CrossRef]
- Gong, J.; Shen, Y.; Zhang, H.; Cao, M.; Guo, M.; He, J.; Zhang, B.; Xiao, C. Gut Microbiota Characteristics of People with Obesity by Meta-Analysis of Existing Datasets. Nutrients 2022, 14, 2993. [Google Scholar] [CrossRef]
- Kou, R.; Wang, J.; Li, A.; Wang, Y.; Zhang, B.; Liu, J.; Sun, Y.; Wang, S. Ameliorating Effects of Bifidobacterium Longum Subsp. Infantis FB3-14 against High-Fat-Diet-Induced Obesity and Gut Microbiota Disorder. Nutrients 2023, 15, 4104. [Google Scholar] [CrossRef]
- Baek, G.H.; Yoo, K.M.; Kim, S.-Y.; Lee, D.H.; Chung, H.; Jung, S.-C.; Park, S.-K.; Kim, J.-S. Collagen Peptide Exerts an Anti-Obesity Effect by Influencing the Firmicutes/Bacteroidetes Ratio in the Gut. Nutrients 2023, 15, 2610. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Wang, Y.-C.; Chiu, C.-C.; Lee, Y.-P.; Hung, S.-W.; Huang, C.-C.; Chiu, C.-F.; Chen, T.-H.; Huang, W.-C.; Chuang, H.-L. Housing Condition-Associated Changes in Gut Microbiota Further Affect the Host Response to Diet-Induced Nonalcoholic Fatty Liver. J. Nutr. Biochem. 2020, 79, 108362. [Google Scholar] [CrossRef]
- Li, Y.; Qi, X.; Wang, Q.; He, Y.; Li, Z.; Cen, X.; Wei, L. Comprehensive Analysis of Key Host Gene-Microbe Networks in the Cecum Tissues of the Obese Rabbits Induced by a High-Fat Diet. Front. Cell. Infect. Microbiol. 2024, 14, 1407051. [Google Scholar] [CrossRef]
- García-Gamboa, R.; Díaz-Torres, O.; Senés-Guerrero, C.; Gradilla-Hernández, M.S.; Moya, A.; Pérez-Brocal, V.; Garcia-Gonzalez, A.; González-Avila, M. Associations between Bacterial and Fungal Communities in the Human Gut Microbiota and Their Implications for Nutritional Status and Body Weigh t. Sci. Rep. 2024, 14, 5703. [Google Scholar] [CrossRef]
- Hassan, N.E.; El Shebini, S.M.; El-Masry, S.A.; Ahmed, N.H.; Kamal, A.N.; Ismail, A.S.; Alian, K.M.; Mostafa, M.I.; Selim, M.; Afify, M.A.S. Brief Overview of Dietary Intake, Some Types of Gut Microbiota, Metabolic Markers and Research Opportunities in Sample of Egyptian Women. Sci. Rep. 2022, 12, 17291. [Google Scholar] [CrossRef]
- Tao, Z.; Wang, Y. The Health Benefits of Dietary Short-Chain Fatty Acids in Metabolic Diseases. Crit. Rev. Food Sci. Nutr. 2024, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gazi, U.; Kocer, G.; Ruh, E.; Holyavkin, C.; Tosun, O.; Celik, M.; Cort Donmez, A.; Birsen, O. Gastric Microbiome Composition in Obese Patients and Normal Weight Subjects with Functional Dyspepsia. J. Infect. Dev. Ctries. 2024, 18, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Lobley, G.E.; Holtrop, G.; Ince, J.; Johnstone, A.M.; Louis, P.; Flint, H.J. Human Colonic Microbiota Associated with Diet, Obesity and Weight Loss. Int. J. Obes. 2008, 32, 1720–1724. [Google Scholar] [CrossRef]
- Schwiertz, A.; Taras, D.; Schäfer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in Lean and Overweight Healthy Subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef]
- Houtman, T.A.; Eckermann, H.A.; Smidt, H.; De Weerth, C. Gut Microbiota and BMI throughout Childhood: The Role of Firmicutes, Bacteroidetes, and Short-Chain Fatty Acid Producers. Sci. Rep. 2022, 12, 3140. [Google Scholar] [CrossRef]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J.; et al. Parabacteroides Distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep. 2019, 26, 222–235.e5. [Google Scholar] [CrossRef]
- Cuffaro, B.; Boutillier, D.; Desramaut, J.; Jablaoui, A.; Werkmeister, E.; Trottein, F.; Waligora-Dupriet, A.-J.; Rhimi, M.; Maguin, E.; Grangette, C. Characterization of Two Parabacteroides Distasonis Candidate Strains as New Live Biotherapeutics against Obesity. Cells 2023, 12, 1260. [Google Scholar] [CrossRef]
- Hasain, Z.; Mokhtar, N.M.; Kamaruddin, N.A.; Mohamed Ismail, N.A.; Razalli, N.H.; Gnanou, J.V.; Raja Ali, R.A. Gut Microbiota and Gestational Diabetes Mellitus: A Review of Host-Gut Microbiota Interactions and Their Therapeutic Potential. Front. Cell. Infect. Microbiol. 2020, 10, 188. [Google Scholar] [CrossRef]
- Ondee, T.; Pongpirul, K.; Janchot, K.; Kanacharoen, S.; Lertmongkolaksorn, T.; Wongsaroj, L.; Somboonna, N.; Ngamwongsatit, N.; Leelahavanichkul, A. Lactiplantibacillus Plantarum Dfa1 Outperforms Enterococcus Faecium Dfa1 on Anti-Obesity in High Fat-Induced Obesity Mice Possibly through the Differences in Gut Dysbiosis Attenuation, despite the Similar Anti-Inflammatory Properties. Nutrients 2021, 14, 80. [Google Scholar] [CrossRef]
- Cömert, T.K.; Akpinar, F.; Erkaya, S.; Durmaz, B.; Durmaz, R. The Effect of Pre-Pregnancy Obesity on Gut and Meconium Microbiome and Relationship with Fetal Growth. J. Matern.-Fetal Neonatal Med. 2022, 35, 10629–10637. [Google Scholar] [CrossRef]
- Suppli, M.P.; Bagger, J.I.; Lelouvier, B.; Broha, A.; Demant, M.; Kønig, M.J.; Strandberg, C.; Lund, A.; Vilsbøll, T.; Knop, F.K. Hepatic Microbiome in Healthy Lean and Obese Humans. JHEP Rep. 2021, 3, 100299. [Google Scholar] [CrossRef]
- Du, J.; Zhang, P.; Luo, J.; Shen, L.; Zhang, S.; Gu, H.; He, J.; Wang, L.; Zhao, X.; Gan, M.; et al. Dietary Betaine Prevents Obesity through Gut Microbiota-Drived microRNA-378a Family. Gut Microbes 2021, 13, 1862612. [Google Scholar] [CrossRef] [PubMed]
- Ashrafian, F.; Keshavarz Azizi Raftar, S.; Lari, A.; Shahryari, A.; Abdollahiyan, S.; Moradi, H.R.; Masoumi, M.; Davari, M.; Khatami, S.; Omrani, M.D.; et al. Extracellular Vesicles and Pasteurized Cells Derived from Akkermansia Muciniphila Protect against High-Fat Induced Obesity in Mice. Microb. Cell Fact. 2021, 20, 219. [Google Scholar] [CrossRef]
- Lin, X.-Q.; Chen, W.; Ma, K.; Liu, Z.-Z.; Gao, Y.; Zhang, J.-G.; Wang, T.; Yang, Y.-J. Akkermansia Muciniphila Suppresses High-Fat Diet-Induced Obesity and Related Metabolic Disorders in Beagles. Molecules 2022, 27, 6074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ni, Y.; Qian, L.; Fang, Q.; Zheng, T.; Zhang, M.; Gao, Q.; Zhang, Y.; Ni, J.; Hou, X.; et al. Decreased Abundance of Akkermansia Muciniphila Leads to the Impairment of Insulin Secretion and Glucose Homeostasis in Lean Type 2 Diabetes. Adv. Sci. 2021, 8, 2100536. [Google Scholar] [CrossRef]
- Davis, J.A.; Collier, F.; Mohebbi, M.; Stuart, A.L.; Loughman, A.; Pasco, J.A.; Jacka, F.N. Obesity, Akkermansia Muciniphila, and Proton Pump Inhibitors: Is There a Link? Obes. Res. Clin. Pract. 2020, 14, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Fitzgibbon, G.; Mills, K.H.G. The Microbiota and Immune-Mediated Diseases: Opportunities for Therapeutic Intervention. Eur. J. Immunol. 2020, 50, 326–337. [Google Scholar] [CrossRef]
- Coimbra, V.O.R.; Crovesy, L.; Ribeiro-Alves, M.; Faller, A.L.K.; Mattos, F.; Rosado, E.L. Gut Microbiota Profile in Adults Undergoing Bariatric Surgery: A Systematic Review. Nutrients 2022, 14, 4979. [Google Scholar] [CrossRef]
- Vallianou, N.; Stratigou, T.; Christodoulatos, G.S.; Tsigalou, C.; Dalamaga, M. Probiotics, Prebiotics, Synbiotics, Postbiotics, and Obesity: Current Evidence, Controversies, and Perspectives. Curr. Obes. Rep. 2020, 9, 179–192. [Google Scholar] [CrossRef]
- He, Z.; Wang, T.; Qiao, L.; Xu, S.; Zhang, S.; Gao, Q.; Zhang, J.; Chen, J.; Lin, C. Anti-Obesity Effects of Bifidobacterium Lactis YGMCC2013 by Promoting Adipocyte Thermogenesis and Beige Remodelling in Association with Gut Microbiota. J. Funct. Foods 2024, 115, 106099. [Google Scholar] [CrossRef]
- Lee, M.; Bok, M.K.; Son, K.; Lee, M.; Park, H.; Yang, J.; Lim, H. Bifidobacterium Lactis IDCC 4301 (B. Lactis FitTM) Supplementation Effects on Body Fat, Serum Triglyceride, and Adipokine Ratio in Obese Women: A Randomized Clinical Trial. Food Funct. 2024, 15, 8448–8458. [Google Scholar] [CrossRef]
- Li, T.; Lin, X.; Mao, X.; Chen, S.; Feng, Z.; Fu, Y.; Zhao, P.; Huang, X.; Ma, Y.; Song, L.; et al. The Prebiotics 2′-Fucosyllactose Prevent High-Fat Diet Induced Obesity via the Promotion of Thermogenesis and Modulation of Gut Microbiota. J. Funct. Foods 2024, 119, 106287. [Google Scholar] [CrossRef]
- Kang, Y.; Ren, P.; Shen, X.; Kuang, X.; Yang, X.; Liu, H.; Yan, H.; Yang, H.; Kang, X.; Ding, Z.; et al. A Newly Synbiotic Combination Alleviates Obesity by Modulating the Gut Microbiota-Fat Axis and Inhibiting the Hepatic TLR4/NF-κB Signaling Pathway. Mol. Nutr. Food Res. 2023, 67, e2300141. [Google Scholar] [CrossRef]
- Radford-Smith, D.E.; Anthony, D.C. Prebiotic and Probiotic Modulation of the Microbiota-Gut-Brain Axis in Depression. Nutrients 2023, 15, 1880. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Yang, J.; Ying, S.; Luo, H.; Zha, L.; Li, Q. Xylooligosaccharide and Akkermansia Muciniphila Synergistically Ameliorate Insulin Resistance by Reshaping Gut Microbiota, Improving Intestinal Barrier and Regulating NKG2D/NKG2DL Signaling in Gestational Diabetes Mellitus Mice. Food Res. Int. 2024, 201, 115634. [Google Scholar] [CrossRef]
- Atazadegan, M.A.; Heidari-Beni, M.; Entezari, M.H.; Sharifianjazi, F.; Kelishadi, R. Effects of Synbiotic Supplementation on Anthropometric Indices and Body Composition in Overweight or Obese Children and Adolescents: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. World J. Pediatr. 2023, 19, 356–365. [Google Scholar] [CrossRef]
- de Abreu Ribeiro Pereira, J.; de Fátima Píccolo Barcelos, M.; Valério Villas Boas, E.; Hilsdorf Píccoli, R.; de Sales Guilarducci, J.; Corrêa Pereira, R.; Pauli, J.R.; Batista Ferreira, E.; Cardoso de Angelis-Pereira, M.; Esper Cintra, D. Combined Effects of Yacon Flour and Probiotic Yogurt on the Metabolic Parameters and Inflammatory and Insulin Signaling Proteins in High-Fat-Diet-Induced Obese Mice. J. Sci. Food Agric. 2022, 102, 7293–7300. [Google Scholar] [CrossRef]
- Kassaian, N.; Feizi, A.; Rostami, S.; Aminorroaya, A.; Yaran, M.; Amini, M. The Effects of 6 Mo of Supplementation with Probiotics and Synbiotics on Gut Microbiota in the Adults with Prediabetes: A Double Blind Randomized Clinical Trial. Nutrition 2020, 79–80, 110854. [Google Scholar] [CrossRef]
- Barouei, J.; Martinic, A.; Bendiks, Z.; Mishchuk, D.; Heeney, D.; Slupsky, C.M.; Marco, M.L. Type 2-Resistant Starch and Lactiplantibacillus Plantarum NCIMB 8826 Result in Additive and Interactive Effects in Diet-Induced Obese Mice. Nutr. Res. 2023, 118, 12–28. [Google Scholar] [CrossRef]
- Wang, B.; Kong, Q.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. A High-Fat Diet Increases Gut Microbiota Biodiversity and Energy Expenditure Due to Nutrient Difference. Nutrients 2020, 12, 3197. [Google Scholar] [CrossRef]
- Du, H.; Han, Y.; Ma, G.; Tan, C.; Hu, Q.; Xiao, H. Dietary Intake of Whole King Oyster Mushroom (Pleurotus Eryngii) Attenuated Obesity via Ameliorating Lipid Metabolism and Alleviating Gut Microbiota Dysbiosis. Food Res. Int. 2024, 184, 114228. [Google Scholar] [CrossRef]
- Meslier, V.; Laiola, M.; Roager, H.M.; De Filippis, F.; Roume, H.; Quinquis, B.; Giacco, R.; Mennella, I.; Ferracane, R.; Pons, N.; et al. Mediterranean Diet Intervention in Overweight and Obese Subjects Lowers Plasma Cholesterol and Causes Changes in the Gut Microbiome and Metabolome Independently of Energy Intake. Gut 2020, 69, 1258–1268. [Google Scholar] [CrossRef]
- Dong, T.S.; Luu, K.; Lagishetty, V.; Sedighian, F.; Woo, S.-L.; Dreskin, B.W.; Katzka, W.; Chang, C.; Zhou, Y.; Arias-Jayo, N.; et al. A High Protein Calorie Restriction Diet Alters the Gut Microbiome in Obesity. Nutrients 2020, 12, 3221. [Google Scholar] [CrossRef]
- Kahleova, H.; Rembert, E.; Alwarith, J.; Yonas, W.N.; Tura, A.; Holubkov, R.; Agnello, M.; Chutkan, R.; Barnard, N.D. Effects of a Low-Fat Vegan Diet on Gut Microbiota in Overweight Individuals and Relationships with Body Weight, Body Composition, and Insulin Sensitivity. A Randomized Clinical Trial. Nutrients 2020, 12, 2917. [Google Scholar] [CrossRef]
- Rinott, E.; Meir, A.Y.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Knights, D.; Tuohy, K.; Scholz, M.U.; Koren, O.; Stampfer, M.J.; et al. The Effects of the Green-Mediterranean Diet on Cardiometabolic Health Are Linked to Gut Microbiome Modifications: A Randomized Controlled Trial. Genome Med. 2022, 14, 29. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, G.; Qian, J.; Xu, Y.; Li, B.; Shi, Y.; Le, G.; Xie, Y. Fecal Microbiota Transplantation from Methionine-Restricted Diet Mouse Donors Reduces Fat Deposition in Obese Mice by Remodeling the Gut Microbiota. Food Biosci. 2024, 59, 104255. [Google Scholar] [CrossRef]
- Rinott, E.; Youngster, I.; Yaskolka Meir, A.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Knights, D.; Tuohy, K.; Fava, F.; Scholz, M.U.; et al. Effects of Diet-Modulated Autologous Fecal Microbiota Transplantation on Weight Regain. Gastroenterology 2021, 160, 158–173.e10. [Google Scholar] [CrossRef]
- Leong, K.S.W.; Jayasinghe, T.N.; Wilson, B.C.; Derraik, J.G.B.; Albert, B.B.; Chiavaroli, V.; Svirskis, D.M.; Beck, K.L.; Conlon, C.A.; Jiang, Y.; et al. Effects of Fecal Microbiome Transfer in Adolescents with Obesity: The Gut Bugs Randomized Controlled Trial. JAMA Netw. Open 2020, 3, e2030415. [Google Scholar] [CrossRef]
- Yu, E.W.; Gao, L.; Stastka, P.; Cheney, M.C.; Mahabamunuge, J.; Torres Soto, M.; Ford, C.B.; Bryant, J.A.; Henn, M.R.; Hohmann, E.L. Fecal Microbiota Transplantation for the Improvement of Metabolism in Obesity: The FMT-TRIM Double-Blind Placebo-Controlled Pilot Trial. PLoS Med. 2020, 17, e1003051. [Google Scholar] [CrossRef]
- Allegretti, J.R.; Kassam, Z.; Mullish, B.H.; Chiang, A.; Carrellas, M.; Hurtado, J.; Marchesi, J.R.; McDonald, J.A.K.; Pechlivanis, A.; Barker, G.F.; et al. Effects of Fecal Microbiota Transplantation with Oral Capsules in Obese Patients. Clin. Gastroenterol. Hepatol. 2020, 18, 855–863.e2. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, B.; Chen, F.; Xia, R.; Zhu, D.; Chen, B.; Lin, A.; Zheng, C.; Hou, D.; Li, X.; et al. Fecal Microbiota Transplantation Reverses Insulin Resistance in Type 2 Diabetes: A Randomized, Controlled, Prospective Study. Front. Cell. Infect. Microbiol. 2023, 12, 1089991. [Google Scholar] [CrossRef]
- Bustamante, J.-M.; Dawson, T.; Loeffler, C.; Marfori, Z.; Marchesi, J.R.; Mullish, B.H.; Thompson, C.C.; Crandall, K.A.; Rahnavard, A.; Allegretti, J.R.; et al. Impact of Fecal Microbiota Transplantation on Gut Bacterial Bile Acid Metabolism in Humans. Nutrients 2022, 14, 5200. [Google Scholar] [CrossRef]
- Ng, S.C.; Xu, Z.; Mak, J.W.Y.; Yang, K.; Liu, Q.; Zuo, T.; Tang, W.; Lau, L.; Lui, R.N.; Wong, S.H.; et al. Microbiota Engraftment after Faecal Microbiota Transplantation in Obese Subjects with Type 2 Diabetes: A 24-Week, Double-Blind, Randomised Controlled Trial. Gut 2022, 71, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Lahtinen, P.; Juuti, A.; Luostarinen, M.; Niskanen, L.; Liukkonen, T.; Tillonen, J.; Kössi, J.; Ilvesmäki, V.; Viljakka, M.; Satokari, R.; et al. Effectiveness of Fecal Microbiota Transplantation for Weight Loss in Patients with Obesity Undergoing Bariatric Surgery: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2247226. [Google Scholar] [CrossRef]
- Zhang, Z.; Mocanu, V.; Deehan, E.C.; Hotte, N.; Zhu, Y.; Wei, S.; Kao, D.H.; Karmali, S.; Birch, D.W.; Walter, J.; et al. Recipient Microbiome-Related Features Predicting Metabolic Improvement Following Fecal Microbiota Transplantation in Adults with Severe Obesity and Metabolic Syndrome: A Secondary Analysis of a Phase 2 Clinical Trial. Gut Microbes 2024, 16, 2345134. [Google Scholar] [CrossRef]
- Gómez-Pérez, A.M.; Muñoz-Garach, A.; Lasserrot-Cuadrado, A.; Moreno-Indias, I.; Tinahones, F.J. Microbiota Transplantation in Individuals with Type 2 Diabetes and a High Degree of Insulin Resistance. Nutrients 2024, 16, 3491. [Google Scholar] [CrossRef]
- Gupta, A.; Osadchiy, V.; Mayer, E.A. Brain-Gut-Microbiome Interactions in Obesity and Food Addiction. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 655–672. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadeh, F.; Fakhri, S.; Shiri Varnamkhasti, B.; Moradi, S.Z.; Khirehgesh, M.R.; Echeverría, J. Phytochemicals from Edible Flowers Prevent Neurodegenerative Diseases via the Gut-Brain Axis. Food Biosci. 2025, 63, 105681. [Google Scholar] [CrossRef]
- Bai, Y.; Zhao, Y.; Jin, J.; Ye, Z.; Fan, H.; Zhao, D.; Gao, S. Jiang Tang San Hao Formula Exerts Its Anti-Diabetic Effect by Affecting the Gut-Microbiota-Brain Axis. Phytomedicine 2024, 135, 156100. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-M.; Zeng, F.-S.; Fu, W.-W.; You, H.-T.; Mu, X.-Y.; Chen, G.-F.; Lv, H.; Li, W.-J.; Xie, M.-Y. White Hyacinth Bean Polysaccharide Ameliorates Diabetes via Microbiota-Gut-Brain Axis in Type 2 Diabetes Mellitus Rats. Int. J. Biol. Macromol. 2023, 253, 127307. [Google Scholar] [CrossRef]
- Qiao, L.; Yang, G.; Wang, P.; Xu, C. The Potential Role of Mitochondria in the Microbiota-Gut-Brain Axis: Implications for Brain Health. Pharmacol. Res. 2024, 209, 107434. [Google Scholar] [CrossRef]
- Asadi, A.; Shadab Mehr, N.; Mohamadi, M.H.; Shokri, F.; Heidary, M.; Sadeghifard, N.; Khoshnood, S. Obesity and Gut-Microbiota-Brain Axis: A Narrative Review. J. Clin. Lab. Anal. 2022, 36, e24420. [Google Scholar] [CrossRef]
- Longo, S.; Rizza, S.; Federici, M. Microbiota-Gut-Brain Axis: Relationships among the Vagus Nerve, Gut Microbiota, Obesity, and Diabetes. Acta Diabetol. 2023, 60, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Agustí, A.; García-Pardo, M.P.; López-Almela, I.; Campillo, I.; Maes, M.; Romaní-Pérez, M.; Sanz, Y. Interplay Between the Gut-Brain Axis, Obesity and Cognitive Function. Front. Neurosci. 2018, 12, 155. [Google Scholar] [CrossRef] [PubMed]
- Cussotto, S.; Sandhu, K.V.; Dinan, T.G.; Cryan, J.F. The Neuroendocrinology of the Microbiota-Gut-Brain Axis: A Behavioural Perspective. Front. Neuroendocrinol. 2018, 51, 80–101. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.O.; Bäckhed, F. Signals from the Gut Microbiota to Distant Organs in Physiology and Disease. Nat. Med. 2016, 22, 1079–1089. [Google Scholar] [CrossRef]
- Lupien, S.J.; McEwen, B.S.; Gunnar, M.R.; Heim, C. Effects of Stress throughout the Lifespan on the Brain, Behaviour and Cognition. Nat. Rev. Neurosci. 2009, 10, 434–445. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The Gut Microbiota-Brain Axis in Behaviour and Brain Disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Sherwin, E.; Bordenstein, S.R.; Quinn, J.L.; Dinan, T.G.; Cryan, J.F. Microbiota and the Social Brain. Science 2019, 366, eaar2016. [Google Scholar] [CrossRef]
- Arnoriaga-Rodríguez, M.; Mayneris-Perxachs, J.; Burokas, A.; Contreras-Rodríguez, O.; Blasco, G.; Coll, C.; Biarnés, C.; Miranda-Olivos, R.; Latorre, J.; Moreno-Navarrete, J.-M.; et al. Obesity Impairs Short-Term and Working Memory through Gut Microbial Metabolism of Aromatic Amino Acids. Cell Metab. 2020, 32, 548–560.e7. [Google Scholar] [CrossRef]
- Hedin, K.A.; Zhang, H.; Kruse, V.; Rees, V.E.; Bäckhed, F.; Greiner, T.U.; Vazquez-Uribe, R.; Sommer, M.O.A. Cold Exposure and Oral Delivery of GLP-1R Agonists by an Engineered Probiotic Yeast Strain Have Antiobesity Effects in Mice. ACS Synth. Biol. 2023, 12, 3433–3442. [Google Scholar] [CrossRef]
- Wang, X.-L.; Chen, W.-J.; Jin, R.; Xu, X.; Wei, J.; Huang, H.; Tang, Y.-H.; Zou, C.-W.; Chen, T.-T. Engineered Probiotics Clostridium Butyricum-pMTL007-GLP-1 Improves Blood Pressure via Producing GLP-1 and Modulating Gut Microbiota in Spontaneous Hypertension Rat Models. Microb. Biotechnol. 2023, 16, 799–812. [Google Scholar] [CrossRef]
- Tettamanzi, F.; Bagnardi, V.; Louca, P.; Nogal, A.; Monti, G.S.; Mambrini, S.P.; Lucchetti, E.; Maestrini, S.; Mazza, S.; Rodriguez-Mateos, A.; et al. A High Protein Diet Is More Effective in Improving Insulin Resistance and Glycemic Variability Compared to a Mediterranean Diet-A Cross-Over Controlled Inpatient Dietary Study. Nutrients 2021, 13, 4380. [Google Scholar] [CrossRef] [PubMed]
| Clinical Trial | Effects | References |
|---|---|---|
| A randomized, placebo-controlled, pilot study in obese patients ([BMI] ≥ 5 kg/m2), n = 22, follow up on baseline and weeks 1, 4, 6, 8, and 12 |
| [68] |
| ||
| A randomized, controlled, prospective study in T2DM patients, n = 31, follow up on week 4 |
| [69] |
| ||
| Dietary-intervention randomized, controlled polyphenols-unprocessed weight-loss trial in abdominally obese or dyslipidemic participants, n = 90, follow up in months 6–14 |
| [65] |
| ||
| A double-blind, randomized, placebo-controlled pilot trial in obese, metabolically healthy patients, n = 22, follow up on week 4 |
| [70] |
| ||
| ||
| A double-blind, randomized, placebo-controlled pilot trial in adults with obesity and mild–moderate insulin resistance, n = 24, follow up on weeks 6 and 12 |
| [67] |
| ||
| A double-blind, randomized, placebo-controlled trial in obese subjects with T2DM, n = 61, follow up on weeks 4, 16, and 24 |
| [71] |
| ||
| A double-blinded, placebo-controlled, multicenter, randomized clinical trial among adult individuals with severe obesity, n = 41, 18 months of follow-up |
| [72] |
| A randomized, placebo-controlled phase 2 clinical trial in adults with obesity and metabolic syndrome, n = 29, follow up on week 6 |
| [73] |
| A phase II, randomized, single-blind, parallel-arm clinical trial in individuals with type 2 diabetes, n = 30, follow up on baseline and at 4 and 12 weeks |
| [74] |
| A randomized, double-masked, placebo-controlled trial, n = 87, follow up on week 26 |
| [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Tao, Z.; Qiao, M.; Shi, L. The Functions of Major Gut Microbiota in Obesity and Type 2 Diabetes. Metabolites 2025, 15, 167. https://doi.org/10.3390/metabo15030167
Liu S, Tao Z, Qiao M, Shi L. The Functions of Major Gut Microbiota in Obesity and Type 2 Diabetes. Metabolites. 2025; 15(3):167. https://doi.org/10.3390/metabo15030167
Chicago/Turabian StyleLiu, Siman, Zhipeng Tao, Mingyu Qiao, and Limin Shi. 2025. "The Functions of Major Gut Microbiota in Obesity and Type 2 Diabetes" Metabolites 15, no. 3: 167. https://doi.org/10.3390/metabo15030167
APA StyleLiu, S., Tao, Z., Qiao, M., & Shi, L. (2025). The Functions of Major Gut Microbiota in Obesity and Type 2 Diabetes. Metabolites, 15(3), 167. https://doi.org/10.3390/metabo15030167








