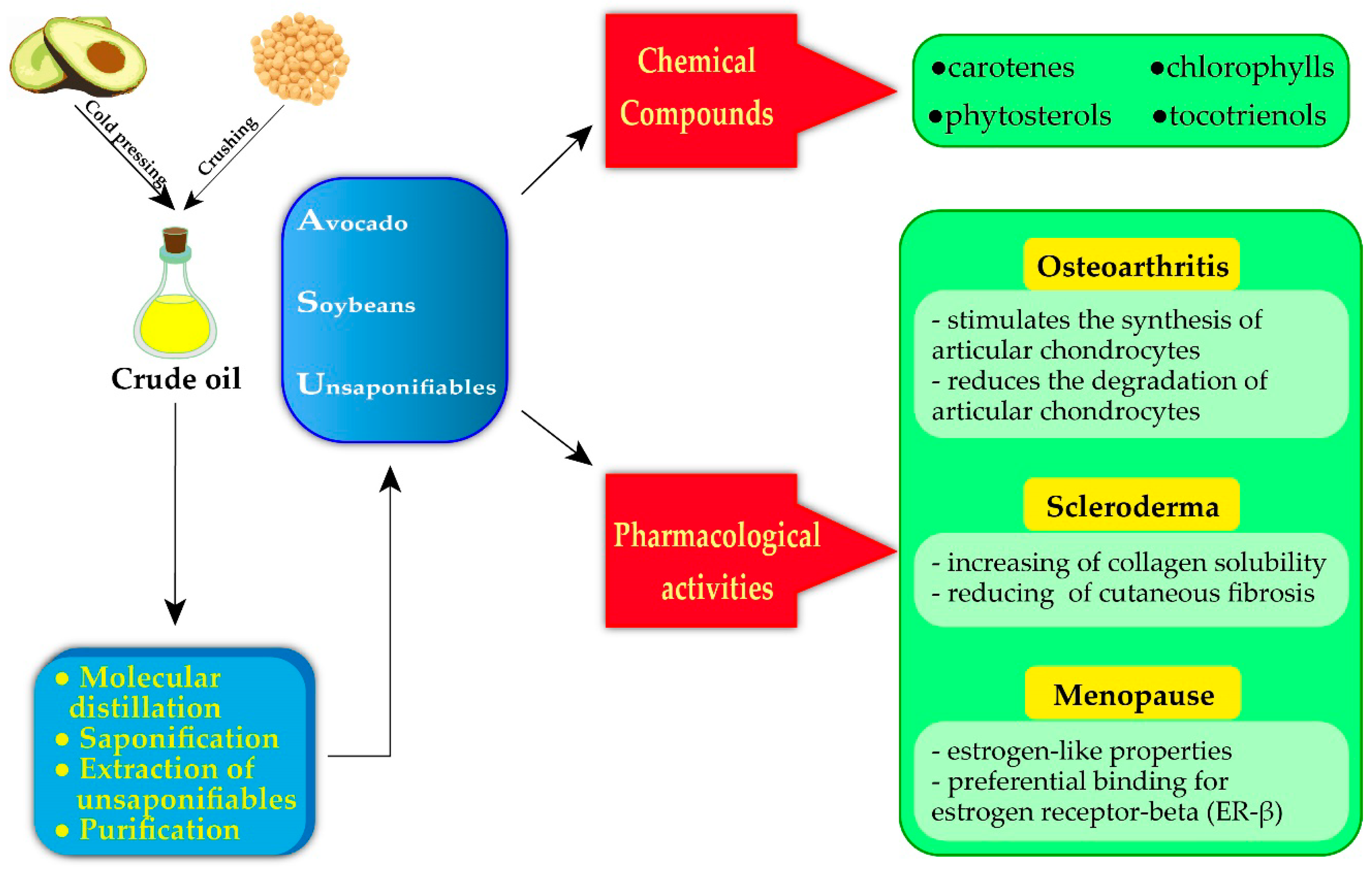
Avocado–Soybean Unsaponifiables: A Panoply of Potentialities to Be Exploited
Abstract
1. Introduction
2. Avocado–Soybean Unsaponifiables: Extraction, Analysis and Chemical Compounds
2.1. Extraction and Analysis of ASU
2.1.1. Extraction Methods of ASU
2.1.2. Analysis Methods of ASU
2.2. Chemical Composition of Avocado–Soybean Unsaponifiables
2.2.1. Tocopherols and Tocotrienols
2.2.2. Phytosterols
2.2.3. Carotenes, Chlorophylls, and Other Unsaponifiable Compounds
2.2.4. Main Components of ASU
3. Avocado–Soybean Unsaponifiables for Medical Purposes
3.1. Osteoarticular Disorders
3.1.1. A Brief Overwiev Of Ostheoarticular Disorders
3.1.2. In Vitro and In Vivo Studies of ASU in Osteoarticular Disorders
3.1.3. Clinical Efficacy of ASU in Osteoarthritis
3.2. Autoimmune Disorders
3.3. Menopause
3.4. Other Pharmacotherapeutic Uses of ASU
4. Avocado–Soybean Unsaponifiables: Safety, Toxicological and Regulatory Aspects
5. Concluding Remarks and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maheu, E.; Cadet, C.; Marty, M.; Moyse, D.; Kerloch, I.; Coste, P.; Dougados, M.; Mazieres, B.; Spector, T.D.; Halhol, H.; et al. Randomised, controlled trial of avocado-soybean unsaponifiable (Piascledine) effect on structure modification in hip osteoarthritis: The ERADIAS study. Ann. Rheum. Dis. 2014, 73, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Pavelka, K.; Coste, P.; Geher, P.; Krejci, G. Efficacy and safety of piascledine 300 versus chondroitin sulfate in a 6 months treatment plus 2 months observation in patients with osteoarthritis of the knee. Clin. Rheumatol. 2010, 29, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Cameron, M.; Chrubasik, S. Oral herbal therapies for treating osteoarthritis. Cochrane Database Syst. Rev. 2014, 22. [Google Scholar] [CrossRef] [PubMed]
- Andriamanalijaona, R.; Benateau, H.; Barre, P.E.; Boumediene, K.; Labbe, D.; Compere, J.F.; Pujol, J.P. Effect of interleukin-1b on transforming growth factor-beta and bone morphogenetic protein-2 expression in human periodontal ligament and alveolar bone cells in culture: Modulation by avocado and soybean unsaponifiables. J. Periodontol. 2006, 77, 1156–1166. [Google Scholar] [CrossRef]
- Au, R.Y.; Al-Talib, T.K.; Au, A.Y.; Phan, P.V.; Frondoza, C.G. Avocado soybean unsaponifiables (ASU) suppress TNF-α, IL-1β, COX-2, iNOS gene expression, and prostaglandin E2 and nitric oxide production in articular chondrocytes and monocyte/macrophages. Osteoarthr. Cartil. 2007, 15, 1249–1255. [Google Scholar] [CrossRef]
- Eser, O.; Songur, A.; Yaman, M.; Cosar, M.; Fidan, H.S.; Sahin, O.; Mollaoglu, H.; Buyukbas, S. The protective effect of avocado soybean unsaponifilables on brain ischemia/reperfusion injury in rat prefrontal cortex. Br. J. Neurosurg. 2011, 25, 701–706. [Google Scholar] [CrossRef]
- Ghasemian, M.; Owlia, S.; Owlia, M.B. Review of anti-inflammatory herbal medicines. Adv. Pharmacol. Sci. 2016, 2016. [Google Scholar] [CrossRef]
- Christiansen, B.A.; Bhatti, S.; Goudarzi, R.; Emami, S. Management of osteoarthritis with avocado/soybean unsaponifiables. Cartilage 2015, 6, 30–44. [Google Scholar] [CrossRef]
- Ernst, E. Avocado–soybean unsaponifiables (ASU) for osteoarthritis–a systematic review. Clin. Rheumatol. 2003, 22, 285–288. [Google Scholar] [CrossRef]
- Hashemibeni, B.; Valiani, A.; Esmaeli, M.; Kazemi, M.; Aliakbari, M.; Iranpour, F.G. Comparison of the efficacy of piascledine and transforming growth factor β1 on chondrogenic differentiation of human adipose-derived stem cells in fibrin and fibrin-alginate scaffolds. Iran. J. Basic Med Sci. 2018, 21, 212. [Google Scholar]
- Cho, S.-J.; Oh, S.-H.; Pridmore, R.D.; Juillerat, M.A.; Lee, C.-H. Purification and characterization of proteases from Bacillus amyloliquefaciens isolated from traditional soybean fermentation starter. J. Agric. Food Chem. 2003, 51, 7664–7670. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.F.; Goudarzi, R.; Yazdi, P.G.; Pedersen, B.A. In vitro effects of arthrocen, an avocado/soy unsaponifiables agent, on inflammation and global gene expression in human monocytes. Int. J. Chem. 2017, 9, 31. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Henrotin, Y. Avocado/Soybean Unsaponifiables (Piacledine® 300) show beneficial effect on the metabolism of osteoarthritic cartilage, synovium and subchondral bone: An overview of the mechanisms. Aims Med Sci. 2018, 5, 33–52. [Google Scholar] [CrossRef]
- Prieto Vidal, N.; Adeseun Adigun, O.; Pham, T.; Mumtaz, A.; Manful, C.; Callahan, G.; Stewart, P.; Keough, D.; Thomas, R. The Effects of Cold Saponification on the Unsaponified Fatty Acid Composition and Sensory Perception of Commercial Natural Herbal Soaps. Molecules 2018, 23, 2356. [Google Scholar] [CrossRef]
- Uquiche, E.; Romero, V.; Ortiz, J.; Del Valle, J. Extraction of oil and minor lipids from cold-press rapeseed cake with supercritical CO2. Braz. J. Chem. Eng. 2012, 29, 585–598. [Google Scholar] [CrossRef]
- EG, B. A rapid method of total lipid extraction and purification. Canj. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Hara, A.; Radin, N.S. Lipid extraction of tissues with a low-toxicity solvent. Anal. Biochem. 1978, 90, 420–426. [Google Scholar] [CrossRef]
- Dewick, P.M.; Fattorusso, E. Chimica, biosintesi e bioattività delle sostanze naturali; Piccin: Padova, Italy, 2012. [Google Scholar]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef]
- Sozen, E.; Demirel, T.; Ozer, N.K. Vitamin E: Regulatory role in the cardiovascular system. Iubmb Life 2019, 71, 507–515. [Google Scholar] [CrossRef]
- Lee, G.; Han, S. The role of vitamin E in immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Ima-Nirwana, S. The role of vitamin E in preventing and treating osteoarthritis-a review of the current evidence. Front. Pharmacol. 2018, 9, 946. [Google Scholar] [CrossRef] [PubMed]
- Peh, H.Y.; Tan, W.D.; Liao, W.; Wong, W.F. Vitamin E therapy beyond cancer: Tocopherol versus tocotrienol. Pharmacol. Ther. 2016, 162, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Khanna, S.; Roy, S.; Packer, L. Molecular Basis of Vitamin E Action Tocotrienol potently inhibits glutamate-induced pp60c-Src Kinase activation and death of ht4 neuronal cells. J. Biol. Chem. 2000, 275, 13049–13055. [Google Scholar] [CrossRef] [PubMed]
- Pearce, B.C.; Parker, R.A.; Deason, M.E.; Qureshi, A.A.; Wright, J.K. Hypocholesterolemic activity of synthetic and natural tocotrienols. J. Med. Chem. 1992, 35, 3595–3606. [Google Scholar] [CrossRef]
- Serbinova, E.; Kagan, V.; Han, D.; Packer, L. Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Free Radic. Biol. Med. 1991, 10, 263–275. [Google Scholar] [CrossRef]
- Bagchi, D.; Preuss, H.G. Phytopharmaceuticals in Cancer Chemoprevention; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Jones, P.J.; MacDougall, D.E.; Ntanios, F.; Vanstone, C.A. Dietary phytosterols as cholesterol-lowering agents in humans. Can. J. Physiol. Pharmacol. 1997, 75, 217–227. [Google Scholar] [CrossRef]
- Moreau, R.A.; Whitaker, B.D.; Hicks, K.B. Phytosterols, phytostanols, and their conjugates in foods: Structural diversity, quantitative analysis, and health-promoting uses. Prog Lipid Res. 2002, 41, 457–500. [Google Scholar] [CrossRef]
- Xu, F.; Rychnovsky, S.D.; Belani, J.D.; Hobbs, H.H.; Cohen, J.C.; Rawson, R.B. Dual roles for cholesterol in mammalian cells. Proc. Natl. Acad. Sci. USA 2005, 102, 14551–14556. [Google Scholar] [CrossRef]
- Kritchevsky, D.; Chen, S.C. Phytosterols—health benefits and potential concerns: A review. Nutr. Res. 2005, 25, 413–428. [Google Scholar] [CrossRef]
- Plat, J.; Baumgartner, S.; Vanmierlo, T.; Lütjohann, D.; Calkins, K.; Burrin, D.; Guthrie, G.; Thijs, C.; Te Velde, A.; Vreugdenhil, A. Plant-based sterols and stanols in health & disease:“Consequences of human development in a plant-based environment?”. Prog. Lipid Res. 2019. [Google Scholar] [CrossRef]
- Pohndorf, R.; Cadaval Jr, T.; Pinto, L. Kinetics and thermodynamics adsorption of carotenoids and chlorophylls in rice bran oil bleaching. J. Food Eng. 2016, 185, 9–16. [Google Scholar] [CrossRef]
- Johnson, E.J. The role of carotenoids in human health. Nutr. Clin. Care 2002, 5, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.-E.; Prasad, K.N.; Kong, K.-W.; Jiang, Y.; Ismail, A. Carotenoids and their isomers: Color pigments in fruits and vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. β-Carotene and other carotenoids in protection from sunlight. Am. J. Clin. Nutr. 2012, 96, 1179S–1184S. [Google Scholar] [CrossRef]
- Gandul-Rojas, B.; Roca, M.; Gallardo-Guerrero, L. Chlorophylls and carotenoids in food products from olive tree. In Products Olive Tree, 1st ed.; Intech: London, UK, 2016; pp. 67–98. [Google Scholar] [CrossRef]
- Canniffe, D.P.; Thweatt, J.L.; Chew, A.G.M.; Hunter, C.N.; Bryant, D.A. A paralog of a bacteriochlorophyll biosynthesis enzyme catalyzes the formation of 1, 2-dihydrocarotenoids in green sulfur bacteria. J. Biol. Chem. 2018, 293, 15233–15242. [Google Scholar] [CrossRef]
- Hoober, J.K.; Eggink, L.L.; Chen, M. Chlorophylls, ligands and assembly of light-harvesting complexes in chloroplasts. Photosynth. Res. 2007, 94, 387–400. [Google Scholar] [CrossRef]
- Bowers, W.F. Chlorophyll in wound healing and suppurative disease. Am. J. Surg. 1947, 73, 37–50. [Google Scholar] [CrossRef]
- Edwards, B. Treatment of chronic leg ulcers with ointment containing soluble chlorophyll. Physiotherapy 1954, 40, 177–179. [Google Scholar] [PubMed]
- Mishra, V.K.; Bacheti, R.; Husen, A. Medicinal uses of chlorophyll: A critical overview. Chlorophyll Struct. Funct. Med. Uses. Hauppauge Nova Sci. Publ. 2012, 177–196. [Google Scholar]
- Egner, P.A.; Stansbury, K.H.; Snyder, E.P.; Rogers, M.E.; Hintz, P.A.; Kensler, T.W. Identification and characterization of chlorin e4 ethyl ester in sera of individuals participating in the chlorophyllin chemoprevention trial. Chem. Res. Toxicol. 2000, 13, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Ferruzzi, M.G.; Blakeslee, J. Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutr. Res. 2007, 27, 1–12. [Google Scholar] [CrossRef]
- Werman, M.; Neeman, I.; Mokady, S. Avocado oils and hepatic lipid metabolism in growing rats. Food Chem. Toxicol. 1991, 29, 93–99. [Google Scholar] [CrossRef]
- Gutfinger, T.; Letan, A. Studies of unsaponifiables in several vegetable oils. Lipids 1974, 9, 658–663. [Google Scholar] [CrossRef]
- Normén, L.; Dutta, P.; Lia, Å.; Andersson, H. Soy sterol esters and β-sitostanol ester as inhibitors of cholesterol absorption in human small bowel. Am. J. Clin. Nutr. 2000, 71, 908–913. [Google Scholar] [CrossRef]
- Veronezi, C.M.; Jorge, N. Effect of Carica papaya and Cucumis melo seed oils on the soybean oil stability. Food Sci. Biotechnol. 2018, 27, 1031–1040. [Google Scholar] [CrossRef]
- Manaf, Y.N.; Rahardjo, A.P.; Yusof, Y.A.; Desa, M.N.; Nusantoro, B.P. Lipid characteristics and tocopherol content of the oils of native avocado cultivars grown in Indonesia. Int. J. Food Prop. 2018, 21, 2758–2771. [Google Scholar] [CrossRef]
- Pokkanta, P.; Sookwong, P.; Tanang, M.; Setchaiyan, S.; Boontakham, P.; Mahatheeranont, S. Simultaneous determination of tocols, γ-oryzanols, phytosterols, squalene, cholecalciferol and phylloquinone in rice bran and vegetable oil samples. Food Chem. 2019, 271, 630–638. [Google Scholar] [CrossRef]
- Li, X.-K.; Ji, W.-J.; Zhao, J.; Wang, S.-J.; Au, C.-T. Ammonia decomposition over Ru and Ni catalysts supported on fumed SiO2, MCM-41, and SBA-15. J. Catal. 2005, 236, 181–189. [Google Scholar] [CrossRef]
- Jorge, T.d.S.; Polachini, T.C.; Dias, L.S.; Jorge, N.; Telis-Romero, J. Physicochemical and rheological characterization of avocado oils. Ciência E Agrotecnologia 2015, 39, 390–400. [Google Scholar] [CrossRef]
- Patel, N.K. Phytotherapeutic Investigation of Major Herbal Steroids to Explore their Potential as an Alternative to Synthetic Steroids. Ph.D. Thesis, Saurashtra University, Rajkot, Gujarat, India, 2011. [Google Scholar]
- Sankowski, A.J.; Łebkowska, U.M.; Ćwikła, J.; Walecka, I.; Walecki, J. Psoriatic arthritis. Pol. J. Radiol. 2013, 78, 7. [Google Scholar] [PubMed]
- Rogoveanu, O.C.; Calina, D.; Cucu, M.G.; Burada, F.; Docea, A.O.; Sosoi, S.; Stefan, E.; Ioana, M.; Burada, E. Association of cytokine gene polymorphisms with osteoarthritis susceptibility. Exp. Ther. Med. 2018, 16, 2659–2664. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.; Paula, L.; Souza, J.; Spin-Neto, R.; Stavropoulos, A.; Marcantonio, R. Effect of avocado/soybean unsaponifiables on ligature-induced bone loss and bone repair after ligature removal in rats. J. Periodontal. Res. 2016, 51, 332–341. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Boileau, C.; Pelletier, J.-P.; Roughley, P.J. Cartilage in normal and osteoarthritis conditions. Best Pract. Res. Clin. Rheumatol. 2008, 22, 351–384. [Google Scholar] [CrossRef]
- Maldonado, M.; Nam, J. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. Biomed Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Duan, L.; Ma, B.; Liang, Y.; Chen, J.; Zhu, W.; Li, M.; Wang, D. Cytokine networking of chondrocyte dedifferentiation in vitro and its implications for cell-based cartilage therapy. Am. J. Transl. Res. 2015, 7, 194. [Google Scholar]
- Wojdasiewicz, P.; Poniatowski, L.; Szukiewicz, D. The role of inflammatory and antiinflammatory cytokines in the pathogenesis of osteoarthritis: Review article. Mediat. Inflammation. Usa Hindawi Publ. Corp. 2014, 1–20. [Google Scholar]
- Lee, A.S.; Ellman, M.B.; Yan, D.; Kroin, J.S.; Cole, B.J.; van Wijnen, A.J.; Im, H.-J. A current review of molecular mechanisms regarding osteoarthritis and pain. Gene 2013, 527, 440–447. [Google Scholar] [CrossRef]
- Scanzello, C.R. Chemokines and inflammation in osteoarthritis: Insights from patients and animal models. J. Orthop. Res. 2017, 35, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Berenbaum, F. The regulation of chondrocyte function by proinflammatory mediators: Prostaglandins and nitric oxide. Clin. Orthop. Relat. Res. 2004, 427, S37–S46. [Google Scholar] [CrossRef] [PubMed]
- Abramson, S.B. Inflammation in osteoarthritis. J. Rheumatol. Suppl. 2004, 70, 70–76. [Google Scholar] [PubMed]
- Henrotin, Y.; Labasse, A.; Jaspar, J.; De Groote, D.; Zheng, S.; Guillou, G.; Reginster, J. Effects of three avocado/soybean unsaponifiable mixtures on metalloproteinases, cytokines and prostaglandin E 2 production by human articular chondrocytes. Clin. Rheumatol. 1998, 17, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.E.; Sanchez, C.; Deberg, M.A.; Piccardi, N.; Guillou, G.B.; Msika, P.; Reginster, J.-Y.L. Avocado/soybean unsaponifiables increase aggrecan synthesis and reduce catabolic and proinflammatory mediator production by human osteoarthritic chondrocytes. J. Rheumatol. 2003, 30, 1825–1834. [Google Scholar]
- Ameye, L.G.; Chee, W.S. Osteoarthritis and nutrition. From nutraceuticals to functional foods: A systematic review of the scientific evidence. Arthritis Res. Ther. 2006, 8, R127. [Google Scholar] [CrossRef]
- Long, L.; Soeken, K.; Ernst, E. Herbal medicines for the treatment of osteoarthritis: A systematic review. Rheumatology 2001, 40, 779–793. [Google Scholar] [CrossRef]
- Blotman, F.; Maheu, E.; Wulwik, A.; Caspard, H.; Lopez, A. Efficacy and safety of avocado/soybean unsaponifiables in the treatment of symptomatic osteoarthritis of the knee and hip. A prospective, multicenter, three-month, randomized, double-blind, placebo-controlled trial. Rev. Du Rhum. (Engl. Ed.) 1997, 64, 825–834. [Google Scholar]
- Morton, A.J.; Campbell, N.B.; J’mai, M.G.; Redding, W.R.; Blikslager, A.T. Preferential and non-selective cyclooxygenase inhibitors reduce inflammation during lipopolysaccharide-induced synovitis. Res. Vet. Sci. 2005, 78, 189–192. [Google Scholar] [CrossRef]
- Beluche, L.A.; Bertone, A.L.; Anderson, D.E.; Rohde, C. Effects of oral administration of phenylbutazone to horses on in vitro articular cartilage metabolism. Am. J. Vet. Res. 2001, 62, 1916–1921. [Google Scholar] [CrossRef]
- Goudarzi, R.; Reid, A.; McDougall, J.J. Evaluation of the novel avocado/soybean unsaponifiable Arthrocen to alter joint pain and inflammation in a rat model of osteoarthritis. PLos ONE 2018, 13, e0191906. [Google Scholar] [CrossRef] [PubMed]
- Gabay, O.; Gosset, M.; Levy, A.; Salvat, C.; Sanchez, C.; Pigenet, A.; Sautet, A.; Jacques, C.; Berenbaum, F. Stress-induced signaling pathways in hyalin chondrocytes: Inhibition by Avocado–Soybean Unsaponifiables (ASU). Osteoarthr. Cartil. 2008, 16, 373–384. [Google Scholar] [CrossRef] [PubMed]
- He, F.-J.; Chen, J.-Q. Consumption of soybean, soy foods, soy isoflavones and breast cancer incidence: Differences between Chinese women and women in Western countries and possible mechanisms. Food Sci. Hum. Wellness 2013, 2, 146–161. [Google Scholar] [CrossRef]
- Miller, M. Dyslipidemia and cardiovascular risk: The importance of early prevention. Qjm Int. J. Med. 2009, 102, 657–667. [Google Scholar] [CrossRef]
- Ownby, S.L.; Fortuno, L.V.; Au, A.Y.; Grzanna, M.W.; Rashmir-Raven, A.M.; Frondoza, C.G. Expression of pro-inflammatory mediators is inhibited by an avocado/soybean unsaponifiables and epigallocatechin gallate combination. J. Inflamm. 2014, 11, 8. [Google Scholar] [CrossRef]
- Altinel, L.; Saritas, Z.K.; Kose, K.C.; Pamuk, K.; Aksoy, Y.; Serteser, M. Treatment with unsaponifiable extracts of avocado and soybean increases TGF-β1 and TGF-β2 levels in canine joint fluid. Tohoku J. Exp. Med. 2007, 211, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Kut-Lasserre, C.; Miller, C.C.; Ejeil, A.; Gogly, B.; Dridi, M.; Piccardi, N.; Guillou, B.; Pellat, B.; Godeau, G. Effect of avocado and soybean unsaponifiables on gelatinase A (MMP-2), stromelysin 1 (MMP-3), and tissue inhibitors of matrix metalloproteinase (TIMP-1 and TIMP-2) secretion by human fibroblasts in culture. J. Periodontol. 2001, 72, 1685–1694. [Google Scholar] [CrossRef]
- Cinelli, M.; Guiducci, S.; Del Rosso, A.; Pignone, A.; Del Rosso, M.; Fibbi, G.; Serratì, S.; Gabrielli, A.; Giacomelli, R.; Piccardi, N. Piascledine modulates the production of VEGF and TIMP-1 and reduces the invasiveness of rheumatoid arthritis synoviocytes. Scand. J. Rheumatol. 2006, 35, 346–350. [Google Scholar] [CrossRef]
- Jablonska, S. Avocado/soybean unsaponifiables in the treatment of scleroderma: Comment on the article by Maheu et al. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 1998, 41, 1705. [Google Scholar] [CrossRef]
- Suthar, A.; Banavalikar, M.; Biyani, M. Pharmacological activities of Genistein, an isoflavone from soy (Glycine max): Part II—Anti-cholesterol activity, effects on osteoporosis & menopausal symptoms. IJEB 2001, 39. [Google Scholar]
- Levis, S.; Strickman-Stein, N.; Ganjei-Azar, P.; Xu, P.; Doerge, D.R.; Krischer, J. Soy isoflavones in the prevention of menopausal bone loss and menopausal symptoms: A randomized, double-blind trial. Arch. Intern. Med. 2011, 171, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Beiraghdar, F.; Kashani, N.; Javan, N.B. Comparison of piascledine (avocado and soybean oil) and hormone replacement therapy in menopausal-induced hot flashing. Iran. J. Pharm. Res. Ijpr 2011, 10, 941. [Google Scholar] [PubMed]
- Nair, P.A. Dermatosis associated with menopause. J. Mid-Life Health 2014, 5, 168. [Google Scholar] [CrossRef] [PubMed]
- Service, U.A.R. USDA National Nutrient Database for Standard Reference; USDA: Washington, DC, USA, 2004.
- Grzanna, M.W.; Ownby, S.L.; Heinecke, L.F.; Au, A.Y.; Frondoza, C.G. Inhibition of cytokine expression and prostaglandin E2 production in monocyte/macrophage-like cells by avocado/soybean unsaponifiables and chondroitin sulfate. J. Complementary Integr. Med. 2010, 7. [Google Scholar] [CrossRef]
- Grzanna, M.W.; Secor, E.J.; Fortuno, L.V.; Au, A.Y.; Frondoza, C.G. Anti-Inflammatory Effect of Carprofen Is Enhanced by Avocado/Soybean Unsaponifiables, Glucosamine and Chondroitin Sulfate Combination in Chondrocyte Microcarrier Spinner Culture. Cartilage 2018, 1947603518783495. [Google Scholar] [CrossRef]
- Tanideh, N.; Zare, Z.; Jamshidzadeh, A.; Lotfi, M.; Azarpira, N.; Sepehrimanesh, M.; Koohi-Hosseinabadi, O. Hydroethanolic extract of Psidium guajava leaf for induced osteoarthritis using a guinea pig model. Biotech. Histochem. 2017, 92, 417–424. [Google Scholar] [CrossRef]
- De Jong, A.; Plat, J.; Mensink, R.P. Metabolic effects of plant sterols and stanols. J. Nutr. Biochem. 2003, 14, 362–369. [Google Scholar] [CrossRef]
- Appelboom, T.; Schuermans, J.; Verbruggen, G.; Henrotin, Y.; Reginster, J.-Y. Symptoms modifying effect of avocado/soybean unsaponifiables (ASU) in knee osteoarthritis. Scand. J. Rheumatol. 2001, 30, 242–247. [Google Scholar]
- Lequesne, M.; Maheu, E.; Cadet, C.; Dreiser, R.L. Structural effect of avocado/soybean unsaponifiables on joint space loss in osteoarthritis of the hip. Arthritis Care Res. Off. J. Am. Coll. Rheumatol. 2002, 47, 50–58. [Google Scholar] [CrossRef]
- Darestani, R.T.; Bakhshi, H.; Sahraee, R. Comparing the efficacy and safety of Diclofenac and Piascledine in patients with knee osteoarthritis. Pajoohandeh J. 2013, 17, 272–278. [Google Scholar]
- Carrillo, J.L.M.; García, F.P.C.; Coronado, O.G.; García, M.A.M.; Cordero, J.F.C. Physiology and Pathology of Innate Immune Response Against Pathogens. In Physiology and Pathology of Immunology; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Aribi, M. Introductory Chapter: Immune system dysfunction and autoimmune diseases. Immunopathog. Immune-Based Ther. Sel. Autoimmune Disord. 2017, 1. [Google Scholar] [CrossRef]
- Maranduba, C.M.d.C.; De Castro, S.B.R.; Souza, G.T.d.; Rossato, C.; da Guia, F.C.; Valente, M.A.S.; Rettore, J.V.P.; Maranduba, C.P.; Souza, C.M.d.; Carmo, A.M.R.d. Intestinal microbiota as modulators of the immune system and neuroimmune system: Impact on the host health and homeostasis. J. Immunol. Res. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Sprague, A.H.; Khalil, R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 2009, 78, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Azadbakht, L.; Shakerhosseini, R.; Atabak, S.; Jamshidian, M.; Mehrabi, Y.; Esmaill-Zadeh, A. Beneficiary effect of dietary soy protein on lowering plasma levels of lipid and improving kidney function in type II diabetes with nephropathy. Eur. J. Clin. Nutr. 2003, 57, 1292. [Google Scholar] [CrossRef]
- Anderson, G.H.; Li, E.; Anthony, S.P.; Ng, L.T.; Bialik, R. Dissociation between plasma and brain amino acid profiles and short-term food intake in the rat. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 1994, 266, R1675–R1686. [Google Scholar] [CrossRef]
- Zhao, J.-h.; Sun, S.-j.; Horiguchi, H.; Arao, Y.; Kanamori, N.; Kikuchi, A.; Oguma, E.; Kayama, F. A soy diet accelerates renal damage in autoimmune MRL/Mp-lpr/lpr mice. Int. Immunopharmacol. 2005, 5, 1601–1610. [Google Scholar] [CrossRef]
- Zhu, C.; Wu, Y.; Jiang, Z.; Zheng, C.; Wang, L.; Yang, X.; Ma, X.; Gao, K.; Hu, Y. Dietary soy isoflavone attenuated growth performance and intestinal barrier functions in weaned piglets challenged with lipopolysaccharide. Int. Immunopharmacol. 2015, 28, 288–294. [Google Scholar] [CrossRef]
- Young, D.; Ibuki, M.; Nakamori, T.; Fan, M.; Mine, Y. Soy-derived di-and tripeptides alleviate colon and ileum inflammation in pigs with dextran sodium sulfate-induced colitis. J. Nutr. 2011, 142, 363–368. [Google Scholar] [CrossRef]
- Santoro, N.; Epperson, C.N.; Mathews, S.B. Menopausal symptoms and their management. Endocrinol. Metab. Clin. 2015, 44, 497–515. [Google Scholar] [CrossRef]
- Baker, F.C.; De Zambotti, M.; Colrain, I.M.; Bei, B. Sleep problems during the menopausal transition: Prevalence, impact, and management challenges. Nat. Sci. Sleep 2018, 10, 73. [Google Scholar] [CrossRef]
- Kuh, D.; Muthuri, S.; Cooper, R.; Moore, A.; Mackinnon, K.; Cooper, C.; Adams, J.; Hardy, R.; Ward, K. Menopause, reproductive life, hormone replacement therapy, and bone phenotype at age 60–64 years: A British birth cohort. J. Clin. Endocrinol. Metab. 2016, 101, 3827–3837. [Google Scholar] [CrossRef] [PubMed]
- Akbari, T.N.; DAVOODABADI, F.M. COMPARATIVE EFFECTS OF FLAXSEED, SOY ON MENOPAUSAL HOT FLASHES. Complementary Med. J. Fac. Nurs. Midwifery Fall 2012, 2, 52–60. [Google Scholar]
- Lewis, J.E.; Nickell, L.A.; Thompson, L.U.; Szalai, J.P.; Kiss, A.; Hilditch, J.R. A randomized controlled trial of the effect of dietary soy and flaxseed muffins on quality of life and hot flashes during menopause. Menopause 2006, 13, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Fontvieille, A.; Dionne, I.; Riesco, E. Long-term exercise training and soy isoflavones to improve quality of life and climacteric symptoms. Climacteric 2017, 20, 233–239. [Google Scholar] [CrossRef]
- Monroe, K.R.; Murphy, S.P.; Henderson, B.E.; Kolonel, L.N.; Stanczyk, F.Z.; Adlercreutz, H.; Pike, M.C. Dietary fiber intake and endogenous serum hormone levels in naturally postmenopausal Mexican American women: The Multiethnic Cohort Study. HNUC 2007, 58, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Merkulova, D.; Onsin, A.; Merkulov, Y.A. Piascledin in the treatment of chronic dorsalgia. Zhurnal Nevrol. I Psikhiatrii Im. Ss Korsakova 2013, 113, 18–22. [Google Scholar]
- Kut, C.; Assoumou, A.; Dridi, M.; Bonnefoix, M.; Gogly, B.; Pellat, B.; Guillou, G.; Godeau, G. Morphometric analysis of human gingival elastic fibres degradation by human leukocyte elastase protective effect of avocado and soybean unsaponifiables (ASU). Pathol. -Biol. 1998, 46, 571–576. [Google Scholar]
- Alekseev, V.; Alekseev, A.; Gol’dzon, G. Nonspecific low-back pain: From symptomatic treatment to pathogenesis-based treatment. Zhurnal Nevrol. I Psikhiatrii Im. Ss Korsakova 2014, 114, 51–55. [Google Scholar]
- Patisaul, H.B.; Jefferson, W. The pros and cons of phytoestrogens. Front. Neuroendocrinol. 2010, 31, 400–419. [Google Scholar] [CrossRef]
- Oryan, A.; Mohammadalipour, A.; Moshiri, A.; Tabandeh, M.R. Avocado/soybean unsaponifiables: A novel regulator of cutaneous wound healing, modelling and remodelling. Int. Wound J. 2015, 12, 674–685. [Google Scholar] [CrossRef]
- Msika, P.; Baudouin, C.; Saunois, A.; Bauer, T. Avocado/soybean unsaponifiables, ASU EXPANSCIENCE™, are strictly different from the nutraceutical products claiming ASU appellation. Osteoarthr. Cartil. 2008, 16, 1275–1276. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Hossain, A.; Ghogomu, E.T.; Mudano, A.S.; Tugwell, P.; Wells, G.A. Biologic or tofacitinib monotherapy for rheumatoid arthritis in people with traditional disease-modifying anti-rheumatic drug (DMARD) failure: A Cochrane Systematic Review and network meta-analysis (NMA). Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Macaigne, G.; Ozon, N.; Dikov, D.; Auriault, M.-L.; Deplus, R. Colite lymphocytaire associée à la prise de Piasclédine®. Gastroentérologie Clin. Et Biol. 2004, 28, 412–413. [Google Scholar] [CrossRef]
- Gosting, D.C.; Doyle, M.E. Food Safety 1990: An Annotated Bibliography of the Literature; Elsevier: Stoneham, MA, USA, 2013. [Google Scholar]

| Form | R1 | R2 | R3 |
| α-Tocopherol | CH3 | CH3 | CH3 |
| β-Tocopherol | CH3 | CH3 | H |
| γ-Tocopherol | H | CH3 | CH3 |
| δ-Tocopherol | H | CH3 | H |
| Basic structure of tocotrienols | 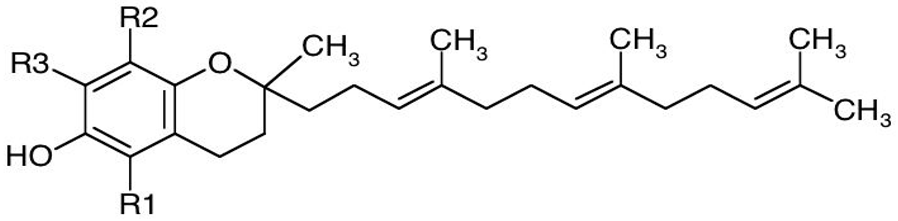 | ||
| Form | R1 | R2 | R3 |
| α-Tocotrienol | CH3 | CH3 | CH3 |
| β-Tocotrienol | CH3 | H | CH3 |
| γ-Tocotrienol | H | CH3 | CH3 |
| δ-Tocotrienol | H | H | CH3 |
| Disease | Main Pharmacological Mechanism | Effects | References | ||
|---|---|---|---|---|---|
| Osteoartrithis | modifies the structure of the articular cartilage | stimulates the synthesis of articular chondrocytes (anabolic effect) | promotion of cartilage repair reduce stiffness and pain | [5,58,63,64,65,67,68,75,76,77,78,79,80,81] | |
| Target tissue organ | Molecular mediators | ||||
| Articular synoviocytes, Chondrocytes Chondrocytes, subchondral bone osteoblasts | Collagen II mRNA Aggrecan proteoglycan TGF-β3, Osteocalcin | ||||
| reduces the degradation of articular chondrocytes (anti-catabolic effects) | inhibition cartilage distruction inhibitory effects on inflammatory and catabolic mediators | ||||
| Target tissue organ | Molecular mediators | ||||
| Chondrocytes, Synoviocytes Chondrocytes Fibroblasts, Chondrocytes Chondrocytes, Monocyte/Macrophage-like cells Nuclear translocation of p65 Subchondral bone osteoblasts | IL-Iβ, IL-4, IL-6 IL-8, MIP-1β, MMP13, TNF-α, Fibronectin MMP-2, MMP-3, COX2, PGE2, iNOS, NO Nf-kB TIMP-1 | ||||
| Scleroderma | potential increasing of collagen solubility and reducing cutaneous fibrosis | reducing clinical symptoms | [82] | ||
| Menopause | estrogen-like properties preferential binding for estrogen receptor-beta (ER-β) than for estrogen receptor-alpha (ER-α). | positive effects in reducing menopausal-related symptoms | [69,83,84,85,86] | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salehi, B.; Rescigno, A.; Dettori, T.; Calina, D.; Docea, A.O.; Singh, L.; Cebeci, F.; Özçelik, B.; Bhia, M.; Dowlati Beirami, A.; et al. Avocado–Soybean Unsaponifiables: A Panoply of Potentialities to Be Exploited. Biomolecules 2020, 10, 130. https://doi.org/10.3390/biom10010130
Salehi B, Rescigno A, Dettori T, Calina D, Docea AO, Singh L, Cebeci F, Özçelik B, Bhia M, Dowlati Beirami A, et al. Avocado–Soybean Unsaponifiables: A Panoply of Potentialities to Be Exploited. Biomolecules. 2020; 10(1):130. https://doi.org/10.3390/biom10010130
Chicago/Turabian StyleSalehi, Bahare, Antonio Rescigno, Tinuccia Dettori, Daniela Calina, Anca Oana Docea, Laxman Singh, Fatma Cebeci, Beraat Özçelik, Mohammed Bhia, Amirreza Dowlati Beirami, and et al. 2020. "Avocado–Soybean Unsaponifiables: A Panoply of Potentialities to Be Exploited" Biomolecules 10, no. 1: 130. https://doi.org/10.3390/biom10010130
APA StyleSalehi, B., Rescigno, A., Dettori, T., Calina, D., Docea, A. O., Singh, L., Cebeci, F., Özçelik, B., Bhia, M., Dowlati Beirami, A., Sharifi-Rad, J., Sharopov, F., C. Cho, W., & Martins, N. (2020). Avocado–Soybean Unsaponifiables: A Panoply of Potentialities to Be Exploited. Biomolecules, 10(1), 130. https://doi.org/10.3390/biom10010130












