Methanol Extract of Dicranopteris linearis Leaves Attenuate Pain via the Modulation of Opioid/NO-Mediated Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Identification of Plant
2.2. Preparation of MEDL
2.3. Drugs and Chemicals for Plant Extraction and Animal Study
2.4. Reagents and Chemicals for Phytoconstituents Analyses Using the Ultra Performance Liquid Chromatography-Electrospray Ionization-High Resolution Mass Spectrometry (UPLC-ESI-HRMS)
2.5. Experimental Animals
2.6. Evaluation of Antinociceptive Potential of MEDL
2.6.1. Acetic Acid-Induced Abdominal Constriction Test
2.6.2. Hot Plate Test
2.6.3. Formalin-induced Paw Licking Test
2.7. Determination of the Muscle Relaxant or Sedative Effects of MEDL
2.8. Investigation on the Possible Mechanisms of Antinociception of MEDL
2.8.1. Role of Transient Receptor Potential Vanilloid 1 (TRPV1) Receptors
2.8.2. Role of Glutamatergic System
2.8.3. Role of Bradykininergic System
2.8.4. Role of Protein Kinase C (PKC)
2.8.5. Role of Opioidergic System
2.8.6. Role of l-Arginine/Nitric Oxide/Cyclic Guanosine Monophosphate (l-arg/NO/cGMP) Pathway
2.9. Phytoconstituents Analyses of MEDL
2.9.1. High-Resolution UPLC-ESI-HRMS Analysis of MEDL
2.9.2. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of MEDL
3. Results
3.1. Antinociceptive Profile of MEDL
3.1.1. Effect of MEDL on Nociceptive Response Assessed Using the Abdominal Constriction Test
3.1.2. Effect of MEDL on Nociceptive Response Assessed Using the Hot Plate Test
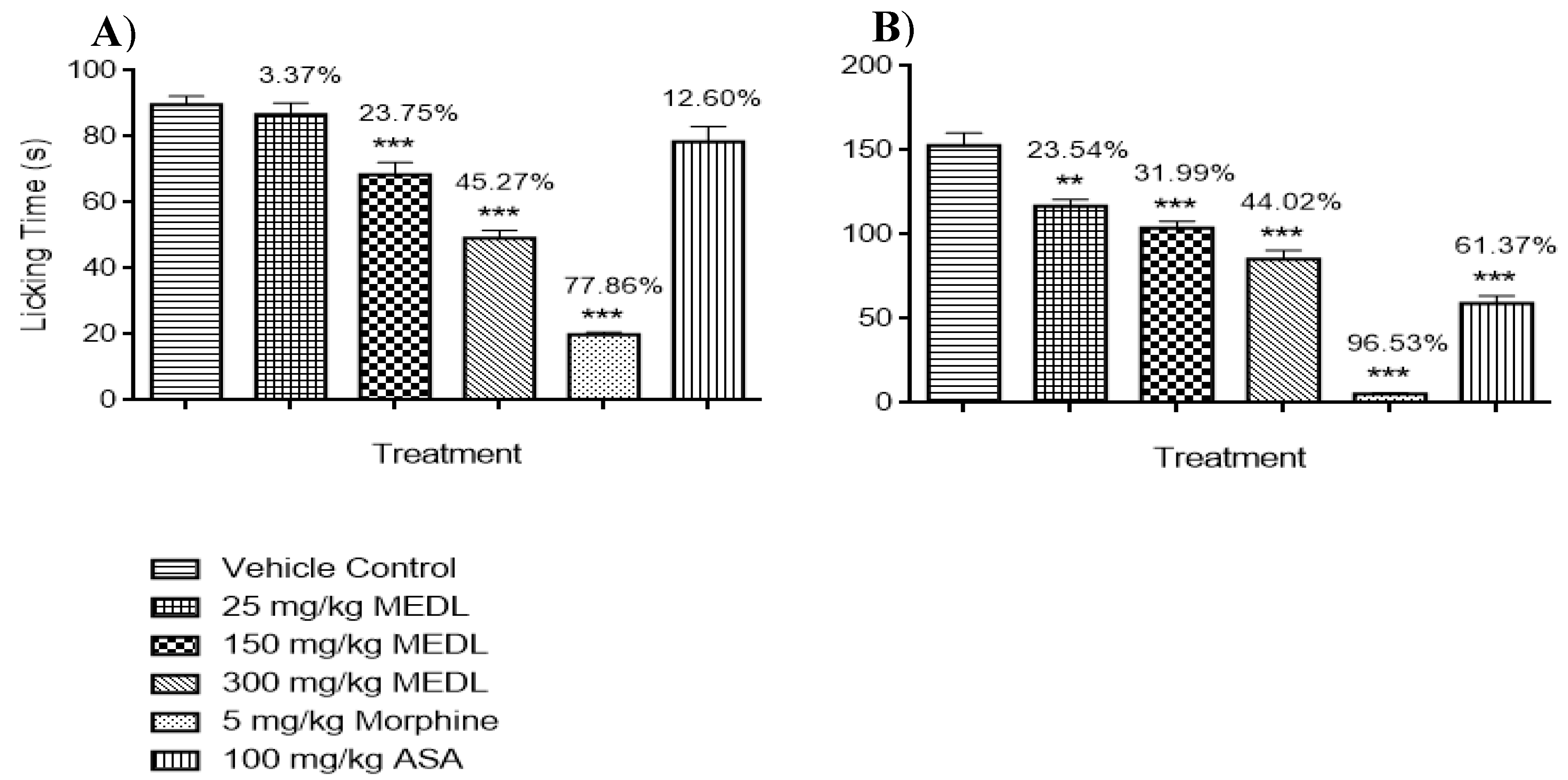
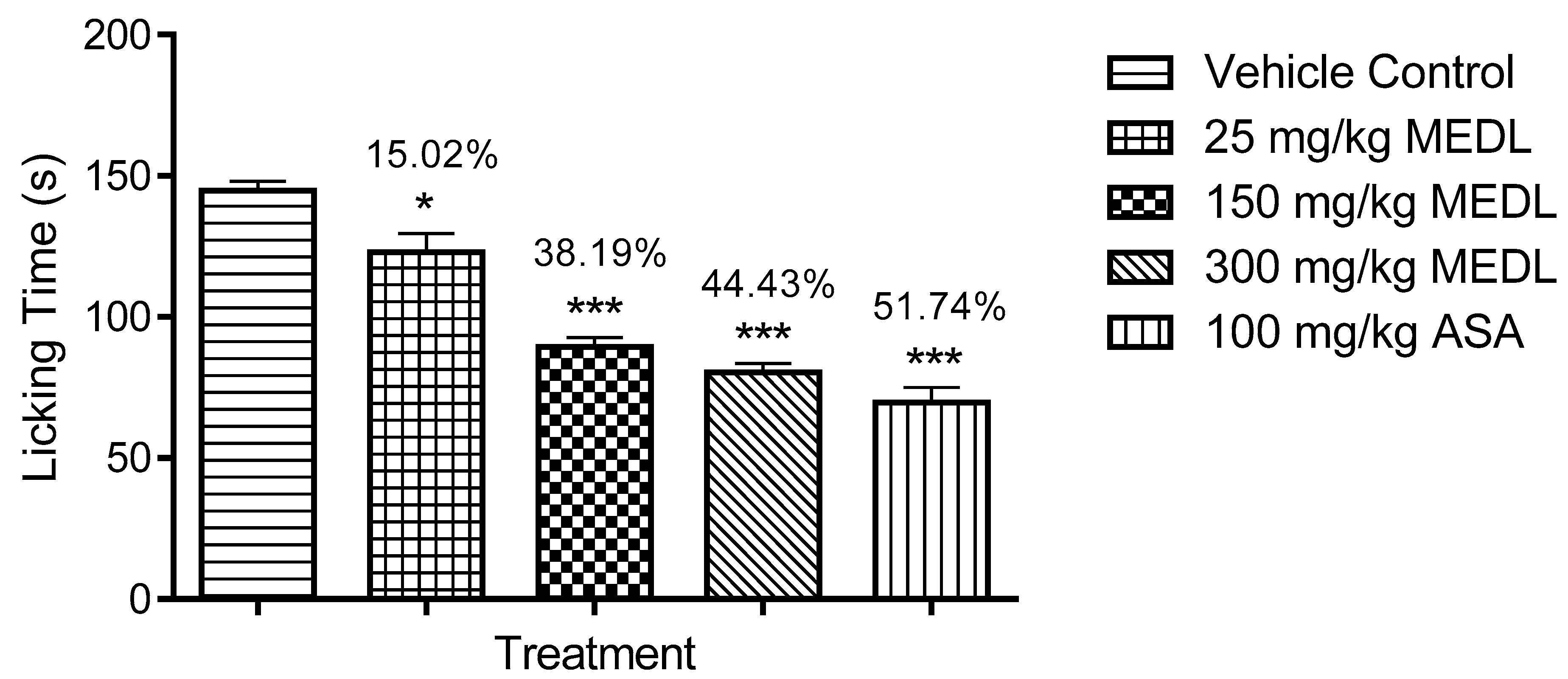
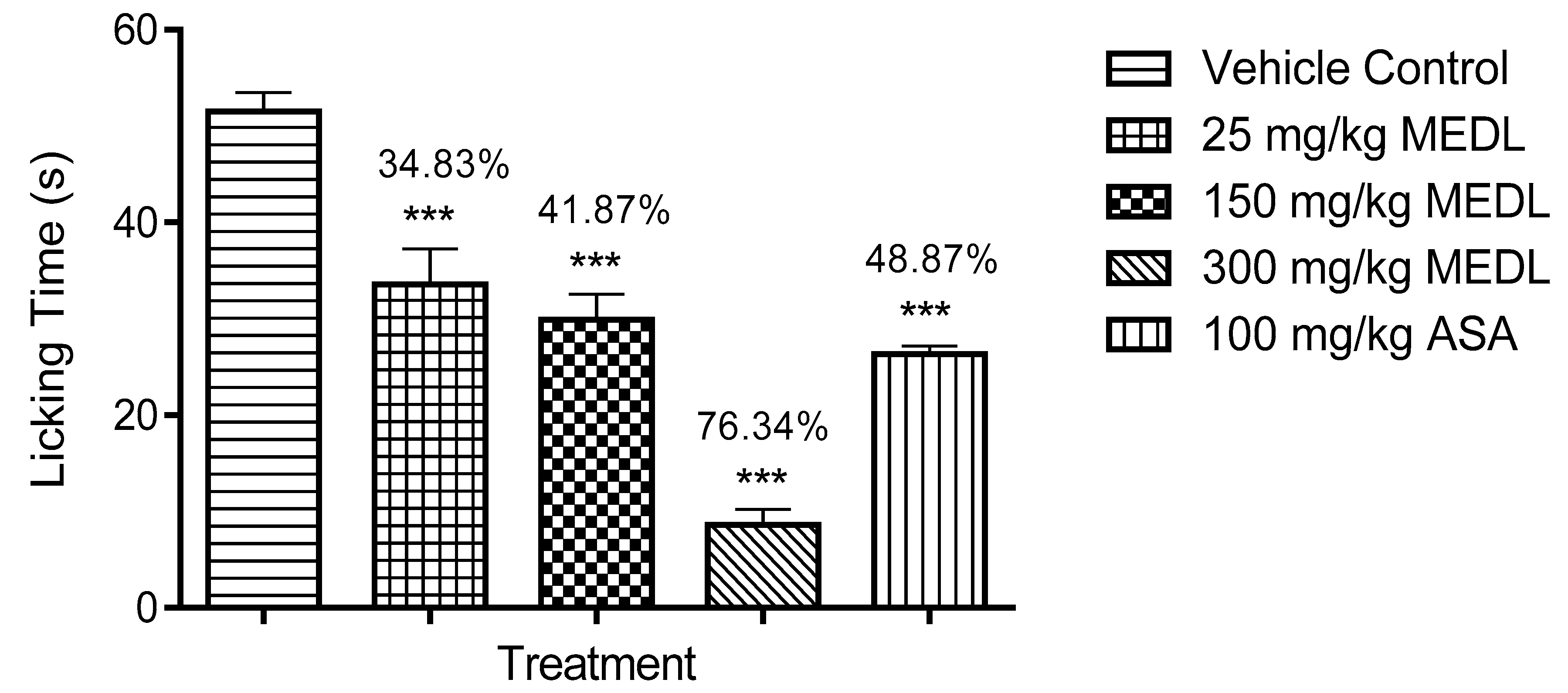
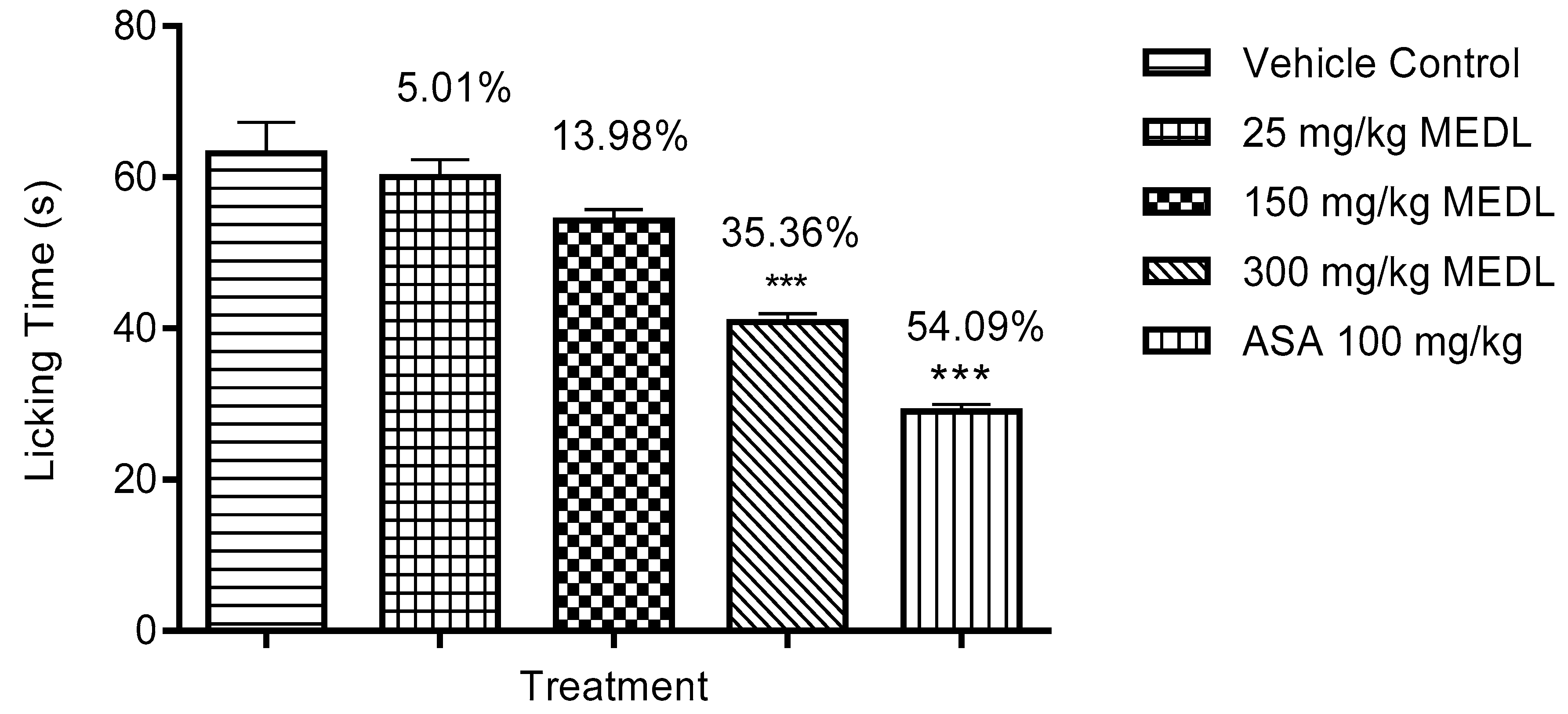
3.1.3. Effect of MEDL on Nociceptive Response Assessed Using the Formalin-induced Paw Licking Test
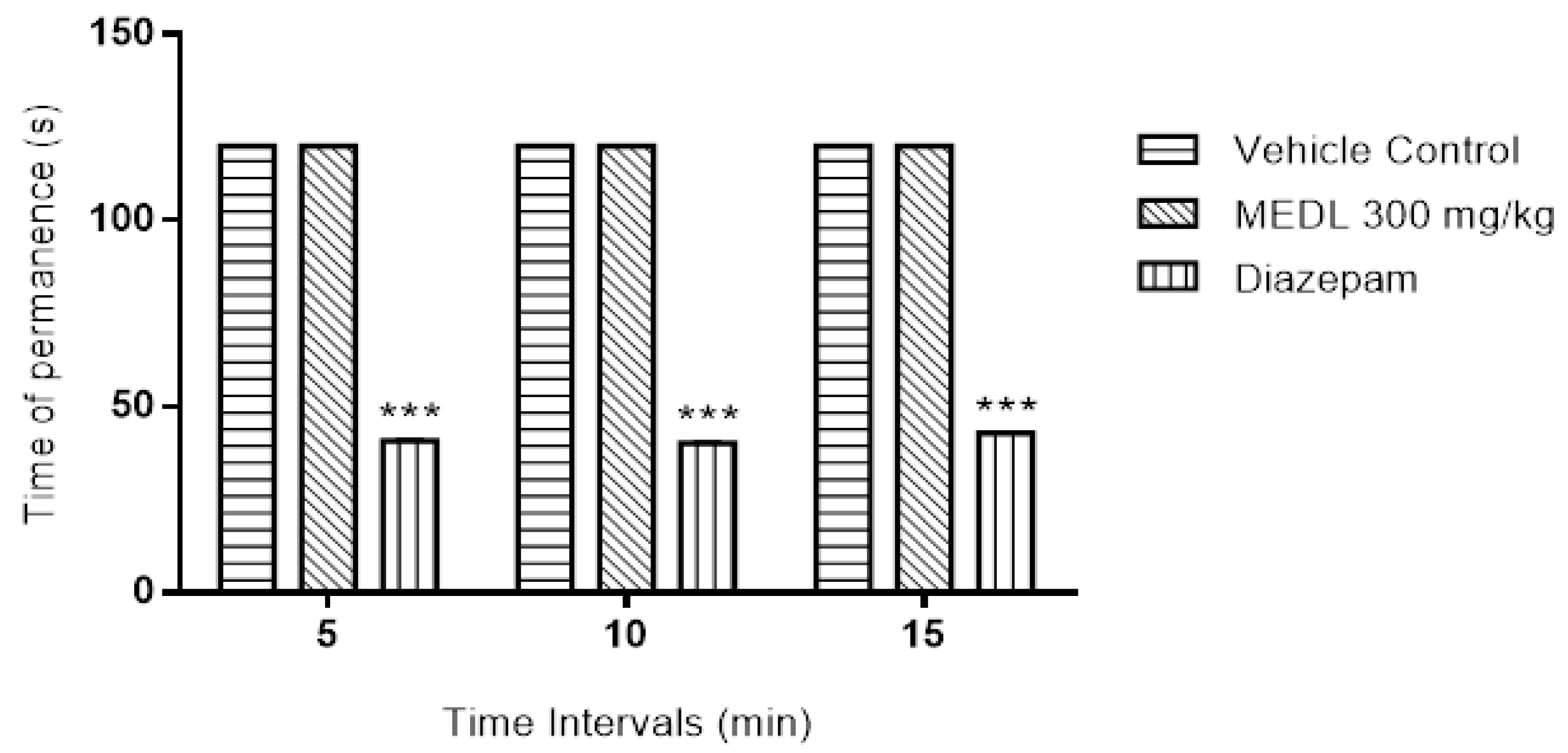
3.2. Effect of MEDL on Motor Coordination Assessed Using the Rotarod Test
3.3. Possible Mechanisms of Antinociception Exhibited by MEDL
3.3.1. Involvement of TRPV1 Receptors in the Modulation of MEDL-induced Antinociceptive Activity
3.3.2. Involvement of the Glutamatergic System in the Modulation of MEDL-induced Antinociceptive Activity
3.3.3. Involvement of the Bradykininergic System in the Modulation of MEDL-induced Antinociceptive Activity
3.3.4. Involvement of PKC Pathway in the Modulation of MEDL-Induced Antinociceptive Activity
3.3.5. Involvement of the Opioidergic System in the Modulation of MEDL-induced Antinociceptive Activity
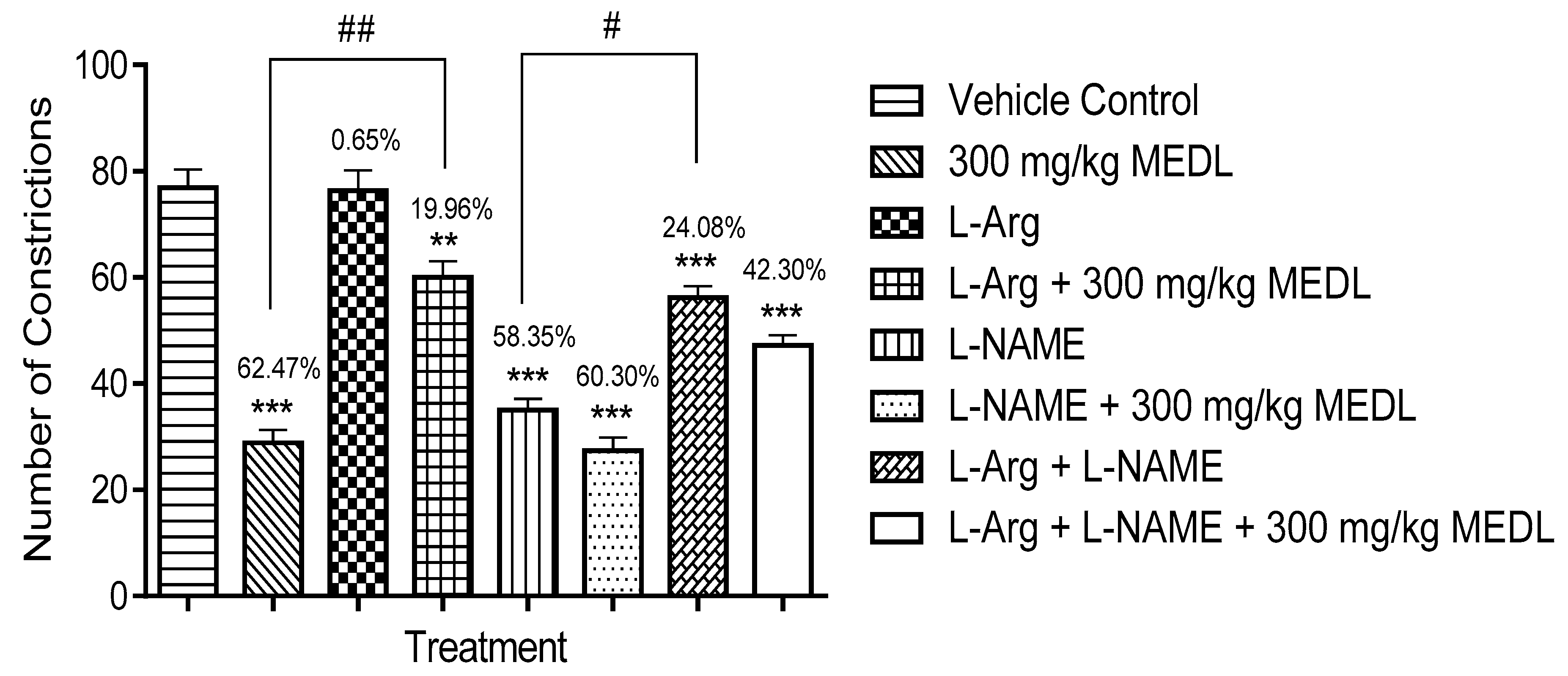
3.3.6. Involvement of the l-arginine/NO/cGMP Pathway in the Modulation of MEDL-induced Antinociceptive Activity
3.4. Phytoconstituents of MEDL
3.4.1. UHPLC-ESI-HRMS Profile of MEDL
3.4.2. GC-MS Profile of MEDL
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Del Vecchio, G.; Spahn, V.; Stein, C. Novel opioid analgesics and side effects. ACS Chem. Neurosci. 2017, 8, 1638–1640. [Google Scholar] [CrossRef]
- Manchikanti, L.; Kaye, A.M.; Kaye, A.D. Current state of opioid therapy and abuse. Curr. Pain Headache Rep. 2016, 20, 34. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [Green Version]
- Vasuda, S.M. Economic importance of pteridophytes. Indian Fern J. 1999, 16, 130–152. [Google Scholar]
- Chin, W.Y. A Guide to Medicinal Plants; Singapore Science Centre: Singapore, 1992; p. 24. [Google Scholar]
- Zakaria, Z.A.; Abdul Ghani, Z.D.F.; Raden Mohd Nor, R.N.S.; Hanan Gopalan, H.K.; Sulaiman, M.R.; Abdullah, F.C. Antinociceptive and anti-inflammatory activities of Dicranopteris linearis leaves chloroform extract in experimental animals. Yakugaku Zasshi 2006, 126, 1197–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakaria, Z.A.; Abdul Ghani, Z.D.F.; Raden Mohd. Nor, R.N.S.; Gopalan, H.K.; Sulaiman, M.R.; Mat Jais, A.M.; Somchit, M.N.; Abdul Kadir, A.; Ripin, J. Antinociceptive, anti-inflammatory, and antipyretic properties of an aqueous extract of Dicranopteris linearis leaves in experimental animal models. J. Nat. Med. 2008, 62, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.; Ursu, O.; Gaulton, A.; Bento, A.P.; Donadi, R.S.; Bologa, C.G.; Karlsson, A.; Al-Lazikani, B.; Hersey, A.; Oprea, T.I.; et al. A comprehensive map of molecular drug targets. Nat. Rev. Drug Discov. 2017, 16, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, Z.A.; Kamisan, F.H.; Omar, M.H.; Mahmood, N.D.; Othman, F.; Abdul Hamid, S.S.; Abdullah, M.N.H. Methanol extract of Dicranopteris linearis L. leaves impedes acetaminophen-induced liver intoxication partly by enhancing the endogenous antioxidant system. BMC Complement. Altern. Med. 2017, 17, 271–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamisan, F.H.; Yahya, F.; Mamat, S.S.; Kamarolzaman, M.F.F.; Mohtarrudin, N.; Teh, L.K.; Salleh, M.Z.; Hussain, M.K.; Zakaria, Z.A. Effect of methanol extract of Dicranopteris linearis against carbon tetrachloride-induced acute liver injury in rats. BMC Complement. Altern. Med. 2014, 14, 123–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, C.; Zhang, J.M. Animal Models of Pain, 1st ed.; Humana Press: Berlin, Germany, 2011. [Google Scholar]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Mohd Sani, M.H.; Zakaria, Z.A.; Balan, T.; Teh, L.K.; Salleh, M.Z. Antinociceptive activity of methanol extract of Muntingia calabura leaves and the mechanisms of action involved. Evid. Based Complement. Altern. Med. 2012, 2012, 10. [Google Scholar] [CrossRef] [Green Version]
- Ong, H.M.; Mohamad, A.S.; Makhtar, N.; Khalid, M.H.; Khalid, S.; Perimal, E.K.; Mastuki, S.N.; Zakaria, Z.A.; Lajis, N.; Israf, D.A.; et al. Antinociceptive activity of methanolic extract of Acmella uliginosa (Sw.) Cass. J. Ethnopharmacol. 2011, 133, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.C.; da Silva, A.V.; Arruda, B.R.; Morais, T.C.; Rios, J.B.; Trevisan, M.T.; Rao, V.S.; Santos, F.A. Peripheral antinociceptive action of mangiferin in mouse models of experimental pain: Role of endogenous opioids, K(ATP)-channels and adenosine. Pharmacol. Biochem. Behav. 2013, 110, 19–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beirith, A.; Santos, A.R.S.; Calixto, J.B. Mechanisms underlying the nociception and paw oedama caused by injection of glutamate into the mouse paw. Brain Res. 2002, 924, 219–228. [Google Scholar] [CrossRef]
- Ferreira, J.; Campos, M.M.; Araujo, R.; Bader, M.; Pesquero, J.B.; Calixto, J.B. The use of kinin B1 and B2 receptor knockout mice and selective antagonists to characterize the nociceptive responses caused by kinins at the spinal level. Neuropharmacology 2002, 43, 1188–1197. [Google Scholar] [CrossRef]
- Savegnago, L.; Pinto, L.G.; Jesse, C.R.; Alves, D.; Rocha, J.B.; Nogueira, C.W.; Zeni, G. Antinociceptive properties of diphenyl diselenide: Evidences for the mechanism of action. Eur. J. Pharmacol. 2007, 555, 129–138. [Google Scholar] [CrossRef]
- Abdul Rahim, M.H.; Zakaria, Z.A.; Mohd Sani, M.H.; Omar, M.H.; Yakob, Y.; Cheema, M.S.; Ching, S.M.; Ahmad, Z.; Abdul Kadir, A. Methanolic extract of Clinacanthus nutans exerts antinociceptive activity via the opioid/nitric oxide-mediated, but cGMP-Independent, Pathways. Evid. Based Complement. Altern. Med. 2016, 2016, 11. [Google Scholar] [CrossRef] [Green Version]
- Jiménez–Andrade, G.Y.; Reyes–García, G.; Sereno, G.; Ceballos–Reyes, G.; Vidal Cantú, G.C.; Granados-Soto, V. Pyritinol reduces nociception and oxidative stress in diabetic rats. Eur. J. Pharmacol. 2008, 590, 170–176. [Google Scholar] [CrossRef]
- Zakaria, Z.A.; Mohamed, A.M.; Mohd. Jamil, N.S.; Rofiee, M.S.; Somchit, M.N.; Zuraini, A.; Arifah, A.K.; Sulaiman, M.R. In vitro cytotoxic and antioxidant properties of the aqueous, chloroform and methanol extracts of Dicranopteris linearis leaves. Afr. J. Biotechnol. 2011, 10, 273–282. [Google Scholar]
- Ribeiro, R.A.; Vale, M.L.; Thomazzi, S.M.; Paschoalato, A.B.; Poole, S.; Ferreira, S.H.; Cunha, F.Q. Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur. J. Pharmacol. 2000, 387, 111–118. [Google Scholar] [CrossRef]
- Wang, Q.S.; Yang, L.; Cui, W.Y.; Chen, L.; Jiang, Y.H. 2014. Anti-Inflammatory and anti-nociceptive activities of methanol extract from aerial part of Phlomis younghusbandii Mukerjee. PLoS ONE 2014, 9, e89149. [Google Scholar] [CrossRef]
- Satyanarayana, P.S.; Jain, N.K.; Singh, A.; Kulkarni, S.K. Isobolographic analysis of interaction between cyclooxygenase inhibitors and tramadol in acetic acid-induced writhing in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2004, 28, 641–649. [Google Scholar]
- Le Bars, D.; Gozariu, M.; Cadden, S.W. Animal models of nociception. Pharmacol. Rev. 2001, 53, 597–652. [Google Scholar] [PubMed]
- Lavich, T.R.; Cordeiro, R.S.B.; Silva, P.M.R.; Martins, M.A. A novel hot-plate test sensitive to hyperalgesic stimuli and non-opioid analgesics. Braz. J. Med. Biol. Res. 2005, 38, 445–451. [Google Scholar] [CrossRef]
- Yin, Z.Y.; Li, L.; Chu, S.S.; Sun, Q.; Ma, Z.L.; Gu, X.P. Antinociceptive effects of dehydrocorydaline in mouse models of inflammatory pain involve the opioid receptor and inflammatory cytokines. Sci Rep. 2016, 6, 27129. [Google Scholar] [CrossRef] [Green Version]
- Almeida, J.R.; Souza, G.R.; Silva, J.C.; Saraiva, S.R.; Júnior, R.G.; Quintans, J.S.; Barreto, R.S.; Bonjardim, L.; Cavalcanti, S.C.; Quintans, L.J., Jr. Borneol, a bicyclic monoterpene alcohol, reduces nociceptive behavior and inflammatory response in mice. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Szallasi, A.; Cruz, F.; Geppetti, P. TRPV1: A therapeutic target for novel analgesic drugs? Trends Mol. Med. 2006, 12, 545–554. [Google Scholar] [CrossRef]
- Taylor, J.; Gupta, S. Neurobiology of persistent pain: Recent advances. In Anesthesia, Critical Pain & Pain: Pain Management; Bakshi, S., Gupta, S., Baheti, D.K., Gehdoo, R.P., Eds.; Jaypee Brothers Medical Publisher Ltd.: New Delhi, India, 2013; Volume 1. [Google Scholar]
- Vetter, I.; Wyse, B.D.; Monteith, G.R.; Roberts-Thomson, S.J.; Cabot, P.J. The mu opioid agonist morphine modulates potentiation of capsaicin-evoked TRPV1 responses through a cyclic AMP-dependent protein kinase A pathway. Mol. Pain 2006, 2, 22. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Quirion, R. Inflammatory mediators modulating the transient receptor potential vanilloid 1 receptor: Therapeutic targets to treat inflammatory and neuropathic pain. Expert Opin. Ther. Targets 2007, 11, 307–320. [Google Scholar] [CrossRef]
- Jin, Y.H.; Takemura, M.; Furuyama, A.; Yonehara, N. Peripheral glutamate receptors are required for hyperalgesia induced by capsaicin. Pain Res. Treat. 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Muñoz, M.; Sánchez-Blázquez, P.; Vicente-Sánchez, A.; Berrocoso, E.; Garzón, J. The mu-opioid receptor and the NMDA receptor associate in PAG neurons: Implications in pain control. Neuropsychopharmacology 2012, 3, 338–349. [Google Scholar] [CrossRef]
- Wu, D.; Lin, X.; Bernloehr, C.; Hildebrandt, T.; Doods, H. Effects of a novel bradykinin B1 receptor antagonist and angiotensin II receptor blockade on experimental myocardial infarction in rats. PLoS ONE 2012, 7, e51151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cayla, C.; Labuz, D.; Machelska, H.; Bader, M.; Schäfer, M.; Stein, C. Impaired nociception and peripheral opioid antinociception in mice lacking both kinin B1 and B2 receptors. Anesthesiology 2012, 116, 448–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikand, P.; Premkumar, L.S. Potentiation of glutamatergic synaptic transmission by protein kinase C-mediated sensitization of TRPV1 at the first sensory synapse. J. Physiol. 2017, 581, 631–647. [Google Scholar] [CrossRef] [PubMed]
- Falkenburger, B.H.; Dickson, E.J.; Hille, B. Quantitative properties and receptor reserve of the DAG and PKC branch of Gq-coupled receptor signaling. J. Gen. Physiol. 2013, 141, 537–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukacs, V.; Yudin, Y.; Hammond, G.R.; Sharma, E.; Fukami, K.; Rohács, T. Distinctive changes in plasma membrane phosphoinositides underlie differential regulation of TRPV1 in nociceptive neurons. J. Neurosci. 2013, 33, 11451–11463. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Kohno, T.; Amaya, F.; Brenner, G.J.; Ito, N.; Allchorne, A.; Ji, R.R.; Woolf, C.J. Bradykinin produces pain hypersensitivity by potentiating spinal cord glutamatergic synaptic transmission. J. Neurosci. 2005, 25, 7986–7992. [Google Scholar] [CrossRef]
- Lai, J.; Luo, M.C.; Chen, Q.; Porreca, F. Pronociceptive actions of dynorphin via bradykinin receptors. Neurosci. Lett. 2008, 437, 175–179. [Google Scholar] [CrossRef] [Green Version]
- Velázquez, K.T.; Mohammad, H.; Sweitzer, S.M. Protein kinase C in pain: Involvement of multiple isoforms. Pharmacol. Res. 2007, 55, 578–589. [Google Scholar] [CrossRef] [Green Version]
- Chuang, H.H.; Prescott, E.D.; Kong, H.; Shields, S.; Jordt, S.E.; Basbaum, A.I.; Chao, M.V.; Julius, D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature 2001, 411, 957–962. [Google Scholar] [CrossRef]
- Ueda, H.; Inoue, M.; Matsumoto, T. Protein Kinase C-mediated inhibition of μ-opioid receptor internalization and its involvement in the development of acute tolerance to peripheral μ-agonist analgesia. J. Neurosci. 2001, 21, 2967–2973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Hasani, R.; Bruchas, M.R. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology 2011, 115, 1363–1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasternak, G.; Pan, Y.X. Mu opioid receptors in pain management. Acta Anaesthesiol. Taiwan 2011, 49, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, P.; Saavedra, G.; Hernández, A.; Paeile, C.; Pelissier, T. Synergistic antinociceptive effects of ketamine and morphine in the orofacial capsaicin test in the rat. Anesthesiology 2003, 99, 969–975. [Google Scholar] [CrossRef]
- Déciga-Campos, M.; López-Muñoz, F.J. Participation of the l-arginine-nitric oxide-cyclic GMP-ATP-sensitive K+ channel cascade in the antinociceptive effect of rofecoxib. Eur. J. Pharmacol. 2004, 484, 193–199. [Google Scholar] [CrossRef]
- Zakaria, Z.A.; Sulaiman, M.R.; Somchit, M.N.; Justin, E.C.; Daud, I.; Mat Jais, A.M. The effects of l-arginine, D-arginine, l-NAME and methylene blue on Haruan (Channa striatus)-induced peripheral antinociception in mice. J. Pharm. Pharm. Sci. 2005, 8, 199–206. [Google Scholar]
- Hafeshjani, Z.K.; Karami, M.; Biglarnia, M. Nitric oxide in the hippocampal cortical area interacts with naloxone in inducing pain. Indian J. Pharmacol. 2012, 44, 443–447. [Google Scholar]
- Vivancos, G.G.; Parada, C.A.; Ferreira, S.H. Opposite nociceptive effects of the l-arginine/NO/cGMP pathway stimulation in dermal and subcutaneous tissues. Br. J. Pharmacol. 2003, 138, 1351–1357. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.D.; Cizkova, D. The role of nitric oxide in nociception. Curr Rev. Pain 2000, 4, 459–466. [Google Scholar] [CrossRef]
- Costa, A.; Galdino, G.; Romero, T.; Silva, G.; Cortes, S.; Santos, R.; Duarte, I. Ang-(1–7) activates the NO/cGMP and ATP-sensitive K+ channels pathway to induce peripheral antinociception in rats. Nitric Oxide 2014, 3, 11–16. [Google Scholar] [CrossRef]
- Freitas, A.C.; Silva, G.C.; Pacheco, D.F.; Pimenta, A.M.; Lemos, V.S.; Duarte, I.D.; de Lima, M.E. The synthetic peptide PnPP-19 induces peripheral antinociception via activation of NO/cGMP/KATP pathway: Role of eNOS and nNOS. Nitric Oxide 2017, 64, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Dubin, A.E.; Petrus, M.J.; Patapoutian, A. TRPV1 and TRPA1 mediate peripheral nitric oxide-induced nociception in mice. PLoS ONE 2009, 4, e7596. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.G.; Chen, S.R.; Cao, X.H.; Li, L.; Pan, H.L. Nitric oxide inhibits nociceptive transmission by differentially regulating glutamate and glycine release to spinal dorsal horn neurons. J. Biol. Chem. 2011, 286, 33190–33202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, A.; Fujita, M.; Shiomi, H. Involvement of endogenous nitric oxide in the mechanism of bradykinin-induced peripheral hyperalgesia. Br. J. Pharmacol. 1996, 117, 407–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galeotti, N.; Ghelardini, C. PKC-dependent signaling pathways within PAG and thalamus contribute to the nitric oxide-induced nociceptive behavior. ISRN Pain 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Duarte, I.D.; dos Santos, I.R.; Lorenzetti, B.B.; Ferreira, S.H. Analgesia by direct antagonism of nociceptor sensitization involves the l-arginine-nitric oxide-cGMP pathway. Eur. J. Pharmacol. 1992, 217, 225–227. [Google Scholar] [CrossRef]
- Nassar, M.I.; Aboutabl, E.-S.A.; Ahmed, R.F.; El-Khrisy, E.D.; Ibrahim, K.M.; Sleem, A.A. Secondary metabolites and bioactivities of Myrtus communis. Pharmacogn. Res. 2010, 2, 325–329. [Google Scholar] [CrossRef] [Green Version]
- Santos, A.R.; De Campos, R.O.; Miguel, O.G.; Cechinel-Filho, V.; Yunes, R.A.; Calixto, J.B. The involvement of K+ channels and Gi/o protein in the antinociceptive action of the gallic acid ethyl ester. Eur. J. Pharmacol. 1999, 379, 7–17. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, D.; Yu, X.; Xie, X.; Wang, L.; Lian, L.; Fei, N.; Chen, J.; Zhu, N.; Wang, G.; et al. The antinociceptive effects of ferulic acid on neuropathic pain: Involvement of descending monoaminergic system and opioid receptors. Oncotarget 2016, 7, 20455–20468. [Google Scholar] [CrossRef] [Green Version]
- Arslan, R.; Aydin, S.; Samur, D.N.; Bektas, N. The possible mechanisms of protocatechuic acid-induced central analgesia. Saudi Pharm. J. 2018, 26, 541–545. [Google Scholar] [CrossRef]
- Mehrotra, A.; Shanbhag, R.; Chamallamudi, M.R.; Singh, V.P.; Mudgal, J. Ameliorative effect of caffeic acid against inflammatory pain in rodents. Eur. J. Pharmacol. 2011, 666, 80–86. [Google Scholar] [CrossRef] [PubMed]
- de Campos Buzzi, F.; Franzoi, C.L.; Antonini, G.; Fracasso, M.; Cechinel Filho, V.; Yunes, R.A.; Niero, R. Antinociceptive properties of caffeic acid derivatives in mice. Eur. J. Med. Chem. 2009, 44, 4596–4602. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, A.; Sivakumar, A. Analgesic, anti-inflammatory and antipyretic evaluations of new isoquinoline derivatives. Int. J. Pharm. Pharm. Sci. 2016, 8, 339–843. [Google Scholar]
- Hernandez-Leon, A.; Fernández-Guasti, A.; González-Trujano, M.E. Rutin antinociception involves opioidergic mechanism and descending modulation of ventrolateral periaqueductal grey matter in rats. Eur. J. Pain 2016, 20, 274–283. [Google Scholar] [CrossRef]
- Krogh, R.; Kroth, R.; Berti, C.; Madeira, A.O.; Souza, M.M.; Cechinel-Filho, V.; Delle-Monache, F.; Yunes, R.A. Isolation and identification of compounds with antinociceptive action from Ipomoea pes-caprae (L.) R. Br. Die Pharm. 1999, 54, 464–466. [Google Scholar]
- Qnais, E.; Raad, D.; Bseiso, Y. Analgesic and anti-Inflammatory effects of an extract and flavonoids from Artemisia Herba-Alba and their mechanisms of action. Neurophysiology 2014, 46, 238–246. [Google Scholar] [CrossRef] [Green Version]
- Islam, S.; Shajib, M.S.; Rashid, R.B.; Khan, M.F.; Al-Mansur, M.A.; Datta, B.K.; Rashid, M.A. Antinociceptive activities of Artocarpus lacucha Buch-ham (Moraceae) and its isolated phenolic compound, catechin, in mice. BMC Complement. Altern. Med. 2019, 19, 214. [Google Scholar] [CrossRef] [Green Version]
- Filho, A.W.; Filho, V.C.; Olinger, L.; de Souza, M.M. Quercetin: Further investigation of its antinociceptive properties and mechanisms of action. Arch. Pharm. Res. 2008, 31, 713–721. [Google Scholar] [CrossRef]
- Anjaneyulu, M.; Chopra, K. Quercetin, a bioflavonoid, attenuates thermal hyperalgesia in a mouse model of diabetic neuropathic pain. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 1001–1005. [Google Scholar] [CrossRef]
- Pinheiro, M.M.; Boylan, F.; Fernandes, P.D. Antinociceptive effect of the Orbignya speciosa Mart. (Babassu) leaves: Evidence for the involvement of apigenin. Life Sci. 2012, 91, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chen, P.; Tang, C.; Wang, Y.; Li, Y.; Zhang, H. Antinociceptive and anti-inflammatory activities of extract and two isolated flavonoids of Carthamus tinctorius L. J. Ethnopharmacol. 2014, 151, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Rauf, A.; Hadda, T.B.; Bawazeer, S.; Abu-Izneid, T.; Khan, H.; Raza, M.; Khan, S.A.; Shah, S.U.; Pervez, S.; et al. Mechanisms underlying anti-hyperalgesic properties of kaempferol-3,7-di-O-α-L-rhamnopyranoside isolated from Dryopteris cycadina. Curr. Top. Med. Chem. 2017, 17, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Chung, S.H. Anti-inflammatory effect of alpha-linolenic acid and its mode of action through the inhibition of nitric oxide production and inducible nitric oxide synthase gene expression via NF-κB and mitogen-activated protein kinase pathways. J. Agric. Food Chem. 2007, 55, 5073–5080. [Google Scholar] [CrossRef]
- Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-inflammatory property of n-hexadecanoic acid: Structural evidence and kinetic assessment. Chem. Biol. Drug Des. 2012, 80, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Jäger, A.K.; Petersen, K.N.; Thomasen, G.; Christensen, S.B. Isolation of linoleic and α-linolenic acids as COX-1 and -2 inhibitors in rose hip. Phytother. Res. 2008, 22, 982–984. [Google Scholar] [CrossRef] [PubMed]





| Treatment | Dose (mg/kg) | Latency of Discomfort (sec) at Respective Time Interval (min) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 60 | 90 | 120 | 150 | 180 | 210 | ||
| Vehicle control | 5.53 ± 0.22 | 5.86 ± 0.19 | 5.90 ± 0.09 | 5.50 ± 0.11 | 5.80 ± 0.25 | 5.62 ± 0.17 | 5.65 ± 0.10 | |
| MEDL | 25 | 6.14 ± 0.16 | 7.17 ± 0.14 | 6.47 ± 0.21 | 6.77 ± 0.07 | 6.38 ± 0.22 | 6.30 ± 0.18 | 6.25 ± 0.15 |
| 150 | 6.30 ± 0.30 | 9.02 ± 0.46 *** | 6.93 ± 0.38 | 6.55 ± 0.13 | 6.20 ± 0.26 | 6.05 ± 0.38 | 6.18 ± 0.36 | |
| 300 | 5.92 ± 0.18 | 10.83 ± 0.74 *** | 8.34 ± 0.25 *** | 8.02 ± 0.31 *** | 6.83 ± 0.26 | 6.43 ± 0.29 | 6.57 ± 0.43 | |
| MOR | 5 | 5.80 ± 0.22 | 12.07 ± 0.90 *** | 10.29 ± 0.39 *** | 9.14 ± 0.36 *** | 8.59 ± 0.43 *** | 7.69 ± 0.21 *** | 7.65 ± 0.31 *** |
| Group | Dose (mg/kg) | Latency of Discomfort (sec) at Respective Time Interval (min) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 min | 60 min | 90 min | 120 min | 150 min | 180 min | 210 min | ||
| Vehicle Control | 5.53 ± 0.22 | 5.86 ± 0.19 | 5.90 ± 0.09 | 5.50 ± 0.11 | 5.80 ± 0.25 | 5.62 ± 0.17 | 5.65 ± 0.10 | |
| MEDL | 300 | 5.92 ± 0.18 | 10.83 ± 0.74 *** | 8.34 ± 0.25 *** | 8.02 ± 0.31 *** | 6.83 ± 0.26 | 6.43 ± 0.29 | 6.57 ± 0.43 |
| NLX + MEDL | 5 + 300 | 6.12 ± 0.22 | 6.21 ± 0.22 ### | 6.30 ± 0.09 ### | 5.88 ± 0.30 ### | 6.03 ± 0.36 | 6.17 ± 0.27 | 5.70 ± 0.28 |
| MOR | 5 | 5.80 ± 0.22 | 12.07 ± 0.90 *** | 10.29 ± 0.39 *** | 9.14 ± 0.36 *** | 8.59 ± 0.43 *** | 7.69 ± 0.21 *** | 7.65 ± 0.31 *** |
| NLX + MOR | 5 + 5 | 6.58 ± 0.24 | 7.08 ± 0.24 ### | 7.40 ± 0.21 ### | 7.58 ± 0.40 # | 7.03 ± 0.36 # | 7.33 ± 0.40 | 7.23 ± 0.40 |
| Tentative Identification | RT (min) | Mol Formula | Exact Mass [M − H]− | ∆ Mass (ppm) | MSn |
|---|---|---|---|---|---|
| Quinic acid | 0.6 | C7H11O6 | 191.05495 | −0.338 | 0 |
| Citric acid | 0.62 | C6H7O7 | 191.01857 | −0.309 | 11,117,367 |
| Gallic acid | 0.65 | C10H9O4 | 169.0.1297 | −1.064 | 12,512,796 |
| Ferulic acid | 0.69 | C7H5O6 | 193.04971 | 0.905 | 87,111,178,134 |
| Protocatechuic acid | 0.87 | C7H5O4 | 153.18720 | 3.169 | 109 |
| Protocatechuic acid-4-O-β-hexoside | 1.1 | C13H15O9 | 315.07184 | 2.481 | 0 |
| Coumaryl-hexoside | 2.89 | C15H17O8 | 325.09268 | 2.633 | 145,163,119 |
| Ferulic acid hexose | 3.50 | C9H7O4 | 355.1033 | 2.651 | 193,178,134 |
| Caffeic acid | 3.52 | C9H7O4 | 179.03387 | −0.085 | 135,107 |
| Liquiritin-O-glucosylapioside | 3.53 | C16H19O10 | 711.2146 | 2.122 | 433,311 |
| Galloylquinic acid | 3.62 | C9H7O3 | 343.06738 | 4.101 | 0 |
| Quercetin-O-diglucoside | 4.28 | C27H29O17 | 625.14069 | 1.223 | 463,301,151 |
| p-Coumaric acid | 4.58 | C14H15O11 | 163.03922 | 1.530 | |
| Rutin isomer ii | 4.86 | C27H29O16 | 609.14557 | 0.918 | 300,301,151 |
| Dichotomain B-i | 5.05 | C21H23O12 | 467.11954 | 2.435 | 112 |
| Isoquercetrin | 5.53 | C21H19O12 | 463.08820 | 2.370 | 300,301,178,151 |
| Dichotamain B-i | 5.82 | C21H23O12 | 467.11945 | 2.242 | 112 |
| Vicenin | 6.1 | C27H29O15 | 593.1033 | 1.321 | 575,473,353 |
| Kaempferol-3-O-galactoside | 6.39 | C21H19O11 | 447.09283 | 1.436 | 284,255 |
| Dichotomain A-i | 6.45 | C23H25O13 | 509.12955 | 1.145 | 474 |
| Astragalin | 6.48 | C21H19O11 | 447.09296 | 1.727 | 28,785 |
| (+)Aromadendrin | 6.82 | C18H17O9 | 287.05521 | 0.681 | 0 |
| Geshoidin | 7.06 | C15H11O7 | 377.08701 | 0.800 | 217,115 |
| Catechin | 7.27 | C15H13O6 | 289.0712 | 1.852 | 87,245 |
| Apigenin-7-O-glucoside | 7.59 | C21H19O9 | 431.09763 | 0.828 | 28,587 |
| Dichotomain A-ii | 7.71 | C23H25O13 | 509.12955 | 0.477 | 474 |
| Quercetin | 9.11 | C15H9O7 | 301.03580 | 1.000 | 178,151 |
| Kaempferol-3-O-glucoside | 9.62 | C30H25O13 | 593.12970 | 1.236 | 285,161 |
| Apigenin | 10.7 | C15H9O5 | 269.04559 | 0.521 | 151,225,228 |
| Kaempferol | 11.17 | C15H9O6 | 285.0394 | 0.125 | 183,257 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakaria, Z.A.; Roosli, R.A.J.; Marmaya, N.H.; Omar, M.H.; Basir, R.; Somchit, M.N. Methanol Extract of Dicranopteris linearis Leaves Attenuate Pain via the Modulation of Opioid/NO-Mediated Pathway. Biomolecules 2020, 10, 280. https://doi.org/10.3390/biom10020280
Zakaria ZA, Roosli RAJ, Marmaya NH, Omar MH, Basir R, Somchit MN. Methanol Extract of Dicranopteris linearis Leaves Attenuate Pain via the Modulation of Opioid/NO-Mediated Pathway. Biomolecules. 2020; 10(2):280. https://doi.org/10.3390/biom10020280
Chicago/Turabian StyleZakaria, Zainul Amiruddin, Rushduddin Al Jufri Roosli, Najihah Hanisah Marmaya, Maizatul Hasyima Omar, Rusliza Basir, and Muhammad Nazrul Somchit. 2020. "Methanol Extract of Dicranopteris linearis Leaves Attenuate Pain via the Modulation of Opioid/NO-Mediated Pathway" Biomolecules 10, no. 2: 280. https://doi.org/10.3390/biom10020280
APA StyleZakaria, Z. A., Roosli, R. A. J., Marmaya, N. H., Omar, M. H., Basir, R., & Somchit, M. N. (2020). Methanol Extract of Dicranopteris linearis Leaves Attenuate Pain via the Modulation of Opioid/NO-Mediated Pathway. Biomolecules, 10(2), 280. https://doi.org/10.3390/biom10020280





