Peritoneal Dialysis Fluid Supplementation with Alanyl-Glutamine Attenuates Conventional Dialysis Fluid-Mediated Endothelial Cell Injury by Restoring Perturbed Cytoprotective Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Experimental PD Fluid Exposure Setting
2.3. Arterioles
2.4. Cell Damage Assay
2.5. Immunofluorescence
2.6. Viability Assay
2.7. Fluorescence Labeling of Proteins and 2-DE
2.8. CBB Staining and In-Gel Digestion
2.9. MALDI and Database Search
2.10. FASP and TMT Labeling of Human Omental Arterioles
2.11. SP3 and TMT Labeling of HUVEC
2.12. Mass Spectrometry Data Analysis
2.13. Enrichment Map Analysis
2.14. Statistical Analysis
2.15. Data Availability
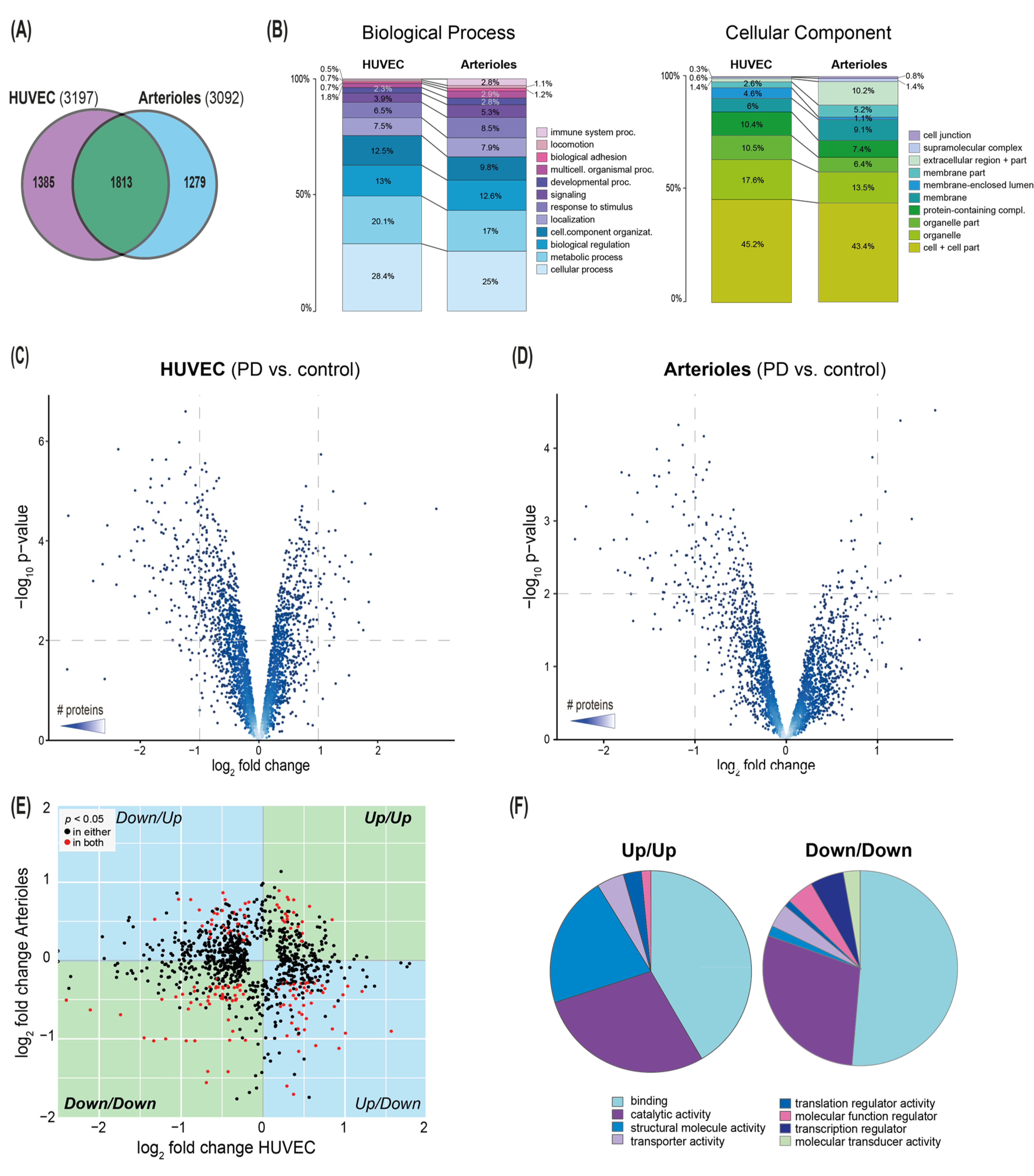
3. Results
3.1. Effect of Conventional PD Fluid on HUVEC
3.2. Cytoprotective Effects of AlaGln in an In Vitro Model of PD Using HUVEC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, P.K.-T.; Chow, K.M.; Van De Luijtgaarden, M.W.; Johnson, D.W.; Jager, K.J.; Mehrotra, R.; Naicker, S.; Pecoits-Filho, R.; Yu, X.Q.; Lameire, N. Changes in the worldwide epidemiology of peritoneal dialysis. Nat. Rev. Nephrol. 2016, 13, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Devuyst, O.; Margetts, P.J.; Topley, N. The Pathophysiology of the Peritoneal Membrane. J. Am. Soc. Nephrol. 2010, 21, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.J. Peritoneal dialysis[mdash]current status and future challenges. Nat. Rev. Nephrol. 2013, 9, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.P.; Aufricht, C. Is there such a thing as biocompatible peritoneal dialysis fluid? Pediatr. Nephrol. 2017, 32, 1835–1843. [Google Scholar] [CrossRef]
- Schilte, M.N.; Celie, J.W.A.M.; Ter Wee, P.M.; Beelen, R.H.J.; Born, J.V.D. Factors contributing to peritoneal tissue remodeling in peritoneal dialysis. Perit. Dial. Int. 2009, 29, 605–617. [Google Scholar] [CrossRef]
- Schaefer, B.; Bartosova, M.; Macher-Goeppinger, S.; Sallay, P.; Vörös, P.; Ranchin, B.; Vondrak, K.; Ariceta, G.; Zaloszyc, A.; Bayazit, A.K.; et al. Neutral pH and low–glucose degradation product dialysis fluids induce major early alterations of the peritoneal membrane in children on peritoneal dialysis. Kidney Int. 2018, 94, 419–429. [Google Scholar] [CrossRef]
- Flessner, M.F.; Choi, J.; Vanpelt, H.; He, Z.; Credit, K.; Henegar, J.; Hughson, M. Correlating structure with solute and water transport in a chronic model of peritoneal inflammation. Am. J. Physiol. Physiol. 2006, 290, 232–240. [Google Scholar] [CrossRef][Green Version]
- Krediet, R.T.; Lindholm, B.; Rippe, B. Pathophysiology of peritoneal membrane failure. Perit. Dial. Int. 2000, 20, 22–42. [Google Scholar] [CrossRef]
- Rippe, C. Peritoneal Angiogenesis in Response to Dialysis Fluid. Contrib. Nephrol. 2009, 163, 60–66. [Google Scholar] [CrossRef]
- Gónzalez-Mateo, G.T.; Pascual-Antón, L.; Carrasco, L.Á.; Martínez-Cabeza, V.; González, I.F.; Selgas, R.; López-Cabrera, M.; Aguilera, A. Angiogenesis and Lymphangiogenesis in Peritoneal Dialysis; IntechOpen: London, UK, 2018; Volume 34, pp. 245–252. [Google Scholar]
- Mortier, S.; De Vriese, A.S.; McLoughlin, R.M.; Topley, N.; Schaub, T.P.; Passlick-Deetjen, J.; Lameire, N.H. Effects of Conventional and New Peritoneal Dialysis Fluids on Leukocyte Recruitment in the Rat Peritoneal Membrane. J. Am. Soc. Nephrol. 2003, 14, 1296–1306. [Google Scholar] [CrossRef][Green Version]
- Schilte, M.N.; Fabbrini, P.; Ter Wee, P.M.; Keuning, E.D.; Zareie, M.; Tangelder, G.-J.; Van Lambalgen, A.A.; Beelen, R.H.; Born, J.V.D. Peritoneal Dialysis Fluid Bioincompatibility and New Vessel Formation Promote Leukocyte-Endothelium Interactions in a Chronic Rat Model for Peritoneal Dialysis. Microcirculation 2010, 17, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Kratochwill, K.; Boehm, M.; Herzog, R.; Lichtenauer, A.M.; Salzer, E.; Lechner, M.; Kuster, L.; Bergmeister, K.; Rizzi, A.; Mayer, B.; et al. Alanyl–glutamine dipeptide restores the cytoprotective stress proteome of mesothelial cells exposed to peritoneal dialysis fluids. Nephrol. Dial. Transplant. 2011, 27, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Ferrantelli, E.; Liappas, G.; Cuenca, M.V.; Keuning, E.D.; Foster, T.L.; Vervloet, M.G.; Lopéz-Cabrera, M.; Beelen, R.H. The dipeptide alanyl-glutamine ameliorates peritoneal fibrosis and attenuates IL-17 dependent pathways during peritoneal dialysis. Kidney Int. 2016, 89, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Kratochwill, K.; Boehm, M.; Herzog, R.; Gruber, K.; Lichtenauer, A.M.; Kuster, L.; Csaicsich, D.; Gleiss, A.; Alper, S.L.; Aufricht, C.; et al. Addition of Alanyl-Glutamine to Dialysis Fluid Restores Peritoneal Cellular Stress Responses—A First-In-Man Trial. PLoS ONE 2016, 11, 1–26. [Google Scholar] [CrossRef]
- Vychytil, A.; Herzog, R.; Probst, P.; Ribitsch, W.; Lhotta, K.; Machold-Fabrizii, V.; Wiesholzer, M.; Kaufmann, M.; Salmhofer, H.; Windpessl, M.; et al. A randomized controlled trial of alanyl-glutamine supplementation in peritoneal dialysis fluid to assess impact on biomarkers of peritoneal health. Kidney Int. 2018, 94, 1227–1237. [Google Scholar] [CrossRef]
- Kratochwill, K.; Lechner, M.; Siehs, C.; Lederhuber, H.C.; Řehulka, P.; Endemann, M.; Kasper, D.C.; Herkner, K.R.; Mayer, B.; Rizzi, A.; et al. Stress Responses and Conditioning Effects in Mesothelial Cells Exposed to Peritoneal Dialysis Fluid. J. Proteome Res. 2009, 8, 1731–1747. [Google Scholar] [CrossRef]
- Kratochwill, K.; Bender, T.O.; Lichtenauer, A.M.; Herzog, R.; Tarantino, S.; Bialas, K.; Jörres, A.; Aufricht, C. Cross-Omics Comparison of Stress Responses in Mesothelial Cells Exposed to Heat- versus Filter-Sterilized Peritoneal Dialysis Fluids. BioMed Res. Int. 2015, 2015, 1–12. [Google Scholar] [CrossRef][Green Version]
- Bender, T.O.; Kratochwill, K.; Böhm, M.; Jörres, A.; Aufricht, C. HSP Induction in Mesothelial Cells by Peritoneal Dialysis Fluid Depends on Biocompatibility Test System. Int. J. Artif. Organs 2011, 34, 405–409. [Google Scholar] [CrossRef]
- Lechner, M.; Kratochwill, K.; Lichtenauer, A.; Řehulka, P.; Mayer, B.; Aufricht, C.; Rizzi, A. A Proteomic View on the Role of Glucose in Peritoneal Dialysis. J. Proteome Res. 2010, 9, 2472–2479. [Google Scholar] [CrossRef]
- Hellinger, R.; Koehbach, J.; Soltis, D.E.; Carpenter, E.J.; Wong, G.K.-S.; Gruber, C.W. Peptidomics of Circular Cysteine-Rich Plant Peptides: Analysis of the Diversity of Cyclotides from Viola tricolor by Transcriptome and Proteome Mining. J. Proteome Res. 2015, 14, 4851–4862. [Google Scholar] [CrossRef]
- Merico, D.; Isserlin, R.; Stueker, O.; Emili, A.; Bader, G.D. Enrichment Map: A Network-Based Method for Gene-Set Enrichment Visualization and Interpretation. PLoS ONE 2010, 5, e13984. [Google Scholar] [CrossRef] [PubMed]
- Tabb, D.L.; Vega-Montoto, L.; Rudnick, P.A.; Variyath, A.M.; Ham, A.-J.L.; Bunk, D.M.; Kilpatrick, L.E.; Billheimer, D.D.; Blackman, R.K.; Cardasis, H.L.; et al. Repeatability and Reproducibility in Proteomic Identifications by Liquid Chromatography−Tandem Mass Spectrometry. J. Proteome Res. 2009, 9, 761–776. [Google Scholar] [CrossRef]
- Basha, B.; Samuel, S.M.; Triggle, C.R.; Ding, H. Endothelial Dysfunction in Diabetes Mellitus: Possible Involvement of Endoplasmic Reticulum Stress? Exp. Diabetes Res. 2012, 2012, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Goveia, J.; Stapor, P.; Carmeliet, P. Principles of targeting endothelial cell metabolism to treat angiogenesis and endothelial cell dysfunction in disease. EMBO Mol. Med. 2014, 6, 1105–1120. [Google Scholar] [CrossRef] [PubMed]
- Aebersold, R.; Agar, J.N.; Amster, I.J.; Baker, M.S.; Bertozzi, C.R.; Boja, E.S.; Costello, C.E.; Cravatt, B.F.; Fenselau, C.; Garcia, B.A.; et al. How many human proteoforms are there? Nat. Chem. Biol. 2018, 14, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Miller, I.; Crawford, J.; Gianazza, E. Protein stains for proteomic applications: Which, when, why? Proteomics 2006, 6, 5385–5408. [Google Scholar] [CrossRef]
- Arentz, G.; Weiland, F.; Oehler, M.K.; Hoffmann, P. State of the art of 2D DIGE. Proteom. Clin. Appl. 2015, 9, 277–288. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Herzog, R.; Boehm, M.; Unterwurzacher, M.; Wagner, A.; Parapatics, K.; Májek, P.; Mueller, A.C.; Lichtenauer, A.; Bennett, K.L.; Alper, S.L.; et al. Effects of Alanyl-Glutamine Treatment on the Peritoneal Dialysis Effluent Proteome Reveal Pathomechanism-Associated Molecular Signatures. Mol. Cell. Proteom. 2018, 17, 516–532. [Google Scholar] [CrossRef]
- D’Apolito, M.; Du, X.; Zong, H.; Catucci, A.; Maiuri, L.; Trivisano, T.; Pettoello-Mantovani, M.; Campanozzi, A.; Raia, V.; Pessin, J.E.; et al. Urea-induced ROS generation causes insulin resistance in mice with chronic renal failure. J. Clin. Investig. 2010, 120, 203–213. [Google Scholar] [CrossRef]
- García-López, E.; Lindholm, B.; Davies, S. An update on peritoneal dialysis solutions. Nat. Rev. Nephrol. 2012, 8, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Bidmon, B.; Endemann, M.; Arbeiter, K.; Ruffingshofer, D.; Regele, H.; Herkner, K.; Eickelberg, O.; Aufricht, C. Overexpression of HSP-72 confers cytoprotection in experimental peritoneal dialysis. Kidney Int. 2004, 66, 2300–2307. [Google Scholar] [CrossRef] [PubMed]
- Adachi, M.; Liu, Y.; Fujii, K.; Calderwood, S.K.; Nakai, A.; Imai, K.; Shinomura, Y. Oxidative Stress Impairs the Heat Stress Response and Delays Unfolded Protein Recovery. PLoS ONE 2009, 4, e7719. [Google Scholar] [CrossRef] [PubMed]
- Quagliaro, L.; Piconi, L.; Assaloni, R.; Martinelli, L.; Motz, E.; Ceriello, A. Intermittent High Glucose Enhances Apoptosis Related to Oxidative Stress in Human Umbilical Vein Endothelial Cells: The Role of Protein Kinase C and NAD(P)H-Oxidase Activation. Diabetes 2003, 52, 2795–2804. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Ron, D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 2011, 13, 184–190. [Google Scholar] [CrossRef]
- Kim, I.; Xu, W.; Reed, J.C. Cell death and endoplasmic reticulum stress: Disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 2008, 7, 1013–1030. [Google Scholar] [CrossRef]
- Herzog, R.; Bender, T.O.; Vychytil, A.; Bialas, K.; Aufricht, C.; Kratochwill, K. DynamicO-LinkedN-Acetylglucosamine Modification of Proteins Affects Stress Responses and Survival of Mesothelial Cells Exposed to Peritoneal Dialysis Fluids. J. Am. Soc. Nephrol. 2014, 25, 2778–2788. [Google Scholar] [CrossRef]
- Boehm, M.; Herzog, R.; Klinglmüller, F.; Lichtenauer, A.M.; Wagner, A.; Unterwurzacher, M.; Beelen, R.H.J.; Alper, S.L.; Aufricht, C.; Kratochwill, K. The Peritoneal Surface Proteome in a Model of Chronic Peritoneal Dialysis Reveals Mechanisms of Membrane Damage and Preservation. Front. Physiol. 2019, 10, 472–489. [Google Scholar] [CrossRef]
- Herzog, R.; Kuster, L.; Becker, J.; Gluexam, T.; Pils, D.; Spittler, A.; Bhasin, M.K.; Alper, S.L.; Vychytil, A.; Aufricht, C.; et al. Functional and Transcriptomic Characterization of Peritoneal Immune-Modulation by Addition of Alanyl-Glutamine to Dialysis Fluid. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Wiesenhofer, F.M.; Herzog, R.; Boehm, M.; Wagner, A.; Unterwurzacher, M.; Kasper, D.C.; Alper, S.L.; Vychytil, A.; Aufricht, C.; Kratochwill, K. Targeted Metabolomic Profiling of Peritoneal Dialysis Effluents Shows Anti-oxidative Capacity of Alanyl-Glutamine. Front. Physiol. 2019, 9, 1961–1974. [Google Scholar] [CrossRef]
- Choi, M.H.; Lee, I.K.; Kim, G.W.; Kim, B.U.; Han, Y.-H.; Yu, D.-Y.; Park, H.S.; Kim, K.Y.; Lee, J.S.; Choi, C.; et al. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nat. Cell Biol. 2005, 435, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, A.P. Structure-functions of HspB1 (Hsp27). Methods Mol. Biol. 2011, 787, 105–119. [Google Scholar] [PubMed]
- Bravo-Cordero, J.J.; Magalhaes, M.A.O.; Eddy, R.J.; Hodgson, L.; Pollard, J.W. Functions of cofilin in cell locomotion and invasion. Nat. Rev. Mol. Cell Biol. 2013, 14, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K. Signaling mechanisms and functional roles of cofilin phosphorylation and dephosphorylation. Cell. Signal. 2013, 25, 457–469. [Google Scholar] [CrossRef]
- Prasain, N.; Stevens, T. The actin cytoskeleton in endothelial cell phenotypes. Microvasc. Res. 2009, 77, 53–63. [Google Scholar] [CrossRef]
- Mattila, P.K.; Lappalainen, P. Filopodia: Molecular architecture and cellular functions. Nat. Rev. Mol. Cell Biol. 2008, 9, 446–454. [Google Scholar] [CrossRef]
- Lin, X.; Amore, A.; LoIacono, E.; Balegno, S.; Manniello, D.; Peruzzi, L.; Camilla, R.; Minieri, V.; Daprà, V.; Qian, J.; et al. Effect of glucose degradation products, glucose-containing dialysate and icodextrin on AQP1 and eNOS expression in cultured endothelial cells. J. Nephrol. 2009, 22, 117–122. [Google Scholar]
- Stenmark, K.R.; Frid, M.; Perros, F. Endothelial-to-Mesenchymal Transition: An Evolving Paradigm and a Promising Therapeutic Target in PAH. Circulation 2016, 133, 1734–1737. [Google Scholar] [CrossRef] [PubMed]
- Dave, J.M.; Bayless, K.J. Vimentin as an Integral Regulator of Cell Adhesion and Endothelial Sprouting. Microcirculation 2014, 21, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Matés, J.M.; Segura, J.A.; Campos-Sandoval, J.A.; Lobo, C.; Alonso, L.; Alonso, F.J.; Márquez, J. Glutamine homeostasis and mitochondrial dynamics. Int. J. Biochem. Cell Biol. 2009, 41, 2051–2061. [Google Scholar] [CrossRef] [PubMed]
- Curi, R. Glutamine, gene expression, and cell function. Front. Biosci. 2007, 12, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Singleton, K.D.; Serkova, N.; Beckey, V.E.; Wischmeyer, P.E. Glutamine attenuates lung injury and improves survival after sepsis: Role of enhanced heat shock protein expression. Crit. Care Med. 2005, 33, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Bartosova, M.; Herzog, R.; Ridinger, D.; Levai, E.; Jenei, H.; Zhang, C.; Gónzalez-Mateo, G.T.; Marinovic, I.; Hackert, T.; Bestvater, F.; et al. Alanyl-Glutamine Restores Tight Junction Organization after Disruption by a Conventional Peritoneal Dialysis Fluid. Biomolecules 2020, 10, 1178. [Google Scholar] [CrossRef] [PubMed]


| Ingenuity Canonical Pathways | Arterioles p-Value | HUVEC p-Value | Arterioles z-Score | HUVEC z-Score | Arterioles Molecules | HUVEC Molecules |
|---|---|---|---|---|---|---|
| Actin Cytoskeleton Sig. | 0.000 * | 0.000 * | −1.265 | 0.000 | KRAS, MYL6, PPP1R12A, MYLK, ARHGEF12, GRB2, ACTA2, MYL9, WASF2, CFL2, PPP1R12B | ACTB, ARHGAP35, ARHGEF1, ARPC2, BCAR1, CFL1, DOCK1, MAPK3, MYH10, MYL12B, PFN1, PFN2, ROCK1, SHC1 |
| Agrin Interactions at Neuromuscular Junction | 0.026 | 0.043 | KRAS, CTTN, ACTA2 | ACTB, AGRN, MAPK3, UTRN | ||
| Aldosterone Sig. in Epithelial Cells | 0.017 | 0.048 | HSPB1, KRAS, HSPA1A/HSPA1B, GRB2, DNAJB4 | DNAJB1, DNAJB6, HSP90AB1, ITPR3, MAPK3, SLC12A2 | ||
| Axonal Guidance Sig. | 0.001 * | 0.003 | TUBB4A, MYL6, KRAS, GNAO1, TUBB3, ARHGEF12, GRB2, ABLIM1, MYL9, PPP3CA, ERAP2, CFL2 | ACE, ARPC2, BCAR1, CFL1, DOCK1, EFNB2, GNAI2, LNPEP, MAPK3, MYL12B, NRP2, PFN1, PFN2, PLXNB2, ROCK1, SHC1, TUBG1 | ||
| Breast Cancer Regulation by Stathmin1 | 0.002 * | 0.019 | TUBB4A, KRAS, ARHGEF17, TUBB3, PPP1R12A, ARHGEF12, GRB2 | ARHGEF1, GNAI2, ITPR3, MAPK3, PPP2R1A, ROCK1, SHC1, TUBG1 | ||
| CXCR4 Sig. | 0.016 | 0.007 | −2.000 | −1.414 | KRAS, MYL6, GNAO1, GRB2, MYL9 | BCAR1, DOCK1, FNBP1, GNAI2, ITPR3, MAPK3, MYL12B, ROCK1 |
| Clathrin-mediated Endocytosis Sig. | 0.000 * | 0.043 | LYZ, APOA4, CTTN, GRB2, ACTA2, RBP4, PPP3CA, EPN1, ITGB4 | ACTB, ARPC2, DAB2, ITGB5, PICALM, RPS27A, TFRC | ||
| EIF2 Sig. | 0.014 | 0.000 * | 0.000 | 0.000 | KRAS, RPL7A, RPS9, GRB2, ACTA2, RPL27 | ACTB, EIF3B, EIF3G, MAPK3, RPL18A, RPL3, RPS27A, RPS27L, RPS5, RPS8, RPSA, SHC1 |
| ERK/MAPK Sig. | 0.031 | 0.043 | −1.342 | −1.633 | HSPB1, KRAS, CREB1, PPP1R12A, GRB2 | BCAR1, DOCK1, MAPK3, PPP2R1A, SHC1, STAT1, YWHAB |
| Ephrin B Sig. | 0.023 * | 0.007 | −2.000 | GNAO1, ABI1, CFL2 | CFL1, EFNB2, GNAI2, MAPK3, ROCK1 | |
| Ephrin Receptor Sig. | 0.004 * | 0.010 | −2.449 | −1.890 | KRAS, CREB1, GNAO1, ABI1, GRB2, CFL2 | ARPC2, BCAR1, CFL1, EFNB2, GNAI2, MAPK3, ROCK1, SHC1 |
| Epithelial Adherens Junction Sig. | 0.000 * | 0.042 | ZYX, TUBB4A, KRAS, MYL6, TUBB3, ACTA2, MYL9, EPN1 | ACTB, ARPC2, CTNNA1, JUP, MYH10, TUBG1 | ||
| Germ Cell-Sertoli Cell Junction Sig. | 0.000 * | 0.008 * | ZYX, TUBB4A, KRAS, TUBB3, GRB2, ACTA2, EPN1, CFL2 | ACTB, BCAR1, CFL1, CTNNA1, FNBP1, JUP, MAPK3, TUBG1 | ||
| ILK Sig. | 0.000 * | 0.000 * | −2.333 | −1.265 | VIM, MYL6, CREB1, TGFB1I1, PPP1R12A, GRB2, ACTA2, MYL9, ITGB4, CFL2, ILKAP | ACTB, CFL1, DOCK1, FLNB, FNBP1, ITGB5, LIMS1, MAPK3, MYH10, PPP2R1A, PTGS2 |
| Integrin Sig. | 0.000 | 0.000 * | −2.121 | −0.500 | ZYX, KRAS, CTTN, PPP1R12A, MYLK, GRB2, ACTA2, MYL9, ITGB4, PPP1R12B, ILKAP | ACTB, ARF3, ARF5, ARPC2, BCAR1, CAV1, DOCK1, FNBP1, ITGB5, LIMS1, MAPK3, MYL12B, PFN1, PFN2, ROCK1, SHC1, TSPAN6 |
| Oncostatin M Sig. | 0.046 | 0.034 | KRAS, GRB2 | MAPK3, SHC1, STAT1 | ||
| Paxillin Sig. | 0.020 | 0.034 | 1.000 | KRAS, GRB2, ACTA2, ITGB4 | ACTB, BCAR1, DOCK1, ITGB5, PTPN12 | |
| Phospholipase C Sig. | 0.000 * | 0.028 | −2.111 | −1.134 | KRAS, MYL6, CREB1, ARHGEF17, PPP1R12A, ARHGEF12, GRB2, MYL9, PPP3CA, MARCKS, PPP1R12B | ARHGEF1, FNBP1, HDAC3, ITPR3, MAPK3, MARCKS, MYL12B, PLD3, SHC1 |
| Protein Kinase A Sig. | 0.002 * | 0.016 | 0.816 | 0.000 | MYL6, AKAP12, ADD1, CREB1, PPP1R12A, MYLK, LIPE, MYL9, PPP3CA, ADD3 | AKAP12, AKAP9, FLNB, GNAI2, ITPR3, MAPK3, MYH10, MYL12B, PTGS2, PTPN12, PTPRB, ROCK1, YWHAB |
| Regulation of Actin-based Motility by Rho | 0.000 * | 0.000 * | 0.000 | 0.378 | MYL6, PPP1R12A, MYLK, ACTA2, MYL9, PPP1R12B | ACTB, ARPC2, CFL1, FNBP1, MYL12B, PFN1, PFN2, ROCK1 |
| Remodeling of Epithelial Adherens Junctions | 0.003 * | 0.026 | ZYX, TUBB4A, TUBB3, ACTA2 | ACTB, ARPC2, CTNNA1, TUBG1 | ||
| RhoA Sig. | 0.000 * | 0.000 * | −1.508 | 0.000 | CDC42EP1, MYL6, CDC42EP4, PPP1R12A, MYLK, ARHGEF12, SEPT9, ACTA2, MYL9, CFL2, PPP1R12B | ACTB, ANLN, ARHGAP35, ARHGEF1, ARPC2, CFL1, CIT, MYL12B, NRP2, PFN1, PFN2, PKN1, ROCK1, SEPTIN5 |
| RhoGDI Sig. | 0.000 * | 0.003 | 0.632 | 0.707 | MYL6, ARHGEF17, GNAO1, PPP1R12A, ARHGEF12, ACTA2, MYL9, WASF2, PPP1R12C, CFL2, PPP1R12B | ACTB, ARHGAP35, ARHGEF1, ARPC2, CFL1, FNBP1, GNAI2, MYL12B, ROCK1 |
| Sertoli Cell-Sertoli Cell Junction Sig. | 0.000 * | 0.035 | SPTBN1, TUBB4A, KRAS, MAP3K20, TUBB3, GUCY1B1, PRKG1, ACTA2, EPN1 | ACTB, BCAR1, CTNNA1, F11R, JUP, MAPK3, TUBG1 | ||
| Sig. by Rho Family GTPases | 0.000 * | 0.001 * | −1.807 | −1.265 | MAP3K20, CDC42EP4, GNAO1, PPP1R12A, ARHGEF12, ACTA2, MYL9, PPP1R12C, CFL2, PPP1R12B, VIM, CDC42EP1, MYL6, ARHGEF17, MYLK, GRB2, SEPT9 | ACTB, ARHGEF1, ARPC2, CFL1, CIT, FNBP1, GNAI2, MAPK3, MYL12B, PKN1, ROCK1, SEPTIN5 |
| Thrombin Sig. | 0.000 * | 0.023 | −2.000 | −2.646 | KRAS, MYL6, CREB1, GNAO1, PPP1R12A, MYLK, ARHGEF12, GRB2, MYL9, PPP1R12B | ARHGEF1, FNBP1, GNAI2, ITPR3, MAPK3, MYL12B, ROCK1, SHC1 |
| Virus Entry via Endocytic Pathways | 0.018 | 0.033 | KRAS, GRB2, ACTA2, ITGB4 | ACTB, CAV1, FLNB, ITGB5, TFRC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herzog, R.; Bartosova, M.; Tarantino, S.; Wagner, A.; Unterwurzacher, M.; Sacnun, J.M.; Lichtenauer, A.M.; Kuster, L.; Schaefer, B.; Alper, S.L.; et al. Peritoneal Dialysis Fluid Supplementation with Alanyl-Glutamine Attenuates Conventional Dialysis Fluid-Mediated Endothelial Cell Injury by Restoring Perturbed Cytoprotective Responses. Biomolecules 2020, 10, 1678. https://doi.org/10.3390/biom10121678
Herzog R, Bartosova M, Tarantino S, Wagner A, Unterwurzacher M, Sacnun JM, Lichtenauer AM, Kuster L, Schaefer B, Alper SL, et al. Peritoneal Dialysis Fluid Supplementation with Alanyl-Glutamine Attenuates Conventional Dialysis Fluid-Mediated Endothelial Cell Injury by Restoring Perturbed Cytoprotective Responses. Biomolecules. 2020; 10(12):1678. https://doi.org/10.3390/biom10121678
Chicago/Turabian StyleHerzog, Rebecca, Maria Bartosova, Silvia Tarantino, Anja Wagner, Markus Unterwurzacher, Juan Manuel Sacnun, Anton M. Lichtenauer, Lilian Kuster, Betti Schaefer, Seth L. Alper, and et al. 2020. "Peritoneal Dialysis Fluid Supplementation with Alanyl-Glutamine Attenuates Conventional Dialysis Fluid-Mediated Endothelial Cell Injury by Restoring Perturbed Cytoprotective Responses" Biomolecules 10, no. 12: 1678. https://doi.org/10.3390/biom10121678
APA StyleHerzog, R., Bartosova, M., Tarantino, S., Wagner, A., Unterwurzacher, M., Sacnun, J. M., Lichtenauer, A. M., Kuster, L., Schaefer, B., Alper, S. L., Aufricht, C., Schmitt, C. P., & Kratochwill, K. (2020). Peritoneal Dialysis Fluid Supplementation with Alanyl-Glutamine Attenuates Conventional Dialysis Fluid-Mediated Endothelial Cell Injury by Restoring Perturbed Cytoprotective Responses. Biomolecules, 10(12), 1678. https://doi.org/10.3390/biom10121678





