Inhibition of Growth of TSC2-Null Cells by a PI3K/mTOR Inhibitor but Not by a Selective MNK1/2 Inhibitor
Abstract
1. Introduction
2. Materials and Methods
2.1. Compounds and Reagents
2.2. In Vitro Cell Culture
2.3. Cell Growth Assays
2.4. Cell Protein Extraction and Immunoblotting
2.5. Lung and Cell Immunofluorescent Analyses
2.6. Statistical Analyses
3. Results
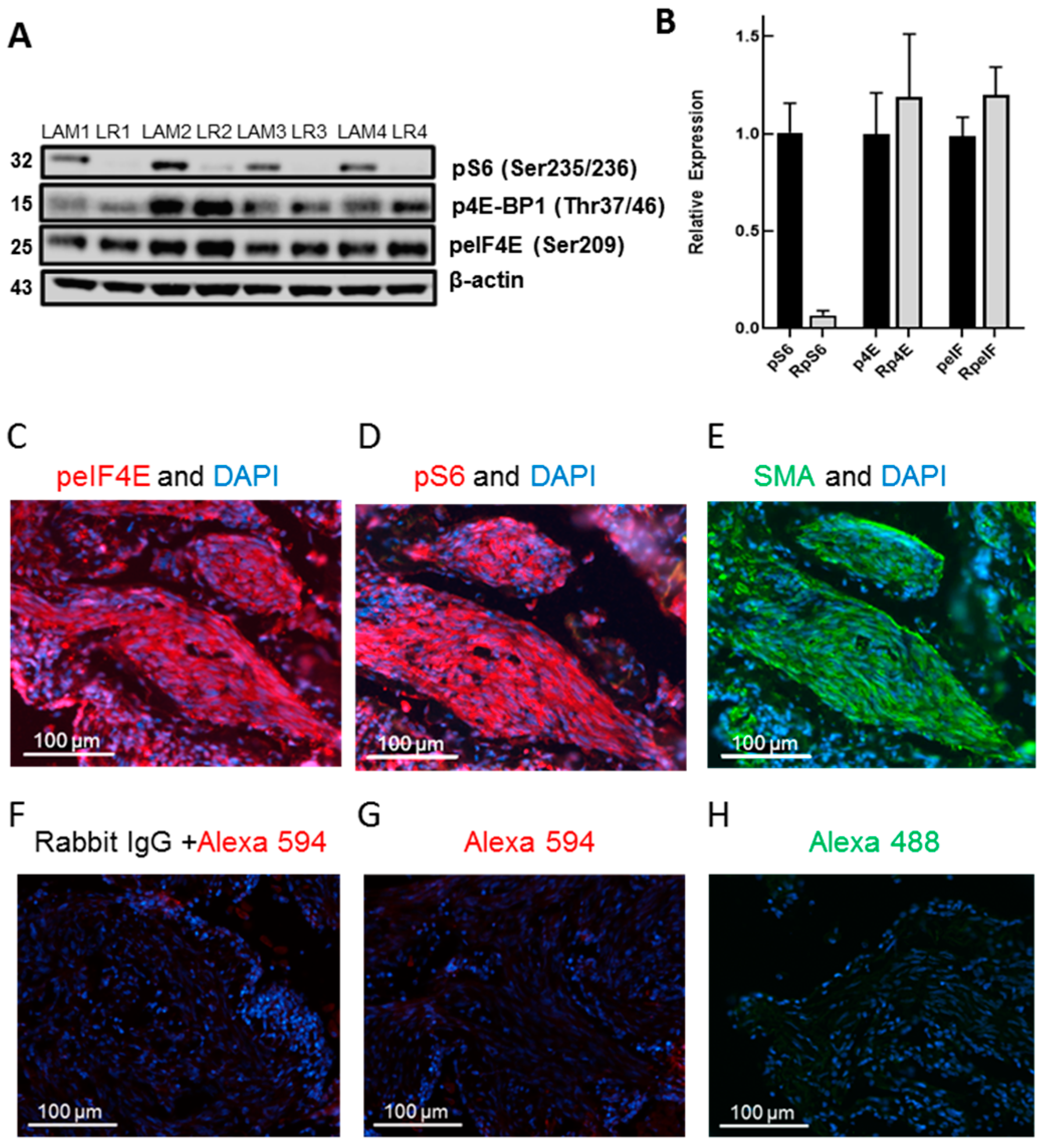
3.1. LAM Lung Cells Express peIF4E and Rapamycin Inhibits pS6 but Not p4E-BP1 or peIF4E
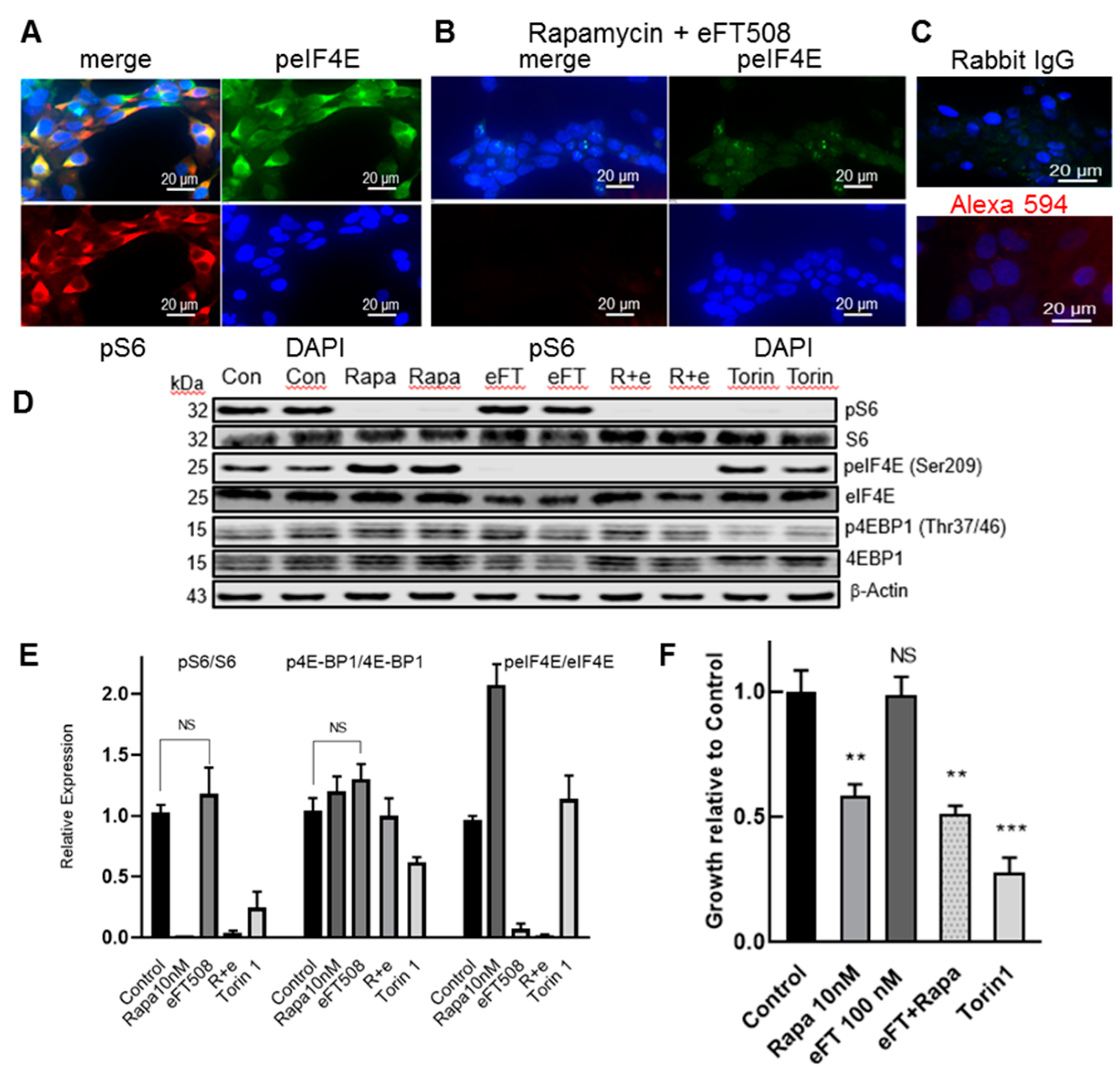
3.2. LAM Lung Cells Express peIF4E and the Selective MNK1/2 Inhibitor eFT508 Reduces peIF4E in LAM Angiomyolipoma Cells but Does Not Inhibit Cell Growth
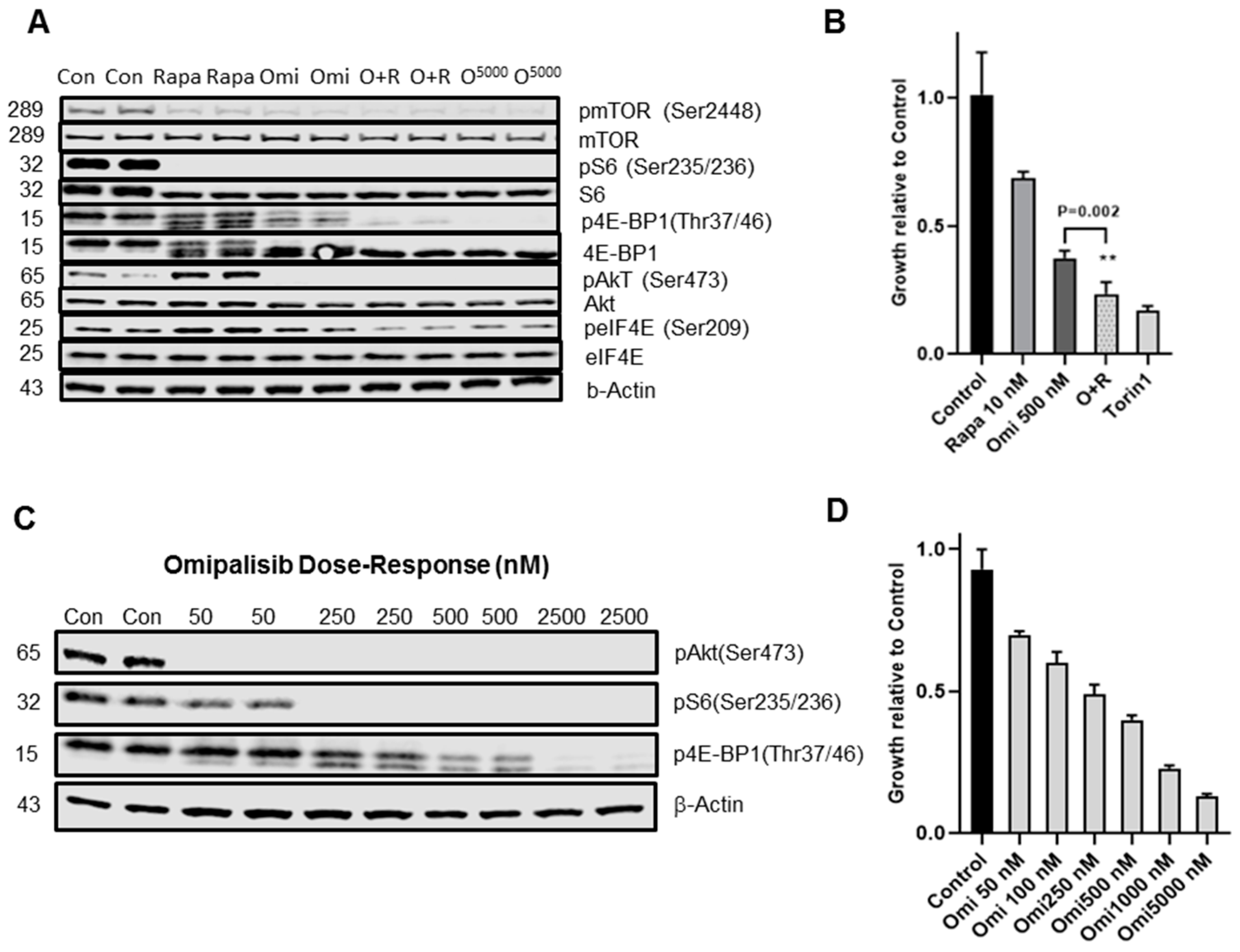
3.3. PI3K/mTOR Inhibitor Omipalisib Inhibits pS6, p4EB-P1, pAkt, and Cell Growth
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Johnson, S.R.; Taveira da Silva, A.M.; Moss, J. Lymphangioleiomyomatosis. Clin. Chest. Med. 2016, 37, 389–403. [Google Scholar] [CrossRef]
- Krymskaya, V.; McCormack, F.X. Lymphangioleiomyomatosis: A monogenic model of malignancy. Annu. Rev. Med. 2017, 68, 69–83. [Google Scholar] [CrossRef]
- Carsillo, T.; Astrinidis, A.; Henske, E.P. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc. Natl. Acad. Sci. USA 2000, 9, 6085–6090. [Google Scholar] [CrossRef]
- Goncharova, E.A.; Goncharov, D.A.; Eszterhas, A.; Hunter, D.S.; Glassberg, M.K.; Yeung, R.S.; Walker, C.L.; Noonan, D.; Kwiatkowski, D.J.; Chou, M.M.; et al. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation: A role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis. J. Biol. Chem. 2002, 277, 30958–30967. [Google Scholar] [CrossRef]
- Yu, J.; Astrinidis, A.; Howard, S.; Henske, E.P. Estradiol and tamoxifen stimulate LAM-associated angiomyolipoma cell growth and activate both genomic and non-genomic signaling pathways. Am. J. Physiol. Lung Cell Mol. Physiol. 2004, 286, 694–700. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR signaling in growth, metabolism and disease. Cell 2017, 168, 960–974. [Google Scholar] [CrossRef]
- McCormack, F.X.; Inoue, Y.; Moss, J.; Singer, L.G.; Strange, C.; Nakata, K.; Barker, A.F.; Chapman, J.T.; Brantly, M.L.; Stocks, J.M.; et al. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N. Engl. J. Med. 2011, 364, 1595–1606. [Google Scholar] [CrossRef]
- Mahalati, K.; Kahan, B.D. Clinical pharmacokinetics of Sirolimus. Clin. Pharm. 2001, 40, 573–585. [Google Scholar] [CrossRef]
- Choo, A.Y.; Yoon, S.O.; Kim, S.G.; Roux, P.P.; Blenis, J. Rapamycin differentially inhibits S6K and 4E-BP1 to mediate cell type specific repression of mRNA. Proc. Natl. Acad. Sci. USA 2008, 105, 17414–17419. [Google Scholar] [CrossRef]
- Sonenberg, N. eIF4E, the cap-binding protein, from basic discovery to translational research. Biochem. Cell Biol. 2008, 86, 178–183. [Google Scholar] [CrossRef]
- De Benedetti, A.; Graff, J.R. eIF-4E expression and its role in malignancies and metastases. Oncogene 2004, 23, 3189–3199. [Google Scholar] [CrossRef]
- Furic, L.; Rong, L.; Larsson, O.; Koumakpayi, I.H.; Yoshida, K.; Brueschke, A.; Petroulakis, E.; Robichaud, N.; Pollak, M.; Gaboury, L.A.; et al. eIF4E phosphorylation promotes tumorigenesis and is associated with prostate cancer progression. Proc. Natl. Acad. Sci. USA 2010, 1107, 14134–14139. [Google Scholar] [CrossRef]
- Reich, S.H.; Sprengeler, P.A.; Chiang, G.G.; Appleman, J.R.; Chen, J.; Clarine, J.; Eam, B.; Ernst, J.T.; Han, Q.; Goel, V.K.; et al. Structure-based design of pyridine-aminal eFT508 targeting dysregulated translation by selective mitogen-activated protein kinase interacting kinases 1 and 2 MNK1/2, inhibition. J. Med. Chem. 2018, 61, 3516–3540. [Google Scholar] [CrossRef]
- Hua, H.; Kong, Q.; Zhang, H.; Wang, J.; Luo, T.; Jiang, Y. Targeting mTOR for cancer therapy. J. Hematol. Oncol. 2019, 12, 71. [Google Scholar] [CrossRef]
- Knight, S.D.; Adams, N.D.; Burgess, J.L.; Chaudhari, A.M.; Darcy, M.G.; Donatelli, C.A.; Luengo, J.I.; Newlander, K.A.; Parrish, C.A.; Ridgers, L.H.; et al. Discovery of GSK2126458. A highly potent inhibitor of PI3K and the mammalian target of rapamycin. ACS Med. Chem. Lett. 2010, 1, 39–43. [Google Scholar] [CrossRef]
- Munster, P.; Aggarwal, R.; Hong, D.; Schellens, J.H.; Van Der Noll, R.; Specht, J.; Witteveen, P.O.; Werner, T.L.; Dees, E.C.; Bergsland, E.; et al. First-in-Human Phase I study of GSK2126458, an oral Pan-Class I phosphatidylinositol-3-kinase inhibitor, in patients with advanced solid tumor malignancies. Clin. Cancer Res. 2016, 22, 1932–1939. [Google Scholar] [CrossRef]
- Lukey, P.T.; Harrison, S.A.; Yang, S.; Man, Y.; Holman, B.F.; Rashidnasab, A.; Azzopardi, G.; Grayer, M.; Simpson, J.K.; Bareille, P.; et al. A randomized, placebo-controlled study of omipalisib PI3K/mTOR, in idiopathic pulmonary fibrosis. Eur. J. Res. 2019, 53, 1801992. [Google Scholar] [CrossRef]
- Goncharova, E.A.; Goncharov, D.A.; James, M.L.; Atochina-Vasserman, E.N.; Stepanova, V.; Hong, S.-B.; Li, H.; Gonzales, L.; Baba, M.; Linehan, W.M.; et al. Folliculin controls lung alveolar enlargement and epithelial cell survival through E-cadherin, LKB1 and AMPK. Cell Rep. 2014, 7, 412–423. [Google Scholar] [CrossRef]
- Maisel, K.; Merrilees, M.J.; Atochina-Vasserman, E.N.; Lian, L.; Obraztsova, K.; Rue, R.; Vasserman, A.N.; Zuo, N.; Angel, L.F.; Gow, A.J.; et al. Immune checkpoint ligand PD-L1 is upregulated in pulmonary lymphangioleiomyomatosis. Am. J. Respir. Cell Mol. Biol. 2018, 59, 723–732. [Google Scholar] [CrossRef]
- Ericson, J.E.; Zimmerman, K.O.; Gonzalez, D.; Melloni, C.; Guptill, J.T.; Hill, K.D.; Wu, H.; Cohen-Wolkowiez, M. A systematic literature review approach to estimate the therapeutic index of selected immunosuppressant drugs following renal transplantation. Ther. Drug Monit. 2017, 39, 13–20. [Google Scholar] [CrossRef]
- Hayashi, T.; Kumasaka, T.; Mitani, K.; Okada, Y.; Kondo, T.; Date, H.; Chen, F.; Oto, T.; Miyoshi, S.; Shiraishi, T.; et al. Bronchial involvement in advanced stage Lymphangioleiomyomatosis: Histopathologic and molecular analyses. Hum. Pathol. 2016, 50, 34–42. [Google Scholar] [CrossRef]
- Fernandez, D.R.; Telarico, T.; Bonilla, E.; Li, Q.; Banerjee, S.; Middleton, F.A.; Phillips, P.E.; Crow, M.K.; Oess, S.; Muller-Esterl, W.; et al. Activation of mammalian target of rapamycin controls the loss of TCRzeta in lupus T cells through HRES-1/Rab4-regulated lysosomal degradation. J. Immun. 2009, 182, 4189–4195. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.-W.; Kelly, R.; Winans, T.; Marchena, I.; Shadakshari, A.; Yu, J.; Dawood, M.; Garcia, R.; Tily, H.; Francis, L.; et al. Sirolimus in patients with clinically active systemic lupus erythematosus resistant to, or intolerant of, conventional medications: A single-arm, open-label, phase 1/2 trial. Lancet 2018, 391, 1186–1196. [Google Scholar] [CrossRef]
- Suto, T.; Karonitsch, T. The immunobiology of mTOR in autoimmunity. J. Autoimmun. 2019, 129, 4451–4463. [Google Scholar] [CrossRef] [PubMed]
- Fajgenbaum, D.C.; Langan, R.-A.; Japp, A.S.; Partridge, H.L.; Pierson, S.K.; Singh, A.; Arenas, D.J.; Ruth, J.R.; Nabel, C.S.; Stone, K.; et al. Identifying and targeting pathogenic PI3K/AKT/mTOR signaling in IL-6 blockade-refractory idiopathic multicentric Castleman disease. J. Clin. Investig. 2019, 129, 4451–4463. [Google Scholar] [CrossRef] [PubMed]
- Lineham, E.; Spencer, J.; Morley, S.J. Dual abrogation of MNK and mTOR: A novel therapeutic approach to the treatment of aggressive cancers. Future Med. Chem. 2017, 9, 1539–1555. [Google Scholar] [CrossRef]
- Ueda, T.; Watanabe-Fukunaga, R.; Fukuyama, H.; Nagata, S.; Fukunaga, R. Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth or development. Mol. Cell. Biol. 2004, 24, 6539. [Google Scholar] [CrossRef]
- Xu, Y.; Poggio, M.; Jin, H.Y.; Shi, Z.; Forester, C.M.; Wang, Y.; Stumpf, C.R.; Xue, L.; Devericks, E.; So, L.; et al. Translation control of the immune checkpoint in cancer and its therapeutic targeting. Nat. Med. 2019, 25, 301–311. [Google Scholar] [CrossRef]
- Liu, H.-J.; Lizotte, P.H.; Du, H.; Speranza, M.C.; Lam, H.C.; Vaughan, S.; Alesi, N.; Wong, K.-K.; Freeman, G.J.; Sharpe, A.H.; et al. TSC2-deficient tumors have evidence of T cell exhaustion and respond to anti-PD1/anti-CTLA4 immunotherapy. JCI Insight 2018, 38, e98674. [Google Scholar] [CrossRef]
- Manning, B.D. Balancing Akt with S6K: Implications for both metabolic diseases and tumorigenesis. J. Cell Biol. 2004, 167, 399–403. [Google Scholar] [CrossRef]
- Himes, B.E.; Obraztsova, K.; Lian, L.; Shumyatcher, M.; Rue, R.; Atochina-Vasserman, E.N.; Hur, S.K.; Bartolomei, M.S.; Evans, J.F.; Krymskaya, V.P. Rapamycin-dependent IGF2 expression in Tsc2-null mouse embryo. PLoS ONE 2018, 135, e1097105. [Google Scholar]
- Terasaki, Y.; Yahiro, K.; Pacheco-Rodriguez, G.; Steagall, W.K.; Stylianou, M.P.; Evans, J.F.; Walker, A.M.; Moss, J. Effects of prolactin on TSC2-null Eker rat cells and in pulmonary Lymphangioleiomyomatosis. Am. J. Respir. Crit. Care Med. 2010, 182, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Aberle, M.R.; Burkhart, R.A.; Tiriac, H.; Damink, S.W.M.O.; DeJong, C.H.; Tuveson, D.A.; Van Dam, R.M. Patient-derived organoid models help define personalized management of gastrointestinal cancer. Br. J. Surg. 2018, 105, e48–e60. [Google Scholar] [CrossRef] [PubMed]
- Graff, J.R.; Konicek, B.W.; Carter, J.H.; Marcusson, E.G. Targeting the eukaryotic initiation factor eIF4E for cancer therapy. Cancer Res. 2008, 68, 631–634. [Google Scholar] [CrossRef]
- Alain, T.; Morita, M.; Fonseca, B.D.; Yanagiya, A.; Siddiqui, N.; Bhat, M.; Zammit, D.; Marcus, V.; Metrakos, P.; Voyer, L.-A.; et al. eIF4E/4E-BP ratio predicts the efficacy of mTOR targeted therapies. Cancer Res. 2012, 72, 6468–6476. [Google Scholar] [CrossRef]
- Selman, C.; Tullet, J.M.; Wieser, D.; Irvine, E.; Lingard, S.J.; Choudhury, A.I.; Claret, M.; Al-Qassab, H.; Carmignac, D.; Ramadani, F.; et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian lifespan. Science 2009, 326, 140–144. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evans, J.F.; Rue, R.W.; Mukhitov, A.R.; Obraztsova, K.; Smith, C.J.; Krymskaya, V.P. Inhibition of Growth of TSC2-Null Cells by a PI3K/mTOR Inhibitor but Not by a Selective MNK1/2 Inhibitor. Biomolecules 2020, 10, 28. https://doi.org/10.3390/biom10010028
Evans JF, Rue RW, Mukhitov AR, Obraztsova K, Smith CJ, Krymskaya VP. Inhibition of Growth of TSC2-Null Cells by a PI3K/mTOR Inhibitor but Not by a Selective MNK1/2 Inhibitor. Biomolecules. 2020; 10(1):28. https://doi.org/10.3390/biom10010028
Chicago/Turabian StyleEvans, Jilly F., Ryan W. Rue, Alexander R. Mukhitov, Kseniya Obraztsova, Carly J. Smith, and Vera P. Krymskaya. 2020. "Inhibition of Growth of TSC2-Null Cells by a PI3K/mTOR Inhibitor but Not by a Selective MNK1/2 Inhibitor" Biomolecules 10, no. 1: 28. https://doi.org/10.3390/biom10010028
APA StyleEvans, J. F., Rue, R. W., Mukhitov, A. R., Obraztsova, K., Smith, C. J., & Krymskaya, V. P. (2020). Inhibition of Growth of TSC2-Null Cells by a PI3K/mTOR Inhibitor but Not by a Selective MNK1/2 Inhibitor. Biomolecules, 10(1), 28. https://doi.org/10.3390/biom10010028



