Increased Arginase Expression and Decreased Nitric Oxide in Pig Donor Lungs after Normothermic Ex Vivo Lung Perfusion
Abstract
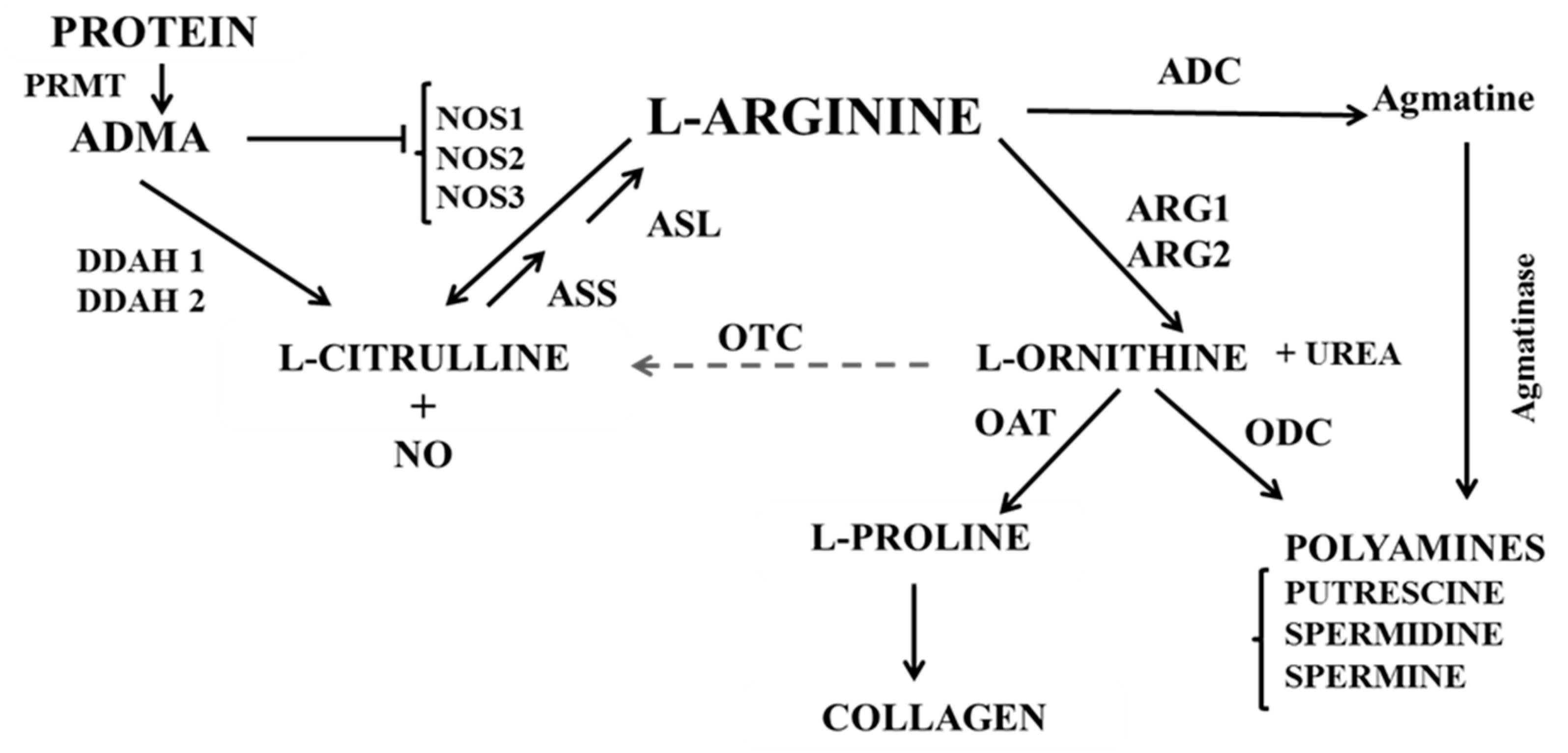
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Tissue Processing and Analyses
3. Statistics
4. Results
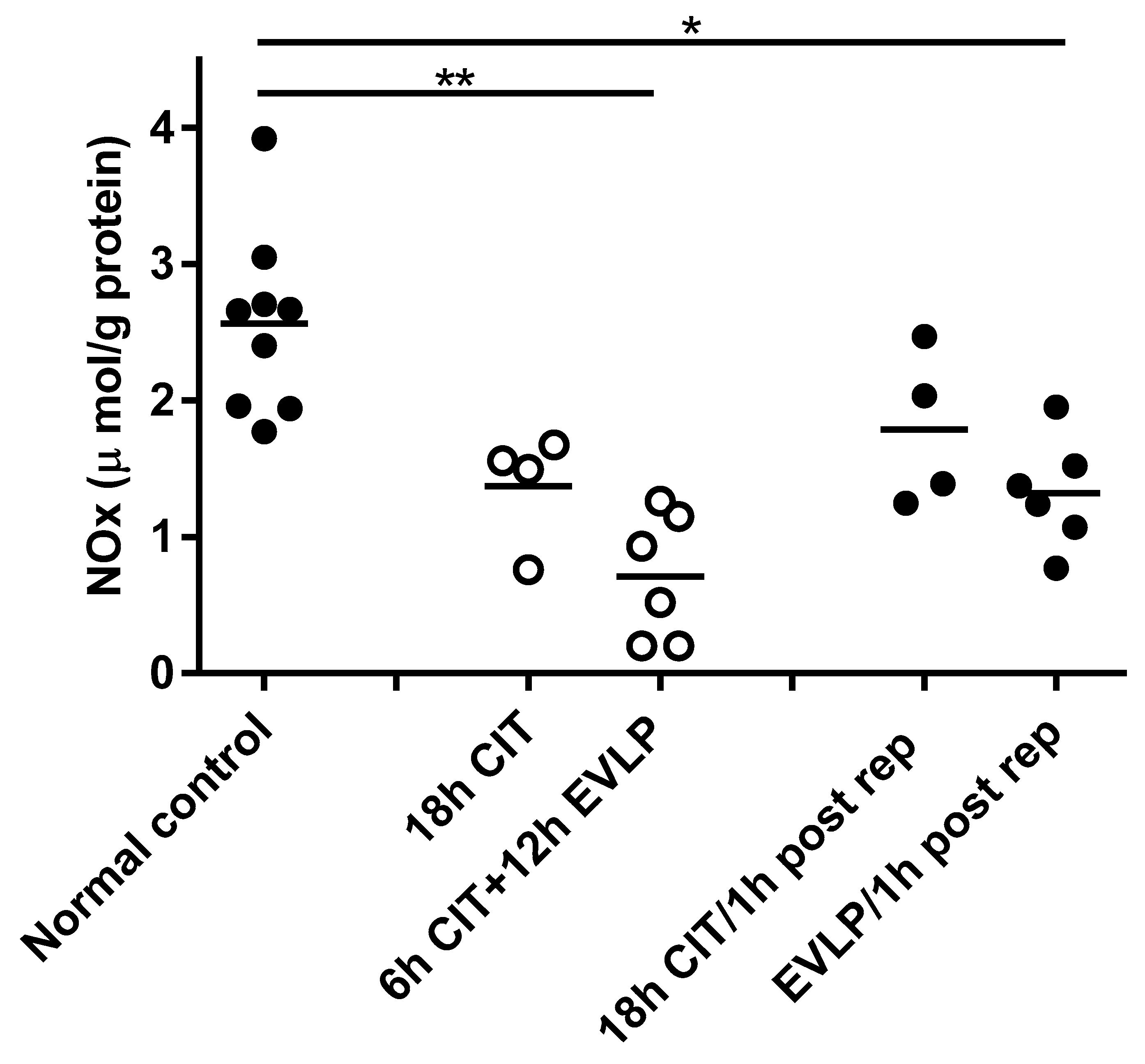
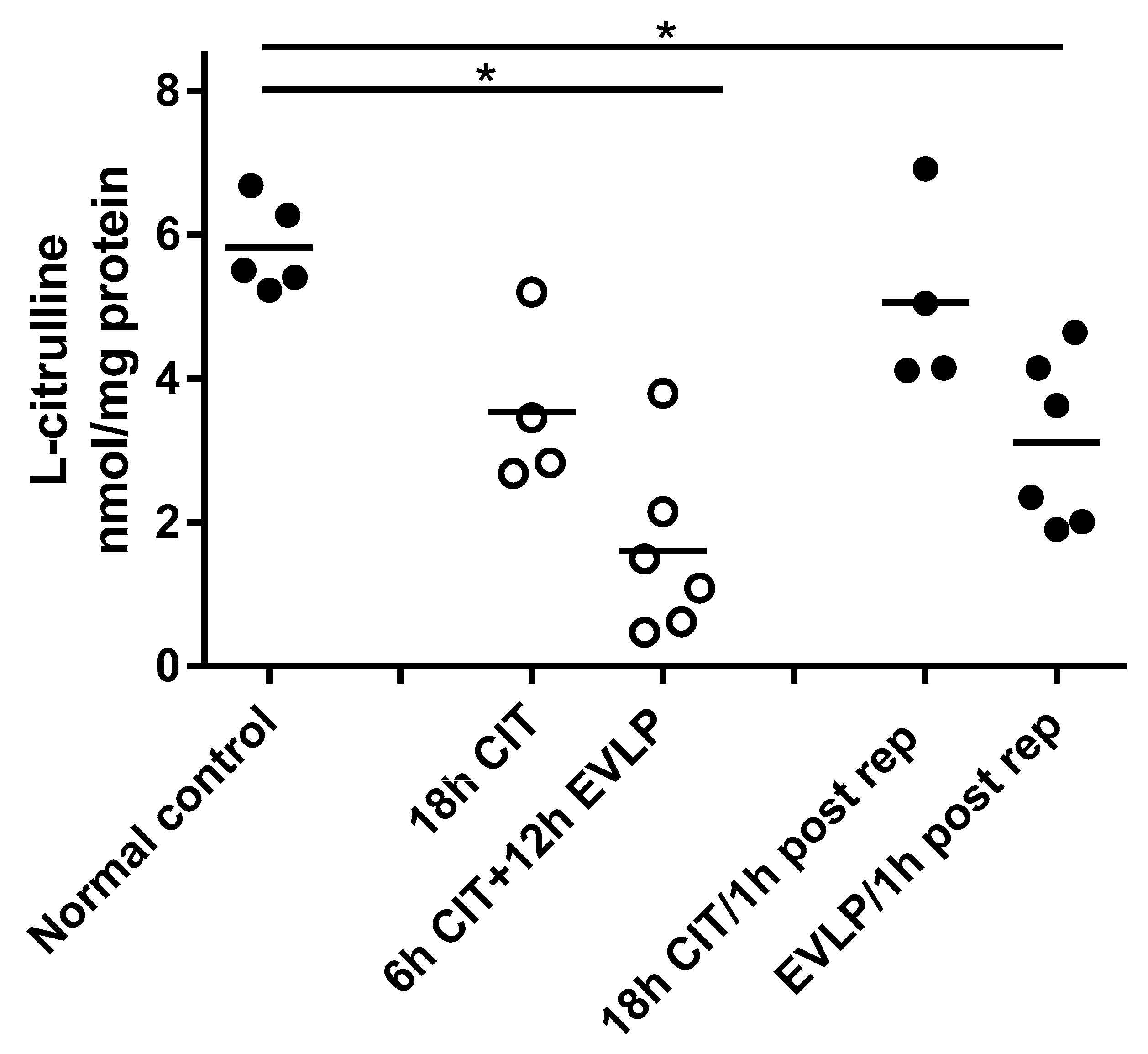
4.1. NOx and l-Citrulline Concentrations in the Lung Are Decreased after EVLP
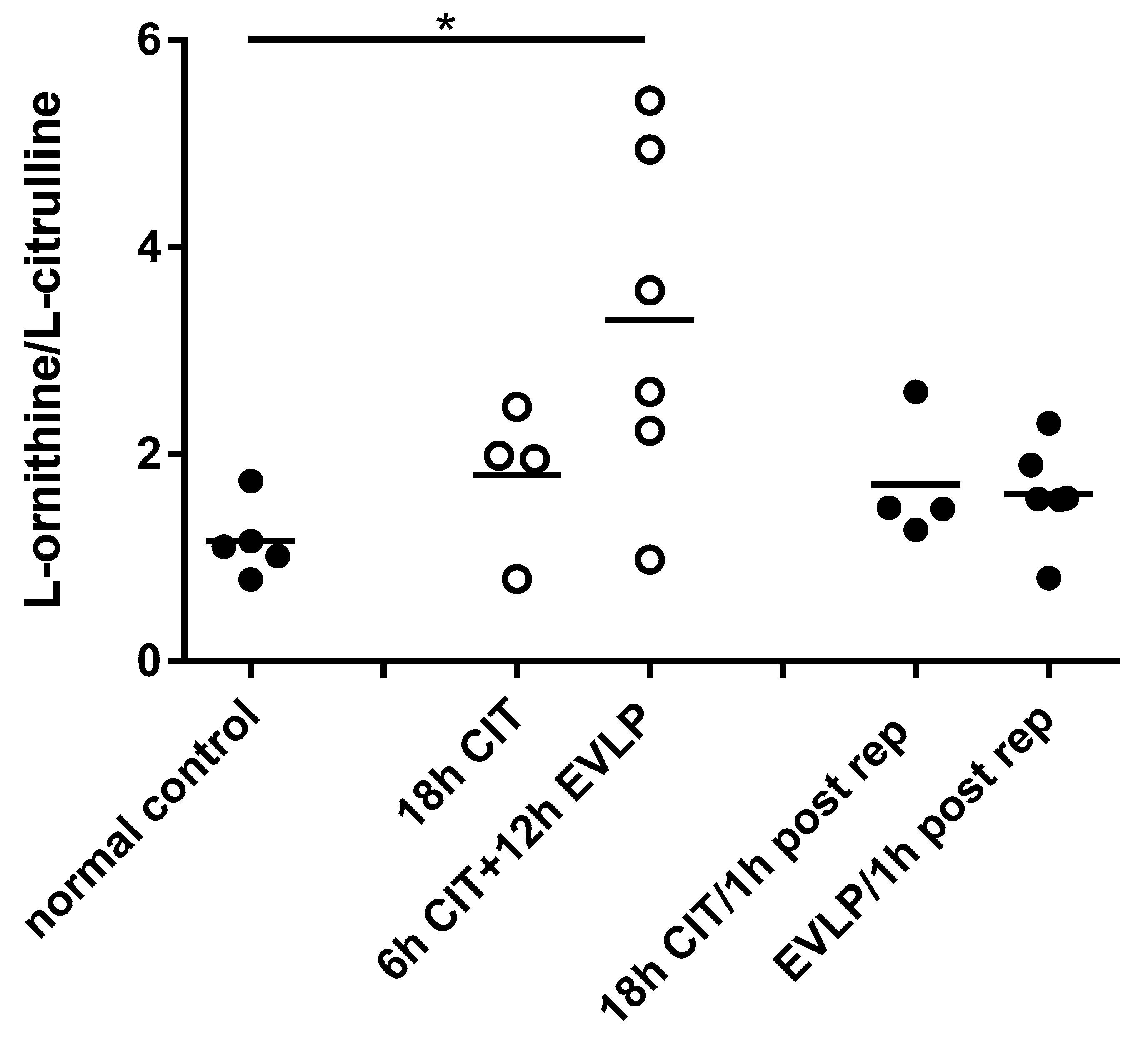
4.2. L-arginine Availability and NOS Impairment
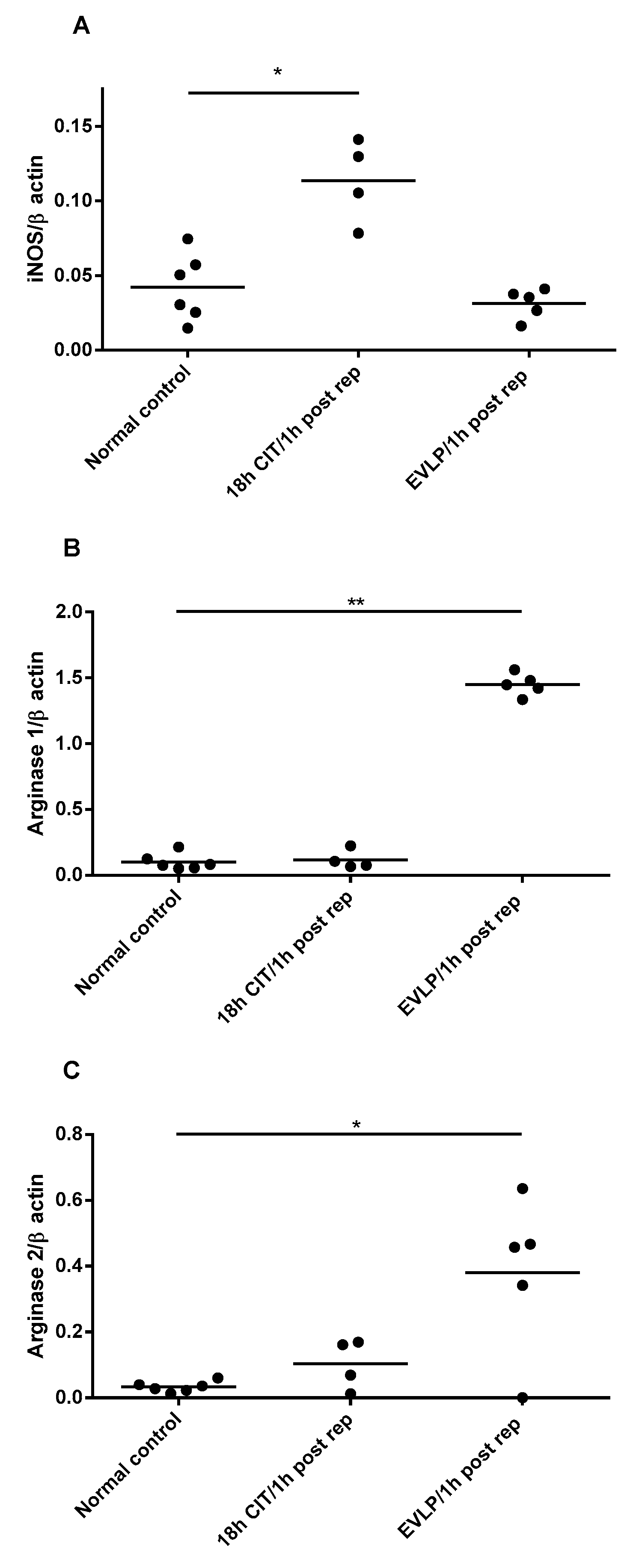
4.3. NOS and Arginase mRNA Expression
5. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ignarro, L.J. Nitric oxide as a unique signaling molecule in the vascular system: A historical overview. J. Physiol. Pharmacol. 2002, 53, 503–514. [Google Scholar] [PubMed]
- Ricciardolo, F. Multiple roles of nitric oxide in the airways. Thorax 2003, 58, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Morris, S.M., Jr. Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998, 336, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Racké, K.; Warnken, M. L-arginine metabolic pathways. Open Nitric Oxide J. 2010, 2, 9–19. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Meininger, C.J.; Hawker, J.R.; Haynes, T.E.; Kepka-Lenhart, D.; Mistry, S.K.; Morris, S.M.; Wu, G. Regulatory role of arginase I and II in nitric oxide, polyamine, and proline syntheses in endothelial cells. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E75–E82. [Google Scholar] [CrossRef]
- Forstermann, U.; Munzel, T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef]
- Lu, Y.T.; Liu, S.F.; Mitchell, J.A.; Malik, A.B.; Hellewell, P.G.; Evans, T.W. The role of endogenous nitric oxide in modulating ischemia-reperfusion injury in the isolated, blood-perfused rat lung. Am. J. Respir. Crit. Care Med. 1998, 157, 273–279. [Google Scholar] [CrossRef]
- Grasemann, H.; Schwiertz, R.; Matthiesen, S.; Racke, K.; Ratjen, F. Increased arginase activity in cystic fibrosis airways. Am. J. Respir. Crit. Care Med. 2005, 172, 1523–1528. [Google Scholar] [CrossRef]
- North, M.L.; Khanna, N.; Marsden, P.A.; Grasemann, H.; Scott, J.A. Functionally important role for arginase 1 in the airway hyperresponsiveness of asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 296, L911–L920. [Google Scholar] [CrossRef]
- Mehl, A.; Grasemann, H. Alterations in L-arginine metabolism after lung transplantation. Open Nitric Oxide J. 2010, 2, 55–63. [Google Scholar] [CrossRef][Green Version]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Den Hengst, W.A.; Gielis, J.F.; Lin, J.Y.; Van Schil, P.E.; De Windt, L.J.; Moens, A.L. Lung ischemia-reperfusion injury: A molecular and clinical view on a complex pathophysiological process. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1283–H1299. [Google Scholar] [CrossRef] [PubMed]
- De Perrot, M.; Liu, M.; Waddell, T.K.; Keshavjee, S. Ischemia-reperfusion-induced lung injury. Am. J. Respir. Crit. Care Med. 2003, 167, 490–511. [Google Scholar] [CrossRef]
- Marczin, N.; Riedel, B.; Gal, J.; Polak, J.; Yacoub, M. Exhaled nitric oxide during lung transplantation. Lancet 1997, 350, 1681–1682. [Google Scholar] [CrossRef][Green Version]
- Yerebakan, C.; Ugurlucan, M.; Bayraktar, S.; Bethea, B.T.; Conte, J.V. Effects of inhaled nitric oxide following lung transplantation. J. Card. Surg. 2009, 24, 269–274. [Google Scholar] [CrossRef]
- Date, H.; Triantafillou, A.N.; Trulock, E.P.; Pohl, M.S.; Cooper, J.D.; Patterson, G.A. Inhaled nitric oxide reduces human lung allograft dysfunction. J. Thorac. Cardiovasc. Surg. 1996, 111, 913–919. [Google Scholar] [CrossRef]
- Moreno, I.; Vicente, R.; Mir, A.; Leon, I.; Ramos, F.; Vicente, J.L.; Barbera, M. Effects of inhaled nitric oxide on primary graft dysfunction in lung transplantation. Transplant. Proc. 2009, 41, 2210–2212. [Google Scholar] [CrossRef]
- Pasero, D.; Martin, E.L.; Davi, A.; Mascia, L.; Rinaldi, M.; Ranieri, V.M. The effects of inhaled nitric oxide after lung transplantation. Minerva Anestesiol. 2010, 76, 353–361. [Google Scholar]
- Normandin, L.; Herve, P.; Brink, C.; Chapelier, A.R.; Dartevelle, P.G.; Mazmanian, G.M. L-arginine and pentoxifylline attenuate endothelial dysfunction after lung reperfusion injury in the rabbit. The Paris-Sud University Lung Transplant Group. Ann. Thorac. Surg. 1995, 60, 646–650. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Lee, J.R.; Laks, H.; Waters, P.F.; Meneshian, A.; Blitz, A.; Johnson, K.; Lam, L.; Chang, P.A. L-arginine administration during reperfusion improves pulmonary function. Ann. Thorac. Surg. 1996, 62, 1580–1586, discussion 1586–1587. [Google Scholar] [CrossRef]
- Steen, S.; Liao, Q.; Wierup, P.N.; Bolys, R.; Pierre, L.; Sjöberg, T. Transplantation of lungs from non–heart-beating donors after functional assessment ex vivo. Ann. Thorac. Surg. 2003, 76, 244–252. [Google Scholar] [CrossRef]
- Cypel, M.; Rubacha, M.; Yeung, J.; Hirayama, S.; Torbicki, K.; Madonik, M.; Fischer, S.; Hwang, D.; Pierre, A.; Waddell, T.K.; et al. Normothermic ex vivo perfusion prevents lung injury compared to extended cold preservation for transplantation. Am. J. Transplant. 2009, 9, 2262–2269. [Google Scholar] [CrossRef]
- Aigner, C.; Slama, A.; Hotzenecker, K.; Scheed, A.; Urbanek, B.; Schmid, W.; Nierscher, F.J.; Lang, G.; Klepetko, W. Clinical ex vivo lung perfusion--pushing the limits. Am. J. Transplant. 2012, 12, 1839–1847. [Google Scholar] [CrossRef]
- Cypel, M.; Yeung, J.C.; Hirayama, S.; Rubacha, M.; Fischer, S.; Anraku, M.; Sato, M.; Harwood, S.; Pierre, A.; Waddell, T.K. Technique for prolonged normothermic ex vivo lung perfusion. J. Heart Lung Transplant. 2008, 27, 1319–1325. [Google Scholar] [CrossRef]
- Nakajima, D.; Chen, F.; Yamada, T.; Sakamoto, J.; Ohsumi, A.; Bando, T.; Date, H. Reconditioning of lungs donated after circulatory death with normothermic ex vivo lung perfusion. J. Heart Lung Transplant. 2012, 31, 187–193. [Google Scholar] [CrossRef]
- Cypel, M.; Yeung, J.C.; Liu, M.; Anraku, M.; Chen, F.; Karolak, W.; Sato, M.; Laratta, J.; Azad, S.; Madonik, M.; et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N. Engl. J. Med. 2011, 364, 1431–1440. [Google Scholar] [CrossRef]
- Sanchez, P.G.; Bittle, G.J.; Burdorf, L.; Pierson, R.N.; Griffith, B.P. State of art: Clinical ex vivo lung perfusion: Rationale, current status, and future directions. J. Heart Lung Transplant. 2012, 31, 339–348. [Google Scholar] [CrossRef]
- Sanchez, P.G.; D’Ovidio, F. Ex-vivo lung perfusion. Curr. Opin. Organ. Transplant. 2012, 17, 490–495. [Google Scholar] [CrossRef]
- Dong, B.M.; Abano, J.B.; Egan, T.M. Nitric oxide ventilation of rat lungs from non-heart-beating donors improves posttransplant function. Am. J. Transplant. 2009, 9, 2707–2715. [Google Scholar] [CrossRef]
- Pierre, A.F.; Xavier, A.M.; Liu, M.; Cassivi, S.D.; Lindsay, T.F.; Marsh, H.C.; Slutsky, A.S.; Keshavjee, S.H. Effect of complement inhibition with soluble complement receptor 1 on pig allotransplant lung function. Transplantation. 1998, 66, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.; de Perrot, M.; Imai, Y.; Yamane, M.; Quadri, S.M.; Segall, L.; Dutly, A.; Sakiyama, S.; Chaparro, A.; Davidson, B.L.; et al. Transbronchial administration of adenoviral-mediated interleukin-10 gene to the donor improves function in a pig lung transplant model. Gene Ther. 2004, 11, 1786–1796. [Google Scholar] [CrossRef]
- Cypel, M.; Liu, M.; Rubacha, M.; Yeung, J.C.; Hirayama, S.; Anraku, M.; Sato, M.; Medin, J.; Davidson, B.L.; de Perrot, M.; et al. Functional repair of human donor lungs by IL-10 gene therapy. Sci. Transl. Med. 2009, 1, 4ra9. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Belik, J.; Stevens, D.; Pan, J.; Shehnaz, D.; Ibrahim, C.; Kantores, C.; Ivanovska, J.; Grasemann, H.; Jankov, R.P. Chronic hypercapnia downregulates arginase expression and activity and increases pulmonary arterial smooth muscle relaxation in the newborn rat. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 297, L777–L784. [Google Scholar] [CrossRef] [PubMed]
- Grasemann, H.; Al-Saleh, S.; Scott, J.A.; Shehnaz, D.; Mehl, A.; Amin, R.; Rafii, M.; Pencharz, P.; Belik, J.; Ratjen, F. Asymmetric dimethylarginine contributes to airway nitric oxide deficiency in patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2011, 183, 1363–1368. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.R.; Poljakovic, M.; Lavrisha, L.; Machado, L.; Kuypers, F.A.; Morris, S.M., Jr. Decreased arginine bioavailability and increased serum arginase activity in asthma. Am. J. Respir. Crit. Care Med. 2004, 170, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Maarsingh, H.; Dekkers, B.G.; Zuidhof, A.B.; Bos, I.S.; Menzen, M.H.; Klein, T.; Flik, G.; Zaagsma, J.; Meurs, H. Increased arginase activity contributes to airway remodelling in chronic allergic asthma. Eur. Respir. J. 2011, 38, 318–328. [Google Scholar] [CrossRef]
- Li, X.; Hanson, C.; Cmarik, J.L.; Ruscetti, S. Neurodegeneration induced by PVC-211 murine leukemia virus is associated with increased levels of vascular endothelial growth factor and macrophage inflammatory protein 1α and is inhibited by blocking activation of microglia. J. Virol. 2009, 83, 4912–4922. [Google Scholar] [CrossRef]
- Liu, M.; Tremblay, L.; Cassivi, S.D.; Bai, X.H.; Mourgeon, E.; Pierre, A.F.; Slutsky, A.S.; Post, M.; Keshavjee, S. Alterations of nitric oxide synthase expression and activity during rat lung transplantation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 278, L1071–L1081. [Google Scholar] [CrossRef]
- Pinsky, D.J.; Naka, Y.; Chowdhury, N.C.; Liao, H.; Oz, M.C.; Michler, R.E.; Kubaszewski, E.; Malinski, T.; Stern, D.M. The nitric oxide/cyclic GMP pathway in organ transplantation: Critical role in successful lung preservation. Proc. Natl. Acad. Sci. USA 1994, 91, 12086–12090. [Google Scholar] [CrossRef]
- van den Berg, M.P.; Meurs, H.; Gosens, R. Targeting arginase and nitric oxide metabolism in chronic airway diseases and their co-morbidities. Curr. Opin. Pharmacol. 2018, 40, 126–133. [Google Scholar] [CrossRef]
- Morris, S.M., Jr. Regulation of enzymes of the urea cycle and arginine metabolism. Annu. Rev. Nutr. 2002, 22, 87–105. [Google Scholar] [CrossRef]
- Que, L.G.; George, S.E.; Gotoh, T.; Mori, M.; Huang, Y.C. Effects of arginase isoforms on NO production by nNOS. Nitric Oxide 2002, 6, 1–8. [Google Scholar] [CrossRef]
- Liu, H.; Drew, P.; Gaugler, A.C.; Cheng, Y.; Visner, G.A. Pirfenidone inhibits lung allograft fibrosis through L-arginine–arginase pathway. Am. J. Transplant. 2005, 5, 1256–1263. [Google Scholar] [CrossRef]
- George, T.J.; Arnaoutakis, G.J.; Beaty, C.A.; Jandu, S.K.; Santhanam, L.; Berkowitz, D.E.; Shah, A.S. A physiologic and biochemical profile of clinically rejected lungs on a normothermic ex vivo lung perfusion platform. J. Surg. Res. 2013, 183, 75–83. [Google Scholar] [CrossRef]
- Yeung, J.C.; Cypel, M.; Machuca, T.N.; Koike, T.; Cook, D.J.; Bonato, R.; Chen, M.; Sato, M.; Waddell, T.K.; Liu, M.; et al. Physiologic assessment of the ex vivo donor lung for transplantation. J. Heart Lung Transplant. 2012, 31, 1120–1126. [Google Scholar] [CrossRef]
- Hsin, M.K.; Cypel, M.; Zamel, R.; Machuca, T.; Chen, M.; Liu, M.; Keshavjee, S. Metabolites in human ex vivo lung perfusates are differentially affected in brain death (BDD) vs. cardiac death donors (DCD) and modified by duration of normothermic perfusion. J. Heart Lung Transplant. 2013, 32, S151–S152. [Google Scholar] [CrossRef]
- Luiking, Y.C.; Engelen, M.P.; Deutz, N.E. Regulation of nitric oxide production in health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 97–104. [Google Scholar] [CrossRef]
- Chen, K.; Pittman, R.N.; Popel, A.S. Nitric oxide in the vasculature: Where does it come from and where does it go? A quantitative perspective. Antioxid. Redox Signal. 2008, 10, 1185–1198. [Google Scholar] [CrossRef]
- Scott-Burden, T. Regulation of nitric oxide production by tetrahydrobiopterin. Circulation 1995, 91, 248–250. [Google Scholar] [CrossRef]
- Schmid, R.A.; Hillinger, S.; Walter, R.; Zollinger, A.; Stammberger, U.; Speich, R.; Schaffner, A.; Weder, W.; Schoedon, G. The nitric oxide synthase cofactor tetrahydrobiopterin reduces allograft ischemia-reperfusion injury after lung transplantation. J. Thorac. Cardiovasc. Surg. 1999, 118, 726–732. [Google Scholar] [CrossRef][Green Version]
| Product Length | Forward Primer 1 | Reverse Primer 1 | |
|---|---|---|---|
| Pig-ARG1: Sus scrofa arginase, liver (ARG1), mRNA | 163 | ACAATCCATCGGGATCATCGGAGC 24 | AGGGACATCAGCAAAGCACAGGT 23 |
| Pig-ARG2: Sus scrofa arginase, type II (ARG2), mRNA | 229 | TGCATTTGACCCTACCCTGGCT 22 | TCCCTCCCTTGTCTGCCCAAAACT 24 |
| Pig –iNOS: Sus scrofa iNOS, mRNA | 187 | TTTCAGGAAGCATCACCCCCGT 22 | TGCATGAGCACAGCGGCAAAGA 22 |
| Pig-eNOS: Sus scrofa nitric oxide synthase 3 (endothelial cell) (NOS3), mRNA | 203 | TGCGATCCTCACCGCTACAACA 22 | TGCTCGTTCTCCAGGTGCTTCA 22 |
| Pig-nNOS: Sus scrofa nitric oxide synthase 1 (neuronal) (NOS1), mRNA | 159 | ACAAAACTCTGCCCCTCGGTGT 22 | ACTTGGACGGGCTGCCATTCTT 22 |
| N | l-arginine nmol/mg Protein | ADMA nmol/mg Protein | l-arginine/ADMA | |
|---|---|---|---|---|
| Normal control | 5 | 30.8 ± 2.0 | 0.16 ± 0.01 | 194.4 ± 20.6 |
| 18 h CIT | 4 | 28.4 ± 2.2 | 0.19 ± 0.04 | 159.7 ± 26.9 |
| 6 h CIT+12 h EVLP | 6 | 42.8 ± 11.6 | 0.22 ± 0.05 | 188.1 ± 19.9 |
| 18 h CIT/1 h post rep | 4 | 35.2 ± 4.2 | 0.16 ± 0.03 | 232.1 ± 26.2 |
| EVLP/1 h post rep | 6 | 32.3 ± 5.2 | 0.13 ± 0.02 | 240.5 ± 17.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavasoli, F.; Liu, M.; Machuca, T.; Bonato, R.; Grant, D.R.; Cypel, M.; Keshavjee, S.; Grasemann, H. Increased Arginase Expression and Decreased Nitric Oxide in Pig Donor Lungs after Normothermic Ex Vivo Lung Perfusion. Biomolecules 2020, 10, 300. https://doi.org/10.3390/biom10020300
Tavasoli F, Liu M, Machuca T, Bonato R, Grant DR, Cypel M, Keshavjee S, Grasemann H. Increased Arginase Expression and Decreased Nitric Oxide in Pig Donor Lungs after Normothermic Ex Vivo Lung Perfusion. Biomolecules. 2020; 10(2):300. https://doi.org/10.3390/biom10020300
Chicago/Turabian StyleTavasoli, Farshad, Mingyao Liu, Tiago Machuca, Riccardo Bonato, David R. Grant, Marcelo Cypel, Shaf Keshavjee, and Hartmut Grasemann. 2020. "Increased Arginase Expression and Decreased Nitric Oxide in Pig Donor Lungs after Normothermic Ex Vivo Lung Perfusion" Biomolecules 10, no. 2: 300. https://doi.org/10.3390/biom10020300
APA StyleTavasoli, F., Liu, M., Machuca, T., Bonato, R., Grant, D. R., Cypel, M., Keshavjee, S., & Grasemann, H. (2020). Increased Arginase Expression and Decreased Nitric Oxide in Pig Donor Lungs after Normothermic Ex Vivo Lung Perfusion. Biomolecules, 10(2), 300. https://doi.org/10.3390/biom10020300





