Metabolic Reprogramming in HIV+ CD4+ T-Cells: Implications for Immune Dysfunction and Therapeutic Targets in M. tuberculosis Co-Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Initiating an Immune Response
2.3. 1H NMR Spectroscopy Method for CD4+ T-Cell Response
2.4. 1H NMR Spectroscopy Method for CD4+/CD8+ T-Cell Response
2.5. Metabolite Annotation and Data Analysis
2.6. Validation Analysis
2.7. INF-γ Quantification
2.8. 25(OH)D3 Quantification
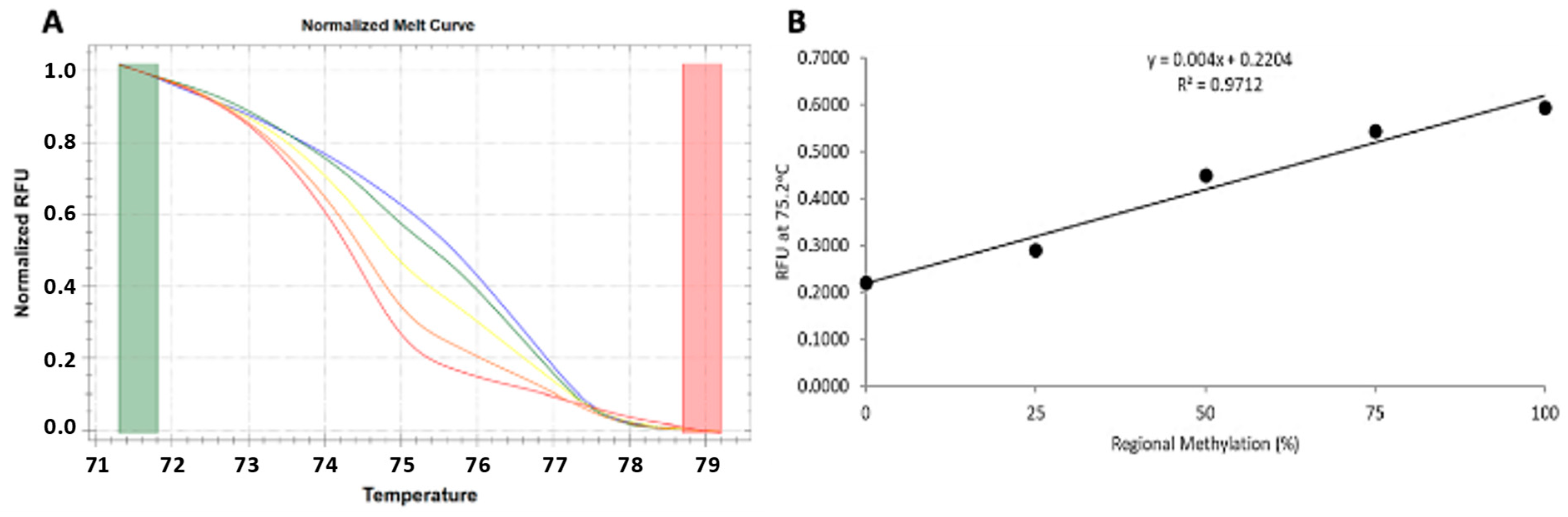
2.9. VDR Methylation Analysis
3. Results
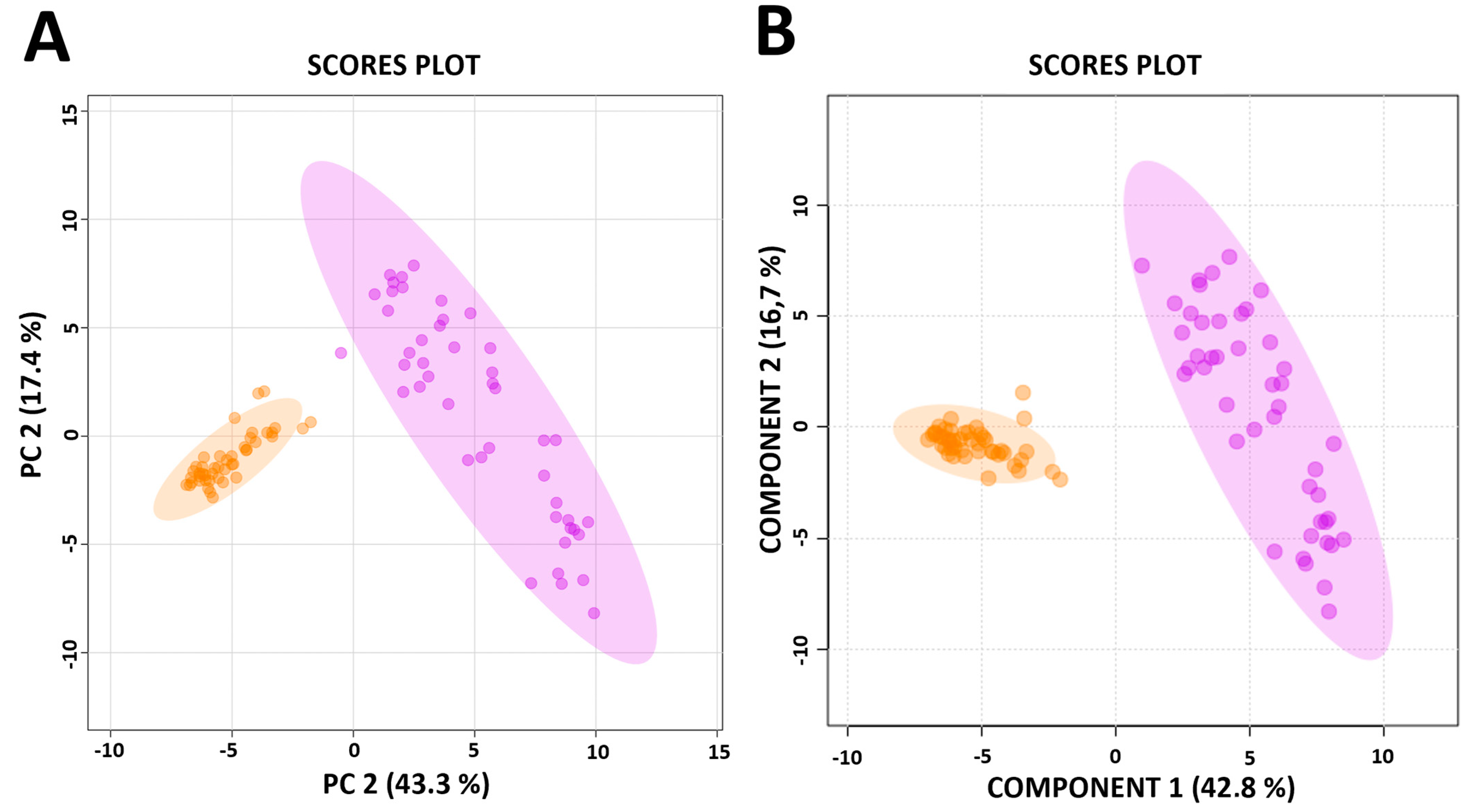
3.1. Distinct Metabolic Profiles Distinguish Between a CD4+ T-Cell Only, and a CD4+ Plus CD8+ T-Cell Response to M.tb
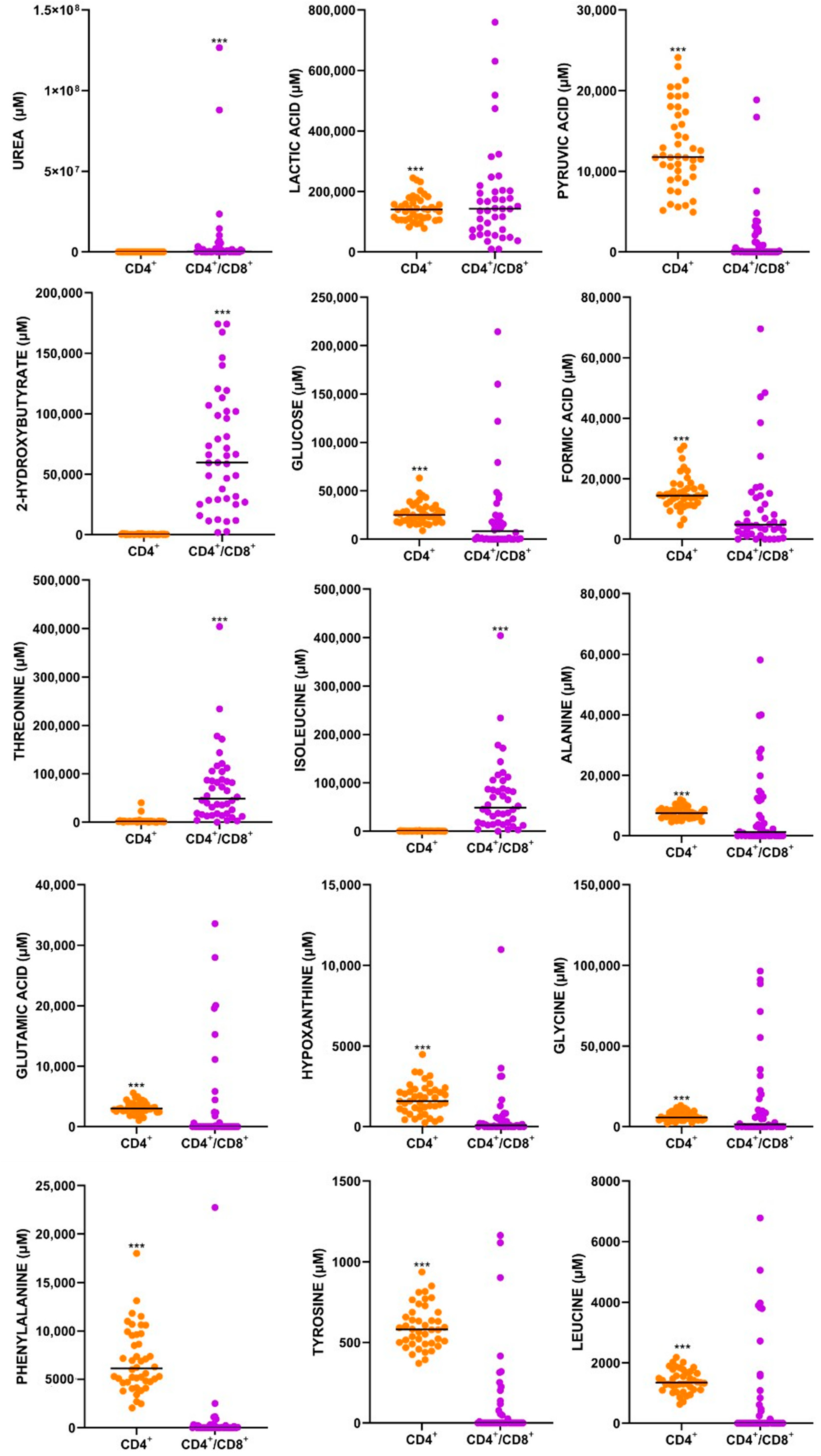
3.2. Pathways Involved in the CD4+ T-Cell Only, and a CD4+ Plus CD8+ T-Cell Response to M.tb
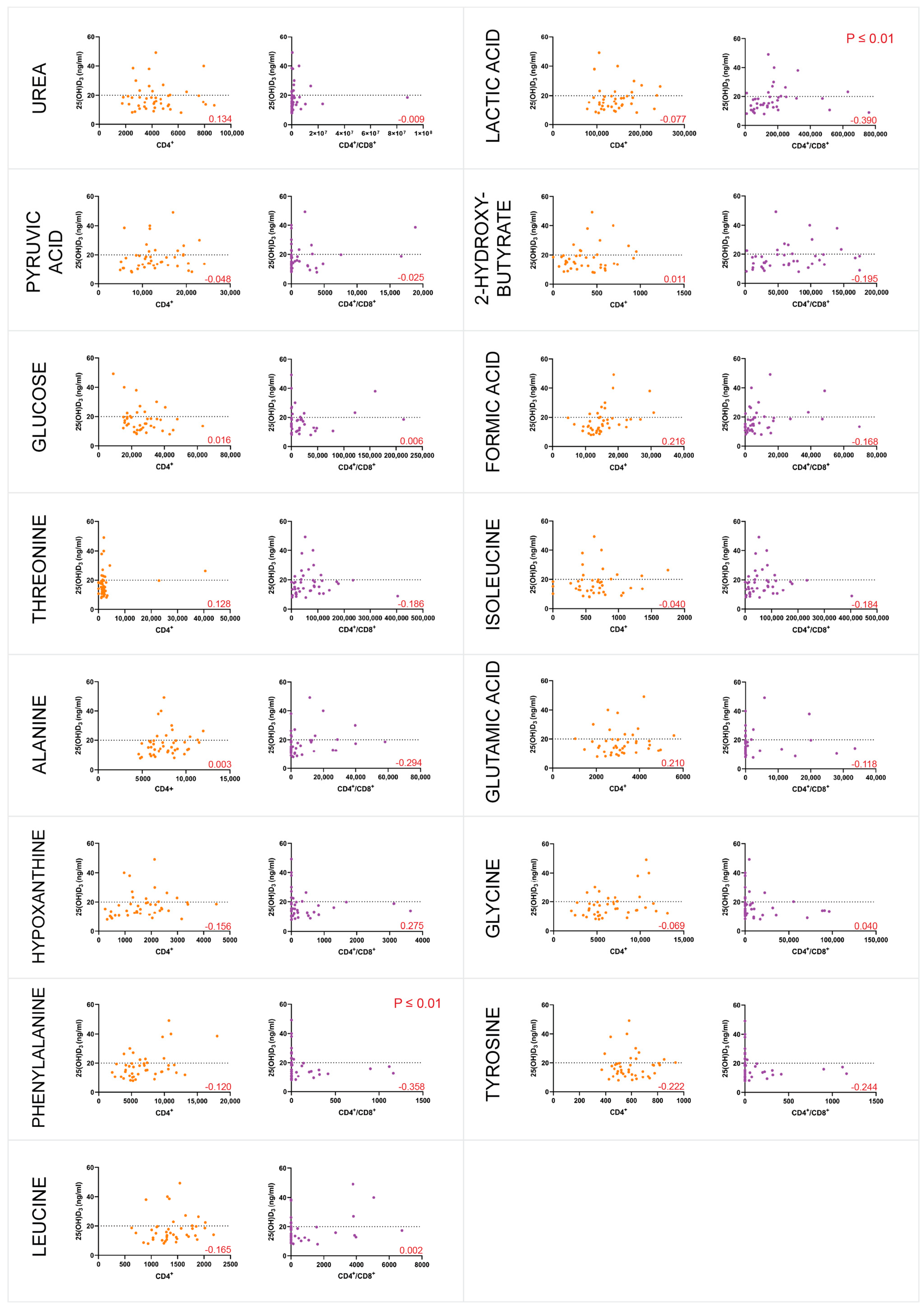
3.3. Impact of LTB Status on IFN-γ Levels and Metabolite Profiles in Newly Diagnosed HIV+ Individuals
3.4. Majority of the Individuals Were Vitamin D-Deficient and VDR-Methylated
4. Discussion
4.1. Metabolites
4.1.1. Glucose
4.1.2. Pyruvic Acid
4.1.3. Lactic Acid
4.1.4. Glutamic Acid
4.1.5. L-Alanine
4.1.6. Glycine
4.1.7. L-Phenylalanine
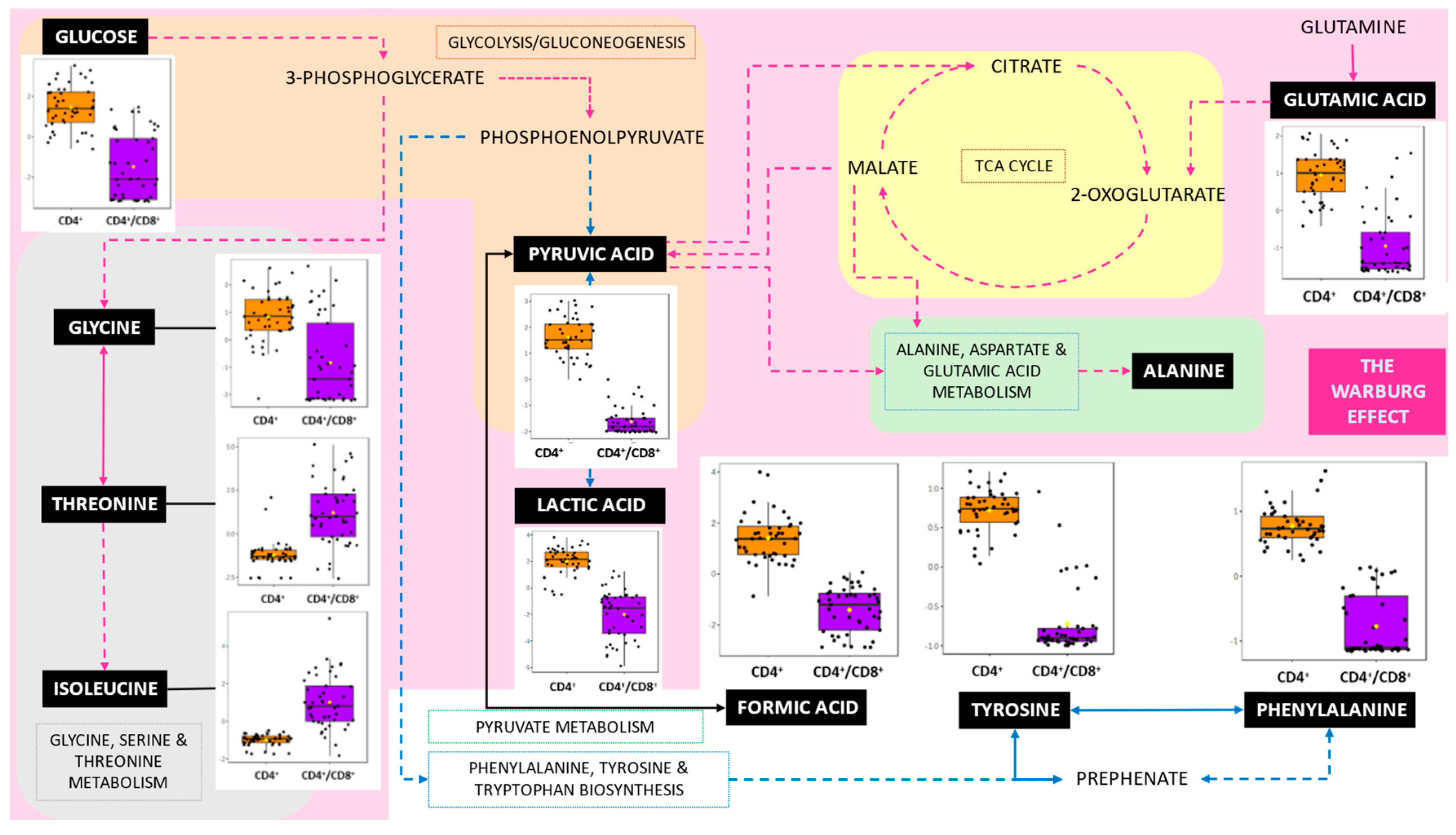
4.2. Pathways
4.2.1. The Warburg Effect, Gluconeogenesis, Glutamate and Pyruvate Metabolism
4.2.2. Amino Sugar Metabolism
4.3. Potential Therapeutic Targets
4.4. Vitamin D Deficiency and VDR Methylation
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2-DG | 2-deoxyglucose |
| APC | Antigen Presenting Cells |
| BCG | Bacillus Calmette–Guérin |
| CD4+ | Cluster of Differentiation 4-Positive |
| CD8+ | Cluster of Differentiation 8-Positive |
| CFP-10 | Culture Filtrate Protein 10 |
| dH2O | Distilled Water |
| ESAT-6 | Early Secreted Antigenic Target 6 |
| HIV | Human Immunodeficiency Virus |
| IGRA | Interferon Gamma Release Assay |
| INF-γ | Interferon Gamma |
| LTB | Latent Tuberculosis |
| M.tb | Mycobacterium tuberculosis |
| MBC | Minimum Bactericidal Concentration |
| MHC | Major Histocompatibility Complex |
| MIC | Minimum Inhibitory Concentration |
| MS-HRM | Methylation-Sensitive High-Resolution Melt Analysis |
| NMR | Nuclear Magnetic Resonance |
| PBMCs | Peripheral Blood Mononuclear Cells |
| PBS | Phosphate Buffer Solution |
| PKM2 | Pyruvate Kinase M2 |
| QFT | QuantiFERON® |
| TSP | Trimethylsilyl-2,2,3,3-Tetradeuteropropionic Acid |
| TXI | Triple Resonance Inverse |
| VDR | Vitamin D receptor |
References
- Parrish, N.M.; Dick, J.D.; Bishai, W.R. Mycobacterium tuberculosis. Trends Microbiol. 1998, 6, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Kanabalan, R.D.; Lee, L.J.; Lee, T.Y.; Chong, P.P.; Hassan, L.; Ismail, R.; Chin, V.K. Human tuberculosis and Mycobacterium tuberculosis complex: A review on genetic diversity, pathogenesis and omics approaches in host biomarkers discovery. Microbiol. Res. 2021, 246, 126674. [Google Scholar] [CrossRef] [PubMed]
- Ndlovu, H.; Marakalala, M.J. Granulomas and Inflammation: Host-Directed Therapies for Tuberculosis. Front. Immunol. 2016, 7, 434. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.L.; Chan, J. Immunology of Tuberculosis [Internet]. 2001. Available online: www.annualreviews.org (accessed on 13 February 2024).
- Stone, J.D.; Stern, L.J. CD8 T Cells, Like CD4 T Cells, Are Triggered by Multivalent Engagement of TCRs by MHC-Peptide Ligands but Not by Monovalent Engagement. J. Immunol. 2006, 176, 1498–1505. [Google Scholar] [CrossRef]
- Zhu, J.; Paul, W.E. CD4 T cells: Fates, functions, and faults. Blood 2008, 112, 1557–1569. [Google Scholar] [CrossRef]
- van de Berg, P.J.; van Leeuwen, E.M.; ten Berge, I.J.; van Lier, R. Cytotoxic human CD4+ T cells. Curr. Opin. Immunol. 2008, 20, 339–343. [Google Scholar] [CrossRef]
- Kitchen, S.G.; Whitmire, J.K.; Jones, N.R.; Galic, Z.; Kitchen, C.M.R.; Ahmed, R.; Zack, J.A. The CD4 molecule on CD8 T lymphocytes directly enhances the immune response to viral and cellular antigens. Proc. Natl. Acad. Sci. USA 2005, 102, 3794–3799. [Google Scholar] [CrossRef]
- Masur, H.; Ognibene, F.P.; Yarchoan, R.; Shelhamer, J.H.; Baird, B.F.; Travis, W.; Suffredini, A.F.; Deyton, L.; Kovacs, J.A.; Falloon, J.; et al. CD4 Counts as Predictors of Opportunistic Pneumonias in Human Immunodeficiency Virus (HIV) Infection. Ann. Intern. Med. 1989, 111, 223–231. [Google Scholar] [CrossRef]
- Meintjes, G.; Lawn, S.D.; Scano, F.; Maartens, G.; French, M.A.; Worodria, W.; Elliott, J.H.; Murdoch, D.; Wilkinson, R.J.; Seyler, C.; et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: Case definitions for use in resource-limited settings. Lancet Infect. Dis. 2008, 8, 516–523. [Google Scholar] [CrossRef]
- Lin, P.L.; Flynn, J.L. CD8 T cells and Mycobacterium tuberculosis infection. Semin. Immunopathol. 2015, 37, 239–249. [Google Scholar] [CrossRef]
- Gulzar, N.; Copeland, K.F. CD8+ T-Cells: Function and Response to HIV Infection. Curr. HIV Res. 2005, 2, 23–37. [Google Scholar] [CrossRef]
- Rozot, V.; Patrizia, A.; Vigano, S.; Mazza-Stalder, J.; Idrizi, E.; Day, C.L.; Perreau, M.; Lazor-Blanchet, C.; Ohmiti, K.; Goletti, D.; et al. Combined Use of Mycobacterium tuberculosis–Specific CD4 and CD8 T-Cell Responses Is a Powerful Diagnostic Tool of Active Tuberculosis. Clin. Infect. Dis. 2015, 60, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Aranow, C. Correspondence: Cynthia Aranow 350 Community Drive Manhasset, NY 11030. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Coussens, A.K.; Martineau, A.R.; Wilkinson, R.J. Anti-Inflammatory and Antimicrobial Actions of Vitamin D in Combating TB/HIV. Scientifica 2014, 2014, 903680. [Google Scholar] [CrossRef] [PubMed]
- Colotta, F.; Jansson, B.; Bonelli, F. Modulation of inflammatory and immune responses by vitamin D. J. Autoimmun. 2017, 85, 78–97. [Google Scholar] [CrossRef]
- Rodríguez, M.; Daniels, B.; Gunawardene, S.; Robbins, G. High Frequency of Vitamin D Deficiency in Ambulatory HIV-Positive Patients. AIDS Res. Hum. Retroviruses 2009, 25, 9–14. [Google Scholar] [CrossRef]
- Maceda, E.B.; Gonçalves, C.C.M.; Andrews, J.R.; Ko, A.I.; Yeckel, C.W.; Croda, J. Serum vitamin D levels and risk of prevalent tuberculosis, incident tuberculosis and tuberculin skin test conversion among prisoners. Sci. Rep. 2018, 8, 997. [Google Scholar] [CrossRef]
- Huang, S.-J.; Wang, X.-H.; Liu, Z.-D.; Cao, W.-L.; Han, Y.; Ma, A.-G.; Xu, S.-F. Vitamin D deficiency and the risk of tuberculosis: A meta-analysis. Drug Des. Dev. Ther. 2017, 11, 91–102. [Google Scholar] [CrossRef]
- Ayelign, B.; Workneh, M.; Molla, M.D.; Dessie, G. Role Of Vitamin-D Supplementation In TB/HIV Co-Infected Patients. Infect. Drug Resist. 2020, 13, 111–118. [Google Scholar] [CrossRef]
- Realegeno, S.; Modlin, R.L. Shedding light on the vitamin D-tuberculosis-HIV connection. Proc. Natl. Acad. Sci. USA 2011, 108, 18861–18862. [Google Scholar] [CrossRef]
- Chawla, A.; Repa, J.J.; Evans, R.M.; Mangelsdorf, D.J. Nuclear Receptors and Lipid Physiology: Opening the X-Files. Science 2001, 294, 1866–1870. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; White, J.H. The pleiotropic actions of vitamin D. BioEssays 2004, 26, 21–28. [Google Scholar] [CrossRef]
- Carlberg, C. Vitamin D Signaling in the Context of Innate Immunity: Focus on Human Monocytes. Front. Immunol. 2019, 10, 2211. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2023; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Xun, J.; Qi, T.; Zou, L.; Tang, Q.; Shen, Y.; Yang, J.; Xie, L.; Ji, Y.; Zhang, R.; Liu, L.; et al. Mycobacterium tuberculosis co-infection is associated with increased surrogate marker of the HIV reservoir. AIDS Res. Ther. 2020, 17, 63. [Google Scholar] [CrossRef] [PubMed]
- Day, J.H.; Grant, A.D.; Fielding, K.L.; Morris, L.; Moloi, V.; Charalambous, S.; Puren, A.J.; Chaisson, R.E.; De Cock, K.M.; Hayes, R.J.; et al. Does Tuberculosis Increase HIV Load? J. Infect. Dis. 2004, 190, 1677–1684. [Google Scholar] [CrossRef]
- Heyderman, R.S.; Makunike, R.; Muza, T.; Odwee, M.; Kadzirange, G.; Manyemba, J.; Muchedzi, C.; Ndemera, B.; Gomo, Z.A.R.; Gwanzura, L.K.Z.; et al. Pleural tuberculosis in Harare, Zimbabwe: The relationship between human immunodeficiency virus, CD4 lymphocyte count, granuloma formation and disseminated disease. Trop. Med. Int. Health 1998, 3, 14–20. [Google Scholar] [CrossRef]
- Di Perri, G.; Cazzadori, A.; Vento, S.; Bonora, S.; Malena, M.; Bontempini, L.; Lanzafame, M.; Allegranzi, B.; Concia, E. Comparative histopathological study of pulmonary tuberculosis in human immunodeficiency virus-infected and non-infected patients. Tuber. Lung Dis. 1996, 77, 244–249. [Google Scholar] [CrossRef]
- Liebenberg, C.; Luies, L.; Williams, A.A. Metabolomics as a Tool to Investigate HIV/TB Co-Infection. Front. Mol. Biosci. 2021, 8, 692823. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; García-Lorda, P. The metabolic puzzle during the evolution of HIV infection. Clin. Nutr. 2001, 20, 379–391. [Google Scholar] [CrossRef]
- Paton, N.; Sangeetha, S.; Earnest, A.; Bellamy, R. The impact of malnutrition on survival and the CD4 count response in HIV-infected patients starting antiretroviral therapy. HIV Med. 2006, 7, 323–330. [Google Scholar] [CrossRef]
- Kell, P.; Sidhu, R.; Qian, M.; Mishra, S.; Nicoli, E.-R.; D’Souza, P.; Tifft, C.J.; Gross, A.L.; Gray-Edwards, H.L.; Martin, D.R.; et al. A pentasaccharide for monitoring pharmacodynamic response to gene therapy in GM1 gangliosidosis. EBioMedicine 2023, 92, 104627. [Google Scholar] [CrossRef]
- MacAllan, D.C.; McNurlan, M.A.; Kurpad, A.V.; De Souza, G.; Shetty, P.S.; Calder, A.G.; Griffin, G.E. Whole Body Protein Metabolism in Human Pulmonary Tuberculosis and Undernutrition: Evidence for Anabolic Block in Tuberculosis. Clin. Sci. 1998, 94, 321–331. [Google Scholar] [CrossRef]
- Cheng, S.-C.; Joosten, L.A.; Netea, M.G. The interplay between central metabolism and innate immune responses. Cytokine Growth Factor Rev. 2014, 25, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Navasardyan, I.; Miwalian, R.; Petrosyan, A.; Yeganyan, S.; Venketaraman, V. HIV–TB Coinfection: Current Therapeutic Approaches and Drug Interactions. Viruses 2024, 16, 321. [Google Scholar] [CrossRef] [PubMed]
- van der Walt, G.; Lindeque, J.Z.; Mason, S.; Louw, R. Sub-cellular metabolomics contributes mitochondria-specific metabolic insights to a mouse model of Leigh syndrome. Metabolites 2021, 11, 658. [Google Scholar] [CrossRef]
- Cantorna, M.; Zhu, Y.; Froicu, M.; Wittke, A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am. Soc. Clin. Nutr. 2004, 80, 1717S–1720S. [Google Scholar] [CrossRef] [PubMed]
- Patterson, B.; Smith, D.; Telford, A.; Tana, A.; Johnstone, D.; Davidson, R.; Martineau, A.R. Vitamin D deficiency predicts latent TB reactivation independent of preventive therapy: A longitudinal study. Int. J. Tuberc. Lung Dis. 2020, 24, 916–921. [Google Scholar] [CrossRef]
- McCartney, C.R.; McDonnell, M.E.; Corrigan, M.D.; Lash, R.W. Vitamin D Insufficiency and Epistemic Humility: An Endocrine Society Guideline Communication. J. Clin. Endocrinol. Metab. 2024, 109, 1948–1954. [Google Scholar] [CrossRef]
- Judge, A.; Dodd, M.S. Metabolism. Essays Biochem. 2020, 64, 607–647. [Google Scholar] [CrossRef]
- MacDonald, M.J. Glucose enters mitochondrial metabolism via both carboxylation and decarboxylation of pyruvate in pancreatic islets. Metabolism 1993, 42, 1229–1231. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.S.; Cherry, C.L.; Sada-Ovalle, I.; Singh, A.; Crowe, S.M. Glucose Metabolism in T Cells and Monocytes: New Perspectives in HIV Pathogenesis. EBioMedicine 2016, 6, 31–41. [Google Scholar] [CrossRef]
- Macintyre, A.N.; Gerriets, V.A.; Nichols, A.G.; Michalek, R.D.; Rudolph, M.C.; DeOliveira, D.; Anderson, S.M.; Abel, E.D.; Chen, B.J.; Hale, L.P.; et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 2014, 20, 61–72. [Google Scholar] [CrossRef]
- Valle-Casuso, J.C.; Angin, M.; Volant, S.; Passaes, C.; Monceaux, V.; Mikhailova, A.; Bourdic, K.; Avettand-Fenoel, V.; Boufassa, F.; Sitbon, M.; et al. Cellular Metabolism Is a Major Determinant of HIV-1 Reservoir Seeding in CD4+ T Cells and Offers an Opportunity to Tackle Infection. Cell Metab. 2019, 29, 611–626.e5. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.L.; Lagunoff, M. Viral activation of cellular metabolism. Virology 2015, 479–480, 609–618. [Google Scholar] [CrossRef]
- Deme, P.; Rubin, L.H.; Yu, D.; Xu, Y.; Nakigozi, G.; Nakasujja, N.; Anok, A.; Kisakye, A.; Quinn, T.C.; Reynolds, S.J.; et al. Immunometabolic Reprogramming in Response to HIV Infection Is Not Fully Normalized by Suppressive Antiretroviral Therapy. Viruses 2022, 14, 1313. [Google Scholar] [CrossRef] [PubMed]
- Mocholi, E.; Russo, L.; Gopal, K.; Ramstead, A.G.; Hochrein, S.M.; Vos, H.R.; Geeven, G.; Adegoke, A.O.; Hoekstra, A.; van Es, R.M.; et al. Pyruvate metabolism controls chromatin remodeling during CD4+ T cell activation. Cell Rep. 2023, 42, 112583. [Google Scholar] [CrossRef]
- Chapman, N.M.; Boothby, M.R.; Chi, H. Metabolic coordination of T cell quiescence and activation. Nat. Rev. Immunol. 2020, 20, 55–70. [Google Scholar] [CrossRef]
- Giron, L.B.; Palmer, C.S.; Liu, Q.; Yin, X.; Papasavvas, E.; Sharaf, R.; Etemad, B.; Damra, M.; Goldman, A.R.; Tang, H.-Y.; et al. Non-invasive plasma glycomic and metabolic biomarkers of post-treatment control of HIV. Nat. Commun. 2021, 12, 3922. [Google Scholar] [CrossRef]
- Lu, J.; Ma, S.; Zhang, W.Y.; Duan, J. Changes in peripheral blood inflammatory factors (TNF-a and IL-6) and intestinal flora in AIDS and HIV-positive individuals. J. Zhejiang Univ. Sci. B 2019, 20, 793–802. [Google Scholar] [CrossRef]
- Chandel, N.S. Glycolysis. Cold Spring Harb. Perspect. Biol. 2021, 13, a040535. [Google Scholar] [CrossRef]
- Dorfman, A. Pathways of Glycolysis. Physiol. Rev. 1943, 23, 124–136. [Google Scholar] [CrossRef]
- Pucino, V.; Certo, M.; Bulusu, V.; Cucchi, D.; Goldmann, K.; Pontarini, E.; Haas, R.; Smith, J.; Headland, S.E.; Blighe, K.; et al. Lactate Buildup at the Site of Chronic Inflammation Promotes Disease by Inducing CD4+ T Cell Metabolic Rewiring. Cell Metab. 2019, 30, 1055–1074.e8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, L.; Kueth, G.; Shao, E.; Wang, X.; Ha, T.; Williams, D.L.; Li, C.; Fan, M.; Yang, K. Lactate’s impact on immune cells in sepsis: Unraveling the complex interplay. Front. Immunol. 2024, 15, 1483400. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, L.; Veliça, P.; Gameiro, P.A.; Cunha, P.P.; Foskolou, I.P.; Rullman, E.; Bargiela, D.; Johnson, R.S.; Rundqvist, H. Lactate exposure shapes the metabolic and transcriptomic profile of CD8+ T cells. Front. Immunol. 2023, 14, 1101433. [Google Scholar] [CrossRef] [PubMed]
- Tu, V.Y.; Ayari, A.; O’connor, R.S. Beyond the Lactate Paradox: How Lactate and Acidity Impact T Cell Therapies against Cancer. Antibodies 2021, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Haas, R.; Smith, J.; Rocher-Ros, V.; Nadkarni, S.; Montero-Melendez, T.; D’acquisto, F.; Bland, E.J.; Bombardieri, M.; Pitzalis, C.; Perretti, M.; et al. Lactate Regulates Metabolic and Pro-inflammatory Circuits in Control of T Cell Migration and Effector Functions. PLoS Biol. 2015, 13, e1002202. [Google Scholar] [CrossRef]
- Wen, J.; Cheng, S.; Zhang, Y.; Wang, R.; Xu, J.; Ling, Z.; Ma, L.; Ai, X.; Sun, B. Lactate anions participate in T cell cytokine production and function. Sci. China Life Sci. 2021, 64, 1895–1905. [Google Scholar] [CrossRef]
- Salinas, T.R.W.; Gosselin, A.; Raymond Marchand, L.; Gabriel, E.M.; Tastet, O.; Goulet, J.-P.; Zhang, Y.; Vlad, D.; Touil, H.; Routy, J.-P.; et al. IL-17A reprograms intestinal epithelial cells to facilitate HIV-1 replication and outgrowth in CD4+ T cells. iScience 2021, 24, 103225. [Google Scholar] [CrossRef]
- De Paoli, P. Immunological effects of interleukin-2 therapy in human immunodeficiency virus-positive subjects. Clin. Diagn. Lab. Immunol. 2001, 8, 671–677. [Google Scholar] [CrossRef]
- Kopperschläger, G.; Kirchberger, J. Methods for the separation of lactate dehydrogenases and clinical significance of the enzyme. J. Chromatogr. B Biomed. Sci. Appl. 1996, 684, 25–49. [Google Scholar] [CrossRef]
- Al-Eisa, E.S.; Alghadir, A.H.; Gabr, S.A. Correlation between vitamin D levels and muscle fatigue risk factors based on physical activity in healthy older adults. Clin. Interv. Aging 2016, 11, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Hegedus, A.; Kavanagh Williamson, M.; Khan, M.B.; Dias Zeidler, J.; Da Poian, A.T.; El-Bacha, T.; Struys, E.A.; Huthoff, H. Evidence for Altered Glutamine Metabolism in Human Immunodeficiency Virus Type 1 Infected Primary Human CD4+T Cells. AIDS Res. Hum. Retroviruses 2017, 33, 1236–1247. [Google Scholar] [CrossRef] [PubMed]
- Datta, P.K.; Deshmane, S.; Khalili, K.; Merali, S.; Gordon, J.C.; Fecchio, C.; Barrero, C.A. Glutamate metabolism in HIV-1 infected macrophages: Role of HIV-1 Vpr. Cell Cycle 2016, 15, 2288–2298. [Google Scholar] [CrossRef]
- Kogan, M.; Rappaport, J. HIV-1 Accessory Protein Vpr: Relevance in the pathogenesis of HIV and potential for therapeutic intervention. Retrovirology 2011, 8, 25. [Google Scholar] [CrossRef]
- Chen, R.; Le Rouzic, E.; Kearney, J.A.; Mansky, L.M.; Benichou, S. Vpr-mediated Incorporation of UNG2 into HIV-1 Particles Is Required to Modulate the Virus Mutation Rate and for Replication in Macrophages. J. Biol. Chem. 2004, 279, 28419–28425. [Google Scholar] [CrossRef] [PubMed]
- Ron-Harel, N.; Ghergurovich, J.M.; Notarangelo, G.; LaFleur, M.W.; Tsubosaka, Y.; Sharpe, A.H.; Rabinowitz, J.D.; Haigis, M.C. T Cell Activation Depends on Extracellular Alanine. Cell Rep. 2019, 28, 3011–3021.e4. [Google Scholar] [CrossRef]
- Khan, N.; Geiger, J.D. Role of Viral Protein U (Vpu) in HIV-1 Infection and Pathogenesis. Viruses 2021, 13, 1466. [Google Scholar] [CrossRef]
- Sugden, S.; Cohen, É.A. Attacking the Supply Lines: HIV-1 Restricts Alanine Uptake to Prevent T Cell Activation. Cell Host Microbe 2015, 18, 514–517. [Google Scholar] [CrossRef]
- Topchyan, P.; Lin, S.; Cui, W. The Role of CD4 T Cell Help in CD8 T Cell Differentiation and Function During Chronic Infection and Cancer. Immune Netw. 2023, 23, e41. [Google Scholar] [CrossRef]
- Liu, S.; Liao, S.; Liang, L.; Deng, J.; Zhou, Y. The relationship between CD4+ T cell glycolysis and their functions. Trends Endocrinol. Metab. 2023, 34, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Borah, K.; Beyß, M.; Theorell, A.; Wu, H.; Basu, P.; Mendum, T.A.; Nöh, K.; Beste, D.J.; McFadden, J. Intracellular Mycobacterium tuberculosis Exploits Multiple Host Nitrogen Sources during Growth in Human Macrophages. Cell Rep. 2019, 29, 3580–3591.e4. [Google Scholar] [CrossRef]
- Zangerle, R.; Kurz, K.; Neurauter, G.; Kitchen, M.; Sarcletti, M.; Fuchs, D. Increased blood phenylalanine to tyrosine ratio in HIV-1 infection and correction following effective antiretroviral therapy. Brain, Behav. Immun. 2010, 24, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Jin, Y.; Fan, Z. The Mechanism of Warburg Effect-Induced Chemoresistance in Cancer. Front. Oncol. 2021, 11, 698023. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Kim, B.; Park, J.; Youn, H.S.; Youn, B.H. The Warburg effect on radio resistance: Survival beyond growth. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2023, 1878, 188988. [Google Scholar] [CrossRef]
- Shi, L.; Eugenin, E.A.; Subbian, S. Immunometabolism in tuberculosis. Front. Immunol. 2016, 7, 150. [Google Scholar] [CrossRef]
- Cumming, B.M.; Pacl, H.T.; Steyn, A.J.C. Relevance of the Warburg Effect in Tuberculosis for Host-Directed Therapy. Front. Cell. Infect. Microbiol. 2020, 10, 576596. [Google Scholar] [CrossRef]
- Engelking, L.R. Gluconeogenesis. In Textbook of Veterinary Physiological Chemistry, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Skarbek, K.; Milewska, M.J. Biosynthetic and synthetic access to amino sugars. Carbohydr. Res. 2016, 434, 44–71. [Google Scholar] [CrossRef]
- Jacob, G.S.; Scudder, P.; Butters, T.D.; Jones, I.; Tiemeier, D.C. Aminosugar Attenuation of HIV Infection. In Natural Products as Antiviral Agents; Springer: Boston, MA, USA, 1992; pp. 137–152. [Google Scholar] [CrossRef]
- Fleet, G.W.; Karpas, A.; Dwek, R.A.; Fellows, L.E.; Tyms, A.; Petursson, S.; Namgoong, S.K.; Ramsden, N.G.; Smith, P.W.; Son, J.C.; et al. Inhibition of HIV replication by amino-sugar derivatives. FEBS Lett. 1988, 237, 128–132. [Google Scholar] [CrossRef]
- Taylor, H.E.; Palmer, C.S. CD4 T Cell Metabolism Is a Major Contributor of HIV Infectivity and Reservoir Persistence. Immunometabolism 2020, 2, e200005. [Google Scholar] [CrossRef]
- Gross, M.I.; Demo, S.D.; Dennison, J.B.; Chen, L.; Chernov-Rogan, T.; Goyal, B.; Janes, J.R.; Laidig, G.J.; Lewis, E.R.; Li, J.; et al. Antitumor Activity of the Glutaminase Inhibitor CB-839 in Triple-Negative Breast Cancer. Mol. Cancer Ther. 2014, 13, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Lake, J.E.; Adams, J.S. Vitamin D in HIV-Infected Patients. Curr. HIV/AIDS Rep. 2011, 8, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Allavena, C.; Delpierre, C.; Cuzin, L.; Rey, D.; Viget, N.; Bernard, J.; Guillot, P.; Duvivier, C.; Billaud, E.; Raffi, F. High frequency of vitamin D deficiency in HIV-infected patients: Effects of HIV-related factors and antiretroviral drugs. J. Antimicrob. Chemother. 2012, 67, 2222–2230. [Google Scholar] [CrossRef] [PubMed]
- Spector Stanger, M. Vitamin D and HIV: Letting the Sun Shine In. Top. Antivir. Med. 2011, 19, 6–10. [Google Scholar]
- Mansueto, P.; Seidita, A.; Vitale, G.; Gangemi, S.; Iaria, C.; Cascio, A. Vitamin D Deficiency in HIV Infection: Not Only a Bone Disorder. BioMed Res. Int. 2015, 2015, 735615. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Waddell, A. The vitamin D receptor turns off chronically activated T cells. Ann. N. Y. Acad. Sci. 2014, 1317, 70–75. [Google Scholar] [CrossRef]
- Chandel, N.; Husain, M.; Goel, H.; Salhan, D.; Lan, X.; Malhotra, A.; McGowan, J.; Singhal, P.C. VDR hypermethylation and HIV-induced T cell loss. J. Leukoc. Biol. 2013, 93, 623–631. [Google Scholar] [CrossRef]
- Vijayan, K.K.V.; Karthigeyan, K.P.; Tripathi, S.P.; Hanna, L.E. Pathophysiology of CD4+ T-Cell Depletion in HIV-1 and HIV-2 Infections. Front. Immunol. 2017, 8, 580. [Google Scholar] [CrossRef]
| Metabolite | Synonym 1 | Confidence Score 2 | p-Value 3 | FDR 3 |
|---|---|---|---|---|
| Urea | 8 | 2.6665 × 10−12 | 6.9329 × 10−12 | |
| L-Lactic Acid | Lactic Acid | 10 | 9.5153 × 10−22 | 5.3014 × 10−21 |
| Pyruvic Acid | 8 | 2.8772 × 10−40 | 1.1221 × 10−38 | |
| 2-Hydroxybutyrate | 9 | 3.2643 × 10−24 | 2.1218 × 10−23 | |
| D-Glucose | Glucose | 10 | 1.2451 × 10−16 | 4.8559 × 10−16 |
| Formic Acid | 9 | 1.049 × 10−25 | 1.0228 × 10−24 | |
| L-Threonine | Threonine | 9 | 5.1955 × 10−12 | 1.2664 × 10−11 |
| L-Isoleucine | Isoleucine | 7 | 1.717 × 10−14 | 6.0875 × 10−14 |
| L-alanine | Alanine | 10 | 1.0802 × 10−12 | 3.5108 × 10−12 |
| L-Glutamic Acid | Glutamic Acid | 10 | 7.1441 × 10−20 | 3.4828 × 10−19 |
| Hypoxanthine | 10 | 8.7547 × 10−25 | 6.8287 × 10−24 | |
| Glycine | 10 | 1.1789 × 10−8 | 2.5542 × 10−8 | |
| L-Phenylalanine | Phenylalanine | 10 | 9.1936 × 10−32 | 1.1952 × 10−30 |
| L-Tyrosine | Tyrosine | 10 | 7.9541 × 10−33 | 1.551 × 10−31 |
| L-Leucine | Leucine | 10 | 4.2008 × 10−17 | 1.8203 × 10−16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayrga, S.; Koorsen, G. Metabolic Reprogramming in HIV+ CD4+ T-Cells: Implications for Immune Dysfunction and Therapeutic Targets in M. tuberculosis Co-Infection. Metabolites 2025, 15, 285. https://doi.org/10.3390/metabo15050285
Ayrga S, Koorsen G. Metabolic Reprogramming in HIV+ CD4+ T-Cells: Implications for Immune Dysfunction and Therapeutic Targets in M. tuberculosis Co-Infection. Metabolites. 2025; 15(5):285. https://doi.org/10.3390/metabo15050285
Chicago/Turabian StyleAyrga, Suheena, and Gerrit Koorsen. 2025. "Metabolic Reprogramming in HIV+ CD4+ T-Cells: Implications for Immune Dysfunction and Therapeutic Targets in M. tuberculosis Co-Infection" Metabolites 15, no. 5: 285. https://doi.org/10.3390/metabo15050285
APA StyleAyrga, S., & Koorsen, G. (2025). Metabolic Reprogramming in HIV+ CD4+ T-Cells: Implications for Immune Dysfunction and Therapeutic Targets in M. tuberculosis Co-Infection. Metabolites, 15(5), 285. https://doi.org/10.3390/metabo15050285






