Lactobacillus johnsonii N6.2 Phospholipids Induce T Cell Anergy upon Cognate Dendritic Cell Interactions
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Generation of BMDCs and Stimulation with L. johnsonii N6.2 Extracted Lipids
2.3. Isolation and Maintenance of Homeostatic Peripheral T Cells
2.4. Co-Culture Experiments
2.5. RT-qPCR Analyses
2.6. Flow Cytometry Analysis
2.7. Statistical Analysis
3. Results
3.1. L. johnsonii N6.2 Phospholipids Reduce the Surface Expression of Maturation Markers in BMDCs
3.2. Expansion of CD8+ CD161+ Memory T Cells from Endogenous Peripheral T Cells
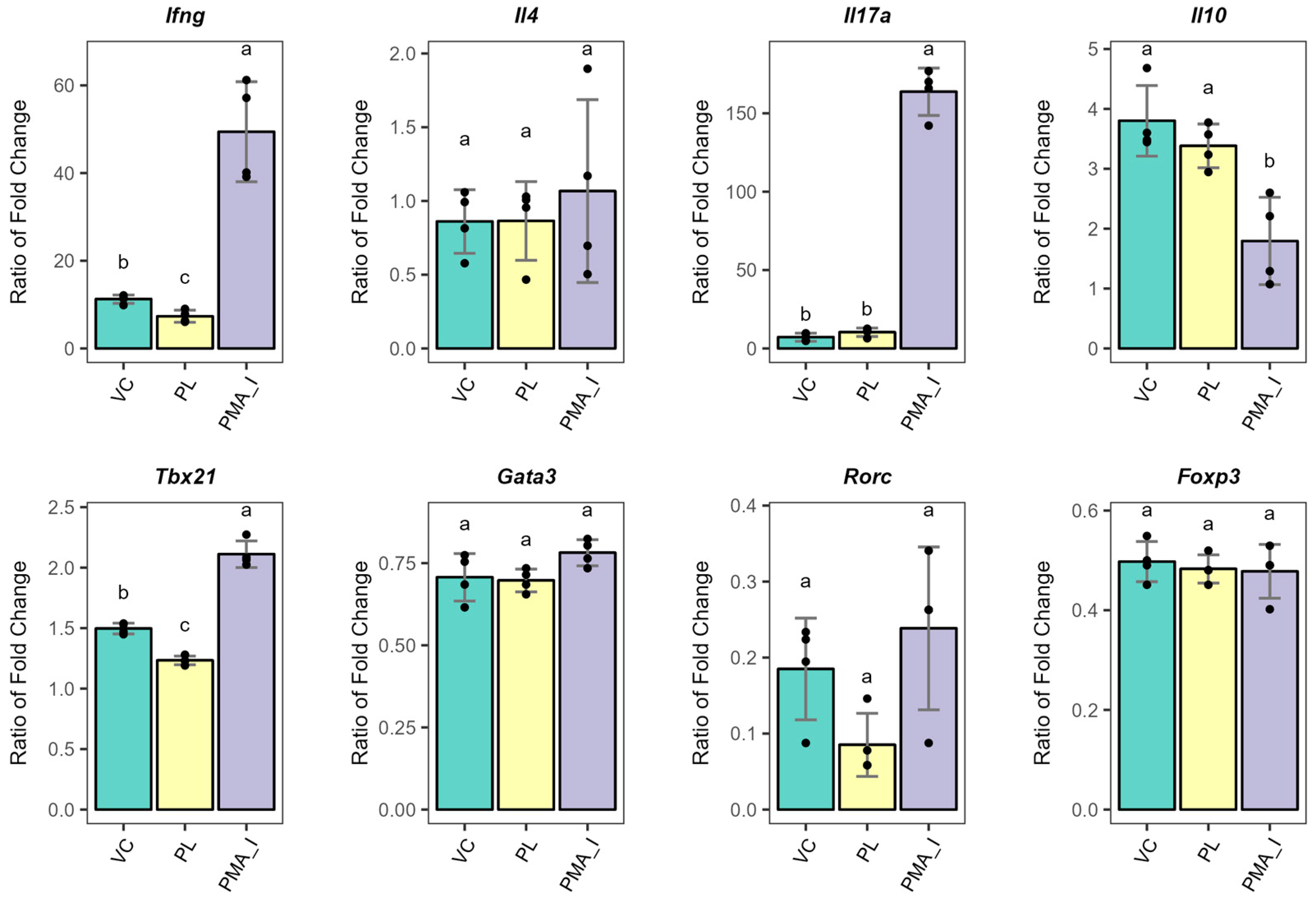
3.3. PL-Stimulated BMDCs Facilitate the Expression of Anergy-Related Genes in T Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| T1D | Type 1 Diabetes |
| BB-DP | Diabetic-prone rats |
| BB-DR | Diabetic-resistant rats |
| MLNs | Mesenteric lymph nodes |
| EVs | Extracellular vesicles |
| AHR | Aryl hydrocarbon receptor |
| ICAM-1 | Intercellular adhesion molecule 1 |
| PAMPs | Pathogen-associated molecular patterns |
| PRR | Pattern recognition receptors |
| BMDCs | Bone marrow dendritic cells |
| LPPs | Lipoproteins |
| LPS | Lipopolysaccharide |
| Ags | Antigens |
| PL | Phospholipids |
| SD | Sprague-Dawley |
| PBMCs | Peripheral blood mononuclear cells |
| MACS | Magnetic activated cell sorting |
| TFs | Transcription factors |
| Abs | Antibodies |
| RT | Room temperature |
| MFI | Median fluorescence intensity |
| TCR | T cell receptor |
| PMA | Phorbol-12-myristate-13-acetate |
| PMA_I | PMA and Ionomycin |
| CL | Cardiolipin |
| PG | Phosphatidylglycerol |
| PE | Phosphatidylethanolamine |
| DCs | Dendritic cells |
| PS | Phosphatidylserine |
| Treg | Regulatory T cells |
| OAS | 2′,5′-oligoadenylate synthetase |
| VC | Vehicle control |
| TL | Total lipids |
References
- Valladares, R.; Sankar, D.; Li, N.; Williams, E.; Lai, K.K.; Abdelgeliel, A.S.; Gonzalez, C.F.; Wasserfall, C.H.; Larkin, J.; Schatz, D.; et al. Lactobacillus johnsonii N6.2 Mitigates the Development of Type 1 Diabetes in BB-DP Rats. PLoS ONE 2010, 5, e10507. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.; Benitez, P.; Ardissone, A.; Wilson, T.D.; Collins, E.L.; Lorca, G.; Li, N.; Sankar, D.; Wasserfall, C.; Neu, J.; et al. Inhibition of Type 1 Diabetes Correlated to a Lactobacillus johnsonii N6.2-Mediated Th17 Bias. J. Immunol. 2011, 186, 3538–3546. [Google Scholar] [CrossRef]
- Marcial, G.E.; Ford, A.L.; Haller, M.J.; Gezan, S.A.; Harrison, N.A.; Cai, D.; Meyer, J.L.; Perry, D.J.; Atkinson, M.A.; Wasserfall, C.H.; et al. Lactobacillus johnsonii N6.2 Modulates the Host Immune Responses: A Double-Blind, Randomized Trial in Healthy Adults. Front. Immunol. 2017, 8, 655. [Google Scholar] [CrossRef]
- Teixeira, L.D.; Harrison, N.A.; da Silva, D.R.; Mathews, C.E.; Gonzalez, C.F.; Lorca, G.L. Nanovesicles from Lactobacillus johnsonii N6.2 Reduce Apoptosis in Human Beta Cells by Promoting AHR Translocation and IL10 Secretion. Front. Immunol. 2022, 13, 899413. [Google Scholar] [CrossRef]
- Cuaycal, A.E.; Teixeira, L.D.; Lorca, G.L.; Gonzalez, C.F. Lactobacillus johnsonii N6.2 Phospholipids Induce Immature-like Dendritic Cells with a Migratory-Regulatory-like Transcriptional Signature. Gut Microbes 2023, 15, 2252447. [Google Scholar] [CrossRef]
- Ernst, R.K.; Chandler, C.E. Bacterial Lipids: Powerful Modifiers of the Innate Immune Response. F1000Research 2017, 6, 1334. [Google Scholar]
- Nigou, J.; Zelle-Rieser, C.; Gilleron, M.; Thurnher, M.; Puzo, G. Mannosylated Lipoarabinomannans Inhibit IL-12 Production by Human Dendritic Cells: Evidence for a Negative Signal Delivered Through the Mannose Receptor. J. Immunol. 2001, 166, 7477–7485. [Google Scholar] [CrossRef]
- Geijtenbeek, T.B.H.; Van Vliet, S.J.; Koppel, E.A.; Sanchez-Hernandez, M.; Vandenbroucke-Grauls, C.M.J.E.; Appelmelk, B.; Van Kooyk, Y. Mycobacteria Target DC-SIGN to Suppress Dendritic Cell Function. J. Exp. Med. 2003, 197, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Cambier, C.J.; Takaki, K.K.; Larson, R.P.; Hernandez, R.E.; Tobin, D.M.; Urdahl, K.B.; Cosma, C.L.; Ramakrishnan, L. Mycobacteria Manipulate Macrophage Recruitment through Coordinated Use of Membrane Lipids. Nature 2013, 505, 218–222. [Google Scholar] [CrossRef]
- Blanc, L.; Gilleron, M.; Prandi, J.; Song, O.-R.; Jang, M.S.; Gicquel, B.; Drocourt, D.; Neyrolles, O.; Brodin, P.; Tiraby, G.; et al. Mycobacterium tuberculosis Inhibits Human Innate Immune Responses via the Production of TLR2 Antagonist Glycolipids. Proc. Natl. Acad. Sci. USA 2017, 114, 11205–11210. [Google Scholar] [CrossRef]
- Chandler, C.E.; Harberts, E.M.; Pelletier, M.R.; Thaipisuttikul, I.; Jones, J.W.; Hajjar, A.M.; Sahl, J.W.; Goodlett, D.R.; Pride, A.C.; Rasko, D.A.; et al. Early Evolutionary Loss of the Lipid A Modifying Enzyme PagP Resulting in Innate Immune Evasion in Yersinia pestis. Proc. Natl. Acad. Sci. USA 2020, 117, 22984–22991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Linke, V.; Overmyer, K.A.; Traeger, L.L.; Kasahara, K.; Miller, I.J.; Manson, D.E.; Polaske, T.J.; Kerby, R.L.; Kemis, J.H.; et al. Genetic Mapping of Microbial and Host Traits Reveals Production of Immunomodulatory Lipids by Akkermansia muciniphila in the Murine Gut. Nat. Microbiol. 2023, 8, 424–440. [Google Scholar] [CrossRef] [PubMed]
- Wieland Brown, L.C.; Penaranda, C.; Kashyap, P.C.; Williams, B.B.; Clardy, J.; Kronenberg, M.; Sonnenburg, J.L.; Comstock, L.E.; Bluestone, J.A.; Fischbach, M.A. Production of α-Galactosylceramide by a Prominent Member of the Human Gut Microbiota. PLoS Biol. 2013, 11, e1001610. [Google Scholar] [CrossRef]
- An, D.; Oh, S.F.; Olszak, T.; Neves, J.F.; Avci, F.Y.; Erturk-Hasdemir, D.; Lu, X.; Zeissig, S.; Blumberg, R.S.; Kasper, D.L. Sphingolipids from a Symbiotic Microbe Regulate Homeostasis of Host Intestinal Natural Killer T Cells. Cell 2014, 156, 123–133. [Google Scholar] [CrossRef]
- von Gerichten, J.; Lamprecht, D.; Opálka, L.; Soulard, D.; Marsching, C.; Pilz, R.; Sencio, V.; Herzer, S.; Galy, B.; Nordström, V.; et al. Bacterial Immunogenic α-Galactosylceramide Identified in the Murine Large Intestine: Dependency on Diet and Inflammation. J. Lipid Res. 2019, 60, 1892–1904. [Google Scholar] [CrossRef]
- Johnson, E.L.; Heaver, S.L.; Waters, J.L.; Kim, B.I.; Bretin, A.; Goodman, A.L.; Gewirtz, A.T.; Worgall, T.S.; Ley, R.E. Sphingolipids Produced by Gut Bacteria Enter Host Metabolic Pathways Impacting Ceramide Levels. Nat. Commun. 2020, 11, 2471. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.F.; Praveena, T.; Song, H.; Yoo, J.S.; Jung, D.J.; Erturk-Hasdemir, D.; Hwang, Y.S.; Lee, C.W.C.; Le Nours, J.; Kim, H.; et al. Host Immunomodulatory Lipids Created by Symbionts from Dietary Amino Acids. Nature 2021, 600, 302–307. [Google Scholar] [CrossRef]
- Bae, M.; Cassilly, C.D.; Liu, X.; Park, S.-M.; Tusi, B.K.; Chen, X.; Kwon, J.; Filipčík, P.; Bolze, A.S.; Liu, Z.; et al. Akkermansia muciniphila Phospholipid Induces Homeostatic Immune Responses. Nature 2022, 608, 168–173. [Google Scholar] [CrossRef]
- Smits, H.H.; Engering, A.; Van Der Kleij, D.; De Jong, E.C.; Schipper, K.; Van Capel, T.M.M.; Zaat, B.A.J.; Yazdanbakhsh, M.; Wierenga, E.A.; Van Kooyk, Y.; et al. Selective Probiotic Bacteria Induce IL-10–Producing Regulatory T Cells in Vitro by Modulating Dendritic Cell Function through Dendritic Cell–Specific Intercellular Adhesion Molecule 3–Grabbing Nonintegrin. J. Allergy Clin. Immunol. 2005, 115, 1260–1267. [Google Scholar] [CrossRef]
- Eslami, S.; Hadjati, J.; Motevaseli, E.; Mirzaei, R.; Farashi Bonab, S.; Ansaripour, B.; Khoramizadeh, M.R. Lactobacillus crispatus Strain SJ-3C-US Induces Human Dendritic Cells (DCs) Maturation and Confers an Anti-Inflammatory Phenotype to DCs. Apmis 2016, 124, 697–710. [Google Scholar] [CrossRef]
- Thakur, B.K.; Saha, P.; Banik, G.; Saha, D.R.; Grover, S.; Batish, V.K.; Das, S. Live and Heat-Killed Probiotic Lactobacillus casei Lbs2 Protects from Experimental Colitis through Toll-like Receptor 2-Dependent Induction of T-Regulatory Response. Int. Immunopharmacol. 2016, 36, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.S.; Lim, S.K.; Jang, J.Y.; Lee, J.; Park, H.K.; Kim, N.; Yun, M.; Shin, M.Y.; Jo, H.E.; Oh, Y.J.; et al. Lactobacillus sakei WIKIM30 Ameliorates Atopic Dermatitis-like Skin Lesions by Inducing Regulatory T Cells and Altering Gut Microbiota Structure in Mice. Front. Immunol. 2018, 9, 1905. [Google Scholar] [CrossRef]
- Lee, J.; Jang, J.Y.; Kwon, M.S.; Lim, S.K.; Kim, N.; Lee, J.; Park, H.K.; Yun, M.; Shin, M.Y.; Jo, H.E.; et al. Mixture of Two Lactobacillus plantarum Strains Modulates the Gut Microbiota Structure and Regulatory T Cell Response in Diet-Induced Obese Mice. Mol. Nutr. Food Res. 2018, 62, 1800329. [Google Scholar] [CrossRef] [PubMed]
- Audiger, C.; Rahman, M.J.; Yun, T.J.; Tarbell, K.V.; Lesage, S. The Importance of Dendritic Cells in Maintaining Immune Tolerance. J. Immunol. 2017, 198, 2223–2231. [Google Scholar] [CrossRef]
- Chancellor, A.; Gadola, S.D.; Mansour, S. The Versatility of the CD1 Lipid Antigen Presentation Pathway. Immunology 2018, 154, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Luciani, C.; Hager, F.T.; Cerovic, V.; Lelouard, H. Dendritic Cell Functions in the Inductive and Effector Sites of Intestinal Immunity. Mucosal Immunol. 2021, 15, 40–50. [Google Scholar] [CrossRef]
- Zhao, Q.; Elson, C.O. Adaptive Immune Education by Gut Microbiota Antigens. Immunology 2018, 154, 28–37. [Google Scholar] [CrossRef]
- Bourque, J.; Hawiger, D. Variegated Outcomes of T Cell Activation by Dendritic Cells in the Steady State. J. Immunol. 2022, 208, 539–547. [Google Scholar] [CrossRef]
- Domogalla, M.P.; Rostan, P.V.; Raker, V.K.; Steinbrink, K. Tolerance through Education: How Tolerogenic Dendritic Cells Shape Immunity. Front. Immunol. 2017, 8, 1764. [Google Scholar] [CrossRef]
- Lutz, M.B.; Backer, R.A.; Clausen, B.E. Revisiting Current Concepts on the Tolerogenicity of Steady-State Dendritic Cell Subsets and Their Maturation Stages. J. Immunol. 2021, 206, 1681–1689. [Google Scholar] [CrossRef]
- Teixeira, L.D.; Kling, D.N.; Lorca, G.L.; Gonzalez, C.F. Lactobacillus johnsonii N6.2 Diminishes Caspase-1 Maturation in the Gastrointestinal System of Diabetes Prone Rats. Benef. Microbes 2018, 9, 527–539. [Google Scholar] [CrossRef]
- Dickson, L.; Bull, I.D.; Gates, P.J.; Evershed, R.P. A Simple Modification of a Silicic Acid Lipid Fractionation Protocol to Eliminate Free Fatty Acids from Glycolipid and Phospholipid Fractions. J. Microbiol. Methods 2009, 78, 249–254. [Google Scholar] [CrossRef]
- Donovan, J.; Brown, P. Blood Collection. Curr. Protoc. Immunol. 2006, 73, 1.7.1–1.7.9. [Google Scholar] [CrossRef] [PubMed]
- Fuss, I.J.; Kanof, M.E.; Smith, P.D.; Zola, H. Isolation of Whole Mononuclear Cells from Peripheral Blood and Cord Blood. Curr. Protoc. Immunol. 2009, 85, 7.1.1–7.1.8. [Google Scholar] [CrossRef]
- Rathmell, J.C.; Farkash, E.A.; Gao, W.; Thompson, C.B. IL-7 Enhances the Survival and Maintains the Size of Naive T Cells. J. Immunol. 2001, 167, 6869–6876. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.T.; Dudl, E.; LeRoy, E.; Murray, R.; Sprent, J.; Weinberg, K.I.; Surh, C.D. IL-7 Is Critical for Homeostatic Proliferation and Survival of Naïve T Cells. Proc. Natl. Acad. Sci. USA 2001, 98, 8732–8737. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.E.; Bishop, K.D.; Phillips, N.E.; Mordes, J.P.; Greiner, D.L.; Rossini, A.A.; Czech, M.P. Early Growth Response Gene-2, a Zinc-Finger Transcription Factor, Is Required for Full Induction of Clonal Anergy in CD4+ T Cells. J. Immunol. 2004, 173, 7331–7338. [Google Scholar] [CrossRef]
- Kalekar, L.A.; Schmiel, S.E.; Nandiwada, S.L.; Lam, W.Y.; Barsness, L.O.; Zhang, N.; Stritesky, G.L.; Malhotra, D.; Pauken, K.E.; Linehan, J.L.; et al. CD4+ T Cell Anergy Prevents Autoimmunity and Generates Regulatory T Cell Precursors. Nat. Immunol. 2016, 17, 304–314. [Google Scholar] [CrossRef]
- Kalekar, L.A.; Mueller, D.L. Relationship between CD4 Regulatory T Cells and Anergy In Vivo. J. Immunol. 2017, 198, 2527–2533. [Google Scholar] [CrossRef]
- Safford, M.; Collins, S.; Lutz, M.A.; Allen, A.; Huang, C.T.; Kowalski, J.; Blackford, A.; Horton, M.R.; Drake, C.; Schwartz, R.H.; et al. Egr-2 and Egr-3 Are Negative Regulators of T Cell Activation. Nat. Immunol. 2005, 6, 472–480. [Google Scholar] [CrossRef]
- Anandasabapathy, N.; Ford, G.S.; Bloom, D.; Holness, C.; Paragas, V.; Seroogy, C.; Skrenta, H.; Hollenhorst, M.; Fathman, C.G.; Soares, L. GRAIL: An E3 Ubiquitin Ligase That Inhibits Cytokine Gene Transcription Is Expressed in Anergic CD4+ T Cells. Immunity 2003, 18, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, S.; Okahashi, N.; Tsugawa, H.; Ogata, Y.; Ikeda, K.; Suda, W.; Arai, H.; Hattori, M.; Arita, M. Elucidation of Gut Microbiota-Associated Lipids Using LC-MS/MS and 16S RRNA Sequence Analyses. iScience 2020, 23, 101841. [Google Scholar] [CrossRef]
- Nagatake, T.; Kishino, S.; Urano, E.; Murakami, H.; Kitamura, N.; Konishi, K.; Ohno, H.; Tiwari, P.; Morimoto, S.; Node, E.; et al. Intestinal Microbe-Dependent Ω3 Lipid Metabolite αKetoA Prevents Inflammatory Diseases in Mice and Cynomolgus Macaques. Mucosal Immunol. 2022, 15, 289–300. [Google Scholar] [CrossRef]
- Li, F.; Hao, X.; Chen, Y.; Bai, L.; Gao, X.; Lian, Z.; Wei, H.; Sun, R.; Tian, Z. The Microbiota Maintain Homeostasis of Liver-Resident ΓδT-17 Cells in a Lipid Antigen/CD1d-Dependent Manner. Nat. Commun. 2017, 8, 13839. [Google Scholar] [CrossRef]
- Monnot, G.C.; Wegrecki, M.; Cheng, T.Y.; Chen, Y.L.; Sallee, B.N.; Chakravarthy, R.; Karantza, I.M.; Tin, S.Y.; Khaleel, A.E.; Monga, I.; et al. Staphylococcal Phosphatidylglycerol Antigens Activate Human T Cells via CD1a. Nat. Immunol. 2022, 24, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.C.; Brown, B.D.; Shay, T.; Gautier, E.L.; Jojic, V.; Cohain, A.; Pandey, G.; Leboeuf, M.; Elpek, K.G.; Helft, J.; et al. Deciphering the Transcriptional Network of the Dendritic Cell Lineage. Nat. Immunol. 2012, 13, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Segura, E. Decoding the Heterogeneity of Human Dendritic Cell Subsets. Trends Immunol. 2020, 41, 1062–1071. [Google Scholar] [CrossRef]
- Dong, G.; Wang, Y.; Xiao, W.; Pacios Pujado, S.; Xu, F.; Tian, C.; Xiao, E.; Choi, Y.; Graves, D.T. FOXO1 Regulates Dendritic Cell Activity through ICAM-1 and CCR7. J. Immunol. 2015, 194, 3745–3755. [Google Scholar] [CrossRef]
- Katakai, T.; Habiro, K.; Kinashi, T. Dendritic Cells Regulate High-Speed Interstitial T Cell Migration in the Lymph Node via LFA-1/ICAM-1. J. Immunol. 2013, 191, 1188–1199. [Google Scholar] [CrossRef]
- Kozlovski, S.; Atrakchi, O.; Feigelson, S.W.; Shulman, Z.; Alon, R. Stable Contacts of Naïve CD4 T Cells with Migratory Dendritic Cells Are ICAM-1-Dependent but Dispensable for Proliferation in Vivo. Cell Adhes. Migr. 2019, 13, 315–321. [Google Scholar] [CrossRef]
- Pui-Yan Ma, V.; Hu, Y.; Kellner, A.V.; Brockman, J.M.; Velusamy, A.; Blanchard, A.T.; Evavold, B.D.; Alon, R.; Salaita, K. The Magnitude of LFA-1/ICAM-1 Forces Fine-Tune TCR-Triggered T Cell Activation. Sci. Adv. 2022, 8, 4485. [Google Scholar] [CrossRef]
- Comrie, W.A.; Li, S.; Boyle, S.; Burkhardt, J.K. The Dendritic Cell Cytoskeleton Promotes T Cell Adhesion and Activation by Constraining ICAM-1 Mobility. J. Cell Biol. 2015, 208, 457–473. [Google Scholar] [CrossRef]
- Schwartz, R.H. T Cell Anergy. Annu. Rev. Immunol. 2003, 21, 305–334. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Fernandez, S.; Pujol-Autonell, I.; Brianso, F.; Perna-Barrull, D.; Cano-Sarabia, M.; Garcia-Jimeno, S.; Villalba, A.; Sanchez, A.; Aguilera, E.; Vazquez, F.; et al. Phosphatidylserine-Liposomes Promote Tolerogenic Features on Dendritic Cells in Human Type 1 Diabetes by Apoptotic Mimicry. Front. Immunol. 2018, 9, 314289. [Google Scholar] [CrossRef] [PubMed]
- Vukman, K.V.; Adams, P.N.; O’Neill, S.M. Fasciola hepatica Tegumental Coat Antigen Suppresses MAPK Signalling in Dendritic Cells and Up-Regulates the Expression of SOCS3. Parasite Immunol. 2013, 35, 234–238. [Google Scholar] [CrossRef]
- Aldridge, A.; O’Neill, S.M. Fasciola hepatica Tegumental Antigens Induce Anergic-like T Cells via Dendritic Cells in a Mannose Receptor-Dependent Manner. Eur. J. Immunol. 2016, 46, 1180–1192. [Google Scholar] [CrossRef]
- Wang, M.; Wu, L.; Weng, R.; Zheng, W.; Wu, Z.; Lv, Z. Therapeutic Potential of Helminths in Autoimmune Diseases: Helminth-Derived Immune-Regulators and Immune Balance. Parasitol. Res. 2017, 116, 2065–2074. [Google Scholar] [CrossRef]
- Bradley, L.M.; Haynes, L.; Swain, S.L. IL-7: Maintaining T-Cell Memory and Achieving Homeostasis. Trends Immunol. 2005, 26, 172–176. [Google Scholar] [CrossRef]
- Rout, N. Enhanced Th1/Th17 Functions of CD161+ CD8+ T Cells in Mucosal Tissues of Rhesus Macaques. PLoS ONE 2016, 11, e0157407. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Uebele, J.; Kumari, N.; Nakayama, H.; Peter, L.; Ticha, O.; Woischnig, A.K.; Schmaler, M.; Khanna, N.; Dohmae, N.; et al. Lipid Moieties on Lipoproteins of Commensal and Non-Commensal Staphylococci Induce Differential Immune Responses. Nat. Commun. 2017, 8, 2246. [Google Scholar] [CrossRef]
- Galdeano, C.M.; De Moreno De Leblanc, A.; Carmuega, E.; Weill, R.; Perdigón, G. Mechanisms Involved in the Immunostimulation by Probiotic Fermented Milk. J. Dairy. Res. 2009, 76, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Zeng, M.Y.; Núñez, G. The Interplay between Host Immune Cells and Gut Microbiota in Chronic Inflammatory Diseases. Exp. Mol. Med. 2017, 49, e339. [Google Scholar] [CrossRef] [PubMed]
- Beliakoff, R.E.; Gonzalez, C.F.; Lorca, G.L. Bile Promotes Lactobacillus johnsonii N6.2 Extracellular Vesicle Production with Conserved Immunomodulatory Properties. Sci. Rep. 2024, 14, 12272. [Google Scholar] [CrossRef]
- da Silva, D.R.; Sharjeel, A.B.; Beliakoff, R.; Teixeira, L.D.; Kima, P.E.; Jones, M.K.; Gonzalez, C.F.; Lorca, G.L. The Sdp-SH3b2 Domain Contained in Lactobacillus johnsonii N6.2-Derived Extracellular Vesicles Inhibit Murine Norovirus Replication. Front. Immunol. 2024, 15, 1490755. [Google Scholar] [CrossRef]
- da Silva, D.R.; Gonzalez, C.F.; Lorca, G. Internalization of Extracellular Vesicles from Lactobacillus johnsonii N6.2 Elicit an RNA Sensory Response in Human Pancreatic Cell Lines. J. Extracell. Biol. 2023, 2, e101. [Google Scholar] [CrossRef]
- Kingma, S.D.K.; Li, N.; Sun, F.; Valladares, R.B.; Neu, J.; Lorca, G.L. Lactobacillus johnsonii N6.2 Stimulates the Innate Immune Response through Toll-Like Receptor 9 in Caco-2 Cells and Increases Intestinal Crypt Paneth Cell Number in BioBreeding Diabetes-Prone Rats. J. Nutr. 2011, 141, 1023–1028. [Google Scholar] [CrossRef]




| Gene | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) |
|---|---|---|
| Cd274 | CAGTCTCCTCGCCTACAGGT | GCTGTGATGGTAAATGCCGC |
| Arg1 | CAGTATTCACCCCGGCTACG | AGTCCTGAAAGTAGCCCTGTCT |
| Hcar2 | ACATGATGACCCGAAACGGC | AGCAGAACAGGATGATGCCC |
| Jag1 | ATGCCTCCTGTCGGGATTTG | CAGTGACCCCCATTCAAGCA |
| Ido1 * | AGCACTGGAGAAGGCACTGT | ACGTGGAAAAAGGTGTCTGG |
| Nos2 ** | CTCACTGTGGCTGTGGTCACCTA | GGGTCTTCGGGCTTCAGGTTA |
| Actb | ACACCCGCCACCAGTTCG | CACGATGGAGGGGAAGACGG |
| Ifng | GTGTCATCGAATCGCACCTGA | GATCTGTGGGTTGTTCACCTCG |
| Il4 | TTACGGCAACAAGGAACACCA | CACCGAGAACCCCAGACTTG |
| Il17a | CCTGGACTCTGAGCCGCAAT | ACTTCCCCTCAGCGTTGACA |
| Il10 | CTGGTAGAAGTGATGCCCCA | GGAGAAATCGATGACAGCGT |
| Tbx21 | GAGCCCACGAGCCATTACAG | CGTATAAGCGGTTCCCTGGC |
| Gata3 | ATGGTCAAGGCAACCACGTC | CATACCTGGCTCCCGTGGTG |
| Rorc | GTACGTGGTGGAGTTCGCC | CGACTTCCATTGCTCCTGCTT |
| Foxp3 | ACCCAGGAAAGACAGCAACCTT | TTCTCACAACCCGGCCACTT |
| Egr2 | CTGCCTGACAGCCTCTACCC | CAATGTTGATCATGCCATCTCCAG |
| Nrp1 | TGGGCTGTGAAGTAGAAGTGCC | CTCCTGTGAGCTGGAAGTCATC |
| Grail | AGCTCTGGGAATTGAGGTGGA | GTTGTCCTCTTCGTGGGGAG |
| Itch | TCGCTGTAGTCGGGGCT | GTGAAATGCATGTTACCGGGAC |
| Il2 | TGTTGCTGGACTTACAGGTGC | ATGTTTCAATTCTGTGGCCTGCTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuaycal, A.E.; Torrez Lamberti, M.F.; Lorca, G.L.; Gonzalez, C.F. Lactobacillus johnsonii N6.2 Phospholipids Induce T Cell Anergy upon Cognate Dendritic Cell Interactions. Metabolites 2025, 15, 284. https://doi.org/10.3390/metabo15050284
Cuaycal AE, Torrez Lamberti MF, Lorca GL, Gonzalez CF. Lactobacillus johnsonii N6.2 Phospholipids Induce T Cell Anergy upon Cognate Dendritic Cell Interactions. Metabolites. 2025; 15(5):284. https://doi.org/10.3390/metabo15050284
Chicago/Turabian StyleCuaycal, Alexandra E., Monica F. Torrez Lamberti, Graciela L. Lorca, and Claudio F. Gonzalez. 2025. "Lactobacillus johnsonii N6.2 Phospholipids Induce T Cell Anergy upon Cognate Dendritic Cell Interactions" Metabolites 15, no. 5: 284. https://doi.org/10.3390/metabo15050284
APA StyleCuaycal, A. E., Torrez Lamberti, M. F., Lorca, G. L., & Gonzalez, C. F. (2025). Lactobacillus johnsonii N6.2 Phospholipids Induce T Cell Anergy upon Cognate Dendritic Cell Interactions. Metabolites, 15(5), 284. https://doi.org/10.3390/metabo15050284








