Naphtho-Gamma-Pyrones Produced by Aspergillus tubingensis G131: New Source of Natural Nontoxic Antioxidants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nuclear Magnetic Resonance
2.2. Analytical HPLC Analysis
2.3. Semi-Preparative HPLC Analysis
2.4. Fungal Material
2.5. Solid State Fermentation
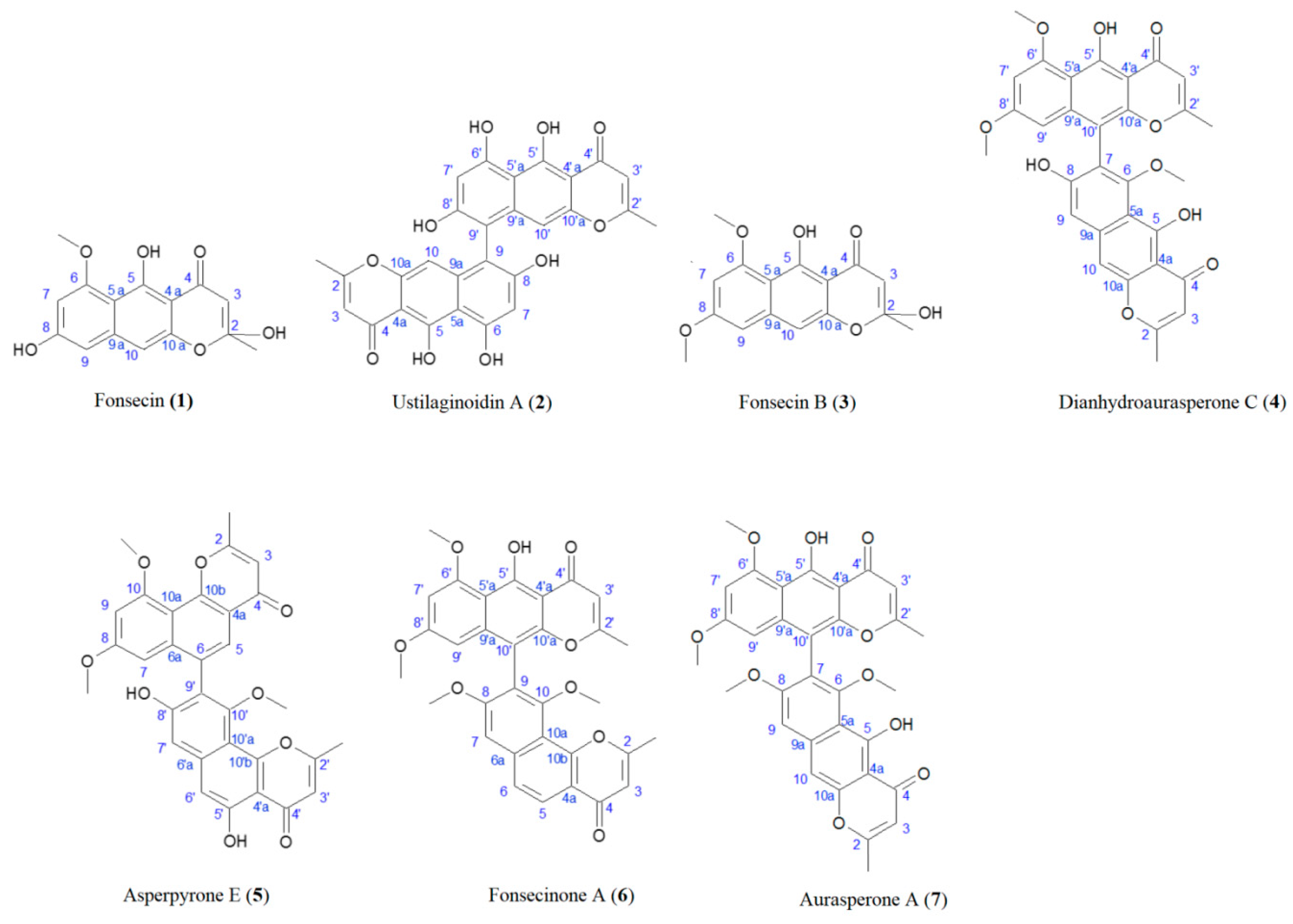
2.6. Isolation of Compounds 1–7
2.7. Radical Scavenging Capacity Assay
2.8. Cell Innocuity and Protective Effect Against H2O2-Mediated Cell Death
2.9. Data Analysis
3. Results
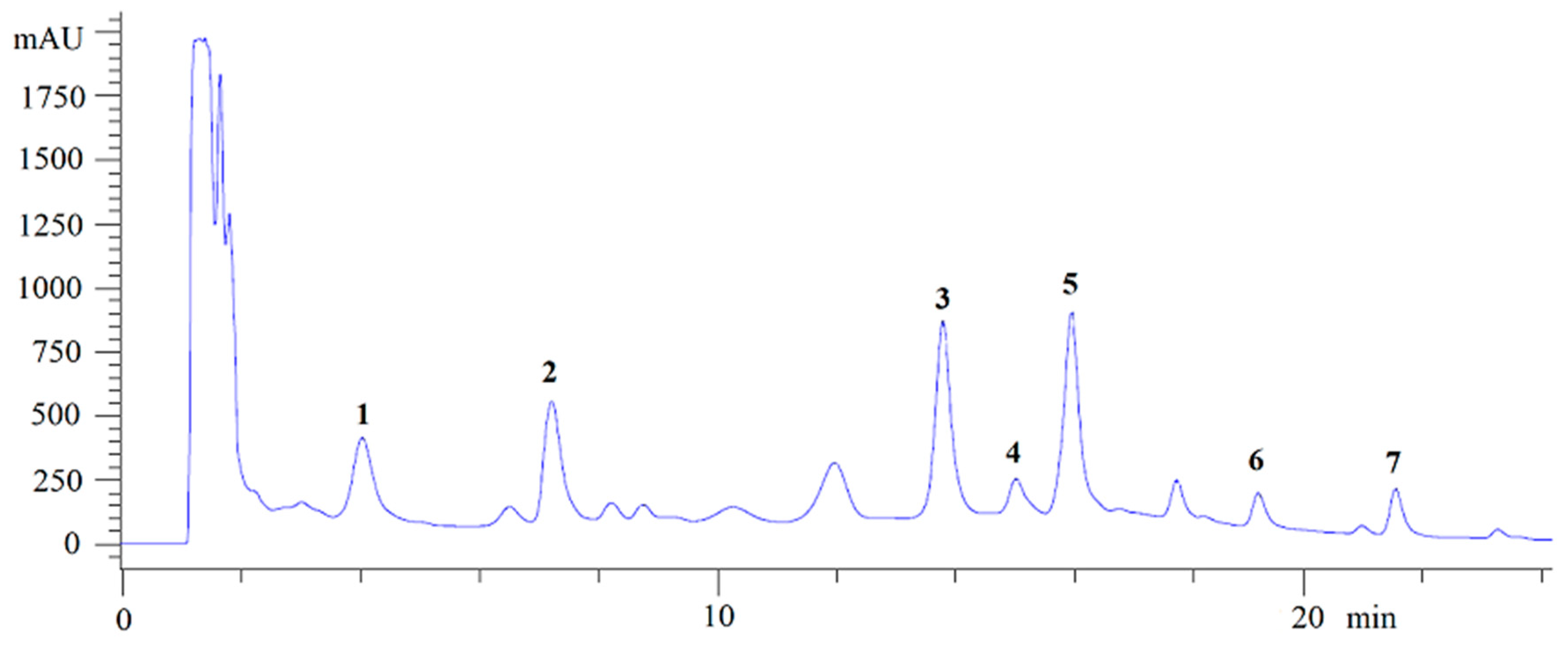
3.1. Isolation of the NγPs
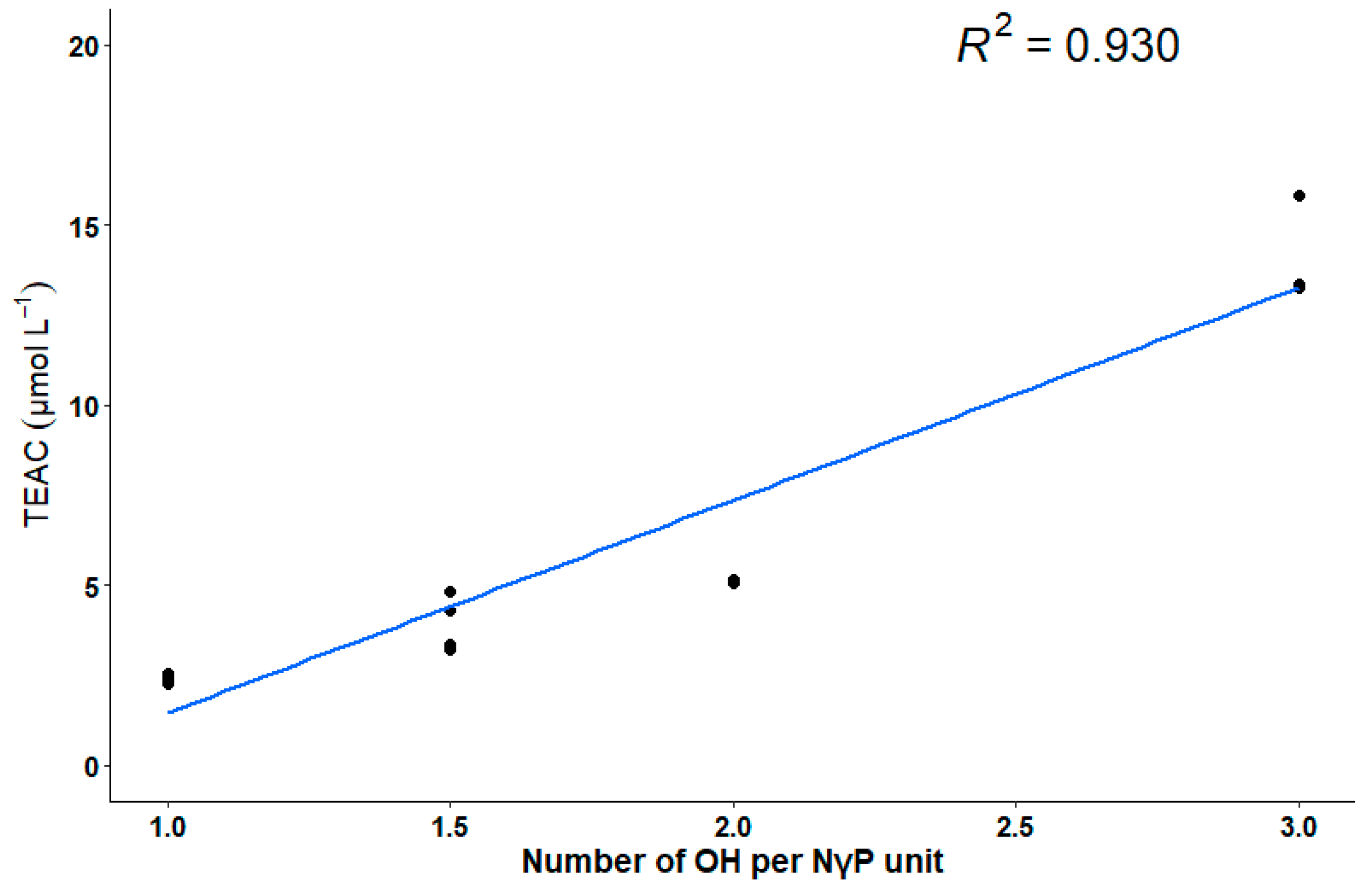
3.2. ABTS Assay
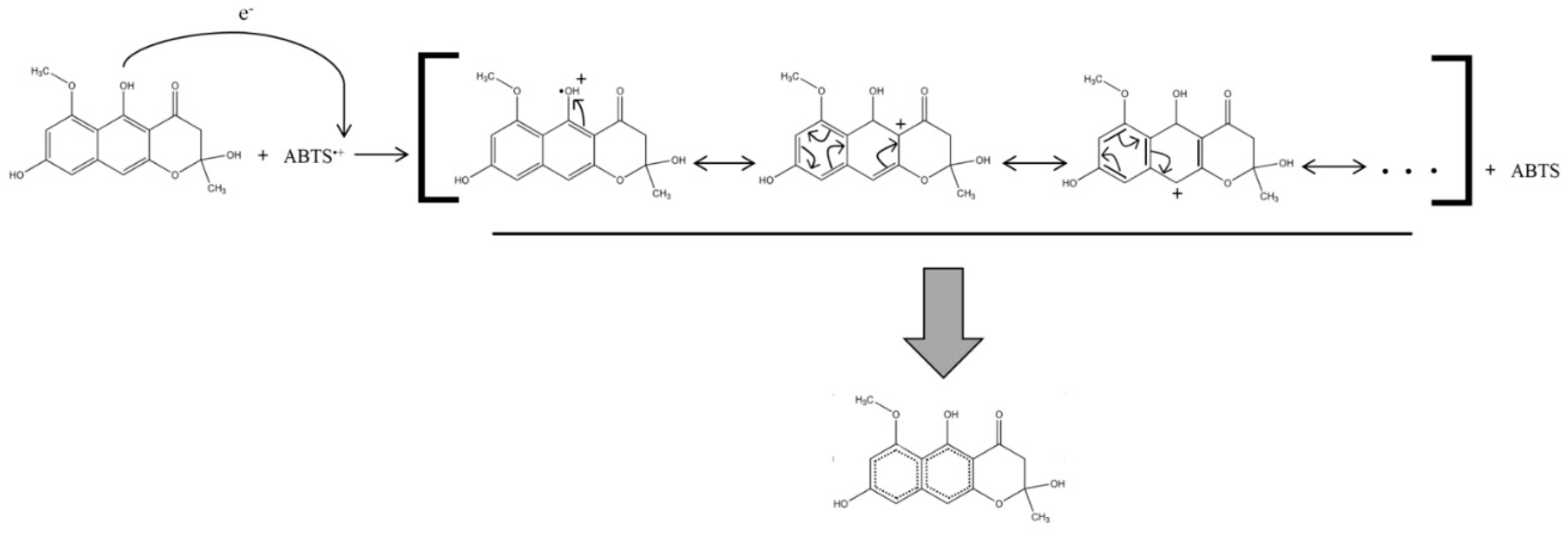
3.3. Structure–Mechanism Relationship
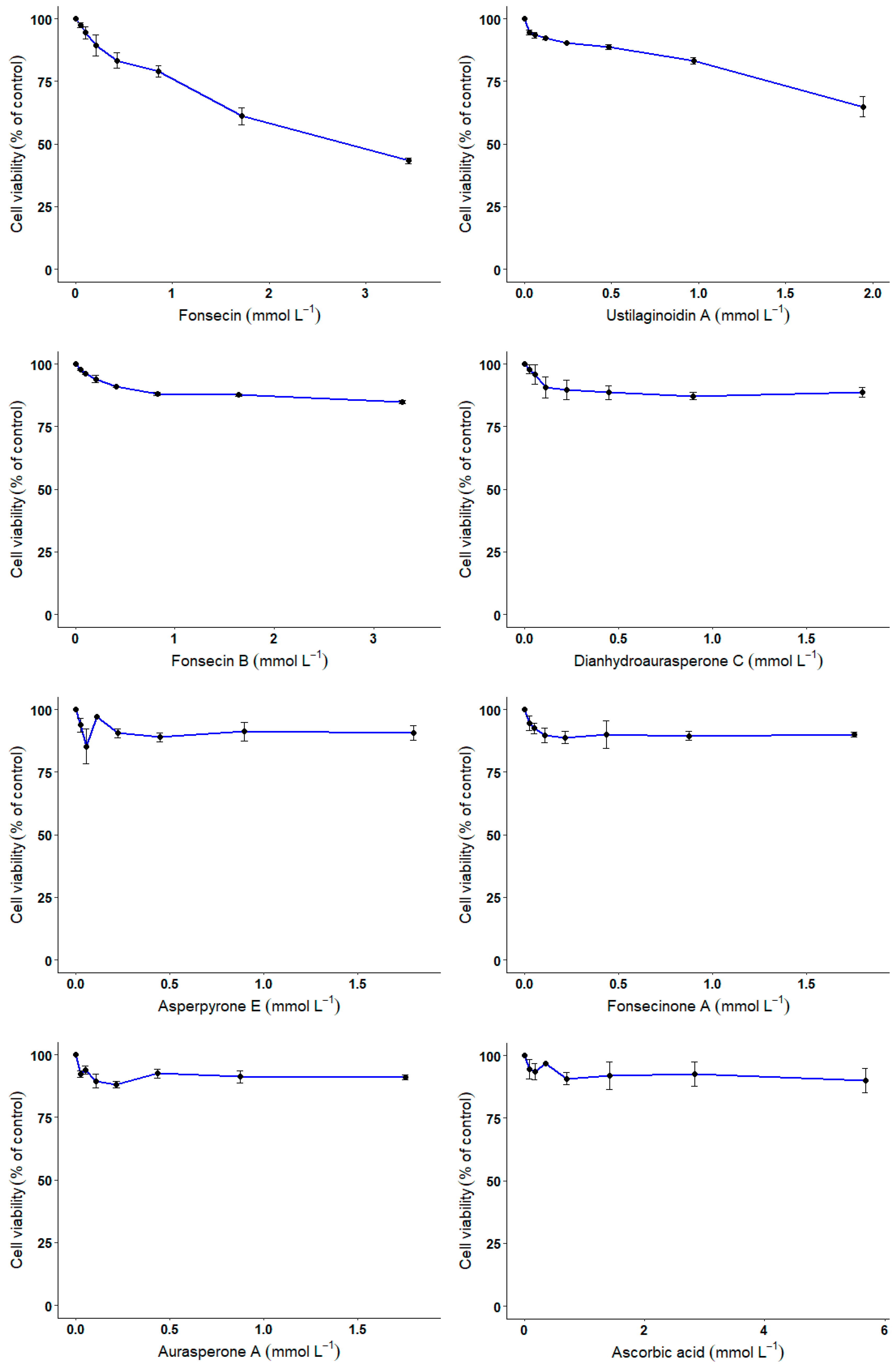
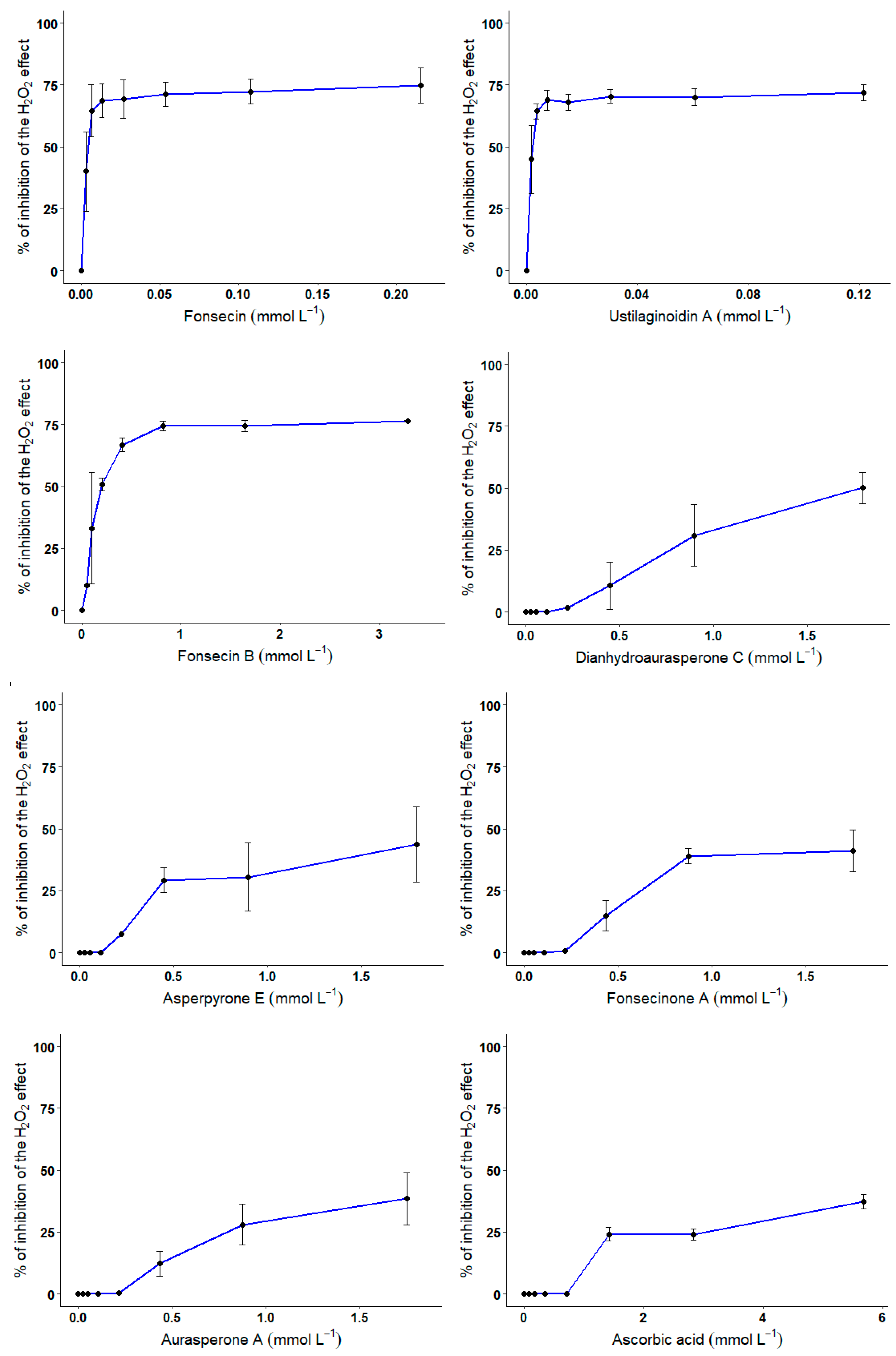
3.4. CHO Cell-Based Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nielsen, K.F.; Mogensen, J.M.; Johansen, M.; Larsen, T.O.; Frisvad, J.C. Review of secondary metabolites and mycotoxins from the Aspergillus niger group. Anal. Bioanal. Chem. 2009, 395, 1225–1242. [Google Scholar] [CrossRef]
- Zhan, J.; Gunaherath, G.M.K.B.; Wijeratne, E.M.K.; Gunatilaka, A.A.L. Asperpyrone D and other metabolites of the plant-associated fungal strain Aspergillus tubingensis. Phytochemistry 2007, 68, 368–372. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.; Tian, J.; Sun, W.; Meng, J.; Wang, X.; Fu, X.; Wang, A.; Lai, D.; Liu, Y.; Zhou, L. Bis-naphtho-γ-pyrones from Fungi and Their Bioactivities. Molecules 2014, 19, 7169–7188. [Google Scholar] [CrossRef] [Green Version]
- Obermaier, S.; Müller, M. Biaryl-Forming Enzymes from Aspergilli Exhibit Substrate-Dependent Stereoselectivity. Biochemistry 2019, 58, 2589–2593. [Google Scholar] [CrossRef]
- Xu, J. Bioactive natural products derived from mangrove-associated microbes. RSC Adv. 2015, 5, 841–892. [Google Scholar] [CrossRef]
- Choque, E.; El Rayess, Y.; Raynal, J.; Mathieu, F. Fungal naphtho-γ-pyrones—secondary metabolites of industrial interest. Appl. Microbiol. Biotechnol. 2015, 99, 1081–1096. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Li, T.; Ma, C. A new pyrone derivative from an endophytic Aspergillus tubingensis of Lycium ruthenicum. Nat. Prod. Res. 2016, 30, 1499–1503. [Google Scholar] [CrossRef]
- Kim, D.O.; Lee, C.Y. Comprehensive Study on Vitamin C Equivalent Antioxidant Capacity (VCEAC) of Various Polyphenolics in Scavenging a Free Radical and its Structural Relationship. Crit. Rev. Food Sci. Nutr. 2004, 44, 253–273. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 212–219. [Google Scholar]
- Cai, X.; Yu, Y.; Li, Q.; Chen, B.K.; Huang, Y.; Zou, X.W.; Tang, J.T.; Huang, B.S. Asperpyrone F, a new dimeric naphtho-γ-pyrone from the edible fungus Pleurotus ostreatus. Nat. Prod. Res. 2018, 1–8. [Google Scholar] [CrossRef]
- Leutou, A.S.; Yun, K.; Son, B.W. Induced production of 6,9-dibromoflavasperone, a new radical scavenging naphthopyranone in the marine-mudflat-derived fungus Aspergillus niger. Arch. Pharm. Res. 2016, 39, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Siriwardane, A.M.D.A.; Kumar, N.S.; Jayasinghe, L.; Fujimoto, Y. Chemical investigation of metabolites produced by an endophytic Aspergillus sp. isolated from Limonia acidissima. Nat. Prod. Res. 2015, 29, 1384–1387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.M.; Wang, B.G. Nigerasperones A~C, New Monomeric and Dimeric Naphtho-γ-pyrones from a Marine Alga-derived Endophytic Fungus Aspergillus niger EN-13. J. Antibiot. (Tokyo) 2007, 60, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Choque, E.; Klopp, C.; Valiere, S.; Raynal, J.; Mathieu, F. Whole-genome sequencing of Aspergillus tubingensis G131 and overview of its secondary metabolism potential. BMC Genom. 2018, 19, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Razafimanjato, H.; Garmy, N.; Guo, X.J.; Varini, K.; Di Scala, C.; Di Pasquale, E.; Taïeb, N.; Maresca, M. The food-associated fungal neurotoxin ochratoxin A inhibits the absorption of glutamate by astrocytes through a decrease in cell surface expression of the excitatory amino-acid transporters GLAST and GLT-1. NeuroToxicology 2010, 31, 475–484. [Google Scholar] [CrossRef]
- Benkhaled, B.T.; Hadiouch, S.; Olleik, H.; Perrier, J.; Ysacco, C.; Guillaneuf, Y.; Gigmes, D.; Maresca, M.; Lefay, C. Elaboration of antimicrobial polymeric materials by dispersion of well-defined amphiphilic methacrylic SG1-based copolymers. Polym. Chem. 2018, 9, 3127–3141. [Google Scholar] [CrossRef]
- Borie, C.; Mondal, S.; Arif, T.; Briand, M.; Lingua, H.; Dumur, F.; Gigmes, D.; Stocker, P.; Barbarat, B.; Robert, V.; et al. Enediynes bearing polyfluoroaryl sulfoxide as new antiproliferative agents with dual targeting of microtubules and DNA. Eur. J. Med. Chem. 2018, 148, 306–313. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Tian, J.; Chen, X.; Sun, W.; Zhu, H.; Li, Q.; Lei, L.; Yao, G.; Xue, Y.; Wang, J.; et al. Fungal naphtho-γ-pyrones: Potent antibiotics for drug-resistant microbial pathogens. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Li, X.B.; Xie, F.; Liu, S.S.; Li, Y.; Zhou, J.C.; Liu, Y.Q.; Yuan, H.Q.; Lou, H.X. Naphtho- γ -pyrones from Endophyte Aspergillus niger Occurring in the Liverwort Heteroscyphus tener (Steph.) Schiffn. Chem. Biodivers. 2013, 10, 1193–1201. [Google Scholar] [CrossRef]
- Shaaban, M.; Shaaban, K.A.; Abdel-Aziz, M.S. Seven naphtho-γ-pyrones from the marine-derived fungus Alternaria alternata: Structure elucidation and biological properties. Org. Med. Chem. Lett. 2012, 2, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carboué, Q.; Claeys-Bruno, M.; Bombarda, I.; Sergent, M.; Jolain, J.; Roussos, S. Experimental design and solid state fermentation: A holistic approach to improve cultural medium for the production of fungal secondary metabolites. Chemom. Intell. Lab. Syst. 2018, 176, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.L.; Tanaka, H. Yellow Pigments of Aspergillus niger and Aspergillus awamori: Part II. Chemical Structure of Aurasperone A. Agric. Biol. Chem. 1966, 30, 683–687. [Google Scholar]
- Priestap, H.A. New naphthopyrones from Aspergillus fonsecaeus. Tetrahedron 1984, 40, 3617–3624. [Google Scholar] [CrossRef]
- Galmarini, O.L.; Stodola, F.H.; Raper, K.B.; Fennell, D.I. Fonsecin, a Naphthopyrone Pigment from a Mutant of Aspergillus fonsecaeus. Nature 1962, 195, 502–503. [Google Scholar] [CrossRef]
- Shibata, S.; Ogihara, Y.; Ohta, A. Metabolic Products of Fungi. XXII. On Ustilaginoidins. (2). The Structure of Ustilaginoidin A. Chem. Pharm. Bull. (Tokyo) 1963, 11, 1179–1182. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, S.I.; Sugita, M.; Yoshimura, A.; Sumizawa, T.; Douzono, H.; Nagata, Y.; Akiyama, S.I. Aspergillus species strain m39 produces two naphtho-γ-pyrones that reverse drug resistance in human KB cells. Int. J. Cancer 1990, 45, 508–513. [Google Scholar] [CrossRef]
- Akiyama, K.; Teraguchi, S.; Hamasaki, Y.; Mori, M.; Tatsumi, K.; Ohnishi, K.; Hayashi, H. New Dimeric Naphthopyrones from Aspergillus niger. J. Nat. Prod. 2003, 66, 136–139. [Google Scholar] [CrossRef]
- Huang, H.B.; Xiao, Z.E.; Feng, X.J.; Huang, C.H.; Zhu, X.; Ju, J.H.; Li, M.F.; Lin, Y.C.; Liu, L.; She, Z.G. Cytotoxic Naphtho-γ-pyrones from the Mangrove Endophytic Fungus Aspergillus tubingensis (GX1-5E). Helv. Chim. Acta 2011, 94, 1732–1740. [Google Scholar] [CrossRef]
- Koyama, K.; Natori, S.; Iitaka, Y. Absolute configurations of chaetochromin A and related bis(naphtho-gamma-pyrone) mold metabolites. Chem. Pharm. Bull. (Tokyo) 1987, 35, 4049–4055. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.; Sun, W.; Meng, J.; Wang, A.; Wang, X.; Tian, J.; Fu, X.; Dai, J.; Liu, Y.; Lai, D.; et al. Bioactive Bis-naphtho-γ-pyrones from Rice False Smut Pathogen Ustilaginoidea virens. J. Agric. Food Chem. 2015, 63, 3501–3508. [Google Scholar] [CrossRef]
- Taniwaki, M.H.; Pitt, J.I.; Iamanaka, B.T.; Sartori, D.; Copetti, M.V.; Balajee, A.; Fungaro, M.H.P.; Frisvad, J.C. Aspergillus bertholletius sp. nov. from Brazil Nuts. PLoS ONE 2012, 7, e42480. [Google Scholar] [CrossRef] [Green Version]
- Frisvad, J.C.; Møller, L.L.H.; Larsen, T.O.; Kumar, R.; Arnau, J. Safety of the fungal workhorses of industrial biotechnology: Update on the mycotoxin and secondary metabolite potential of Aspergillus niger, Aspergillus oryzae, and Trichoderma reesei. Appl. Microbiol. Biotechnol. 2018, 102, 9481–9515. [Google Scholar] [CrossRef] [Green Version]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Amić, D.; Davidović-Amić, D.; Bešlo, D.; Trinajstić, N. Structure-radical scavenging activity relationships of flavonoids. Croat. Chem. Acta 2003, 76, 55–61. [Google Scholar]
- Foo, S.C.; Yusoff, F.M.; Ismail, M.; Basri, M.; Yau, S.K.; Khong, N.M.H.; Chan, K.W.; Ebrahimi, M. Antioxidant capacities of fucoxanthin-producing algae as influenced by their carotenoid and phenolic contents. J. Biotechnol. 2017, 241, 175–183. [Google Scholar] [CrossRef]
- Paudel, P.; Jung, H.A.; Choi, J.S. Anthraquinone and naphthopyrone glycosides from Cassia obtusifolia seeds mediate hepatoprotection via Nrf2-mediated HO-1 activation and MAPK modulation. Arch. Pharm. Res. 2018, 41, 677–689. [Google Scholar] [CrossRef]
- Rona, C.; Vailati, F.; Berardesca, E. The cosmetic treatment of wrinkles. J. Cosmet. Dermatol. 2004, 3, 26–34. [Google Scholar] [CrossRef]
- Ratz-Lyko, A.; Arct, J.; Pytkowska, K. Methods for evaluation of cosmetic antioxidant capacity. Skin Res. Technol. 2012, 18, 421–430. [Google Scholar] [CrossRef]
- Zhang, H.; Yin, M.; Huang, L.; Wang, J.; Gong, L.; Liu, J.; Sun, B. Evaluation of the Cellular and Animal Models for the Study of Antioxidant Activity: A Review. J. Food Sci. 2017, 82, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Beckman, K.B.; Ames, B.N. The Free Radical Theory of Aging Matures. Physiol. Rev. 1998, 78, 547–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toyokuni, S. Reactive oxygen species-induced molecular damage and its application in pathology. Pathol. Int. 1999, 49, 91–102. [Google Scholar] [CrossRef]
- de Haan, J.B.; Bladier, C.; Lotfi-Miri, M.; Taylor, J.; Hutchinson, P.; Crack, P.J.; Hertzog, P.; Kola, I. Fibroblasts derived from Gpx1 knockout mice display senescent-like features and are susceptible to H2O2-mediated cell death. Free Radic. Biol. Med. 2004, 36, 53–64. [Google Scholar] [CrossRef]
- Zdolsek, J.; Zhang, H.; Roberg, K.; Brunk, U.; Sies, H. H2O2-Mediated Damage to Lysosomal Membranes of J-774 Cells. Free Radic. Res. Commun. 1993, 18, 71–85. [Google Scholar] [CrossRef]
- Festa, F.; Aglitti, T.; Duranti, G.; Ricordy, R.; Perticone, P.; Cozzi, R. Strong antioxidant activity of ellagic acid in mammalian cells in vitro revealed by the comet assay. Anticancer Res. 2001, 21, 3903–3908. [Google Scholar]
- Lopez de Leon, L.; Caceres, I.; Bornot, J.; Choque, E.; Raynal, J.; Taillandier, P.; Mathieu, F. Influence of the culture conditions on the production of NGPs by Aspergillus tubingensis. J. Microbiol. Biotechnol. 2019, 29, 1412–1423. [Google Scholar] [CrossRef] [Green Version]
| Compounds | Number of OH Per NγP Unit | ABTS | AOA DPPH Essay | |||
|---|---|---|---|---|---|---|
| TEAC (µmol L−1), Tested Concentration: 10 µmol L−1 (in This Work) | IC50 (µmol L−1) [11] | Radical Scavenging (%), Tested Concentration: 50 µg L−1 [13] | [12] | Radical Scavenging (%), Tested Concentration: 250,000 µg L−1 [10] | ||
| 6,9-Dibromoflavasperone | 1 | 21.0 | ||||
| Ascorbic acid | 13.23 ± 0.05 a | 20.0 | 90.5 | |||
| Asperpyrone A | 1 | N | ||||
| Asperpyrones B | 1 | 31.9 | ||||
| Asperpyrone C | 1 | N | 32.9 | |||
| Asperpyrone E | 1 | 3.29 ± 0.11 c | ||||
| Asperpyrone F | 1.5 | 32.8 | ||||
| Aurasperone A | 1 | 2.4 ± 0.20 d | N | N | 38.6 | |
| Aurasperone B | 2 | 0.01 | 48.1 | |||
| Aurasperone E | 1 | 33.4 | ||||
| BHT | 80.4 | |||||
| Dianhydroaurasperone C | 1.5 | 4.57 ± 0.36 b,c | N | |||
| Flavasperone | 1 | 25.0 | N | |||
| Fonsecin | 3 | 13.32 ± 0.08 a | 0.02 | |||
| Fonsecin B | 2 | 5.12 ± 0.08 b | ||||
| Fonsecinone A | 1 | 2.36 ± 0.07 d | N | 36.9 | ||
| Fonsecinones B | 1.5 | 13.7 | 38.7 | |||
| Fonsecinone C | 1.5 | N | ||||
| Fonsecinone D | 1.5 | 37.5 | N | |||
| Nigerone A | 2 | N | ||||
| Nigerone B | 2 | N | ||||
| Nigerone C | 2 | 41.6 | ||||
| Rubrofusarin B | 1 | N | ||||
| TMC-256A1 | 2 | 0.30 | ||||
| Ustilaginoidin A | 3 | 14.59 ± 1.75 a | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carboué, Q.; Maresca, M.; Herbette, G.; Roussos, S.; Hamrouni, R.; Bombarda, I. Naphtho-Gamma-Pyrones Produced by Aspergillus tubingensis G131: New Source of Natural Nontoxic Antioxidants. Biomolecules 2020, 10, 29. https://doi.org/10.3390/biom10010029
Carboué Q, Maresca M, Herbette G, Roussos S, Hamrouni R, Bombarda I. Naphtho-Gamma-Pyrones Produced by Aspergillus tubingensis G131: New Source of Natural Nontoxic Antioxidants. Biomolecules. 2020; 10(1):29. https://doi.org/10.3390/biom10010029
Chicago/Turabian StyleCarboué, Quentin, Marc Maresca, Gaëtan Herbette, Sevastianos Roussos, Rayhane Hamrouni, and Isabelle Bombarda. 2020. "Naphtho-Gamma-Pyrones Produced by Aspergillus tubingensis G131: New Source of Natural Nontoxic Antioxidants" Biomolecules 10, no. 1: 29. https://doi.org/10.3390/biom10010029






