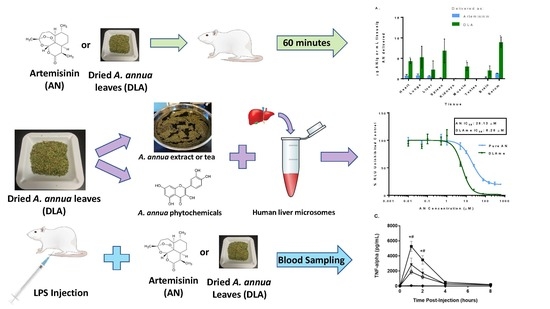
Dried Leaf Artemisia Annua Improves Bioavailability of Artemisinin via Cytochrome P450 Inhibition and Enhances Artemisinin Efficacy Downstream
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Reagents
2.3. In Vitro P450 Inhibition
2.4. Animals
2.5. In Vivo Artemisinin Tissue Distribution
2.6. Tissue Extraction
2.7. Serum and Urine Extractions
2.8. Fecal Extractions
2.9. Preparation of DLA Extracts and Teas
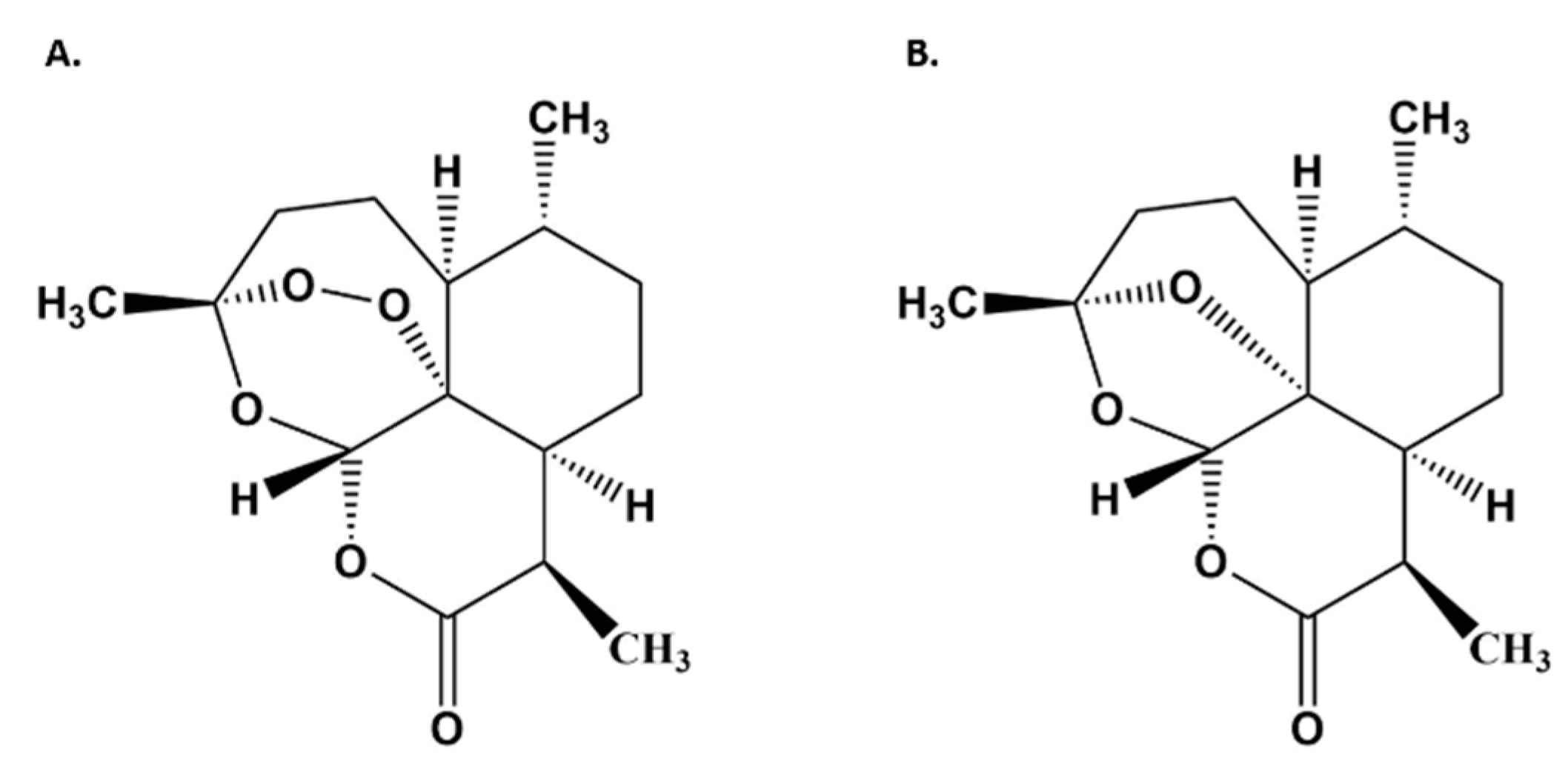
2.10. Artemisinin and Deoxyartemisinin Analyses
2.11. Attenuation of Inflammation in Vivo
2.12. Statistical Analysis
3. Results
3.1. Inhibition of CYP2B6 and CYP3A4 by DLA
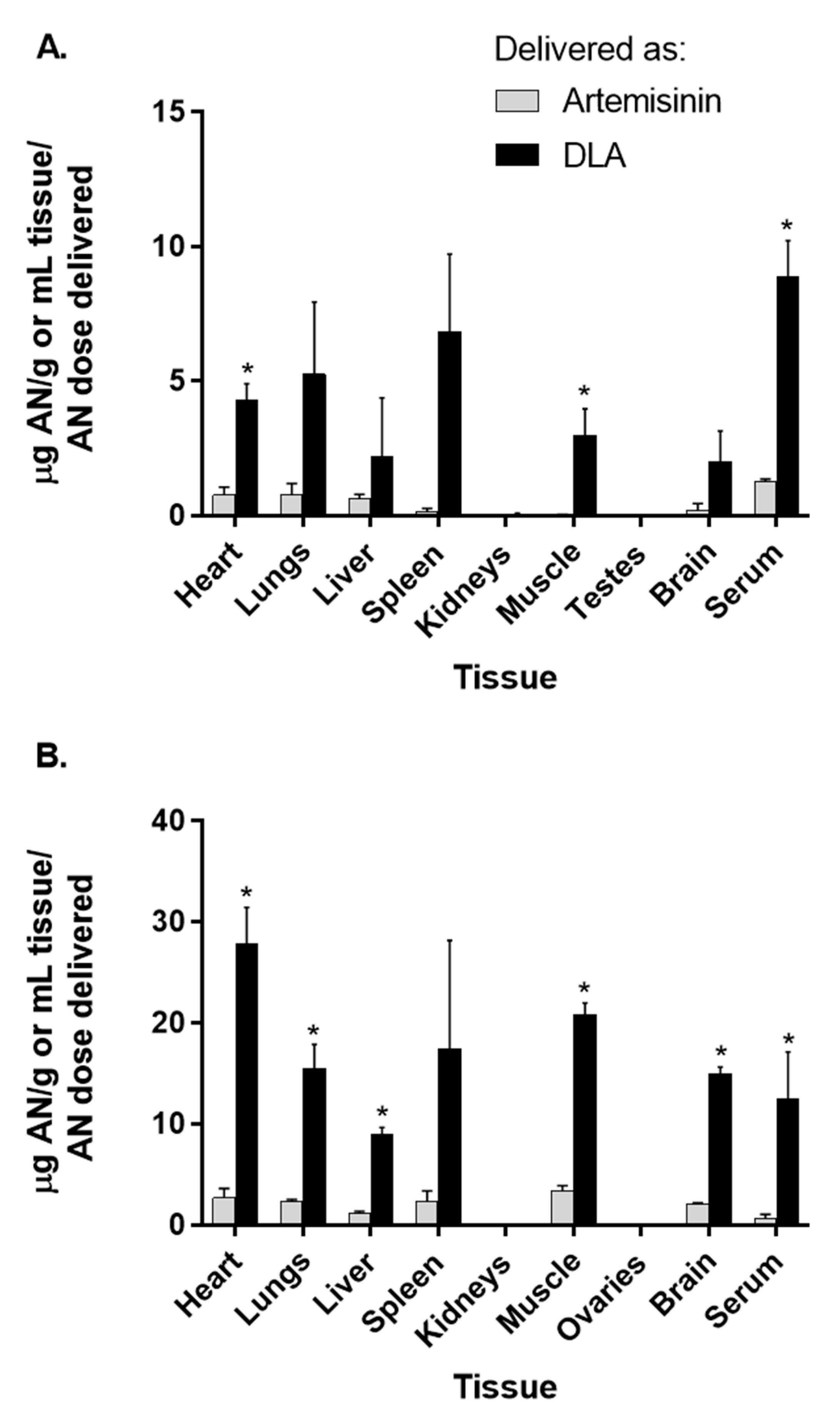
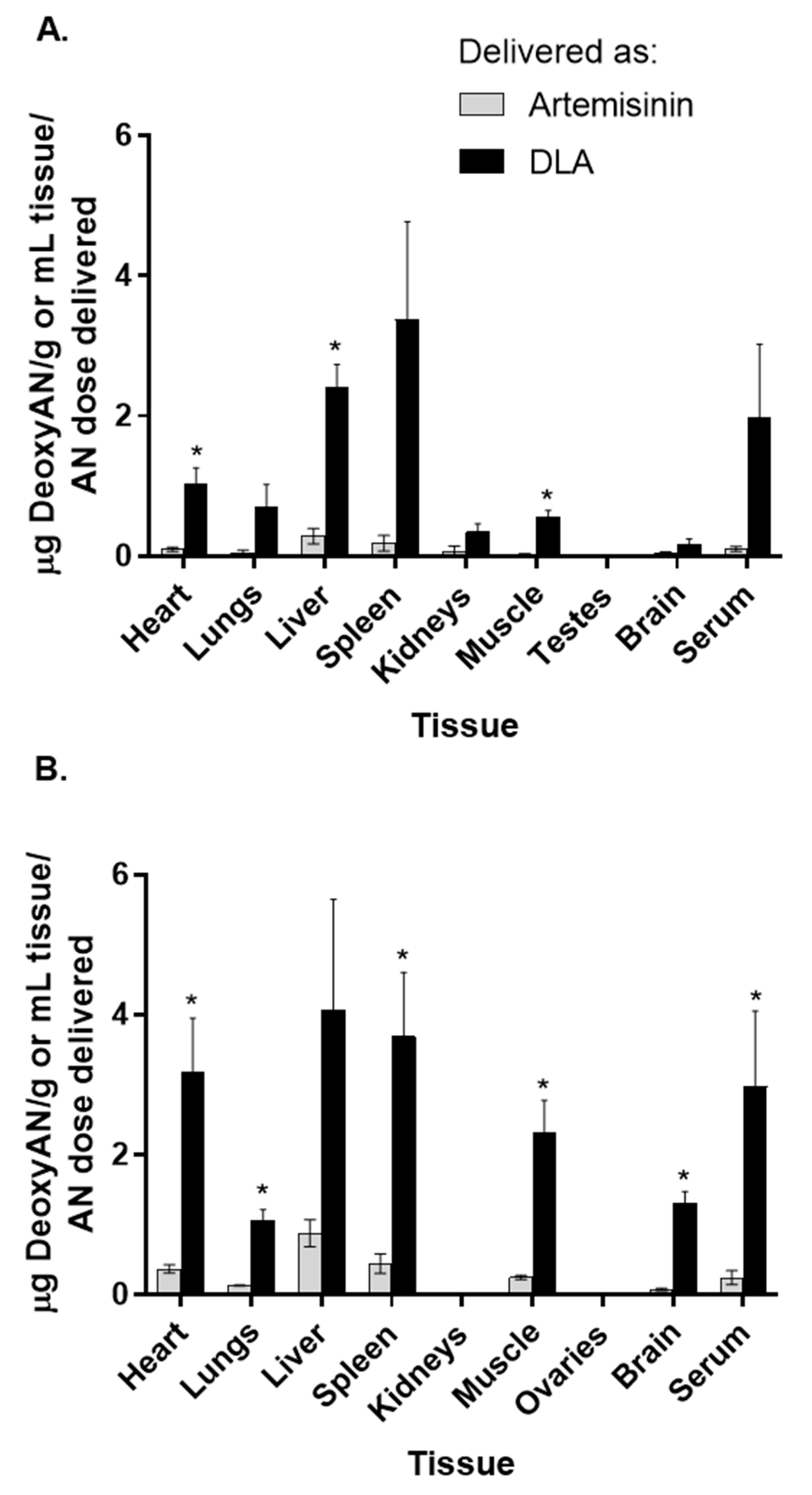
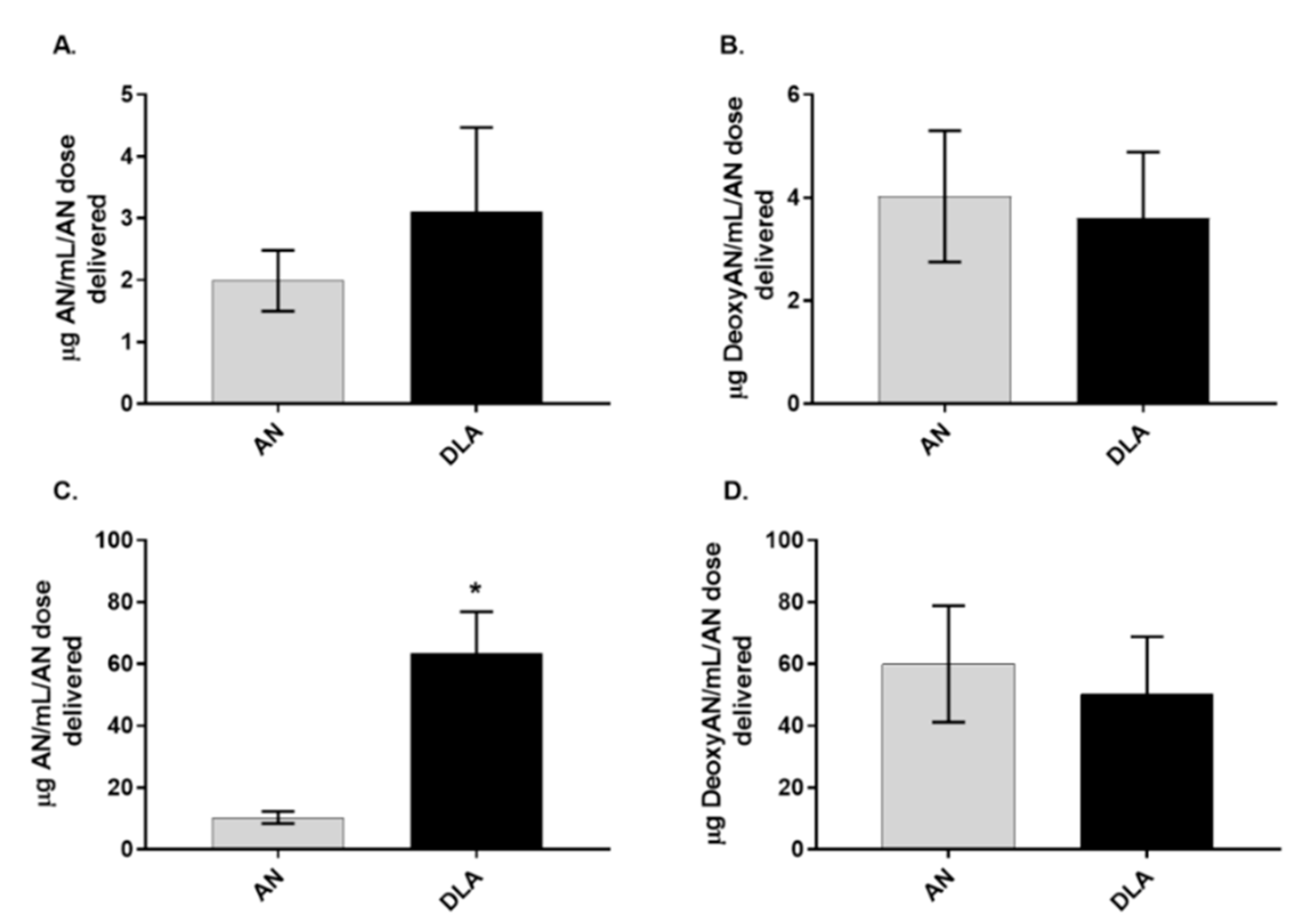
3.2. Artemisinin Delivered as DLA Increased Bioavailability in Some Tissues
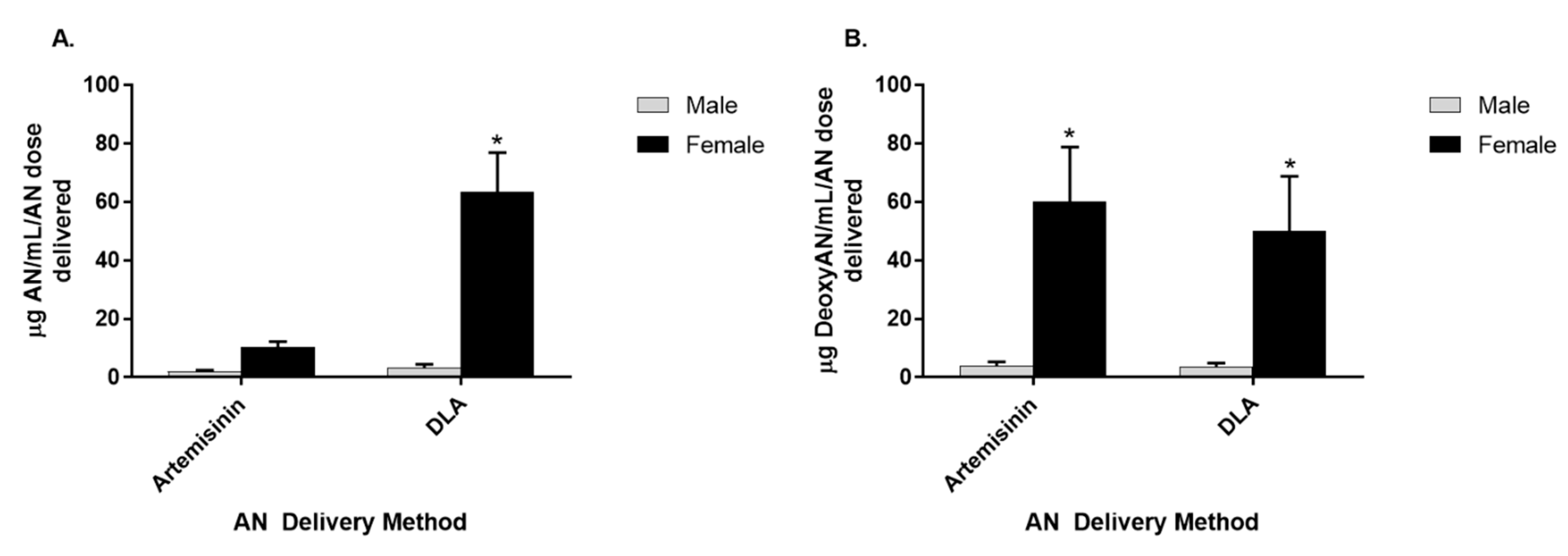
3.3. Artemisinin Elimination through the Urine is Gender-Dependent
3.4. Artemisinin Elimination through Feces
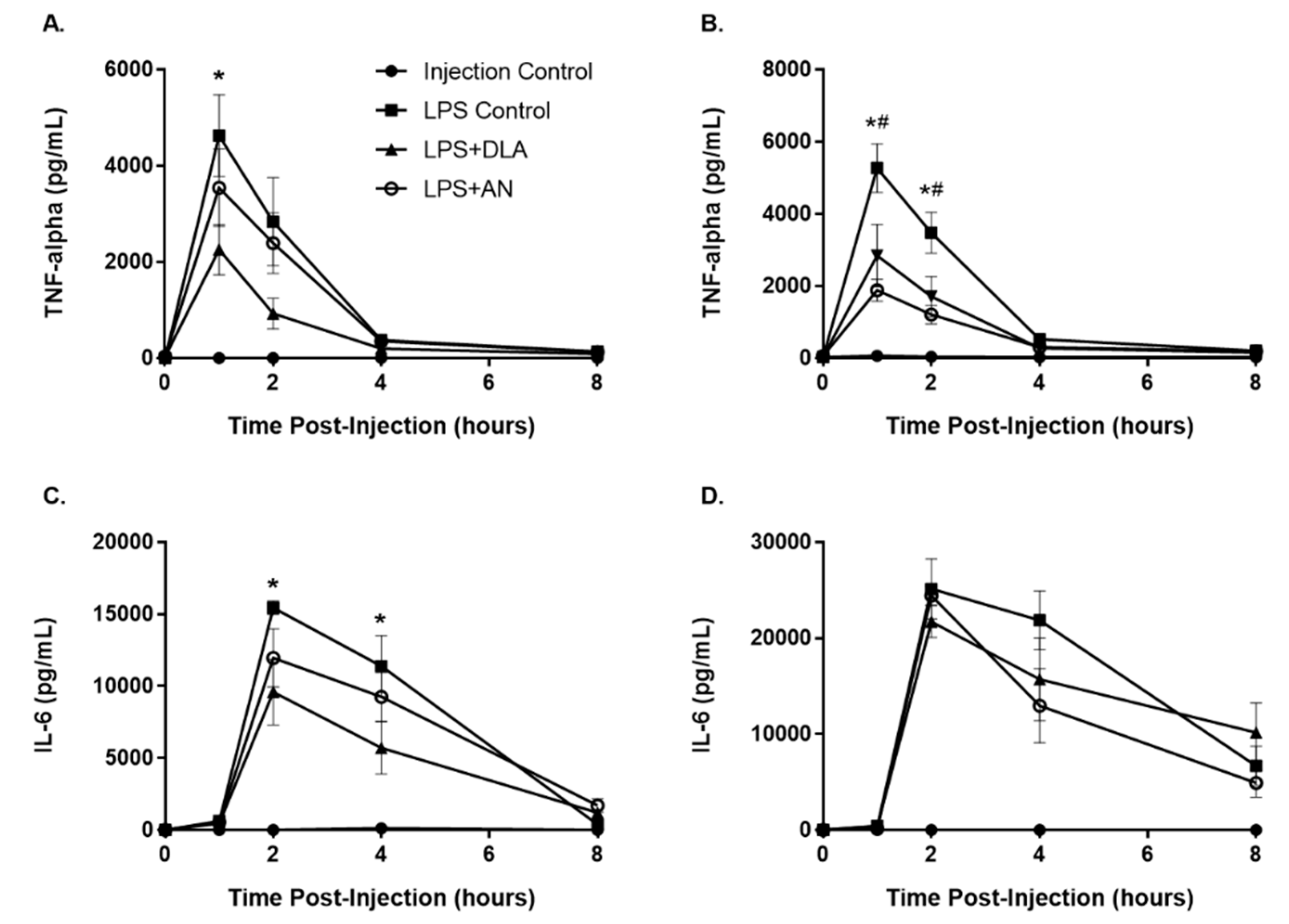
3.5. Attenuation of Inflammation by Artemisinin and DLA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Malaria. Available online: http://www.who.int/mediacentre/factsheets/fs094/en/ (accessed on 23 December 2019).
- WHO. Guidelines for the treatment of malaria. Available online: http://apps.who.int/iris/bitstream/10665/162441/1/9789241549127_eng.pdf?ua=1&ua=1 (accessed on 23 December 2019).
- Olsson, M.E.; Olofsson, L.M.; Lindahl, A.L.; Lundgren, A.; Brodelius, M.; Brodelius, P.E. Localization of enzymes of artemisinin biosynthesis to the apical cells of glandular secretory trichomes of Artemisia annua L. Phytochemistry 2009, 70, 1123–1128. [Google Scholar] [CrossRef]
- Hsu, E. The history of qing hao in the Chinese materia medica. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Munyangi, J.; Cornet-Vernet, L.; Idumbo, M.; Lu, C.; Lutgen, P.; Perronne, C.; Ngombe, N.; Bianga, J.; Mupenda, B.; Lalukala, P.; et al. Artemisia annua and Artemisia afra tea infusions vs. artesunate-amodiaquine (ASAQ) in treating Plasmodium falciparum malaria in a large scale, double blind, randomized clinical trial. Phytomedicine 2019, 57, 49–56. [Google Scholar] [CrossRef] [PubMed]
- ICIPE. Whole-leaf Artemisia Annua-based Antimalarial Drug: Report on Proof-of-Concepts Studies; ICIPE: Nairobi, Kenya, 2005. [Google Scholar]
- Desrosiers, M.R.; Weathers, P.J. Effect of leaf digestion and artemisinin solubility for use in oral consumption of dried Artemisia annua leaves to treat malaria. J. Ethnopharmacol. 2016, 190, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Desrosiers, M.R.; Weathers, P.J. Artemisinin permeability via Caco-2 cells increases after simulated digestion of Artemisia annua leaves. J. Ethnopharmacol. 2018, 210, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Weathers, P.J.; Arsenault, P.R.; Covello, P.S.; McMickle, A.; Teoh, K.H.; Reed, D.W. Artemisinin production in Artemisia annua: Studies in planta and results of a novel delivery method for treating malaria and other neglected diseases. Phytochem. Rev. 2011, 10, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Svensson, U.S.H.; Ashton, M. Identification of the human cytochrome P450 enzymes involved in the in vitro metabolism of artemisinin. Br. J. Clin. Pharmacol. 1999, 48, 528–535. [Google Scholar] [CrossRef]
- Lee, I.S.; Hufford, C.D. Metabolism of antimalarial sesquiterpene lactones. Pharmacol. Ther. 1990, 48, 345–355. [Google Scholar] [CrossRef]
- Weathers, P.J.; Elfawal, M.A.; Towler, M.J.; Acquaah-Mensah, G.K.; Rich, S.M. Pharmacokinetics of artemisinin delivered by oral consumption of Artemisia annua dried leaves in healthy vs. Plasmodium chabaudi-infected mice. J. Ethnopharmacol. 2014, 153, 732–736. [Google Scholar] [CrossRef]
- Räth, K.; Taxis, K.; Walz, G.; Gleiter, C.H.; Li, S.M.; Heide, L. Pharmacokinetic study of artemisinin after oral intake of a traditional preparation of Artemisia annua L.(annual wormwood). Am. J. Trop. Med. Hyg. 2004, 70, 128–132. [Google Scholar] [CrossRef]
- Xinyi, N.; Liyi, H.O.; Zhihong, R.; Zhenyu, S. Metabolic fate of Qinghaosu in rats; a new TLC densitometric method for its determination in biological material. Eur. J. Drug Metab. Pharmacokinet. 1985, 10, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Ashton, M. Quantitative in vivo and in vitro sex differences in artemisinin metabolism in rat. Xenobiotica 1999, 29, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.S.; Li, J.; Wang, Z.; Mi, C.; Ma, J.; Piao, L.X.; Xu, G.H.; Li, X.; Jin, X. Artemisinin inhibits inflammatory response via regulating NF-κB and MAPK signaling pathways. Immunopharmacol. Immunotoxicol. 2017, 39, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Choi, W.J.; Lee, S.; Kim, W.J.; Lee, D.C.; Sohn, U.D.; Shin, H.S.; Kim, W. Anti-inflammatory, Antioxidant and Antimicrobial Effects of Artemisinin Extracts from Artemisia annua L. Kor. J. Physiol. Pharmacol. 2015, 19, 21–27. [Google Scholar] [CrossRef]
- Rathee, P.; Chaudhary, H.; Rathee, S.; Rathee, D.; Kumar, V.; Kohli, K. Mechanism of action of flavonoids as anti-inflammatory agents: a review. Inflamm. Allergy Drug Targets 2009, 8, 229–235. [Google Scholar] [CrossRef]
- De Cássia da Silveira e Sá, R.; Andrade, L.N.; de Sousa, D.P. A review on anti-inflammatory activity of monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar] [CrossRef]
- Li, Y.J.; Guo, Y.; Yang, Q.; Weng, X.G.; Yang, L.; Wang, Y.J.; Chen, Y.; Zhang, D.; Li, Q.; Liu, X.C.; et al. Flavonoids casticin and chrysosplenol D from Artemisia annua L. inhibit inflammation in vitro and in vivo. Toxicol. Appl. Pharmacol. 2015, 286, 151–158. [Google Scholar] [CrossRef]
- Juergens, U.R.; Engelen, T.; Racké, K.; Stöber, M.; Gillissen, A.; Vetter, H. Inhibitory activity of 1, 8-cineol (eucalyptol) on cytokine production in cultured human lymphocytes and monocytes. Pulm. Pharmacol. Ther. 2004, 17, 281–287. [Google Scholar] [CrossRef]
- Melillo de Magalhães, P.; Dupont, I.; Hendrickx, A.; Joly, A.; Raas, T.; Dessy, S.; Sergent, T.; Schneider, Y.-J. Anti-inflammatory effect and modulation of cytochrome P450 activities by Artemisia annua tea infusions in human intestinal Caco-2 cells. Food Chem. 2012, 134, 864–871. [Google Scholar] [CrossRef]
- Weathers, P.J.; Jordan, N.J.; Lasin, P.; Towler, M.J. Simulated digestion of dried leaves of Artemisia annua consumed as a treatment (pACT) for malaria. J. Ethnopharmacol. 2014, 151, 858–863. [Google Scholar] [CrossRef]
- Weathers, P.J.; Towler, M.J. The flavonoids casticin and artemetin are poorly extracted and are unstable in an Artemisia annua tea infusion. Planta Med. 2012, 78, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Arvouet-Grand, A.; Vennat, B.; Pourrat, A.; Legret, P. Standardization of propolis extract and identification of principal constituents. J. Pharm. Belg. 1994, 49, 462–468. [Google Scholar] [PubMed]
- Weathers, P.J.; Towler, M.J. Changes in key constituents of clonally propagated Artemisia annua L. during preparation of compressed leaf tablets for possible therapeutic use. Ind. Crop. Prod. 2014, 62, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Gruessner, B.M.; Cornet-Vernet, L.; Desrosiers, M.R.; Lutgen, P.; Towler, M.J.; Weathers, P.J. It is not just artemisinin: Artemisia sp. for treating diseases including malaria and schistosomiasis. Phytochem. Rev. 2019. [Google Scholar] [CrossRef]
- Munyangi, J.; Cornet-Vernet, L.; Idumbo, M.; Lu, C.; Lutgen, P.; Perronne, C.; Ngombe, N.; Bianga, J.; Mupenda, B.; Lalukala, P.; et al. Effect of Artemisia annua and Artemisia afra tea infusions on schistosomiasis in a large clinical trial. Phytomedicine 2018, 51, 233–240. [Google Scholar] [CrossRef]
- Titulaer, H.A.C.; Zuidema, J.; Kager, P.A.; Wetsteyn, J.; Lugt, C.B.; Merkus, F. The pharmacokinetics of artemisinin after oral, intramuscular and rectal administration to volunteers. J. Pharm. Pharmacol. 1990, 42, 810–813. [Google Scholar] [CrossRef]
- Towler, M.J.; Weathers, P.J. Variations in key artemisinic and other metabolites throughout plant development in Artemisia annua L. for potential therapeutic use. Ind. Crop. Prod. 2015, 67, 185–191. [Google Scholar] [CrossRef]
- Bison, S.; Carboni, L.; Arban, R.; Bate, S.; Gerrard, P.A.; Razzoli, M. Differential behavioral, physiological, and hormonal sensitivity to LPS challenge in rats. Int. J. Interferon Cytokine Mediat. Res. 2009, 1, 1–13. [Google Scholar] [CrossRef]
- Ferreira, J.F.S.; Luthria, D.L.; Sasaki, T.; Heyerick, A. Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules 2010, 15, 3135–3170. [Google Scholar] [CrossRef]
- Lau, A.J.; Chang, T.K.H. Inhibition of Human CYP2B6-Catalyzed Bupropion Hydroxylation by Ginkgo biloba Extract: Effect of Terpene Trilactones and Flavonols. Drug Metab. Dispos. 2009, 37, 1931. [Google Scholar] [CrossRef]
- Wei, S.; Ji, H.; Yang, B.; Ma, L.; Bei, Z.; Li, X.; Dang, H.; Yang, X.; Liu, C.; Wu, X.; et al. Impact of chrysosplenetin on the pharmacokinetics and anti-malarial efficacy of artemisinin against Plasmodium berghei as well as in vitro CYP450 enzymatic activities in rat liver microsome. Malar. J. 2015, 14, 432. [Google Scholar] [CrossRef] [PubMed]
- Sergent, T.; Dupont, I.; Van Der Heiden, E.; Scippo, M.L.; Pussemier, L.; Larondelle, Y.; Schneider, Y.J. CYP1A1 and CYP3A4 modulation by dietary flavonoids in human intestinal Caco-2 cells. Toxicol. Lett. 2009, 191, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Piao, Y.J.; Kang, K.W. Effects of quercetin on the bioavailability of doxorubicin in rats: Role of CYP3A4 and P-gp inhibition by quercetin. Arch. Pharm. Res. 2011, 34, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Alin, M.H.; Bjorkman, A. Concentration and time dependency of artemisinin efficacy against Plasmodium falciparum in vitro. Am. J. Trop. Med. Hyg. 1994, 50, 771–776. [Google Scholar] [CrossRef] [PubMed]
- WHO. Position Statement. Effectiveness of Non-Pharmaceutical Forms of Artemisia annua L. against malaria. Available online: https://www.who.int/malaria/diagnosis_treatment/position_statement_herbal_remedy_artemisia_annua_l.pdf?ua=1 (accessed on 23 December 2019).
- WHO. The Use of Non-pharmaceutical Forms of Artemisia. Available online: https://www.who.int/publications-detail/the-use-of-non-pharmaceutical-forms-of-artemisia (accessed on 23 December 2019).
| Phytochemical or Extract | CYP2B6 IC50 (µM) | CYP3A4 IC50 (µM) |
|---|---|---|
| DLAme | 6.07 ** | 4.93 # |
| DLAtea | 2.31 ** | 5.67 # |
| A. aframe | 7.89 ** | 5.18 # |
| A. afratea | 11.27 ** | 5.59 # |
| Arteannuin B | 9.36 ** | 12.33 # |
| Quercetin | 19.86 * | 4.59 # |
| Artemisinin | 27.75 | >600 |
| Chrysosplenol-D | 52.06 | 22.96 # |
| Chrysosplenetin | 74.67 | 21.68 # |
| Kaempferol | >600 | 33.84 # |
| Deoxyartemisinin | 110.86 | >600 |
| Camphor (+) | 119.01 | >600 |
| Artemisinic Acid | 122.83 | 239.5 # |
| Camphor (-) | 141.93 | >600 |
| Scopoletin | >600 | 410.19 # |
| Chlorogenic Acid | >600 | >600 |
| Artemisinin Treated | DLA Treated | |||||||
|---|---|---|---|---|---|---|---|---|
| Artemisinin (µg AN/g tissue/g AN delivered ± SD) | Deoxyartemisinin (µg DeoxyAN/g tissue/g AN delivered ± SD) | Artemisinin (µg AN/g tissue/g AN delivered ± SD) | Deoxyartemisinin (µg DeoxyAN/g tissue/g AN delivered ± SD) | |||||
| Tissue | Male | Female | Male | Female | Male | Female | Male | Female |
| Heart | 0.77 ± 0.49 | 2.70 ± 1.87 | 0.097 ± 0.050 | 0.37 ± 0.12 * | 4.30 ± 1.06 | 27.81 ± 7.25 * | 1.02 ± 0.42 | 3.18 ± 1.54 |
| Lungs | 0.79 ± 0.69 | 2.37 ± 0.26 * | 0.055 ± 0.054 | 0.13 ± 0.018 * | 5.26 ± 4.64 | 15.56 ± 4.63 * | 0.70 ± 0.65 | 1.06 ± 0.31 |
| Liver | 0.62 ± 0.30 | 1.23 ± 0.26 * | 0.29 ± 0.19 | 0.88 ± 0.39 | 2.19 ± 3.79 | 8.96 ± 1.40 * | 2.42 ± 0.55 | 4.06 ± 3.18 |
| Spleen | 0.14 ± 0.24 | 2.37 ± 2.05 | 0.19 ± 0.20 | 0.44 ± 0.28 | 6.82 ± 5.03 | 17.49 ± 21.37 | 3.38 ± 2.79 | 3.69 ± 1.83 |
| Kidneys | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.074 ± 0.12 | 0.00 ± 0.00 | 0.048 ± 0.096 | 0.00 ± 0.00 | 0.35 ± 0.23 | 0.00 ± 0.00* |
| Muscle | 0.019 ± 0.034 | 3.41 ± 0.85 * | 0.025 ± 0.018 | 0.25 ± 0.049 * | 2.99 ± 1.68 | 20.84 ± 2.26 * | 0.57 ± 0.15 | 2.31 ± 0.93 * |
| Testes/Ovaries | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Brain | 0.22 ± 0.38 | 2.05 ± 0.35 * | 0.038 ± 0.043 | 0.075 ± 0.034 | 2.02 ± 1.96 | 15.00 ± 1.31 * | 0.18 ± 0.12 | 1.30 ± 0.36 * |
| Serum | 1.29 ± 0.11 | 0.67 ± 0.75 | 0.10 ± 0.061 | 0.24 ± 0.20 | 8.90 ± 2.63 | 12.49 ± 9.34 | 1.97 ± 2.10 | 2.97 ± 2.17 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desrosiers, M.R.; Mittleman, A.; Weathers, P.J. Dried Leaf Artemisia Annua Improves Bioavailability of Artemisinin via Cytochrome P450 Inhibition and Enhances Artemisinin Efficacy Downstream. Biomolecules 2020, 10, 254. https://doi.org/10.3390/biom10020254
Desrosiers MR, Mittleman A, Weathers PJ. Dried Leaf Artemisia Annua Improves Bioavailability of Artemisinin via Cytochrome P450 Inhibition and Enhances Artemisinin Efficacy Downstream. Biomolecules. 2020; 10(2):254. https://doi.org/10.3390/biom10020254
Chicago/Turabian StyleDesrosiers, Matthew R., Alexis Mittleman, and Pamela J. Weathers. 2020. "Dried Leaf Artemisia Annua Improves Bioavailability of Artemisinin via Cytochrome P450 Inhibition and Enhances Artemisinin Efficacy Downstream" Biomolecules 10, no. 2: 254. https://doi.org/10.3390/biom10020254
APA StyleDesrosiers, M. R., Mittleman, A., & Weathers, P. J. (2020). Dried Leaf Artemisia Annua Improves Bioavailability of Artemisinin via Cytochrome P450 Inhibition and Enhances Artemisinin Efficacy Downstream. Biomolecules, 10(2), 254. https://doi.org/10.3390/biom10020254