Intracellular Staphylococcus aureus Elicits the Production of Host Very Long-Chain Saturated Fatty Acids with Antimicrobial Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Levels and Labelling Pattern of Saturated Fatty Acids in HeLa Cells in Response to Intracellular MRSA Infection
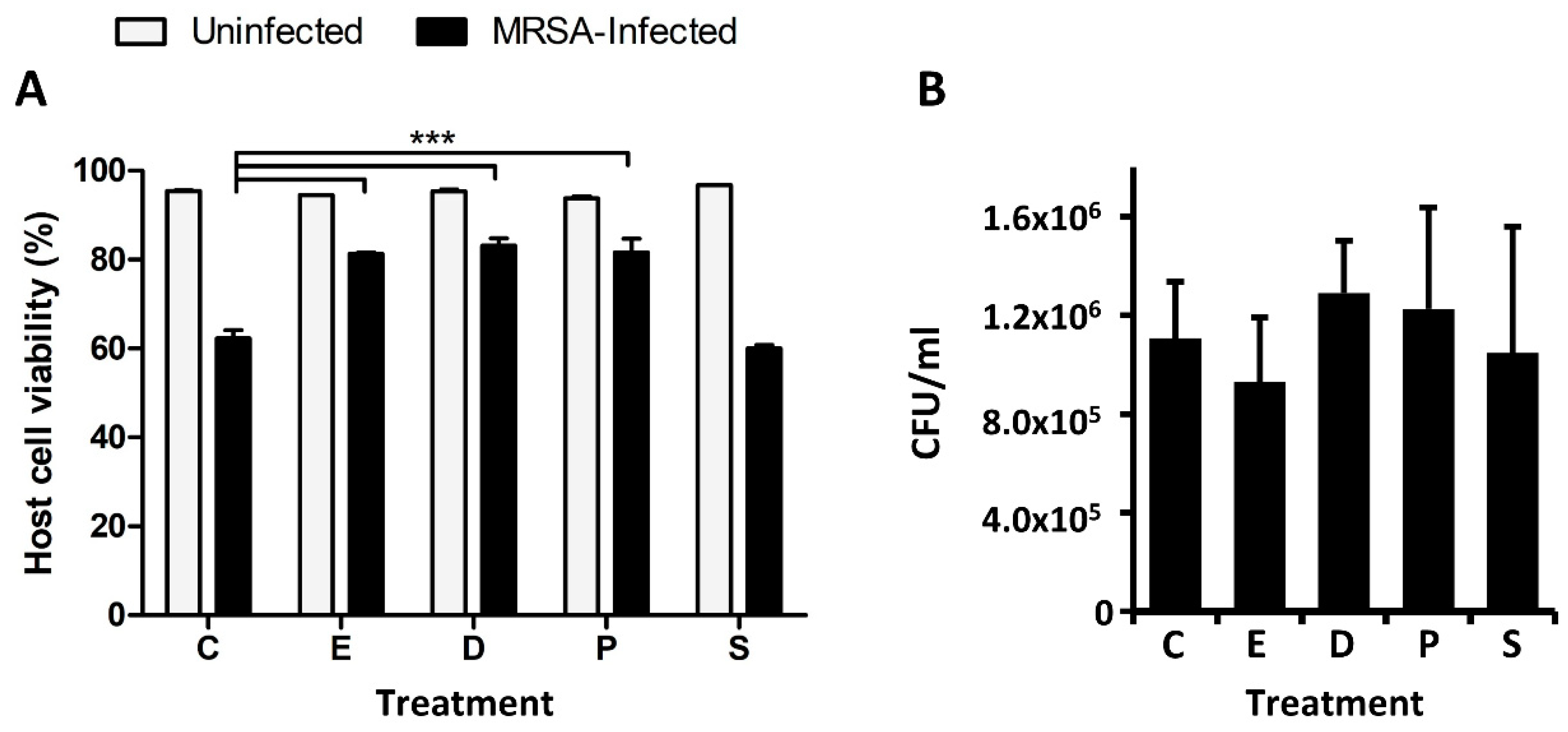
2.2. Docosanoic, Eicosanoic and Palmitic Acids Showed a Cytoprotective Role in MRSA-Infected HeLa Cells
3. Materials and Methods
3.1. Bacterial Strains, Cell Lines and Culture Conditions
3.2. Intracellular Infection Assays
3.3. Host Cell and Bacterial Viability Assays
3.4. Samples Preparation for Intracellular Metabolome Analysis
3.5. Gas Chromatography-Mass Spectrometry (GC-MS)
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef]
- Wertheim, H.F.; Melles, D.C.; Vos, M.C.; van Leeuwen, W.; van Belkum, A.; Verbrugh, H.A.; Nouwen, J.L. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 2005, 5, 751–762. [Google Scholar] [CrossRef]
- Lowy, F.D. Is Staphylococcus aureus an intracellular pathogen? Trends Microbiol. 2000, 8, 341–343. [Google Scholar] [CrossRef]
- Sendi, P.; Proctor, R.A. Staphylococcus aureus as an intracellular pathogen: The role of small colony variants. Trends Microbiol. 2009, 17, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Garzoni, C.; Kelley, W.L. Staphylococcus aureus: New evidence for intracellular persistence. Trends Microbiol. 2009, 17, 59–65. [Google Scholar] [CrossRef]
- Fraunholz, M.; Sinha, B. Intracellular Staphylococcus aureus: Live-in and let die. Front. Cell. Infect. Microbiol. 2012, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lehar, S.M.; Pillow, T.; Xu, M.; Staben, L.; Kajihara, K.K.; Vandlen, R.; DePalatis, L.; Raab, H.; Hazenbos, W.L.; Hiroshi Morisaki, J.; et al. Novel antibody–antibiotic conjugate eliminates intracellular S. aureus. Nature 2015, 527, 323–328. [Google Scholar] [CrossRef]
- Abu Kwaik, Y.; Bumann, D. Host delivery of favorite meals for intracellular pathogens. PLoS Pathog. 2015, 11, e1004866. [Google Scholar] [CrossRef]
- Dandekar, T.; Eisenreich, W. Host-adapted metabolism and its regulation in bacterial pathogens. Front. Cell. Infect. Microbiol. 2015, 5, 27–28. [Google Scholar] [CrossRef]
- Eisenreich, W.; Heesemann, J.; Rudel, T.; Goebel, W. Metabolic host responses to infection by intracellular bacterial pathogens. Front. Cell. Infect. Microbiol. 2013, 3, 1–22. [Google Scholar] [CrossRef]
- Olive, A.J.; Sassetti, C.M. Metabolic crosstalk between host and pathogen: Sensing, adapting and competing. Nat. Rev. Microbiol. 2016, 14, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Santano, N.; Ellis, J.K.; Mateos, L.M.; Calle, Y.; Keun, H.C.; Behrends, V.; Letek, M. Intracellular Staphylococcus aureus modulates host central carbon metabolism to activate autophagy. mSphere 2018, 3, e00374-18. [Google Scholar] [CrossRef] [PubMed]
- Eisenreich, W.; Dandekar, T.; Heesemann, J.; Goebel, W. Carbon metabolism of intracellular bacterial pathogens and possible links to virulence. Nat. Rev. Microbiol. 2010, 8, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Escoll, P.; Song, O.R.O.-R.; Viana, F.; Steiner, B.; Lagache, T.; Olivo-Marin, J.-C.J.C.; Impens, F.; Brodin, P.; Hilbi, H.; Buchrieser, C. Legionella pneumophila modulates mitochondrial dynamics to trigger metabolic repurposing of infected macrophages. Cell Host Microbe 2017, 22, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Kentner, D.; Martano, G.; Callon, M.; Chiquet, P.; Brodmann, M.; Burton, O.; Wahlander, A.; Nanni, P.; Delmotte, N.; Grossmann, J.; et al. Shigella reroutes host cell central metabolism to obtain high-flux nutrient supply for vigorous intracellular growth. Proc. Natl. Acad. Sci. USA 2014, 111, 9929–9934. [Google Scholar] [CrossRef]
- Pandey, A.K.; Sassetti, C.M. Mycobacterial persistence requires the utilization of host cholesterol. Proc. Natl. Acad. Sci. USA 2008, 105, 4376–4380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gierok, P.; Harms, M.; Methling, K.; Hochgräfe, F.; Lalk, M. Staphylococcus aureus infection reduces nutrition uptake and nucleotide biosynthesis in a human airway epithelial cell line. Metabolites 2016, 6, 41. [Google Scholar] [CrossRef]
- Quehenberger, O.; Armando, A.M.; Dennis, E.A. High sensitivity quantitative lipidomics analysis of fatty acids in biological samples by gas chromatography-mass spectrometry. Biochim. Biophys. Acta 2011, 1811, 648–656. [Google Scholar] [CrossRef]
- Porter, E.; Ma, D.C.; Alvarez, S.; Faull, K.F. Antimicrobial lipids: Emerging effector molecules of innate host defense. World J. Immunol. 2015, 5, 51. [Google Scholar] [CrossRef] [Green Version]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef]
- Kabara, J.O.N.J.; Swieczkowski, D.M.; Conley, A.J.; Truant, J.P. Fatty acids and derivatives as antimicrobial agents. Antimicrob. Agents Chemother. 1972, 2, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sen, S.; Sirobhushanam, S.; Johnson, S.R.; Song, Y.; Tefft, R.; Gatto, C.; Wilkinson, B.J. Growth-environment dependent modulation of Staphylococcus aureus Branched-Chain to Straight-Chain fatty acid ratio and incorporation of unsaturated fatty acids. PLoS ONE 2016, 11, e0165300. [Google Scholar] [CrossRef] [PubMed]
- Tredwell, G.D.; Keun, H.C. ConvISA: A simple, convoluted method for isotopomer spectral analysis of fatty acids and cholesterol. Metab. Eng. 2015, 32, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Argus, J.P.; Wilks, M.Q.; Zhou, Q.D.; Hsieh, W.Y.; Khialeeva, E.; Hoi, X.P.; Bui, V.; Xu, S.; Yu, A.K.; Wang, E.S.; et al. Development and application of FASA, a model for quantifying fatty acid metabolism using stable isotope labeling. Cell Rep. 2018, 25, 2919–2934.e8. [Google Scholar] [CrossRef] [PubMed]
- Pietrocola, F.; Galluzzi, L.; Bravo-San Pedro, J.M.; Madeo, F.; Kroemer, G. Acetyl coenzyme A: A central metabolite and second messenger. Cell Metab. 2015, 21, 805–821. [Google Scholar] [CrossRef]
- Bravo-Santano, N.; Calle, Y.; Behrends, V.; Letek, M. Hijacking of host cellular functions in Staphylococcus aureus infections. In Proceedings of the Oral Presentation at the International Symposium on Staphylococci and Staphylococcal Infections, University of Copenhagen, Copenhagen, Denmark, 23–26 August 2018. [Google Scholar]
- Jakobsson, A.; Westerberg, R.; Jacobsson, A. Fatty acid elongases in mammals: Their regulation and roles in metabolism. Prog. Lipid Res. 2006, 45, 237–249. [Google Scholar] [CrossRef]
- Thormar, H. Lipids and Essential Oils; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2010; ISBN 9780470741788. [Google Scholar]
- Yoon, B.K.; Jackman, J.A.; Valle-Gonz, E.R.; Cho, N. Antibacterial free fatty acids and monoglycerides: Biological activities, experimental testing, and therapeutic applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef]
- Watanabe, T.; Yamamoto, Y.; Miura, M.; Konno, H.; Yano, S.; Nonomura, Y. Systematic analysis of selective bactericidal activity of fatty acids against Staphylococcus aureus with minimum inhibitory concentration and minimum bactericidal concentration. J. Oleo Sci. 2019, 68, 291–296. [Google Scholar] [CrossRef]
- Cartron, M.L.; England, S.R.; Chiriac, A.I.; Josten, M.; Turner, R.; Rauter, Y.; Hurd, A.; Sahl, H.G.; Jones, S.; Foster, S.J. Bactericidal activity of the human skin fatty acid cis-6-hexadecanoic acid on Staphylococcus aureus. Antimicrob. Agents Chemother. 2014, 58, 3599–3609. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kawamura, Y.; Yamazaki, Y.; Kijima, T.; Morikawa, T.; Nonomura, Y. Palmitoleic acid calcium salt: A lubricant and bactericidal powder from natural lipids. J. Oleo Sci. 2015, 64, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Morikawa, T.; Kawai, T.; Nonomura, Y. Selective bactericidal activity of divalent metal salts of lauric acid. ACS Omega 2017, 2, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Yamamoto, Y.; Nonomura, Y. Effect of pH on bactericidal activities of calcium laurate. J. Oleo Sci. 2018, 67, 859–862. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kijima, T.; Morikawa, T.; Nonomura, Y. Lubricant and bactericidal properties of calcium salts of fatty acids: Effect of degree of unsaturation. J. Oleo Sci. 2015, 64, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Wang, Y.; Nakatsuji, T.; Liu, Y.; Zouboulis, C.C.; Gallo, R.L.; Zhang, L.; Hsieh, M.; Huang, C. An innate bactericidal oleic acid effective against skin infection of methicillin-resistant Staphylococcus aureus: A therapy concordant with evolutionary medicine. J. Microbiol. Biotechnol. 2011, 21, 391–399. [Google Scholar] [PubMed]
- Pornpattananangkul, D.; Fu, V.; Thamphiwatana, S.; Zhang, L.; Chen, M.; Vecchio, J.; Gao, W.; Huang, C.-M.; Zhang, L. In vivo treatment of Propionibacterium acnes infection with liposomal lauric acids. Adv. Healthc. Mater. 2013, 2, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Obonyo, M.; Zhang, L.; Thamphiwatana, S.; Pornpattananangkul, D.; Fu, V.; Zhang, L. Antibacterial activities of liposomal linolenic acids against antibiotic-resistant Helicobacter pylori. Mol. Pharm. 2012, 9, 2677–2685. [Google Scholar] [CrossRef]
- Thamphiwatana, S.; Gao, W.; Obonyo, M.; Zhang, L. In vivo treatment of Helicobacter pylori infection with liposomal linolenic acid reduces colonization and ameliorates inflammation. Proc. Natl. Acad. Sci. USA 2014, 111, 17600–17605. [Google Scholar] [CrossRef]
- Seabra, C.L.; Nunes, C.; Gomez-Lazaro, M.; Correia, M.; Machado, J.C.; Gonçalves, I.C.; Reis, C.A.; Reis, S.; Martins, M.C.L. Docosahexaenoic acid loaded lipid nanoparticles with bactericidal activity against Helicobacter pylori. Int. J. Pharm. 2017, 73, 128–137. [Google Scholar] [CrossRef]
- Kitahara, T.; Koyama, N.; Matsuda, J.; Aoyama, Y.; Hirakata, Y.; Kamihira, S.; Kohno, S.; Nakashima, M.; Sasaki, H. Antimicrobial activity of saturated fatty acids and fatty amines against Methicillin-Resistant Staphylococcus aureus. Biol. Pharm. Bull. 2004, 27, 1321–1326. [Google Scholar] [CrossRef]
- Knapp, H.R.; Melly, M.A. Bactericidal effects of polyunsaturated fatty acids. J. Infect. Dis. 1986, 154, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Kim, Y.-S.; Shin, D.-H. Antimicrobial synergistic effect of linolenic acid and monoglyceride against Bacillus cereus and Staphylococcus aureus. J. Agric. Food Chem. 2002, 50, 2193–2199. [Google Scholar] [CrossRef] [PubMed]
- Greenway, D.; Dyke, K. Mechanism of the inhibitory action of linoleic acid on the growth of Staphylococcus aureus. J. Gen. Microbiol. 1979, 115, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Yoo, J.-S.; Lee, T.-G.; Cho, H.-Y.; Kim, Y.-H.; Kim, W.-G. Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett. 2005, 579, 5157–5162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, U.N. Arachidonic acid and other unsaturated fatty acids and some of their metabolites function as endogenous antimicrobial molecules: A review. J. Adv. Res. 2018, 11, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Cheung Lam, A.H.; Sandoval, N.; Wadhwa, R.; Gilkes, J.; Do, T.Q.; Ernst, W.; Chiang, S.-M.; Kosina, S.; Xu, H.H.; Fujii, G.; et al. Assessment of free fatty acids and cholesteryl esters delivered in liposomes as novel class of antibiotic. BMC Res. Notes 2016, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.M. Phenotype switching is a natural consequence of Staphylococcus aureus replication. J. Bacteriol. 2012, 194, 5404–5412. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Santano, N.; Stölting, H.; Cooper, F.; Bileckaja, N.; Majstorovic, A.; Ihle, N.; Mateos, L.M.; Calle, Y.; Behrends, V.; Letek, M. Host-directed kinase inhibitors act as novel therapies against intracellular Staphylococcus Aureus. Sci. Rep. 2019, 9, 4876. [Google Scholar] [CrossRef]
- Sellick, C.A.; Hansen, R.; Maqsood, A.R.; Dunn, W.B.; Stephens, G.M.; Goodacre, R.; Dickson, A.J. Effective quenching processes for physiologically valid metabolite profiling of suspension cultured mammalian cells. Anal. Chem. 2009, 81, 174–183. [Google Scholar] [CrossRef]
- Behrends, V.; Giskeødegård, G.F.; Bravo-Santano, N.; Letek, M.; Keun, H.C. Acetaminophen cytotoxicity in HepG2 cells is associated with a decoupling of glycolysis from the TCA cycle, loss of NADPH production, and suppression of anabolism. Arch. Toxicol. 2019, 93, 341–353. [Google Scholar] [CrossRef]
- Kind, T.; Wohlgemuth, G.; Lee, D.Y.; Lu, Y.; Palazoglu, M.; Shahbaz, S.; Fiehn, O. FiehnLib—Mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal. Chem. 2009, 81, 10038–10048. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.E. An integrated method for spectrum extraction and compound identification from gas chromatography/mass spectrometry data. J. Am. Soc. Mass Spectrom. 1999, 10, 770–781. [Google Scholar] [CrossRef] [Green Version]
- Behrends, V.; Tredwell, G.D.; Bundy, J.G. A software complement to AMDIS for processing GC-MS metabolomic data. Anal. Biochem. 2011, 415, 206–208. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bravo-Santano, N.; Ellis, J.K.; Calle, Y.; Keun, H.C.; Behrends, V.; Letek, M. Intracellular Staphylococcus aureus Elicits the Production of Host Very Long-Chain Saturated Fatty Acids with Antimicrobial Activity. Metabolites 2019, 9, 148. https://doi.org/10.3390/metabo9070148
Bravo-Santano N, Ellis JK, Calle Y, Keun HC, Behrends V, Letek M. Intracellular Staphylococcus aureus Elicits the Production of Host Very Long-Chain Saturated Fatty Acids with Antimicrobial Activity. Metabolites. 2019; 9(7):148. https://doi.org/10.3390/metabo9070148
Chicago/Turabian StyleBravo-Santano, Natalia, James K. Ellis, Yolanda Calle, Hector C. Keun, Volker Behrends, and Michal Letek. 2019. "Intracellular Staphylococcus aureus Elicits the Production of Host Very Long-Chain Saturated Fatty Acids with Antimicrobial Activity" Metabolites 9, no. 7: 148. https://doi.org/10.3390/metabo9070148
APA StyleBravo-Santano, N., Ellis, J. K., Calle, Y., Keun, H. C., Behrends, V., & Letek, M. (2019). Intracellular Staphylococcus aureus Elicits the Production of Host Very Long-Chain Saturated Fatty Acids with Antimicrobial Activity. Metabolites, 9(7), 148. https://doi.org/10.3390/metabo9070148






