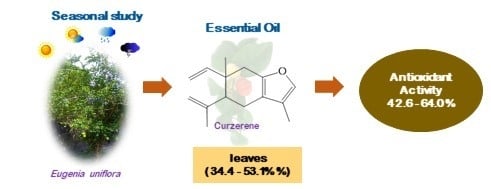
Seasonal and Antioxidant Evaluation of Essential Oil from Eugenia uniflora L., Curzerene-Rich, Thermally Produced in Situ
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Climatic Data
2.2. Essential Oils Extraction and Composition
2.3. Antioxidant Assay
2.4. Statistical Analysis
3. Results and Discussion
3.1. Essential Oil Yield vs Environmental Conditions
3.2. Oil Composition and Environmental Conditions
3.3. Antioxidant Capacity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WCSP (2020). World Checklist of Selected Plant Families. Facilitated by the Royal Botanic Gardens, Kew. Available online: http://wcsp.science.kew.org (accessed on 18 February 2020).
- Van der Merwe, M.M.; Van Wyk, A.E.; Botha, A.M. Molecular phylogenetic analysis of Eugenia L. (Myrtaceae), with emphasis on southern African taxa. Plant. Syst. Evol. 2005, 251, 21–34. [Google Scholar] [CrossRef]
- Eugenia in Flora do Brasil 2020 em Construção. Available online: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB10560 (accessed on 18 February 2020).
- Rahimifard, N.; Sabzevari, O.; Shoeibi, S.; Pakzad, S.R.; Ajdary, S.; Hajimehdipoor, H.; Bagheri, F.; Safaee, M. Antifungal activity of the essential oil of Eugenia caryophyllata on Candida albicans, Aspergillus niger and Aspergillus flavus. Biomed. Pharmacol. J. Vol. 2008, 1, 43–46. [Google Scholar]
- Lawal, O.A.; Ogunwande, I.A.; Owolabi, M.S.; Opoku, A.R.; Oyedeji, A.O. Chemical Composition, Antibacterial Activity, and Brine Shrimp Lethality Test of Essential Oil from the Leaves of Eugenia natalitia. Chem. Nat. Compd. 2016, 52, 731–733. [Google Scholar] [CrossRef]
- Feitosa, C.M.; Barbosa, A.D.R.; Melo, C.H.S.D.; Freitas, R.M.; Fontes, J.E.D.N.; Costa, E.V.; Rashed, K.N.Z.; Costa Júnior, J.S.D. Antioxidant and anticholinesterase activities of the essential oil of Eugenia dysenterica DC. Afr. J. Pharm. Pharmacol. 2017, 11, 241–249. [Google Scholar]
- Wilson, P.G. Conspectus of the genus Eugenia (Myrtaceae) in the Philippines. Gard. Bull. Singapore 2009, 60, 399–410. [Google Scholar]
- Costa, A.G.V.; Garcia-Diaz, D.F.; Jimenez, P.; Silva, P.I. Bioactive compounds and health benefits of exotic tropical red-black berries. J. Funct. Foods 2013, 5, 539–549. [Google Scholar] [CrossRef]
- Gallucci, S.; Neto, A.P.; Porto, C.; Barbizan, D.; Costa, I.; Marques, K.; Benevides, P.; Figueiredo, R. Essential Oil of Eugenia uniflora L.: An Industrial Perfumery Approach. J. Essent. Oil Res. 2010, 22, 176–179. [Google Scholar] [CrossRef]
- Vendruscolo, G.S.; Rates, S.M.K.; Mentz, L.A. Dados químicos e farmacológicos sobre as plantas utilizadas como medicinais pela comunidade do bairro Ponta Grossa, Porto Alegre, Rio Grande do Sul. Rev. Bras. Farmacogn. 2005, 15, 361–372. [Google Scholar] [CrossRef] [Green Version]
- Stefanello, M.É.A.; Pascoal, A.C.R.F.; Salvador, M.J. Essential Oils from Neotropical Myrtaceae: Chemical Diversity and Biological Properties. Chem. Biodivers. 2011, 8, 73–94. [Google Scholar] [CrossRef]
- Bender, D.A. Benders’ Dictionary of Nutrition and Food Technology, 8th ed.; Woodhead Publishing: Sawston, UK, 2006; Available online: http://www.sciencedirect.com/science/article/pii/B9781845690519500028 (accessed on 14 February 2020).
- Lima, B.G.; Tietbohl, L.A.C.; Fernandes, C.P.; Cruz, R.A.S.; da Botas, G.S.; Santos, M.G.; Silva-Filho, M.V.; Rocha, L. Chemical composition of essential oils and anticholinesterasic activity of Eugenia sulcata spring ex mart. Lat. Am. J. Pharm. 2012, 31, 152–155. [Google Scholar]
- Coradin, L.; Siminski, A.; Reis, A. Espécies Nativas da Flora Brasileira de Valor Econômico Atual ou Potencial: Plantas para o Futuro—Região Sul, 1st ed.; Ministério do Meio Ambiente: Brasília, Brazil, 2011; pp. 170–177. ISBN 9788577381531. [Google Scholar]
- Queiroz, J.M.G.; Suzuki, M.C.M.; Motta, A.P.R.; Nogueira, J.M.R.; Carvalho, E.M.D. Aspectos populares e científicos do uso de espécies de Eugenia como fitoterápico. Rev. Fitos 2015, 9, 87–100. [Google Scholar]
- Figueiredo, P.L.B.; Pinto, L.C.; da Costa, J.S.; da Silva, A.R.C.; Mourão, R.H.V.; Montenegro, R.C.; da Silva, J.K.R.; Maia, J.G.S. Composition, antioxidant capacity and cytotoxic activity of Eugenia uniflora L. chemotype-oils from the Amazon. J. Ethnopharmacol. 2019, 232, 30–38. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Guo, J.; Wang, Q.; Zhu, S.; Gao, S.; Yang, C.; Wei, M.; Pan, X.; Zhu, W.; et al. Cytotoxic and tumor effects of curzerene from Curcuma longa. Planta. Med. 2017, 83, 23–29. [Google Scholar] [PubMed] [Green Version]
- Baldovini, N.; Tomi, F.; Casanova, J. Identification and quantitative determination of furanodiene, a heat-sensitive compound, in essential oil by 13C-NMR. Phytochem. Anal. 2001, 12, 58–63. [Google Scholar] [CrossRef]
- Melo, R.M.; Corrêa, V.F.S.; Amorim, A.C.L.; Miranda, A.L.P.; Rezende, C.M. Identification of Impact Aroma Compounds in Eugenia uniflora L. (Brazilian Pitanga) Leaf Essential Oil. J. Braz. Chem. Soc. 2007, 18, 179–183. [Google Scholar] [CrossRef] [Green Version]
- National Institute of Standards and Technology (NIST). Mass Spectral Library (NIST/EPA/NIH, v.2.0d); The NIST Mass Spectrometry Data Center: Gaithersburg, MD, USA, 2014. [Google Scholar]
- Mondello, L. FFNSC 2: Flavors and Fragrances of Natural and Synthetic Compounds, Mass Spectral Database; John Wiley & Sons Inc.: New York, NY, USA, 2011; ISBN 1118145836. [Google Scholar]
- Van Den Dool, H.; Kratz, P. A generalization of the retention index system including linear temperature programmed gas–liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, EUA, 2007. [Google Scholar]
- Choi, H.S.; Song, H.S.; Ukeda, H.; Sawamura, M. Radical-scavenging activities of citrus essential oils and their components: Detection using 1,1-diphenyl-2-icrylhydrazyl. J. Agric. Food Chem. 2000, 48, 4156–4161. [Google Scholar] [CrossRef]
- Da Silva, J.K.R.; Andrade, E.H.A.; Barreto, L.; da Silva, N.; Ribeiro, A.; Montenegro, R.; Maia, J. Chemical Composition of Four Essential Oils of Eugenia from the Brazilian Amazon and Their Cytotoxic and Antioxidant Activity. Medicines 2017, 4, 51. [Google Scholar] [CrossRef] [Green Version]
- Marengo, J.A.; Nobre, C.A. Clima da região Amazônica. In Tempo e Clima no Brasil, 1st ed.; Cavalcanti, I.F.A., Ferreira, N.J., Silva, M.G.A.J.D., Dias, M.A.F.D.S., Eds.; Oficina de Textos: São Paulo, Brazil, 2009. [Google Scholar]
- Gobbo-Neto, L.; Lopes, N.P. Plantas medicinais: Fatores de influência no conteúdo de metabólitos secundários. Quim. Nova 2007, 30, 374–381. [Google Scholar] [CrossRef]
- Ribeiro, P.H.S.; Dos Santos, M.L.; Da Camara, C.A.G.; Born, F.S.; Fagg, C.W. Seasonal chemical compositions of the essential oils of two Eugenia species and their acaricidal properties. Quim. Nova 2016, 39, 38–43. [Google Scholar]
- Defaveri, A.C.A.; Sato, A.; Borré, L.B.; Aguiar, D.L.M.; San Gil, R.A.S.; Arruda, R.C.O.; Riehl, C.A.S. Eugenia neonitida Sobral and Eugenia rotundifolia Casar. (Cyrtaceae) essential oils: Composition, seasonality influence, antioxidant activity and leaf histochemistry. J. Braz. Chem. Soc. 2011, 22, 1531–1538. [Google Scholar] [CrossRef]
- Maia, J.G.S.; Andrade, E.H.A.; da Silva, M.H.L.; Zoghbi, M.G.B. A new chemotype of Eugenia uniflora L. from North Brazil. J. Essent. Oil Res. 1999, 11, 727–729. [Google Scholar] [CrossRef]
- Becker, N.A.; Volcão, L.M.; Camargo, T.M.; Freitag, R.A.; Ribeiro, G.A. Biological properties of Eugenia uniflora L. essential oil: Chemical composition and antimicrobial activity against gram negative bacteria. VITTALLE Rev. Ciências da Saúde 2017, 29, 22–30. [Google Scholar] [CrossRef]
- Costa, D.P.; Santos, S.C.; Seraphin, J.C.; Ferri, P.H. Seasonal variability of essential oils of Eugenia uniflora leaves. J. Braz. Chem. Soc. 2009, 20, 1287–1293. [Google Scholar] [CrossRef]
- Santos, F.R.; Braz-Filho, R.; Castro, R.N. Influência da idade das folhas de Eugenia uniflora L. na composição química do óleo essencial. Quim. Nova 2015, 38, 762–768. [Google Scholar]
- Ogunwande, I.A.; Olawore, N.O.; Ekundayo, O.; Walker, T.M.; Schmidt, J.M.; Setzer, W.N. Studies on the essential oils composition, antibacterial and cytotoxicity of Eugenia uniflora L. Int. J. Aromather. 2005, 15, 147–152. [Google Scholar] [CrossRef]
- Koleva, I.I.; van Beek, T.A.; Linssen, J.P.H.; de Groot, A.; Evstatieva, L.N. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem. Anal. 2002, 13, 8–17. [Google Scholar] [CrossRef]
- Li, Z.-H.; Wang, Q.; Ruan, X.; Pan, C.-D.; Jiang, D.-A. Phenolics and Plant Allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef] [Green Version]
- Victoria, F.N.; Lenardão, E.J.; Savegnago, L.; Perin, G.; Jacob, R.G.; Alves, D.; Silva, W.P.D.; Motta, A.D.S.D.; Nascente, P.D.S. Essential oil of the leaves of Eugenia uniflora L.: Antioxidant and antimicrobial properties. Food Chem. Toxicol. 2012, 50, 2668–2674. [Google Scholar] [CrossRef] [Green Version]






| Oil Yields (%) | 1.6 | 1.3 | 3.1 | 1.9 | 2.0 | 1.0 | 2.2 | 0.8 | 1.6 | 1.2 | 1.9 | 1.2 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oil Constituents (%) * | RIC | RIL | January | February | March | April | May | June | July | August | September | October | November | December |
| Hexan-3-one | 785 | 782 a | 0.1 | 0.1 | ||||||||||
| Hexan-2-one | 788 | 786 b | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | ||||||
| Hexan-3-ol | 792 | 795 b | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |||||
| Hexan-2-ol | 795 | 802 a | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | ||||||
| Hexen-2E-al | 847 | 846 a | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.3 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 |
| Hexen-3Z-ol | 849 | 850 a | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |||||||
| Myrcene | 991 | 988 a | 0.1 | 0.5 | 0.3 | 0.2 | 0.4 | 1.1 | 0.1 | 0.3 | 0.2 | 0.5 | 1.6 | 0.2 |
| β-Phellandrene | 1028 | 1025 a | 0.1 | 0.1 | 0.3 | 0.7 | ||||||||
| Limonene | 1028 | 1024 a | 0.3 | 0.2 | 0.2 | 0.5 | 0.1 | 0.2 | 0.2 | 0.1 | ||||
| (Z)-β-ocimene | 1036 | 1032 a | 0.1 | 0.5 | 0.4 | 0.1 | 0.1 | 0.6 | 0.1 | 0.4 | 0.1 | 0.6 | 2.0 | 0.2 |
| (E)-β-ocimene | 1046 | 1044 a | 0.2 | 1.1 | 1.0 | 0.2 | 0.2 | 1.0 | 0.1 | 1.1 | 0.2 | 1.5 | 4.3 | 0.6 |
| Linalool | 1099 | 1095 a | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | ||||||
| Hexen-3Z-yl butanoate | 1185 | 1184 a | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | ||||||
| δ-Elemene | 1336 | 1335 a | 0.5 | 0.4 | 0.9 | 0.4 | 0.3 | 0.1 | 0.7 | 0.6 | 0.6 | 1.2 | 1.6 | 0.7 |
| Isoledene | 1374 | 1374 a | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | ||||
| α-Copaene | 1376 | 1374 a | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |||||||
| β-Elemene | 1385 | 1389 a | 2.8 | 3.3 | 4.2 | 3.5 | 3.3 | 1.8 | 5.8 | 2.6 | 4.4 | 5.0 | 4.4 | 3.8 |
| α-Gurjunene | 1411 | 1409 a | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.2 | 0.1 | |||
| E-Caryophyllene | 1420 | 1417 a | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.3 | 0.1 | 0.2 | 0.3 | 0.3 | 0.3 | |
| β-Copaene | 1428 | 1430 a | 0.1 | 0.1 | 0.1 | |||||||||
| γ-Elemene | 1434 | 1434 a | 2.1 | 1.4 | 2.7 | 1.3 | 1.2 | 0.8 | 2.8 | 1.7 | 2.3 | 3.7 | 2.9 | 2.6 |
| Aromadendrene | 1440 | 1439 a | 0.4 | 0.2 | 0.4 | 0.3 | 0.2 | 0.6 | 0.4 | 0.4 | 0.4 | 0.3 | ||
| Selina-5,11-diene | 1444 | 1445 b | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |||||
| Cadina-3,5-diene | 1451 | 1452 b | 0.1 | 0.1 | ||||||||||
| cis-Muurola-3,5-diene | 1452 | 1448 a | 0.1 | 0.1 | 0.1 | |||||||||
| α-Humulene | 1454 | 1452 a | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |||||||
| allo-Aromadendrene | 1460 | 1458 a | 0.5 | 0.2 | 0.3 | |||||||||
| 9-epi-E-Caryophyllene | 1462 | 1464 a | 0.5 | 0.4 | 0.7 | 0.5 | 1.0 | 0.8 | 0.7 | 0.8 | 0.5 | |||
| Selina-4,11-diene | 1476 | 1476 b | 0.4 | 0.2 | 0.4 | 0.2 | 0.5 | 0.2 | 0.4 | 0.4 | 0.5 | 0.3 | ||
| Germacrene D | 1482 | 1478 a | 0.8 | 2.5 | 0.6 | 0.4 | 0.5 | 0.9 | 1.5 | 2.7 | 2.8 | 1.7 | ||
| γ-Muurolene | 1481 | 1478 a | 1.4 | 1.0 | ||||||||||
| β-Selinene | 1487 | 1481 a | 0.8 | 0.5 | 0.6 | 0.6 | 0.6 | 0.6 | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | |
| δ-Selinene | 1492 | 1492 a | 0.2 | 0.1 | 0.3 | 0.1 | 0.2 | 0.1 | 0.1 | 0.3 | 0.4 | 0.2 | ||
| Viridiflorene | 1496 | 1496 a | 2.4 | 0.8 | 2.7 | 1.4 | 0.8 | 0.2 | 2.8 | 0.8 | 3.2 | 1.4 | 4.1 | 1.9 |
| Curzerene | 1503 | 1499 a | 35.8 | 48.7 | 43.6 | 34.4 | 45.0 | 49.1 | 41.1 | 53.1 | 41.0 | 44.0 | 36.2 | 41.4 |
| α-Muurolene | 1502 | 1500 a | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.2 | ||||||
| Germacrene A | 1504 | 1508 a | 0.1 | 0.2 | ||||||||||
| α-Bulnesene | 1511 | 1509 a | 0.2 | 0.2 | 0.2 | 0.9 | 0.1 | |||||||
| γ-Cadinene | 1516 | 1513 a | 0.1 | 0.1 | 0.1 | 0.1 | 0.3 | 0.1 | 0.1 | |||||
| 7-epi-α-Selinene | 1519 | 1520 a | 0.1 | 0.1 | 0.1 | |||||||||
| δ-Cadinene | 1525 | 1522 a | 0.5 | 0.2 | 0.5 | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.5 | 0.5 | 0.5 |
| γ-Cuprenene | 1536 | 1532 a | 0.1 | 0.1 | ||||||||||
| Selina-4(15),7(11)-diene | 1538 | 1540 b | 0.3 | 0.3 | 0.1 | |||||||||
| Selina-3,7(11)-diene | 1542 | 1546 b | 0.1 | 0.2 | 0.2 | |||||||||
| Germacrene B | 1559 | 1559 a | 4.8 | 3.9 | 6.6 | 3.5 | 3.5 | 2.8 | 0.1 | 5.9 | 7.5 | 5.0 | 6.0 | |
| Maaliol | 1566 | 1566 a | 0.7 | |||||||||||
| Spathulenol | 1578 | 1577 a | 1.7 | 4.2 | 0.8 | 5.4 | 7.0 | 6.9 | 1.2 | 3.3 | 1.6 | 1.7 | 0.5 | 3.4 |
| Globulol | 1583 | 1590 a | 2.7 | 4.1 | 3.6 | 1.5 | 7.4 | 2.1 | 5.1 | |||||
| Viridiflorol | 1592 | 1592 a | 2.7 | 1.5 | 1.9 | 2.4 | 2.0 | 1.0 | 4.7 | 0.8 | 6.2 | 1.3 | 1.8 | 1.9 |
| Cubeban-11-ol | 1594 | 1595 a | 1.1 | 0.5 | 0.7 | 0.6 | 0.5 | 0.7 | 0.6 | |||||
| Rosifoliol | 1603 | 1600 a | 1.1 | 0.5 | 0.6 | 0.5 | 0.6 | 0.2 | 0.5 | 0.6 | 0.6 | |||
| trans-β-Elemenone | 1604 | 1602 a | 1.9 | 0.8 | 0.9 | 1.3 | 1.0 | 1.7 | 0.4 | 0.8 | 0.9 | 1.1 | ||
| Junenol | 1618 | 1618 a | 0.1 | 0.2 | ||||||||||
| γ-Eudesmol | 1632 | 1630 a | 0.1 | 0.2 | 0.2 | |||||||||
| 8-isobutyryloxy-Isoborneol | 1638 | 1632 a | 0.4 | |||||||||||
| epi-α-Murrolol | 1641 | 1640 a | 0.6 | 0.8 | 0.9 | |||||||||
| α-Muurolol | 1646 | 1644 a | 0.1 | 0.3 | 0.3 | |||||||||
| α-Cadinol | 1655 | 1652 a | 3.1 | 1.8 | 1.1 | 2.2 | 0.2 | |||||||
| Atractylone | 1657 | 1657 a | 1.1 | 1.4 | 0.9 | 1.3 | 1.2 | 1.3 | 0.1 | 0.5 | 0.7 | 0.8 | 0.8 | |
| Intermedeol | 1666 | 1665 a | 0.1 | 0.2 | ||||||||||
| Germacrone | 1696 | 1693 a | 7.5 | 4.2 | 4.8 | 5.6 | 5.2 | 10.5 | 0.2 | 4.2 | 3.8 | 5.4 | ||
| Monoterpene hydrocarbons | 0.5 | 2.4 | 1.8 | 0.7 | 0.9 | 3.2 | 0.4 | 2.0 | 0.7 | 2.9 | 8.6 | 1.1 | ||
| Oxygenated monoterpenes | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | ||||||||
| Sesquiterpene hydrocarbons | 17.4 | 13.0 | 23.5 | 13.2 | 11.3 | 6.4 | 17.1 | 9.0 | 20.8 | 27.1 | 25.8 | 20.4 | ||
| Oxygenated sesquiterpenes | 52.9 | 64.5 | 53.5 | 78.8 | 68.7 | 70.9 | 55.7 | 60.0 | 54.4 | 55.8 | 49.6 | 56.8 | ||
| Others | 0.5 | 0.6 | 0.4 | 0.5 | 0.5 | 0.7 | 0.4 | 0.2 | 0.2 | 0.3 | 0.4 | 0.1 | ||
| Total | 71.3 | 80.6 | 79.2 | 73.2 | 81.4 | 81.6 | 73.7 | 71.2 | 76.2 | 86.2 | 84.5 | 78.4 | ||
| Months | Antioxidant Capacity | |
|---|---|---|
| Trolox Equivalent (mg TE/g) | Inhibition (%) * | |
| January | 436.3 ± 2.3 | 64.2 ± 0.3 b |
| February | 378.2 ± 6.2 | 55.7 ± 0.9 c |
| March | 393.2 ± 4.8 | 57.9 ± 0.7 c |
| April | 371.1 ± 7.1 | 57.3 ± 0.4 c,d |
| May | 373.6 ± 13.1 | 55.0 ± 1.9 c,d,e |
| June | 339.4 ± 1.6 | 51.1 ± 3.1 a |
| July | 400.3 ± 3.4 | 59.6 ± 1.2 c,d,f |
| August | 186.9 ± 7.7 | 42.6 ± 0.3 h |
| September | 286.6 ± 4.0 | 57.9 ± 0.4 c,d,e,f,g |
| October | 322.3 ± 3.4 | 47.4 ± 0.5 a |
| November | 326.5 ± 10.3 | 48.1 ± 1.5 a |
| December | 435.0 ± 0.6 | 64.0 ± 0.1 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Costa, J.S.; Barroso, A.S.; Mourão, R.H.V.; da Silva, J.K.R.; Maia, J.G.S.; Figueiredo, P.L.B. Seasonal and Antioxidant Evaluation of Essential Oil from Eugenia uniflora L., Curzerene-Rich, Thermally Produced in Situ. Biomolecules 2020, 10, 328. https://doi.org/10.3390/biom10020328
da Costa JS, Barroso AS, Mourão RHV, da Silva JKR, Maia JGS, Figueiredo PLB. Seasonal and Antioxidant Evaluation of Essential Oil from Eugenia uniflora L., Curzerene-Rich, Thermally Produced in Situ. Biomolecules. 2020; 10(2):328. https://doi.org/10.3390/biom10020328
Chicago/Turabian Styleda Costa, Jamile S., Adenilson S. Barroso, Rosa Helena V. Mourão, Joyce Kelly R. da Silva, José Guilherme S. Maia, and Pablo Luis B. Figueiredo. 2020. "Seasonal and Antioxidant Evaluation of Essential Oil from Eugenia uniflora L., Curzerene-Rich, Thermally Produced in Situ" Biomolecules 10, no. 2: 328. https://doi.org/10.3390/biom10020328
APA Styleda Costa, J. S., Barroso, A. S., Mourão, R. H. V., da Silva, J. K. R., Maia, J. G. S., & Figueiredo, P. L. B. (2020). Seasonal and Antioxidant Evaluation of Essential Oil from Eugenia uniflora L., Curzerene-Rich, Thermally Produced in Situ. Biomolecules, 10(2), 328. https://doi.org/10.3390/biom10020328