Indoleacetic Acid Levels in Wheat and Rice Seedlings under Oxygen Deficiency and Subsequent Reoxygenation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Conditions
2.2. Imposition of Anoxic and Post-Anoxic Conditions, IAA Treatment
2.3. Quantification of Endogenous IAA
2.4. Electrolyte Leakage Test
2.5. Evans Blue Staining
2.6. Thiobarbituric Acid Reactive Substances Assay
2.7. Xylenol Orange Assay
2.8. Statistics
3. Results
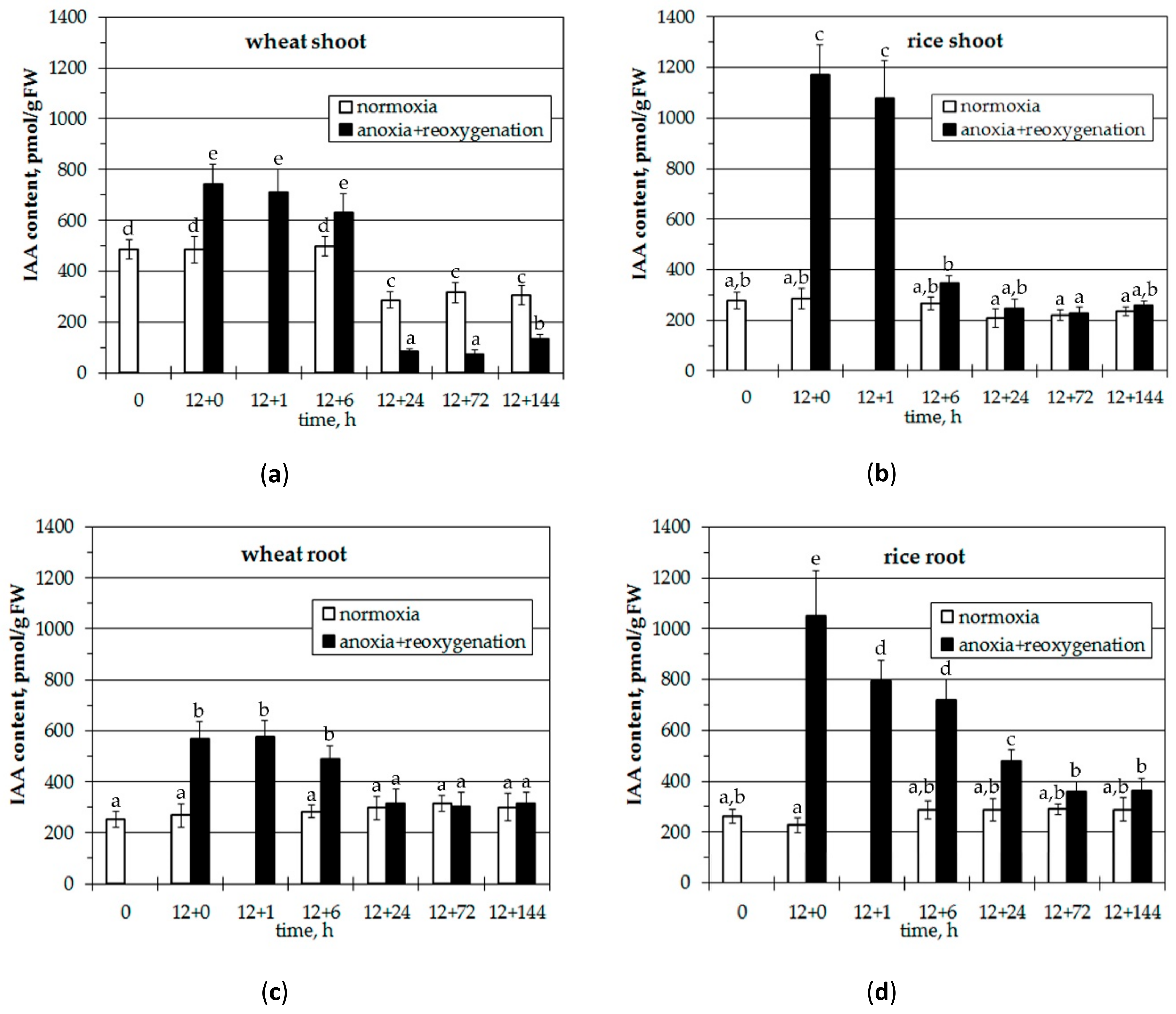
3.1. Effects of Anoxia and Reoxygenation on IAA Content in Wheat and Rice
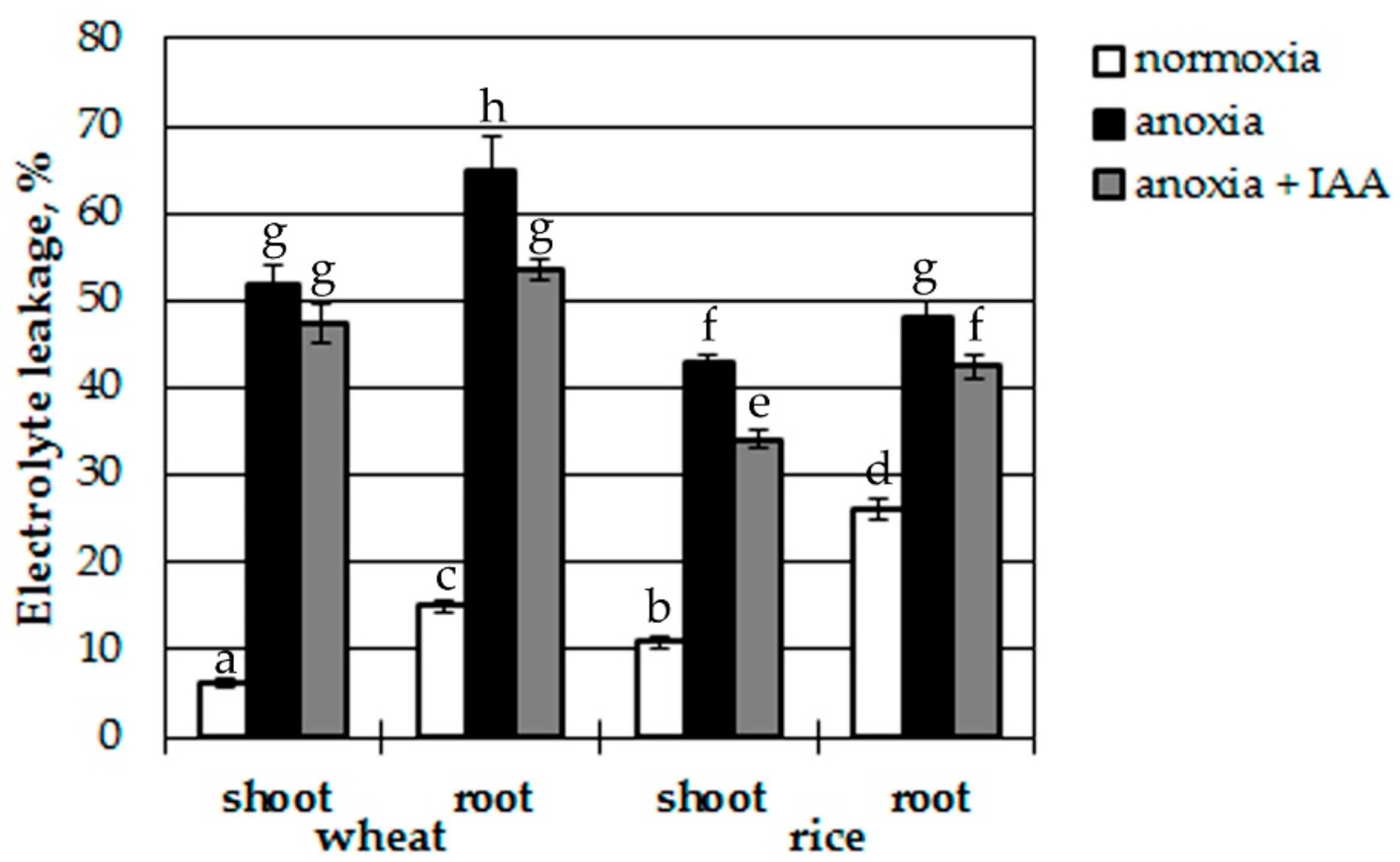
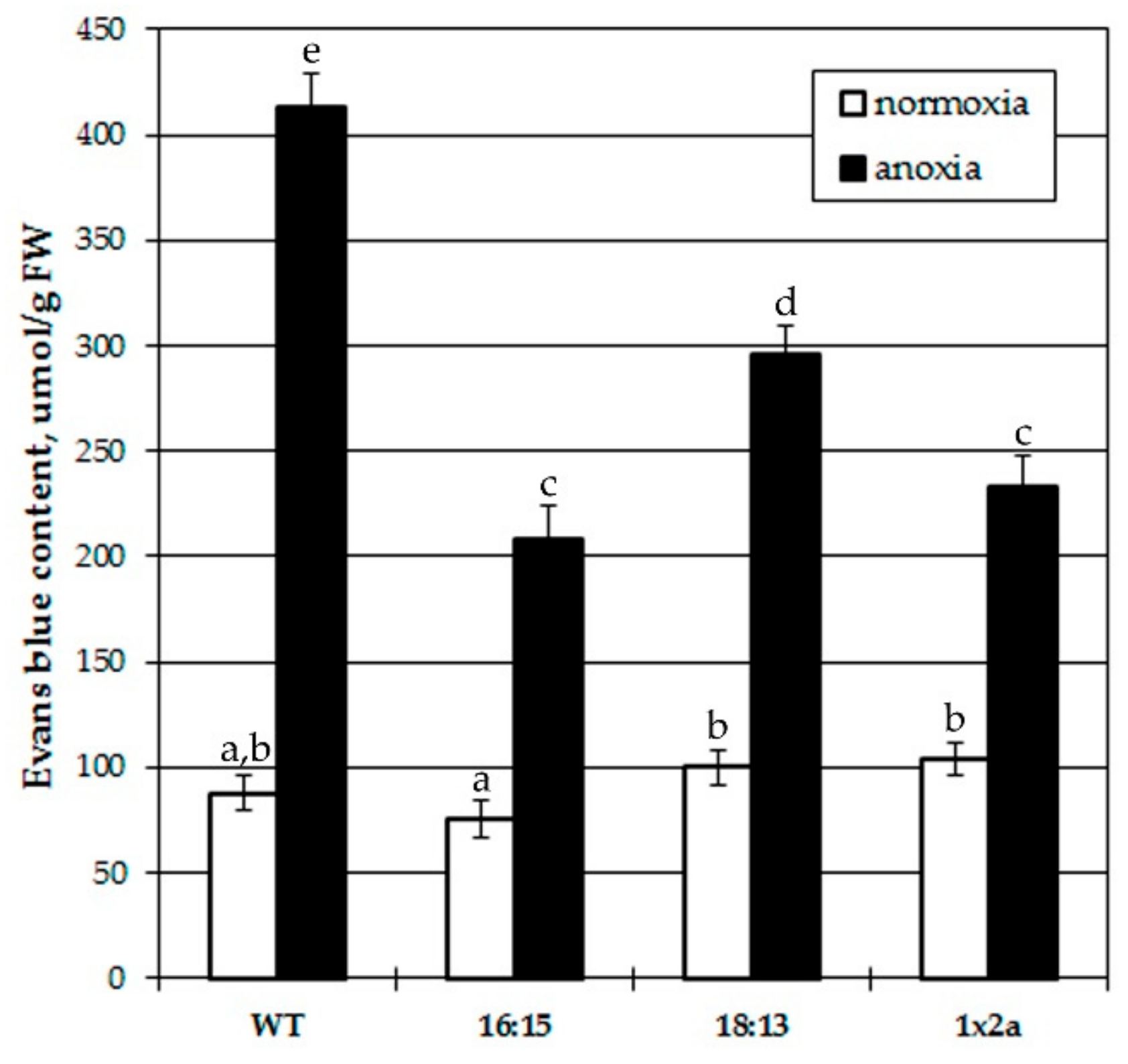
3.2. Effects of Pretreatment with IAA and IAA Overproduction on Anoxia-Induced Plant Damage
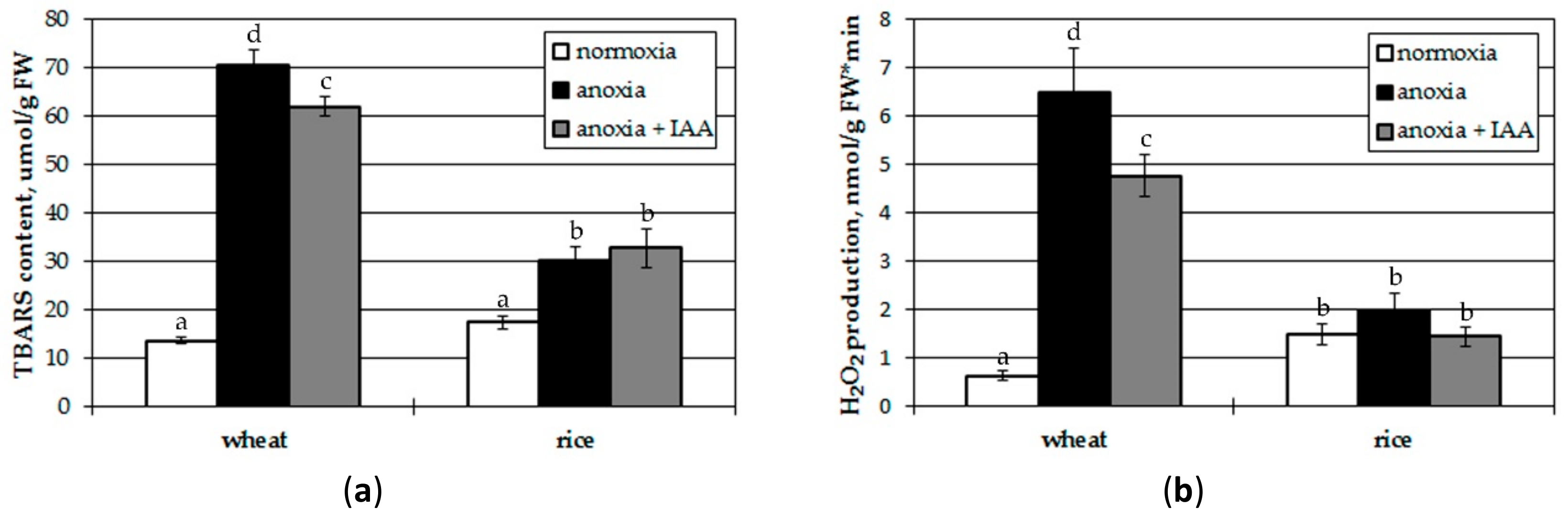
3.3. Effects of Plant Pretreatment with IAA on Oxidative Processes after Anoxia
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tardieu, F.; Tuberosa, R. Dissection and modelling of abiotic stress tolerance in plants. Curr. Opin. Plant Biol. 2010, 13, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Voesenek, L.A.C.J.; Bailey-Serres, J. Flooding tolerance: O2 sensing and survival strategies. Curr. Opin. Plant Biol. 2013, 16, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.; Voesenek, L.A.C.J. Flooding stress: Acclimations and genetic diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef] [Green Version]
- Voesenek, L.A.C.J.; Bailey-Serres, J. Flood adaptive traits and processes: An overview. New Phytol. 2015, 206, 57–73. [Google Scholar] [CrossRef]
- Chirkova, T.; Yemelyanov, V. The study of plant adaptation to oxygen deficiency in Saint Petersburg University. Bio. Comm. 2018, 63, 17–31. [Google Scholar] [CrossRef] [Green Version]
- Bailey-Serres, J.; Voesenek, L.A.C.J. Life in the balance: A signaling network controlling survival of flooding. Curr. Opin. Plant Biol. 2010, 13, 489. [Google Scholar] [CrossRef]
- Dennis, E.S.; Dolferus, R.; Ellis, M.; Rahman, M.; Wu, Y.; Hoeren, F.U.; Grover, A.; Ismond, K.P.; Good, A.G.; Peacock, W.J. Molecular strategies for improving waterlogging tolerance in plants. J. Exp. Bot. 2000, 51, 89–97. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Lee, S.C.; Brinton, E. Waterproofing crops: Effective flooding survival strategies. Plant Physiol. 2012, 160, 1698–1709. [Google Scholar] [CrossRef] [Green Version]
- Yemelyanov, V.V.; Shishova, M.F. The role of phytohormones in the control of plant adaptation to oxygen depletion. In Phytohormones and Abiotic Stress Tolerance in Plants; Khan, N.A., Nazar, R., Iqbal, A., Anjum, N.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 229–248. [Google Scholar] [CrossRef]
- Voesenek, L.A.C.J.; Sasidharan, R. Ethylene–And oxygen signalling–Drive plant survival during flooding. Plant Biol. 2013, 15, 426–435. [Google Scholar] [CrossRef]
- Tamang, B.G.; Fukao, T. Plant adaptation to multiple stresses during submergence and following desubmergence. Int. J. Mol. Sci. 2015, 16, 30164–30180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Qi, X.; Zhang, M.; Chen, X.; Nakazono, M. Roles of phytohormones in morphological and anatomical responses of plants to flooding stress. In Plant Hormones under Challenging Environmental Factors; Ahammed, G.J., Yu, J.-Q., Eds.; Springer Science + Business Media: Dordrecht, Germany, 2016; pp. 117–132. [Google Scholar] [CrossRef]
- Hattori, Y.; Nagai, K.; Furukawa, S.; Song, X.J.; Kawano, R.; Sakakibara, H.; Wu, J.; Matsumoto, T.; Yoshimura, A.; Kitano, H.; et al. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature 2009, 460, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Xu, K.; Ronald, P.C.; Bailey-Serres, J. A variable cluster of ethylene response factor-Like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell 2006, 18, 2021–2034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Licausi, F.; Weits, D.A.; Pant, B.D.; Scheible, W.R.; Geigenberger, P.; van Dongen, J.T. Hypoxia responsive gene expression is mediated by various subsets of transcription factors and miRNAs that are determined by the actual oxygen availability. New Phytol. 2011, 190, 442–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerpen, L.; Niccolini, L.; Licausi, F.; van Dongen, J.T.; Weits, D.A. Hypoxic conditions in crown galls induce plant anaerobic responses that support tumor proliferation. Front Plant Sci. 2019, 10, 56. [Google Scholar] [CrossRef]
- Shukla, V.; Lombardi, L.; Iacopino, S.; Pencik, A.; Novak, O.; Perata, P.; Giuntoli, B.; Licausi, F. Endogenous hypoxia in lateral root primordial controls root architecture by antagonizing auxin signaling in Arabidopsis. Mol. Plant. 2019, 12, 538–551. [Google Scholar] [CrossRef] [Green Version]
- Visser, E.J.W.; Bogemann, G.M.; Blom, C.W.P.M.; Voesenek, L.A.C.J. Ethylene accumulation in waterlogged Rumex plants promotes formation of adventitious roots. J. Exp. Bot. 1996, 47, 403–410. [Google Scholar] [CrossRef] [Green Version]
- Vidoz, M.L.; Loreti, E.; Mensuali, A.; Alpi, A.; Perata, P. Hormonal interplay during adventitious root formation in flooded tomato plants. Plant J. 2010, 63, 551–562. [Google Scholar] [CrossRef]
- Wample, R.L.; Reid, D.M. The role of endogenous auxins and ethylene in the formation of adventitious roots and hypocotyl hypertrophy in flooded sunflower plants (Helianthus annus L.). Physiol. Plant. 1979, 45, 219–226. [Google Scholar] [CrossRef]
- Yamamoto, F.; Kozlowski, T.T. Regulation by auxin and ethylene of responses of Acer negundo seedlings to flooding of the soil. Environ. Exp. Bot. 1987, 27, 329–340. [Google Scholar] [CrossRef]
- Visser, E.J.W.; Heijink, C.J.; van Hout, K.J.G.M.; Voesenek, L.A.C.J.; Barendse, G.W.M.; Blom, C.W.P.M. Regulatory role of auxin in adventitious root formation in two spices of Rumex, differing in their sensitivity to waterlogging. Physiol. Plant. 1995, 93, 116–122. [Google Scholar] [CrossRef] [Green Version]
- Visser, E.J.W.; Cohen, J.D.; Barendse, G.W.M.; Blom, C.W.P.M.; Voesenek, L.A.C.J. An ethylene-Mediated increase in sensitivity to auxin induces adventitious root formation in flooded Rumex palustris Sm. Plant Physiol. 1996, 112, 1687–1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, M.C.; Benschop, J.J.; Vreeburg, R.A.; Wagemaker, C.A.; Moritz, T.; Peeters, A.J.; Voesenek, L.A. The roles of ethylene, auxin, abscisic acid, and gibberellin in the hyponastic growth of submerged Rumex palustris petioles. Plant Physiol. 2004, 136, 2948–2960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Jung, J.W.; Kim, S.K.; Hwang, Y.S.; Lee, J.S.; Kim, S.H. Ethylene-Induced opposite redistributions of calcium and auxin are essential components in the development of tomato petiolar epinastic curvature. Plant Physiol. Biochem. 2008, 46, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Walters, J.; Osborne, D.J. Ethylene and auxin-Induced cell growth in relation to auxin transport and metabolism and ethylene production in the semi-Aquatic plant. Regnellidium Diphyllum Planta 1979, 146, 309–317. [Google Scholar] [CrossRef]
- Summers, J.E.; Jackson, M.B. Anaerobic promotion of stem extension in Potamogeton pectinatus. Roles for carbone dioxide, acidification and hormones. Physiol. Plant. 1996, 96, 615–622. [Google Scholar] [CrossRef]
- Ridge, I.; Osborne, D.J. Wall extensibility, wall pH and tissue osmolality—Significance for auxin and ethylene-Enhanced petiole growth in semi-aquatic plants. Plant Cell Environ. 1989, 12, 383–393. [Google Scholar] [CrossRef]
- Horton, R.F.; Samarakoon, A.B. Petiole growth in the celery-Leaved crowfoot (Ranunculus sceleratus L.): Effects of auxin-transport inhibitors. Aquat. Bot. 1982, 13, 97–104. [Google Scholar] [CrossRef]
- Smulders, M.J.M.; Horton, R.F. Ethylene promotes elongation growth and auxin promotes radial growth in Ranunculus sceleratus petioles. Plant Physiol. 1991, 96, 806–811. [Google Scholar] [CrossRef] [Green Version]
- Rijnders, J.G.H.M.; Barendse, G.W.M.; Blom, C.W.P.M.; Voesenek, L.A.C.J. The contrasting role of auxin in submergence-Induced petiole elongation in two species from frequently flooded wetlands. Physiol. Plant 1996, 96, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Cox, M.C.H.; Peeters, A.J.M.; Voesenek, L.A.C.J. The stimulating effects of ethylene and auxin on petiole elongation and on hyponastic curvature are independent processes in submerged Rumex palustris. Plant Cell Environ. 2006, 29, 282–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsura, N.; Suge, H. Does ethylene induce elongation of the rice coleoptile through auxin? Plant Cell Physiol. 1979, 20, 1147–1150. [Google Scholar] [CrossRef]
- Jackson, M.B. Ethylene-Promoted elongation: An adaptation to submergence stress. Ann. Bot. 2008, 101, 229–248. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, S.; Lombardi, L.; Bertani, A. The effect of growth substances on rice germination and growth under anoxia. Plant Physiol. (Life Sci. Adv.) 1993, 12, 9–15. [Google Scholar]
- Hoson, T.; Masuda, Y.; Pilet, P.E. Auxin content in air and water grown rice coleoptiles. J. Plant Physiol. 1992, 139, 685–689. [Google Scholar] [CrossRef]
- Yemelyanov, V.V.; Chirkova, T.V. Free forms of phytohormones in plants with different tolerance to the lack of oxygen under aeration and anaerobiosis. Bio. Comm. 1996, 41, 73–81. (In Russian) [Google Scholar]
- Bakhtenko, E.Y.; Skorobogatova, I.V.; Karsunkina, N.P.; Platonov, A.V. Hormonal balance of wheat (Triticum aestivum L.) and oat (Avena sativa L.) under submergence and reparation. Agrochemistry 2008, 8, 33–41. (In Russian) [Google Scholar]
- Mapelli, S.; Rocchi, P.; Bertany, A. ABA and IAA in rice seedlings under anaerobic conditions. Biol. Plant. 1986, 28, 57–61. [Google Scholar] [CrossRef]
- Emel’yanov, V.V.; Kirchikhina, N.A.; Lastochkin, V.V.; Chirkova, T.V. Hormonal status in wheat and rice seedlings under anoxia. Russ. J. Plant Physiol. 2003, 50, 827–834. [Google Scholar] [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Janků, M.; Luhová, L.; Petřivalský, M. On the origin and fate of reactive oxygen species in plant cell compartments. Antioxidants 2019, 8, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faseela, P.; Sinisha, A.K.; Brestič, M.; Puthur, J.T. Chlorophyll a fluorescence parameters as indicators of a particular abiotic stress in rice. Photosynthetica 2019, 57, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Blokhina, O.B.; Chirkova, T.V.; Fagerstedt, K.V. Anoxic stress leads to hydrogen peroxide formation in plant cells. J. Exp. Bot. 2001, 52, 1179–1190. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, S.; Premkumar, A.; Rasmussen, U.; Schulz, A.; Lager, I. Phospholipases AtPLDζ1 and AtPLDζ2 function differently in hypoxia. Physiol. Plant. 2018, 162, 98–108. [Google Scholar] [CrossRef] [Green Version]
- Blokhina, O.; Fagerstedt, K.V. Oxidative metabolism, ROS and NO under oxygen deprivation. Plant Physiol. Biochem. 2010, 48, 359–373. [Google Scholar] [CrossRef]
- Sitbon, F.; Hennion, S.; Sundberg, B.; Little, C.H.; Olsson, O.; Sandberg, G. Transgenic tobacco plants coexpressing the Agrobacterium tumefaciens iaaM and iaaH genes display altered growth and indoleacetic acid metabolism. Plant Physiol. 1992, 99, 1062–1069. [Google Scholar] [CrossRef] [Green Version]
- Eklöf, S.; Astot, C.; Sitbon, F.; Moritz, T.; Olsson, O.; Sandberg, G. Transgenic tobacco plants co-Expressing Agrobacterium iaa and ipt genes have wild-type hormone levels but display both auxin- and cytokinin-Overproducing phenotypes. Plant J. 2000, 23, 279–284. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Veselov, S.Y.; Kudoyarova, G.R.; Egutkin, N.L.; Gyuli-Zade, V.G.; Mustafina, A.R.; Kof, E.K. Modified solvent partitioning scheme providing increased specificity and rapidity of immunoassay for indole 3-acetic acid. Physiol. Plant. 1992, 86, 93–96. [Google Scholar] [CrossRef]
- Chirkova, T.V.; Zhukova, T.M.; Goncharova, N.N.; Belonogova, V.A. Opredelenie stepeni pronitsaemosti membran kak sposob diagnostiki rasteniy na ustoychivost’ k nedostatku kisloroda [Determination of the membrane permeability level as the method of plant diagnostic for resistance to oxygen deficiency]. Fiziol. Rast. Genet. 1991, 23, 68–75. (In Russian) [Google Scholar]
- Baker, C.J.; Mock, N.M. An improved method for monitoring cell-Death in cell-suspension and leaf disc assays using Evans blue. Plant Cell Tissue Organ Cult. 1994, 39, 7–12. [Google Scholar] [CrossRef]
- Bozhkov, P.V.; Filonova, L.H.; von Arnold, S. A key developmental switch during Norway spruce somatic embryogenesis is induced by withdrawal of growth regulators and is associated with cell death and extracellular acidification. Biotechnol. Bioeng. 2002, 77, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Chirkova, T.V.; Novitskaya, L.O.; Blokhina, O.B. Lipid peroxidation and antioxidant systems under anoxia in plants differing in their tolerance to oxygen deficiency. Russ. J. Plant Physiol. 1998, 45, 55–62. [Google Scholar]
- Rubin, B.A.; Merzlyak, M.N.; Juferova, S.G. Oxidation of lipid components in isolated chloroplasts under lighting. The substrates and products of lipid peroxidation. Sov. Plant Physiol. 1976, 23, 254–261. [Google Scholar]
- Minibayeva, F.; Kolesnikov, O.; Chasov, A.; Beckett, R.P.; Lüttge, S.; Vylegzhanina, N.; Buck, F.; Böttger, M. Wound-Induced apoplastic peroxidase activities: Their roles in the production and detoxification of reactive oxygen species. Plant Cell Environ. 2009, 32, 497–508. [Google Scholar] [CrossRef]
- Gay, C.; Gebicki, J.M. A critical evaluation of the effect of sorbitol on the ferric-Xylenol orange hydroperoxide assay. Anal. Biochem. 2000, 284, 217–220. [Google Scholar] [CrossRef]
- Olatunji, D.; Geelen, D.; Verstraeten, I. Control of endogenous auxin levels in plant root development. Int. J. Mol. Sci. 2017, 18, 2587. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Lin, J.E.; Harris, C.; Campos Mastrotti Pereira, F.; Wu, F.; Blakeslee, J.J.; Peer, W.A. DAO1 catalyzes temporal and tissue-Specific oxidative inactivation of auxin in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2016, 113, 11010–11015. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Jansen, M.J.; Zhang, Q.; Sergeeva, L.; Ligterink, W.; Mariani, C.; Rieu, I.; Visser, E.J.W. A disturbed auxin signaling affects adventitious root outgrowth in Solanum dulcamara under complete submergence. J. Plant Physiol. 2018, 224–225, 11–18. [Google Scholar] [CrossRef]
- Lastochkin, V.V.; Yemelyanov, V.V.; Chirkova, T.V. Aktivnost’ peroksidazy v prorostkakh pshenitsy i risa v svyazi s vozdeystviem anoksii [Peroxidase activity in wheat and rice seedlings under anoxia]. Bio. Comm. 2000, 45, 59–64. (In Russian) [Google Scholar]
- Tognetti, V.B.; Mühlenbock, P.; Van Breusegem, F. Stress homeostasis–The redox and auxin perspective. Plant Cell Environ. 2012, 35, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Ivanchenko, M.G.; den Os, D.; Monshausen, G.B.; Dubrovsky, J.G.; Bednarova, A.; Krishnan, N. Auxin increases the hydrogen peroxide (H2O2) concentration in tomato(Solanum lycopersicum) root tips while inhibiting root growth. Ann. Bot. 2013, 112, 1107–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peer, W.A.; Cheng, Y.; Murphy, A.S. Evidence of oxidative attenuation of auxin signaling. J. Exp. Bot. 2013, 64, 2629–2639. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.H.; Umemura, K.; Kawano, T. Indole-3-Acetic acid-Induced oxidative burst and an increase in cytosolic calcium ion concentration in rice suspension culture. Biosci. Biotech. Bioch. 2016, 80, 1546–1554. [Google Scholar] [CrossRef] [Green Version]
- Huang, A.; Wang, Y.; Liu, Y.; Wang, G.; She, X. Reactive oxygen species regulate auxin levels to mediate adventitious root induction in Arabidopsis hypocotyl cuttings. J. Integr. Plant Biol. 2019. [Google Scholar] [CrossRef]
- Liszkay, A.; van der Zalm, E.; Schopfer, P. Production of reactive oxygen intermediates (O2−, H2O2, and ˙OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiol. 2004, 136, 3114–3123. [Google Scholar] [CrossRef] [Green Version]
- Pavlů, J.; Novák, J.; Koukalová, V.; Luklová, M.; Brzobohatý, B.; Černý, M. Cytokinin at the crossroads of abiotic stress signalling pathways. Int. J. Mol. Sci. 2018, 19, 2450. [Google Scholar] [CrossRef] [Green Version]
- Sohag, A.A.M.; Tahjib-Ul-Arif, M.; Brestič, M.; Afrin, S.; Sakil, M.d.A.; Hossain, M.d.T.; Hossain, M.A.; Hossain, M.d.A. Exogenous salicylic acid and hydrogen peroxide attenuate drought stress in rice. Plant Soil Environ. 2020. [Google Scholar] [CrossRef] [Green Version]
- Ishizawa, K.; Esashi, Y. Co-operation of ethylene and auxin in the growth regulation of rice coleoptile segments. J. Exp. Bot. 1983, 34, 74–82. [Google Scholar] [CrossRef]
- Horton, R.F. The effect of ethylene and other regulators on coleotile growth of rice under anoxia. Plant Sci. 1991, 79, 57–62. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Effects of plant hormones on the activity of alcohol dehydrogenase in lettuce seedlings. J. Plant Physiol. 2000, 157, 223–225. [Google Scholar] [CrossRef]
- Mapelli, S.; Locatelli, F. The relation of rice coleoptiles, auxin-Binding protein, and protein synthesis to anoxia and indoleacetic acid. Russ. J. Plant Physiol. 1995, 42, 624–629. [Google Scholar]
- Dharmasiri, N.; Dharmasiri, S.; Estelle, M. The F-box protein TIR1 is an auxin receptor. Nature 2005, 435, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Kant, S.; Bi, Y.M.; Zhu, T.; Rothstein, S.J. SAUR39, a Small Auxin-Up RNA gene, acts as a negative regulator of auxin synthesis and transport in rice. Plant Physiol. 2009, 151, 691–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Peng, S.; Xian, Z.; Lin, D.; Hu, G.; Yang, L.; Ren, M.; Li, Z. Overexpression of a tomato miR171 target gene SlGRAS24 impacts multiple agronomical traits via regulating gibberellin and auxin homeostasis. Plant Biotechnol. J. 2017, 15, 472–488. [Google Scholar] [CrossRef]
- Hou, Y.; Jiang, F.; Zheng, X.; Wu, Z. Identification and analysis of oxygen responsive microRNAs in the root of wild tomato (S. habrochaites). BMC Plant Biol. 2019, 19, 100. [Google Scholar] [CrossRef] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yemelyanov, V.V.; Lastochkin, V.V.; Chirkova, T.V.; Lindberg, S.M.; Shishova, M.F. Indoleacetic Acid Levels in Wheat and Rice Seedlings under Oxygen Deficiency and Subsequent Reoxygenation. Biomolecules 2020, 10, 276. https://doi.org/10.3390/biom10020276
Yemelyanov VV, Lastochkin VV, Chirkova TV, Lindberg SM, Shishova MF. Indoleacetic Acid Levels in Wheat and Rice Seedlings under Oxygen Deficiency and Subsequent Reoxygenation. Biomolecules. 2020; 10(2):276. https://doi.org/10.3390/biom10020276
Chicago/Turabian StyleYemelyanov, Vladislav V., Victor V. Lastochkin, Tamara V. Chirkova, Sylvia M. Lindberg, and Maria F. Shishova. 2020. "Indoleacetic Acid Levels in Wheat and Rice Seedlings under Oxygen Deficiency and Subsequent Reoxygenation" Biomolecules 10, no. 2: 276. https://doi.org/10.3390/biom10020276
APA StyleYemelyanov, V. V., Lastochkin, V. V., Chirkova, T. V., Lindberg, S. M., & Shishova, M. F. (2020). Indoleacetic Acid Levels in Wheat and Rice Seedlings under Oxygen Deficiency and Subsequent Reoxygenation. Biomolecules, 10(2), 276. https://doi.org/10.3390/biom10020276






