Macrophage Cholesterol Efflux Downregulation Is Not Associated with Abdominal Aortic Aneurysm (AAA) Progression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Lipid, Apolipoprotein and Lipoprotein Analyses
2.3. Macrophage Cholesterol Efflux Assays
2.4. Statistical Analysis
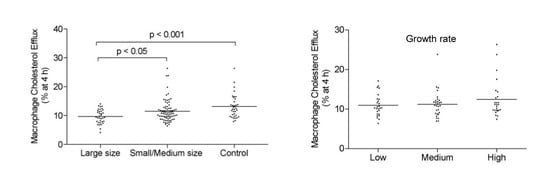
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hirsch, A.T.; Haskal, Z.J.; Hertzer, N.R.; Bakal, C.W.; Creager, M.A.; Halperin, J.L.; Hiratzka, L.F.; Murphy, W.R.; Olin, J.W.; Puschett, J.B.; et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006, 113, e463–e654. [Google Scholar] [PubMed] [Green Version]
- Ashton, H.A.; Buxton, M.J.; Day, N.E.; Kim, L.G.; Marteau, T.M.; Scott, R.A.; Thompson, S.G.; Walker, N.M. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: A randomised controlled trial. Lancet 2002, 360, 1531–1539. [Google Scholar] [PubMed]
- Moll, F.L.; Powell, J.T.; Fraedrich, G.; Verzini, F.; Haulon, S.; Waltham, M.; van Herwaarden, J.A.; Holt, P.J.; van Keulen, J.W.; Rantner, B.; et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur. J. Vasc. Endovasc. Surg. 2011, 41 (Suppl. 1), S1–S58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kent, K.C.; Zwolak, R.M.; Egorova, N.N.; Riles, T.S.; Manganaro, A.; Moskowitz, A.J.; Gelijns, A.C.; Greco, G. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J. Vasc. Surg. 2010, 52, 539–548. [Google Scholar] [CrossRef] [Green Version]
- Wanhainen, A. How to define an abdominal aortic aneurysm—Influence on epidemiology and clinical practice. Scand. J. Surg. 2008, 97, 105–109; discussion 109. [Google Scholar] [CrossRef]
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef] [Green Version]
- Periard, D.; Guessous, I.; Mazzolai, L.; Haesler, E.; Monney, P.; Hayoz, D. Reduction of small infrarenal abdominal aortic aneurysm expansion rate by statins. Vasa 2012, 41, 35–42. [Google Scholar] [CrossRef]
- Lindholt, J.S.; Bjorck, M.; Michel, J.B. Anti-platelet treatment of middle-sized abdominal aortic aneurysms. Curr. Vasc. Pharmacol. 2013, 11, 305–313. [Google Scholar] [CrossRef]
- Wang, Y.D.; Liu, Z.J.; Ren, J.; Xiang, M.X. Pharmacological Therapy of Abdominal Aortic Aneurysm: An Update. Curr. Vasc. Pharmacol. 2018, 16, 114–124. [Google Scholar] [CrossRef]
- Sakalihasan, N.; Limet, R.; Defawe, O.D. Abdominal aortic aneurysm. Lancet 2005, 365, 1577–1589. [Google Scholar] [CrossRef]
- Michel, J.B.; Martin-Ventura, J.L.; Egido, J.; Sakalihasan, N.; Treska, V.; Lindholt, J.; Allaire, E.; Thorsteinsdottir, U.; Cockerill, G.; Swedenborg, J. Novel aspects of the pathogenesis of aneurysms of the abdominal aorta in humans. Cardiovasc. Res. 2011, 90, 18–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajamaki, K.; Lappalainen, J.; Oorni, K.; Valimaki, E.; Matikainen, S.; Kovanen, P.T.; Eklund, K.K. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: A novel link between cholesterol metabolism and inflammation. PLoS ONE 2010, 5, e11765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tall, A.R.; Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 2015, 15, 104–116. [Google Scholar] [CrossRef] [Green Version]
- Harrison, S.C.; Holmes, M.V.; Burgess, S.; Asselbergs, F.W.; Jones, G.T.; Baas, A.F.; van’t Hof, F.N.; de Bakker, P.I.W.; Blankensteijn, J.D.; Powell, J.T.; et al. Genetic Association of Lipids and Lipid Drug Targets With Abdominal Aortic Aneurysm: A Meta-analysis. JAMA Cardiol. 2018, 3, 26–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, L.C.; Roetker, N.S.; Lutsey, P.L.; Alonso, A.; Guan, W.; Pankow, J.S.; Folsom, A.R.; Steffen, L.M.; Pankratz, N.; Tang, W. Evaluation of the relationship between plasma lipids and abdominal aortic aneurysm: A Mendelian randomization study. PLoS ONE 2018, 13, e0195719. [Google Scholar] [CrossRef] [PubMed]
- Burillo, E.; Lindholt, J.S.; Molina-Sanchez, P.; Jorge, I.; Martinez-Pinna, R.; Blanco-Colio, L.M.; Tarin, C.; Torres-Fonseca, M.M.; Esteban, M.; Laustsen, J.; et al. ApoA-I/HDL-C levels are inversely associated with abdominal aortic aneurysm progression. Thromb. Haemost. 2015, 113, 1335–1346. [Google Scholar] [PubMed]
- Lee-Rueckert, M.; Escola-Gil, J.C.; Kovanen, P.T. HDL functionality in reverse cholesterol transport—Challenges in translating data emerging from mouse models to human disease. Biochim. Biophys. Acta 2016, 1861, 566–583. [Google Scholar] [CrossRef]
- Martinez-Lopez, D.; Cedo, L.; Metso, J.; Burillo, E.; Garcia-Leon, A.; Canyelles, M.; Lindholt, J.S.; Torres-Fonseca, M.; Blanco-Colio, L.M.; Vazquez, J.; et al. Impaired HDL (High-Density Lipoprotein)-Mediated Macrophage Cholesterol Efflux in Patients With Abdominal Aortic Aneurysm-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2750–2754. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Carrio, J.; Lindholt, J.S.; Canyelles, M.; Martinez-Lopez, D.; Tondo, M.; Blanco-Colio, L.M.; Michel, J.B.; Escola-Gil, J.C.; Suarez, A.; Martin-Ventura, J.L. IgG Anti-High Density Lipoprotein Antibodies Are Elevated in Abdominal Aortic Aneurysm and Associated with Lipid Profile and Clinical Features. J. Clin. Med. 2019, 9, 67. [Google Scholar] [CrossRef] [Green Version]
- Escola-Gil, J.C.; Lee-Rueckert, M.; Santos, D.; Cedo, L.; Blanco-Vaca, F.; Julve, J. Quantification of In Vitro Macrophage Cholesterol Efflux and In Vivo Macrophage-Specific Reverse Cholesterol Transport. Methods Mol. Biol. 2015, 1339, 211–233. [Google Scholar]
- Forsdahl, S.H.; Singh, K.; Solberg, S.; Jacobsen, B.K. Risk factors for abdominal aortic aneurysms: A 7-year prospective study: The Tromso Study, 1994–2001. Circulation 2009, 119, 2202–2208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Lopez, D.; Camafeita, E.; Cedo, L.; Roldan-Montero, R.; Jorge, I.; Garcia-Marques, F.; Gomez-Serrano, M.; Bonzon-Kulichenko, E.; Blanco-Vaca, F.; Blanco-Colio, L.M.; et al. APOA1 oxidation is associated to dysfunctional high-density lipoproteins in human abdominal aortic aneurysm. EBioMedicine 2019, 43, 43–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, A.J.; Woollard, K.J.; Hoang, A.; Mukhamedova, N.; Stirzaker, R.A.; McCormick, S.P.; Remaley, A.T.; Sviridov, D.; Chin-Dusting, J. High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 2071–2077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, S.G.; Brown, L.C.; Sweeting, M.J.; Bown, M.J.; Kim, L.G.; Glover, M.J.; Buxton, M.J.; Powell, J.T. Systematic review and meta-analysis of the growth and rupture rates of small abdominal aortic aneurysms: Implications for surveillance intervals and their cost-effectiveness. Health Technol. Assess. 2013, 17, 1–118. [Google Scholar] [CrossRef]
- Torsney, E.; Pirianov, G.; Charolidi, N.; Shoreim, A.; Gaze, D.; Petrova, S.; Laing, K.; Meisinger, T.; Xiong, W.; Baxter, B.T.; et al. Elevation of plasma high-density lipoproteins inhibits development of experimental abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2678–2686. [Google Scholar] [CrossRef] [Green Version]
- Burillo, E.; Tarin, C.; Torres-Fonseca, M.M.; Fernandez-Garcia, C.E.; Martinez-Pinna, R.; Martinez-Lopez, D.; Llamas-Granda, P.; Camafeita, E.; Lopez, J.A.; Vega de Ceniga, M.; et al. Paraoxonase-1 overexpression prevents experimental abdominal aortic aneurysm progression. Clin. Sci. (Lond.) 2016, 130, 1027–1038. [Google Scholar] [CrossRef]
- Lindholt, J.S.; Kristensen, K.L.; Burillo, E.; Martinez-Lopez, D.; Calvo, C.; Ros, E.; Martin-Ventura, J.L.; Sala-Vila, A. Arachidonic Acid, but Not Omega-3 Index, Relates to the Prevalence and Progression of Abdominal Aortic Aneurysm in a Population-Based Study of Danish Men. J. Am. Heart Assoc. 2018, 7, e007790. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Garcia, C.E.; Burillo, E.; Lindholt, J.S.; Martinez-Lopez, D.; Pilely, K.; Mazzeo, C.; Michel, J.B.; Egido, J.; Garred, P.; Blanco-Colio, L.M.; et al. Association of ficolin-3 with abdominal aortic aneurysm presence and progression. J. Thromb. Haemost. 2017, 15, 575–585. [Google Scholar] [CrossRef] [Green Version]

| Parameters | Large Size (n = 38) | Small/Medium Size (n = 81) | Control (n = 39) | ANOVA or Chi-Square p-Value |
|---|---|---|---|---|
| Age (y) | 69.71 ± 2.88 | 69.65 ± 2.81 | 68.87 ± 2.69 | ns |
| BMI (%) | 28.03 ± 2.42 † | 27.41 ± 3.48 † | 25.80 ± 2.55 | <0.01 |
| Total cholesterol (mmol/L) | 4.52 ± 0.71 † | 4.87 ± 0.88 | 5.24 ± 0.77 | <0.001 |
| TG (mmol/L) | 0.98 ± 0.40 # | 1.44 ± 0.66 † | 1.10 ± 0.36 | <0.001 |
| ApoA-I (g/L) | 1.53 ± 0.28 † | 1.59 ± 0.32 † | 1.80 ± 0.32 | <0.001 |
| HDLc (mmol/L) | 1.09 ± 0.43 | 1.09 ± 0.40 | 1.22 ± 0.42 | ns |
| LDLc (mmol/L) | 2.98 ± 0.81 † | 3.13 ± 0.90 | 3.52 ± 0.89 | <0.05 |
| VLDLc (mmol/L) | 0.45 ± 0.19 # | 0.65 ± 0.26 † | 0.50 ± 0.16 | <0.001 |
| Aortic diameter (mm) | 62.52 ± 15.35 †,# | 36.35 ± 4.54 † | 18.16 ± 2.90 | <0.001 |
| DBP (mm Hg) | 91.00 ± 13.62 † | 88.00 ± 12.30 † | 80.97 ± 11.36 | <0.01 |
| Lowest ABI | 0.99 ± 0.11 † | 0.95 ± 0.19 † | 1.10 ± 0.09 | <0.001 |
| Smoking | 8 (2%) | 32 (41%) | 15 (40%) | ns |
| Diabetes | 3 (8%) | 10 (13%) | 4 (8%) | ns |
| Arterial hypertension | 15 (40%) | 41 (52%) | 23 (61%) | ns |
| Previous CVD | 1 (18%) | 11 (14%) | 7 (3%) | ns |
| Statin use | 16 (42%) | 41 (52%) | 20 (53%) | ns |
| Low-dose aspirin | 8 (50%) | 35 (44%) | 19 (22%) | ns |
| Coefficients | |||
|---|---|---|---|
| Model | Standardized Coefficients | t | p-Value |
| Beta | |||
| Age | 0.050 | 0.634 | 0.527 |
| BMI | 0.145 | 1.622 | 0.107 |
| Smoke | 0.027 | 0.325 | 0.745 |
| Statins | 0.103 | 1.273 | 0.205 |
| DBP | 0.275 | 3.457 | 0.001 |
| MCE capacity | −0.115 | −1.295 | 0.198 |
| Dependent variable: aortic baseline diameter | |||
| Growth Rate | ApoA-I | HDLc | BMI | |
|---|---|---|---|---|
| MCE capacity in small/medium size AAA group | 0.11 (−0.11–0.32) | 0.36 (0.16–0.54) | 0.37 (0.16–0.55) | −0.36 (−0.54–(−0.15)) |
| p-value | ns | <0.001 | <0.001 | <0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canyelles, M.; Tondo, M.; Lindholt, J.S.; Santos, D.; Fernández-Alonso, I.; de Gonzalo-Calvo, D.; Blanco-Colio, L.M.; Escolà-Gil, J.C.; Martín-Ventura, J.L.; Blanco-Vaca, F. Macrophage Cholesterol Efflux Downregulation Is Not Associated with Abdominal Aortic Aneurysm (AAA) Progression. Biomolecules 2020, 10, 662. https://doi.org/10.3390/biom10040662
Canyelles M, Tondo M, Lindholt JS, Santos D, Fernández-Alonso I, de Gonzalo-Calvo D, Blanco-Colio LM, Escolà-Gil JC, Martín-Ventura JL, Blanco-Vaca F. Macrophage Cholesterol Efflux Downregulation Is Not Associated with Abdominal Aortic Aneurysm (AAA) Progression. Biomolecules. 2020; 10(4):662. https://doi.org/10.3390/biom10040662
Chicago/Turabian StyleCanyelles, Marina, Mireia Tondo, Jes S. Lindholt, David Santos, Irati Fernández-Alonso, David de Gonzalo-Calvo, Luis Miguel Blanco-Colio, Joan Carles Escolà-Gil, José Luís Martín-Ventura, and Francisco Blanco-Vaca. 2020. "Macrophage Cholesterol Efflux Downregulation Is Not Associated with Abdominal Aortic Aneurysm (AAA) Progression" Biomolecules 10, no. 4: 662. https://doi.org/10.3390/biom10040662
APA StyleCanyelles, M., Tondo, M., Lindholt, J. S., Santos, D., Fernández-Alonso, I., de Gonzalo-Calvo, D., Blanco-Colio, L. M., Escolà-Gil, J. C., Martín-Ventura, J. L., & Blanco-Vaca, F. (2020). Macrophage Cholesterol Efflux Downregulation Is Not Associated with Abdominal Aortic Aneurysm (AAA) Progression. Biomolecules, 10(4), 662. https://doi.org/10.3390/biom10040662