The Biological Function of Extracellular Vesicles during Fertilization, Early Embryo—Maternal Crosstalk and Their Involvement in Reproduction: Review and Overview
Abstract
:1. Introduction
2. Extracellular Vesicles and Biogenesis
3. Methods for EV Isolation and Characterization
4. Extracellular Vesicle Composition and their Molecular Cargo Function
5. Extracellular Vesicle in Animal Reproduction and Embryo-Maternal Cross-Talk
6. Extracellular Vesicle Molecular Cargo in Animal Reproduction and Embryo-Maternal Cross-Talk
7. Extracellular Vesicles as Biomarkers in Reproductive Medicine
7.1. Biomarkers for Female Reproductive Cancer
7.2. Biomarkers for Female Fertility
7.3. Biomarkers for Embryo Quality
7.4. Biomarkers for Placenta Quality
7.5. Biomarker for Early Abortion
8. EVs and Therapeutic Action
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ng, Y.H.; Rome, S.; Jalabert, A.; Forterre, A.; Singh, H.; Hincks, C.L.; Salamonsen, L.A. Endometrial exosomes/microvesicles in the uterine microenvironment: A new paradigm for embryo-endometrial cross talk at implantation. PLoS ONE 2013, 8, e58502. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Greening, D.W.; Zhu, H.J.; Takahashi, N.; Simpson, R.J. Extracellular vesicle isolation and characterization: Toward clinical application. J. Clin. Invest. 2016, 126, 1152–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Andronico, F.; Battaglia, R.; Ragusa, M.; Barbagallo, D.; Purrello, M.; Di Pietro, C. Extracellular vesicles in human oogenesis and implantation. Int. J. Mol. Sci. 2019, 20, 2162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almiñana, C.; Bauersachs, S. Extracellular vesicles: Multi-signal messengers in the gametes/embryo-oviduct cross-talk. Theriogenology 2020, 150, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Bridi, A.; Perecin, F.; Silveira, J.C.D. Extracellular vesicles mediated early embryo-maternal interactions. Int. J. Mol. Sci. 2020, 21, 1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurung, S.; Greening, D.W.; Catt, S.; Salamonsen, L.; Evans, J. Exosomes and soluble secretome from hormone-treated endometrial epithelial cells direct embryo implantation. Mol. Hum. Reprod. 2020, 26, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Vilella, F.; Moreno-Moya, J.M.; Balaguer, N.; Grasso, A.; Herrero, M.; Martinez, S.; Marcilla, A.; Simon, C. Hsa-miR-30d, secreted by the human endometrium, is taken up by the pre-implantation embryo and might modify its transcriptome. Development 2015, 142, 3210–3221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balaguer, N.; Moreno, I.; Herrero, M.; González, M.; Simón, C.; Vilella, F. Heterogeneous nuclear ribonucleoprotein C1 may control miR-30d levels in endometrial exosomes affecting early embryo implantation. Mol. Hum. Reprod. 2018, 24, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Greening, D.W.; Nguyen, H.P.; Elgass, K.; Simpson, R.J.; Salamonsen, L.A. Human endometrial exosomes contain hormone-specific cargo modulating trophoblast adhesive capacity: Insights into endometrial-embryo interactions. Biol. Reprod. 2016, 94, 38. [Google Scholar] [CrossRef]
- Evans, J.; Rai, A.; Nguyen, H.P.T.; Poh, Q.H.; Elglass, K.; Simpson, R.J.; Salamonsen, L.A.; Greening, D.W. Human endometrial extracellular vesicles functionally prepare human trophectoderm model for implantation: Understanding bidirectional maternal-embryo communication. Proteomics 2019, 19, e1800423. [Google Scholar] [CrossRef]
- Simon, C.; Greening, D.W.; Bolumar, D.; Balaguer, N.; Salamonsen, L.A.; Vilella, F. Extracellular vesicles in human reproduction in health and disease. Endocr. Rev. 2018, 39, 292–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnett, L.A.; Nowak, R.A. Exosomes mediate embryo and maternal interactions at implantation and during pregnancy. Front. Biosci. (Schol Ed.) 2016, 8, 79–96. [Google Scholar] [CrossRef] [Green Version]
- Crawford, N. The presence of contractile proteins in platelet microparticles isolated from human and animal platelet-free plasma. Br. J. Haematol. 1971, 21, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Stegmayr, B.; Ronquist, G. Promotive effect on human sperm progressive motility by prostasomes. Urol. Res. 1982, 10, 253–257. [Google Scholar] [CrossRef]
- Trams, E.G.; Lauter, C.J.; Salem, N., Jr.; Heine, U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim. Biophys. Acta 1981, 645, 63–70. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Hammond, J.R.; Turbide, C. Vesicle formation during reticulocyte maturation. association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar]
- Makarova, K.S.; Yutin, N.; Bell, S.D.; Koonin, E.V. Evolution of diverse cell division and vesicle formation systems in Archaea. Nat. Rev. Microbiol. 2010, 8, 731–741. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Lee, J.; Park, J.; Gho, Y.S. Gram-negative and Gram-positive bacterial extracellular vesicles. Semin. Cell Dev. Biol. 2015, 40, 97–104. [Google Scholar] [CrossRef]
- Oliveira, D.L.; Nakayasu, E.S.; Joffe, L.S.; Guimarães, A.J.; Sobreira, T.J.; Nosanchuk, J.D.; Cordero, R.J.; Frases, S.; Casadevall, A.; Almeida, I.C.; et al. Characterization of yeast extracellular vesicles: Evidence for the participation of different pathways of cellular traffic in vesicle biogenesis. PLoS ONE 2010, 5, e11113. [Google Scholar] [CrossRef] [Green Version]
- Harding, C.; Heuser, J.; Stahl, P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 1983, 97, 329–339. [Google Scholar] [CrossRef]
- Pan, B.T.; Teng, K.; Wu, C.; Adam, M.; Johnstone, R.M. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J. Cell Biol. 1985, 101, 942–948. [Google Scholar] [CrossRef] [Green Version]
- McConnell, R.E.; Higginbotham, J.N.; Shifrin, D.A., Jr; Tabb, D.L.; Coffey, R.J.; Tyska, M.J. The enterocyte microvillus is a vesicle-generating organelle. J. Cell Biol. 2009, 185, 1285–1298. [Google Scholar] [CrossRef] [Green Version]
- Muralidharan-Chari, V.; Clancy, J.; Plou, C.; Romao, M.; Chavrier, P.; Raposo, G.; D’Souza-Schorey, C. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr. Biol. 2009, 19, 1875–1885. [Google Scholar] [CrossRef] [Green Version]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of extracellular vesicles: General methodologies and latest trends. Biomed. Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef]
- Böing, A.N.; van der Pol, E.; Grootemaat, A.E.; Coumans, F.A.; Sturk, A.; Nieuwland, R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles 2014, 3, 23430. [Google Scholar] [CrossRef] [PubMed]
- Deregibus, M.C.; Figliolini, F.; D’Antico, S.; Manzini, P.M.; Pasquino, C.; De Lena, M.; Tetta, C.; Brizzi, M.F.; Camussi, G. Charge-based precipitation of extracellular vesicles. Int. J. Mol. Med. 2016, 38, 1359–1366. [Google Scholar] [CrossRef] [Green Version]
- Kenigsberg, S.; Wyse, B.A.; Librach, C.L.; da Silveira, J. Protocol for exosome isolation from small volume of ovarian follicular fluid: Evaluation of ultracentrifugation and commercial kits. Methods Mol. Biol. 2017, 1660, 321–341. [Google Scholar] [CrossRef]
- Brennan, K.; Martin, K.; FitzGerald, S.P.; O’Sullivan, J.; Wu, Y.; Blanco, A.; Richardson, C.; Mc Gee, M.M. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci. Rep. 2020, 10, 1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakai, W.; Yoshida, T.; Diez, D.; Miyatake, Y.; Nishibu, T.; Imawaka, N.; Naruse, K.; Sadamura, Y.; Hanayama, R. A novel affinity-based method for the isolation of highly purified extracellular vesicles. Sci. Rep. 2016, 6, 33935. [Google Scholar] [CrossRef] [Green Version]
- Sidhom, K.; Obi, P.O.; Saleem, A. A Review of exosomal isolation methods: Is size exclusion chromatography the best option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lyden, D. Asymmetric-flow field-flow fractionation technology for exomere and small extracellular vesicle separation and characterization. Nat. Protoc. 2019, 14, 1027–1053. [Google Scholar] [CrossRef]
- Zhang, Q.; Higginbotham, J.N.; Jeppesen, D.K.; Yang, Y.P.; Li, W.; McKinley, E.T.; Graves-Deal, R.; Ping, J.; Britain, C.M.; Dorsett, K.A.; et al. Transfer of functional cargo in exomeres. Cell Rep. 2019, 27, 940–954. [Google Scholar] [CrossRef] [Green Version]
- Witwer, K.W.; Buzás, E.I.; Bemis, L.T.; Bora, A.; Lässer, C.; Lötvall, J.; Nolte-’t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J.; et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2013, 2. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Deng, W.; Klinke, D.J., 2nd. Exosomes: Improved methods to characterize their morphology, RNA content, and surface protein biomarkers. Nanoparticle Tracking Analysis (NTA) is commonly used to determine EV concentration and diameter. Analyst 2015, 140, 6631–6642. [Google Scholar] [CrossRef] [Green Version]
- Cizmar, P.; Yuana, Y. Detection and characterization of extracellular vesicles by transmission and cryo-transmission electron microscopy. Methods Mol. Biol. 2017, 1660, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Szatanek, R.; Baj-Krzyworzeka, M.; Zimoch, J.; Lekka, M.; Siedlar, M.; Baran, J. The Methods of choice for extracellular vesicles (EVs) characterization. Int. J. Mol. Sci. 2017, 18, 1153. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Ng, W.B.; Bernt, W.; Cho, N.J. Validation of size estimation of nanoparticle tracking analysis on polydisperse macromolecule assembly. Sci. Rep. 2019, 9, 2639. [Google Scholar] [CrossRef] [Green Version]
- Noble, J.M.; Roberts, L.M.; Vidavsky, N.; Chiou, A.E.; Fischbach, C.; Paszek, M.J.; Estroff, L.A.; Kourkoutis, L.F. Direct comparison of optical and electron microscopy methods for structural characterization of extracellular vesicles. J. Struct. Biol. 2020, 210, 107474. [Google Scholar] [CrossRef]
- Menck, K.; Bleckmann, A.; Schulz, M.; Ries, L.; Binder, C. Isolation and characterization of microvesicles from peripheral blood. J. Vis. Exp. 2017, 119, 55057. [Google Scholar] [CrossRef] [Green Version]
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New technologies for analysis of extracellular vesicles. Chem. Rev. 2018, 118, 1917–1950. [Google Scholar] [CrossRef]
- Greening, D.W.; Simpson, R.J. Understanding extracellular vesicle diversity - current status. Expert Rev. Proteom. 2018, 15, 887–910. [Google Scholar] [CrossRef]
- Claridge, B.; Kastaniegaard, K.; Stensballe, A.; Greening, D.W. Post-translational and transcriptional dynamics—Regulating extracellular vesicle biology. Expert Rev. Proteom. 2019, 16, 17–31. [Google Scholar] [CrossRef]
- Kreimer, S.; Belov, A.M.; Ghiran, I.; Murthy, S.K.; Frank, D.A.; Ivanov, A.R. Mass-spectrometry-based molecular characterization of extracellular vesicles: Lipidomics and proteomics. J. Proteome Res. 2015, 14, 2367–2384. [Google Scholar] [CrossRef]
- Haraszti, R.A.; Didiot, M.C.; Sapp, E.; Leszyk, J.; Shaffer, S.A.; Rockwell, H.E.; Gao, F.; Narain, N.R.; DiFiglia, M.; Kiebish, M.A.; et al. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J. Extracell. Vesicles 2016, 5, 32570. [Google Scholar] [CrossRef]
- Record, M.; Silvente-Poirot, S.; Poirot, M.; Wakelam, M.J.O. Extracellular vesicles: Lipids as key components of their biogenesis and functions. J. Lipid Res. 2018, 59, 1316–1324. [Google Scholar] [CrossRef] [Green Version]
- Vagner, T.; Chin, A.; Mariscal, J.; Bannykh, S.; Engman, D.M.; Di Vizio, D. Protein composition reflects extracellular vesicle heterogeneity. Proteomics 2019, 19, e1800167. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Desrochers, L.M.; Bordeleau, F.; Reinhart-King, C.A.; Cerione, R.A.; Antonyak, M.A. Microvesicles provide a mechanism for intercellular communication by embryonic stem cells during embryo implantation. Nat. Commun. 2016, 7, 11958. [Google Scholar] [CrossRef] [Green Version]
- Collino, F.; Deregibus, M.C.; Bruno, S.; Sterpone, L.; Aghemo, G.; Viltono, L.; Tetta, C.; Camussi, G. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS ONE 2010, 5, e11803. [Google Scholar] [CrossRef]
- Lange-Consiglio, A.; Lazzari, B.; Perrini, C.; Pizzi, F.; Stella, A.; Cremonesi, F.; Capra, E. MicroRNAs of Equine amniotic mesenchymal cell-derived microvesicles and their involvement in anti-inflammatory processes. Cell Transpl. 2018, 27, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Donker, R.B.; Mouillet, J.F.; Chu, T.; Hubel, C.A.; Stolz, D.B.; Morelli, A.E.; Sadovsky, Y. The expression profile of C19MC microRNAs in primary human trophoblast cells and exosomes. Mol. Hum. Reprod. 2012, 18, 417–424. [Google Scholar] [CrossRef] [Green Version]
- Tosar, J.P.; Gambaro, F.; Sanguinetti, J.; Bonilla, B.; Witwer, K.W.; Cayota, A. Assessment of small RNA sorting into different extracellular fractions revealed by high-throughput sequencing of breast cell lines. Nucleic Acids Res. 2015, 43, 5601–5616. [Google Scholar] [CrossRef] [Green Version]
- Mateescu, B.; Kowal, E.J.; van Balkom, B.W.; Bartel, S.; Bhattacharyya, S.N.; Buzás, E.I.; Buck, A.H.; de Candia, P.; Chow, F.W.; Das, S.; et al. Obstacles and opportunities in the functional analysis of extracellular vesicle RNA—An ISEV position paper. J. Extracell. Vesicles 2017, 6, 1286095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chevillet, J.R.; Kang, Q.; Ruf, I.K.; Briggs, H.A.; Vojtech, L.N.; Hughes, S.M.; Cheng, H.H.; Arroyo, J.D.; Meredith, E.K.; Gallichotte, E.N.; et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc. Natl. Acad. Sci. USA 2014, 111, 14888–14893. [Google Scholar] [CrossRef] [Green Version]
- Lange-Consiglio, A.; Lazzari, B.; Pizzi, F.; Stella, A.; Girani, A.; Quintè, A.; Cremonesi, F.; Capra, E. different culture times affect microRNA cargo in equine amniotic mesenchymal cells and their microvesicles. Tissue Eng, Part C Methods 2018, 24, 596–604. [Google Scholar] [CrossRef]
- Willms, E.; Cabañas, C.; Mäger, I.; Wood, M.J.A.; Vader, P. Extracellular vesicle heterogeneity: Subpopulations.; isolation techniques.; and diverse functions in cancer progression. Front. Immunol. 2018, 9, 738. [Google Scholar] [CrossRef] [Green Version]
- Margolis, L.; Sadovsky, Y. The biology of extracellular vesicles: The known unknowns. PLoS Biol. 2019, 17, e3000363. [Google Scholar] [CrossRef]
- Buzás, E.I.; Tóth, E.Á.; Sódar, B.W.; Szabó-Taylor, K.É. Molecular interactions at the surface of extracellular vesicles. Semin. Immunopathol. 2018, 40, 453–464. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Yan, J.; Shen, X.; Sun, Y.; Thulin, M.; Cai, Y.; Wik, L.; Shen, Q.; Oelrich, J.; Qian, X.; et al. Profiling surface proteins on individual exosomes using a proximity barcoding assay. Nat. Commun. 2019, 10, 3854. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3. [Google Scholar] [CrossRef] [Green Version]
- Horibe, S.; Tanahashi, T.; Kawauchi, S.; Murakami, Y.; Rikitake, Y. Mechanism of recipient cell-dependent differences in exosome uptake. BMC Cancer 2018, 18, 47. [Google Scholar] [CrossRef] [Green Version]
- Kanada, M.; Bachmann, M.H.; Hardy, J.W.; Frimannson, D.O.; Bronsart, L.; Wang, A.; Sylvester, M.D.; Schmidt, T.L.; Kaspar, R.L.; Butte, M.J.; et al. Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc. Natl. Acad. Sci. USA 2015, 112, E1433–E1442. [Google Scholar] [CrossRef] [Green Version]
- Ono, R.; Yasuhiko, Y.; Aisaki, K.I.; Kitajima, S.; Kanno, J.; Hirabayashi, Y. Exosome-mediated horizontal gene transfer occurs in double-strand break repair during genome editing. Commun. Biol. 2019, 2, 57. [Google Scholar] [CrossRef]
- Kahlert, C.; Melo, S.A.; Protopopov, A.; Tang, J.; Seth, S.; Koch, M.; Zhang, J.; Weitz, J.; Chin, L.; Futreal, A.; et al. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. Biol. Chem. 2014, 289, 3869–3875. [Google Scholar] [CrossRef] [Green Version]
- Thakur, B.K.; Zhang, H.; Becker, A.; Matei, I.; Huang, Y.; Costa-Silva, B.; Zheng, Y.; Hoshino, A.; Brazier, H.; Xiang, J.; et al. Double-stranded DNA in exosomes: A novel biomarker in cancer detection. Cell Res. 2014, 24, 766–769. [Google Scholar] [CrossRef] [Green Version]
- Bai, R.; Latifi, Z.; Kusama, K.; Nakamura, K.; Shimada, M.; Imakawa, K. Induction of immune-related gene expression by seminal exosomes in the porcine endometrium. Biochem. Biophys. Res. Commun. 2018, 495, 1094–1101. [Google Scholar] [CrossRef]
- Al-Dossary, A.A.; Strehler, E.E.; Martin-Deleon, P.A. Expression and secretion of plasma membrane Ca2þ-ATPase 4a (PMCA4a) during murine estrus: Association with oviductal exosomes and uptake in sperm. PLoS ONE 2013, 8, e80181. [Google Scholar] [CrossRef] [Green Version]
- Bathala, P.; Fereshteh, Z.; Li, K.; Al-Dossary, A.A.; Galileo, D.S.; Martin-DeLeon, P.A. Oviductal extracellular vesicles (oviductosomes, OVS) are conserved in humans: Murine OVS play a pivotal role in sperm capacitation and fertility. Mol. Hum. Reprod. 2018, 24, 143–157. [Google Scholar] [CrossRef]
- Ferraz, M.d.A.M.M.; Carothers, A.; Dahal, R.; Noonan, M.J.; Songsasen, N. Oviductal extracellular vesicles interact with the spermatozoon’s head and mid-piece and improves its motility and fertilizing ability in the domestic cat. Sci. Rep. 2019, 9, 9484. [Google Scholar] [CrossRef]
- Franchi, A.; Moreno-Irusta, A.; Domínguez, E.M.; Adre, A.J.; Giojalas, L.C. Extracellular vesicles from oviductal isthmus and ampulla stimulate the induced acrosome reaction and signaling events associated with capacitation in bovine spermatozoa. J. Cell Biochem. 2020, 121, 2877–2888. [Google Scholar] [CrossRef]
- Almiñana, C.; Corbin, E.; Tsikis, G.; Alcântara-Neto, A.S.; Labas, V.; Reynaud, K.; Galio, L.; Uzbekov, R.; Garanina, A.S.; Druart, X.; et al. Oviduct extracellular vesicles protein content and their role during oviduct–embryo cross-talk. Reproduction 2017, 154, 253–268. [Google Scholar] [CrossRef]
- Lopera-Vasquez, R.; Hamdi, M.; Maillo, V.; Gutierrez-Adan, A.; Bermejo-Alvarez, P.; Ramírez, M.Á.; Yáñez-Mó, M.; Rizos, D. Effect of bovine oviductal extracellular vesicles on embryo development and quality in vitro. Reproduction 2017, 153, 461–470. [Google Scholar] [CrossRef] [Green Version]
- da Silveira, J.C.; Andrade, G.M.; Del Collado, M.; Sampaio, R.V.; Sangalli, J.R.; Silval, L.A.; Pinaffi, F.V.L.; Jardim, I.B.; Cesar, M.C.; Coutinho, L.L.; et al. Supplementation with small-extracellular vesicles from ovarian follicular fluid during in vitro production modulates bovine embryo development. PLoS ONE 2017, 12, e0179451. [Google Scholar] [CrossRef]
- Bidarimath, M.; Khalaj, K.; Kridli, R.T.; Kan, F.W.K.; Koti, M.; Tayade, C. Extracellular vesicle mediated intercellular communication at the porcine maternal-fetal interface: A new paradigm for conceptus-endometrial cross-talk. Sci. Rep. 2017, 7, 40476. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Kusama, K.; Bai, R.; Sakurai, T.; Isuzugawa, K.; Godkin, J.D.; Suda, Y.; Imakawa, K. Induction of IFNT-stimulated genes by conceptus-derived exosomes during the attachment period. PLoS ONE 2016, 11, e0158278. [Google Scholar] [CrossRef] [Green Version]
- Salomon, C.; Kobayashi, M.; Ashman, K.; Sobrevia, L.; Murray, M.D.; Rice, G.E. Hypoxia-induced changes in the bioactivity of cytotrophoblast-derived exosomes. PLoS ONE 2013, 8, e79636. [Google Scholar] [CrossRef] [Green Version]
- Kusama, K.; Nakamura, K.; Bai, R.; Nagaoka, K.; Sakurai, T.; Imakawa, K. Intrauterine exosomes are required for bovine conceptus implantation. Biochem. Biophys. Res. Commun. 2018, 495, 1370–1375. [Google Scholar] [CrossRef]
- Wang, X.; Tian, F.; Chen, C.; Feng, Y.; Sheng, X.; Guo, Y.; Ni, H. Exosome-derived uterine microRNAs isolated from cows with endometritis impede blastocyst development. Reprod. Biol. 2019, 19, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Riou, C.; Brionne, A.; Cordeiro, L.; Harichaux, G.; Gargaros, A.; Labas, V.; Gautron, J.; Gérard, N. Avian uterine fluid proteome: Exosomes and biological processes potentially involved in sperm survival. Mol. Reprod. Dev. 2020, 87, 454–470. [Google Scholar] [CrossRef]
- Pons-Rejraji, H.; Artonne, C.; Sion, B.; Brugnon, F.; Canis, M.; Janny, L.; Grizard, G. Prostasomes: Inhibitors of capacitation and modulators of cellular signaling in human sperm. Int. J. Androl. 2011, 34, 568–580. [Google Scholar] [CrossRef]
- Piehl, L.L.; Fischman, M.L.; Hellman, U.; Cisale, H.; Miranda, P.V. Boar seminal plasma exosomes: Effect on sperm function and protein identification by sequencing. Theriogenology 2013, 79, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Da Silveira, J.C.; Veeramachaneni, D.N.R.; Winger, Q.A.; Carnevale, E.M.; Bouma, G.J. Cell-secreted vesicles in equine ovarian follicular fluid contain mirnas and proteins: A possible new form of cell communication within the ovarian follicle. Biol. Reprod 2012, 86, 71. [Google Scholar] [CrossRef] [PubMed]
- Sang, Q.; Yao, Z.; Wang, H.; Feng, R.; Wang, H.; Zhao, X.; Xing, Q.; Jin, L.; He, L.; Wu, L.; et al. Identification of microRNAs in human follicular fluid: Characterization of microRNAs that govern steroidogenesis in vitro and are associated with polycystic ovary syndrome in vivo. J. Clin. Endocrinol. Metab. 2013, 98, 3068–3079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, G.; Meirelles, F.; Perecin, F.; da Silveira, J. Cellular and extracellular vesicular origins of miRNAs within the bovine ovarian follicle. Reprod. Domest. Anim. 2017, 52, 1036–1045. [Google Scholar] [CrossRef]
- Spitschak, M.; Hoeflich, A. Potential functions of IGFBP-2 for ovarian folliculogenesis and steroidogenesis. Front. Endocrinol. 2018, 9, 119. [Google Scholar] [CrossRef] [Green Version]
- Bourdiec, A.; Akoum, A. Embryo implantation: Role of interleukin 1 family members. Med. Sci. (Paris) 2014, 30, 644–650. [Google Scholar] [CrossRef] [Green Version]
- Bourdiec, A.; Shao, R.; Rao, C.V.; Akoum, A. Human chorionic gonadotropin triggers angiogenesis via the modulation of endometrial stromal cell responsiveness to interleukin 1: A new possible mechanism underlying embryo implantation. Biol. Reprod. 2012, 87, 6. [Google Scholar] [CrossRef]
- Stavreus-Evers, A.; Koraen, L.; Scott, J.E.; Zhang, P.; Westlund, P. Distribution of cyclooxygenase-1, cyclooxygenase-2, and cytosolic phospholipase A2 in the luteal phase human endometrium and ovary. Fertil. Steril. 2005, 83, 156–162. [Google Scholar] [CrossRef]
- Jessmon, P.; Leach, R.E.; Armant, D.R. Diverse functions of HBEGF during pregnancy. Mol. Reprod. Dev. 2009, 76, 1116–1127. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Shi, G.; Li, M.; Fan, H.; Ma, H.; Sheng, L. Correlation of IL-1 and HB-EGF with endometrial receptivity. Exp. Ther. Med. 2018, 16, 5130–5136. [Google Scholar] [CrossRef] [Green Version]
- Merviel, P.; Challier, J.C.; Carbillon, L.; Foidart, J.M.; Uzan, S. The role of integrins in human embryo implantation. Fetal Diagn, Ther. 2001, 16, 364–371. [Google Scholar] [CrossRef]
- Salleh, N.; Giribabu, N. Leukemia inhibitory factor: Roles in embryo implantation and in nonhormonal contraception. Sci. World J. 2014, 2014, 201514. [Google Scholar] [CrossRef] [Green Version]
- Mardpour, S.; Hamidieh, A.A.; Taleahmad, S.; Sharifzad, F.; Taghikhani, A.; Baharvand, H. Interaction between mesenchymal stromal cell-derived extracellular vesicles and immune cells by distinct protein content. J. Cell Physiol. 2019, 234, 8249. [Google Scholar] [CrossRef]
- Burns, G.W.; Brooks, K.E.; Spencer, T.E. Extracellular vesicles originate from the conceptus and uterus during early pregnancy in sheep. Biol. Reprod. 2016, 94, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kshirsagar, S.K.; Alam, S.M.; Jasti, S.; Hodes, H.; Nauser, T.; Gilliam, M.; Billstrand, C.; Hunt, J.S.; Petroff, M.G. Immunomodulatory molecules are released from the first trimester and term placenta via exosomes. Placenta 2012, 33, 982–990. [Google Scholar] [CrossRef] [Green Version]
- Mincheva-Nilsson, L.; Baranov, V. Placenta-derived exosomes and syncytiotrophoblast microparticles and their role in human reproduction: Immune modulation for pregnancy success. Am. J. Reprod Immunol. 2014, 72, 440–457. [Google Scholar] [CrossRef]
- Giacomini, E.; Alleva, E.; Fornelli, G.; Quartucci, A.; Privitera, L.; Vanni, V.S.; Viganò, P. Embryonic extracellular vesicles as informers to the immune cells at the maternal-fetal interface. Clin. Exp. Immunol. 2019, 198, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Lokossou, A.G.; Toudic, C.; Nguyen, P.T.; Elissee, X.; Vargas, A.; Rassart, É.; Lafond, J.; Leduc, L.; Bourgault, S.; Gilbert, C.; et al. Endogenous retrovirus-encoded syncytin-2 contributes to exosome-mediated immunosuppression of t cells. Biol. Reprod. 2020, 102, 185–198. [Google Scholar] [CrossRef]
- Pillay, P.; Moodley, K.; Moodley, J.; Mackraj, I. Placenta-derived exosomes: Potential biomarkers of preeclampsia. Int. J. Nanomed. 2017, 12, 8009–8023. [Google Scholar] [CrossRef] [Green Version]
- Chang, X.; Yao, J.; He, Q.; Liu, M.; Duan, T.; Wang, K. Exosomes from women with preeclampsia induced vascular dysfunction by delivering sFlt (Soluble Fms-Like Tyrosine Kinase)-1 and sEng (Soluble Endoglin) to endothelial cells. Hypertension 2018, 72, 1381–1390. [Google Scholar] [CrossRef]
- Gézsi, A.; Kovács, Á.; Visnovitz, T.; Buzás, E.I. Systems Biology approaches to investigating the roles of extracellular vesicles in human diseases. Exp. Mol. Med. 2019, 51, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Burkova, E.; Grigor’eva, A.E.; Bulgakov, D.V.; Dmitrenok, P.S.; Vlassov, V.V.; Ryabchikova, E.I.; Sedykh, S.E.; Nevinsky, G.A. Extra purified exosomes from human placenta contain an unpredictable small number of different major proteins. Int. J. Mol. Sci. 2019, 20, 2434. [Google Scholar] [CrossRef] [Green Version]
- Tong, M.; Kleffmann, T.; Pradhan, S.; Johansson, C.L.; DeSousa, J.; Stone, P.R.; James, J.L.; Chen, Q.; Chamley, L.W. Proteomic characterization of macro-, micro- and nano-extracellular vesicles derived from the same first trimester placenta: Relevance for feto-maternal communication. Hum. Reprod. 2016, 31, 687–699. [Google Scholar] [CrossRef]
- Almiñana, C.; Tsikis, G.; Labas, V.; Uzbekov, R.; da Silveira, J.C.; Bauersachs, S.; Mermillod, P. Deciphering the oviductal extracellular vesicles content across the estrous cycle: Implications for the gametes-oviduct interactions and the environment of the potential embryo. BMC Genom. 2018, 19, 622. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, G.S.; Reese, K.L.; Galileo, D.S.; Martin-DeLeon, P.A. Investigating the role of murine epididymosomes and uterosomes in GPI-linked protein transfer to sperm using SPAM1 as a model. Mol. Reprod. Dev. 2008, 75, 1627–1636. [Google Scholar] [CrossRef]
- Szekeres-Bartho, J.; Šućurović, S.; Mulac-Jeričević, B. The Role of extracellular vesicles and PIBF in embryo-maternal immune-interactions. Front. Immunol. 2018, 9, 2890. [Google Scholar] [CrossRef]
- Herrera-Van Oostdam, A.S.; Salgado-Bustamante, M.; López, J.A.; Herrera-Van Oostdam, D.A.; López-Hernández, Y. Placental exosomes viewed from an ’omics’ perspective: Implications for gestational diabetes biomarkers identification. Biomark. Med. 2019, 13, 675–684. [Google Scholar] [CrossRef]
- Santonocito, M.; Vento, M.; Guglielmino, M.R.; Battaglia, R.; Wahlgren, J.; Ragusa, M.; Barbagallo, D.; Borzì, P.; Rizzari, S.; Maugeri, M.; et al. Molecular characterization of exosomes and their microRNA cargo in human follicular fluid: Bioinformatic analysis reveals that exosomal microRNAs control pathways involved in follicular maturation. Fertil. Steril. 2014, 102, 1751–1761. [Google Scholar] [CrossRef]
- de Ávila, A.C.F.C.M.; Bridi, A.; Andrade, G.M.; Del Collado, M.; Sangalli, J.R.; Nociti, R.P.; da Silva Junior, W.A.; Bastien, A.; Robert, C.; Meirelles, F.V.; et al. Estrous cycle impacts microRNA content in extracellular vesicles that modulate bovine cumulus cell transcripts during in vitro maturation. Biol. Reprod. 2020, 102, 362–375. [Google Scholar] [CrossRef]
- Fereshteh, Z.; Schmidt, S.A.; Al-Dossary, A.A.; Accerbi, M.; Arighi, C.; Cowart, J.; Song, J.L.; Green, P.J.; Choi, K.; Yoo, S.; et al. Murine Oviductosomes (OVS) microRNA profiling during the estrous cycle: Delivery of OVS-borne micro-RNAs to sperm where miR-34c-5p localizes at the centrosome. Sci. Rep. 2018, 8, 16094. [Google Scholar] [CrossRef]
- Lange-Consiglio, A.; Perrini, C.; Albini, G.; Modina, S.; Lodde, V.; Orsini, E.; Esposti, P.; Cremonesi, F. Oviductal microvesicles and their effect on in vitro maturation of canine oocytes. Reproduction 2017, 154, 167–180. [Google Scholar] [CrossRef]
- Bauersachs, S.; Mermillod, P.; Almiñana, C. The oviductal extracellular vesicles RNA cargo regulates the bovine embryonic transcriptome. Int. J. Mol. Sci. 2020, 21, 1303. [Google Scholar] [CrossRef] [Green Version]
- Qu, P.; Zhao, Y.; Wang, R.; Zhang, Y.; Li, L.; Fan, J.; Liu, E. Extracellular vesicles derived from donor oviduct fluid improved birth rates after embryo transfer in mice. Reprod. Fertil. Dev. 2019, 31, 324–332. [Google Scholar] [CrossRef] [Green Version]
- Cuman, C.; Van Sinderen, M.; Gantier, M.P.; Rainczuk, K.; Sorby, K.; Rombauts, L.; Osianlis, T.; Dimitriadis, E. Human blastocyst secreted microRNA regulate endometrial epithelial cell adhesion. EBioMedicine 2015, 2, 1528–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battaglia, R.; Palini, S.; Vento, M.E.; La Ferlita, A.; Lo Faro, M.J.; Caroppo, E.; Borzì, P.; Falzone, L.; Barbagallo, D.; Ragusa, M.; et al. Identification of extracellular vesicles and characterization of miRNA expression profiles in human blastocoel fluid. Sci. Rep. 2019, 9, 84. [Google Scholar] [CrossRef]
- Stefanski, A.L.; Martinez, N.; Peterson, L.K.; Callahan, T.J.; Treacy, E.; Luck, M.; Friend, S.F.; Hermesch, A.; Maltepe, E.; Phang, T.; et al. Murine trophoblast-derived and pregnancy-associated exosome-enriched extracellular vesicle microRNAs: Implications for placenta driven effects on maternal physiology. PLoS ONE 2019, 14, e0210675. [Google Scholar] [CrossRef] [Green Version]
- Tan, Q.; Shi, S.; Liang, J.; Zhang, X.; Cao, D.; Wang, Z. MicroRNAs in small extracellular vesicles indicate successful embryo implantation during early pregnancy. Cells 2020, 9, 645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burns, G.W.; Brooks, K.E.; O’Neil, E.V.; Hagen, D.E.; Behura, S.K.; Spencer, T.E. Progesterone effects on extracellular vesicles in the sheep uterus. Biol. Reprod. 2018, 98, 612–622. [Google Scholar] [CrossRef]
- Es-Haghi, M.; Godakumara, K.; Häling, A.; Lättekivi, F.; Lavrits, A.; Viil, J.; Andronowska, A.; Nafee, T.; James, V.; Jaakma, Ü.; et al. Specific trophoblast transcripts transferred by extracellular vesicles affect gene expression in endometrial epithelial cells and may have a role in embryo-maternal crosstalk. Cell Commun. Signal. 2019, 17, 146. [Google Scholar] [CrossRef] [Green Version]
- Simon, B.; Bolumar, D.; Amadoz, A.; Jimenez-Almazán, J.; Valbuena, D.; Vilella, F.; Moreno, I. Identification and characterization of extracellular vesicles and its DNA cargo secreted during murine embryo development. Genes 2020, 11, 203. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Muller, V.; Milde-Langosch, K.; Trillsch, F.; Pantel, K.; Schwarzenbach, H. Diagnostic and prognostic relevance of circulating exosomal miR-373, miR-200a, miR-200b and miR-200c in patients with epithelial ovarian cancer. Oncotarget 2016, 7, 16923–16935. [Google Scholar] [CrossRef] [Green Version]
- Martinez, R.M.; Hauser, R.; Liang, L.; Mansur, A.M.; Dioni, L.; Racowsky, C.; Bollati, V.; Baccarelli, A.A.; Machtinger, R. Urinary concentrations of phenols and phthalate metabolites reflect extracellular vesicle microRNA expression in follicular fluid. Environ. Int. 2019, 123, 20–28. [Google Scholar] [CrossRef]
- Parks, J.C.; McCallie, B.R.; Patton, A.L.; Al-Safi, Z.A.; Polotsky, A.J.; Griffin, D.K.; Schoolcraft, W.B.; Katz-Jaffe, M.G. The impact of infertility diagnosis on embryo-endometrial dialogue. Reproduction 2018, 155, 543–552. [Google Scholar] [CrossRef]
- Pallinger, E.; Bognar, Z.; Bodis, J.; Csabai, T.; Farkas, N.; Godony, K.; Varnagy, A.; Buzas, E.; Szekeres-Bartho, J. A simple and rapid flow cytometry-based assay to identify a competent embryo prior to embryo transfer. Sci. Rep. 2017, 7, 39927. [Google Scholar] [CrossRef]
- Vargas, A.; Zhou, S.; Ethier-Chiasson, M.; Flipo, D.; Lafond, J.; Gilbert, C.; Barbeau, B. Syncytin proteins incorporated in placenta exosomes are important for cell uptake and show variation in abundance in serum exosomes from patients with preeclampsia. FASEB J. 2014, 28, 3703–3719. [Google Scholar] [CrossRef]
- Li, H.; Ouyang, Y.; Sadovsky, E.; Parks, W.T.; Chu, T.; Sadowsky, Y. Unique microRNA signals in plasma exosomes from pregnancies complicated by preeclampsia. Hypertension 2020, 75, 762–771. [Google Scholar] [CrossRef]
- Jayabalan, N.; Lai, A.; Nair, S.; Guanzon, D.; Scholz-Romero, K.; Palma, C.; McIntyre, H.D.; Lappas, M.; Salomon, C. Quantitative proteomics by SWATH-MS suggest an association between circulating exosomes and maternal metabolic changes in gestational diabetes mellitus. Proteomics 2019, 19, e1800164. [Google Scholar] [CrossRef] [Green Version]
- Nair, S.; Jayabalan, N.; Guanzon, D.; Palma, C.; Scholz-Romero, K.; Elfeky, O.; Zuñiga, F.; Ormazabal, V.; Diaz, E.; Rice, G.E.; et al. Human placental exosomes in gestational diabetes mellitus carry a specific set of miRNAs associated with skeletal muscle insulin sensitivity. Clin. Sci. (Lond) 2018, 132, 2451–2467. [Google Scholar] [CrossRef]
- Pohler, K.G.; Green, J.A.; Moley, L.A.; Gunewardena, S.; Hung, W.T.; Payton, R.R.; Hong, X.; Christenson, L.K.; Geary, T.W.; Smith, M.F. Circulating microRNA as candidates for early embryonic viability in cattle. Mol. Reprod. Dev. 2017, 84, 731–743. [Google Scholar] [CrossRef]
- Larrea, E.; Sole, C.; Manterola, L.; Goicoechea, I.; Armesto, M.; Arestin, M.; Caffarel, M.M.; Araujo, A.M.; Araiz, M.; Fernandez-Mercado, M.; et al. New concepts in cancer biomarkers: Circulating miRNAs in liquid biopsies. Int. J. Mol. Sci. 2016, 17, 627. [Google Scholar] [CrossRef] [Green Version]
- Barger, J.F.; Rahman, M.A.; Jackson, D.; Acunzo, M.; Nana-Sinkam, S.P. Extracellular miRNAs as biomarkers in cancer. Food Chem. Toxicol. 2016, 98, 66–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Li, J.; Huang, B.; Liu, J.; Chen, X.; Chen, X.M.; Xu, Y.M.; Huang, L.F.; Wang, X.Z. Exosomes: Novel biomarkers for clinical diagnosis. Sci. World J. 2015, 2015, 657086. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable bloodbased markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, I.H.; Aguilar, H.A.; PaezPaez, J.S.; Wu, X.; Pan, L.; Wendt, M.K.; Iliuk, A.B.; Zhang, Y.; Tao, W.A. Analytical pipeline for discovery and verification of glycoproteins from plasma-derived extracellular vesicles as breast cancer biomarkers. Anal. Chem. 2018, 90, 6307–6313. [Google Scholar] [CrossRef]
- Thind, A.; Wilson, C. Exosomal miRNAs as cancer biomarkers and therapeutic targets. J. Extracell. Vesicles 2016, 5, 31292. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An endocrine society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- Machtinger, R.; Gaskins, A.J.; Racowsky, C.; Mansur, A.; Adir, M.; Baccarelli, A.A.; Calafat, A.M.; Hauser, R. Urinary concentrations of biomarkers of phthalates and phthalate alternatives and IVF outcomes. Environ. Int. 2018, 111, 23–31. [Google Scholar] [CrossRef]
- Rodgers, R.J.; Irving-Rodgers, H.F. Formation of the ovarian follicular antrum and follicular fluid. Biol. Reprod. 2010, 82, 1021–1029. [Google Scholar] [CrossRef] [Green Version]
- Zuccotti, M.; Merico, V.; Cecconi, S.; Redi, C.A.; Garagna, S. What does it take to make a developmentally competent mammalian egg? Hum. Reprod. Update 2011, 17, 525–540. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H.; Seo, Y.M.; Kim, E.Y.; Lee, S.Y.; Kwon, J.; Ko, J.J.; Lee, K.A. The miR-125 family is an important regulator of the expression and maintenance of maternal effect genes during preimplantational embryo development. Open Biol. 2016, 6, 160181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kropp, J.; Khatib, H. Characterization of microRNA in bovine in vitro culture media associated with embryo quality and development. J. Dairy Sci. 2015, 98, 6552–6563. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Liu, C.; Jiang, H.; Gao, Y.; Xu, M.; Wang, J.; Liu, S.; Fu, Y.; Sun, X.; Xu, J.; et al. Regulatory role of miRNA-375 in expression of BMP15/GDF9 receptors and its effect on proliferation and apoptosis of bovine cumulus cells. Cell. Physiol. Biochem. 2017, 41, 439–450. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, B.; Chen, H.; Xu, M.; Sun, X.; Xu, J.; Gao, Y.; Chen, C.; Jiang, H.; Zhang, J. Effects of MiR-375-BMPR2 as a key factor downstream of BMP15/GDF9 on the Smad1/5/8 and Smad2/3 signaling pathways. Cell. Physiol. Biochem. 2018, 46, 213–225. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Li, M.; Wang, Y.; Liu, Y.; Yan, C.; Pan, J.; Liu, J.; Cui, S. miR-375 mediates CRH signaling pathway in inhibiting E2 synthesis in porcine ovary. Reproduction 2017, 153, 63–73. [Google Scholar] [CrossRef] [Green Version]
- International Embryo Transfer Society. Manual of the international embryo transfer society. In IETS Manual; Stringfellow, D.A., Seidel, S.M., Eds.; The Society: Savoy, IL, USA, 1998. [Google Scholar]
- Gardner, D.K.; Schoolcraf, W.B. Culture and transfer of human blastocysts. Curr. Opin. Obstet. Gynecol. 1999, 11, 307–311. [Google Scholar] [CrossRef]
- Veeck, L.L.; Bodine, R.; Clarke, R.N.; Berrios, R.; Libraro, J.; Moschini, R.M.; Zaninovic, N.; Rosenwaks, Z. High pregnancy rates can be achieved after freezing and thawing human blastocysts. Fertil. Steril. 2004, 82, 1418–1427. [Google Scholar] [CrossRef]
- Stephenson, E.L.; Braude, P.R.; Mason, C. International community consensus standard for reporting derivation of humanembryonic stem cell lines. Regen. Med. 2007, 2, 349–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, R.T.; Upham, K.M.; Forman, E.J.; Zhao, T.; Treff, N.R. Cleavage-stage biopsy significantly impairs human embryonic implantation potential while blastocyst biopsy does not: A randomized and paired clinical trial. Fertil. Steril. 2013, 100, 624–630. [Google Scholar] [CrossRef]
- Gutiérrez-Mateo, C.; Sánchez-García, J.F.; Fischer, J.; Tormasi, S.; Cohen, J.; Munné, S.; Wells, D. Preimplantation genetic diagnosis of single-gene disorders: Experience with more than 200 cycles conducted by a reference laboratory in the United States. Fertil. Steril. 2009, 92, 1544–1556. [Google Scholar] [CrossRef]
- Palini, S.; Galluzzi, L.; De Stefani, S.; Bianchi, M.; Wells, D.; Magnani, M.; Bulletti, C. Genomic DNA in human blastocoelefluid. Reprod. Biomed. Online 2013, 26, 603–610. [Google Scholar] [CrossRef] [Green Version]
- Hammond, E.R.; McGillivray, B.C.; Wicker, S.M.; Peek, J.C.; Shelling, A.N.; Stone, P.; Chamley, L.W.; Cree, L.M. Characterizing nuclear and mitochondrial DNA in spent embryo culture media: Genetic contamination identified. Fertil. Steril. 2017, 107, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Rosenbluth, E.M.; Shelton, D.N.; Wells, L.M.; Sparks, A.E.; Van Voorhis, B.J. Human embryos secrete microRNAs into culture media–a potential biomarker for implantation. Fertil. Steril. 2014, 101, 1493–1500. [Google Scholar] [CrossRef]
- Galliano, D.; Pellicer, A. MicroRNA and implantation. Fertil. Steril. 2014, 101, 1531–1544. [Google Scholar] [CrossRef] [PubMed]
- Giacomini, E.; Vago, R.; Sanchez, A.M.; Podini, P.; Zarovni, N.; Murdica, V.; Rizzo, R.; Bortolotti, D.; Candiani, M.; Viganò, P. Secretome of in vitro cultured human embryos contains extracellular vesicles that are uptaken by the maternal side. Sci. Rep. 2017, 7, 5210. [Google Scholar] [CrossRef]
- Andrade, G.M.; Bomfim, M.M.; del Collado, M.; Meirelles, F.V.; Perecin, F.; da Silveira, J.C. Oxygen tension modulates extracellular vesicles and its miRNA contents in bovine embryo culture medium. Mol. Reprod. Dev. 2019, 86, 1067–1080. [Google Scholar] [CrossRef]
- Cimadomo, D.; Rienzi, L.; Giancani, A.; Alviggi, E.; Dusi, L.; Canipari, R.; Noli, L.; Ilic, D.; Khalaf, Y.; Ubaldi, F.M.; et al. Definition and validation of a custom protocol to detect miRNAs in the spent media after blastocyst culture: Searching for biomarkers of implantation. Hum. Reprod. 2019, 34, 1746–1761. [Google Scholar] [CrossRef]
- Tannetta, D.; Dragovic, R.; Alyahyaei, Z.; Southcombe, J. Extracellular vesicles and reproduction–promotion of successful pregnancy. Cell. Mol. Immunol. 2014, 11, 548–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabapatha, A.; Gercel-Taylor, C.; Taylor, D.D. Specific isolation of placenta-derived exosomes from the circulation of pregnant women and their immunoregulatory consequences. Am. J. Reprod. Immunol. 2006, 56, 345–355. [Google Scholar] [CrossRef]
- Ouyang, Y.; Mouillet, J.F.; Coyne, C.B.; Sadovsky, Y. Review: Placenta-specific micro- RNAs in exosomesegood things come in nanopackages. Placenta 2014, 35, S69–S73. [Google Scholar] [CrossRef] [Green Version]
- Pap, E.; Pallinger, E.; Falus, A.; Kiss, A.A.; Kittel, A.; Kovács, P.; Buzás, E.I. T lymphocytes are targets for platelet- and trophoblast-derived microvesicles during pregnancy. Placenta 2008, 29, 826–832. [Google Scholar] [CrossRef]
- Salomon, C.; Scholz-Romero, K.; Kobayashi, M.; Smith, M.; Duncombe, M.; Hanes, G.; Mitchell, S.; Rice, M.D.; Gregory, E. Oxygen tension regulates glucose-induced biogenesis and release of different subpopulations of exosome vesicles from trophoblast cells: A gestational age profile of placental exosomes in maternal plasma with gestational diabetes mellitus. Placenta 2015, 36, 488. [Google Scholar] [CrossRef]
- Mitchell, M.D.; Peiris, H.N.; Kobayashi, M.; Koh, Y.Q.; Duncombe, G.; Illanes, S.E.; Rice, G.E.; Salomon, C. Placental exosomes in normal and complicated pregnancy. Am. J. Obstet. Gynecol. 2015, S173–S181. [Google Scholar] [CrossRef]
- Bazer, F.W. Pregnancy recognition signaling mechanisms in ruminants and pigs. J. Anim. Sci. Biotechnol. 2013, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Godkin, J.; Bazer, F.; Thatcher, W.; Roberts, R. Proteins released by cultured day 15-16 conceptuses prolong luteal maintenance when introduced into the uterine lumen of cyclic ewes. J. Reprod. Fertil. 1984, 71, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Roberts, R.; Imakawa, K.; Niwano, Y.; Kazemi, M.; Malathy, P.-V.; Hansen, T.; Glass, A.A.; Kronenberg, L. Interferon production by the preimplantation sheep embryo. J. Interferon Res. 1989, 9, 175–187. [Google Scholar] [CrossRef]
- De Bem, T.H.C.; da Silveira, J.C.; Sampaio, R.V.; Sangalli, J.R.; Oliveira, M.L.F.; Ferreira, R.M.; Silva, L.A.; Perecin, F.; King, W.A.; Meirelles, F.V.; et al. Low levels of exosomal-miRNAs in maternal blood are associated with early pregnancy loss in cloned cattle. Sci. Rep. 2017, 7, 14319. [Google Scholar] [CrossRef]
- Andaloussi, S.E.L.; Mager, I.; Breakefield, X.O.; Wood, M.J. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357. [Google Scholar] [CrossRef]
- Yang, T.; Martin, P.; Fogarty, B.; Brown, A.; Schurman, K.; Phipps, R.; Yin, V.P.; Lockman, P.; Bai, S. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio Rerio. Pharm. Res. 2015, 32, 2003–2014. [Google Scholar] [CrossRef]
- Pascucci, L.; Coccè, V.; Bonomi, A.; Ami, D.; Ceccarelli, P.; Ciusani, E.; Viganò, L.; Locatelli, A.; Sisto, F.; Doglia, S.M.; et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: A new approach for drug delivery. J. Control. Release 2014, 192, 262–270. [Google Scholar] [CrossRef]
- Tian, Y.; Li, S.; Song, J.; Ji, T.; Zhu, M.; Anderson, G.J.; Wei, J.; Nie, G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014, 35, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wei, F.; Schafer, C.; Wong, D.T.W. Detection of tumor cell-specific mRNA and protein in exosome-like microvesicles from blood and saliva. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Xu, R.; Rai, A.; Chen, M.; Suwakulsiri, W.; Greening, D.W.; Simpson, R.J. Extracellular vesicles in cancer - implications for future improvements in cancer care. Nat. Rev. Clin. Oncol. 2018, 15, 617–638. [Google Scholar] [CrossRef]
- Vinaiphat, A.; Sze, S.K. Clinical implications of extracellular vesicles in neurodegenerative diseases. Expert Rev. Mol. Diagn. 2019, 1–12. [Google Scholar] [CrossRef]
- Lelek, J.; Zuba-Surma, E.K. Perspectives for future use of extracellular vesicles from umbilical cord- and adipose tissue-derived mesenchymal stem/stromal cells in regenerative therapies-synthetic review. Int. J. Mol. Sci. 2020, 21, 799. [Google Scholar] [CrossRef] [Green Version]
- Riazifar, M.; Pone, E.J.; Lötvall, J.; Zhao, W. Stem cell extracellular vesicles: Extended messages of regeneration. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 125–154. [Google Scholar] [CrossRef] [Green Version]
- Lange-Consiglio, A.; Funghi, F.; Cantile, C.; Idda, A.; Cremonesi, F.; Riccaboni, P. Case report: Use of amniotic microvesicles for regenerative medicine treatment of a mare with chronic endometritis. Front. Vet. Sci. 2020, 7, 347. [Google Scholar] [CrossRef]
- Burnouf, T.; Agrahari, V.; Agrahari, V. Extracellular vesicles as nanomedicine: Hopes and hurdles in clinical translation. Int. J. Nanomed. 2019, 14, 8847–8859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gimona, M.; Pachler, K.; Laner-Plamberger, S.; Schallmoser, K.; Rohde, E. Manufacturing of human extracellular vesicle-based therapeutics for clinical use. Int. J. Mol Sci. 2017, 18, 1190. [Google Scholar] [CrossRef]
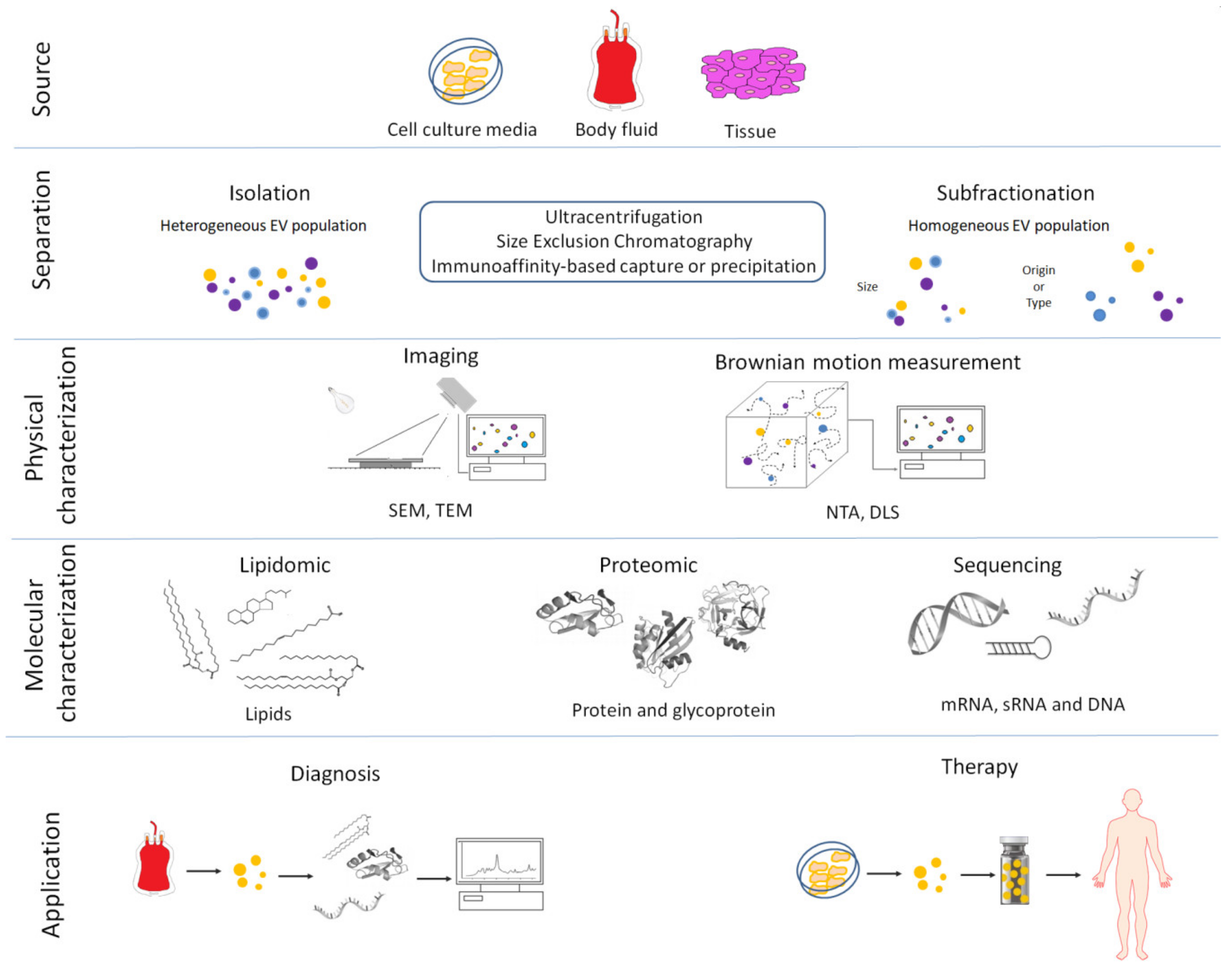
| Method | Principle | Advantages | Disadvantages |
|---|---|---|---|
| Differential centrifugation | EVs isolation after different consequent centrifugation steps (from 300g to 100,000 g): depletion of cells, platelets and large apoptotic bodies by low-speed centrifugation steps. Larger EVs are pelleted at 10,000 20,000 g range. Smaller EVs are then pelleted at high speed (100,000 120,000 g). | Most used, intermediated recovery and specificity | Time consuming and extravesicular proteins complexes/aggregates, lipoprotein particles, and other contaminants may also sediment |
| Density gradient centrifugation | EVs isolation through density gradients of sucrose or iohexol or iodixanol | High purity (EVs float upward or move downwards into an overlaid density gradient) | Applicable to small volume (usually after differential centrifugation), sucrose or iohexol or iodixanol can influence downstream application |
| Filtration | EVs filtration with different molecular weight cutoff | Recovery and purity depend on the consequent centrifugation step and the cutoff of centrifugal filter employ | Low specificity, EV populations may adhere to the filter or filtering may cause deformation and breakup of large vesicles |
| Precipitation | EVs are precipitated with organic solvent or in presence of different chemical compound such as polyethylene glycol (PEG), sodium acetate or protamine | High recovery, fast and easy | Low specificity Coprecipitation of numerous non-EV contaminants such as lipoproteins. Rigorous assessment of contaminating particle is recommended |
| Size Exclusion Chromatography | EVs are separated by their ability to pass through a resin packed in a column | Well separated EVs from protein complexes biofluids | Not suitable for large volume |
| Affinity isolation | EVs bind specific antibodies against exosome-specific surface proteins or EV-binding molecules | High purity | Low recovery, requires specific antibody |
| Microfluidic devices | Microfluidics-based on-chip EVs isolation based on immunoaffinity, membrane filtration, nanowire-based traps, nano-sized deterministic lateral displacement, viscoelastic flow and acoustic isolation | high-throughput and low processing time | Not suitable for large volume |
| Nanoscale flow cytometric sorting | Fluorescent labelled EVs are sorted using flow cytometer | Very specific and high purity | Laborious and time consuming |
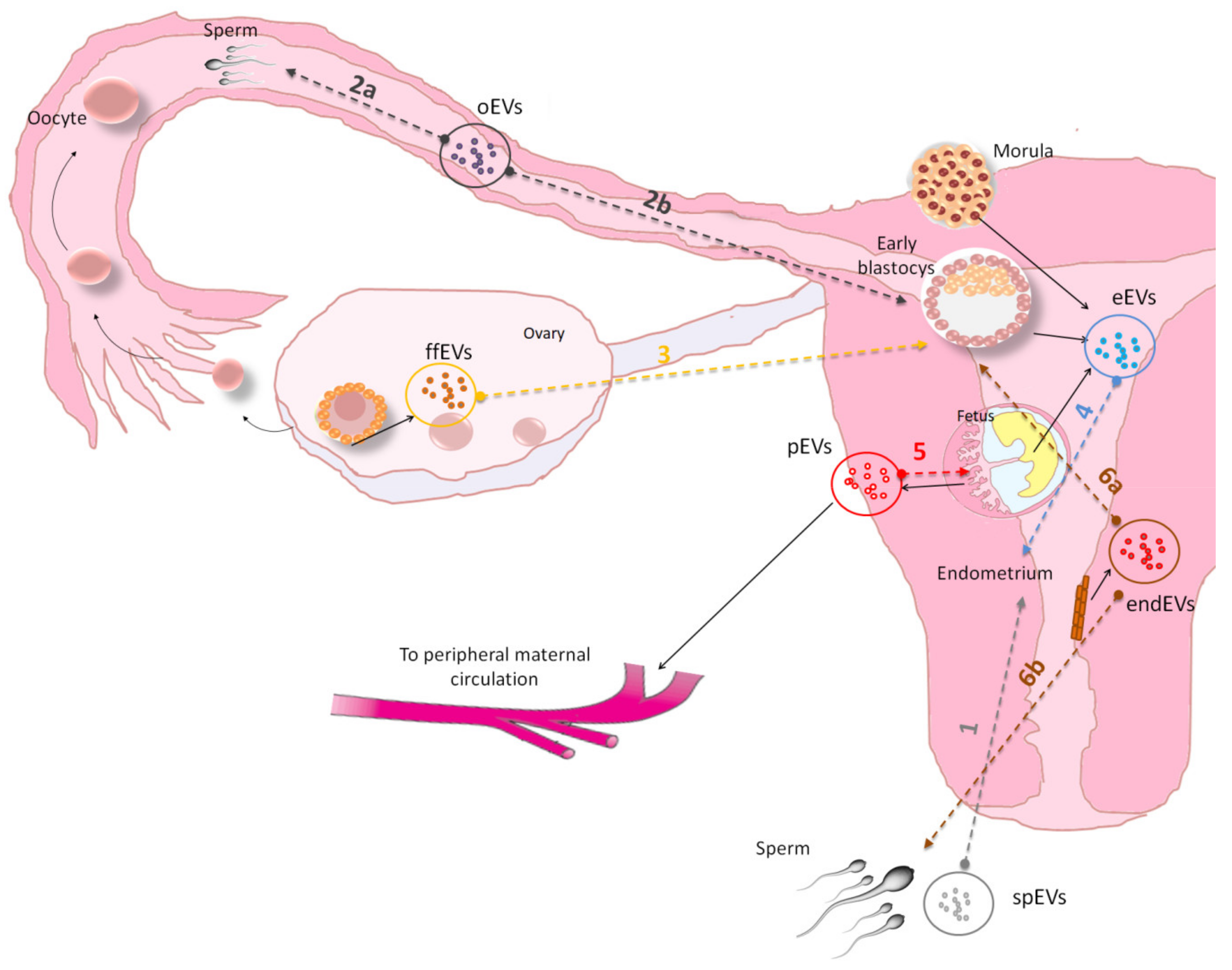
| Ref. Figure 2 | EVs Isolated from | Species | Isolation Method | Target Cell | Physical Characterization | Main Results | References |
|---|---|---|---|---|---|---|---|
| 1 | seminal plasma | sus scrofa | polymer precipitation | endometrial epithelial cells | TEM, NTA | induction of immune-related gene expression in endometrial epithelial cells EECs | [68] |
| 2a | oviduct | mus musculus | ultracentrifugation | Sperm | TEM | PMCA4 sperm up-take from exosomes released in female luminal fluids | [69] |
| 2a | oviduct | mus musculus | ultracentrifugation | sperm | TEM | oEVs transfer increase PMCA1 and PMCA4 activity in sperm | [70] |
| 2a | oviduct | felis catus | polymer precipitation | Sperm | NTA | oEVs contain protein related to energy metabolism, sperm functionality and enhance sperm motility and fertility in vitro | [71] |
| 2a | oviduct | bos taurus | ultracentrifugation | Sperm | DLS | oEVs induced acrosome reaction and signalingevents associated with sperm capacitation | [72] |
| 2b | oviduct | bos taurus | ultracentrifugation | Embryo | TEM | oEVs were internalized in embryo, increasing blastocyst rate and embryo quality | [73] |
| 2b | oviduct | bos taurus | ultracentrifugation | Embryo | NTA, TEM | oEVs increased embryo quality and altered expression of SNRPN | [74] |
| 3 | follicular fluid | bos taurus | ultracentrifugation | Embryo | tRPS, TEM | FF isolated EVs caused transcription and epigenetic alteration in embryos | [75] |
| 4 | embryo | sus scrofa | ultracentrifugation and precipitation | endometrial epithelial cells | TEM | evidence on embryo endometrial cross-talk mediated by EVs. EVs released by trophectoderm-cells increase the expression of miRNAs in maternal endothelial cells related to angiogenesis signaling | [76] |
| 4 | uterine flushings (UFs) from pregnant ewes | ovis aries | polymer precipitation | endometrial epithelial cells | TEM, NTA | Conceptus-derived EVs induce the expression of Interferon-Stimulated Genes ISG in bovine EECs culture | [77] |
| 5 | cytotrophoblast cell-derived exosome | homo sapiens | ultracentrifugation | extravillous trophoblasts (EVT) | TEM | Exosomes regulate intercellular communication between placental cells and EVT cell invasion in an oxygen-dependent manner | [78] |
| 6a | Endometrial tissue and uterine fluid | homo sapiens | ultracentrifugation | Embryo | tRPS, FC | Endometrial derived EVs contain specific miRNA that may contribute to the endometrial-embryo cross talk and embryo implantation | [1] |
| 6a | endometrial epithelial cell | homo sapiens/mus musculus | ultracentrifugation | Embryo | TEM | Endometrial derived EVs transport miRNAs influencing embryo transcriptomic for genes related to embryonic adhesion phenomenon | [8] |
| 6a | Uterine Fluid | bos taurus | polymer precipitation | Embryo | TEM, NTA | EVs from uterine fluid regulate bovine conceptus implantation | [79] |
| 6a | Uterine Fluid | bos taurus | polymer precipitation | Embryo | EM | EVs from uterine fluid of cows with endometritis impact blastocyst development | [80] |
| 6b | Uterine Fluid | gallus gallus | ultracentrifugation | Sperm | TEM | Uterine fluid EV contain proteins that may play an essential role in the preservation of sperm functions | [81] |
| Biomarkers for: | EVs Isolated from | Species | Isolation Method | Main Results | References |
|---|---|---|---|---|---|
| female reproductive cancer | serum | homo sapiens | polymer precipitation | EV miRNAs increase in the serum of epithelial ovarian cancer patients | [123] |
| female fertility | follicular fluid | homo sapiens | ultracentrifugation | EV-miRNAs in follicular fluid are associated with urinary concentrations of phenols and phthalate metabolites | [124] |
| female fertility | medium of blastocysts and endometrial cell co-cultures | homo sapiens | polymer precipitation | EV-bound secreted miRNAs are altered in co-culture experiments with blastocysts and endometrial cells isolated from patients diagnosed with AMA or endometriosis | [125] |
| embryo quality | medium of embryo cultures | homo sapiens | no isolation | DNA content in EVs isolated from embryo culture is linked to successful implantation | [126] |
| placenta quality | primary cytotrophoblasts and serum | homo sapiens | polymer precipitation and ultracentrifugation | serum EVs from patients with preeclampsia showed alteration in syncytin content | [127] |
| placenta quality | plasma | homo sapiens | ultracentrifugation | microRNAs from plasma EVs are altered in preeclampsia | [128] |
| placenta quality | plasma | homo sapiens | polymer precipitation and ultracentrifugation | EVs from preeclampsia patients delivered antiangiogenic factors to endothelial cells | [102] |
| placenta quality | plasma | homo sapiens | Ultracentrifugation and size exclusion chromatography | Proteomic analysis of plasma EVs revealed protein alterations related to gestational diabetes mellitus | [129] |
| placenta quality | condition media of chorionic villi | homo sapiens | Ultracentrifugation | Gestational diabetes mellitus alters miRNA profile of EVs isolated from chorionic villi explant cultures | [130] |
| early abortion | serum | bos taurus | Ultracentrifugation | EVs from serum contain microRNAs related to embryonic mortality in cows | [131] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capra, E.; Lange-Consiglio, A. The Biological Function of Extracellular Vesicles during Fertilization, Early Embryo—Maternal Crosstalk and Their Involvement in Reproduction: Review and Overview. Biomolecules 2020, 10, 1510. https://doi.org/10.3390/biom10111510
Capra E, Lange-Consiglio A. The Biological Function of Extracellular Vesicles during Fertilization, Early Embryo—Maternal Crosstalk and Their Involvement in Reproduction: Review and Overview. Biomolecules. 2020; 10(11):1510. https://doi.org/10.3390/biom10111510
Chicago/Turabian StyleCapra, Emanuele, and Anna Lange-Consiglio. 2020. "The Biological Function of Extracellular Vesicles during Fertilization, Early Embryo—Maternal Crosstalk and Their Involvement in Reproduction: Review and Overview" Biomolecules 10, no. 11: 1510. https://doi.org/10.3390/biom10111510
APA StyleCapra, E., & Lange-Consiglio, A. (2020). The Biological Function of Extracellular Vesicles during Fertilization, Early Embryo—Maternal Crosstalk and Their Involvement in Reproduction: Review and Overview. Biomolecules, 10(11), 1510. https://doi.org/10.3390/biom10111510





