The Lipid Composition of the Exo-Metabolome from Haemonchus contortus
Abstract
1. Introduction
2. Methods
2.1. Experimental Infection of Sheep with Haemonchus contortus
2.2. Culture of H. contortus Adult Worms for Exo-Metabolome Collection
2.3. Global Lipidomic Analysis on H. contortus Culture Medium
2.4. Samples Preparation and Lipid Extraction
2.5. Liquid Chromatography–Mass Spectrometry (LC-MS) Analysis Condition
2.6. Lipid Annotation
2.7. Data Normalization
2.8. Statistical Analysis
3. Results
3.1. Lipids Identification
3.2. Lipid Classification Based on Carbon Composition
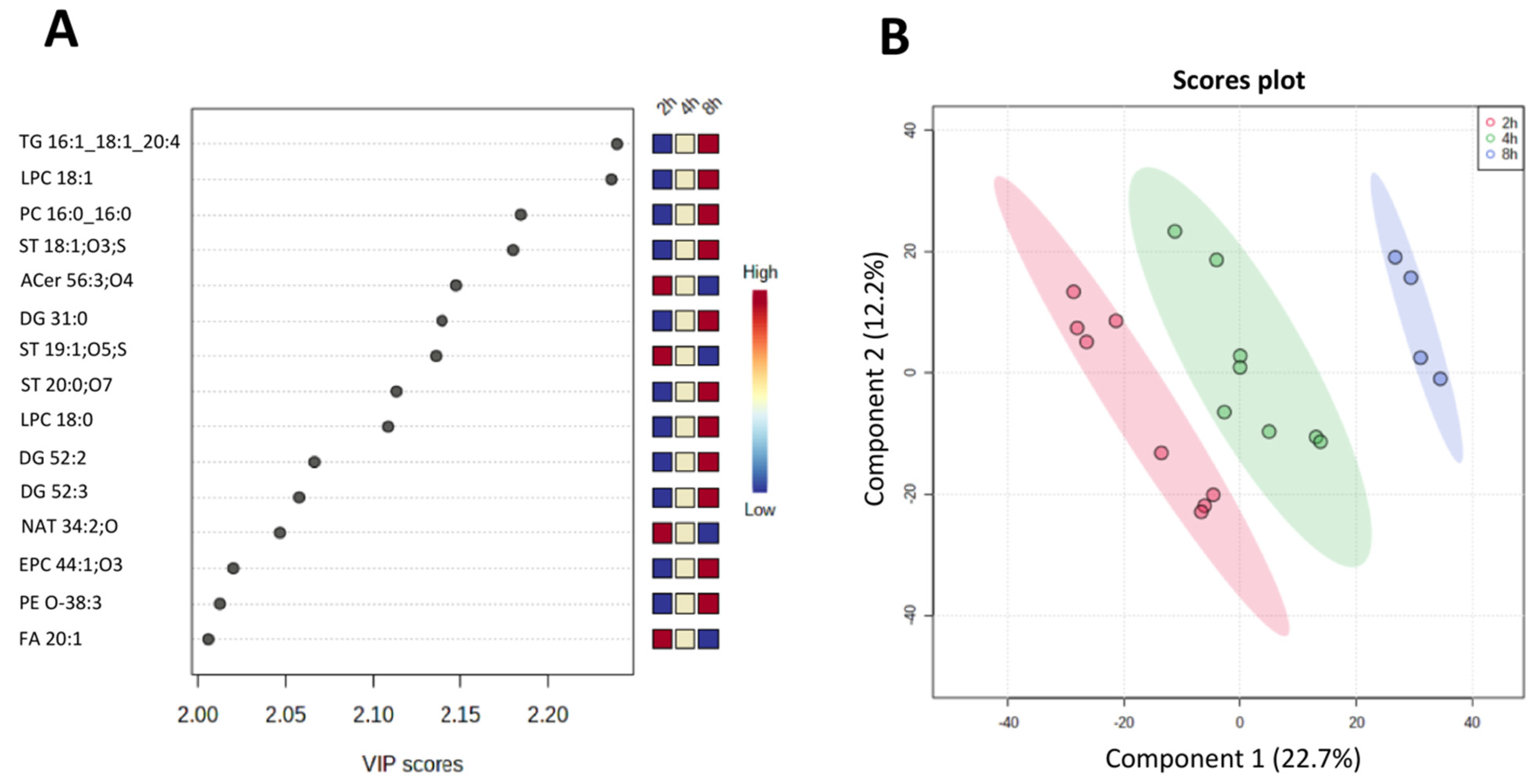
3.3. Statistical and Multivariate Analyses
4. Discussion
5. Conclusions
6. Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vercruysse, J.; Charlier, J.; Van Dijk, J.; Morgan, E.R.; Geary, T.; von Samson-Himmelstjerna, G.; Claerebout, E. Control of helminth ruminant infections by 2030. Parasitology 2018, 145, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Emery, D.L.; Hunt, P.W.; Le Jambre, L.F. Haemonchus contortus: The then and now, and where to from here? Int. J. Parasitol. 2016, 46, 755–769. [Google Scholar] [CrossRef] [PubMed]
- Besier, R.B.; Kahn, L.P.; Sargison, N.D.; Van Wyk, J.A. The pathophysiology, ecology and epidemiology of Haemonchus contortus infection in small ruminants. Adv. Parasitol. 2016, 93, 95–143. [Google Scholar] [CrossRef] [PubMed]
- Bordes, L.; Dumont, N.; Lespine, A.; Souil, E.; Sutra, J.F.; Prévot, F.; Grisez, C.; Romanos, L.; Dailledouze, A.; Jacquiet, P. First report of multiple resistance to eprinomectin and benzimidazole in Haemonchus contortus on a dairy goat farm in France. Parasitol. Int. 2020, 76, 102063. [Google Scholar] [CrossRef]
- Geurden, T.; Chartier, C.; Fanke, J.; Di Regalbono, A.F.; Traversa, D.; von Samson-Himmelstjerna, G.; Demeler, J.; Vanimisetti, H.B.; Bartram, D.J.; Denwood, M.J. Anthelmintic resistance to ivermectin and moxidectin in gastrointestinal nematodes of cattle in Europe. Int. J. Parasitol. Drugs Drug Resist. 2015, 5, 163–171. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Deng, Q.; Umair, S.; Savoian, M.S.; Knight, J.S.; Pernthaner, A.; Simpson, H.V. Excretory/secretory products of adult Haemonchus contortus and Teladorsagia circumcincta which increase the permeability of Caco-2 cell monolayers are neutralised by antibodies from immune hosts. Vet. Parasitol. 2016, 221, 104–110. [Google Scholar] [CrossRef]
- Wangchuk, P.; Kouremenos, K.; Eichenberger, R.M.; Pearson, M.; Susianto, A.; Wishart, D.S.; McConville, M.J.; Loukas, A. Metabolomic profiling of the excretory–secretory products of hookworm and whipworm. Metabolomics 2019, 15, 101. [Google Scholar] [CrossRef]
- Karanu, F.N.; Rurangirwa, F.R.; McGuire, T.C.; Jasmer, D.P. Haemonchus contortus: Identification of proteases with diverse characteristics in adult worm excretory-secretory products. Exp. Parasitol. 1993, 77, 362–371. [Google Scholar] [CrossRef]
- Bu, Y.; Cao, M.; Tian, X.; Lu, M.; Li, J.; Mao, D.; Yu, L.; Memon, M.A.; Li, C.; Xu, L.; et al. HcFAR, a functional inhibitor of goat TGF-β1 identified from excretory and secretory products of Haemonchus contortus. Vet Parasitol. 2020, 286, 109236. [Google Scholar] [CrossRef]
- Dong, C.; Reilly, D.K.; Bergame, C.; Dolke, F.; Srinivasan, J.; von Reuss, S.H. Comparative ascaroside profiling of Caenorhabditis exometabolomes reveals species-specific (ω) and (ω-2)-hydroxylation downstream of peroxisomal β-oxidation. J. Org. Chem. 2018, 83, 7109–7120. [Google Scholar] [CrossRef]
- Britton, C.; Laing, R.; Devaney, E. Small RNAs in parasitic nematodes—Forms and functions. Parasitology 2020, 147, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Wangchuk, P.; Lavers, O.; Wishart, D.S.; Loukas, A. Excretory/Secretory metabolome of the zoonotic roundworm parasite Toxocara canis. Biomolecules 2020, 10, 1157. [Google Scholar] [CrossRef] [PubMed]
- Drurey, C.; Maizels, R.M. Helminth extracellular vesicles: Interactions with the host immune system. Mol. Immunol. 2021, 137, 124–133. [Google Scholar] [CrossRef]
- Maizels, R.M.; Smits, H.H.; McSorley, H.J. Modulation of host immunity by helminths: The expanding repertoire of parasite effector molecules. Immunity 2018, 49, 801–818. [Google Scholar] [CrossRef] [PubMed]
- Rehman, Z.U.; Knight, J.S.; Koolaard, J.; Simpson, H.V.; Pernthaner, A. Immunomodulatory effects of adult Haemonchus contortus excretory/secretory products on human monocyte-derived dendritic cells. Parasite Immunol. 2015, 37, 657–669. [Google Scholar] [CrossRef]
- Gadahi, J.A.; Wang, S.; Bo, G.; Ehsan, M.; Yan, R.; Song, X.; Xu, L.; Li, X. Proteomic analysis of the excretory and secretory proteins of Haemonchus contortus (HcESP) binding to goat PBMCs in vivo revealed stage-specific binding profiles. PLoS ONE. 2016, 11, e0159796. [Google Scholar] [CrossRef]
- Burren, C.H.; Ehrlich, I.; Johnson, P. Excretion of lipids by the liver fluke (Fasciola hepatica). Lipids 1967, 2, 353–356. [Google Scholar] [CrossRef]
- Niedfeld, G.; Pezzani, B.; Minvielle, M.; Basualdo Farjat, J.A. Presence of lipids in the secretory/excretory product from Toxocara canis. Vet. Parasitol. 1993, 51, 155–158. [Google Scholar] [CrossRef]
- Giera, M.; Kaisar, M.M.; Derks, R.J.; Steenvoorden, E.; Kruize, Y.C.; Hokke, C.H.; Yazdanbakhsh, M.; Everts, B. The Schistosoma mansoni lipidome: Leads for immunomodulation. Anal Chim. Acta 2018, 1037, 107–118. [Google Scholar] [CrossRef]
- Wang, T.; Nie, S.; Reid, G.E.; Gasser, R.B. Helminth lipidomics: Technical aspects and future prospects. Curr. Res. Parasitol. Vector Borne Dis. 2021, 1, 100018. [Google Scholar] [CrossRef]
- van Meer, G.; de Kroon, A.I.P.M. Lipid map of the mammalian cell. J. Cell Sci. 2011, 124, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.A.; Kohlwein, S.D.; Carman, G.M. Metabolism and regulation of glycerolipids in the yeast Saccharomyces cerevisiae. Genetics 2012, 190, 317–349. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Devaiah, S.; Zhang, W.; Welti, R. Signaling functions of phosphatidic acid. Prog. Lipid Res. 2006, 45, 250–278. [Google Scholar] [CrossRef] [PubMed]
- Daugschies, A.; Joachim, A. Eicosanoids in parasites and parasitic infections. Adv. Parasitol 2000, 46, 181–240. [Google Scholar] [CrossRef]
- Laan, L.C.; Williams, A.R.; Stavenhagen, K.; Giera, M.; Kooij, G.; Vlasakov, I.; Kalay, H.; Kringel, H.; Nejsum, P.; Thamsborg, S.M.; et al. The whipworm (Trichuris suis) secretes prostaglandin E2 to suppress proinflammatory properties in human dendritic cells. FASEB J. 2017, 31, 719–731. [Google Scholar] [CrossRef]
- Wang, T.; Nie, S.; Ma, G.; Korhonen, P.K.; Koehler, A.V.; Ang, C.S.; Reid, G.E.; Williamson, N.A.; Gasser, R.B. The developmental lipidome of Haemonchus contortus. Int. J. Parasitol. 2018, 48, 887–895. [Google Scholar] [CrossRef]
- Wang, T.; Guangxu, M.; Shuai, N.; Williamson, N.A.; Reid, G.E.; Gasser, R.B. Lipid composition and abundance in the reproductive and alimentary tracts of female Haemonchus contortus. Parasites Vectors 2020, 13, 338. [Google Scholar] [CrossRef]
- Ranjan, S.; Wang, G.T.; Hirschlein, C.; Simkins, K.L. Selection for resistance to macrocyclic lactones by Haemonchus contortus in sheep. Vet. Parasitol. 2020, 103, 109–117. [Google Scholar] [CrossRef]
- Paras, K.L.; George, M.M.; Vidyashankar, A.N.; Kaplan, R.M. Comparison of fecal egg counting methods in four livestock species. Vet. Parasitol. 2018, 257, 21–27. [Google Scholar] [CrossRef]
- Witola, W.H.; Matthews, K.; McHugh, M. In vitro anthelmintic efficacy of inhibitors of phosphoethanolamine methyltransferases in Haemonchus contortus. Int. J. Parasitol. Drugs Drug Resist. 2016, 6, 44–53. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Zardini Buzatto, A.; Jabar, M.A.; Nizami, I.; Dasouki, M.; Li, L.; Abdel Rahman, A.M. Lipidome alterations induced by cystic fibrosis, CFTR mutation, and lung function. J. Proteome Res. 2021, 20, 549–564. [Google Scholar] [CrossRef] [PubMed]
- Zardini Buzatto, A.; Kwon, B.K.; Li, L. Development of a NanoLC-MS workflow for high-sensitivity global lipidomic analysis. Anal. Chim. Acta 2020, 1139, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Rakusanova, S.; Fiehn, O.; Cajka, T. Toward building mass spectrometry-based metabolomics and lipidomics atlases for biological and clinical research. TrAC Trends Anal. Chem. 2023, 158, 116825. [Google Scholar] [CrossRef]
- Sud, M.; Fahy, E.; Cotter, D.; Dennis, E.A.; Subramaniam, S. LIPID MAPS-nature lipidomics gateway: An online resource for students and educators interested in lipids. J. Chem. Educ. 2012, 89, 291–292. [Google Scholar] [CrossRef]
- Liebisch, G.; Fahy, E.; Aoki, J.; Dennis, E.A.; Durand, T.; Ejsing, C.S.; Fedorova, M.; Feussner, I.; Griffiths, W.J.; Köfeler, H.; et al. Update on LIPID MAPS classification, nomenclature, and shorthand notation for MS-derived lipid structures. J. Lipid Res. 2020, 61, 1539–1555. [Google Scholar] [CrossRef]
- Breitkopf, S.B.; Ricoult, S.J.H.; Yuan, M.; Xu, Y.; Peake, D.A.; Manning, B.D.; Asara, J.M. A relative quantitative positive/negative ion switching method for untargeted lipidomics via high resolution LC-MS/MS from any biological source. Metabolomics 2017, 13, 30. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of Extracellular Vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Gu, H.Y.; Marks, N.D.; Winter, A.D.; Weir, W.; Tzelos, T.; McNeilly, T.N.; Britton, C.; Devaney, E. Conservation of a MicroRNA cluster in parasitic nematodes and profiling of miRNAs in excretory-secretory products and microvesicles of Haemonchus contortus. PLoS Neglected Trop. Dis. 2017, 11, e0006056. [Google Scholar] [CrossRef]
- Simbari, F.; McCaskill, J.; Coakley, G.; Millar, M.; Maizels, R.M.; Fabriás, G.; Casas, J.; Buck, A.H. Plasmalogen enrichment in exosomes secreted by a nematode parasite versus those derived from its mouse host: Implications for exosome stability and biology. J. Extracell. Vesicles 2016, 5, 30741. [Google Scholar] [CrossRef]
- Franchini, G.R.; Pórfido, J.L.; Shimabukuro, M.I.; Burusco, M.F.R.; Bélgamo, J.A.; Smith, B.O.; Kennedy, M.W.; Córsico, B. The Unusual lipid binding proteins of parasitic helminths and their potential roles in parasitism and as therapeutic targets. Prostaglandins Leukot. Essent. Fat. Acids 2015, 93, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Yatsuda, A.P.; Krijgsveld, J.; Cornelissen, A.W.C.A.; Heck, A.J.R.; de Vries, E. Comprehensive analysis of the secreted proteins of the parasite Haemonchus contortus reveals extensive sequence variation and differential immune recognition. J. Biol. Chem. 2003, 278, 16941–16951. [Google Scholar] [CrossRef]
- Kennedy, M.W. The polyprotein lipid binding proteins of nematodes. Biochim. Biophys. Acta 2000, 1476, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Rustan, A.C.; Drevon, C.A. Fatty Acids: Structures and properties. In ELS, 1st ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005. [Google Scholar] [CrossRef]
- Feingold, K.R. Lipid and Lipoprotein Metabolism. Endocrinol. Metab. Clin. N. Am. 2022, 51, 437–458. [Google Scholar] [CrossRef] [PubMed]
- Kozan, D.W.; Derrick, J.T.; Ludington, W.B.; Farber, S.A. From worms to humans: Understanding intestinal lipid metabolism via model organisms. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2023, 1868, 159290. [Google Scholar] [CrossRef]
- Zhang, J.; Hashmi, S.; Cheema, F.; Al-Nasser, N.; Bakheet, R.; Parhar, R.S.; Al-Mohanna, F.; Gaugler, R.; Hussain, M.M.; Hashmi, S. Regulation of lipoprotein assembly, secretion and fatty acid β-oxidation by Krüppel-like transcription factor, Klf-3. J. Mol. Biol. 2013, 425, 2641–2655. [Google Scholar] [CrossRef]
- Wang, T.; Ma, G.; Ang, C.S.; Korhonen, P.K.; Koehler, A.V.; Young, N.D.; Nie, S.; Williamson, N.A.; Gasser, R.B. High throughput LC-MS/MS-based proteomic analysis of excretory-secretory products from short-term in vitro culture of Haemonchus contortus. J. Proteom. 2019, 204, 103375. [Google Scholar] [CrossRef]
- Stringfellow, F. Cultivation of Haemonchus contortus (Nematoda: Trichostrongylidae) from infective larvae to the adult male and the egg-laying female. J. Parasitol. 1986, 72, 339. [Google Scholar] [CrossRef]
- Quinn, W.J.; Wan, M.; Shewale, S.V.; Gelfer, R.; Rader, D.J.; Birnbaum, M.J.; Titchenell, P.M. MTORC1 stimulates phosphatidylcholine synthesis to promote triglyceride secretion. J. Clin. Investig. 2017, 127, 4207–4215. [Google Scholar] [CrossRef]
- Na, H.; Zhang, P.; Chen, Y.; Zhu, X.; Liu, Y.; Liu, Y.; Xie, K.; Xu, N.; Yang, F.; Yu, Y.; et al. Identification of lipid droplet structure-like/resident proteins in Caenorhabditis elegans. Biochim. Biophys. Acta 2015, 1853, 2481–2491. [Google Scholar] [CrossRef]
- Fleming, M.W. Ascaris suum: Role of ecdysteroids in molting. Exp. Parasitol. 1985, 60, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Chitwood, D.J. Biochemistry and function of nematode steroids. Crit. Rev. Biochem. Mol. Biol. 1999, 34, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Mercer, J.G.; Munn, A.E.; Rees, H.H. Caenorhabditis elegans: Occurrence and metabolism of ecdysteroidsin adults and dauer larvae. Comp. Biochem. Physiol. 1988, 90, 261–267. [Google Scholar] [CrossRef] [PubMed]
- O’Hanlon, G.M.; Cleator, M.; Mercer, J.G.; Howells, R.E.; Rees, H.H. Metabolism and fate of ecdysteroids in the nematodes Ascaris suum and Parascaris equorum. Mol. Biochem. Parasitol. 1991, 47, 179–187. [Google Scholar] [CrossRef]
- Fleming, M.W. Ecdysteroids during development in the ovine parasitic nematode, Haemonchus contortus. Comp. Biochem. Physiol. B 1993, 104, 653–655. [Google Scholar] [CrossRef]
- Entchev, E.V.; Kurzchalia, T.V. Requirement of sterols in the life cycle of the nematode Caenorhabditis elegans. Semin. Cell Dev. Biol. 2005, 16, 175–182. [Google Scholar] [CrossRef]
- Merris, M.; Wadsworth, W.G.; Khamrai, U.; Bittman, R.; Chitwood, D.J.; Lenard, J. Sterol effects and sites of sterol accumulation in Caenorhabditis elegans: Developmental requirement for 4alpha-methyl sterols. J. Lipid Res. 2003, 44, 172–181. [Google Scholar] [CrossRef]
- Futerman, A.H.; Hannun, Y.A. The complex life of simple sphingolipids. EMBO 2004, 5, 777–782. [Google Scholar] [CrossRef]
- Hänel, V.; Pendleton, C.; Witting, M. The Sphingolipidome of the model organism Caenorhabditis elegans. Chem. Phys. Lipids 2019, 222, 15–22. [Google Scholar] [CrossRef]
- Chan, J.P.; Sieburth, D. Localized sphingolipid signaling at presynaptic terminals is regulated by calcium influx and promotes recruitment of priming factors. J. Neurosci. 2012, 32, 17909–17920. [Google Scholar] [CrossRef]
- Mosbech, M.B.; Kruse, R.; Harvald, E.B.; Olsen, A.S.B.; Gallego, S.F.; Hannibal-Bach, H.K.; Ejsing, C.S.; Færgeman, N.J. Functional loss of two ceramide synthases elicits autophagy-dependent lifespan extension in C. elegans. PLoS ONE 2013, 8, e70087. [Google Scholar] [CrossRef] [PubMed]
- Soeda, S.; Hiraiwa, M.; O’Brien, J.S.; Kishimoto, Y. Binding of cerebrosides and sulfatides to saposins A-D. J. Biol. Chem. 1993, 268, 18519–18523. [Google Scholar] [CrossRef] [PubMed]
- Sarwal, R.; Sanyal, S.N.; Khera, S. Lipid metabolism in Trichuris Globulosa (Nematoda). J. Helminthol. 1989, 63, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Wangchuk, P.; Constantinoiu, C.; Eichenberger, M.R.; Field, M.; Loukas, A. Characterization of tapeworm metabolites and their reported biological activities. Molecules 2019, 24, 1480. [Google Scholar] [CrossRef]
- Wewer, V.; Makepeace, B.L.; Tanya, V.N.; Peisker, H.; Pfarr, K.; Hoerauf, A.; Dörmann, P. Lipid profiling of the filarial nematodes Onchocerca volvulus, Onchocerca ochengi and Litomosoides sigmodontis reveals the accumulation of nematode-specific ether phospholipids in the host. Int. J. Parasitol. 2017, 47, 903–912. [Google Scholar] [CrossRef]
- Lucanic, M.; Held, J.M.; Vantipalli, M.C.; Klang, I.M.; Graham, J.B.; Gibson, B.W.; Lithgow, G.J. N-Acylethanolamine signaling mediates the effect of diet on lifespan in Caenorhabditis elegans. Nature 2011, 473, 226–229. [Google Scholar] [CrossRef]
- Batugedara, H.M.; Argueta, D.; Jang, J.C.; Lu, D.; Macchietto, M.; Kaur, J.; Ge, S.; Dillman, A.R.; DiPatrizio, N.V.; Nair, M.G. Host- and helminth-derived endocannabinoids that have effects on host immunity are generated during infection. Infect. Immun. 2018, 86, e00441-18. [Google Scholar] [CrossRef]
- Galles, C.; Prez, G.M.; Penkov, S.; Boland, S.; Porta, E.O.; Altabe, S.G.; Labadie, G.R.; Schmidt, U.; Knölker, H.J.; Kurzchalia, T.V.; et al. Endocannabinoids in Caenorhabditis elegans are essential for the mobilization of cho-lesterol from internal reserves. Sci. Rep. 2018, 8, 6398. [Google Scholar] [CrossRef]
- Dall, K.B.; Havelund, J.F.; Harvald, E.B.; Witting, M.; Faergeman, N.J. HLH-30-dependent rewiring of metabolism during starvation in C. elegans. Aging Cell 2021, 20, e13342. [Google Scholar] [CrossRef]
- Hou Shangming, N.; Taubert, S. Function and regulation of lipid biology in Caenorhabditis elegans Aging. Front. Physiol. 2012, 3, 143. [Google Scholar] [CrossRef]
- Liu, Z.; Kariya, M.J.; Chute, C.D.; Pribadi, A.K.; Leinwand, S.G.; Tong, A.; Curran, K.P.; Bose, N.; Schroeder, F.C.; Srinivasan, J.; et al. Predator-secreted sulfolipids induce defensive responses in C. elegans. Nat. Commun. 2018, 9, 1128. [Google Scholar] [CrossRef] [PubMed]
- Wilinski, D.; Winzeler, J.; Duren, W.; Persons, J.L.; Holme, K.J.; Mosquera, J.; Khabiri, M.; Kinchen, J.M.; Freddolino, P.L.; Karnovsky, A.; et al. Rapid metabolicshifts occur during the transition between hunger and satiety in Drosophila melanogaster. Nat. Commun. 2019, 10, 4052. [Google Scholar] [CrossRef] [PubMed]
- Bansal, P.; Gaur, S.N.; Arora, N. Lysophosphatidylcholine plays critical role in allergic airway disease manifestation. Sci. Rep. 2016, 6, 27430. [Google Scholar] [CrossRef] [PubMed]
- Yeshi, K.; Creek, D.J.; Anderson, D.; Ritmejerytė, E.; Becker, L.; Loukas, A.; Wangchuk, P. Metabolomes and lipidomes of the infective stages of the gastrointestinal nematodes, Nippostrongylus brasiliensis and Trichuris muris. Metabolites 2020, 10, 446. [Google Scholar] [CrossRef]
- White, R.; Sotillo, J.; Ancarola, M.E.; Borup, A.; Boysen, A.T.; Brindley, P.J.; Buzás, E.I.; Cavallero, S.; Chaiyadet, S.; Chalmers, I.W.; et al. Special considerations for studies of extracellular vesicles from parasitic helminths: A community-led roadmap to increase rigour and reproducibility. J. Extracell. Vesicles 2023, 12, e12298. [Google Scholar] [CrossRef]

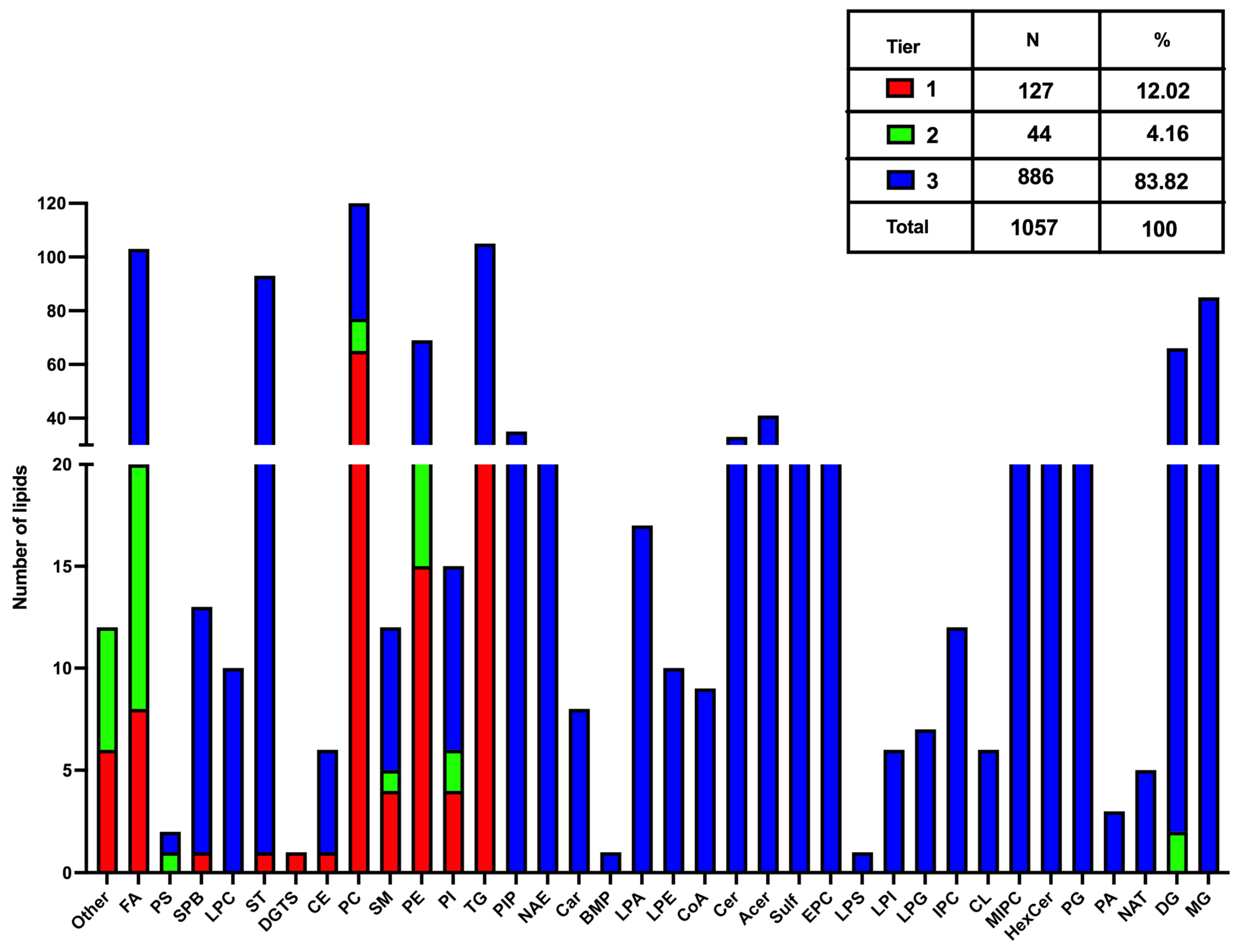


| Saturated | Unsaturated | |||||
|---|---|---|---|---|---|---|
| Medium Chain (10–14 C) | Long Chain (15–20 C) | Very Long Chain (>20 C) | Medium Chain (10–14 C) | Long Chain (15–20 C) | Very Long Chain (>20 C) | |
| Fatty Acyls | ||||||
| FA | 2 | 10 | 14 | 11 | 35 | 31 |
| NAE | 0 | 0 | 1 | 2 | 6 | 18 |
| Car | 0 | 0 | 0 | 3 | 2 | 3 |
| NAT | 0 | 0 | 0 | 3 | 0 | 2 |
| CoA | 0 | 0 | 6 | 0 | 0 | 3 |
| Glycerolipids | ||||||
| DG | 0 | 0 | 1 | 0 | 4 | 61 |
| TG | 0 | 0 | 1 | 0 | 23 | 81 |
| MG | 0 | 2 | 1 | 33 | 24 | 25 |
| DGTS | 0 | 1 | 0 | 0 | 0 | 0 |
| Sterols | ||||||
| ST | 0 | 0 | 0 | 0 | 43 | 50 |
| CE | 0 | 0 | 0 | 0 | 4 | 2 |
| Glycerophospholipids | ||||||
| CL | 0 | 0 | 0 | 0 | 0 | 6 |
| PA | 0 | 0 | 0 | 0 | 0 | 3 |
| PC | 0 | 0 | 3 | 4 | 69 | 44 |
| PE | 0 | 0 | 1 | 0 | 22 | 46 |
| PS | 0 | 0 | 0 | 0 | 0 | 2 |
| PI | 0 | 0 | 0 | 0 | 5 | 10 |
| PIP | 0 | 0 | 2 | 0 | 0 | 33 |
| PG | 0 | 0 | 2 | 0 | 0 | 26 |
| LPA | 3 | 0 | 0 | 6 | 4 | 4 |
| LPC | 1 | 5 | 0 | 1 | 2 | 1 |
| LPI | 0 | 0 | 0 | 2 | 0 | 4 |
| LPE | 0 | 0 | 0 | 0 | 3 | 7 |
| BMP | 0 | 0 | 0 | 0 | 1 | 0 |
| LPG | 0 | 0 | 0 | 1 | 1 | 5 |
| LPS | 0 | 0 | 0 | 0 | 0 | 1 |
| Sphingolipids | ||||||
| SPB | 0 | 0 | 0 | 1 | 12 | 0 |
| Cer | 0 | 0 | 0 | 0 | 0 | 33 |
| Acer | 0 | 0 | 0 | 0 | 0 | 41 |
| Sulf | 0 | 0 | 0 | 0 | 0 | 22 |
| HexCer | 0 | 0 | 0 | 0 | 0 | 28 |
| SM | 0 | 0 | 0 | 1 | 3 | 8 |
| EPC | 0 | 0 | 0 | 0 | 0 | 24 |
| IPC | 0 | 0 | 0 | 0 | 0 | 12 |
| MIPC | 0 | 0 | 0 | 0 | 0 | 22 |
| Total | 6 | 18 | 32 | 68 | 263 | 658 |
| Grand Total = 1045 (100%) | 0.6% | 1.7% | 3.1% | 6.5% | 25.2% | 63.0% |
| Lipid Subclass | 2 h vs. RPMI (0 h) | 4 h vs. 2 h | 8 h vs. 4 h | |||
|---|---|---|---|---|---|---|
| Fold Change (Mean ± SD) | N | Fold Change (Mean ± SD) | N | Fold Change (Mean ± SD) | N | |
| Sphingolipids | ||||||
| Sphingomyelins (SMs) | 7.5 ± 0.2 | 2 | 2.2 ± 0.9 | 2 | ||
| Ceramides (Cers) | 4.4 ± 3.7 | 6 | 0.55 | 1 | 0.7 ± 0.4 | 5 |
| Acylceramides (Acers) | 7.6 ± 0.0 | 2 | 1.4 ± 0.7 | 3 | ||
| Hexosylceramides (HexCers) | 7.4 ± 0.0 | 2 | 3.9 | 1 | ||
| Ceramide phosphoethanolamine (EPC) | 2.3 ± 1.8 | 3 | ||||
| Sulfatides (Sulfs) | 7.4 ± 0.0 | 9 | 2.0 ± 0.6 | 4 | ||
| Glycerolipids | ||||||
| Triacylglycerols (TGs) | 6.2 ± 0.6 | 7 | 3.1 | 1 | 1.7 ± 0.5 | 9 |
| Diacylglycerols (DGs) | 5.8 ± 3.4 | 12 | 1.9 | 1 | 2.3 ± 0.7 | 13 |
| Monoacylglycerols (MGs) | 0.4 ± 0.0 | 2 | ||||
| Glycerophospholipids | ||||||
| Phosphatidic acid (PA) | 1.5 | 1 | ||||
| Phosphatidylcholines (PCs) | 6.5 ± 0.2 | 57 | 2.6 ± 1.3 | 24 | ||
| Phosphatidylinositols (PIs) | 6.6 ± 1.8 | 7 | 2.1 ± 0.9 | 2 | ||
| Phosphatidyl-inositol phosphates (PIPs) | 3.2 | 1 | ||||
| Phosphatidylethanolamines (PEs) | 6.5 ± 1.5 | 29 | 3.2 ± 1.0 | 18 | ||
| Phosphatidylserines (PSs) | 3.0 ± 1.1 | 5 | ||||
| Phosphatidylglycerolipid (PG) | 1.6 | 1 | ||||
| Lysophosphatidylcholines (LPC) | 2.9 ± 4.6 | 3 | 1.7 | 1 | 2.0 ± 0.0 | 2 |
| Lysophosphatidic acid (LPA) | 0.2 | 1 | ||||
| Lysophosphatidylethanolamine (LPE) | 0.3 | 1 | 1.6 | 1 | ||
| Lysophosphatidylglycerol (LPG) | 0.6 | 1 | ||||
| Cardiolipin (CL) | 3.4 | 1 | ||||
| Fatty Acyls | ||||||
| Fatty Acids and conjugates (FAs) | 4.1 ± 5.5 | 26 | 2.5 ± 1.6 | 8 | ||
| Fatty Acyl carnitines (Cars) | 1.7 | 1 | 0.3 | 1 | ||
| N-acyl taurines (NATs) | 0.3 ± 0.0 | 2 | ||||
| N-acyl ethanolamines (NAEs) | 2.2 ± 4.1 | 14 | 1.2 ± 1.2 | 2 | 0.5 ± 0.2 | 3 |
| Fatty acyl CoEnzyme A (CoA) | 1.8 ± 0.0 | 2 | ||||
| Sterol Lipids | ||||||
| Sterols (STs) | 1.8 | 11 | ||||
| Cholesteryl esters (CEs) | 1.7 ± 1.0 | 18 | 2.0 ± 2.0 | 1 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godoy, P.; Rezanezhad Dizaji, B.; Zardini Buzatto, A.; Sanchez, L.; Li, L. The Lipid Composition of the Exo-Metabolome from Haemonchus contortus. Metabolites 2025, 15, 193. https://doi.org/10.3390/metabo15030193
Godoy P, Rezanezhad Dizaji B, Zardini Buzatto A, Sanchez L, Li L. The Lipid Composition of the Exo-Metabolome from Haemonchus contortus. Metabolites. 2025; 15(3):193. https://doi.org/10.3390/metabo15030193
Chicago/Turabian StyleGodoy, Pablo, Behrouz Rezanezhad Dizaji, Adriana Zardini Buzatto, Laura Sanchez, and Liang Li. 2025. "The Lipid Composition of the Exo-Metabolome from Haemonchus contortus" Metabolites 15, no. 3: 193. https://doi.org/10.3390/metabo15030193
APA StyleGodoy, P., Rezanezhad Dizaji, B., Zardini Buzatto, A., Sanchez, L., & Li, L. (2025). The Lipid Composition of the Exo-Metabolome from Haemonchus contortus. Metabolites, 15(3), 193. https://doi.org/10.3390/metabo15030193








