Acupuncture on ST36, CV4 and KI1 Suppresses the Progression of Methionine- and Choline-Deficient Diet-Induced Nonalcoholic Fatty Liver Disease in Mice
Abstract
:1. Introduction
2. Results
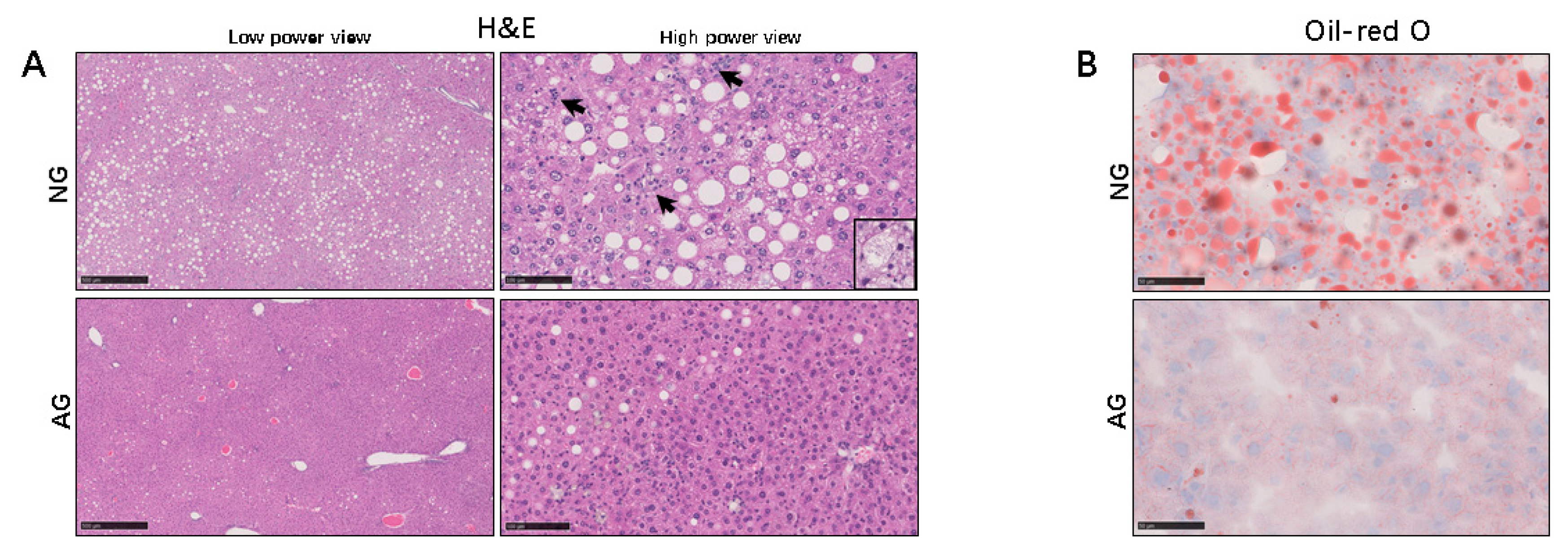
2.1. Lipid Accumulation was Significantly Reduced in the Livers of AG Mice with NAFLD Induced by an MCD Diet
2.2. Acupuncture Treatment Inhibits the Inflammation Reaction during the Progression of MCD Diet–Induced NAFLD
2.3. Acupuncture Treatment Changed the Lipid Profiles and Regulated Lipid Metabolism in the Liver with NAFLD Induced by an MCD Diet
2.4. Acupuncture Treatment Improved Oxidative Stress Induced by Lipid Accumulation of the NAFLD Liver in Mice
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Protocol
4.2. Acupuncture Manipulation
4.3. Ethics
4.4. Histopathology
4.5. IHC
4.6. Western Blotting
4.7. Analyses of Lipid Contents from the Liver
4.8. Real Time Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.9. The Measurement of the TBARS Levels
4.10. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marjot, T.; Moolla, A.; Cobbold, J.F.; Hodson, L.; Tomlinson, J.W. Non-alcoholic fatty liver disease in adults: Current concepts in etiology, outcomes and management. Endocr. Rev. 2019, bnz009. [Google Scholar] [CrossRef]
- Bugianesi, E.; Gentilcore, E.; Manini, R.; Natale, S.; Vanni, E.; Villanova, N.; David, E.; Rizzetto, M.; Marchesini, G. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am. J. Gastroenterol. 2005, 100, 1082–1090. [Google Scholar] [CrossRef]
- Neuschwander-Tetri, B.A.; Brunt, E.M.; Wehmeier, K.R.; Oliver, D.; Bacon, B.R. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology 2003, 38, 1008–1017. [Google Scholar] [CrossRef]
- Abd El-Kader, S.M.; El-Den Ashmawy, E.M. Non-alcoholic fatty liver disease: The diagnosis and management. World J. Hepatol. 2015, 7, 846–858. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, Y.; Wang, X.L.; Shang, X.; Qi, Z.G.; Xue, J.; Zhao, X.; Deng, M.; Xie, M.L. PPARalpha/gamma agonists and antagonists differently affect hepatic lipid metabolism, oxidative stress and inflammatory cytokine production in steatohepatitic rats. Cytokine 2015, 75, 127–135. [Google Scholar] [CrossRef]
- Pham, D.D.; Bruelle, C.; Thi Do, H.; Pajanoja, C.; Jin, C.; Srinivasan, V.; Olkkonen, V.M.; Eriksson, O.; Jauhiainen, M.; Lalowski, M.; et al. Caspase-2 and p75 neurotrophin receptor (p75NTR) are involved in the regulation of SREBP and lipid genes in hepatocyte cells. Cell Death Dis. 2019, 10, 537. [Google Scholar] [CrossRef]
- Liu, Q.; Pan, R.; Ding, L.; Zhang, F.; Hu, L.; Ding, B.; Zhu, L.; Xia, Y.; Dou, X. Rutin exhibits hepatoprotective effects in a mouse model of non-alcoholic fatty liver disease by reducing hepatic lipid levels and mitigating lipid-induced oxidative injuries. Int. Immunopharmacol. 2017, 49, 132–141. [Google Scholar] [CrossRef]
- Zhang, B.; Li, M.; Zou, Y.; Guo, H.; Zhang, B.; Xia, C.; Zhang, H.; Yang, W.; Xu, C. NFkappaB/Orai1 Facilitates Endoplasmic Reticulum Stress by Oxidative Stress in the Pathogenesis of Non-alcoholic Fatty Liver Disease. Front. Cell Dev. Biol. 2019, 7, 202. [Google Scholar] [CrossRef]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Dong, W.; Yang, W.; Li, F.; Guo, W.; Qian, C.; Wang, F.; Li, C.; Lin, L.; Lin, R. Electroacupuncture Improves Synaptic Function in SAMP8 Mice Probably via Inhibition of the AMPK/eEF2K/eEF2 Signaling Pathway. Evid. Based Complement. Altern. Med. 2019, 8260815. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.Y.; Li, X.H.; Wu, M.X. Effect of electroacupuncture at Wnt/beta-catenin signaling pathway on inhibiting cartilage degeneration in rats with knee osteoarthritis. Zhongguo Zhen Jiu 2019, 39, 1081–1086. [Google Scholar] [PubMed]
- Guo, Z.Q.; Huang, Y.; Jiang, H.; Wang, W.B. Randomized clinical trials of early acupuncture treatment of limb paralysis in traumatic brain injury patients and its mechanism. Zhen Ci Yan Jiu 2019, 44, 589–593. [Google Scholar] [PubMed]
- Gao, Y.; Chen, R.; Liang, F. Mechanisms of acupuncture for non-alcoholic fatty liver disease: Researches progress and prospects. Zhongguo Zhen Jiu 2018, 38, 109–113. [Google Scholar] [PubMed]
- Liang, F.; Koya, D. Acupuncture: Is it effective for treatment of insulin resistance? Diabetes Obes. Metab. 2010, 12, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Liang, C.M.; Cui, J.W.; Pan, L.; Hu, H.; Fang, H.J. Acupuncture improves hepatic lipid metabolism by suppressing oxidative stress in obese nonalcoholic fatty liver disease rats. Zhen Ci Yan Jiu 2019, 44, 189–194. [Google Scholar] [PubMed]
- Dela Peña, A.; Leclercq, I.; Field, J.; George, J.; Jones, B.; Farrell, G. NF-kappaB activation, rather than TNF, mediates hepatic inflammation in a murine dietary model of steatohepatitis. Gastroenterology 2005, 129, 1663–1674. [Google Scholar] [CrossRef]
- Nawata, A.; Noguchi, H.; Mazaki, Y.; Kurahashi, T.; Izumi, H.; Wang, K.Y.; Guo, X.; Uramoto, H.; Kohno, K.; Taniguchi, H.; et al. Overexpression of Peroxiredoxin 4 Affects Intestinal Function in a Dietary Mouse Model of Nonalcoholic Fatty Liver Disease. PLoS ONE 2016, 11, e0152549. [Google Scholar] [CrossRef]
- Li, W.; Tai, Y.; Zhou, J.; Gu, W.; Bai, Z.; Zhou, T.; Zhong, Z.; McCue, P.A.; Sang, N.; Ji, J.Y.; et al. Repression of endometrial tumor growth by targeting SREBP1 and lipogenesis. Cell Cycle 2012, 11, 2348–2358. [Google Scholar] [CrossRef] [Green Version]
- Korbecki, J.; Bobinski, R.; Dutka, M. Self-regulation of the inflammatory response by peroxisome proliferator-activated receptors. Inflamm. Res. 2019, 68, 443–458. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.W.; Li, Q.Q.; Li, F.; Fu, Q.N.; Zeng, X.H.; Liu, C.Z. The holistic effects of acupuncture treatment. Evid. Based Complement. Alternat. Med. 2014, 2014, 739708. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.K.; Yang, Z.J.; Chen, F. Effect of electroacupuncture stimulation of “Housanli” (ST 36) and “Zhongwan” (CV 12) on serum leptin and hepatocellular JAK 2-STAT 3 signaling in obese rats. Acupunct. Res. 2015, 40, 1–5. [Google Scholar]
- Kim, S.; Zhang, X.; O’Buckley, S.C.; Cooter, M.; Park, J.J.; Nackley, A.G. Acupuncture Resolves Persistent Pain and Neuroinflammation in a Mouse Model of Chronic Overlapping Pain Conditions. J. Pain 2018, 19, 1384 e1–1384 e14. [Google Scholar] [CrossRef]
- Li, Z.X.; Zhang, H.H.; Lan, D.C.; Chen, X.Z.; Sun, J. Progress of researches on mechanisms of acupuncture therapy for insulin resistance. Zhen Ci Yan Jiu 2019, 44, 231–234. [Google Scholar] [PubMed]
- Fan, X.L.; Yu, M.L.; Fu, S.P.; Zhuang, Y.; Lu, S.F. Effectiveness of Acupuncture in Treatment of Simple Obesity in Animal Models: A Systematic Review and Meta-Analysis. Evid. Based Complement. Altern. Med. 2019, 2019, 5459326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.H.; Huang, W.; Wei, D.; Ding, D.G.; Liu, Y.R.; Wang, J.J.; Zhou, Z.Y. Mechanisms of Acupuncture Therapy for Simple Obesity: An Evidence-Based Review of Clinical and Animal Studies on Simple Obesity. Evid. Based Complement. Altern. Med. 2019, 2019, 5796381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado, M.V.; Cortez-Pinto, H. Non-alcoholic fatty liver disease: What the clinician needs to know. World J. Gastroenterol. 2014, 20, 12956–12980. [Google Scholar] [CrossRef] [PubMed]
- Kubes, P.; Mehal, W.Z. Sterile inflammation in the liver. Gastroenterology 2012, 143, 1158–1172. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, K.; Zhong, G.; Huang, Y.; Li, S.; Qu, S.; Zhang, J. Acupuncture Decreases NF-kappaB p65, miR-155, and miR-21 and Increases miR-146a Expression in Chronic Atrophic Gastritis Rats. Evid. Based Complement. Altern. Med. 2016, 2016, 9404629. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Jin, C.Y.; Kim, C.H.; Yoo, Y.H.; Choi, S.H.; Kim, G.Y.; Yoon, H.M.; Park, H.T.; Choi, Y.H. Isorhamnetin alleviates lipopolysaccharide-induced inflammatory responses in BV2 microglia by inactivating NF-kappaB, blocking the TLR4 pathway and reducing ROS generation. Int. J. Mol. Med. 2019, 43, 682–692. [Google Scholar]
- Anderson, N.; Borlak, J. Molecular mechanisms and therapeutic targets in steatosis and steatohepatitis. Pharmacol. Rev. 2008, 60, 311–357. [Google Scholar] [CrossRef] [Green Version]
- Park, J.W.; Jeong, G.; Kim, S.J.; Kim, M.K.; Park, S.M. Predictors reflecting the pathological severity of non-alcoholic fatty liver disease: comprehensive study of clinical and immunohistochemical findings in younger Asian patients. J. Gastroenterol. Hepatol. 2007, 22, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.D.; Bobinski, F.; Sato, K.L.; Kolker, S.J.; Sluka, K.A.; Santos, A.R. IL-10 cytokine released from M2 macrophages is crucial for analgesic and anti-inflammatory effects of acupuncture in a model of inflammatory muscle pain. Mol. Neurobiol. 2015, 51, 19–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, F.Y.; Huo, Z.J.; Zhang, L.; Guo, J.; Chen, H.; Liu, T.; Peng, B.; Hong, P.X.; Peng, Y.Y.; Fan, Y.F.; et al. The Effects of Needling Fenglong (ST40) and Neiguan (PC6) on IL-17 of ApoE-Gene-Knockout Mice’s Liver. Evid. Based Complement. Altern. Med. 2014, 2014, 691863. [Google Scholar] [CrossRef] [PubMed]
- Browning, J.D.; Horton, J.D. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Investig. 2004, 114, 147–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, S.H. Nonalcoholic fatty liver disease: Molecular mechanisms for the hepatic steatosis. Clin. Mol. Hepatol. 2013, 19, 210–215. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Yang, L.; McCall, S.; Huang, J.; Yu, X.X.; Pandey, S.K.; Bhanot, S.; Monia, B.P.; Li, Y.X.; Diehl, A.M. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology 2007, 45, 1366–1374. [Google Scholar] [CrossRef]
- Caballero, F.; Fernandez, A.; De Lacy, A.M.; Fernandez-Checa, J.C.; Caballeria, J.; Garcia-Ruiz, C. Enhanced free cholesterol, SREBP-2 and StAR expression in human NASH. J. Hepatol. 2009, 50, 789–796. [Google Scholar] [CrossRef]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [Green Version]
- Bell, M.; Wang, H.; Chen, H.; McLenithan, J.C.; Gong, D.W.; Yang, R.Z.; Yu, D.; Fried, S.K.; Quon, M.J.; Londos, C.; et al. Consequences of lipid droplet coat protein downregulation in liver cells: Abnormal lipid droplet metabolism and induction of insulin resistance. Diabetes 2008, 57, 2037–2045. [Google Scholar] [CrossRef] [Green Version]
- Basaranoglu, M.; Kayacetin, S.; Yilmaz, N.; Kayacetin, E.; Tarcin, O.; Sonsuz, A. Understanding mechanisms of the pathogenesis of nonalcoholic fatty liver disease. World J. Gastroenterol. 2010, 16, 2223–2226. [Google Scholar] [CrossRef]
- Tessari, P.; Coracina, A.; Cosma, A.; Tiengo, A. Hepatic lipid metabolism and non-alcoholic fatty liver disease. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; He, Y.; Zhang, S.; Pu, D.; Wu, J. Effect of transcutaneous electrical acupoint stimulation on protecting against radiotherapy- induced ovarian damage in mice. J. Ovarian Res. 2019, 12, 65. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xu, X.; Chen, B.; Rong, J.; Jiang, H. Photoacoustic imaging of acupuncture effect in small animals. Biomed. Opt. Express 2015, 6, 433–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Guo, X.; Hamada, T.; Yokoyama, S.; Nakamura, Y.; Zheng, J.; Kurose, N.; Ishigaki, Y.A.-O.; Uramoto, H.; Tanimoto, A.; et al. Protective Effects of Peroxiredoxin 4 (PRDX4) on Cholestatic Liver Injury. Int. J. Mol. Sci. 2018, 19, 2509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nabeshima, A.; Yamada, S.; Guo, X.; Tanimoto, A.; Wang, K.Y.; Shimajiri, S.; Kimura, S.; Tasaki, T.; Noguchi, H.; Kitada, S.; et al. Peroxiredoxin 4 protects against nonalcoholic steatohepatitis and type 2 diabetes in a nongenetic mouse model. Antioxid Redox Signal 2013, 19, 1983–1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Noguchi, H.; Ishii, N.; Homma, T.; Hamada, T.; Hiraki, T.; Zhang, J.; Matsuo, K.; Yokoyama, S.; Ishibashi, H.; et al. The Association of Peroxiredoxin 4 with the Initiation and Progression of Hepatocellular Carcinoma. Antioxid. Redox Signal. 2019, 30, 1271–1284. [Google Scholar] [CrossRef]





| Steatosis Score | |||
|---|---|---|---|
| Score | NG | AG | P |
| 0 | 0 | 11 | <0.001 |
| 1 | 8 | 6 | |
| 2 | 4 | 0 | |
| 3 | 5 | 0 | |
| Inflammation Score | |||
| Score | NG | AG | P |
| 0 | 0 | 5 | 0.003 |
| 1 | 12 | 12 | |
| 2 | 4 | 0 | |
| 3 | 1 | 0 | |
| Ballooning Score | |||
| Score | NG | AG | P |
| 0 | 5 | 16 | <0.001 |
| 1 | 10 | 1 | |
| 2 | 2 | 0 | |
| 3 | 0 | 0 | |
| NAFLD Score | |||
| Score | NG | AG | P |
| 0–3 | 7 | 17 | <0.001 |
| 4–6 | 8 | 0 | |
| 7–9 | 6 | 0 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, X.; Guo, X.; Zhang, J.; Moriya, J.; Kobayashi, J.; Yamaguchi, R.; Yamada, S. Acupuncture on ST36, CV4 and KI1 Suppresses the Progression of Methionine- and Choline-Deficient Diet-Induced Nonalcoholic Fatty Liver Disease in Mice. Metabolites 2019, 9, 299. https://doi.org/10.3390/metabo9120299
Meng X, Guo X, Zhang J, Moriya J, Kobayashi J, Yamaguchi R, Yamada S. Acupuncture on ST36, CV4 and KI1 Suppresses the Progression of Methionine- and Choline-Deficient Diet-Induced Nonalcoholic Fatty Liver Disease in Mice. Metabolites. 2019; 9(12):299. https://doi.org/10.3390/metabo9120299
Chicago/Turabian StyleMeng, Xiangjin, Xin Guo, Jing Zhang, Junji Moriya, Junji Kobayashi, Reimon Yamaguchi, and Sohsuke Yamada. 2019. "Acupuncture on ST36, CV4 and KI1 Suppresses the Progression of Methionine- and Choline-Deficient Diet-Induced Nonalcoholic Fatty Liver Disease in Mice" Metabolites 9, no. 12: 299. https://doi.org/10.3390/metabo9120299
APA StyleMeng, X., Guo, X., Zhang, J., Moriya, J., Kobayashi, J., Yamaguchi, R., & Yamada, S. (2019). Acupuncture on ST36, CV4 and KI1 Suppresses the Progression of Methionine- and Choline-Deficient Diet-Induced Nonalcoholic Fatty Liver Disease in Mice. Metabolites, 9(12), 299. https://doi.org/10.3390/metabo9120299





