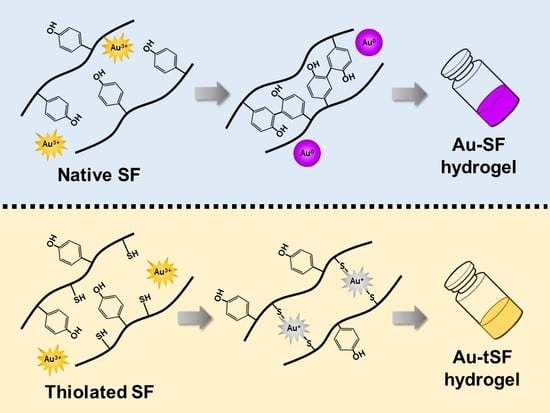
Exploring the Gelation Mechanisms and Cytocompatibility of Gold (III)-Mediated Regenerated and Thiolated Silk Fibroin Hydrogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SF and tSF Solutions
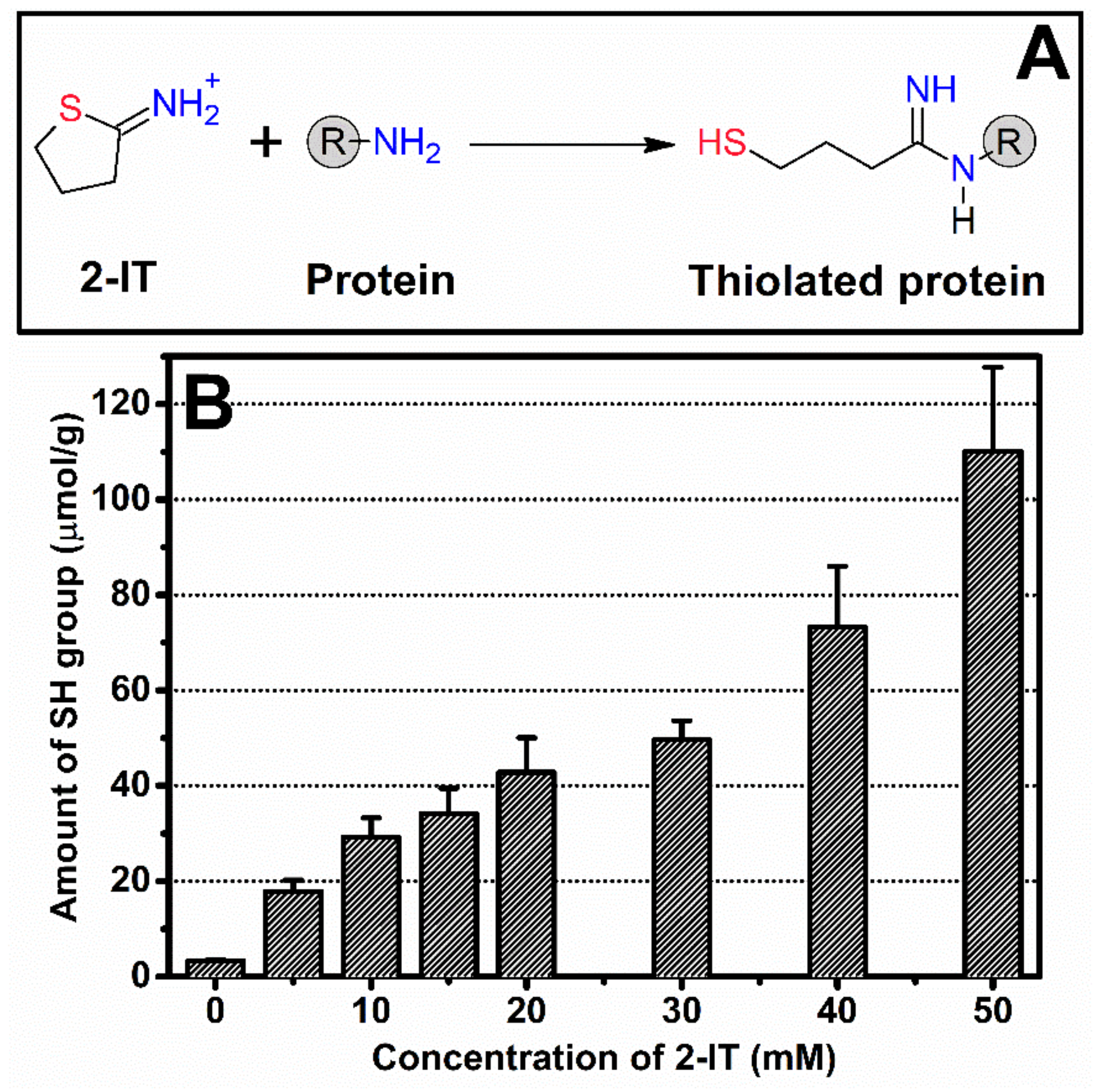
2.3. Quantification of Sulfhydryl Groups
2.4. Gelation of SF and tSF with Au3+
2.5. Determination of Bonds and AuNPs Formation in Au-SF or Au-tSF Hydrogels
2.6. XPS Analysis
2.7. Micromorphological Assessment
2.8. Analysis of Viscoelastic Properties
2.9. Cell Culture
2.10. Cytocompatibility Evaluation
2.11. Statistical Analysis
3. Results
3.1. Thiolation of SF
3.2. Formation of Au-SF and Au-tSF Hydrogels
3.3. Formation of Dityrosine, Au-S Bonds, and AuNPs
3.4. XPS Analysis of Au3+-Mediated SF and tSF Hydrogels
3.5. SEM Analysis
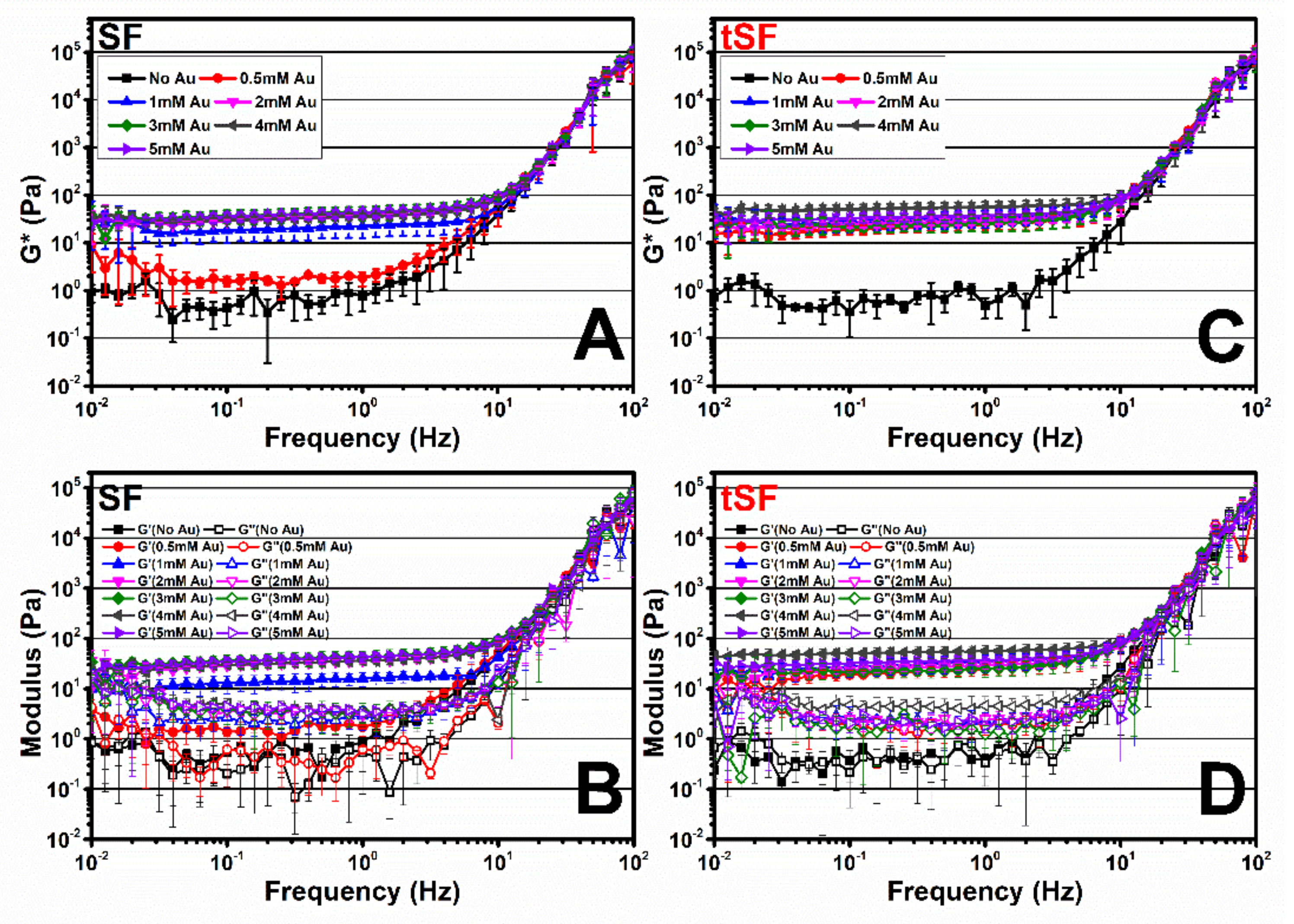
3.6. Viscoelastic Properties of Au3+-SF and tSF Gels
3.7. Cytocompatibility Evaluation of Au3+-SF and tSF Hydrogels
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Murphy, A.R.; Romero, I.S. 8-Biochemical and biophysical properties of native Bombyx mori silk for tissue engineering applications. In Silk Biomaterials for Tissue Engineering and Regenerative Medicine; Kundu, S.C., Ed.; Woodhead Publishing: Cambridge, UK, 2014; pp. 219–238. [Google Scholar]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, A.; Chen, J.; Collette, A.L.; Kim, U.-J.; Altman, G.H.; Cebe, P.; Kaplan, D.L. Mechanisms of Silk Fibroin Sol-Gel Transitions. J. Phys. Chem. B 2006, 110, 21630–21638. [Google Scholar] [CrossRef]
- Kaewprasit, K.; Kobayashi, T.; Damrongsakkul, S. Thai silk fibroin gelation process enhancing by monohydric and polyhydric alcohols. Int. J. Biol. Macromol. 2018, 118, 1726–1735. [Google Scholar] [CrossRef]
- Chantong, N.; Damrongsakkul, S.; Ratanavaraporn, J. Gelation Process and Physicochemical Properties of Thai Silk Fibroin Hydrogels Induced by Various Anionic Surfactants for Controlled Release of Curcumin. J. Surfactants Deterg. 2019, 22, 1395–1407. [Google Scholar] [CrossRef]
- Laomeephol, C.; Guedes, M.; Ferreira, H.; Reis, R.L.; Kanokpanont, S.; Damrongsakkul, S.; Neves, N.M. Phospholipid-induced silk fibroin hydrogels and their potential as cell carriers for tissue regeneration. J. Tissue Eng. Regen. Med. 2020, 14, 160–172. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, S.; Kundu, S.C. Silk protein-based hydrogels: Promising advanced materials for biomedical applications. Acta Biomater. 2016, 31, 17–32. [Google Scholar] [CrossRef]
- Shaw, C.F. Gold-Based Therapeutic Agents. Chem. Rev. 1999, 99, 2589–2600. [Google Scholar] [CrossRef]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef]
- Yadav, P.; Singh, S.P.; Rengan, A.K.; Shanavas, A.; Srivastava, R. Gold laced bio-macromolecules for theranostic application. Int. J. Biol. Macromol. 2018, 110, 39–53. [Google Scholar] [CrossRef]
- Lakshmeesha Rao, B.; Gowda, M.; Asha, S.; Byrappa, K.; Narayana, B.; Somashekar, R.; Wang, Y.; Madhu, L.N.; Sangappa, Y. Rapid synthesis of gold nanoparticles using silk fibroin: Characterization, antibacterial activity, and anticancer properties. Gold Bull. 2017, 50, 289–297. [Google Scholar] [CrossRef]
- Si, S.; Bhattacharjee, R.R.; Banerjee, A.; Mandal, T.K. A Mechanistic and Kinetic Study of the Formation of Metal Nanoparticles by Using Synthetic Tyrosine-Based Oligopeptides. Chem. A Eur. J. 2006, 12, 1256–1265. [Google Scholar] [CrossRef]
- Jung, Y.L.; Park, J.H.; Kim, M.I.; Park, H.G. Label-free colorimetric detection of biological thiols based on target-triggered inhibition of photoinduced formation of AuNPs. Nanotechnology 2015, 27, 055501. [Google Scholar] [CrossRef]
- Xu, Y.; Sherwood, J.; Qin, Y.; Crowley, D.; Bonizzoni, M.; Bao, Y. The role of protein characteristics in the formation and fluorescence of Au nanoclusters. Nanoscale 2014, 6, 1515–1524. [Google Scholar] [CrossRef]
- Kaewprasit, K.; Promboon, A.; Kanokpanont, S.; Damrongsakkul, S. Physico-chemical properties and in vitro response of silk fibroin from various domestic races. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1639–1647. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, W.; Itoh, H.; Naka, K.; Ni, Q.; Yamane, H.; Chujo, Y. Preparation of a novel core–shell nanostructured gold colloid–silk fibroin bioconjugate by the protein redox technique at room temperature. Chem. Commun. 2001, 23, 2518–2519. [Google Scholar] [CrossRef]
- Casuso, P.; Pérez-San Vicente, A.; Iribar, H.; Gutiérrez-Rivera, A.; Izeta, A.; Loinaz, I.; Cabañero, G.; Grande, H.-J.; Odriozola, I.; Dupin, D. Aurophilically cross-linked “dynamic” hydrogels mimicking healthy synovial fluid properties. Chem. Commun. 2014, 50, 15199–15201. [Google Scholar] [CrossRef] [Green Version]
- Casuso, P.; Odriozola, I.; Pérez-San Vicente, A.; Loinaz, I.; Cabañero, G.; Grande, H.-J.; Dupin, D. Injectable and Self-Healing Dynamic Hydrogels Based on Metal(I)-Thiolate/Disulfide Exchange as Biomaterials with Tunable Mechanical Properties. Biomacromolecules 2015, 16, 3552–3561. [Google Scholar] [CrossRef]
- Monteiro, N.; Martins, A.; Pires, R.; Faria, S.; Fonseca, N.A.; Moreira, J.N.; Reis, R.L.; Neves, N.M. Immobilization of bioactive factor-loaded liposomes on the surface of electrospun nanofibers targeting tissue engineering. Biomater. Sci. 2014, 2, 1195–1209. [Google Scholar] [CrossRef]
- Hu, X.; Kaplan, D.; Cebe, P. Determining Beta-Sheet Crystallinity in Fibrous Proteins by Thermal Analysis and Infrared Spectroscopy. Macromolecules 2006, 39, 6161–6170. [Google Scholar] [CrossRef]
- Malencik, D.A.; Anderson, S.R. Dityrosine as a product of oxidative stress and fluorescent probe. Amino Acids 2003, 25, 233–247. [Google Scholar] [CrossRef]
- Karpenko, A.; Leppelt, R.; Plzak, V.; Behm, R.J. The role of cationic Au3+ and nonionic Au0 species in the low-temperature water–gas shift reaction on Au/CeO2 catalysts. J. Catal. 2007, 252, 231–242. [Google Scholar] [CrossRef]
- Castner, D.G.; Hinds, K.; Grainger, D.W. X-ray Photoelectron Spectroscopy Sulfur 2p Study of Organic Thiol and Disulfide Binding Interactions with Gold Surfaces. Langmuir 1996, 12, 5083–5086. [Google Scholar] [CrossRef]
- Singh, R.; Kats, L.; Blättler, W.A.; Lambert, J.M. Formation of N-Substituted 2-Iminothiolanes When Amino Groups in Proteins and Peptides Are Modified by 2-Iminothiolane. Anal. Biochem. 1996, 236, 114–125. [Google Scholar] [CrossRef] [Green Version]
- Mulvaney, P. Surface Plasmon Spectroscopy of Nanosized Metal Particles. Langmuir 1996, 12, 788–800. [Google Scholar] [CrossRef]
- Schedle, A.; Samorapoompichit, P.; Rausch-Fan, X.H.; Franz, A.; Füreder, W.; Sperr, W.R.; Sperr, W.; Ellinger, A.; Slavicek, R.; Boltz-Nitulescu, G.; et al. Response of L-929 Fibroblasts, Human Gingival Fibroblasts, and Human Tissue Mast Cells to Various Metal Cations. J. Dent. Res. 1995, 74, 1513–1520. [Google Scholar] [CrossRef]
- Dickerson, M.B.; Sandhage, K.H.; Naik, R.R. Protein- and Peptide-Directed Syntheses of Inorganic Materials. Chem. Rev. 2008, 108, 4935–4978. [Google Scholar] [CrossRef]


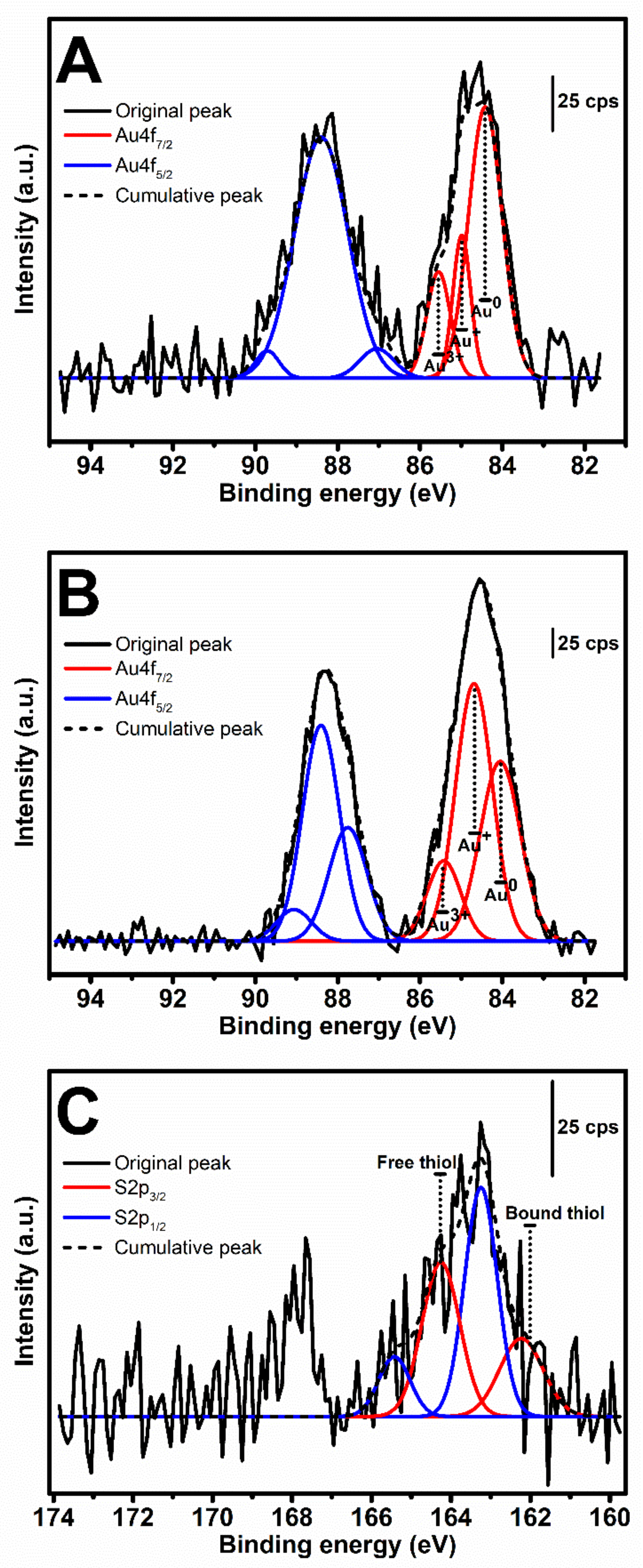



| XPS Region | Chemical State | 1 mM Au + 3% SF | 1 mM Au + 3% tSF | ||
|---|---|---|---|---|---|
| BE (eV) | Relative Amount (%) | BE (eV) | Relative Amount (%) | ||
| Au4f7/2 | Au0 | 84.4 | 31.7 | 84.0 | 21.9 |
| Au+ | 84.9 | 8.9 | 84.6 | 28.9 | |
| Au3+ | 85.5 | 8.8 | 85.4 | 8.3 | |
| S2p3/2 | Bound S | N/A | 162.2 | 18.2 | |
| Free S | 164.2 | 31.4 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laomeephol, C.; Ferreira, H.; Yodmuang, S.; Reis, R.L.; Damrongsakkul, S.; Neves, N.M. Exploring the Gelation Mechanisms and Cytocompatibility of Gold (III)-Mediated Regenerated and Thiolated Silk Fibroin Hydrogels. Biomolecules 2020, 10, 466. https://doi.org/10.3390/biom10030466
Laomeephol C, Ferreira H, Yodmuang S, Reis RL, Damrongsakkul S, Neves NM. Exploring the Gelation Mechanisms and Cytocompatibility of Gold (III)-Mediated Regenerated and Thiolated Silk Fibroin Hydrogels. Biomolecules. 2020; 10(3):466. https://doi.org/10.3390/biom10030466
Chicago/Turabian StyleLaomeephol, Chavee, Helena Ferreira, Supansa Yodmuang, Rui L. Reis, Siriporn Damrongsakkul, and Nuno M. Neves. 2020. "Exploring the Gelation Mechanisms and Cytocompatibility of Gold (III)-Mediated Regenerated and Thiolated Silk Fibroin Hydrogels" Biomolecules 10, no. 3: 466. https://doi.org/10.3390/biom10030466
APA StyleLaomeephol, C., Ferreira, H., Yodmuang, S., Reis, R. L., Damrongsakkul, S., & Neves, N. M. (2020). Exploring the Gelation Mechanisms and Cytocompatibility of Gold (III)-Mediated Regenerated and Thiolated Silk Fibroin Hydrogels. Biomolecules, 10(3), 466. https://doi.org/10.3390/biom10030466