Kinetic Study of the Biodegradation of Acephate by Indigenous Soil Bacterial Isolates in the Presence of Humic Acid and Metal Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Soil Sample Collection and Isolation of Microorganisms
2.3. Molecular Characterization of Bacterial Isolates
2.4. UV—Visible Spectrophotometric Study for Cell Growth
2.5. Biodegradation of Acephate
2.6. Characterization of Degradation Metabolites of Acephate by Using ESI-MS and FTIR Analysis
2.7. Degradation Kinetics
2.8. Statistical Analysis
3. Results
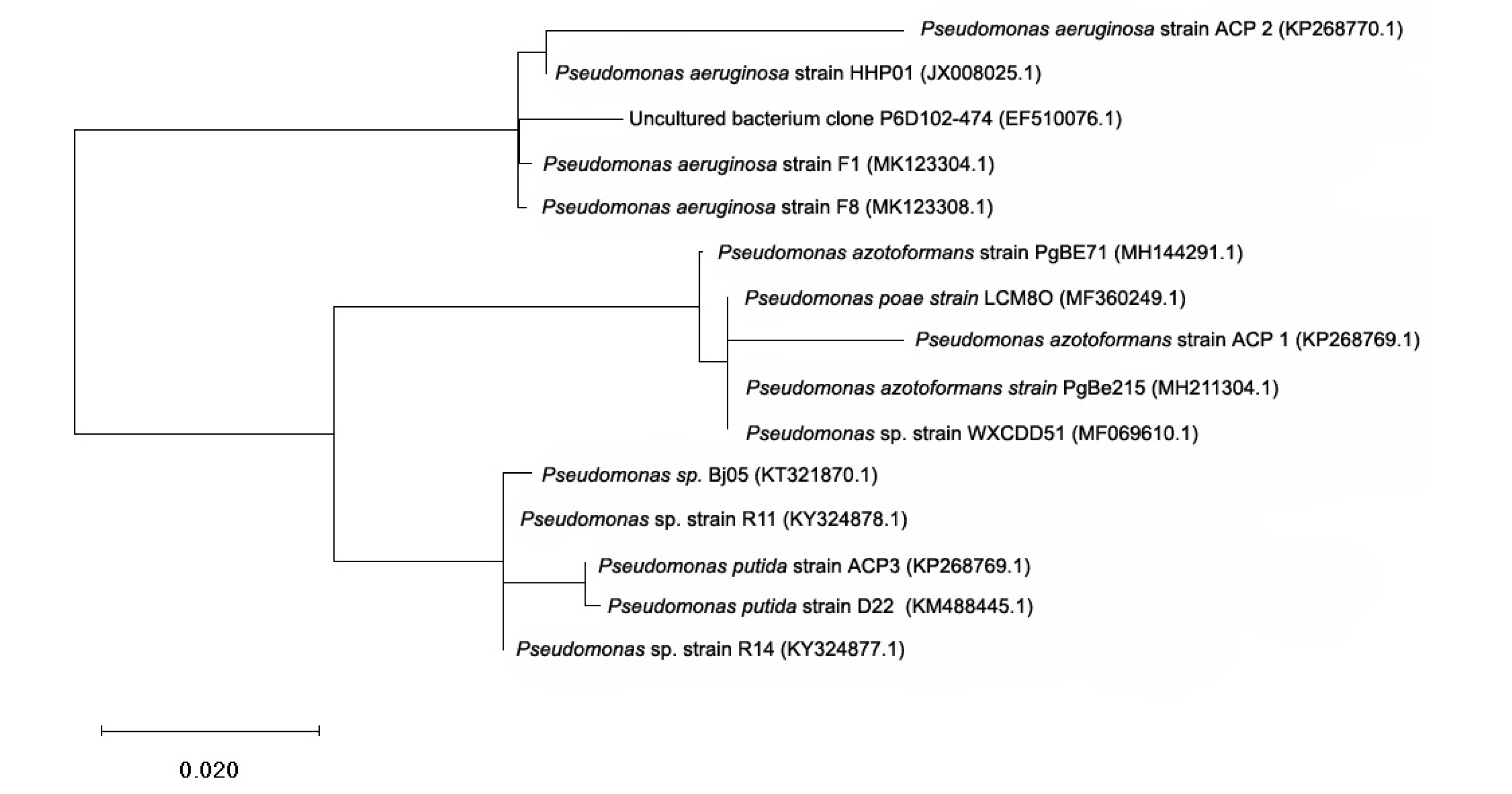
3.1. Isolation and Identification of Acephate Degrading Bacterial Strain
3.2. Cell Growth Studies Using UV—Visible Spectrophotometric Technique
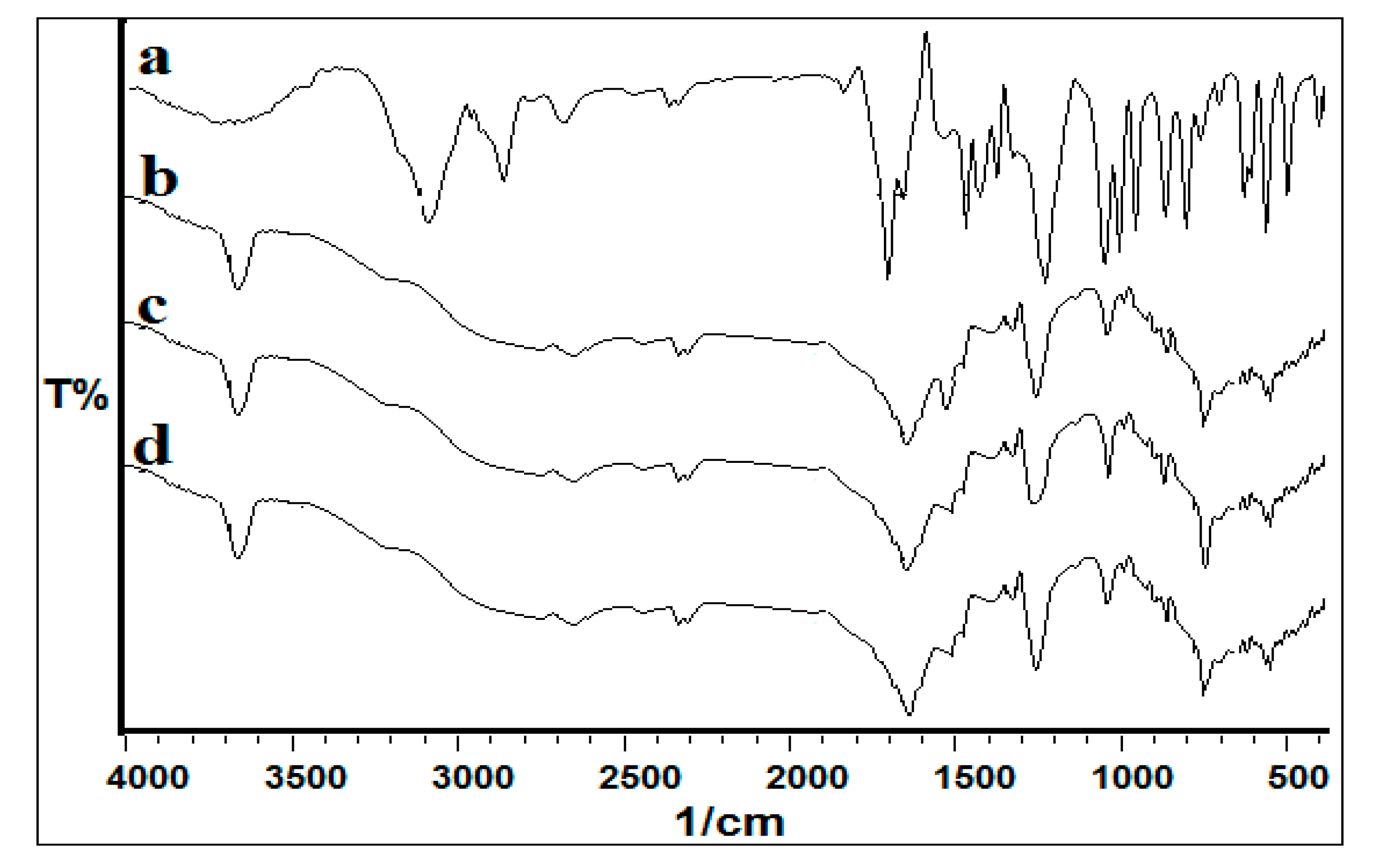
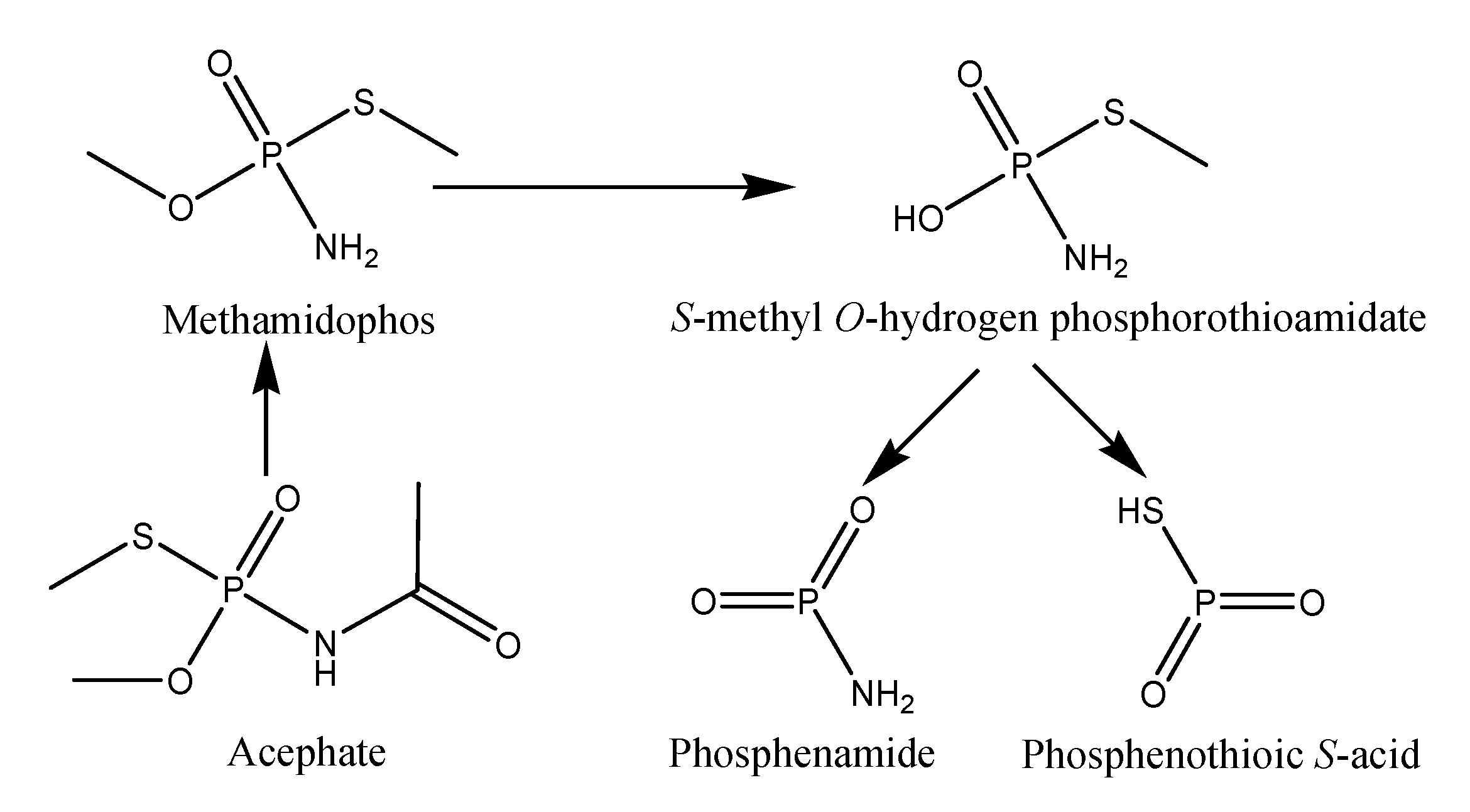
3.3. Biodegradation Products Identification and Confirmation by Using MS and FTIR Studies
3.4. Biodegradation Kinetics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, S.; Kumar, V.; Upadhyay, N.; Singh, J. The effects of Fe (II), Cu (II) and humic acid on biodegradation of atrazine. J. Environ. Chem. Eng. 2019, 103539. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Chauhan, A.; Datta, S.; Wani, A.B.; Singh, N.; Singh, J. Toxicity, degradation and analysis of the herbicide atrazine. Environ. Chem. Lett. 2018, 16, 211–237. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Singh, S.; Singh, J. Influence of humic acid, iron and copper on microbial degradation of fungicide Carbendazim. Biocatal. Agric. Biotechnol. 2019, 20, 101196. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Singh, J. Kinetic study of the biodegradation of glyphosate by indigenous soil bacterial isolates in presence of humic acid, Fe (III) and Cu (II) ions. J. Environ. Chem. Eng. 2019, 7, 103098. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Kapoor, D.; Kumar, S.; Singh, S.; Dhanjal, D.S.; Datta, S.; Samuel, J.; Dey, P.; Wang, S.; et al. Revealing on hydrogen sulfide and nitric oxide signals co-ordination for plant growth under stress conditions. Physiol. Plant 2020, 168, 301–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Kumar, V.; Datta, S.; Dhanjal, D.S.; Sharma, K.; Samuel, J.; Singh, J. Current advancement and future prospect of biosorbents for bioremediation. Sci. Total Environ. 2019, 709, 135895. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Upadhyay, N.; Singh, J.; Singla, S.; Datta, S. Efficient biodegradation of acephate by Pseudomonas pseudoalcaligenes PS-5 in the presence and absence of heavy metal ions [Cu(II) and Fe(III)], and humic acid. 3 Biotech. 2017, 7, 262. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Singh, S.; Kaur, S.; Upadhyay, N. Unexpected formation of N′-phenyl-thiophosphorohydrazidic acid O,S-dimethyl ester from acephate: Chemical, biotechnical and computational study. 3 Biotech. 2016, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Ramu, S.; Seetharamana, B. Biodegradation of acephate and methamidophos by a soil bacterium Pseudomonas aeruginosa strain Is-6. J. Environ. Sci. Health B 2014, 49, 23–34. [Google Scholar] [CrossRef]
- Pinjari, B.; Novikov, B.; Rezenom, Y.H.; Russell, D.H.; Wales, M.E.; Siddavattam, D. Mineralization of Acephate a Recalcitrant Organophosphate Insecticide is Initiated by a Pseudomonad in Environmental Samples. PLoS ONE 2012, 7, 31963–31970. [Google Scholar] [CrossRef]
- Kumar, V.; Upadhyay, N.; Wasit, A.B.; Singh, S.; Kaur, P. Spectroscopic Methods for the Detection of Organophosphate Pesticides—A Preview. Current World Environ. 2013, 8, 313–319. [Google Scholar] [CrossRef]
- Wang, L.; Wen, Y.; Guo, X.; Wang, G.; Li, S.; Jiang, J. Degradation of methamidophos by Hyphomicrobium species MAP-1 and the biochemical degradation pathway. Biodegradation 2010, 21, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.K.; Wong, M.H.; Mohd-Tahir, N.; Hansen, H.C.B. Degradation and mineralization kinetics of acephate in humid tropic soils of Malaysia. Chemosphere 2010, 79, 434–440. [Google Scholar] [PubMed]
- Kumar, V.; Upadhyay, N.; Singh, J.; Singh, S.; Kaur, P. Thin-Layer Chromatography: Comparative Estimation of Soil’s Atrazine. Current World Environ. 2013, 8, 469–472. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Upadhyay, N.; Kumar, V.; Kaur, S.; Singh, J.; Singh, S.; Datta, S. Environmental Exposure and Health Risks of the Insecticide Monocrotophos-A Review. J. Bio. Env. Sci. 2014, 5, 111–120. [Google Scholar]
- Manzanilla-Cano, J.A.; Barceló-Quintal, M.H.; Reyes-Salas, E.O. Electrochemical monitoring of methylparathion degradation in an acid aqueous medium in presence of Cu(II). J. Environ. Sci. Health B 2004, 39, 577–588. [Google Scholar] [CrossRef]
- Manzanilla-Canom, J.A.; Barceló-Quintal, M.H.; Rendón-Osorio, R.B.; Flores-Rodríguez, J. Effect of Fe (III) on acid degradation of methylparathion. J. Environ. Sci. Health B 2007, 425, 15–22. [Google Scholar]
- Shehata, A.A.; Kühnert, M.; Haufe., S.; Krüger, M. Neutralization of the antimicrobial effect of glyphosate by humic acid in vitro. Chemosphere 2014, 104, 258–261. [Google Scholar] [CrossRef]
- Mazzeia, P.; Oschkinatb, H.; Piccolo, A. Reduced activity of alkaline phosphatase due to host–guest interactions with humic superstructures. Chemosphere 2013, 93, 1972–1979. [Google Scholar] [CrossRef]
- Mazzei, P.; Piccolo, A. Quantitative Evaluation of Noncovalent Interactions between Glyphosate and Dissolved Humic Substances by NMR Spectroscopy. Environ. Sci. Tech. 2012, 46, 5939–5946. [Google Scholar] [CrossRef]
- Sarkouhi, M.; Shamsipur, M.; Hassan, J. Metal ion promoted degradation mechanism of chlorpyrifos and phoxim. Arab. J. Chem. 2016, 9, 43–47. [Google Scholar] [CrossRef] [Green Version]


| Time Period (days) | ||||
|---|---|---|---|---|
| Experimental Conditions | 3 | 7 | 14 | 30 |
| Cell Growth (%) with Pseudomonas aeruginosa | ||||
| Inoculum + acephate | 14 ± 0.85 a,b | 25 ± 2.44 a,b* | 42 ± 3.44 a,b* | 77 ± 4.55 a,b |
| Inoculum + acephate + Cu(II) | 10 ± 0.71 a,b* | 22 ± 1.05 a,b | 43 ± 3.21 a,b* | 67 ± 3.72 a,b* |
| Inoculum + acephate + Fe(III) | 10 ± 0.79 a*,b* | 26 ± 2.85 a,b* | 39 ± 2.97 a*,b | 66 ± 3.44 a,b* |
| Inoculum + acephate + HA | 8 ± 0.57 a*,b | 28 ± 2.45 a*,b | 47 ± 3.01 a,b | 74 ± 4.22 a,b |
| Cell Growth (%) with Pseudomonas putida | ||||
| Inoculum + acephate | 11 ± 0.65 a,b* | 27 ± 1.58 a,b* | 54 ± 2.95 a,b | 74 ± 4.55 a,b |
| Inoculum + acephate + Cu(II) | 9 ± 0.25 a*,b | 24 ± 1.58 a,b* | 39 ± 2.33 a,b | 64 ± 3.09 a*,b |
| Inoculum + acephate + Fe(III) | 7 ± 0.38 a,b | 25 ± 1.11 a,b* | 33 ± 2.08 a,b | 60 ± 4.27 a*,b |
| Inoculum + acephate + HA | 11 ± 0.61 a,b* | 26 ± 1.44 a,b* | 44 ± 2.81 a,b | 71 ± 4.92 a,b |
| Cell Growth (%) with Pseudomonas azotoformans | ||||
| Inoculum + acephate | 12 ± 0.66 a,b | 22 ± 1.25 a,b* | 41 ± 2.58 a,b | 69 ± 4.11 a,b* |
| Inoculum + acephate + Cu(II) | 9 ± 0.40 a*,b | 18 ± 1.03 a,b | 37 ± 2.08 a,b | 64 ± 3.55 a*,b |
| Inoculum + acephate + Fe(III) | 10 ± 0.53 a*,b | 23 ± 1.44 a,b* | 39 ± 2.35 a*,b | 61 ± 3.77 a*,b |
| Inoculum + acephate + HA | 8 ± 0.33 a*,b | 28 ± 1.42 a*,b | 45 ± 2.48 a,b | 69 ± 4.02 a,b* |
| Experimental Conditions | K | t1/2 (days) | Equation of line | r2 |
|---|---|---|---|---|
| Pseudomonas aeruginosa | ||||
| Inoculum + acephate | 0.021 | 14.33 a,b | y = −0.021x + 2.024 | 0.98 |
| Inoculum + acephate + Cu(II) | 0.016 | 18.81 a,b | y = −0.016x + 2.002 | 0.94 |
| Inoculum + acephate + Fe(III) | 0.015 | 20.06 a,b | y = −0.015x + 1.991 | 0.96 |
| Inoculum + acephate + HA | 0.020 | 15.05 a,b | y = −0.02x + 2.009 | 0.97 |
| Pseudomonas putida | ||||
| Inoculum + acephate | 0.019 | 15.84 a,b | y = −0.019x + 2.009 | 0.97 |
| Inoculum + acephate + Cu(II) | 0.014 | 21.50 a,b | y = −0.014x + 1.993 | 0.98 |
| Inoculum + acephate + Fe(III) | 0.013 | 23.15 a*,b | y = −0.013x + 1.992 | 0.96 |
| Inoculum + acephate + HA | 0.017 | 17.70 a*,b | y = −0.017x + 1.999 | 0.95 |
| Pseudomonas azotoformans | ||||
| Inoculum + acephate | 0.018 | 16.72 a,b | y = −0.018x + 2.012 | 0.97 |
| Inoculum + acephate + Cu(II) | 0.015 | 20.06 a,b | y = −0.015x + 2.011 | 0.98 |
| Inoculum + acephate + Fe(III) | 0.013 | 23.15 a*,b | y = −0.013x + 1.983 | 0.96 |
| Inoculum + acephate + HA | 0.017 | 17.70 a*,b | y = −0.017x + 1.992 | 0.97 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.; Kumar, V.; Singla, S.; Sharma, M.; Singh, D.P.; Prasad, R.; Thakur, V.K.; Singh, J. Kinetic Study of the Biodegradation of Acephate by Indigenous Soil Bacterial Isolates in the Presence of Humic Acid and Metal Ions. Biomolecules 2020, 10, 433. https://doi.org/10.3390/biom10030433
Singh S, Kumar V, Singla S, Sharma M, Singh DP, Prasad R, Thakur VK, Singh J. Kinetic Study of the Biodegradation of Acephate by Indigenous Soil Bacterial Isolates in the Presence of Humic Acid and Metal Ions. Biomolecules. 2020; 10(3):433. https://doi.org/10.3390/biom10030433
Chicago/Turabian StyleSingh, Simranjeet, Vijay Kumar, Sourav Singla, Minaxi Sharma, Dhananjaya P. Singh, Ram Prasad, Vijay Kumar Thakur, and Joginder Singh. 2020. "Kinetic Study of the Biodegradation of Acephate by Indigenous Soil Bacterial Isolates in the Presence of Humic Acid and Metal Ions" Biomolecules 10, no. 3: 433. https://doi.org/10.3390/biom10030433










