The Influence of Selected Gastrointestinal Parasites on Apoptosis in Intestinal Epithelial Cells
Abstract
:1. Introduction
2. Apoptosis
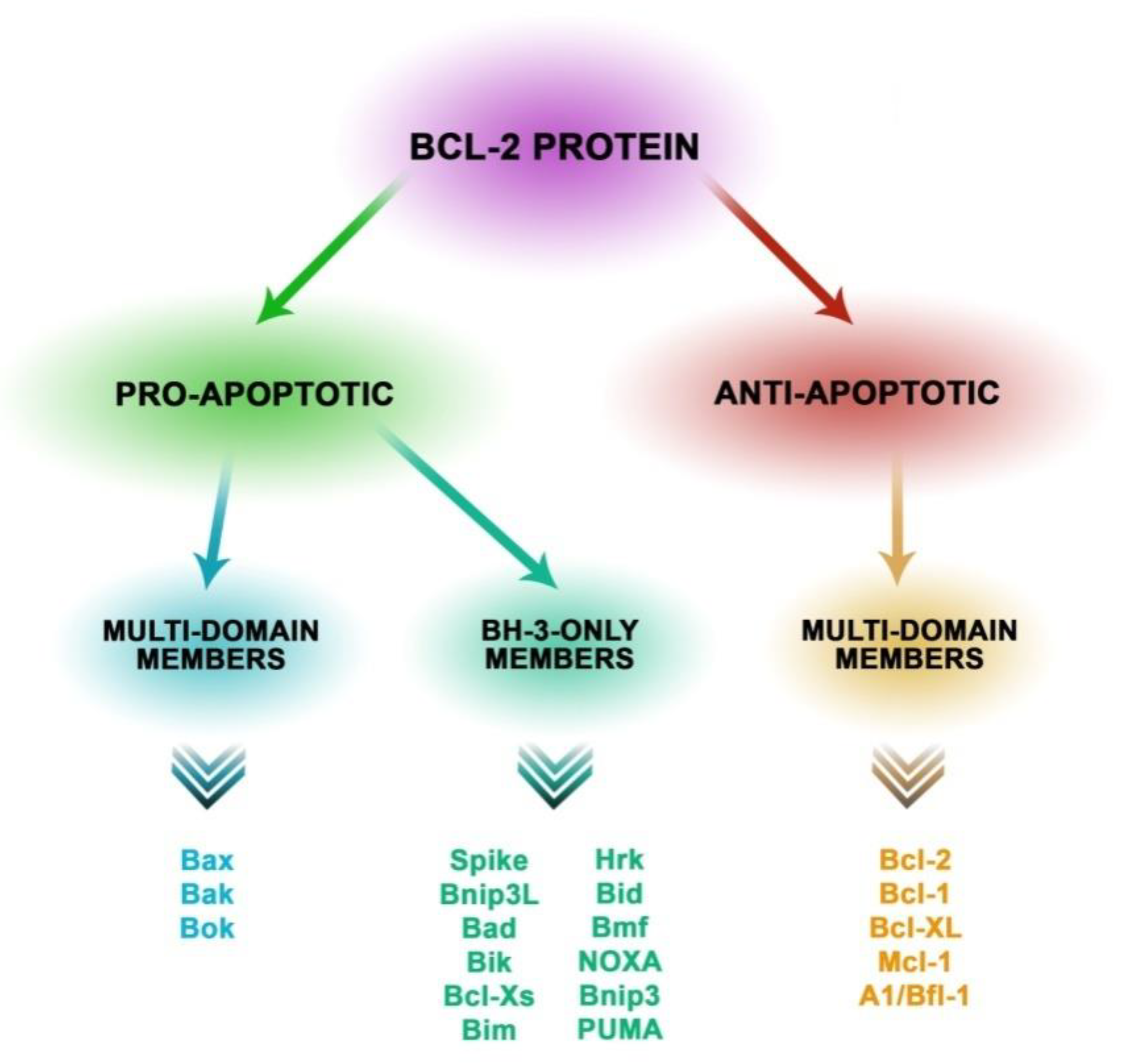
2.1. Proteins Involved in the Mechanisms of Apoptosis
2.2. Pathways of Apoptosis and the Execution Phase
2.3. Physiological Role of Apoptosis in the Intestinal Epithelium
3. The Effect of Intestinal Parasites on Apoptosis in Intestinal Epithelial Cells
3.1. Blastocystis sp.
3.2. Giardia sp.
3.3. Cryptosporidium sp.
3.4. Trichuris sp.
3.5. Entamoeba Histolytica
3.6. Nippostrongylus Brasiliensis
3.7. Heligmosomoides Polygyrus
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rutkowska, J. Regulation of parasite-host immune response interactions on the cellular level. Now Lek 2007, 76, 251–255. [Google Scholar]
- Wędrychowicz, H. Evasion of host immunity by parasites. Kosmos 2005, 54, 39–48. [Google Scholar]
- Bienvenu, A.L.; Gonzales-Rey, E.; Picot, S. Apoptosis by parasitic diseases. Parasites Vectors 2010, 3, 106. [Google Scholar] [CrossRef] [Green Version]
- James, E.R.; Green, D.R. Manipulation of apoptosis in the host-parasite interaction. Trends Parasitol. 2004, 20, 280–287. [Google Scholar] [CrossRef]
- Baś, M.; Cywińska, A.; Sokołowska, J.; Krzyżowska, M. Apoptosis–programmed cell death. Part III. The role of apoptosis in physiology and pathology. Zycie Weter. 2004, 79, 671–675. [Google Scholar]
- Hacker, G. The morphology of apoptosis. Cell Tissue Res. 2000, 301, 5–7. [Google Scholar] [CrossRef] [PubMed]
- White, M.K.; Cinti, C. A morphologic approach to detect apoptosis based on electron microscopy. Methods Mol. Biol. 2004, 285, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Stępień, A.; Izdebska, M.; Grzanka, A. The types of cell death. Postepy Hig. Med. Dosw. 2007, 61, 420–428. [Google Scholar]
- Hordyjewska, A.; Pasternak, K. Apoptotic Death of the Cell. Adv. Clin. Exp. Med. 2005, 14, 545–554. [Google Scholar]
- Nijhawan, D.; Honarpour, N.; Wang, X. Apoptosis in neural development and disease. Annu. Rev. Neurosci. 2000, 23, 73–87. [Google Scholar] [CrossRef]
- Oppenheim, R.W.; Flavell, R.A.; Vinsant, S.; Prevette, D.; Kuan, C.Y.; Rakic, P. Programmed cell death of developing mammalian neurons after genetic deletion of caspases. J. Neurosci. 2001, 21, 4752–4760. [Google Scholar] [CrossRef] [Green Version]
- Thomson, C.B. Apoptosis in the pathogenesis and treatment of disease. Science 1995, 267, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Ollson, M.; Zhivotovsky, B. Caspases and cancer. Cell Death Differ. 2011, 18, 1441–1449. [Google Scholar] [CrossRef] [Green Version]
- Cohen, G.M. Caspases: The executioners of apoptosis. Biochem. J. 1997, 325, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, T.J.; Han, L.H.; Cong, R.S.; Liang, J. Caspase family proteases and apoptosis. Acta Biochim. Biophys. Sin. 2005, 37, 71727. [Google Scholar] [CrossRef] [Green Version]
- LDeveraux, Q.L.; Reed, J.C. IAP family proteins-suppressors of apoptosis. Genes Dev. 1999, 13, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Ravagnan, L.; Gurbuxani, S.; Susin, S.A.; Maisse, C.; Daugas, E.; Zamzami, N.; Mak, T.; Jäättelä, M.; Penninger, J.M.; Garrido, C.; et al. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat. Cell Biol. 2001, 3, 839–843. [Google Scholar] [CrossRef]
- Lewis, J.; Devin, A.; Miller, A.; Lin, Y.; Rodriguez, Y.; Neckers, L.; Liu, Z.G. Disruption of Hsp90 function results in degradation of the death domain kinase, receptor-interacting protein (RIP), and blockage of tumor necrosis factor-induced nuclear factor-kappaB activation. J. Biol. Chem. 2000, 275, 10519–10526. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Mahfouz, R.Z.; Sharma, R.K.; Sarkar, O.; Mangrola, D.; Mathur, P.P. Potential biological role of poly (ADP-ribose) polymerase (PARP) in male gametes. Reprod. Biol. Endocrinol. 2009, 7, 143. [Google Scholar] [CrossRef] [Green Version]
- Kelly, P.N.; Strasser, A. The role of Bcl-2 and its pro-survival relatives in tumorigenesis and cancer therapy. Cell Death Differ. 2011, 18, 1414–1424. [Google Scholar] [CrossRef] [Green Version]
- Chonghaile, T.N.; Letai, A. Mimicking the BH3 domain to kill cancer cells. Oncogene 2008, 27, S149–S157. [Google Scholar] [CrossRef] [Green Version]
- Gavathiosis, E.; Suzuki, M.; Davis, M.C.; Pitter, K.; Bird, G.H.; Katz, S.G.; Tu, H.C.; Kim, H.; Cheng, E.H.; Tjandra, N.; et al. Bax activation is initiated at a novel interaction site. Nature 2008, 455, 1076–1081. [Google Scholar] [CrossRef] [Green Version]
- Miyashita, T.; Krajewski, S.; Krajewska, M.; Wang, H.G.; Lin, H.K.; Liebermann, D.A.; Hoffman, B.; Reed, J.C. Tumor suppressor p53 is a regulator of Bcl-2 and Bax gene expression in vitro and in vivo. Oncogene 1994, 9, 1799–1805. [Google Scholar]
- Hartman, M.Ł.; Czyż, M. BH3 mimetics as a strategy to complement anticancer therapies. Postepy Hig. Med. Dosw. 2012, 66, 67–77. [Google Scholar]
- Sznarkowska, A.; Olszewski, R.; Zawadzka-Pankau, J. Pharmacological activation of tumor suppresor, wild-type p53 as a promising strategy to fight cancer. Postepy Hig. Med. Dosw. 2010, 64, 396–407. [Google Scholar]
- Favaloro, B.; Allocati, N.; Graziano, V.; Di Ilio, C.; De Laurenzi, V. Role of apoptosis in disease. Aging 2012, 4, 330–349. [Google Scholar] [CrossRef] [PubMed]
- Monian, P.; Jiang, X. Clearing the final hurdles to mitochondrial apoptosis: Regulation post cytochrome c release. Exp. Oncol. 2012, 34, 185–191. [Google Scholar] [PubMed]
- Dlamini, Z.; Mbita, Z.; Zungu, M. Genealogy, expression, and molecular mechanisms in apoptosis. Pharmacol. Ther. 2004, 101, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Reubold, T.F.; Eschenburg, S. A molecular view on signal transduction by the apoptosome. Cell Signal. 2012, 24, 1420–1425. [Google Scholar] [CrossRef]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Paduch, R.; Klatka, M.; Klatka, J. Types of cel death. Pomeranian J. Life Sci. 2015, 61, 411–418. [Google Scholar] [PubMed]
- Frisch, S.M.; Francis, H. Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol. 1994, 124, 619–626. [Google Scholar] [CrossRef] [Green Version]
- Ruifrok, A.C.C.; Mason, K.A.; Lozano, G.; Thames, H.D. Spatial and temporal patterns of expression of epidermal growth factor, transforming growth factor alpha and transforming growth factor beta 1–3 and their receptor in mouse jejunum after radiation treatment. Radiat. Res. 1997, 147, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ina, K.; Itoh, J.; Fukushima, K.; Kusugami, K.; Yamaguchi, T.; Kyokane, K.; Imada, A.; Binion, D.G.; Musso, A.; West, G.A.; et al. Resistance of Crohn’s disease T cells to multiple apoptotic signale is associated with a Bcl-2/Bax mucosal imbalance. J. Immunol. 1999, 163, 1081–1090. [Google Scholar]
- Wang, Y.; George, S.P.; Roy, S.; Pham, E.; Esmaeilniakooshkghazi, A.; Khurana, S. Both the anti- and pro-apoptotic functions of villi regulate cell turnover and intestinal homeostasis. Sci. Rep. 2016, 6, 35491. [Google Scholar] [CrossRef] [Green Version]
- Buret, A.G.; Bhargava, A. Modulatory mechanisms of enterocyte apoptosis by viral, bacterial and parasitic pathogens. Crit. Rev. Microbiol. 2014, 40, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kapczuk, P.; Kosik-Bogacka, D.; Łanocha-Arendarczyk, N.; Gutowska, I.; Kupnicka, P.; Chlubek, D.; Baranowska-Bosiacka, I. Selected Molecular Mechanisms Involved in the Parasite-Host System Hymenolepis diminuta-Rattus norvegicus. Int. J. Mol. Sci. 2018, 17, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wawrzyniak, I.; Poirier, P.; Viscogliosi, E.; Dionigia, M.; Texier, C.; Delbac, F.; Alaoui, H.E. Blastocystis, an unrecognized parasite: An overview of pathogenesis and diagnosis. Ther. Adv. Infect. Dis. 2013, 1, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Morozińska-Gogol, J. Unwanted strangers—Alien parasites in Poland. Kosmos 2015, 64, 89–101. [Google Scholar]
- Pociecha, A.; Solarz, W.; Najberek, K.; Wilk-Woźniak, E. Native, alien, cosmopolitan, or cryptogenic? A Framework for clarifying the origin status of rotifers, Aquat. Biol. 2016, 24, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Pojmańska, T.; Niewiadomska, K. Parasites-inconvenient element in the structure of ecosystem food web. Kosmos 2010, 59, 99–110. [Google Scholar]
- Prokopowicz, D.; Wasiluk, A.; Rogalska, M. Opportunistic parasitic infections affecting human beings. Kosmos 2005, 54, 109–113. [Google Scholar]
- Duda, A.; Kosik-Bogacka, D.; Łanocha, N.; Szymański, S. Blastocystis hominis–komensal czy patogen? Rocz PAM 2014, 60, 23–28. [Google Scholar]
- Kaya, S.; SesliCetin, E.; Cicioglu Aridogan, B.; Arikan, S.; Demirci, M. Pathogenicity of Blastocysitis hominis, a clinical reevaluation. Türk. Parazitol. Derg. 2007, 31, 184–187. [Google Scholar]
- Pasqui, A.L.; Sarini, E.; Saletti, M.; Guzzo, C.; Puccetti, L.; Auteri, A. Chronic urticaria and Blastocystis hominis infection. A case report. Eur. Rev. Med. Pharmacol. Sci. 2004, 8, 117–120. [Google Scholar]
- Tan, K.S. New Insights on Classification, Identification, and Clinical Relevance of Blastocystis spp. Clin. Microbiol. Rev. 2008, 21, 639–665. [Google Scholar] [CrossRef] [Green Version]
- Chandramathi, S.; Suresh, K.; Kuppusamy, U.R. Elevated levels of urinary hyaluronidase in humans infected with intestinal parasites. Ann. Trop. Med. Parasitol. 2010, 104, 449–452. [Google Scholar] [CrossRef]
- Chandramathi, S.; Suresh, K.; Mahmood, A.A.; Kuppusamy, U.R. Urinary hyaluronidase activity in rats infected with Blastocystis hominis-evidence for invasion? Parasitol. Res. 2010, 106, 1459–1463. [Google Scholar] [CrossRef]
- Hu, K.C.; Lin, C.C.; Wang, T.E.; Liu, C.Y.; Chen, M.J.; Chang, W.H. Amoebic liver abscess or is it? Gut 2008, 57, 627–683. [Google Scholar] [CrossRef]
- Patino, W.D.; Cavuoti, D.; Benerjee, S.K.; Swartz, K.; Ashfag, R.; Gokaslan, T. Cytologic diagnosis of Blastocystis hominis in peritoneal fluid: A case report. Acta Cytol. 2008, 52, 718–720. [Google Scholar] [CrossRef] [PubMed]
- Mirza, H.; Wu, Z.; Teo, J.D.; Tan, K.S. Statin pleotropy prevents rho kinase-mediated intestinal epithelial barrier compromise induced by Blastocystis cysteine proteases. Cell. Microbiol. 2012, 14, 1474–1484. [Google Scholar] [CrossRef]
- Puthia, M.K.; Sio, S.W.S.; Lu, J.; Tan, K.S.W. Blastocystis ratti induces contact-independent apoptosis, F-actin rearrangement, and barrier function disruption in IEC-6 cells. Infect. Immun. 2006, 74, 4114–4123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirza, H.; Tan, K.S. Blastocystis exhibits inter- and intra-subtype variation in cysteine protease activity. Parasitol. Res. 2009, 104, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Mirza, H.; Teo, J.D.; Tan, K.S. Strain-dependent induction of human enterocyte apoptosis by Blastocystis disrupts epithelial barrier and ZO-1 organization in a caspase 3- and 9-dependent manner. Biomed. Res. Int. 2014, 2014, 209163. [Google Scholar] [CrossRef] [Green Version]
- Solaymani-Mohammadi, S.; Singer, S.M. Giardia duodenalis: The double-edged sword of immune responses in giardiasis. Exp. Parasitol. 2010, 126, 292–297. [Google Scholar] [CrossRef] [Green Version]
- Wiercińska-Drapało, A. Giardiosis—Clinical manifestations, diagnosis and therapy. Gastroenterol. Klin. 2010, 2, 98–102. [Google Scholar]
- Leońska-Duniec, A.; Adamska, M. Biology, epidemiology, and diagnostics of pathogenic waterborne protozoan parasites. Wiad. Parazytol. 2010, 56, 125–132. [Google Scholar]
- Panaro, M.A.; Cianciulli, A.; Mitolo, V.; Mitolo, C.I.; Acquafredda, A.; Brandonisio, O.; Cavallo, P. Caspase-dependent apoptosis of the HCT-epithelial cell line induced by the parasite Giardia intestinalis. FEMS Immunol. Med. Microbiol. 2007, 51, 302–309. [Google Scholar] [CrossRef] [Green Version]
- Buret, A.A.; Mitchell, K.; Muench, D.G.; Scott, K.G. Giardia lamblia disrupts tight junctional ZO-1 and increases permeability in nontransformed human small intestinal epithelilal monolayers: Effects of epidermal growth factor. Parasitology 2002, 125, 11–19. [Google Scholar] [CrossRef]
- Chin, A.C.; Teoh, D.A.; Scott, K.G.; Meddings, J.B.; Macnaughton, W.K.; Buret, A.G. Strain-dependent induction of enterocyte apoptosis by Giardia lamblia disrupts epithelial barrier function in a caspase-3-dependent manner. Infect. Immun. 2002, 70, 3673–3680. [Google Scholar] [CrossRef] [Green Version]
- Roxstrom-Lindquist, K.; Ringqvist, E.; Palm, D.; Svärd, S. Giardia lamblia-induced changes in gene expression in differentiated Caco-2 human intestinal epithelial cells. Infect. Immun. 2005, 73, 8204–8208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troeger, H.; Epple, H.J.; Schneider, T.; Wahnschaffe, U.; Ullrich, R.; Burchard, G.D.; Jelinek, T.; Zeitz, M.; Fromm, M.; Schulzke, J.D. Effect of chronic Giardia lamblia infection on epithelial transport and barrier function in human duodenum. Gut 2007, 56, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Cevallos, A.M.; Carnaby, S.; James, M.; Farthing, J.G. Small intestinal injury in a neonatal rat model of giardiasis is strain-dependent. Gastroenterology 1995, 109, 766–773. [Google Scholar] [CrossRef]
- Bojar, H.; Kłapeć, T. Water as a potential source of human and animal infection with protozoa of the genera Cryptosporidium and Giardia. MONZ 2011, 17, 45–51. [Google Scholar]
- Rożej, W.; Gołąb, E.; Waloch, M.; Wąsik, M.; Sadkowska-Todys, M.; Czeriński, M.; Dzbeński, T.H. The occurrence of cryptosporidium in a group of children and adults with diarrhoea of undetermined earlier aetiology. Prz. Epidemiol. 2010, 64, 35–39. [Google Scholar]
- Wesołowska, M.; Gąsiorowski, J.; Jankowski, S. The opportunistic protozoa in immunocompromised individuals. Adv. Clin. Exp. Med. 2005, 14, 349–355. [Google Scholar]
- Buret, A.G.; Chin, A.C.; Scott, K.G. Infection of human and bovine epithelial cells with Cryptosporidium andersoni induces apoptosis and disrupts tight junctional ZO-1: Effects of epidermal growth factor. Int. J. Parasitol. 2003, 33, 1363–1371. [Google Scholar] [CrossRef]
- Chen, X.M.; Gores, G.J.; Paya, C.V.; LaRusso, N.F. Cryptosporidium parvum induces apoptosis in biliary epithelia by a Fas/Fas ligand-dependent mechanism. Am. J. Physiol. 1999, 277, G599–G608. [Google Scholar] [CrossRef]
- McCole, D.F.; Eckmann, L.; Laurent, F.; Kagnoff, M.F. Intestinal epithelial cell apoptosis following Cryptosporidium parvum infection. Infect. Immun. 2000, 68, 1710–1713. [Google Scholar] [CrossRef] [Green Version]
- Mele, R.; Gomez Morales, MA.; Tosini, F.; Pozio, F. Cryptosporidium parvum at different developmental stages modulates host cell apoptosis in vitro. Infect. Immun. 2004, 72, 6061–6067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojcius, D.M.; Perfettini, J.L.; Bonnin, A.; Laurent, F. Caspase-dependent apoptosis during infection with Cryptosporidium parvum. Microbes Infect. 1999, 1, 1163–1168. [Google Scholar] [CrossRef]
- Sasahara, T.; Maruyama, H.; Aoki, M.; Kikuno, R.; Sekiguchi, T.; Takahashi, A.; Satoh, Y.; Kitasato, H.; Takayama, Y.; Inoue, M. Apoptosis of intestinal crypt epithelium after Cryptosporidium parvum infection. J. Infect. Chemother. 2003, 9, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Enomoto, S.; Lancto, C.A.; Abrahamsen, M.S.; Rutherford, M.S. Inhibition of apoptosis in Cryptosporidium parvum-infected intestinal epithelial cells is dependent on survivin. Infect. Immun. 2008, 76, 3784–3792. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Deng, M.; Lancto, C.A.; Abrahamsen, M.S.; Rutherford, M.S.; Enomoto, S. Biphasic modulation of apoptotic pathways in Cryptosporidium parvum-infected human intestinal epithelial cells. Infect. Immun. 2009, 77, 837–849. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Chatterjee, I.; Anbazhagan, A.N.; Jayawardena, D.; Priyamvada, S.; Alrefai, W.A.; Sun, J.; Borthakur, A.; Dudeja, P.K. Cryptosporidium parvum disrupts intestinal epithelial barrier function via altering expression of key tight junction and adherens junction proteins. Cell. Microbiol. 2018, 20, e12830. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, L.; Zhu, H.; Zhang, L.; Wang, R.; Zhang, Z.; Huang, J.; Zhang, S.; Jian, F.; Ning, C.; et al. MicroRNA expression profile of HCT-8 cells in the early phase of Cryptosporidium parvum infection. BMC Genom. 2019, 20, 37. [Google Scholar] [CrossRef] [Green Version]
- Ghai, R.R.; Simons, N.D.; Chapman, C.A.; Omeja, P.A.; Davies, T.J.; Ting, N.; Goldberg, T.L. Hidden Population Structure and Cross-species Transmission of Whipworms (Trichuris sp.) in Humans and Non-human Primates in Uganda. PLoS Negl. Trop. Dis. 2014, 8, e3256. [Google Scholar] [CrossRef] [Green Version]
- Phosuk, I.; Sanpool, O.; Thanchomnang, T.; Sadaow, L.; Rodpai, R.; Anamnart, W.; Janwan, P.; Wijit, A.; Laymanivong, S.; Pa Aung, W.P.; et al. Molecular Identification of Trichuris suis and Trichuris trichiura Eggs in Human Populations from Thailand, Lao PDR, and Myanmar. Am. J. Trop. Med. Hyg. 2018, 98, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Zhao, B.; Hoberg, E.P.; Li, M.; Zhou, X.; Gu, X.; Lai, W.; Peng, X.; Yang, G. Genetic characterisation and phylogenetic status of whipworms (Trichuris spp.) from captive non-human primates in China, determined by nuclear and mitochondrial sequencing. Parasites Vectors 2018, 11, 516. [Google Scholar] [CrossRef]
- Hasnain, S.Z.; Thornton, D.J.; Grencis, R.K. Changes in the mucosal barrier during acute and chronic Trichuris muris infection. Parasite Immunol. 2011, 33, 45–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cliffe, L.J.; Potten, C.S.; Booth, C.E.; Grencis, R.K. An Increase in Epithelial Cell Apoptosis Is Associated with Chronic Intestinal Nematode infection. Infect. Immun. 2007, 75, 1556–1564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cliffe, L.J.; Humphreys, N.E.; Lane, T.E.; Potten, C.S.; Booth, C.; Grencis, R.K. Accelerated intestinal epithelial cell turnover: A new mechanism of parasite expulsion. Science 2005, 308, 1463–1465. [Google Scholar] [CrossRef] [PubMed]
- Levison, S.E.; Mc Laughlin, J.T.; Zeef, L.A.; Fisher, P.; Grencis, R.K.; Pennock, J.L. Colonic transcriptional profiling in resistance and susceptibility to trichuriasis: Phenotyping a chronic colitis and lessons for iatrogenic helminthosis. Inflamm. Bowel Dis. 2010, 16, 2065–2079. [Google Scholar] [CrossRef] [PubMed]
- Hayes, K.S.; Bancroft, A.J.; Grencis, R.K. The role of TNF-alpha in Trichuris muris infection I: Influence of TNF-alpha receptor usage, gender, and IL-13. Parasite Immunol. 2007, 29, 575–582. [Google Scholar] [CrossRef]
- Hayes, K.S.; Cliffe, L.J.; Bancroft, A.J.; Forman, S.P.; Thompson, S.; Booth, C.; Grencis, R.K. Chronic Trichuris muris infection causes neoplastic change in the intestine and exacerbates tumour formation in APC min/+ mice. PLoS Negl. Trop. Dis. 2017, 11, e0005708. [Google Scholar] [CrossRef] [Green Version]
- Boettner, D.R.; Petri, W.A. Entamoeba histolytica activates host cell caspases during contact-dependent cell killing. Curr. Top. Microbiol. Immunol. 2005, 289, 175–184. [Google Scholar] [CrossRef]
- Kantor, M.; Abrantes, A.; Estevez, A.; Schiller, A.; Torrent, J.; Gascon, J.; Hernandez, R.; Ochner, C. Entamoeba histolytica: Updates in Clinical Manifestation, Pathogenesis, and Vaccine Development. Can. J. Gastroenterol. Hepatol. 2018, 2018, 4601420. [Google Scholar] [CrossRef] [Green Version]
- Stanley, S.L., Jr. Amoebiasis. Lancet 2003, 361, 1025–1034. [Google Scholar] [CrossRef]
- Tanyuksel, M.; Petri, W.A., Jr. Laboratory diagnosis of amebiasis. Clin. Microbiol. 2003, 16, 713–729. [Google Scholar] [CrossRef] [Green Version]
- El-Ashram, S.; Al Nasr, I.; Mehmood, R.; Çağdaş, D.; Hu, M.; He, L.; Suo, X. Cell death offers exceptional cellular and molecular windows for pharmacological interventions in protozoan parasites. Integr. Mol. Med. 2017, 4, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Huston, C.D.; Houpt, E.R.; Mann, B.J.; Hahn, C.S.; Petri, W.A., Jr. Caspase 3-dependent killing of host cells by the parasite Entamoeba histolytica. Cell. Microbiol. 2000, 2, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Ragland, B.D.; Ashley, L.S.; Vaux, D.L.; Petri, W.A., Jr. Entamoeba histolytica: Target cells killed by trophozoites undergo DNA fragmentation which is not blocked by Bcl-2. Exp. Parasitol. 1994, 79, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Seydel, K.B.; Stanley, S.L., Jr. Entamoeba histolytica induces host cell death in amebic liver abscess by a non-Fas-dependent, non-tumor necrosis factor alpha-dependent pathway of apoptosis. Infect. Immun. 1998, 66, 2980–2983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, S.M.; Cho, K.N.; Guo, X.; Fendig, K.; Oosman, M.N.; Whitehead, R.; Cohn, S.M.; Houpt, E.R. Epithelial cell apoptosis facilitates Entamoeba histolytica infection in the gut. Am. J. Pathol. 2010, 176, 1316–1322. [Google Scholar] [CrossRef]
- Stadnyk, A.W.; McElroy, P.J.; Gauldie, J.; Befus, A.D. Characterization of Nippostrongylus brasiliensis infection in different strains of mice. J. Parasitol. 1990, 76, 377–382. [Google Scholar] [CrossRef]
- Mercer, J.G.; Mitchell, P.I.; Moar, K.M.; Bissett, A.; Geissler, S.; Bruce, K.; Chappell, L.H. Anorexia in rats infected with the nematode, Nippostrongylus brasiliensis: Experimental manipulations. Parasitology 2000, 120, 641–647. [Google Scholar] [CrossRef]
- Camberis, M.; Le Gros, G.; Urban, J., Jr. Animal model of Nippostrongylus brasiliensis and Heligmosomoides polygyrus. Curr. Protoc. Immunol. 2003, 19, 12. [Google Scholar] [CrossRef]
- Marsland, B.J.; Kurrer, M.; Reissmann, R.; Harris, N.L.; Kopf, M. Nippostrongylus brasiliensis infection leads to the development of emphysema associated with the induction of alternatively activated macrophages. Eur. J. Immunol. 2008, 38, 479–488. [Google Scholar] [CrossRef]
- Swain, K.; Routray, A.; Panigrahi, S.; Prakash, A.R.; Sahoo, S.; Ganguly, S. Nippostrongylus Brasiliensis, an Experimental Model: A Review. IJCP 2016, 2, 2. [Google Scholar] [CrossRef]
- Hyoh, Y.; Nishida, M.; Tegoshi, T.; Yamada, M.; Uchikawa, R.; Matsuda, S.; Arizono, N. Enhancement of apoptosis with loss of cellular adherence in the villus epithelium of the small intestine after infection with the nematode Nippostrongylus brasiliensis in rats. Parasitology 1999, 119, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Healer, J.; Ashall, F.; Maizels, R.M. Characterization of proteolytic enzymes from larval and adult Nippostrongylus brasiliensis. Parasitology 1991, 103, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, M.; Yamada, M.; Uchikawa, R.; Arizono, N. Immunocytochemical localization of secretory acetylcholinesterase of the parasitic nematode Nippostrongylus brasiliensis. Cell Tissue Res. 1995, 280, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, A.; Uchikawa, R.; Matsuda, S.; Yamada, M.; Tegoshi, T.; Arizono, N. Up-regulation of Fas (CD95) and induction of apoptosis in intestinal epithelial cells by nematode-derived molecules. Infect. Immun. 2002, 70, 4002–4008. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, L.A.; Filbey, K.J.; Maizels, R.M. Immunity to the model intestinal helminth parasite Heligmosomoides polygyrus. Semin. Immunopathol. 2012, 34, 829–846. [Google Scholar] [CrossRef] [Green Version]
- Rzepecka, J.; Lucius, R.; Doligalska, M.; Beck, S.; Rausch, S.; Hartmann, S. Screening for immunomodulatory proteins of the intestinal parasitic nematode Heligmosomoides polygyrus. Parasite Immunol. 2006, 28, 463–472. [Google Scholar] [CrossRef]
- Doligalska, M.; Donskow-Schmelter, K.; Rzepecka, J.; Drela, N. Reduced apoptosis in BALB/c mice infected with Heligmosomoides polygyrus. Parasite Immunol. 2007, 29, 283–291. [Google Scholar] [CrossRef]
- Donskow-Schmelter, K.; Doligalska, M.; Rzepecka, J.; Jedlina-Panasiuk, L. Heligmosomoides polygyrus: Decreased apoptosis in fast responder FVB mice during infection. Exp. Parasitol. 2007, 117, 149–156. [Google Scholar] [CrossRef]
- Doligalska, M.; Brodaczewska, K.; Donskow-Łysoniewska, K. The antiapoptotic activity of Heligmosomoides polygyrus antigen fractions. Parasite Immunol. 2012, 34, 589–603. [Google Scholar] [CrossRef]
- Donskow-Łysoniewska, K.; Brodaczewska, K.; Doligalska, M. Heligmosomoides polygyrus antigens inhibit the intrinsic pathway of apoptosis by overexpression of survivin and Bcl-2 protein in CD4 T cells. Prion 2013, 7, 319–327. [Google Scholar] [CrossRef] [Green Version]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapczuk, P.; Kosik-Bogacka, D.; Kupnicka, P.; Metryka, E.; Simińska, D.; Rogulska, K.; Skórka-Majewicz, M.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. The Influence of Selected Gastrointestinal Parasites on Apoptosis in Intestinal Epithelial Cells. Biomolecules 2020, 10, 674. https://doi.org/10.3390/biom10050674
Kapczuk P, Kosik-Bogacka D, Kupnicka P, Metryka E, Simińska D, Rogulska K, Skórka-Majewicz M, Gutowska I, Chlubek D, Baranowska-Bosiacka I. The Influence of Selected Gastrointestinal Parasites on Apoptosis in Intestinal Epithelial Cells. Biomolecules. 2020; 10(5):674. https://doi.org/10.3390/biom10050674
Chicago/Turabian StyleKapczuk, Patrycja, Danuta Kosik-Bogacka, Patrycja Kupnicka, Emilia Metryka, Donata Simińska, Karolina Rogulska, Marta Skórka-Majewicz, Izabela Gutowska, Dariusz Chlubek, and Irena Baranowska-Bosiacka. 2020. "The Influence of Selected Gastrointestinal Parasites on Apoptosis in Intestinal Epithelial Cells" Biomolecules 10, no. 5: 674. https://doi.org/10.3390/biom10050674
APA StyleKapczuk, P., Kosik-Bogacka, D., Kupnicka, P., Metryka, E., Simińska, D., Rogulska, K., Skórka-Majewicz, M., Gutowska, I., Chlubek, D., & Baranowska-Bosiacka, I. (2020). The Influence of Selected Gastrointestinal Parasites on Apoptosis in Intestinal Epithelial Cells. Biomolecules, 10(5), 674. https://doi.org/10.3390/biom10050674






