Secondary Metabolites from Croton Species and Their Biological Activity on Cell Cycle Regulators
Abstract
:1. Introduction
Review Methodology
2. Overview of Ethnopharmacological Uses of Croton Secondary Metabolites
3. Molecular Targets of Croton Metabolites in Cancer
Cell Cycle Arrest and Apoptotic Induction
4. Perspectives and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDK | Cyclin-dependent Kinase |
| SMs | Secondary metabolites |
| RB | Retinoblastoma protein |
References
- Villaseñor, J.L. Checklist of the native vascular plants of México. Rev. Mex. Biodivers. 2016, 87, 559–902. [Google Scholar] [CrossRef]
- Govaerts, R.; Frodin, D.G.; Radcliffe-Smith, A.; Carter, S. World Checklist and Bibliography of Euphorbiaceae (with Pandaceae); The Royal Botanic Gardens, Kew: Richmond, UK, 2000; Available online: http://catalog.hathitrust.org/api/volumes/oclc/44041175.html (accessed on 29 May 2024).
- Berry, P.E.; Hipp, A.L.; Wurdack, K.J.; Van Ee, B.; Riina, R. Molecular Phylogenetics of the Giant Genus Croton and Tribe Crotoneae (Euphorbiaceae Sensu Stricto) Using ITS and TRNL-TRNF DNA Sequence Data. Am. J. Bot. 2005, 92, 1520–1534. [Google Scholar] [CrossRef] [PubMed]
- Webster, G.L. A Provisional Synopsis of the Sections of the Genus Croton (Euphorbiaceae). Taxon 1993, 42, 793–823. [Google Scholar] [CrossRef]
- Osathanunkul, M.; Suwannapoom, C.; Ounjai, S.; Rora, J.A.; Madesis, P.; De Boer, H. Refining DNA Barcoding Coupled High Resolution Melting for Discrimination of 12 Closely Related Croton Species. PLoS ONE 2015, 10, e0138888. [Google Scholar] [CrossRef]
- van Ee, B.; Riina, R.; Berry, P. A Revised Infrageneric Classification and Molecular Phylogeny of New World Croton (Euphorbiaceae). Taxon 2011, 60, 791–823. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Ortiz-Sánchez, E.; Domínguez, F.; López-Toledo, G.; Chávez, M.; Ortiz-Tello, A.D.J.; García-Carrancá, A. Antitumor Effect of Croton lechleri Mull. Arg. (Euphorbiaceae). J. Ethnopharmacol. 2012, 140, 438–442. [Google Scholar] [CrossRef]
- Steinmann, V.W. A Synopsis of Croton (Euphorbiaceae) in Michoacán, Mexico. Taxonomy 2021, 1, 395–424. [Google Scholar] [CrossRef]
- Sharma, V.; Kumar, D.; Dev, K.; Sourirajan, A. Anticancer Activity of Essential Oils: Cell Cycle Perspective. S. Afr. J. Bot. 2023, 157, 641–647. [Google Scholar] [CrossRef]
- Salatino, A.; Salatino, M.L.; Negri, G. Traditional Uses, Chemistry and Pharmacology of Croton Species (Euphorbiaceae). J. Braz. Chem. Soc. 2007, 18, 11–33. [Google Scholar] [CrossRef]
- Jura-Morawiec, J.; Tulik, M. Dragon’s Blood Secretion and Its Ecological Significance. Chemoecology 2016, 26, 101–105. [Google Scholar] [CrossRef]
- Ramón, F.; Williamson, J.S.; Rodríguez, S.V.; Angeles, G.; Portugal, V.O. Bark Anatomy in Croton draco Var. Draco (Euphorbiaceae). Am. J. Bot. 2009, 96, 2155–2167. [Google Scholar] [CrossRef]
- Casao, T.d.R.L.; Pinheiro, C.G.; Sarandy, M.M.; Zanatta, A.C.; Vilegas, W.; Novaes, R.D.; Gonçalves, R.V.; Viana Leite, J.P. Croton urucurana Baillon Stem Bark Ointment Accelerates the Closure of Cutaneous Wounds in Knockout IL-10 Mice. J. Ethnopharmacol. 2020, 261, 113042. [Google Scholar] [CrossRef] [PubMed]
- Murillo, R.M.; Jakupovic, J.; Rivera, J.; Castro, V.H. Diterpenes and Other Constituents from Croton draco (Euphorbiaceae). Rev. Biol. Trop. 2001, 49, 259–264. [Google Scholar] [PubMed]
- Bezerra, F.W.F.; Salazar, M.d.L.A.R.; Freitas, L.C.; de Oliveira, M.S.; dos Santos, I.R.C.; Dias, M.N.C.; Gomes-Leal, W.; Andrade, E.H.d.A.; Ferreira, G.C.; de Carvalho, R.N. Chemical Composition, Antioxidant Activity, Anti-Inflammatory and Neuroprotective Effect of Croton matourensis Aubl. Leaves Extracts Obtained by Supercritical CO2. J. Supercrit. Fluids 2020, 165, 104992. [Google Scholar] [CrossRef]
- Martins, A.O.B.P.B.; Rodrigues, L.B.; Cesário, F.R.A.S.; de Oliveira, M.R.C.; Tintino, C.D.M.; Castro, F.F.E.; Alcântara, I.S.; Fernandes, M.N.M.; de Albuquerque, T.R.; da Silva, M.S.A.; et al. Anti-Edematogenic and Anti-Inflammatory Activity of the Essential Oil from Croton rhamnifolioides Leaves and Its Major Constituent 1,8-Cineole (Eucalyptol). Biomed. Pharmacother. 2017, 96, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Tintino, C.D.d.M.; Pessoa, R.T.; Fernandes, M.N.M.; Alcântara, I.S.; da Silva, B.A.F.; de Oliveira, M.R.C.; Martins, A.O.B.P.B.; da Silva, M.d.S.; Tintino, S.R.; Rodrigues, F.F.G.; et al. Anti-Inflammatory and Anti-Edematogenic Action of the Croton campestris A. St.-Hil (Euphorbiaceae) Essential Oil and the Compound β-Caryophyllene in in Vivo Models. Phytomedicine 2018, 41, 82–95. [Google Scholar] [CrossRef]
- Gupta, D.; Bleakley, B.; Gupta, R.K. Dragon’s Blood: Botany, Chemistry and Therapeutic Uses. J. Ethnopharmacol. 2007, 115, 361–380. [Google Scholar] [CrossRef]
- Block, S.; Stévigny, C.; De Pauw-Gillet, M.C.; de Hoffmann, E.; Llabrès, G.; Adjakidjé, V.; Quetin-Leclercq, J. Ent-Trachyloban-3β-Ol, a New Cytotoxic Diterpene from Croton zambesicus. Planta Med. 2002, 68, 647–649. [Google Scholar] [CrossRef]
- Ngamrojnavanich, N.; Sirimongkon, S.; Roengsumran, S.; Petsom, A.; Kamimura, H. Inhibition of Na+,K+-ATPase Activity by (-)-Ent-Kaur-16-En-19-Oic Acid and Its Derivatives. Planta Med. 2003, 69, 555–556. [Google Scholar] [CrossRef]
- Pierre-Ruphin, F.; Baholy, R.; Sylver, S.; Aubin Yves Oscar, R.; Mahamoud, A.; Francois Raymond, F.; Marcelin, S.; Jeannelle Rakotoniriana, H.; Amélie, R.; Koto-te-Nyiwa Ngbolua, K. GC-FID and GC/MS Analyses and Antimicrobial Activity of Croton greveanus, C. borarium and C. geayi (Euphorbiaceae) Essential Oils from Madagascar. J. Pharmacogn. Phytochem. 2016, 5, 188–197. [Google Scholar]
- Vigor, C.; Fabre, N.; Fourasté, I.; Moulis, C. Three Clerodane Diterpenoids from Croton eluteria Bennett. Phytochemistry 2001, 57, 1209–1212. [Google Scholar] [CrossRef] [PubMed]
- García-Díaz, J.; Tuenter, E.; Escalona-Arranz, J.C.; Llauradó Maury, G.; Cos, P.; Pieters, L. Antimicrobial Activity of Leaf Extracts and Isolated Constituents of Croton linearis. J. Ethnopharmacol. 2019, 236, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Okokon, J.E.; Nwafor, P.A. Antimicrobial Activity of Root Extract and Crude Fractions of Croton zambesicus. Pak. J. Pharm. Sci. 2010, 23, 114–118. [Google Scholar] [PubMed]
- Panda, S.K.; Dutta, S.K.; Bastia, A.K. Antibacterial Activity of Croton roxburghii Balak. against the Enteric Pathogens. J. Adv. Pharm. Technol. Res. 2010, 1, 419–422. [Google Scholar] [CrossRef]
- Sá Firmino, N.C.; Alexandre, F.S.O.; de Vasconcelos, M.A.; Pinheiro, A.A.; Arruda, F.V.S.; Guedes, M.L.S.; Silveira, E.R.; Teixeira, E.H. Diterpenes Isolated from Croton blanchetianus Baill: Potential Compounds in Prevention and Control of the Oral Streptococci Biofilms. Ind. Crops Prod. 2019, 131, 371–377. [Google Scholar] [CrossRef]
- Ahmed, B.; Alam, T.; Varshney, M.; Khan, A. Hepatoprotective Activity of Two Plants Belonging to the Apiaceae and the Euphorbiaceae Family. J. Ethnopharmacol. 2002, 79, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Grassi-Kassisse, D.M.; Wolf-Nunes, V.; Miotto, A.M.; Farias-Silva, E.; Brito, A.R.M.S.; Nunes, D.S.; Spadari-Bratfisch, R.C. Sensitivity to β-Adrenoceptor Agonists of Adipocytes from Rats Treated with an Aqueous Extract of Croton cajucara Benth. J. Pharm. Pharmacol. 2010, 55, 253–257. [Google Scholar] [CrossRef]
- Ravanelli, N.; Santos, K.P.; Motta, L.B.; Lago, J.H.G.; Furlan, C.M. Alkaloids from Croton echinocarpus Baill.: Anti-HIV Potential. S. Afr. J. Bot. 2016, 102, 153–156. [Google Scholar] [CrossRef]
- Terefe, E.M.; Okalebo, F.A.; Derese, S.; Muriuki, J.; Batiha, G.E.S. In Vitro Cytotoxicity and Anti-HIV Activity of Crude Extracts of Croton macrostachyus, Croton megalocarpus and Croton dichogamus. J. Exp. Pharmacol. 2021, 13, 971–979. [Google Scholar] [CrossRef]
- Mekonnen, A.N.; Asrade Atnafie, S.; Wahab Atta, M.A. Evaluation of Antiulcer Activity of 80% Methanol Extract and Solvent Fractions of the Root of Croton macrostachyus Hocsht: Ex Del. (Euphorbiaceae) in Rodents. Evid.-Based Complement. Altern. Med. 2020, 2020, 2809270. [Google Scholar] [CrossRef]
- Okokon, J.E.; Nwafor, P.A. Antiulcer and Anticonvulsant Activity of Croton Zambesicus. Pak. J. Pharm. Sci. 2009, 22, 384–390. [Google Scholar] [PubMed]
- Vidal, C.S.; Oliveira Brito Pereira Bezerra Martins, A.; de Alencar Silva, A.; de Oliveira, M.R.C.; Ribeiro-Filho, J.; de Albuquerque, T.R.; Coutinho, H.D.M.; da Silva Almeida, J.R.G.; Quintans, L.J.; de Menezes, I.R.A. Gastroprotective Effect and Mechanism of Action of Croton rhamnifolioides Essential Oil in Mice. Biomed. Pharmacother. 2017, 89, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Dey, P.; Sarkar, P.; Karmakar, S.; Tae, I.H.; Kim, K.S.; Park, J.H.; Lee, S.H.; Lee, B.M.; Renthlei, L.; et al. Protective Effects of Croton hookeri on Streptozotocin-Induced Diabetic Nephropathy. Food Chem. Toxicol. 2020, 135, 110873. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.R.; Di Naso, F.C.; Porawski, M.; Marcolin, É.; Kretzmann, N.A.; Ferraz, A.D.B.F.; Richter, M.F.; Marroni, C.A.; Marroni, N.P. Treatment with Aqueous Extract from Croton cajucara Benth Reduces Hepatic Oxidative Stress in Streptozotocin-Diabetic Rats. J. Biomed. Biotechnol. 2012, 902351. [Google Scholar] [CrossRef] [PubMed]
- Torrico, F.; Cepeda, M.; Guerrero, G.; Melendez, F.; Blanco, Z.; Canelón, D.J.; Diaz, B.; Compagnone, R.S.; Suárez, A.I. Hypoglycaemic Effect of Croton cuneatus in Streptozotocin-Induced Diabetic Rats. Rev. Bras. Farmacogn. 2007, 17, 166–169. [Google Scholar] [CrossRef]
- Torrico, F.; Ramos, K.; Morales, A.; Guarirapa, L.; Cando, A.; Guerrero, G.; Márquez, G.; Compagnone, R.S.; Suárez, A.I. Evaluation of Acute Toxicity, Analgesic and Hipoglicaemic Activity of Aqueous Extract of Croton Pungens in Experimental Animals. Sci. J. Exp. Fac. Sci. 2013, 21, 181–191. [Google Scholar]
- Tufer, S.; Engidawork, E.; Ayele, A.G.; Bashea, C. Evaluation of the Diuretic Activity of Aqueous and 80% Methanol Extracts of Croton macrostachyus (Euphorbiaceae) Leaves in Saline-Loaded Rats. J. Exp. Pharmacol. 2021, 13, 213–221. [Google Scholar] [CrossRef]
- Guerrero, M.F.; Carrón, R.; Martín, M.L.; Román, L.S.; Reguero, M.T. Antihypertensive and Vasorelaxant Effects of Aqueous Extract from Croton Schiedeanus Schlecht in Rats. J. Ethnopharmacol. 2001, 5, 33–36. [Google Scholar] [CrossRef]
- Ortiz, A.P.; Puebla, P.; Guerrero, M.F. Vascular Interactions of Croton schiedeanus Major Flavonoids in Isolated Aortic Rings from Wistar Rats. Vitae 2021, 28, 343923. [Google Scholar] [CrossRef]
- Hort, M.A.; Straliotto, M.R.; Duz, M.S.; Netto, P.M.; Souza, C.B.; Schulz, T.; Horst, H.; Pizzolatti, M.G.; de Bem, A.F.; Ribeiro-do-Valle, R.M. Cardioprotective Effects of a Proanthocyanidin-Rich Fraction from Croton celtidifolius Baill: Focus on Atherosclerosis. Food Chem. Toxicol. 2012, 50, 3769–3775. [Google Scholar] [CrossRef]
- Peres, M.T.L.P.; Delle Monache, F.; Cruz, B.; Pizzolatti, M.G.; Yunes, R.A. Chemical Composition and Antimicrobial Activity of Croton urucurana Baillon (Euphorbiaceae). J. Ethnopharmacol. 1997, 56, 223–226. [Google Scholar] [CrossRef]
- Suárez, A.I.; Compagnone, R.S.; Salazar-Bookaman, M.M.; Tillett, S.; Delle Monache, F.; Di Giulio, C.; Bruges, G. Antinociceptive and Anti-Inflammatory Effects of Croton malambo Bark Aqueous Extract. J. Ethnopharmacol. 2003, 88, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Ali Al-Hakami, I.; Raweh, S.; El-Shaibany, A.; Humaid, A.; Elaasser, M. A Review of Biological Activities of Genus Croton. PSM Microbiol. 2022, 7, 12–18. [Google Scholar]
- Guerra Júnior, J.I.; Ferreira, M.R.A.; de Oliveira, A.M.; Soares, L.A.L. Croton Sp.: A Review about Popular Uses, Biological Activities and Chemical Composition. Res. Soc. Dev. 2022, 11, e57311225306. [Google Scholar] [CrossRef]
- Moremi, M.P.; Makolo, F.; Viljoen, A.M.; Kamatou, G.P. A Review of Biological Activities and Phytochemistry of Six Ethnomedicinally Important South African Croton Species. J. Ethnopharmacol. 2021, 280, 114416. [Google Scholar] [PubMed]
- Huang, M.; Lu, J.-J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef]
- Bailon-Moscoso, N.; Cevallos-Solorzano, G.; Romero-Benavides, J.; Ramirez Orellana, M. Natural Compounds as Modulators of Cell Cycle Arrest: Application for Anticancer Chemotherapies. Curr. Genom. 2017, 18, 106–131. [Google Scholar] [CrossRef]
- Maciel, M.A.M.; Pinto, A.C.; Arruda, A.C.; Pamplona, S.G.S.R.; Vanderlinde, F.A.; Lapa, A.J.; Echevarria, A.; Grynberg, N.F.; Côlus, I.M.S.; Farias, R.A.F.; et al. Ethnopharmacology, Phytochemistry and Pharmacology: A Successful Combination in the Study of Croton cajucara. J. Ethnopharmacol. 2000, 70, 41–55. [Google Scholar] [CrossRef]
- Galvão Freire, A.C.; Da Silva Melo, P.; Aoyama, H.; Haun, M.; Durán, N.; Ferreira, C.V. Cytotoxic Effect of the Diterpene Lactone Dehydrocrotonin from Croton cajucara on Human Promyelocytic Leukemia Cells. Planta Med. 2003, 69, 67–69. [Google Scholar] [CrossRef]
- Sandoval, M.; Okuhama, N.N.; Clark, M.; Angeles, F.M.; Lao, J.; Bustamante, S.; Miller, M.J.S. Sangre de Grado Croton palanostigma Induces Apoptosis in Human Gastrointestinal Cancer Cells. J. Ethnopharmacol. 2002, 80, 121–129. [Google Scholar] [CrossRef]
- Rossi, D.; Bruni, R.; Bianchi, N.; Chiarabelli, C.; Gambari, R.; Medici, A.; Lista, A.; Paganetto, G. Evaluation of the Mutagenic, Antimutagenic and Antiproliferative Potential of Croton lechleri (Muell. Arg.) Latex. Phytomedicine 2003, 10, 139–144. [Google Scholar] [CrossRef]
- Montopoli, M.; Bertin, R.; Chen, Z.; Bolcato, J.; Caparrotta, L.; Froldi, G. Croton Lechleri Sap and Isolated Alkaloid Taspine Exhibit Inhibition against Human Melanoma SK23 and Colon Cancer HT29 Cell Lines. J. Ethnopharmacol. 2012, 144, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Fayad, W.; Fryknäs, M.; Brnjic, S.; Olofsson, M.H.; Larsson, R.; Linder, S. Identification of a Novel Topoisomerase Inhibitor Effective in Cells Overexpressing Drug Efflux Transporters. PLoS ONE 2009, 4, e7238. [Google Scholar] [CrossRef]
- Tzintzarov, A.; Boyadzhieva, S.S.; Coelho, J.A.P.; Tsvetanova, F.; Petrova, M.; Stoev, G.; Yankov, D.S.; Ugrinova, I.; Stateva, R.P. Novel Insights into the Biological Activity of Croton lechleri Twigs Extracts and Advancements in Their Sustainable Recovery. Molecules 2024, 29, 4161. [Google Scholar] [CrossRef] [PubMed]
- Block, S.; Gerkens, P.; Peulen, O.; Jolois, O.; Mingeot-Leclercq, M.P.; De Pauw-Gillet, M.C.; Quetin-Leclercq, J. Induction of Apoptosis in Human Promyelocytic Leukemia Cells by a Natural Trachylobane Diterpene. Anticancer Res. 2005, 25, 363–368. [Google Scholar]
- Kuo, P.C.; Shen, Y.C.; Yang, M.L.; Wang, S.H.; Tran, D.T.; Nguyen, X.D.; Chiang, P.C.; Lee, K.H.; Lee, E.J.; Wu, T.S. Crotonkinins A and B and Related Diterpenoids from Croton tonkinensis as Anti-Inflammatory and Antitumor Agents. J. Nat. Prod. 2007, 70, 1906–1909. [Google Scholar] [CrossRef]
- Lee, H.-M.; Kuo, P.-C.; Chen, W.-H.; Chen, P.-J.; Lam, S.-H.; Su, Y.-C.; Chen, C.-H. Diterpenoid from Croton tonkinensis as a Potential Radiation Sensitizer in Oral Squamous Cell Carcinoma: An In Vitro Study. Int. J. Mol. Sci. 2024, 25, 11839. [Google Scholar] [CrossRef]
- Pudhom, K.; Vilaivan, T.; Ngamrojanavanich, N.; Dechangvipart, S.; Sommit, D.; Petsom, A.; Roengsumran, S. Furanocembranoids from the Stem Bark of Croton oblongifolius. J. Nat. Prod. 2007, 70, 659–661. [Google Scholar] [CrossRef]
- Neiva, T.D.J.C.; De Moraes, A.C.R.; Buchele, C.; Pizzolatti, M.G.; D’Amico, E.A.; Fries, D.M.; Da Rocha, T.R.F. Antiplatelet Activity of Croton celditifolius. Rev. Bras. Cienc. Farm./Braz. J. Pharm. Sci. 2008, 44, 127–132. [Google Scholar] [CrossRef]
- Bezerra, D.P.; Marinho Filho, J.D.B.; Alves, A.P.N.N.; Pessoa, C.; De Moraes, M.O.; Pessoa, O.D.L.; Torres, M.C.M.; Silveira, E.R.; Viana, F.A.; Costa-Lotufo, L.V. Antitumor Activity of the Essential Oil from the Leaves of Croton regelianus and Its Component Ascaridole. Chem. Biodivers. 2009, 6, 1224–1231. [Google Scholar] [CrossRef]
- Santos, H.S.; Barros, F.W.A.; Albuquerque, M.R.J.R.; Bandeira, P.N.; Pessoa, C.; Braz-Filho, R.; Monte, F.J.Q.; Leal-Cardoso, J.H.; Lemos, T.L.G. Cytotoxic Diterpenoids from Croton argyrophylloides. J. Nat. Prod. 2009, 72, 1884–1887. [Google Scholar] [CrossRef]
- Rakotonandrasana, O.L.; Raharinjato, F.H.; Rajaonarivelo, M.; Dumontet, V.; Martin, M.T.; Bignon, J.; Rasoanaivo, P. Cytotoxic 3,4- Seco-Atisane Diterpenoids from Croton barorum and Croton goudotii. J. Nat. Prod. 2010, 73, 1730–1733. [Google Scholar] [CrossRef]
- Omosa, L.K.; Midiwo, J.O.; Masila, V.M.; Gisacho, B.M.; Munayi, R.; Francisca-Kamakama; Chemutai, K.P.; Elhaboob, G.; Saeed, M.E.M.; Hamdoun, S.; et al. Cytotoxicity of 91 Kenyan Indigenous Medicinal Plants towards Human CCRF-CEM Leukemia Cells. J. Ethnopharmacol. 2016, 179, 177–196. [Google Scholar] [CrossRef] [PubMed]
- Bhavana, J.; Kalaivani, M.K.; Sumathy, A. Cytotoxic and Pro-Apoptotic Activities of Leaf Extract of Croton bonplandianus Baill. against Lung Cancer Cell Line A549. Indian J. Exp. Biol. 2016, 54, 379–385. Available online: http://nopr.niscpr.res.in/handle/123456789/34322 (accessed on 30 June 2024).
- Suresh, M.; Alfonisan, M.; Alturaiki, W.; Al Aboody, M.S.; Alfaiz, F.A.; Premanathan, M.; Vijayakumar, R.; Umamagheswari, K.; Al Ghamdi, S.; Alsagaby, S.A. Investigations of Bioactivity of Acalypha indica (L.), Centella asiatica (L.) and Croton bonplandianus (Baill) against Multidrug Resistant Bacteria and Cancer Cells. J. Herb. Med. 2021, 28, 100359. [Google Scholar] [CrossRef]
- de Matos Cândido-Bacani, P.; Ezan, F.; de Oliveira Figueiredo, P.; Matos, M.d.F.C.; Rodrigues Garcez, F.; Silva Garcez, W.; Baffet, G. [1–9-NαC]-Crourorb A1 Isolated from Croton urucurana Latex Induces G2/M Cell Cycle Arrest and Apoptosis in Human Hepatocarcinoma Cells. Toxicol. Lett. 2017, 273, 44–54. [Google Scholar] [CrossRef]
- Mfotie Njoya, E.; Eloff, J.N.; McGaw, L.J. Croton Gratissimus Leaf Extracts Inhibit Cancer Cell Growth by Inducing Caspase 3/7 Activation with Additional Anti-Inflammatory and Antioxidant Activities. BMC Complement. Altern. Med. 2018, 18, 305. [Google Scholar] [CrossRef]
- Amaral, R.G.; Andrade, L.N.; Severino, P.; de Araújo, S.S.; Santos, M.I.S.; Dias, A.S.; de Moraes Filho, M.O.; Do O’Pessoa, C.; Carvalho, A.A.; Thomazzi, S.M. Investigation of the Possible Antioxidant and Anticancer Effects of Croton argyrophyllus (Euphorbiaceae). Chem. Eng. Trans. 2018, 64, 253–258. [Google Scholar] [CrossRef]
- Premprasert, C.; Yoenyongsawad, S.; Tewtrakul, S.; Wungsintaweeku, J. Plaunotol from Croton stellatopilosus Ohba Inhibited Cell Growth and Induced Apoptosis in Human Cancer Cell Lines. Songklanakarin J. Sci. Technol. 2019, 41, 846–855. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, C.; Yao, S.; Lai, H.; Li, R.; Xu, J.; Wang, Q.; Fan, X.X.; Wu, Q.B.; Leung, E.L.H.; et al. Discovery of a Novel Protein Kinase C Activator from Croton tiglium for Inhibition of Non-Small Cell Lung Cancer. Phytomedicine 2019, 65, 153100. [Google Scholar] [CrossRef]
- Niu, Q.L.; Sun, H.; Liu, C.; Li, J.; Liang, C.X.; Zhang, R.R.; Ge, F.R.; Liu, W. Croton tiglium Essential Oil Compounds Have Anti-Proliferative and pro-Apoptotic Effects in A549 Lung Cancer Cell Lines. PLoS ONE 2020, 15, e0231437. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, R.-R.; Wang, Y.-M.; Zhang, J.; Wang, Q.; Cheng, A.-W.; Guo, X.; Wang, X.-K.; Sun, J.-Y. Supercritical CO2 Fluid Extraction of Croton crassifolius Geisel Root: Chemical Composition and Anti-Proliferative, Autophagic, Apoptosis-Inducing, and Related Molecular Effects on A549 Tumour Cells. Phytomedicine 2019, 61, 152846. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, R.R.; Sun, J.Y.; Liu, Y.; Yuan, X.S.; Chen, Y.Y.; Sun, H.; Liu, C. The Molecular Mechanism of Action for the Potent Antitumor Component Extracted Using Supercritical Fluid Extraction from Croton crassifolius Root. J. Ethnopharmacol. 2024, 327, 117835. [Google Scholar] [CrossRef] [PubMed]
- Cândido, A.C.S.; Scalon, S.P.Q.; Silva, C.B.; Simionatto, E.; Morel, A.F.; Stüker, C.Z.; Matos, M.F.C.; Peres, M.T.L.P. Chemical Composition and Phytotoxicity of Essential Oils of Croton doctoris S. Moore (Euphorbiaceae). Braz. J. Biol. 2021, 82, e231957. [Google Scholar] [CrossRef]
- Bhargav, E.; Salamma, S.; Chandana, B.; Harika, S.; Jyothi, M.V.; Rao, B.R.P. Bio Active Compound Analysis of Croton scabiosus Bedd: By HPTLC, GC-MS and Evaluation of Anthelmintic Activity and Anticancer Potential on Lung (A549) and Breast (MCF-7) Cancer Cell Lines. J. Pharm. Res. Int. 2021, 45–53. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, Y.; Wang, C.; Yu, J.; Yue, J.; Zhou, B. Cytotoxic Diterpenoids from Croton kongensis Inhibiting Tumor Proliferation and Migration. Bioorg. Chem. 2024, 152, 107739. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Martínez, C.; Lallena, M.J.; Sanfeliciano, S.G.; de Dios, A. Cyclin Dependent Kinase (CDK) Inhibitors as Anticancer Drugs: Recent Advances (2015–2019). Bioorg. Med. Chem. Lett. 2019, 29, 126637. [Google Scholar] [CrossRef]
- Vermeulen, K.; Van Bockstaele, D.R.; Berneman, Z.N. The Cell Cycle: A Review of Regulation, Deregulation and Therapeutic Targets in Cancer. Cell Prolif. 2003, 36, 131–149. [Google Scholar] [CrossRef]
- Dickson, M.A.; Schwartz, G.K. Development of Cell-Cycle Inhibitors for Cancer Therapy. Curr. Oncol. 2009, 16, 36–43. [Google Scholar] [CrossRef]
- Qie, S.; Diehl, J.A. Cyclin D1, Cancer Progression, and Opportunities in Cancer Treatment. J. Mol. Med. 2016, 94, 1313–1326. [Google Scholar] [CrossRef]
- Cooke, M.; Magimaidas, A.; Casado-Medrano, V.; Kazanietz, M.G. Protein Kinase C in Cancer: The Top Five Unanswered Questions. Mol. Carcinog. 2017, 56, 1531–1542. [Google Scholar] [CrossRef]
- Poli, A.; Ramazzotti, G.; Matteucci, A.; Manzoli, L.; Lonetti, A.; Suh, P.G.; McCubrey, J.A.; Cocco, L. A Novel DAG-Dependent Mechanism Links PKCa and Cyclin B1 Regulating Cell Cycle Progression. Oncotarget 2014, 5, 11526–11540. [Google Scholar] [CrossRef] [PubMed]
- Canedo-Téxon, A.; Ramón-Farias, F.; Monribot-Villanueva, J.L.; Villafán, E.; Alonso-Sánchez, A.; Pérez-Torres, C.A.; Ángeles, G.; Guerrero-Analco, J.A.; Ibarra-Laclette, E. Novel Findings to the Biosynthetic Pathway of Magnoflorine and Taspine through Transcriptomic and Metabolomic Analysis of Croton draco (Euphorbiaceae). BMC Plant Biol. 2019, 19, 560. [Google Scholar] [CrossRef]
- Milanowski, D.J.; Winter, R.E.K.; Elvin-Lewis, M.P.F.; Lewis, W.H. Geographic Distribution of Three Alkaloid Chemotypes of Croton lechleri. J. Nat. Prod. 2002, 65, 814–819. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Q.; Wang, N.; Dai, B.; Chen, Y.; He, L. Effects of Taspine on Proliferation and Apoptosis by Regulating Caspase-3 Expression and the Ratio of BAX/BCL-2 in A431 Cells. Phytother. Res. 2011, 25, 357–364. [Google Scholar] [CrossRef]
- Kontro, M.; Kumar, A.; Majumder, M.; Eldfors, S.; Parsons, A.; Pemovska, T.; Saarela, J.; Yadav, B.; Malani, D.; Fløisand, Y.; et al. HOX Gene Expression Predicts Response to BCL-2 Inhibition in Acute Myeloid Leukemia. Leukemia 2017, 31, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Yang, L.; Shi, X.; Gong, Z.; Yu, R.; Zhang, D.; Zhang, Y.; Ma, W. TPD7 Inhibits the Growth of Cutaneous T Cell Lymphoma H9 Cell through Regulating IL-2R Signalling Pathway. J. Cell. Mol. Med. 2019, 24, 984–995. [Google Scholar] [CrossRef]
- Belletti, B.; Nicoloso, M.S.; Schiappacassi, M.; Berton, S.; Lovat, F.; Wolf, K.; Canzonieri, V.; D’Andrea, S.; Zucchetto, A.; Friedl, P.; et al. Stathmin Activity Influences Sarcoma Cell Shape, Motility, and Metastatic Potential. Mol. Biol. Cell 2008, 19, 2003–2013. [Google Scholar] [CrossRef]
- Cambray-Deakin, M.A.; Burgoyne, R.D. Acetylated and Detyrosinated Alpha-Tubulins are Co-localized in Stable Microtubules in Rat Meningeal Fibroblasts. Cell Motil. Cytoskelet. 1987, 8, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Perdiz, D.; Mackeh, R.; Poüs, C.; Baillet, A. The Ins and Outs of Tubulin Acetylation: More Than Just a Post-Translational Modification? Cell. Signal. 2011, 23, 763–771. [Google Scholar] [CrossRef]
- Ong, P.S.; Wang, L.Z.; Dai, X.; Tseng, S.H.; Loo, S.J.; Sethi, G. Judicious Toggling of mTOR Activity to Combat Insulin Resistance and Cancer: Current Evidence and Perspectives. Front. Pharmacol. 2016, 25, 395. [Google Scholar] [CrossRef]
- Hennessy, B.; Smith, D.; Ram, P.T.; Lu, Y.; Mills, G.B. Exploiting the PI3K/AKT Pathway for Cancer Drug Discovery. Nat. Rev. Drug Discov. 2005, 4, 988–1004. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, C.; Um, J.-Y.; Sethi, G.; Ahn, K.S. Casticin-Induced Inhibition of Cell Growth and Survival are Mediated through the Dual Modulation of Akt/mTOR Signaling Cascade. Cancers 2019, 11, 254. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.C.; Hung, S.K.; Liao, H.F.; Lee, C.C.; Lin, H.Y.; Lai, H.C.; Li, S.C.; Ho, H.C.; Huang, H.B.; Su, Y.C. RAD001 Enhances the Radiosensitivity of SCC4 Oral Cancer Cells by Inducing Cell Cycle Arrest at the G2/M Checkpoint. Anticancer Res. 2014, 34, 2927–2935. [Google Scholar]
- Shamloo, B.; Usluer, S. P21 in Cancer Research. Cancers 2019, 11, 1178. [Google Scholar] [CrossRef] [PubMed]
- Lara-Gonzalez, P.; Westhorpe, F.G.; Taylor, S.S. The Spindle Assembly Checkpoint. Curr. Biol. 2012, 22, R966–R980. [Google Scholar] [CrossRef]
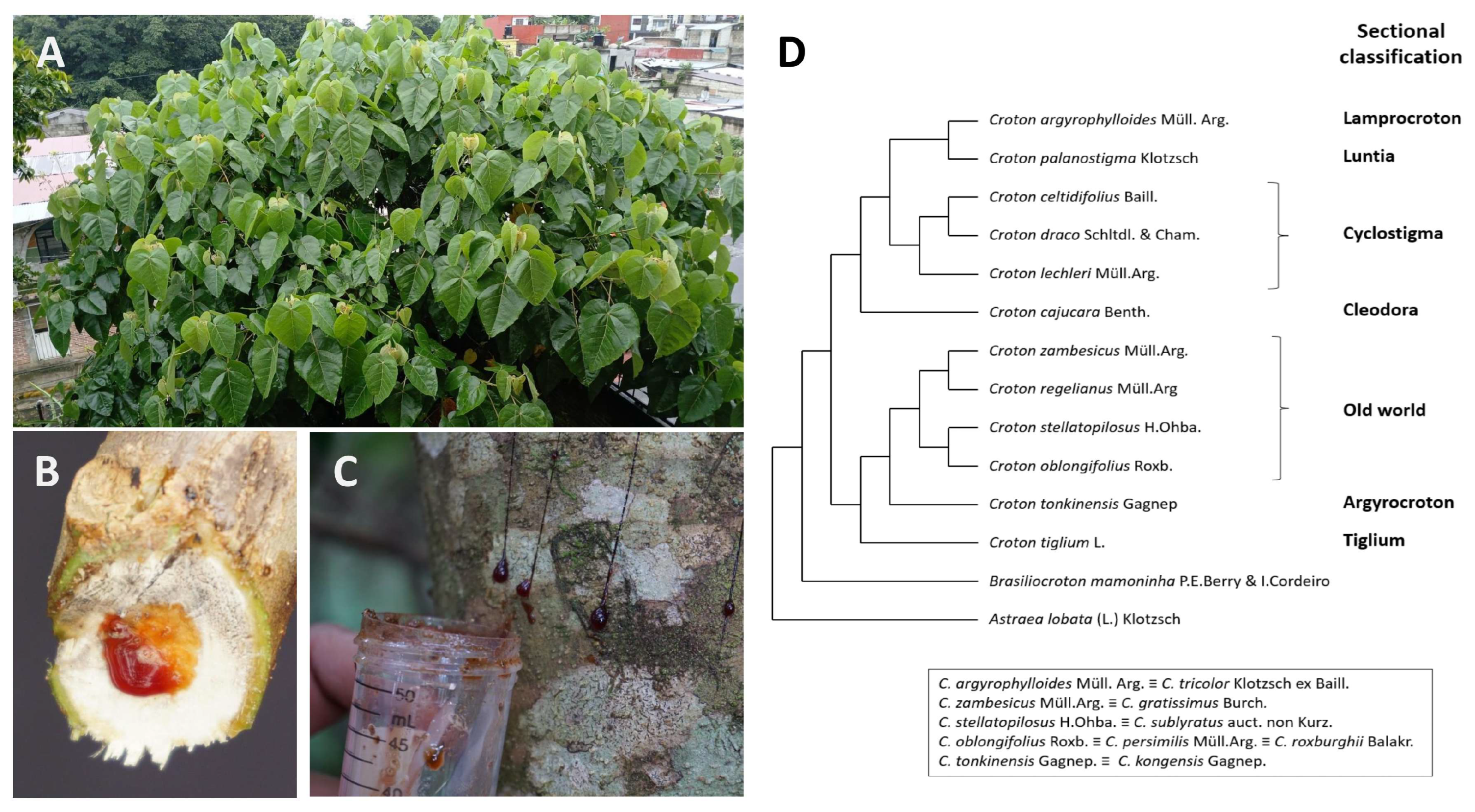
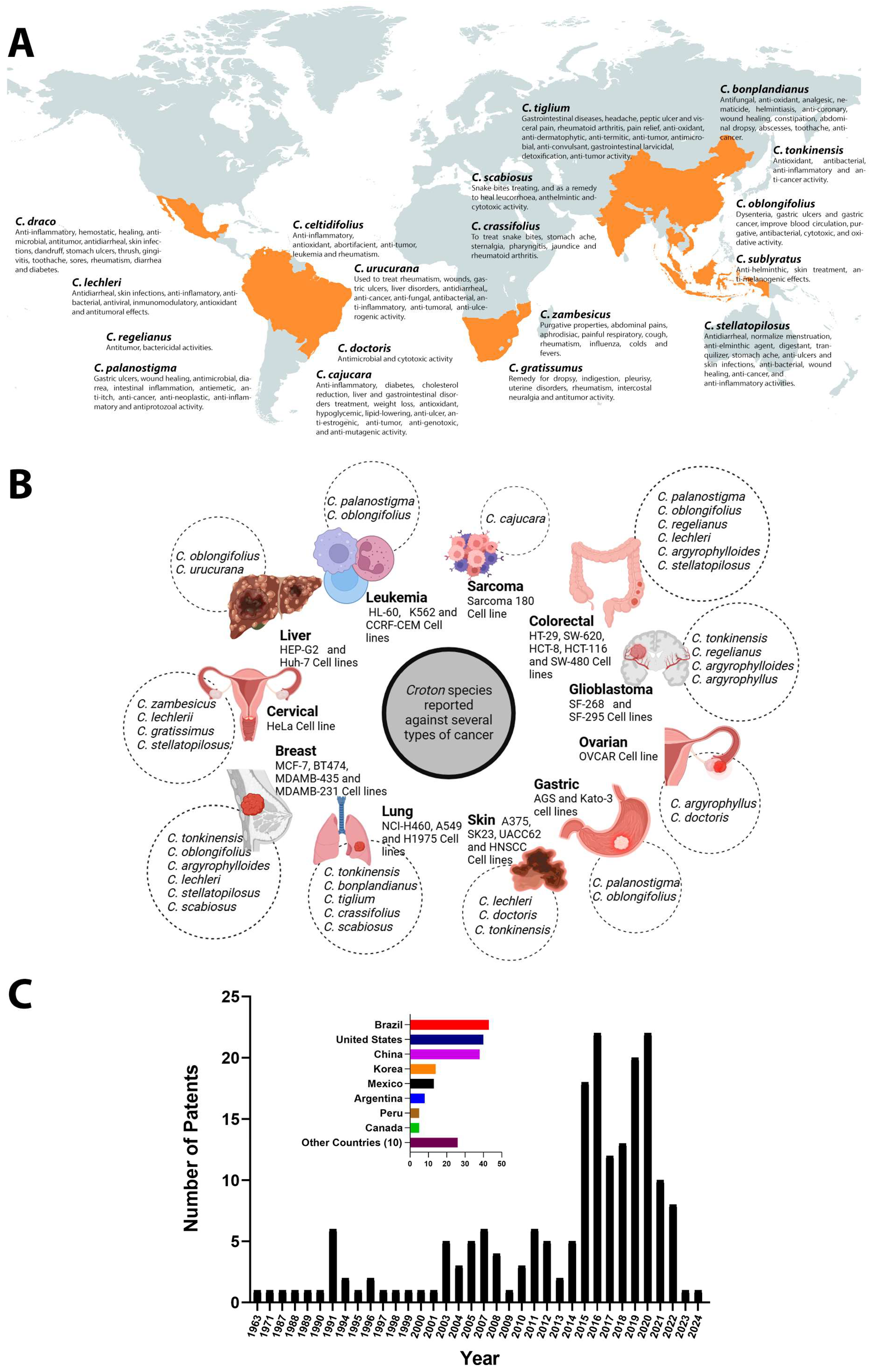

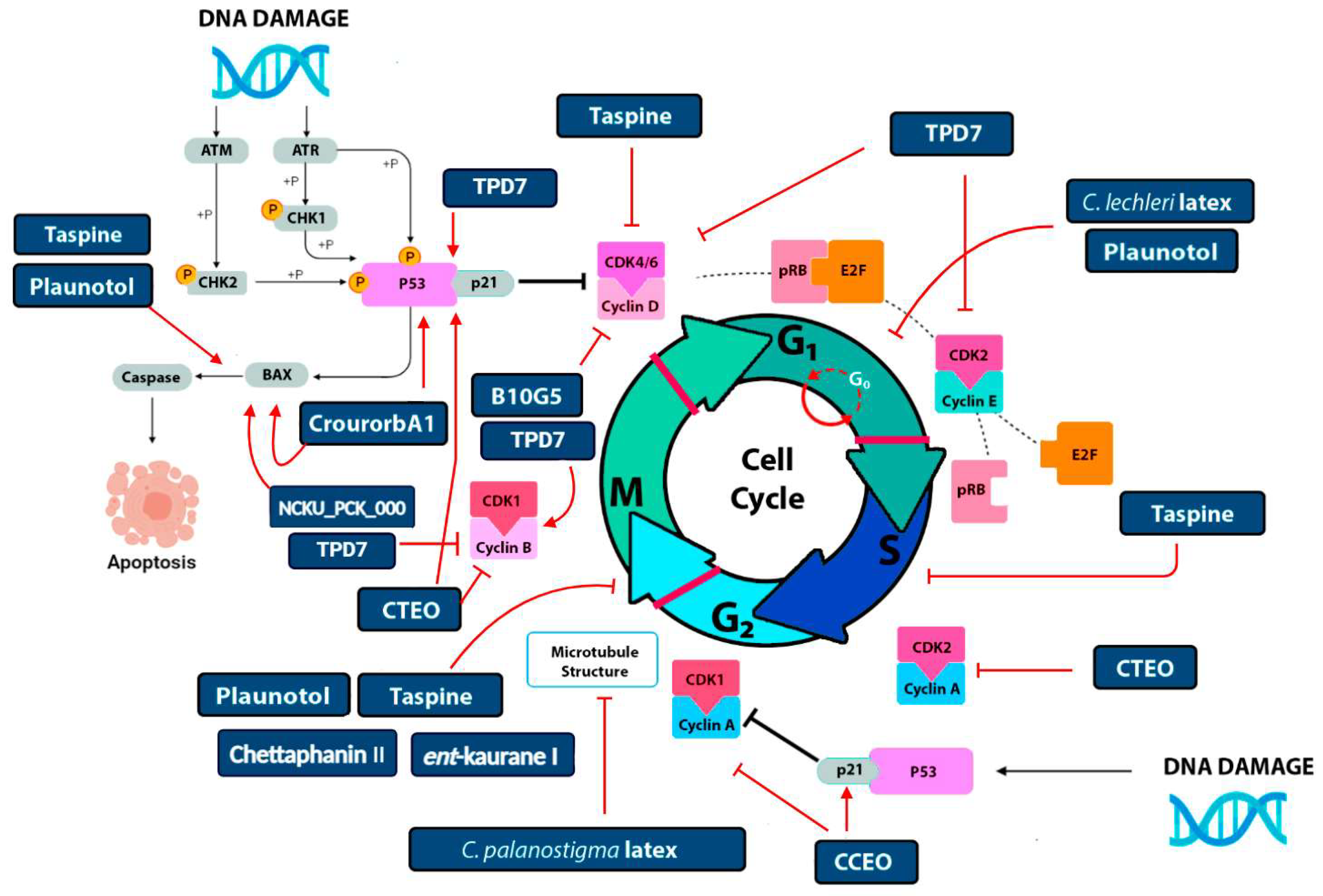
| Species | Molecule/Extraction Method/Dose | Study Model | Biological Activity/Target Regulation | Ref. |
|---|---|---|---|---|
| C. cajucara | DCTN and CTN/Hexane and Methanol/80 and 120 mg/Kg | Sarcoma-180 cells and Ehrlich carcinoma | Tumor inhibition, TNF-α | [49] |
| t-DCTN/-/180 µM | HL-60 cells | Cytotoxic activity | [50] | |
| C. palanostigma | Latex/Aqueous extract/100 µL/mL | HT-29, and HT-84 cells | Apoptosis, β-tubulin | [51] |
| C. lechleri | Latex/DMSO/2.5 µg/mL | K562 cells | Cellular proliferation inhibition | [52] |
| Latex and taspine/Dichloromethane–Methanol/1 to 10 µg/mL | SK23 cells | Apoptotic activity α-tubulin, F-actin | [53] | |
| Taspine/-/10 mg/Kg | HCT-116 model | Apoptosis in vivo, Topoisomerase I, and II inhibitor | [54] | |
| Rutin and vitexin/Methanol/17 µg/mL | HeLa, SW-480, and MDAMB-231 cells | Apoptotic activity | [7] | |
| Twig extracts/Soxhlet ethanol and pressurized ethanol/13.31 to 249.8 µg/mL | HaCat and A375 cells | Apoptotic activity Cell cycle arrest | [55] | |
| C. zambesicus | Ent-18 hidroxy-traquilobano-3β-ol/Dichloromethane extract/7.3 µg/mL | HeLa and HL-60 cells | Caspase-3/CPP32 | [56] |
| C. tonkinensis | Crotonkinins A-B/Methanolic extract/0.61 to 1.45 µg/mL | MCF-7, A549, and KB cells | Cellular proliferation inhibition | [57] |
| NCKU_PCKuo_0001/-/10 µM | HNSCC cell line | Cell viability and AKT/mTOR downregulation | [58] | |
| C. oblongifolius | Furanocembranoids/Hexane fraction/5.6 to 9.5 µ g/mL | BT474, HEP-G2, Kato-3, and SW-620 cells | Cytotoxic activity | [59] |
| C. celtidifolius | Catequin and gallocatequin/Aqueous ethanol extract/200 µg/mL | Human platelets | Platelet aggregation inhibition | [60] |
| C. regelianus | Ascaridole and essential oils/Hidrodistilled extract/6.3 to 18.4 µg/mL | HL-60, SF-295, and HCT-8 cells | Cytotoxic activity | [61] |
| C. argyrophylloides | Ent-kaurene 1-3/Ethanol extract/1.5 to 8.2 µg/mL | HL-60, MDAMB-435, SF-295, and HCT-8 cells | Cytotoxic activity | [62] |
| C. barorum | Crotobarin and crotogoudin/Ethyl acetate extract/4 µM | Murine P388 cells | Cytotoxic activity Cell cycle arrest (G2/M) | [63] |
| C. sylvaticus | Root bark/Dichloromethane–Methanol/10 µg/mL | CCRF-CEM cells | Cytotoxic activity | [64] |
| C. bonplandianus | Leaf extract/Acetone extract/15.68 µg/mL | A549 cells | Cytotoxic and apoptotic activities | [65] |
| Leaf extract/Ethanolic fraction/86.33 µg/mL | K562 cells | Cytotoxic activity | [66] | |
| C. urucurana | Crourorb A1/Ethyl acetate/35.75 µg/mL | Huh-7 cells | CDK1, Cyclin B1, MAP Kinases JNK signaling | [67] |
| C. gratissimus | Leaf extracts/Acetone and ethanol/152.3 to 462.88 µg/mL | HeLa Cells | Cytotoxic activity and Caspase-3 and -7 activation | [68] |
| C. argyrophyllus | Essential oil/Hydrodistilled extract/14.81 to 32.79 µg/mL | MDAMB-435 and OVCAR cells | Cytotoxic activity | [69] |
| C. stellatopilosus | Plaunotol/Hexane extract/65.47 to 80.90 µg/mL | MCF7, KB, HeLa, and HT-29 cells | TNF-α, BCL-2, BAK/BAX | [70] |
| C. tiglium | B10G5/-/0.11 to 20 µM | A549 and H1975 cells | PKC activation, Cyclin B1, Cyclin D1, PARP | [71] |
| CTEO/Supercritical CO2 fluid extract/48.38 µg/mL | A549 cells | Proliferation inhibition, cell cycle arrest. Cyclin and CDK inhibition. | [72] | |
| C. crassifolius | CCEO/Supercritical CO2 fluid extract/25 µg/mL | A549 cells | Apoptosis, Cyclin B1, Cyclin A, CDK1 | [73] |
| Chettaphanin II/Supercritical CO2 fluid extract/8.58 µM | A549 cells | BAX, BCL-2, Cyt-C, Cell cycle arrest, mTOR/PI3K/Akt signaling | [74] | |
| C. doctoris | Essential oils/Hexane fraction/13.4–21.8 µg/mL | UACC62 and OVCAR cells | Cytotoxic activity | [75] |
| C. scabiosus | Bark extracts/Chloroform and ethyl acetate/187.33 to 201.89 µg/mL | A549 and MCF7 cells | Cytotoxic activity | [76] |
| C. kongensis | Ent-kaurane I/Ethyl acetate extract/1 to 4 µM | MDA-MB-231 cells | Apoptotic activity and regulation of STAT3 and FAK signal pathways | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamillo-Vásquez, J.A.; Pérez-Torres, C.-A.; Ibarra-Laclette, E.; Ramón-Farías, F.; Nicasio-Torres, P.; Alatorre-Cobos, F. Secondary Metabolites from Croton Species and Their Biological Activity on Cell Cycle Regulators. Metabolites 2025, 15, 216. https://doi.org/10.3390/metabo15040216
Alamillo-Vásquez JA, Pérez-Torres C-A, Ibarra-Laclette E, Ramón-Farías F, Nicasio-Torres P, Alatorre-Cobos F. Secondary Metabolites from Croton Species and Their Biological Activity on Cell Cycle Regulators. Metabolites. 2025; 15(4):216. https://doi.org/10.3390/metabo15040216
Chicago/Turabian StyleAlamillo-Vásquez, Jorge Augusto, Claudia-Anahí Pérez-Torres, Enrique Ibarra-Laclette, Feliza Ramón-Farías, Pilar Nicasio-Torres, and Fulgencio Alatorre-Cobos. 2025. "Secondary Metabolites from Croton Species and Their Biological Activity on Cell Cycle Regulators" Metabolites 15, no. 4: 216. https://doi.org/10.3390/metabo15040216
APA StyleAlamillo-Vásquez, J. A., Pérez-Torres, C.-A., Ibarra-Laclette, E., Ramón-Farías, F., Nicasio-Torres, P., & Alatorre-Cobos, F. (2025). Secondary Metabolites from Croton Species and Their Biological Activity on Cell Cycle Regulators. Metabolites, 15(4), 216. https://doi.org/10.3390/metabo15040216








