Probiotic Supplementation Improves Glucose Homeostasis and Modulates Interleukin (IL)-21 and IL-22 Levels in Pediatric Patients with Type 1 Diabetes: A Randomized Placebo-Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Size
2.2. Randomization and Study Population
2.3. Baseline Clinical Assessment and Laboratory Investigations
2.4. Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Baseline Clinical and Laboratory Data of the Enrolled Participants with T1DM
3.2. Effect of Probiotic Supplementation on Glycemic Control, Lipid Profile, and IL-21 and IL-22 Levels
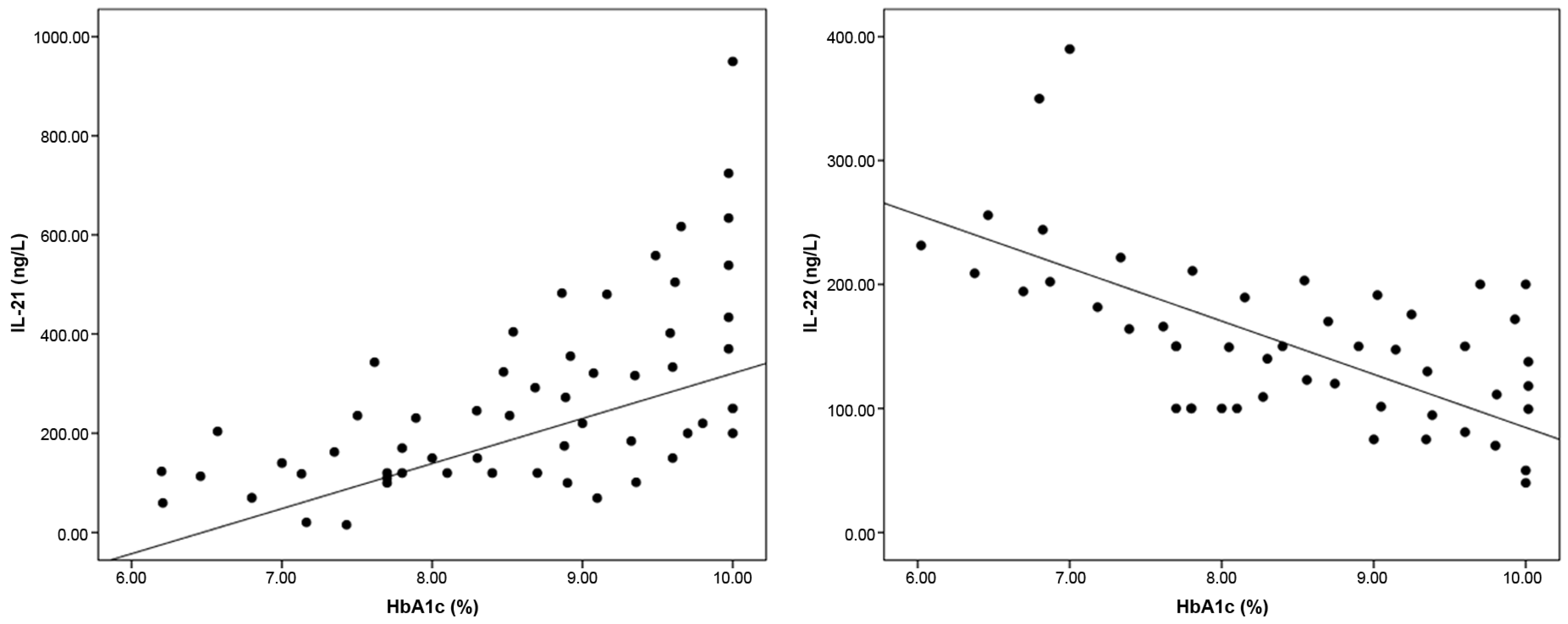
3.3. Correlation Between Baseline ILs and Other Studied Variables Among T1DM Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borody, T.J.; Campbell, J. Fecal microbiota transplantation: Techniques, applications, and issues. Gastroenterol. Clin. 2012, 41, 781–803. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ota, N.; Manzanillo, P.; Kates, L.; Zavala-Solorio, J.; Eidenschenk, C.; Zhang, J.; Lesch, J.; Lee, W.P.; Ross, J.; et al. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature 2014, 514, 237–241. [Google Scholar] [CrossRef] [PubMed]
- McLean, M.H.; Dieguez, D.; Miller, L.M.; Young, H.A. Does the microbiota play a role in the pathogenesis of autoimmune diseases? Gut 2015, 64, 332–341. [Google Scholar] [CrossRef]
- Knip, M.; Siljander, H. The role of the intestinal microbiota in type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2016, 12, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Ejtahed, H.S.; Mohtadi-Nia, J.; Homayouni-Rad, A.; Niafar, M.; Asghari-Jafarabadi, M.; Mofid, V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 2012, 28, 539–543. [Google Scholar] [CrossRef]
- Fijan, S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef]
- Liu, Y.; Alookaran, J.J.; Rhoads, J.M. Probiotics in autoimmune and inflammatory disorders. Nutrients 2018, 10, 1537. [Google Scholar] [CrossRef]
- Hummel, S.; Veltman, K.; Cichon, C.; Sonnenborn, U.; Schmidt, M.A. Differential targeting of the E-cadherin/β-catenin complex by Gram-positive probiotic lactobacilli improves epithelial barrier function. Appl. Environ. Microbiol. 2012, 78, 1140–1147. [Google Scholar] [CrossRef]
- Azad, M.; Kalam, A.; Sarker, M.; Wan, D. Immunomodulatory effects of probiotics on cytokine profiles. BioMed Res. Int. 2018, 18, 7–11. [Google Scholar] [CrossRef]
- Shah, N.J.; Swami, O.C. Role of probiotics in diabetes: A review of their rationale and efficacy. Diabetes 2017, 5, 104–110. [Google Scholar] [CrossRef]
- Schreiber, F.; Arasteh, J.M.; Lawley, T.D. Pathogen resistance mediated by IL-22 signaling at the epithelial–microbiota interface. J. Mol. Biol. 2015, 427, 3676–3682. [Google Scholar] [CrossRef]
- Hasnain, S.Z.; Borg, D.J.; Harcourt, B.E.; Tong, H.; Sheng, Y.H.; Ng, C.P.; Das, I.; Wang, R.; Chen, A.C.; Loudovaris, T.; et al. Glycemic control in diabetes is restored by therapeutic manipulation of cytokines that regulate beta cell stress. Nat. Med. 2014, 20, 1417–1426. [Google Scholar] [CrossRef]
- Sajiir, H.; Wong, K.Y.; Müller, A.; Keshvari, S.; Burr, L.; Aiello, E.; Mezza, T.; Giaccari, A.; Sebastiani, G.; Dotta, F.; et al. Pancreatic beta-cell IL-22 receptor deficiency induces age-dependent dysregulation of insulin biosynthesis and systemic glucose homeostasis. Nat. Commun. 2024, 15, 4527. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, A.P.; Van Belle, T.; Wurster, A.L.; Suto, A.; Michaud, M.; Zhang, D.; Grusby, M.J.; von Herrath, M. Interleukin-21 is required for the development of type 1 diabetes in NOD mice. Diabetes 2009, 58, 1144–1155. [Google Scholar] [CrossRef]
- Pelletier, M.; Girard, D. Biological functions of interleukin-21 and its role in inflammation. Sci. World J. 2007, 7, 1715–1735. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.C.; Simons, H.Z.; Thompson, W.S.; Cutler, A.J.; Dopico, X.C.; Smyth, D.J.; Mashar, M.; Schuilenburg, H.; Walker, N.M.; Dunger, D.B.; et al. IL-21 production by CD4+ effector T cells and frequency of circulating follicular helper T cells are increased in type 1 diabetes patients. Diabetologia 2015, 58, 781–790. [Google Scholar] [CrossRef]
- Schroderus, A.M.; Poorbaugh, J.; McElyea, S.; Beasley, S.; Zhang, L.; Näntö-Salonen, K.; Rintamäki, R.; Pihlajamäki, J.; Knip, M.; Veijola, R.; et al. Evaluation of plasma IL-21 as a potential biomarker for type 1 diabetes progression. Front. Immunol. 2023, 14, 1157265. [Google Scholar] [CrossRef]
- Cao, A.T.; Yao, S.; Gong, B.; Nurieva, R.I.; Elson, C.O.; Cong, Y. Interleukin (IL)-21 promotes intestinal IgA response to microbiota. Mucosal Immunol. 2015, 8, 1072–1082. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomized trials. Ann. Intern. Med. 2010, 152, 726–732. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43, S14–S31. [Google Scholar] [CrossRef] [PubMed]
- Ostadrahimi, A.; Taghizadeh, A.; Mobasseri, M.; Farrin, N.; Payahoo, L.; Gheshlaghi, Z.B.; Vahedjabbari, M. Effect of Probiotic Fermented Milk (Kefir) on Glycemic Control and Lipid Profile In Type 2 Diabetic Patients: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Iran. J. Public. Health 2015, 44, 228–237. [Google Scholar] [PubMed] [PubMed Central]
- Cengiz, E.; Danne, T.; Ahmad, T.; Ayyavoo, A.; Beran, D.; Ehtisham, S.; Fairchild, J.; Jarosz-Chobot, P.; Ng, S.M.; Paterson, M.; et al. ISPAD Clinical Practice Consensus Guidelines 2022: Insulin treatment in children and adolescents with diabetes. Pediatr. Diabetes 2022, 23, 1277–1296. [Google Scholar] [CrossRef]
- Flynn, J.T.; Kaelber, D.C.; Baker-Smith, C.M.; Blowey, D.; Carroll, A.E.; Daniels, S.R.; de Ferranti, S.D.; Dionne, J.M.; Falkner, B.; Flinn, S.K.; et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics 2017, 140, e20171904. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.A.; Roy, A.; Burrell, A.; Fairchild, C.J.; Fuldeore, M.J.; Ollendorf, D.A.; Wong, P.K. Medication compliance and persistence: Terminology and definitions. Value Health 2008, 11, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Bibbo, S.; Dorea, M.P.; Pesa, G.M.; Delitalaa, G.; Delitalab, A.P. Is there a role for gut microbiota in type 1 diabetes pathogenesis? Ann. Med. 2017, 49, 11–22. [Google Scholar] [CrossRef]
- Peng, J.; Narasimhan, S.; Marchesi, J.R.; Benson, A.; Wong, F.S.; Wen, L. Long term effect of gut microbiota transfer on diabetes development. J. Autoimmun. 2014, 53, 85–94. [Google Scholar] [CrossRef]
- Tonucci, L.B.; Dos Santos, K.M.; de Oliveira, L.L.; Ribeiro, S.M.; Martino, H.S. Clinical application of probiotics in type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled study. Clin. Nutr. 2017, 36, 85–92. [Google Scholar] [CrossRef]
- Takiishi, T.; Korf, H.; Van Belle, T.L.; Robert, S.; Grieco, F.A.; Caluwaerts, S.; Galleri, L.; Spagnuolo, I.; Steidler, L.; Van Huynegem, K.; et al. Reversal of autoimmune diabetes by restoration of antigen-specific tolerance using genetically modified Lactococcus lactis in mice. J. Clin. Investig. 2012, 122, 1717–1725. [Google Scholar] [CrossRef]
- Robert, S.; Gysemans, C.; Takiishi, T.; Korf, H.; Spagnuolo, I.; Sebastiani, G.; Van Huynegem, K.; Steidler, L.; Caluwaerts, S.; Demetter, P.; et al. Oral delivery of glutamic acid decarboxylase (GAD)-65 and IL10 by Lactococcus lactis reverses diabetes in recent-onset NOD mice. Diabetes 2014, 63, 2876–2887. [Google Scholar] [CrossRef]
- Sato, J.; Kanazawa, A.; Azuma, K.; Ikeda, F.; Goto, H.; Komiya, K.; Kanno, R.; Tamura, Y.; Asahara, T.; Takahashi, T.; et al. Probiotic reduces bacterial translocation in type 2 diabetes mellitus: A randomised controlled study. Sci. Rep. 2017, 7, 12115. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.I.; Park, H.O.; Kang, J.H. Effect of Lactobacillus gasseri BNR17 on blood glucose levels and body weight in a mouse model of type 2 diabetes. J. Appl. Microbiol. 2009, 107, 1681–1686. [Google Scholar] [CrossRef]
- Moroti, C.; Magri, L.F.S.; de Rezende Costa, M.; Cavallini, D.C.U.; Sivieri, K. Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis. 2012, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Yamano, T.; Tanida, M.; Niijima, A.; Maeda, K.; Okumura, N.; Fukushima, Y.; Nagai, K. Effects of the probiotic strain Lactobacillus johnsonii strain La1 on autonomic nerves and blood glucose in rats. Life Sci. 2006, 79, 1963–1967. [Google Scholar] [CrossRef] [PubMed]
- Calcinaro, F.; Dionisi, S.; Marinaro, M.; Candeloro, P.; Bonato, V.; Marzotti, S.; Corneli, R.B.; Ferretti, E.; Gulino, A.; Grasso, F.; et al. Oral probiotic administration induces interleukin-10 production and prevents spontaneous autoimmune diabetes in the non-obese diabetic mouse. Diabetologia 2005, 48, 1565–1575. [Google Scholar] [CrossRef]
- Dolpady, J.; Sorini, C.; Di Pietro, C.; Cosorich, I.; Ferrarese, R.; Saita, D.; Clementi, M.; Canducci, F.; Falcone, M. Oral probiotic VSL#3 prevents autoimmune diabetes by modulating microbiota and promoting indoleamine 2,3-dioxygenase-enriched tolerogenic intestinal environment. J. Diabetes Res. 2016, 2016, 7569431. [Google Scholar] [CrossRef]
- Harisa, G.I.; Taha, E.I.; Khalil, A.F.; Salem, M.M. Oral administration of Lactobacillus acidophilus restores nitric oxide level in diabetic rats. Aust. J. Basic. Appl. Sci. 2009, 3, 2963–2969. [Google Scholar]
- Honda, K.; Moto, M.; Uchida, N.; He, F.; Hashizume, N. Antidiabetic effects of lactic acid bacteria in normal and type 2 diabetic mice. J. Clin. Biochem. Nutr. 2012, 51, 96–101. [Google Scholar] [CrossRef]
- Kim, S.W.; Park, K.Y.; Kim, B.; Kim, E.; Hyun, C.K. Lactobacillus rhamnosus GG improves insulin sensitivity and reduces adiposity in high-fat diet-fed mice through enhancement of adiponectin production. Biochem. Biophys. Res. Commun. 2013, 431, 258–263. [Google Scholar] [CrossRef]
- Al-Salami, H.; Butt, G.; Fawcett, J.P.; Tucker, I.G.; Golocorbin-Kon, S.; Mikov, M. Probiotic treatment reduces blood glucose levels and increases systemic absorption of gliclazide in diabetic rats. Eur. J. Drug Metab. Pharmacokinet. 2008, 33, 101–106. [Google Scholar] [CrossRef]
- Pickup, J.C. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 2004, 27, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Neyrinck, A.M.; Fava, F.; Knauf, C.; Burcelin, R.G.; Tuohy, K.M.; Gibson, G.R.; Delzenne, N.M. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 2007, 50, 2374–2383. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.H.; Lu, Y.C.; Ou, C.C.; Lin, S.L.; Tsai, C.C.; Huang, C.T.; Lin, M.Y. Lactobacillus plantarum MYL26 induces endotoxin tolerance phenotype in Caco-2 cells. BMC Microbiol. 2013, 13, 190. [Google Scholar] [CrossRef]
- Datta, S.; Sarvetnick, N.E. IL-21 limits peripheral lymphocyte numbers through T cell homeostatic mechanisms. PLoS ONE 2008, 3, e3118. [Google Scholar] [CrossRef] [PubMed]
- Spolski, R.; Kashyap, M.; Robinson, C.; Yu, Z.; Leonard, W.J. IL-21 signaling is critical for the development of type I diabetes in the NOD mouse. Proc. Natl. Acad. Sci. USA 2008, 105, 14028–14033. [Google Scholar] [CrossRef]
- Abadpour, S.; Halvorsen, B.; Sahraoui, A.; Korsgren, O.; Aukrust, P.; Scholz, H. Interleukin-22 reverses human islet dysfunction and apoptosis triggered by hyperglycemia and LIGHT. J. Mol. Endocrinol. 2018, 60, 171–183. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, X.M.; Zhang, Q.X.; Shen, Z.; Tian, F.W.; Zhang, H.; Sun, Z.H.; Zhang, H.P.; Chen, W. Influence of consumption of probiotics on the plasma lipid profile: A meta-analysis of randomised controlled trials. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 844–850. [Google Scholar] [CrossRef]
- Mazloom, Z.; Yousefinejad, A.; Dabbaghmanesh, M.H. Effect of probiotics on lipid profile, glycemic control, insulin action, oxidative stress, and inflammatory markers in patients with type 2 diabetes: A clinical trial. Iran. J. Med. Sci. 2013, 38, 38–43. [Google Scholar] [PubMed] [PubMed Central]
- Sudha, M.R.; Chauhan, P.; Dixit, K.; Babu, S.; Jamil, K. Probiotics as complementary therapy for hypercholesterolemia. Biol. Med. 2009, 1, 1–13. [Google Scholar]

| Variable | Probiotics | Placebo | p-Value b | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline (n = 35) | At 6 Months (n = 33) | Percent Change | p-Value a | Baseline (n = 35) | At 6 Months (n = 34) | Percent Change | p-Value a | ||
| Age (years) | 8.3 ± 2.6 | - | - | - | 8.7 ± 2.1 | - | - | - | 0.449 * |
| Males, n (%) | 21 (60.0) | - | - | - | 17 (48.6) | - | - | - | 0.337 † |
| Disease duration (years) | 6.8 ± 3.3 | - | - | - | 6.8 ± 3.1 | - | - | - | 0.941 * |
| BMI SDS | 0.32 (−1.96–1.01) | 0.53 (−1.76–1.21) | 22.43 ± 12.16 | 0.152 | 0.29 (−1.13–0.93) | 0.7 (−0.04–1.16) | 25.31 ± 11.57 | 0.317 | 0.066 |
| Systolic BP percentile | 75.4 ± 11.3 | 67.2 ± 9.7 | −11.2 ± 5.1 | 0.002 | 77.1 ± 10.2 | 81.4 ± 12.1 | 5.7 ± 1.3 | 0.124 | <0.001 |
| Diastolic BP percentile | 80.2 ± 12.6 | 72.6 ± 10.7 | −9.5 ± 3.2 | 0.008 | 81.1 ± 12.1 | 84.8 ± 13.2 | 4.2 ± 1.1 | 0.235 | 0.001 |
| Insulin dose (IU/Kg/day) | 1.18 ± 0.21 | 1.12 ± 0.11 | −2.9 ± 2.1 | 0.194 | 1.21 ± 0.25 | 1.27 ± 0.3 | 0.9 ± 0.3 | 0.481 | 0.258 |
| FBG (mg/dL) | 170.5 ± 31.9 | 111.8 ± 17.9 | −33.1 ± 12.6 | <0.001 | 177.1 ± 31.2 | 182.4 ± 33.3 | 4.8 ± 2.8 | 0.393 | <0.001 |
| RBG (mg/dL) | 313.3 ± 72.9 | 226.2 ± 41.1 | −25.5 ± 15.6 | <0.001 | 308.6 ± 34.7 | 313.1 ± 41.3 | 1.66 ± 1.3 | 0.384 | 0.001 |
| HbA1c (%) HbA1c (mmol/mol) | 9.12 ± 1.6 75.3 ± 9.7 | 7.73 ± 1.44 60.8 ± 8.1 | −13.5 ± 8.7 −18.5 ± 9.8 | <0.001 <0.001 | 9.09 ± 1.6 77.6 ± 8.9 | 9.26 ± 1.66 79.3 ± 9.9 | 3.4 ± 1.8 2.9 ± 1.0 | 0.569 0.316 | <0.001 <0.001 |
| Triglycerides (mg/dL) | 0.50 ± 0.07 | 0.48 ± 0.07 | −2.5 ± 2.0 | 0.205 | 0.49 ± 0.07 | 0.46 ± 0.09 | −3.3 ± 2.6 | 0.200 | 0.340 |
| Total cholesterol (mg/dL) | 72.0 ± 17.6 | 61.9 ± 16.0 | −3.9 ± 3.45 | <0.001 | 73.4 ± 11.4 | 74.9 ± 10.5 | 1.8 ± 0.33 | 0.338 | 0.067 |
| HDL cholesterol (mg/dL) | 119.5 ± 27.1 | 95.9 ± 13.0 | −15.8 ± 22.5 | <0.001 | 111.8 ± 15.5 | 116.0 ± 13.4 | 5.6 ± 2.9 | 0.081 | 0.001 |
| LDL cholesterol (mg/dL) | 52.7 ± 13.2 | 59.23 ± 11.39 | 23.9 ± 11.0 | 0.056 | 55.6 ± 11.4 | 54.1 ± 14.2 | −0.01 ± 13.8 | 0.825 | 0.186 |
| IL 21 (ng/L) | 180 (150–250) | 50 (50–100) | −61.4 ± 25.8 | <0.001 | 170 (120–220) | 190 (170–250) | 50.2 ± 21.7 | 0.075 | <0.001 |
| IL 22 (ng/L) | 108 (70–150) | 250 (200–375) | 236.0 ± 62.4 | <0.001 | 120 (100–170) | 102 (57–152) | −7.03 ± 4.5 | 0.219 | <0.001 |
| Dependent Variable | Independent Variables | Unstandardized Coefficients | Standardized Coefficients | p-Value | |
|---|---|---|---|---|---|
| B | Standard Error | Beta | |||
| IL-21 | FBG (mg/dL) | 1.097 | 0.300 | 0.384 | 0.002 |
| HbA1c (%) | 24.386 | 4.195 | 0.303 | 0.011 | |
| Total cholesterol (mg/dL) | 1.253 | 0.987 | 0.249 | 0.009 | |
| IL-22 | Diastolic BP percentile | −29.409 | 20.418 | −0.158 | 0.155 |
| FBG (mg/dL) | −0.573 | 0.227 | −0.28 | 0.014 | |
| HbA1c (%) | −6.791 | 0.619 | −0.265 | 0.026 | |
| Total cholesterol (mg/dL) | −0.691 | 0.321 | −0.239 | 0.035 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adly, A.A.M.; Ismail, E.A.R.; Abd-Elgawad, M.M.; Salah, N.Y. Probiotic Supplementation Improves Glucose Homeostasis and Modulates Interleukin (IL)-21 and IL-22 Levels in Pediatric Patients with Type 1 Diabetes: A Randomized Placebo-Controlled Trial. Metabolites 2025, 15, 288. https://doi.org/10.3390/metabo15050288
Adly AAM, Ismail EAR, Abd-Elgawad MM, Salah NY. Probiotic Supplementation Improves Glucose Homeostasis and Modulates Interleukin (IL)-21 and IL-22 Levels in Pediatric Patients with Type 1 Diabetes: A Randomized Placebo-Controlled Trial. Metabolites. 2025; 15(5):288. https://doi.org/10.3390/metabo15050288
Chicago/Turabian StyleAdly, Amira Abdel Moneam, Eman Abdel Rahman Ismail, Mahasen Mohamed Abd-Elgawad, and Nouran Yousef Salah. 2025. "Probiotic Supplementation Improves Glucose Homeostasis and Modulates Interleukin (IL)-21 and IL-22 Levels in Pediatric Patients with Type 1 Diabetes: A Randomized Placebo-Controlled Trial" Metabolites 15, no. 5: 288. https://doi.org/10.3390/metabo15050288
APA StyleAdly, A. A. M., Ismail, E. A. R., Abd-Elgawad, M. M., & Salah, N. Y. (2025). Probiotic Supplementation Improves Glucose Homeostasis and Modulates Interleukin (IL)-21 and IL-22 Levels in Pediatric Patients with Type 1 Diabetes: A Randomized Placebo-Controlled Trial. Metabolites, 15(5), 288. https://doi.org/10.3390/metabo15050288








