Hemoglobin Reassembly of Antimicrobial Fragments from the Midgut of Triatoma infestans
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Bacteria Inoculation and Intestinal Content Collection
2.3. Sample Fractionation
2.3.1. Acid and Solid Phase Extractions
2.3.2. Reverse Phase High-Performance Liquid Chromatography
2.4. Liquid Growth Inhibition Assay
2.5. Mass Spectrometry (LC/MS)
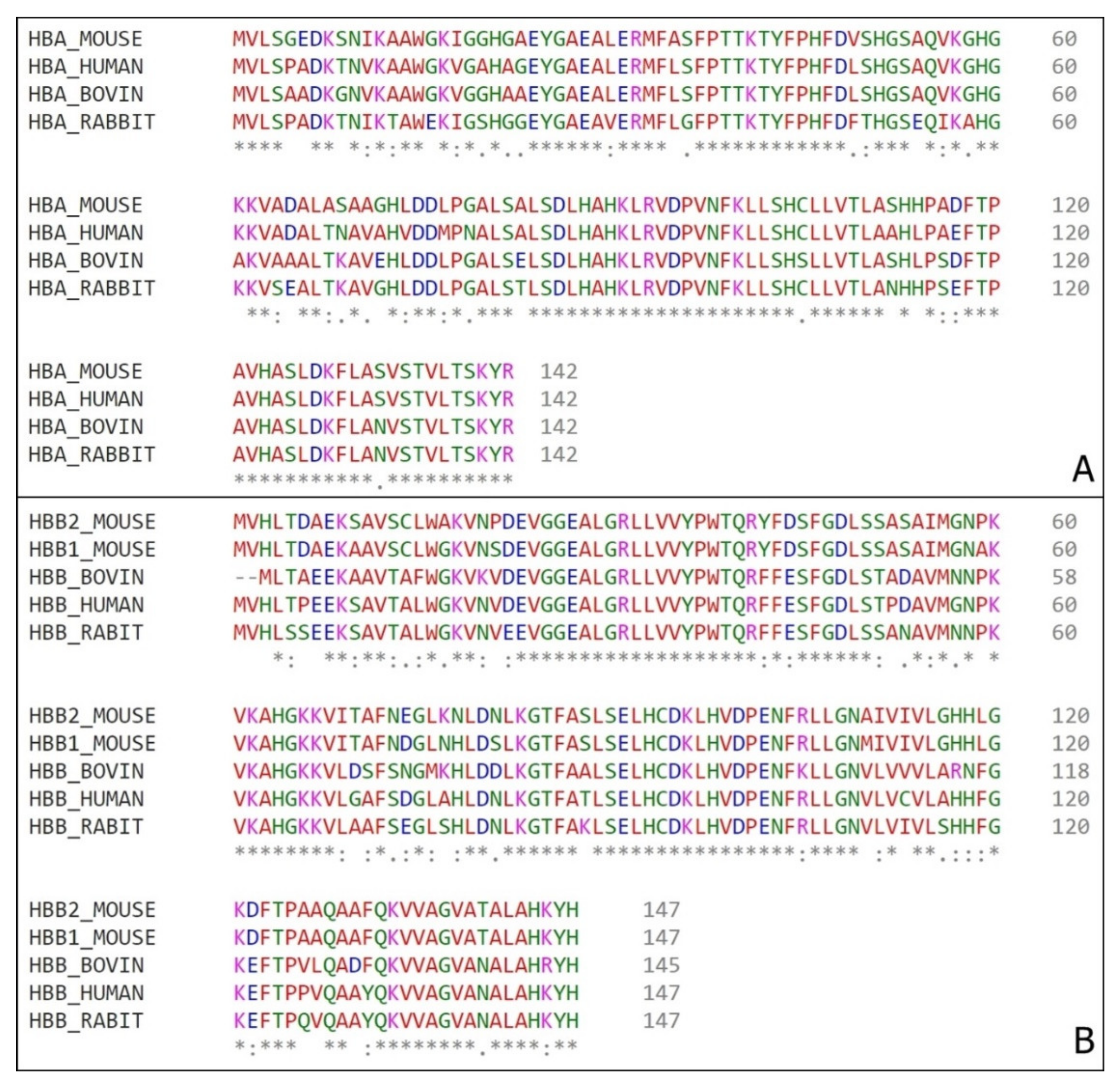
2.6. Computational Analysis and Sequences Alignment
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marengo-Rowe, A.J. Structure-function relations of human hemoglobins. Proc. (Bayl Univ Med. Cent.) 2006, 19, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Hobson, D.; Hirsch, J.G. The Antibacterial Activity of Hemoglobin. J. Exp. Med. 1958, 107, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, V.T.; Karelin, A.A.; Philippova, M.M.; Nazimov, I.V.; Pletnev, V.Z. Hemoglobin as a source of endogenous bioactive peptides: The concept of tissue-specific peptide pool. Biopolymers 1997, 43, 171–188. [Google Scholar] [CrossRef]
- Schally, A.V.; Baba, Y.; Arimura, A.; Redding, T.W.; White, W.F. Evidence for peptide nature of LH and FSH-releasing hormones, Biochem. Biophys. Res. Commun. 1971, 42, 50–56. [Google Scholar] [CrossRef]
- Schally, A.V.; Baba, Y.; Nair, R.M.G. The Amino acid sequence of a peptide with growth hormone-releasing activity isolated from porcine hypotalamus. J. Biol. Chem. 1971, 246, 6647–6650. [Google Scholar]
- Schally, A.V.; Huang, W.Y.; Redding, T.W.; Coy, D.H.; Chihara, K.; Chang, R.C.C.; Raymond, V.; Labrie, F. Isolation, structural elucidation and synthesis of a tetradecapeptide with in vitro ACTH-releasing activity corresponding to residues 33–46 of the α-chain of porcine hemoglobin. Biochem. Biophys. Res. Commun 1978, 82, 582–588. [Google Scholar] [CrossRef]
- Chang, R.C.C.; Huang, W.Y.; Redding, T.W.; Arimura, A.; Coy, D.H.; Schally, A.V. Isolation and structure of several peptides from porcine hypothalamic. Biochem. Biophys. Acta 1980, 625, 266–273. [Google Scholar]
- Brantl, V.; Gramsch, C.; Lottspeich, F.; Mertz, R.; Jaeger, K.-H.; Herz, A. Novel opioid peptides derived from hemoglobin: Hemorphins. Eur. J. Pharmacol. 1986, 125, 309–310. [Google Scholar] [CrossRef]
- Glamsta, E.-L.; Marklund, A.; Hellman, U.; Wemstedt, C.; Terenius, L.; Nyberg, F. Isolation and characterization of a hemoglobinderived opioid peptide from the human pituitary gland. Regul. Pept. 1991, 34, 169–179. [Google Scholar] [CrossRef]
- Glamsta, E.-L.; Meyrson, B.; Silbening, J.; Terenius, L.; Nyberg, F. Isolation of a hemoglobin-derived opioid peptide from cerebrosoinal fluid of patients with cerebrovascular bleedings. B&hem. Biophys. Res. Commun. 1992, 184, 1060–1066. [Google Scholar]
- Erchegyi, J.; Kastin, A.J.; Zadina, J.E.; Qiu, X.-D. Isolation of a heptapeptide Val-Val-Tyr-Pro-Trp-Thr-Gln (valorphin) with some opiate activity. Int. J. Pept. Protein Res. 1992, 39, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Lantz, I.; Glamsta, E.-L.; Talback, L.; Nyberg, F. Hemorphins derived from hemoglobin have an inhibitory action on anniotensin converting enzyme activity. FEBS Lett. 1991, 287, 39–41. [Google Scholar] [CrossRef]
- Barkhudaryan, N.A.; Kellermann, J.; Galoyan, A.A.; Lottspeich, F. High molecular weight aspartic endopeptidase generates a coronaro- constrictory peptide from the p-chain of hemoglobin. FEBS Lett. 1993, 329, 215–218. [Google Scholar] [CrossRef]
- Mak, P.; W´ojcik, K.; Silberring, J.; Dubin, A. Antimicrobal peptides from heme-containing proteins: Hemocidins. Antonie van Leeuwenhoek 2000, 77, 197–200. [Google Scholar] [CrossRef]
- Mak, P.; Wojcik, K.; Silberring, J.; Dubin, A. Antimicrobial peptides derived from heme-containing proteins: Hemocidins. Antonie Van Leeuwenhoek 2004, 77, 197–207. [Google Scholar] [CrossRef]
- Fogaça, A.C.; da Silva, P.I., Jr.; Miranda, M.T.; Bianchi, A.G.; Miranda, A.; Ribolla, P.E.; Daffre, S. Antimicrobial activity of a bovine hemoglobin fragment in the tick Boophilus microplus. J. Biol Chem. 1999, 274, 25330–25334. [Google Scholar]
- Nakajima, Y.; Ogihara, K.; Taylor, D.; Yamakawa, M. Antibacterial hemoglobin fragments from the midgut of the soft tick, Ornithodoros moubata (Acari: Argasidae). J. Med. Entomol. 2003, 40, 78–81. [Google Scholar] [CrossRef]
- Belmonte, R.; Cruz, C.E.; Pires, J.R.; Daffre, S. Purification and characterization of Hb 98–114: A novel hemoglobin-derived antimicrobial peptide from the midgut of Rhipicephalus (Boophilus) microplus. Peptides 2012, 37, 120–127. [Google Scholar] [CrossRef]
- Diniz, L.C.L.; Miranda, A.; da Silva, P.I., Jr. Human Antimicrobial Peptide Isolated from Triatoma infestans Haemolymph, Trypanosoma cruzi-Transmitting Vector. Front. Cell Infect. Microbiol. 2018, 8, 354. [Google Scholar] [CrossRef]
- Bulet, P. Strategies for the discovery, isolation, and characterization of natural bioactive peptides from the immune system of invertebrates. Methods Mol. Biol. 2008, 494, 9–29. [Google Scholar]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Hetru, C.; Bulet, P. Strategies for the isolation and characterization of antimicrobial peptides of invertebrates. Methods Mol. Biol. 1997, 78, 35–49. [Google Scholar] [PubMed]
- Poppel, A.K.; Vogel, H.; Wiesner, J.; Vilcinskas, A. Antimicrobial peptides expressed in medicinal maggots of the blow fly Lucilia sericata show combinatorial activity against bacteria. Antimicrob. Agents Chemother. 2015, 59, 2508–2514. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.I., Jr.; Daffre, S.; Bulet, P. Isolation and characterization of gomesin, an 18-residue cysteine-rich defense peptide from the spider Acanthoscurria gomesiana hemocytes with sequence similarities to horseshoe crab antimicrobial peptides of the tachyplesin family. J. Biol. Chem. 2000, 275. [Google Scholar] [CrossRef] [PubMed]
- Lorenzini, D.M.; Da Silva, P.I., Jr.; Fogaca, A.C.; Bulet, P.; Daffre, S. Acanthoscurrin: A novel glycine-rich antimicrobial peptide constitutively expressed in the hemocytes of the spider Acanthoscurria gomesiana. Dev. Comp. Immunol. 2003, 27, 781–791. [Google Scholar] [CrossRef]
- Riciluca, K.C.; Sayegh, R.S.; Melo, R.L.; Silva, P.I., Jr. Rondonin an antifungal peptide from spider (Acanthoscurria rondoniae) haemolymph. Results Immunol. 2012, 2, 66–71. [Google Scholar] [CrossRef]
- Universal Protein. Available online: www.uniprot.org (accessed on 22 May 2019).
- National Center for Biotechnology Information. Available online: www.ncbi.nlm.nih.gov (accessed on 22 May 2019).
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Peptide Property Calculator. Available online: http://pepcalc.com/ (accessed on 16 November 2019).
- APD3: Antimicrobial Peptide Calculator and Predictor. Available online: http://aps.unmc.edu/AP/prediction/prediction_main.php (accessed on 16 November 2019).
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, 1087–1093. [Google Scholar] [CrossRef]
- Barrett, F.M. Absorption of fluid from the anterior midgut of Rhodnius. J. Insect Physiol. 1982, 28, 335–341. [Google Scholar] [CrossRef]
- De Azambuja, P.; Guimares, J.A.; Garcia, E.S. Haemolytic factor from the crop of Rhodnius prolixus: Evidence and partial characterisation. J. Insect Physiol. 1983, 29, 833–837. [Google Scholar] [CrossRef]
- Lehane, M.J. Managing the blood meal. In The biology of blood-sucking insects. Cambridge University Press: Cambridge, UK, 2005; pp. 84–115. [Google Scholar]
- Schaub, G.A. Kissing bugs. In Encyclopedia of Parasitology, 3rd ed.; Mehlhorn, H., Ed.; Springer-Verlag: Heidelberg, Germany, 2008; pp. 684–686. [Google Scholar]
- Albritton, A.B. Standard Value in Blood; W.B. Saunders: Philadelphia, PA, USA, 1961; p. 19. [Google Scholar]
- Altman, P.L.; Dittmer, D.S. Blood and other body fluids. Respiration and Circulation; MDI: Federation of American Societies of Experimental Biology: Rockville, MA, USA, 1971; Volume 540. [Google Scholar]
- Terra, W.R.; Ferreira, C. Insect digestive enzymes – properties, compartmentalization and function. Comp. Biochem. Physiol. B 1994, 109, 1–62. [Google Scholar] [CrossRef]
- Terra, W.R.; Ferreira, C.; Garcia, E.S. Origin, distribution, properties and functions of them major Rhodnius prolixus midgut hydrolases. Insect Biochem. 1988, 18, 423–434. [Google Scholar] [CrossRef]
- Lopez-Ordoñez, T.; Rodriguez, M.H.; Hernandez-Hernandez, F.D. Characterization of a cDNA encoding a cathepsin L-like protein of Rhodnius prolixus. Insect Mol. Biol. 2011, 10, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Kollien, A.H.; Waniek, P.J.; Nisbet, A.J.; Billingsley, P.F.; Schaub, G.A. Activity and sequence characterization of two cysteine proteases in the digestive tract of the reduviid bug Triatoma infestans. Insect Mol. Biol. 2004, 13, 569–579. [Google Scholar] [CrossRef]
- Barrett, A.J.; Rawlings, N.D.; Wössner, J.F. Handbook of Proteolytic Enzymes, 2nd ed.; Elsevier: London, UK, 2004; p. 2368. [Google Scholar]
- Balczun, C.; Pausch, J.; Schaub, G. Blood digestion in triatomines—a review. Mitt. Dtsch. Ges. Allg. Angew. Entomol. 2012, 18, 331–334. [Google Scholar]
- Billingsley, P.F.; Downe, A.E.R. Cellular localization of aminopeptidase in the midgut of Rhodnius prolixus Stål (Hemiptera, Reduviidae) during blood digestion. Cell Tissue Res. 1985, 241, 421–428. [Google Scholar] [CrossRef]
- Ferreira, C.; Ribeiro, A.F.; Garcia, E.S.; Terra, W.R. Digestive enzymes trapped between and associated with the double plasma membranes of Rhodnius prolixus posterior midgut cells. Insect Biochem. 1988, 18, 521–530. [Google Scholar] [CrossRef]
- Mihajlovic, M.; Lazaridis, T. Antimicrobial peptides bind more strongly to membrane pores. Biochim. Biophys. Acta 2010, 1798, 1494–1502. [Google Scholar] [CrossRef]
- Powers, J.P.S.; Hancock, R.E.W. The relationship between peptide structure and bacterial activity. Peptides 2003, 24, 1681–1691. [Google Scholar] [CrossRef]
- Liepke, C.; Baxmann, S.; Heine, C.; Breithaupt, N.; Ständker, L.; Forssmann, W.-G. Human hemoglobin-derived peptides exhibit antimicrobial activity: A class of host defense peptides. J. Chromatogr. B. 2003, 791, 345–356. [Google Scholar] [CrossRef]
- Catiau, L.; Traisnel, J.; Chihib, N.-E.; Le Flem, G.; Blanpain, A.; Melnyk, O.; Guillochon, D.; Nedjar-Arroume, N. RYH: A minimal peptidic sequence obtained from beta-chain hemoglobin exhibiting an antimicrobial activity. Peptides 2011, 32, 1463–1468. [Google Scholar]
- Catiau, L.; Traisnel, J.; Delval-Dubois, V.; Chihib, N.-E.; Guillochon, D.; Nedjar-Arroume, N. Minimal antimicrobial peptidic sequence from hemoglobin alpha-chain: KYR. Peptides 2011, 32, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Froidevaux, R.; Krier, F.; Nedjar-Arroume, N.; Vercaigne-Marko, D.; Kosciarz, E.; Ruckebusch, C.; Dhulster, P.; Guillochon, D. Antibacterial activity of a pepsin-derived bovine hemoglobin fragment. FEBS Lett. 2001, 491. [Google Scholar] [CrossRef]
- Daoud, R.; Dubois, V.; Bors-Dodita, L.; Nedjar-Arroume, N.; Krier, F.; Chihib, N.-E.; Mary, P.; Kouach, M.; Briand, G.; Guillochon, D. New antibacterial peptide derived from bovine hemoglobin. Peptides 2005, 26, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Bah, C.S.F.; Carne, A.; McConnell, M.A.; Mros, S.; Bekhit, A.E.-D.A. Production of bioactive peptide hydrolysates from deer, sheep, pig and cattle red blood cell fractions using plant and fungal protease preparations. Food Chem. 2016, 202, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Nedjar-Arroume, N.; Dubois-Delval, V.; Miloudi, K.; Daoud, R.; Krier, F.; Kouach, M.; Briand, G.; Guillochon, D. Isolation and characterization of four antibacterial peptides from bovine hemoglobin. Peptides 2006, 27, 2082–2089. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xu, M.; Hang, B.; Wang, L.; Wang, Q.; Chen, J.; Song, T.; Fu, D.; Wang, Z.; Wang, S.; et al. Isolation and characterization of an antimicrobial peptide from bovine hemoglobin a subunit. World J. Microbiol. Biotechnol. 2010, 27, 767–771. [Google Scholar] [CrossRef]
- Roque, A.L.R.; Jansen, A.M. Reservatórios do Trypanosoma cruzi e sua relação com os vetores; Vetores da doença de chagas no Brasil [online].; Galvão, C., Ed.; Sociedade Brasileira de Zoologia: Curitiba, Brazil, 2014; pp. 76–88. [Google Scholar]






| Chromatogram Label | Retention Time | Activity |
|---|---|---|
| ↓ | 24.80 | M. luteus |
| ↓ | 28.28 | M. luteus |
| ↓ | 35.50 | M. luteus |
| ↓ | 43.00 | M. luteus |
| ↓ | 56.40 | M. luteus |
| ↓ | 64.50 | M. luteus |
| ↓ | 75.30 | M. luteus |
| ↓ | 88.80 | M. luteus |
| ¢ | 48.10 | M. luteus and E. coli |
| ¢ | 61.70 | M. luteus and E. coli |
| ¢ | 93.70 | M. luteus and E. coli |
| ¢ | 94.8 | M. luteus and E. coli |
| £ | 81.80 | M. luteus and S. aureus |
| Fraction | Retention Time | Fragment |
|---|---|---|
| A1 | 24.80 | VLSGEDKSN (α 2-10) |
| A1 | 24.80 | LASHHPAD (α 110-117) |
| A2 | 64.50 | ASFPTTKTYFPHF (α 45-57) |
| A2 | 64.50 | DALASAAGHLDDLPGALSALSDLHAHKLRVD (α 75–95) |
| A2 | 64.50 | LASHHPADFTPAVHASLDKFLASVST (α 119–145) |
| A3 | 81.80 | ASFPTTKTYFPHFD (α 45–58) |
| A3 | 81.80 | ALASAAGHLDDLPGALSALSDLHAHKLRVDPVNFKLLSH (α 76–104) |
| A3 | 81.80 | LLVTLASHHPADFTPAVHASLDKFLASVSTVL (α 106–147) |
| Fraction | Retention Time | Sequence |
|---|---|---|
| B1 | 28.28 | HLTDAEKSA (β2_ 2-10) |
| B1 | 28.28 | GDLSSASAIMGN (β2_ 46-57) |
| B2 | 35.50 | VHLTDAEKSA (β2_1-10) |
| B2 | 35.50 | AKVNPDEVGGEA (β2_16-27) |
| B2 | 35.50 | SSASAIMGNPKVKAHGKKVITA (β2_ 49-70) |
| B2 | 35.50 | EGLKNLDN (β2_73-80) |
| B2 | 35.50 | FTPAAQAAFQKVVAG (β2_122-136) |
| B3 | 43.00 | VHLTDAEKSAVS (β2_1-12) |
| B3 | 43.00 | PKVKAHGKKVITAFN (β2_58-72) |
| B3 | 43.00 | IVLGHHLGKDFTPAAQAA (β2_112-129) |
| B4 | 48.10 | VHLTDAEKAAVSC (β1_ 2-14) |
| B4 | 48.10 | AKVKAHGKKVITAFNDGLNHLDSLKGTFAS (β1_ 59-88) |
| B4 | 48.10 | AGVATALAHKYH (β1_135-147) |
| B5 | 56.40 | VVYPWTQ (β1_34-40) |
| B5 | 56.40 | AKVKAHGKKVITA (β1_ 59-71) |
| B5 | 56.40 | GLNHLDSLKGTF (β1_75-86) |
| B5 | 56.40 | SELHCDKLHVD (β1_90-100) |
| B6 | 61.70 | VHLTDAEKAAVSCL (β1_2-15) |
| B6 | 61.70 | SDEVGGEALGRLL (β1_21-33) |
| B6 | 61.70 | SELHCDKLHVD (β1_ 90-100) |
| B6 | 61.70 | FRLLGNM β (β1_104-110) |
| B6 | 61.70 | FQKVVAGVATALAHKYH (β1_131-147) |
| B7 | 93.70 | PENFRLLG (β2_100-107) |
| B7 | 93.70 | IVLGHHLGKDFTPAAQAAFQKVVAGVATALAHKYH (β2_112-146) |
| Fraction | Retention Time | Fragment |
|---|---|---|
| A1 | 24.80 | VLSGEDKSN (α 2–10) |
| A2 | 64.50 | LPGALSALSDLHAHKLRVD (α 77–95) |
| A3 | 81.80 | LPGALSALSDLHAHKLRVD (α 77–95) |
| B1 | 28.28 | HLTDAEKSA (β2_ 2–10) |
| B2 | 35.50 | VHLTDAEKSA (β2_1–10) |
| B3 | 43.00 | AKVKAHGKKVITAFND (β1_59–74) |
| B4 | 48.10 | AKVKAHGKKVITAFNDGLN (β1_ 59–77) |
| B5 | 56.40 | GLNHLDSLKG (β1_75–84) |
| B6 | 61.70 | FQKVVAGVATALAHKYH (β1_131–147) |
| B7 | 93.70 | TPAAQAAFQKVVAGVATALAHKYH (β2_123–147) |
| Fraction | Fragment | Charge pH 7 | Total Hydrophobic Ratio | Same Surface brk H.R.* |
|---|---|---|---|---|
| A2 | α 75-95 | -2.7 | 48% | 12 |
| A2 | α 119-145 | -0.7 | 46% | 8 |
| A3 | α 76-104 | -0.6 | 48% | 14 |
| A3 | α 106-147 | -0.7 | 53% | 14 |
| B1 | β2_2-10 | -0.9 | 33% | 3 |
| B1 | β2_46-57 | -1 | 41% | 4 |
| B2 | β2_1-10 | -0.9 | 40% | 3 |
| B2 | β2_49-70 | 4.1 | 40% | 6 |
| B2 | β2_73-80 | -1 | 25% | 2 |
| B2 | β2_122-136 | 1 | 60% | 6 |
| B3 | β2_1-12 | -0.9 | 41% | 3 |
| B3 | β2_58-72 | 4.1 | 40% | 4 |
| B3 | β2_112-129 | 0.2 | 50% | 6 |
| B4 | β1_2-14 | -1 | 53% | 4 |
| B4 | β1_59-88 | 3.2 | 40% | 8 |
| B4 | β1_135-147 | 1.2 | 50% | 4 |
| B5 | β1_59-71 | 4.1 | 46% | 3 |
| B5 | β1_75-86 | 0.1 | 33% | 4 |
| B6 | β1_2-15 | -1 | 57% | 6 |
| B6 | β1_21-33 | -2 | 38% | 3 |
| B6 | β1_104-110 | 1 | 57% | 3 |
| B6 | β1_131-147 | 2.2 | 52% | 6 |
| B7 | β2_100-107 | 0 | 37% | 2 |
| B7 | β2_112-146 | 2.4 | 51% | 14 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diniz, L.C.L.; da Silva Junior, P.I. Hemoglobin Reassembly of Antimicrobial Fragments from the Midgut of Triatoma infestans. Biomolecules 2020, 10, 261. https://doi.org/10.3390/biom10020261
Diniz LCL, da Silva Junior PI. Hemoglobin Reassembly of Antimicrobial Fragments from the Midgut of Triatoma infestans. Biomolecules. 2020; 10(2):261. https://doi.org/10.3390/biom10020261
Chicago/Turabian StyleDiniz, Laura Cristina Lima, and Pedro Ismael da Silva Junior. 2020. "Hemoglobin Reassembly of Antimicrobial Fragments from the Midgut of Triatoma infestans" Biomolecules 10, no. 2: 261. https://doi.org/10.3390/biom10020261
APA StyleDiniz, L. C. L., & da Silva Junior, P. I. (2020). Hemoglobin Reassembly of Antimicrobial Fragments from the Midgut of Triatoma infestans. Biomolecules, 10(2), 261. https://doi.org/10.3390/biom10020261





