SGLT2 Inhibition by Intraperitoneal Dapagliflozin Mitigates Peritoneal Fibrosis and Ultrafiltration Failure in a Mouse Model of Chronic Peritoneal Exposure to High-Glucose Dialysate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Peritoneal Samples
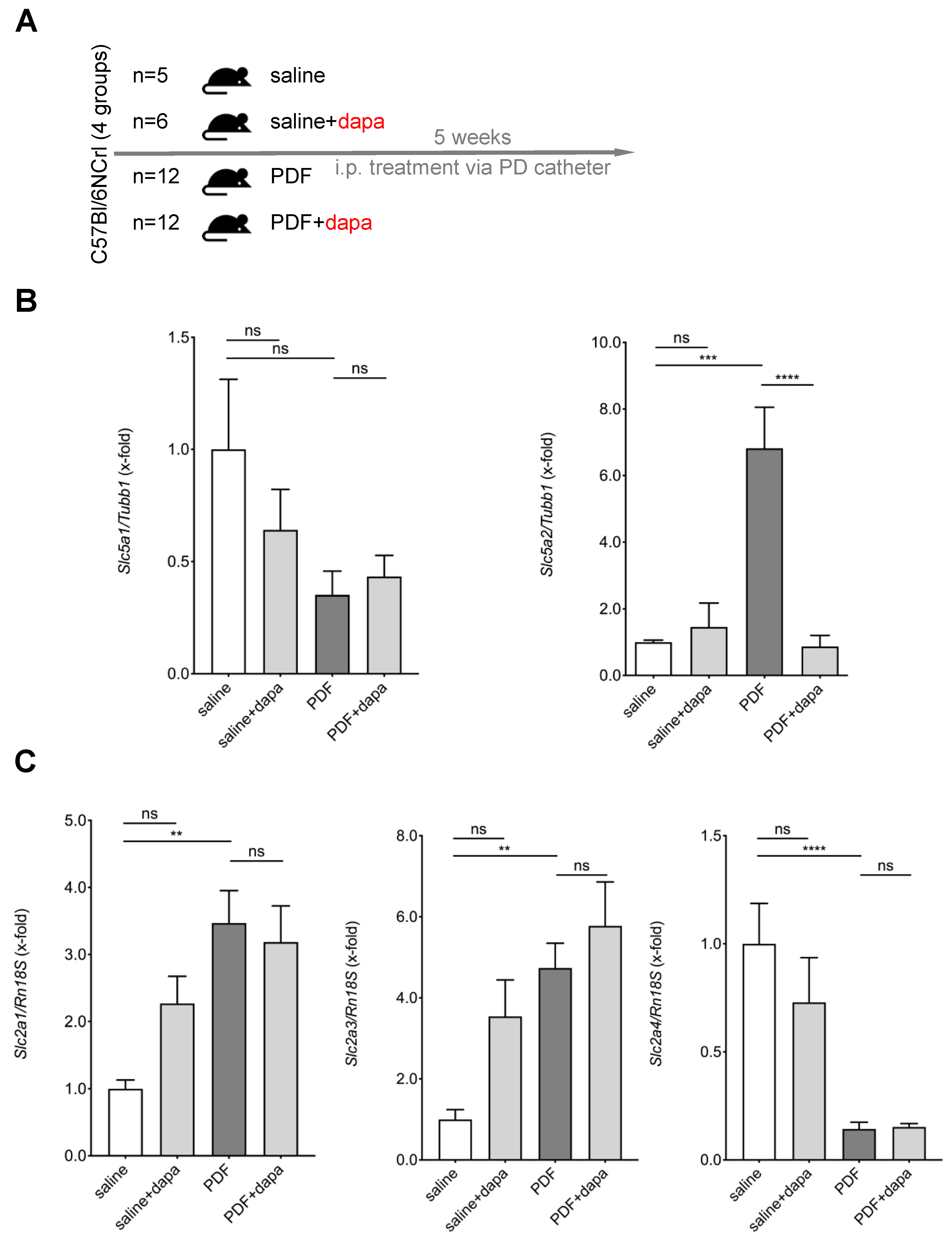
2.2. Peritoneal Dialysis Fluid Exposure Model in Mice
2.3. Chemical Analyses of Blood and Urine, Peritoneal Ultrafiltration, and Transport Studies
2.4. Flow Cytometry and ELISA Measurements in Peritoneal Effluents
2.5. Morphological, Immunofluorescence, and Immunohistochemical Analysis of Peritoneum
2.6. RNA Extraction and Real-Time Quantitative PCR
2.7. HPMC, Immortalized Mouse Peritoneal Cells MPMC, and RAW264.7 Macrophage Cell Culture and Treatment
3. Statistical Analysis
4. Key Resources
5. Results
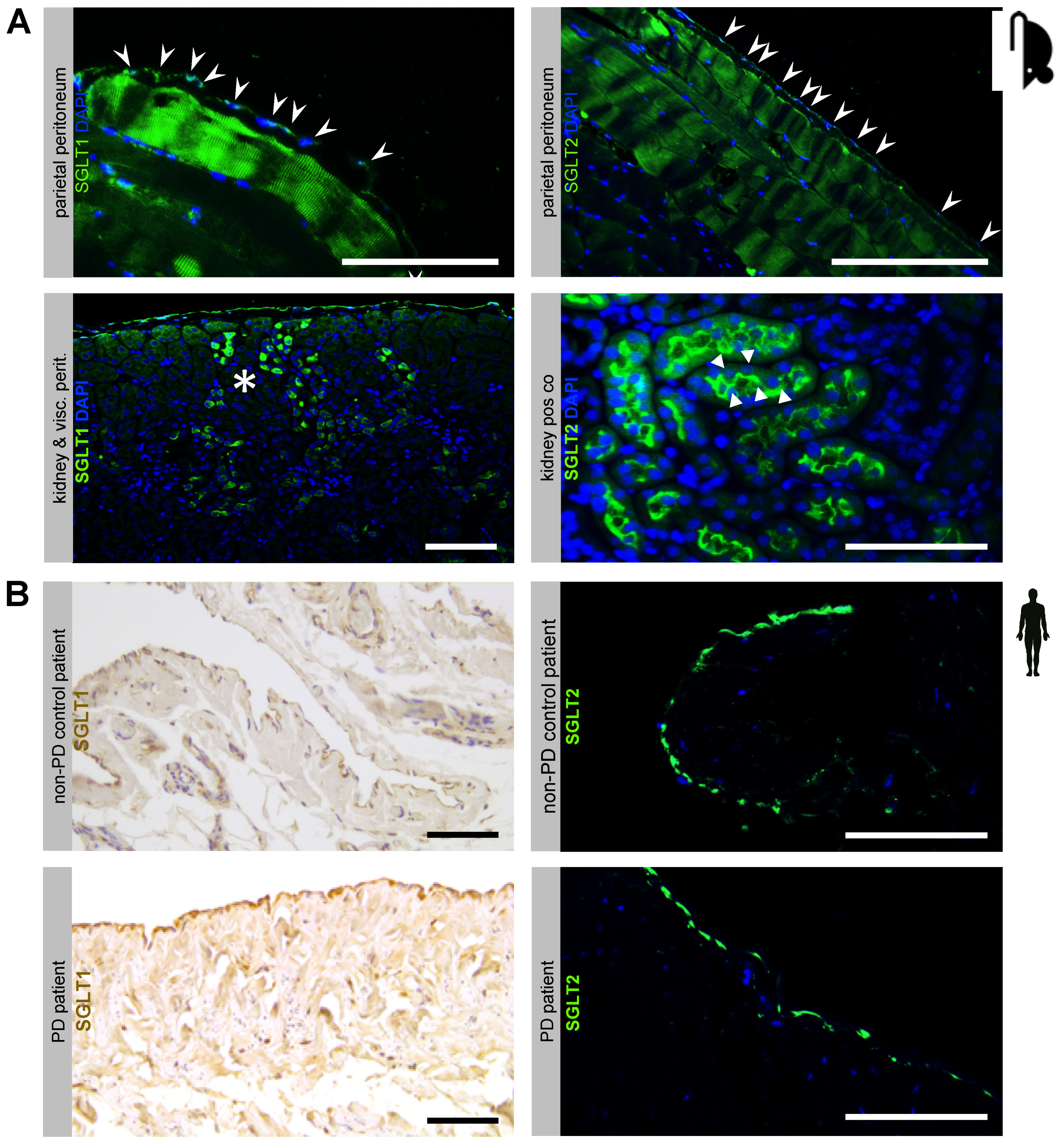
5.1. Sodium-Dependent Glucose Transporters Are Expressed in the Murine and Human Peritoneal Membrane
5.2. Chronic PDF-Induced SGLT2 Upregulation Is Abrogated by Intraperitoneal Dapagliflozin Treatment
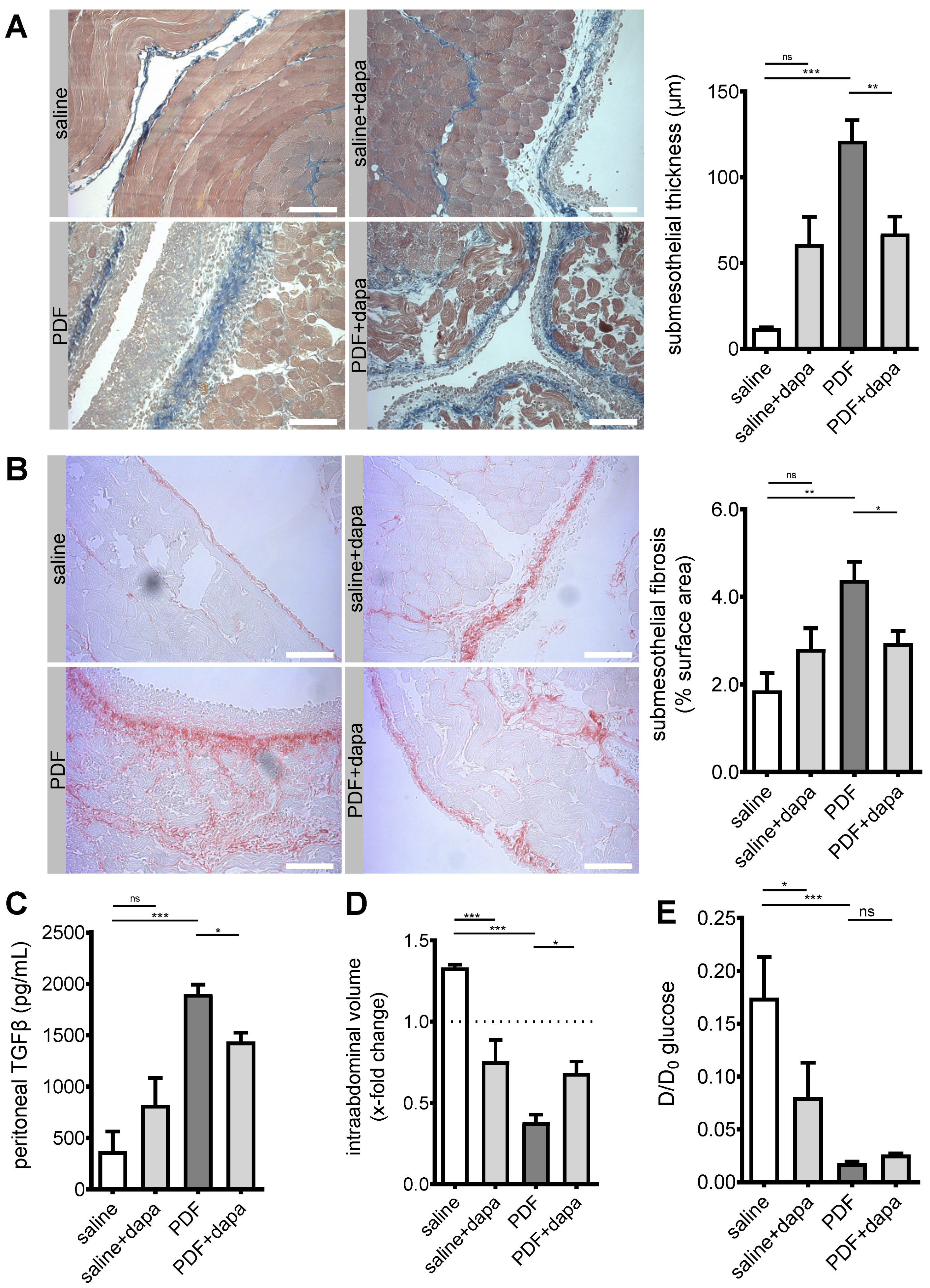
5.3. Peritoneal Fibrosis and Ultrafiltration Failure Are Ameliorated by Dapagliflozin
5.4. Dapagliflozin Reduces Submesothelial Microvessel Density in a Non-VEGF-Dependent Manner
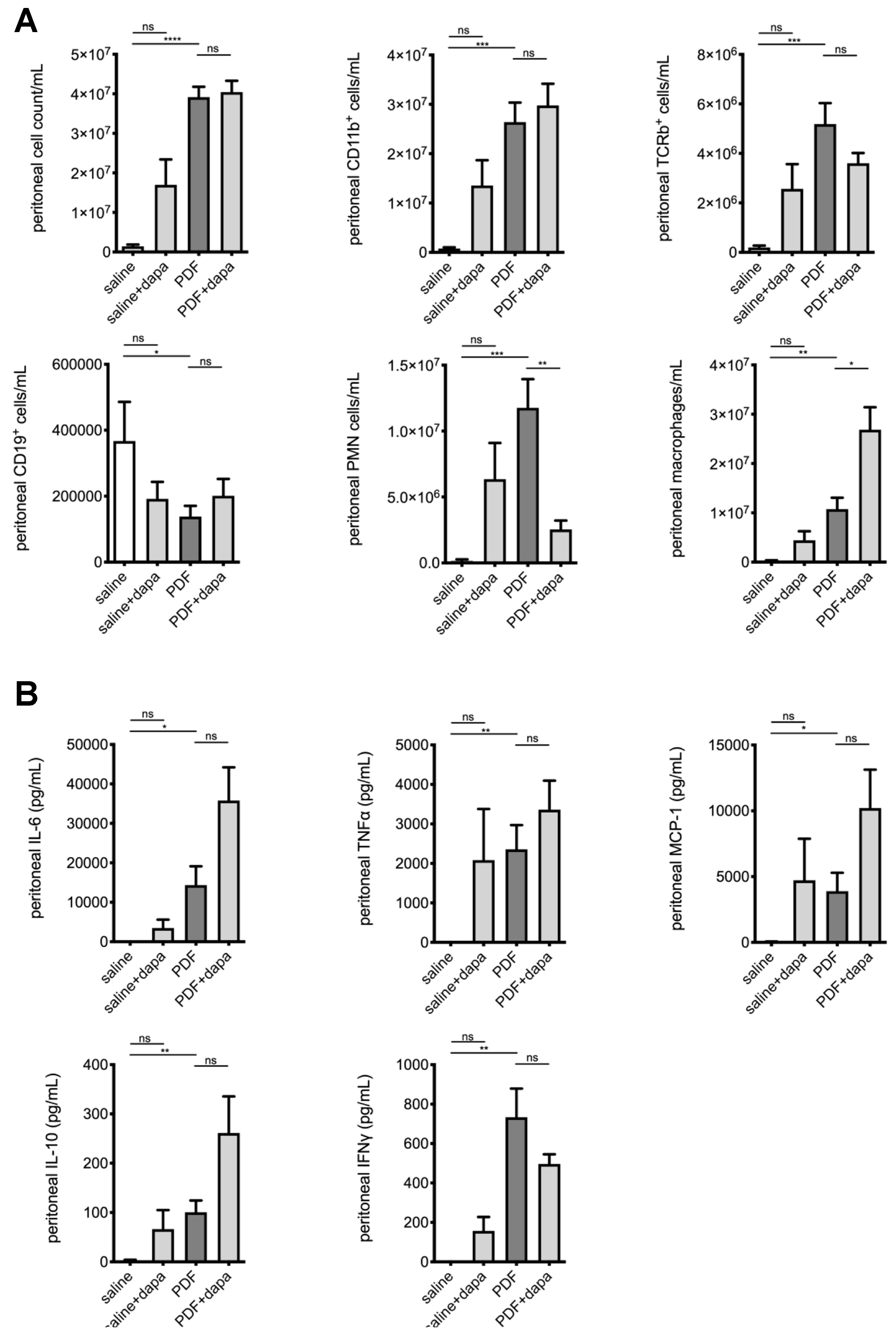
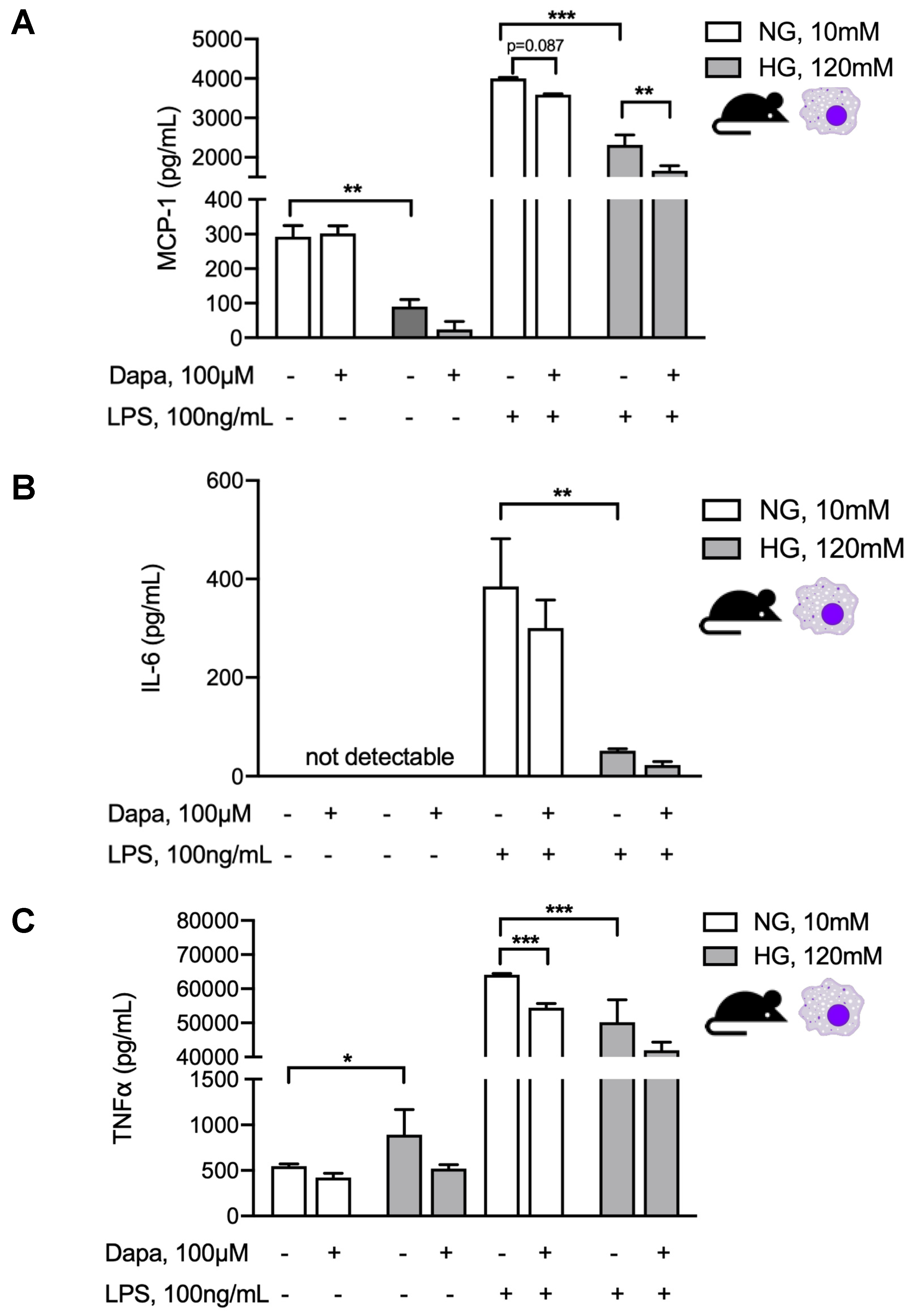
5.5. Dapagliflozin Modulates Intraperitoneal Inflammatory Response
5.6. Dapagliflozin Abrogates Proinflammatory Signaling in Murine and Human Peritoneal Mesothelial Cells and Exerts Glucose-Independent Anti-Inflammatory Effects on Murine Peritoneal Macrophages
6. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Devuyst, O.; Margetts, P.J.; Topley, N. The pathophysiology of the peritoneal membrane. J. Am. Soc. Nephrol. 2010, 21, 1077–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balzer, M.S. Molecular pathways in peritoneal fibrosis. Cell. Signal. 2020, 75, 109778. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M.E. Peritoneal dialysis: Diabetes of the peritoneal cavity. J. Lab. Clin. Med. 1999, 134, 103–104. [Google Scholar] [CrossRef]
- Wang, L.; Balzer, M.S.; Rong, S.; Menne, J.; von Vietinghoff, S.; Dong, L.; Gueler, F.; Jang, M.S.; Xu, G.; Timrott, K.; et al. Protein kinase C alpha inhibition prevents peritoneal damage in a mouse model of chronic peritoneal exposure to high-glucose dialysate. Kidney Int. 2016, 89, 1253–1267. [Google Scholar] [CrossRef] [PubMed]
- Holmes, C.; Mujais, S. Glucose sparing in peritoneal dialysis: Implications and metrics. Kidney Int. 2006, 70, S104–S109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroppel, B.; Fischereder, M.; Wiese, P.; Segerer, S.; Huber, S.; Kretzler, M.; Heiss, P.; Sitter, T.; Schlondorff, D. Expression of glucose transporters in human peritoneal mesothelial cells. Kidney Int. 1998, 53, 1278–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debray-Garcia, Y.; Sanchez, E.I.; Rodriguez-Munoz, R.; Venegas, M.A.; Velazquez, J.; Reyes, J.L. Diabetes and exposure to peritoneal dialysis solutions alter tight junction proteins and glucose transporters of rat peritoneal mesothelial cells. Life Sci. 2016, 161, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Nakano, D.; Guan, Y.; Hitomi, H.; Uemura, A.; Masaki, T.; Kobara, H.; Sugaya, T.; Nishiyama, A. A sodium-glucose cotransporter 2 inhibitor attenuates renal capillary injury and fibrosis by a vascular endothelial growth factor-dependent pathway after renal injury in mice. Kidney Int. 2018, 94, 524–535. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Wu, Y.; Tian, M.; Sjostrom, C.D.; Johansson, U.; Peng, X.R.; Smith, D.M.; Huang, Y. Dapagliflozin slows the progression of the renal and liver fibrosis associated with type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E563–E576. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Xue, M.; Li, X.; Han, F.; Liu, X.; Xu, L.; Lu, Y.; Cheng, Y.; Li, T.; et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc. Diabetol. 2019, 18, 15. [Google Scholar] [CrossRef]
- Schaefer, B.; Bartosova, M.; Macher-Goeppinger, S.; Sallay, P.; Voros, P.; Ranchin, B.; Vondrak, K.; Ariceta, G.; Zaloszyc, A.; Bayazit, A.K.; et al. Neutral pH and low-glucose degradation product dialysis fluids induce major early alterations of the peritoneal membrane in children on peritoneal dialysis. Kidney Int. 2018, 94, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, B.; Bartosova, M.; Macher-Goeppinger, S.; Ujszaszi, A.; Wallwiener, M.; Nyarangi-Dix, J.; Sallay, P.; Burkhardt, D.; Querfeld, U.; Pfeifle, V.; et al. Quantitative Histomorphometry of the Healthy Peritoneum. Sci. Rep. 2016, 6, 21344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balzer, M.S.; Helmke, A.; Ackermann, M.; Casper, J.; Dong, L.; Hiss, M.; Kiyan, Y.; Rong, S.; Timrott, K.; von Vietinghoff, S.; et al. Protein kinase C beta deficiency increases glucose-mediated peritoneal damage via M1 macrophage polarization and up-regulation of mesothelial protein kinase C alpha. Nephrol. Dial. Transpl. 2019, 34, 947–960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Dai, H.; Peng, L. Involvement of STAT3 Signaling in High Glucose-Induced Epithelial Mesenchymal Transition in Human Peritoneal Mesothelial Cell Line HMrSV5. Kidney Blood Press. Res. 2019, 44, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fan, J.; Zheng, C.; Yin, P.; Wu, H.; Li, X.; Luo, N.; Yu, X.; Chen, C. SGLT-2 inhibitors reduce glucose absorption from peritoneal dialysis solution by suppressing the activity of SGLT-2. Biomed. Pharmacother. 2019, 109, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.J.; Phillips, L.; Naish, P.F.; Russell, G.I. Peritoneal glucose exposure and changes in membrane solute transport with time on peritoneal dialysis. J. Am. Soc. Nephrol. 2001, 12, 1046–1051. [Google Scholar]
- Ha, H.; Yu, M.R.; Lee, H.B. High glucose-induced PKC activation mediates TGF-beta 1 and fibronectin synthesis by peritoneal mesothelial cells. Kidney Int. 2001, 59, 463–470. [Google Scholar] [CrossRef] [Green Version]
- Bartosova, M.; Schaefer, B.; Bermejo, J.L.; Tarantino, S.; Lasitschka, F.; Macher-Goeppinger, S.; Sinn, P.; Warady, B.A.; Zaloszyc, A.; Parapatics, K.; et al. Complement Activation in Peritoneal Dialysis-Induced Arteriolopathy. J. Am. Soc. Nephrol. 2017, 29, 268–282. [Google Scholar] [CrossRef]
- Deng, D.; Yan, N. GLUT, SGLT, and SWEET: Structural and mechanistic investigations of the glucose transporters. Protein Sci. 2016, 25, 546–558. [Google Scholar] [CrossRef] [Green Version]
- Wright, E.M.; Loo, D.D.; Hirayama, B.A. Biology of human sodium glucose transporters. Physiol. Rev. 2011, 91, 733–794. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.Q.; Keating, A.F. Functional properties and genomics of glucose transporters. Curr. Genom. 2007, 8, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Fischereder, M.; Schroppel, B.; Wiese, P.; Fink, M.; Banas, B.; Schmidbauer, S.; Schlondorff, D. Regulation of glucose transporters in human peritoneal mesothelial cells. J. Nephrol. 2003, 16, 103–109. [Google Scholar] [PubMed]
- Witters, L.A.; Vater, C.A.; Lienhard, G.E. Phosphorylation of the glucose transporter in vitro and in vivo by protein kinase C. Nature 1985, 315, 777–778. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.E.; Ma, J.; Sacharidou, A.; Mi, W.; Salato, V.K.; Nguyen, N.; Jiang, Y.; Pascual, J.M.; North, P.E.; Shaul, P.W.; et al. A Protein Kinase C Phosphorylation Motif in GLUT1 Affects Glucose Transport and is Mutated in GLUT1 Deficiency Syndrome. Mol. Cell 2015, 58, 845–853. [Google Scholar] [CrossRef] [Green Version]
- Hodrea, J.; Balogh, D.B.; Hosszu, A.; Lenart, L.; Besztercei, B.; Koszegi, S.; Sparding, N.; Genovese, F.; Wagner, L.J.; Szabo, A.J.; et al. Reduced O-GlcNAcylation and tubular hypoxia contribute to the antifibrotic effect of SGLT2 inhibitor dapagliflozin in the diabetic kidney. Am. J. Physiol. Ren. Physiol. 2020, 318, F1017–F1029. [Google Scholar] [CrossRef]
- Huang, F.; Zhao, Y.; Wang, Q.; Hillebrands, J.L.; van den Born, J.; Ji, L.; An, T.; Qin, G. Dapagliflozin Attenuates Renal Tubulointerstitial Fibrosis Associated with Type 1 Diabetes by Regulating STAT1/TGFbeta1 Signaling. Front. Endocrinol. 2019, 10, 441. [Google Scholar] [CrossRef] [Green Version]
- Castoldi, G.; Carletti, R.; Ippolito, S.; Colzani, M.; Barzaghi, F.; Stella, A.; Zerbini, G.; Perseghin, G.; di Gioia, C.R.T. Renal Anti-Fibrotic Effect of Sodium Glucose Cotransporter 2 Inhibition in Angiotensin II-Dependent Hypertension. Am. J. Nephrol. 2020, 51, 119–129. [Google Scholar] [CrossRef]
- Lee, T.M.; Chang, N.C.; Lin, S.Z. Dapagliflozin, a selective SGLT2 Inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts. Free Radic. Biol. Med. 2017, 104, 298–310. [Google Scholar] [CrossRef]
- Flessner, M.F.; Fenstermacher, J.D.; Dedrick, R.L.; Blasberg, R.G. A distributed model of peritoneal-plasma transport: Tissue concentration gradients. Am. J. Physiol. Ren. Physiol. 1985, 248, F425–F435. [Google Scholar] [CrossRef]
- Martus, G.; Bergling, K.; Simonsen, O.; Goffin, E.; Morelle, J.; Öberg, C.M. Novel Method for Osmotic Conductance to Glucose in Peritoneal Dialysis. Kidney Int. Rep. 2020, 5, 1974–1981. [Google Scholar] [CrossRef]
- Eich, G.; Bartosova, M.; Tischer, C.; Wlodkowski, T.T.; Schaefer, B.; Pichl, S.; Kraewer, N.; Ranchin, B.; Vondrak, K.; Liebau, M.C.; et al. Bicarbonate buffered peritoneal dialysis fluid upregulates angiopoietin-1 and promotes vessel maturation. PLoS ONE 2017, 12, e0189903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatanaka, T.; Ogawa, D.; Tachibana, H.; Eguchi, J.; Inoue, T.; Yamada, H.; Takei, K.; Makino, H.; Wada, J. Inhibition of SGLT2 alleviates diabetic nephropathy by suppressing high glucose-induced oxidative stress in type 1 diabetic mice. Pharmacol. Res. Perspect. 2016, 4, e00239. [Google Scholar] [CrossRef] [PubMed]
- Leng, W.; Ouyang, X.; Lei, X.; Wu, M.; Chen, L.; Wu, Q.; Deng, W.; Liang, Z. The SGLT-2 Inhibitor Dapagliflozin Has a Therapeutic Effect on Atherosclerosis in Diabetic ApoE(-/-) Mice. Mediat. Inflamm. 2016, 2016, 6305735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.X.; Levi, J.; Luo, Y.; Myakala, K.; Herman-Edelstein, M.; Qiu, L.; Wang, D.; Peng, Y.; Grenz, A.; Lucia, S.; et al. SGLT2 Protein Expression Is Increased in Human Diabetic Nephropathy: SGLT2 Protein Inhibition Decreases Renal Lipid Accumulation, Inflammation, and the Development of Nephropathy in Diabetic Mice. J. Biol. Chem. 2017, 292, 5335–5348. [Google Scholar] [CrossRef] [Green Version]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef]
- Han, J.H.; Oh, T.J.; Lee, G.; Maeng, H.J.; Lee, D.H.; Kim, K.M.; Choi, S.H.; Jang, H.C.; Lee, H.S.; Park, K.S.; et al. The beneficial effects of empagliflozin, an SGLT2 inhibitor, on atherosclerosis in ApoE (-/-) mice fed a western diet. Diabetologia 2017, 60, 364–376. [Google Scholar] [CrossRef] [Green Version]
- Vallon, V.; Gerasimova, M.; Rose, M.A.; Masuda, T.; Satriano, J.; Mayoux, E.; Koepsell, H.; Thomson, S.C.; Rieg, T. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am. J. Physiol. Ren. Physiol. 2014, 306, F194–F204. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Nagata, N.; Nagashimada, M.; Zhuge, F.; Ni, Y.; Chen, G.; Mayoux, E.; Kaneko, S.; Ota, T. SGLT2 Inhibition by Empagliflozin Promotes Fat Utilization and Browning and Attenuates Inflammation and Insulin Resistance by Polarizing M2 Macrophages in Diet-induced Obese Mice. EBioMedicine 2017, 20, 137–149. [Google Scholar] [CrossRef] [Green Version]


| Reagent or Resource | Source | Identifier |
|---|---|---|
| Antibodies for Immunofluorescence and Immunohistochemistry | ||
| Anti-SGLT1 | Millipore | 07-1417 |
| Anti-SGLT2 | Abcam | ab85626 |
| anti-CD31 (clone SZ31) | Dianova | DIA310 |
| Antibodies for Flow Cytometry | ||
| Anti-CD11b | Biolegend | clone M1/70 |
| Anti-F4/80 | Biolegend | clone BM8 |
| Anti-CD19 | Biolegend | clone 6D5 |
| Anti-Gr1 | Biolegend | clone RB6-8C5 |
| Anti-TCRb | Biolegend | clone H57-597 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Dapagliflozin | Sigma-Aldrich | Cat#461432-26-8 |
| RPMI-1640 | Gibco | Cat#21875-034 |
| RIPA buffer | Cell signaling | Cat#9806 |
| SYBR Green PCR Master Mix | Applied Biosystem | Cat#KK4605 |
| LightCycler 480 RNA Master Hydrolysis probes | Roche | Cat#04991885001 |
| Fluoromount-G | Southern Biotech | Cat# 0100-01 |
| Critical Commercial Assays | ||
| cDNA Reverse Transcription Kit | Promega | Cat#C1181 Cat#U1515 Cat#M1705 |
| Rneasy Mini Kit | Qiagen | Cat#74106 |
| Oligonucleotides | ||
| TaqMan Primers for qPCR | Eurofins | N/A |
| Slc5a1 PROBE AAAAAATCGCCTGTGTCCTCCCTGAAGA Slc5a1 SENSE GGAATGATCAGCCGGATTCTAT Slc5a1 ANTISENSE TGTGCCGCAGTATTTCTGACA Slc5a2 PROBE TCCAGTCCCCGGCTCCAGGC Slc5a2 SENSE AATGTGCAATGGAGATGGAAGA Slc5a2 ANTISENSE CATCCCACAGAACCAAAGCA | ||
| SybrGreen Primers for qPCR | Eurofins | N/A |
| Slc5a1_#1_fwd TGGGCTGGATATTTGTCCCGA Slc5a1_#1_rev CAAACCGCTTCCGCAGATACTT Slc5a1_#2_fwd CACCGAGGGCTGACTCATTC Slc5a1_#2_rev TGATCCGTACACCAGTACCAC Slc5a2_#1_fwd TGGTGTTGGCTTGTGGTCTA Slc5a2_#1_rev ATGTTGCTGGCGAACAGAGA Slc5a2_#2_fwd ATGGAGCAACACGTAGAGGC Slc5a2_#2_rev ATGACCAGCAGGAAATAGGCA | ||
| Software | ||
| Aperio Image Scope | Leica | https://www.leicabiosystems.com/digital-pathology/manage/aperio-imagescope/ |
| FlowJo | FlowJo, LLC | https://www.flowjo.com |
| ImageJ | NIH | https://imagej.nih.gov/ij |
| Prism 7 | Graphpad Software | https://www.graphpad.com/scientific-software/prism |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balzer, M.S.; Rong, S.; Nordlohne, J.; Zemtsovski, J.D.; Schmidt, S.; Stapel, B.; Bartosova, M.; von Vietinghoff, S.; Haller, H.; Schmitt, C.P.; et al. SGLT2 Inhibition by Intraperitoneal Dapagliflozin Mitigates Peritoneal Fibrosis and Ultrafiltration Failure in a Mouse Model of Chronic Peritoneal Exposure to High-Glucose Dialysate. Biomolecules 2020, 10, 1573. https://doi.org/10.3390/biom10111573
Balzer MS, Rong S, Nordlohne J, Zemtsovski JD, Schmidt S, Stapel B, Bartosova M, von Vietinghoff S, Haller H, Schmitt CP, et al. SGLT2 Inhibition by Intraperitoneal Dapagliflozin Mitigates Peritoneal Fibrosis and Ultrafiltration Failure in a Mouse Model of Chronic Peritoneal Exposure to High-Glucose Dialysate. Biomolecules. 2020; 10(11):1573. https://doi.org/10.3390/biom10111573
Chicago/Turabian StyleBalzer, Michael S., Song Rong, Johannes Nordlohne, Jan D. Zemtsovski, Sonja Schmidt, Britta Stapel, Maria Bartosova, Sibylle von Vietinghoff, Hermann Haller, Claus P. Schmitt, and et al. 2020. "SGLT2 Inhibition by Intraperitoneal Dapagliflozin Mitigates Peritoneal Fibrosis and Ultrafiltration Failure in a Mouse Model of Chronic Peritoneal Exposure to High-Glucose Dialysate" Biomolecules 10, no. 11: 1573. https://doi.org/10.3390/biom10111573
APA StyleBalzer, M. S., Rong, S., Nordlohne, J., Zemtsovski, J. D., Schmidt, S., Stapel, B., Bartosova, M., von Vietinghoff, S., Haller, H., Schmitt, C. P., & Shushakova, N. (2020). SGLT2 Inhibition by Intraperitoneal Dapagliflozin Mitigates Peritoneal Fibrosis and Ultrafiltration Failure in a Mouse Model of Chronic Peritoneal Exposure to High-Glucose Dialysate. Biomolecules, 10(11), 1573. https://doi.org/10.3390/biom10111573





