Biochemical and Behavioral Characterization of IN14, a New Inhibitor of HDACs with Antidepressant-Like Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
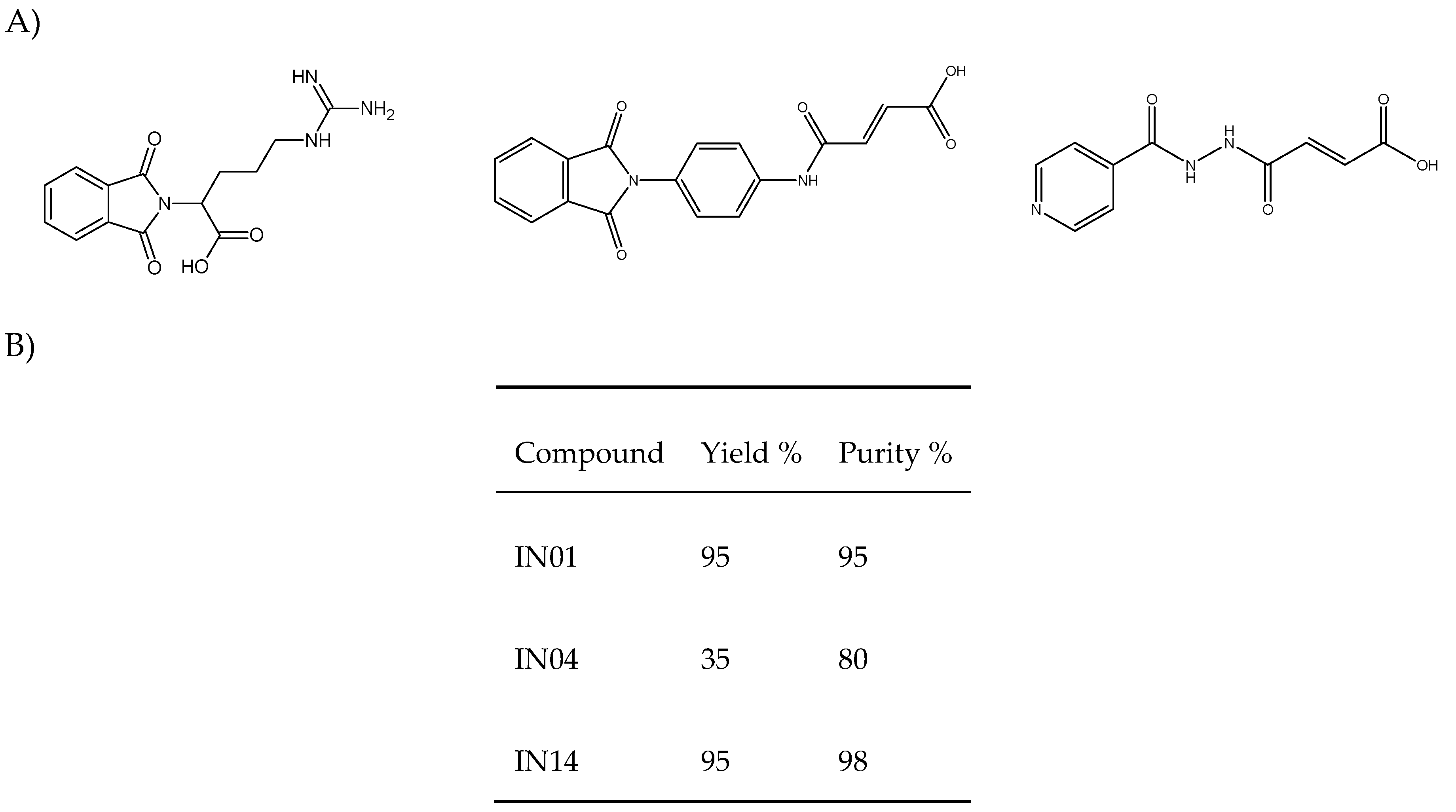
2.2. Chemicals and Reagents
2.3. Artemia Salina Toxicity Test
2.3.1. Hatching of Artemia Salina
2.3.2. Toxicity of HDAC Inhibitors to A. Salina
2.4. In vitro ELISA-Based HDAC Activity Assay
2.5. Pharmacokinetics of IN14
2.5.1. Mice Treatment
2.5.2. Rodent Biofluid Harvesting and Purification
2.5.3. UPLC–ESI-TOF-MS Analysis
2.6. Behavioral Assays
2.6.1. Treatment
2.6.2. Forced-Swimming Test
2.6.3. Elevated T Maze
2.7. Data Analysis
3. Results
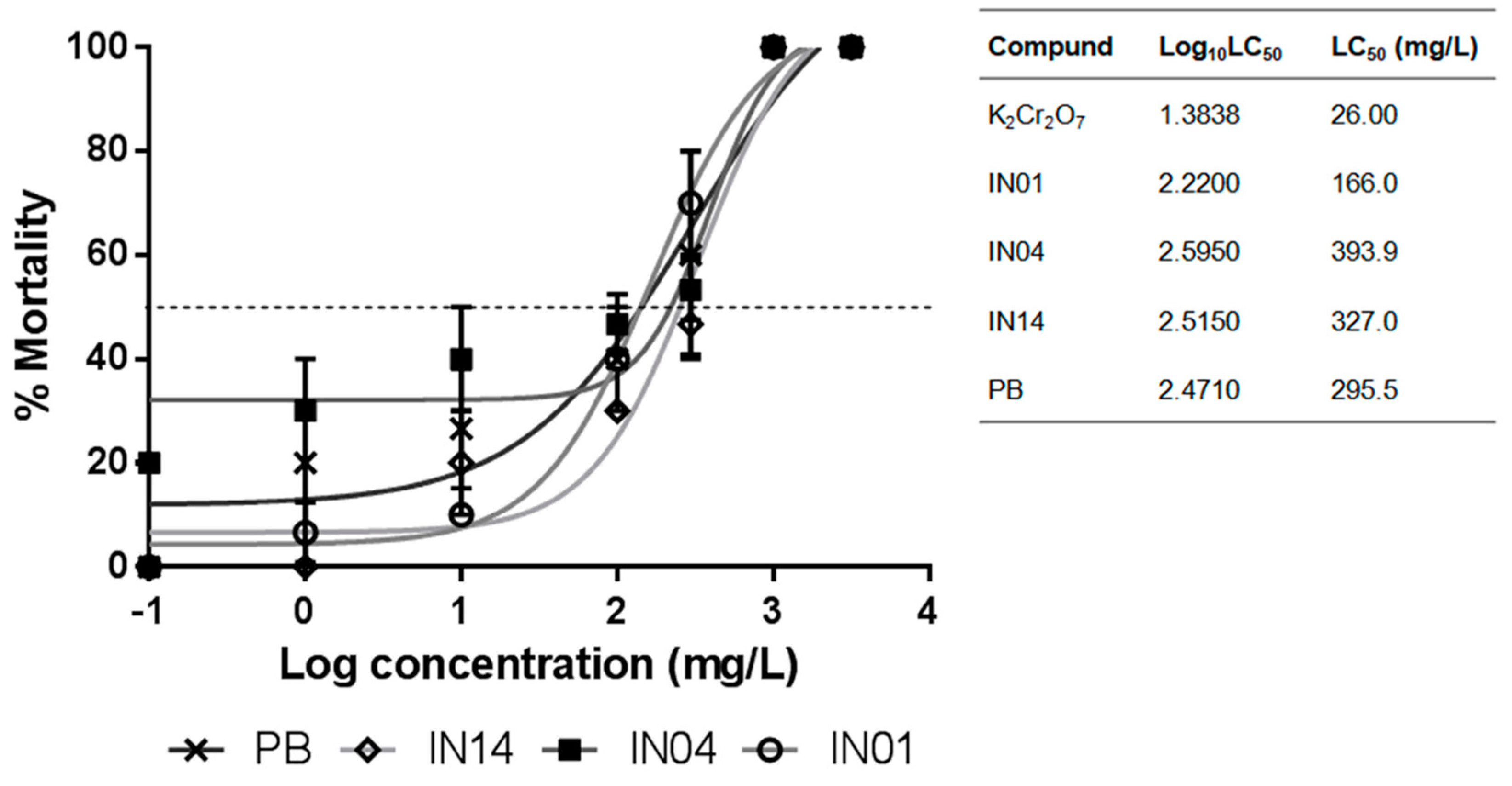
3.1. LC50 of IN01, IN04 and IN14
3.2. Inhibitory Activity of IN01, IN04 and IN14 Against Class I HDACs.
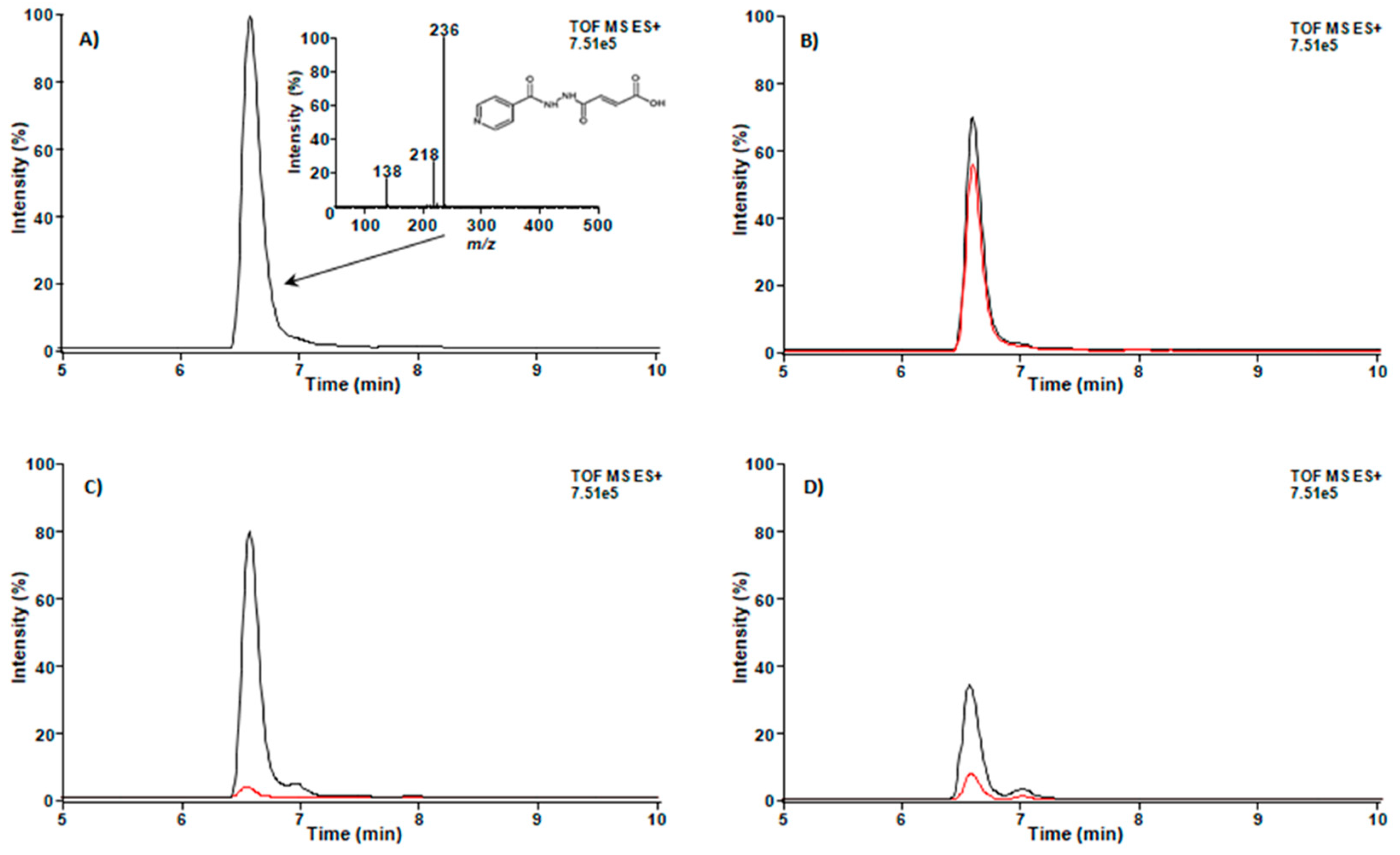
3.3. Compound IN14 Crosses the BBB
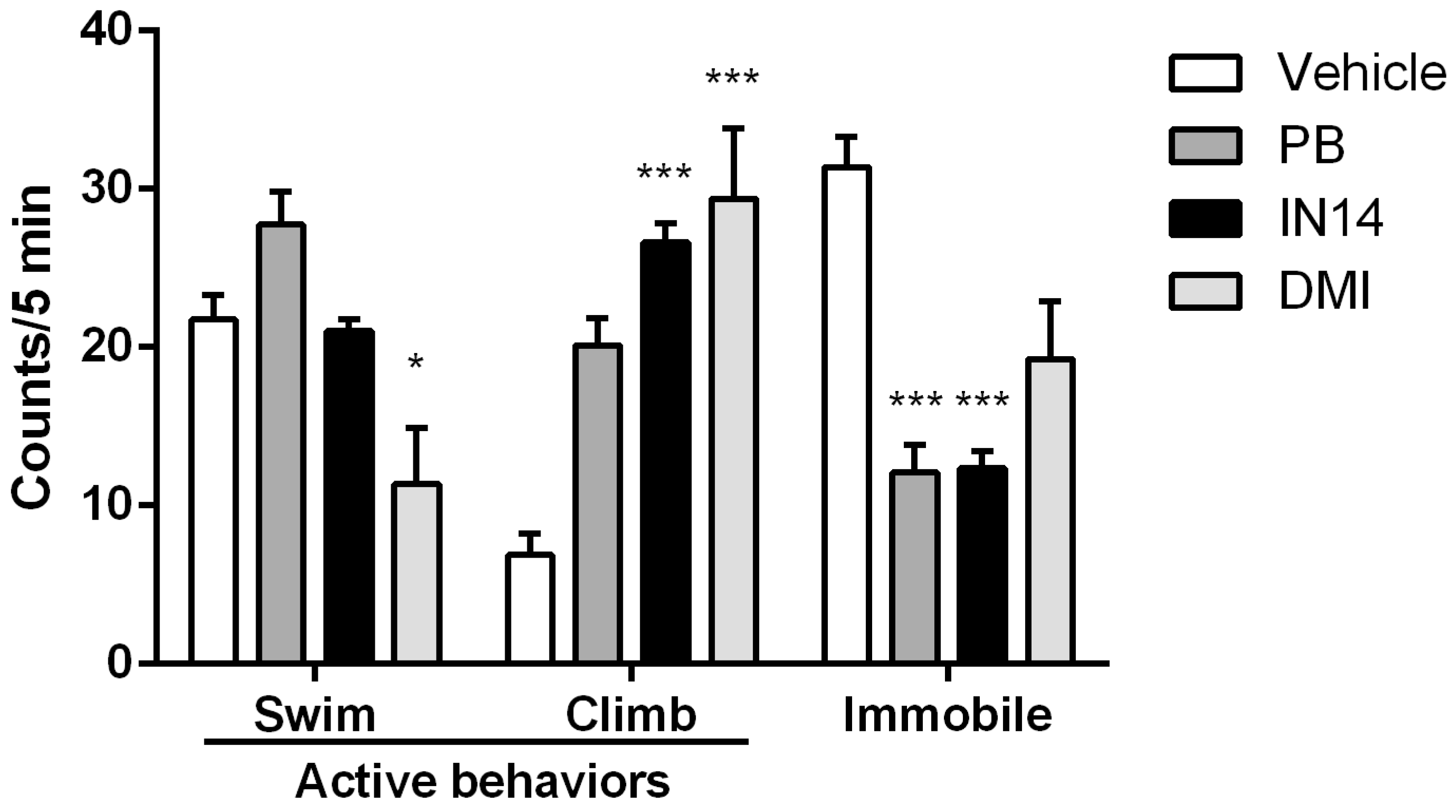
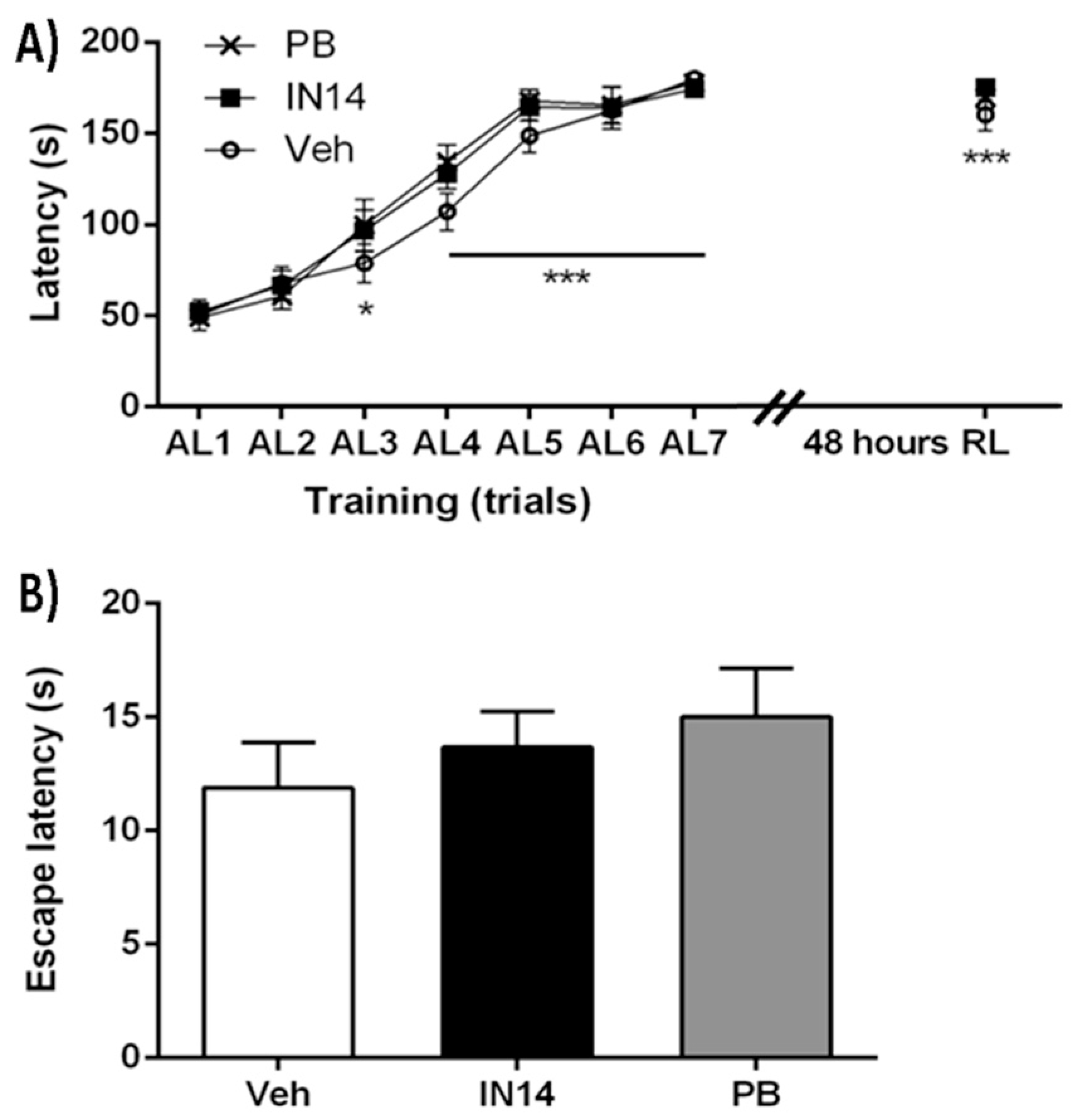
3.4. Behavioral Assays
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Levenson, J.M.; O’Riordan, K.J.; Brown, K.D.; Trinh, M.A.; Molfese, D.L.; Sweatt, J.D. Regulation of histone acetylation during memory formation in the hippocampus. J. Biol. Chem. 2004, 279, 40545–40559. [Google Scholar] [CrossRef]
- Fischer, A.; Sananbenesi, F.; Wang, X.; Dobbin, M.; Tsai, L.H. Recovery of learning and memory is associated with chromatin remodelling. Nature 2007, 447, 178–182. [Google Scholar] [CrossRef]
- Lattal, K.M.; Barrett, R.M.; Wood, M.A. Systemic or Intrahippocampal Delivery of Histone Deacetylase Inhibitors Facilitates Fear Extinction. Behav. Neurosci. 2007, 121, 1125–1131. [Google Scholar] [CrossRef]
- Barrett, R.M.; Wood, M.A. Beyond transcription factors: The role of chromatin modifying enzymes in regulating transcription required for memory. Learn. Mem. 2008, 15, 460–467. [Google Scholar] [CrossRef]
- Monsey, M.S.; Ota, K.T.; Akingbade, I.F.; Hong, E.S.; Schafe, G.E. Epigenetic Alterations are Critical for Fear Memory Consolidation and Synaptic Plasticity in The Lateral Amygdala. Plos ONE 2011, 6. [Google Scholar] [CrossRef]
- Nestler, E.J. Epigenetic mechanisms in psychiatry. Biol. Psychiatry 2009, 65, 189–190. [Google Scholar] [CrossRef]
- Lee, S.; Jeong, J.; Kwak, Y.; Park, S.K. Depression research: Where are we now? Mol. Brain 2010, 3, 1–10. [Google Scholar] [CrossRef]
- Misztak, P.; Panczyszyn-Trzewik, P.; Sowa-Kucma, M. Histone deacetylases (HDACs) as therapeutic target for depressive disorders. Pharm. Rep. 2018, 70, 398–408. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente Revenga, M.; Ibi, D.; Cuddy, T.; Toneatti, R.; Kurita, M.; Ijaz, M.K.; Miles, M.F.; Wolstenholme, J.T.; Gonzalez-Maeso, J. Correction: Chronic clozapine treatment restrains via HDAC2 the performance of mGlu2 receptor agonism in a rodent model of antipsychotic activity. Neuropsychopharmacology 2019, 44, 455–456. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente Revenga, M.; Ibi, D.; Cuddy, T.; Toneatti, R.; Kurita, M.; Ijaz, M.K.; Miles, M.F.; Wolstenholme, J.T.; Gonzalez-Maeso, J. Chronic clozapine treatment restrains via HDAC2 the performance of mGlu2 receptor agonism in a rodent model of antipsychotic activity. Neuropsychopharmacology 2019, 44, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.J.; Huang, H.Y.; Hsieh-Li, H.M. MGCD0103, a selective histone deacetylase inhibitor, coameliorates oligomeric Abeta25-35 -induced anxiety and cognitive deficits in a mouse model. CNS Neurosci. Ther. 2019, 25, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Citraro, R.; Leo, A.; De Caro, C.; Nesci, V.; Gallo Cantafio, M.E.; Amodio, N.; Mattace Raso, G.; Lama, A.; Russo, R.; Calignano, A.; et al. Effects of Histone Deacetylase Inhibitors on the Development of Epilepsy and Psychiatric Comorbidity in WAG/Rij Rats. Mol. Neurobiol. 2020, 57, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, E.; Nestler, E.J.; Allis, C.D.; Sassone-Corsi, P. Decoding the Epigenetic Language of Neuronal Plasticity. Neuron 2008, 60, 961–974. [Google Scholar] [CrossRef] [PubMed]
- De Sa Nogueira, D.; Merienne, K.; Befort, K. Neuroepigenetics and addictive behaviors: Where do we stand? Neurosci. Biobehav. Rev. 2018. [Google Scholar] [CrossRef]
- Sweatt, J.D. The emerging field of neuroepigenetics. Neuron 2013, 80, 624–632. [Google Scholar] [CrossRef]
- Verdone, L.; Caserta, M.; Mauro, E.D. Role of histone acetylation in the control of gene expression. Biochem. Cell Biol. 2005, 83, 344–353. [Google Scholar] [CrossRef]
- Kurdistani, S.K.; Grunstein, M. Histone acetylation and deacetylation in yeast. Nat. Rev. Mol. Cell Biol. 2003, 4, 276–284. [Google Scholar] [CrossRef]
- Goldberg, A.D.; Allis, C.D.; Bernstein, E. Epigenetics: A Landscape Takes Shape. Cell 2007, 128, 635–638. [Google Scholar] [CrossRef]
- Covington, H.E.; Maze, I.; Laplant, Q.C.; Vialou, V.F.; Ohnishi, N.; Berton, O.; Fass, D.M.; Renthal, W.; Iii, A.J.R.; Wu, Y.; et al. Antidepressant Actions of HDAC Inhibitors. J. Neurosci. 2009, 29, 11451–11460. [Google Scholar] [CrossRef]
- Lin, H.; Geng, X.; Dang, W.; Wu, B.; Dai, Z.; Li, Y.; Yang, Y.; Zhang, H.; Shi, J. Molecular mechanisms associated with the antidepressant effects of the class i histone deacetylase inhibitor MS-275 in the rat ventrolateral orbital cortex. Brain Res. 2012, 1447, 119–125. [Google Scholar] [CrossRef]
- Yamawaki, Y.; Fuchikami, M.; Morinobu, S.; Segawa, M.; Matsumoto, T.; Yamawaki, S. Antidepressant-like effect of sodium butyrate (HDAC inhibitor) and its molecular mechanism of action in the rat hippocampus. World J. Biol. Psychiatry 2012, 13, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.S.; Haggarty, S.J.; Giacometti, E.; Dannenberg, J.H.; Joseph, N.; Gao, J.; Nieland, T.J.F.; Zhou, Y.; Wang, X.; Mazitschek, R.; et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature 2009, 459, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Gräff, J.; Woldemichael, B.T.; Berchtold, D.; Dewarrat, G.; Mansuy, I.M. Dynamic histone marks in the hippocampus and cortex facilitate memory consolidation. Nat. Commun. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pacheco, H.; Espinosa-Raya, J.; Picazo, O.; Roldán-Roldán, G.; Viñas-Bravo, O.; Ramírez-Galicia, G. Design (Docking and QSAR Studies) and synthesis of histone deacetylase 2 (HDAC2) inhibitors series. Med. Chem. Res. 2018, 27, 206–223. [Google Scholar] [CrossRef]
- Nunes, B.S.; Carvalho, F.D.; Guilhermino, L.M.; Van Stappen, G. Use of the genus Artemia in ecotoxicity testing. Environ. Pollut. 2006, 144, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pacheco, H. Diseño, síntesis y evaluación de efectos bioquímicos y conductuales de posibles inhibidores de la desacetilación de histonas; Instituto Politécnico Nacional: Ciudad de México, México, 2015. [Google Scholar]
- Porsolt, R.D.; Deniel, M.; Jalfre, M. Forced swimming in rats: Hypothermia, immobility and the effects of imipramine. Eur. J. Pharmacol. 1979, 57, 431–436. [Google Scholar] [CrossRef]
- Farzin, D.; Mansouri, N. Antidepressant-like effect of harmane and other beta-carbolines in the mouse forced swim test. Eur. Neuropsychopharmacol. 2006, 16, 324–328. [Google Scholar] [CrossRef]
- Yankelevitch-Yahav, R.; Franko, M.; Huly, A.; Doron, R. The forced swim test as a model of depressive-like behavior. J. Vis. Exp. Jove 2015. [Google Scholar] [CrossRef]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef]
- Treit, D.; Menard, J.; Royan, C. Anxiogenic stimuli in the elevated plus-maze. Pharmacol. Biochem. Behav. 1993, 44, 463–469. [Google Scholar] [CrossRef]
- Moshi, M.J.; Innocent, E.; Magadula, J.J.; Otieno, D.F.; Weisheit, A.; Mbabazi, P.K.; Nondo, R.S.O. Brine shrimp toxicity of some plants used as traditional medicines in Kagera Region, north western Tanzania. Tanzan. J. Health Res. 2010, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Vigushin, D.M.; Ali, S.; Pace, P.E.; Mirsaidi, N.; Ito, K.; Adcock, I.; Coombes, R.C. Trichostatin A is a histone deacetylase inhibitor with potent antitumor activity against breast cancer in vivo. Clin. Cancer Res. 2001, 7, 971–976. [Google Scholar] [PubMed]
- Koutsounas, I.; Giaginis, C.; Theocharis, S. Histone deacetylase inhibitors and pancreatic cancer: Are there any promising clinical trials? World J. Gastroenterol. 2013, 19, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Harkin, A.; Houlihan, D.D.; Kelly, J.P. Reduction in preference for saccharin by repeated unpredictable stress in mice and its prevention by imipramine. J. Psychopharmacol. 2002, 16, 115–123. [Google Scholar] [CrossRef]
- Frenois, F.; Moreau, M.; O’Connor, J.; Lawson, M.; Micon, C.; Lestage, J.; Kelley, K.W.; Dantzer, R.; Castanon, N. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology 2007, 32, 516–531. [Google Scholar] [CrossRef]
- Painsipp, E.; Köfer, M.J.; Sinner, F.; Holzer, P. Prolonged depression-like behavior caused by immune challenge: Influence of mouse strain and social environment. Plos ONE 2011, 6. [Google Scholar] [CrossRef]
- Bechtholt-Gompf, A.J.; Smith, K.L.; John, C.S.; Kang, H.H.; Carlezon, W.A.; Cohen, B.M.; Öngür, D. CD-1 and Balb/cJ mice do not show enduring antidepressant-like effects of ketamine in tests of acute antidepressant efficacy. Psychopharmacology 2011, 215, 689–695. [Google Scholar] [CrossRef]
- Gundersen, B.B.; Blendy, J.A. Effects of the histone deacetylase inhibitor sodium butyrate in models of depression and anxiety. Neuropharmacology 2009, 57, 67–74. [Google Scholar] [CrossRef]
- Meylan, E.M.; Halfon, O.; Magistretti, P.J.; Cardinaux, J.R. The HDAC inhibitor SAHA improves depressive-like behavior of CRTC1-deficient mice: Possible relevance for treatment-resistant depression. Neuropharmacology 2016, 107, 111–121. [Google Scholar] [CrossRef]
- Detke, M.J.; Rickels, M.; Lucki, I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology 1995, 121, 66–72. [Google Scholar] [CrossRef]
- McQuown, S.C.; Wood, M.A. Epigenetic regulation in substance use disorders. Curr. Psychiatry Rep. 2010, 12, 145–153. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sandi, C.; Pinto, R.M.; Al-Mahdawi, S.; Ezzatizadeh, V.; Barnes, G.; Jones, S.; Rusche, J.R.; Gottesfeld, J.M.; Pook, M.A. Prolonged treatment with pimelic o-aminobenzamide HDAC inhibitors ameliorates the disease phenotype of a Friedreich ataxia mouse model. Neurobiol. Dis. 2011, 42, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.J.; Mahgoub, M.; Na, E.S.; Pranav, H.; Monteggia, L.M. Loss of Histone Deacetylase 2 Improves Working Memory and Accelerates Extinction Learning. J. Neurosci. 2013, 33, 6401–6411. [Google Scholar] [CrossRef] [PubMed]
- Sakharkar, A.J.; Zhang, H.; Tang, L.; Baxstrom, K.; Shi, G.; Moonat, S.; Pandey, S.C. Effects of histone deacetylase inhibitors on amygdaloid histone acetylation and neuropeptide y expression: A role in anxiety-like and alcohol-drinking behaviours. Int. J. Neuropsychopharmacol. 2014, 17, 1207–1220. [Google Scholar] [CrossRef]
- Schmauss, C. An HDAC-dependent epigenetic mechanism that enhances the efficacy of the antidepressant drug fluoxetine. Sci. Rep. 2015, 5, 4–11. [Google Scholar] [CrossRef]
- Asth, L.; Lobão-Soares, B.; André, E.; Soares, V.d.P.; Gavioli, E.C. The elevated T-maze task as an animal model to simultaneously investigate the effects of drugs on long-term memory and anxiety in mice. Brain Res. Bull. 2012, 87, 526–533. [Google Scholar] [CrossRef]
- Villain, H.; Florian, C.; Roullet, P. HDAC inhibition promotes both initial consolidation and reconsolidation of spatial memory in mice. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Pacheco, H.; Picazo, O.; López-Torres, A.; Morin, J.-P.; Castro-Cerritos, K.V.; Zepeda, R.C.; Roldán-Roldán, G. Biochemical and Behavioral Characterization of IN14, a New Inhibitor of HDACs with Antidepressant-Like Properties. Biomolecules 2020, 10, 299. https://doi.org/10.3390/biom10020299
Martínez-Pacheco H, Picazo O, López-Torres A, Morin J-P, Castro-Cerritos KV, Zepeda RC, Roldán-Roldán G. Biochemical and Behavioral Characterization of IN14, a New Inhibitor of HDACs with Antidepressant-Like Properties. Biomolecules. 2020; 10(2):299. https://doi.org/10.3390/biom10020299
Chicago/Turabian StyleMartínez-Pacheco, Heidy, Ofir Picazo, Adolfo López-Torres, Jean-Pascal Morin, Karla Viridiana Castro-Cerritos, Rossana Citlali Zepeda, and Gabriel Roldán-Roldán. 2020. "Biochemical and Behavioral Characterization of IN14, a New Inhibitor of HDACs with Antidepressant-Like Properties" Biomolecules 10, no. 2: 299. https://doi.org/10.3390/biom10020299
APA StyleMartínez-Pacheco, H., Picazo, O., López-Torres, A., Morin, J.-P., Castro-Cerritos, K. V., Zepeda, R. C., & Roldán-Roldán, G. (2020). Biochemical and Behavioral Characterization of IN14, a New Inhibitor of HDACs with Antidepressant-Like Properties. Biomolecules, 10(2), 299. https://doi.org/10.3390/biom10020299




