Connexin-46 Contained in Extracellular Vesicles Enhance Malignancy Features in Breast Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Extracellular Vesicles Isolation
2.3. Western Blot
2.4. Nanoparticle Tracking Analysis (NTA)
2.5. Transmission Electron Microscopy (TEM)
2.6. Membrane Labeling of EVs
2.7. Microscopy Imaging of EV Uptake
2.8. Extracellular Vesicles DNA Loading
2.9. Measurement of EVs Uptake
2.10. Migration Assay
2.11. Transwell Invasion Assay
2.12. Statistical Analysis
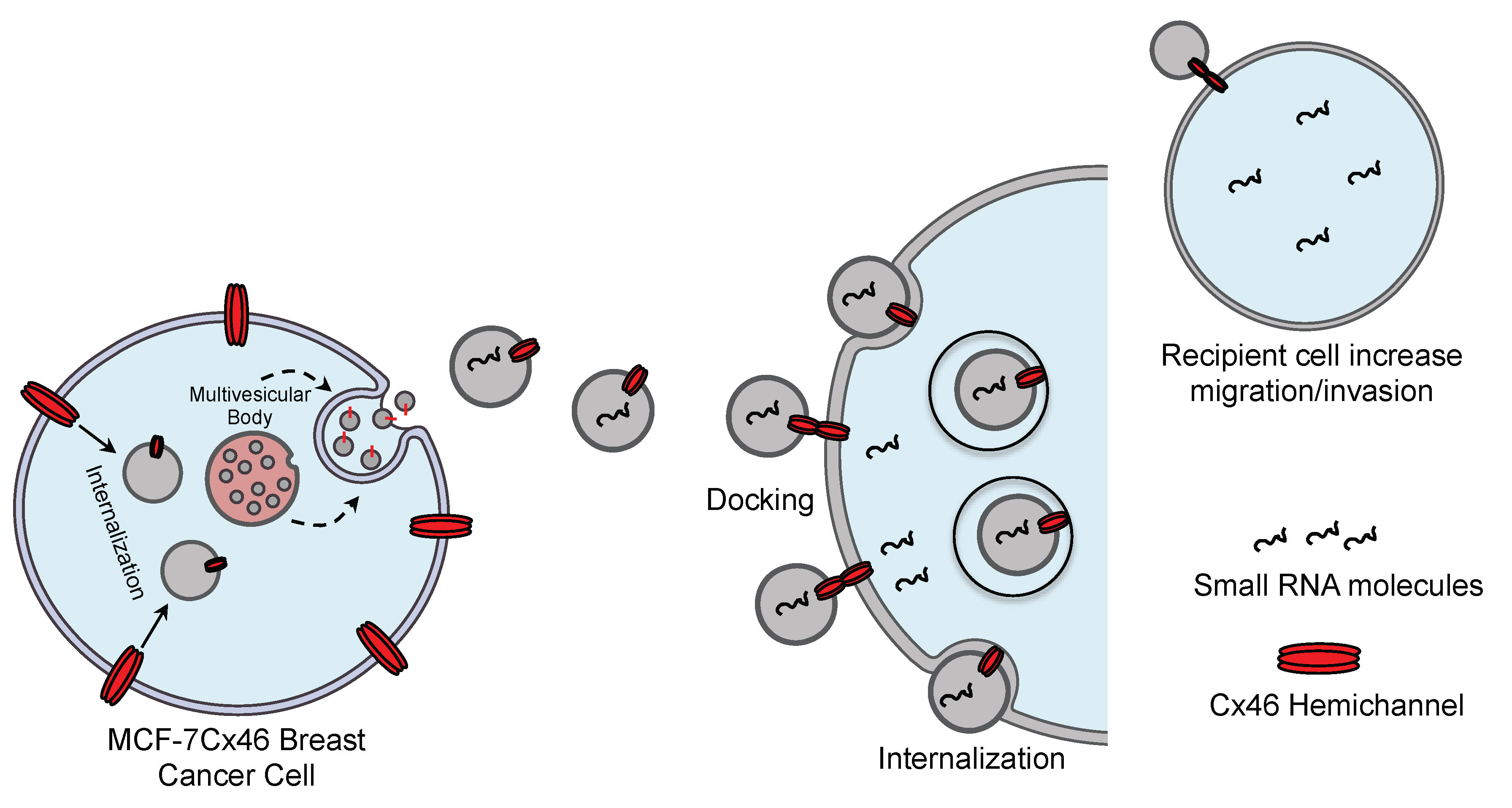
3. Results
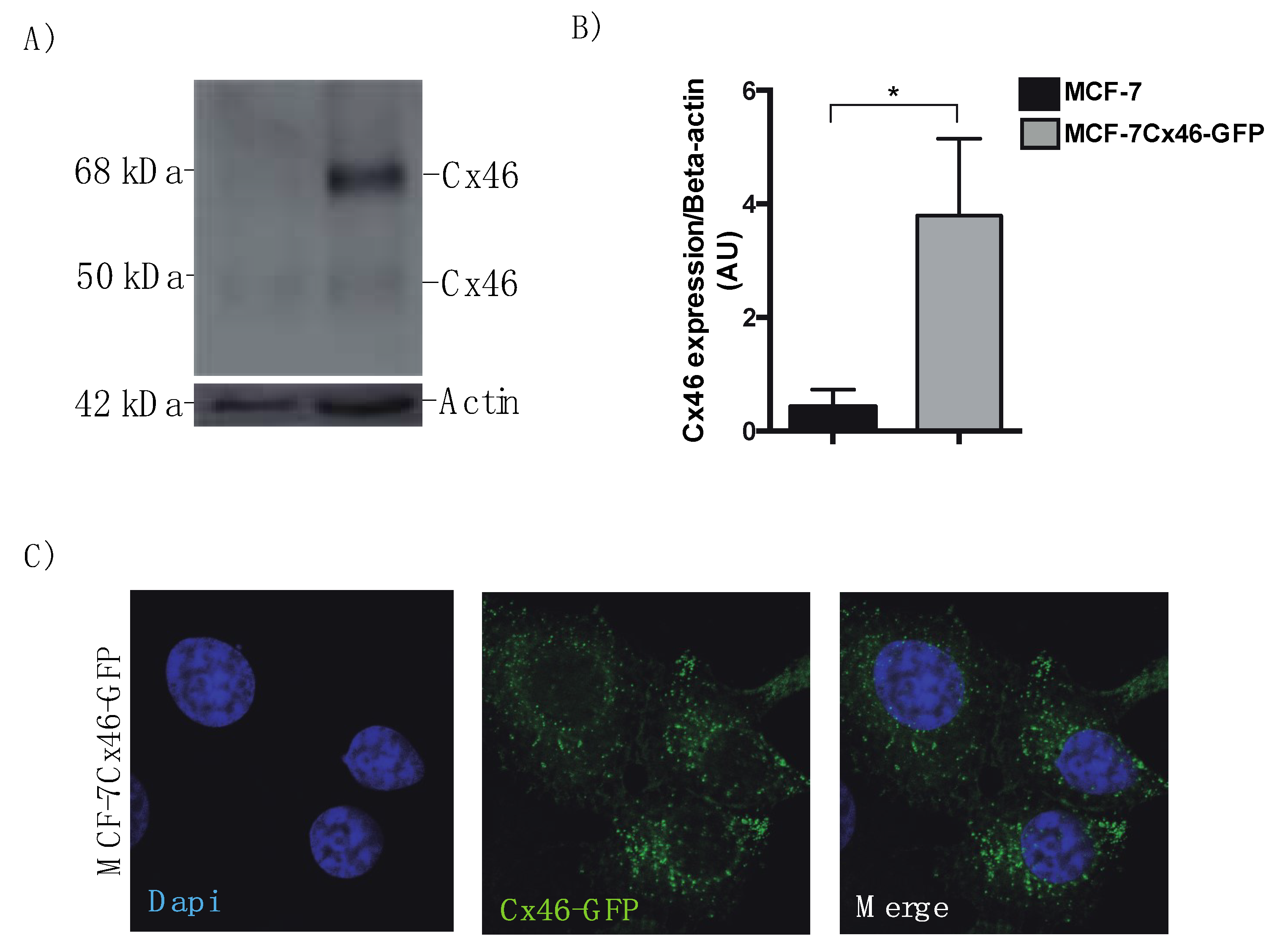
3.1. Cx46 is Contained in EVs Released from MCF-7 Cells Overexpressing Cx46
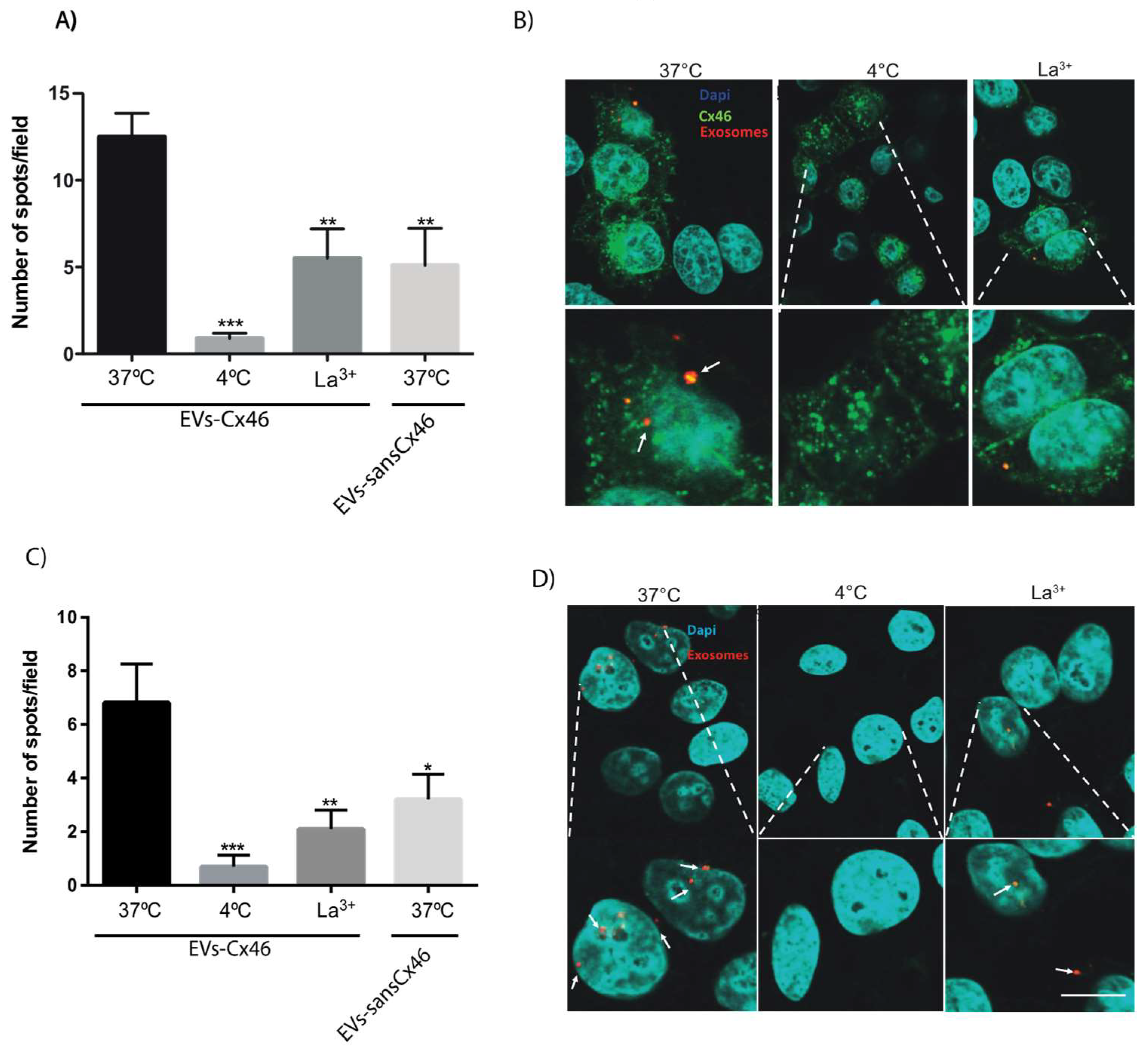
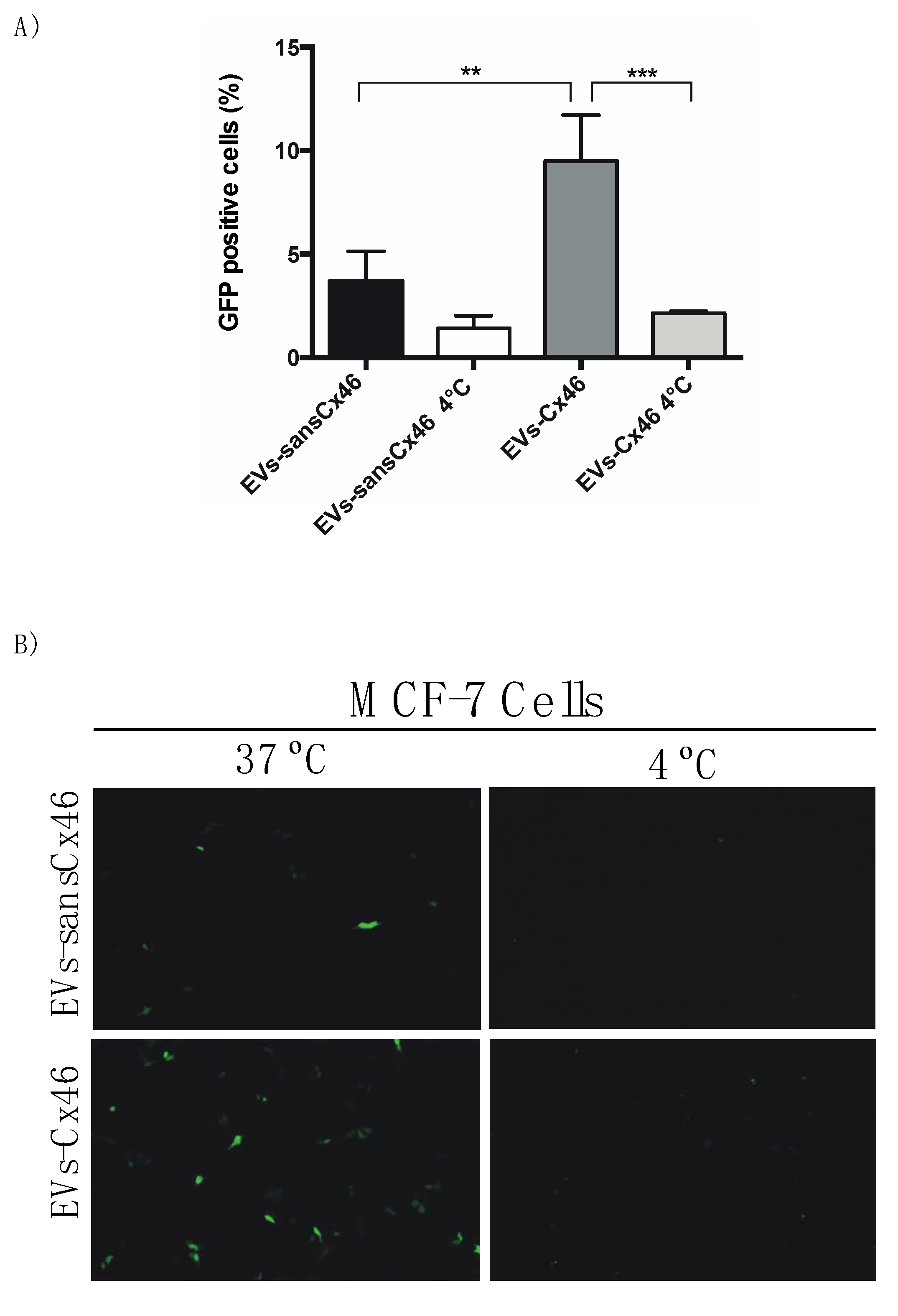
3.2. The Presence of Cx46 on EVs Facilitates its Interaction/Internalization and Delivery of DNA in the Recipient Cell
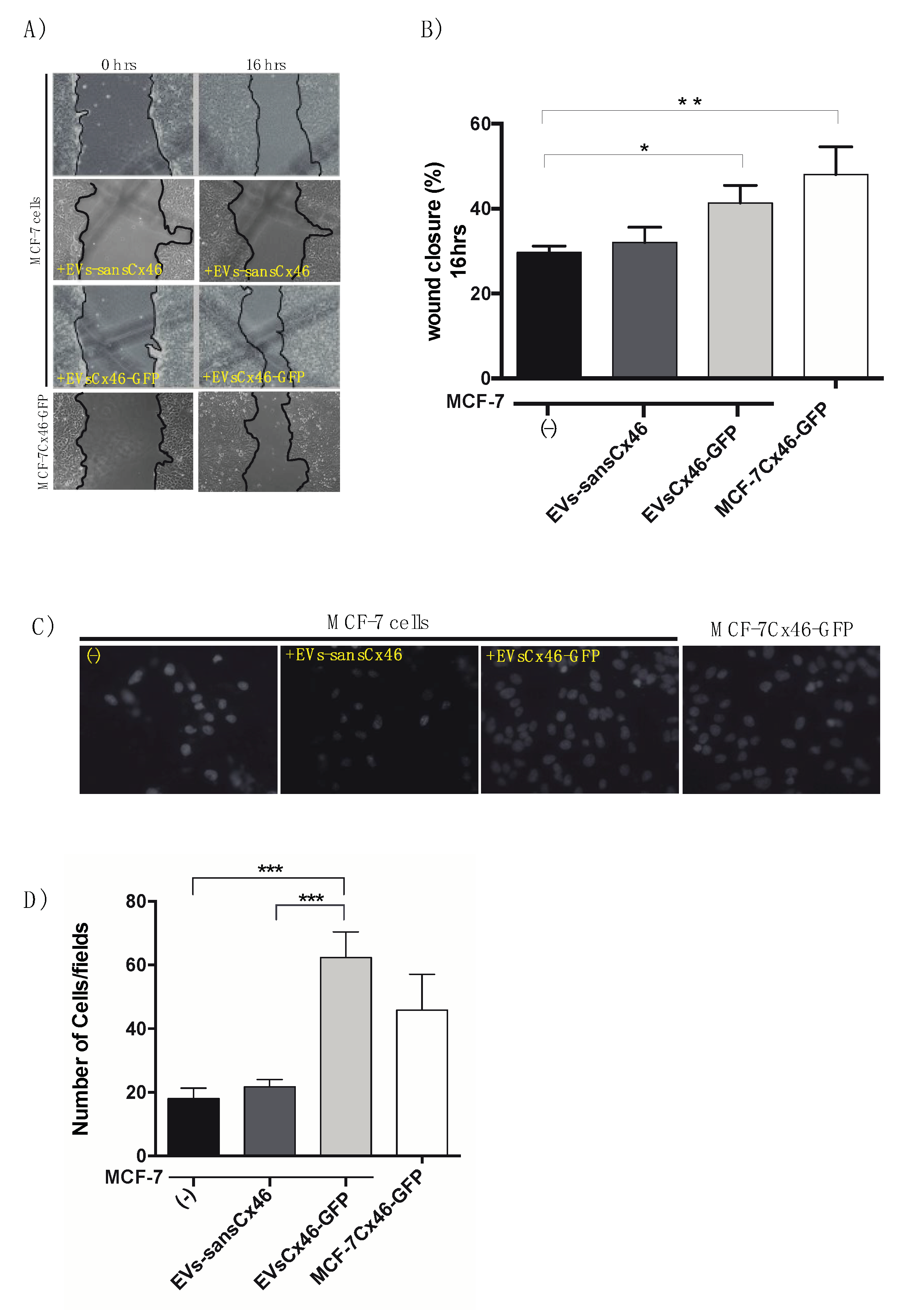
3.3. EVs Containing Cx46 increase Migration/Invasion in MCF-7 Recipient Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saez, J.; Berthoud, V.M.; Mar, I.; Beyer, E.C. Plasma Membrane Channels Formed by Connexins: Their Regulation and Functions. Physiol. Rev. 2003, 83, 1359–1400. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.H. Cell communication across gap junctions: A historical perspective and current developments. Biochem. Soc. Trans. 2015, 43, 450–459. [Google Scholar] [CrossRef]
- Söhl, G.; Willecke, K. Gap junctions and the connexin protein family. Cardiovasc. Res. 2004, 62, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, D.A.; Paul, D.L. Gap junctions. Cold Spring Harb. Perspect. Biol. 2009, 1, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Aasen, T.; Mesnil, M.; Naus, C.C.; Lampe, P.D.; Laird, D.W. Gap junctions and cancer: Communicating for 50 years. Nat. Rev. Cancer 2016, 16, 775–788. [Google Scholar] [CrossRef]
- Oyamada, M.; Takebe, K.; Oyamada, Y. Regulation of connexin expression by transcription factors and epigenetic mechanisms. Biochim. Biophys. Acta Biomembr. 2013, 1828, 118–133. [Google Scholar] [CrossRef]
- Pfenniger, A.; Wohlwend, A.; Kwak, B.R. Mutations in connexin genes and disease. 2010, 41, 103–116. [Google Scholar] [CrossRef]
- Dobrowolski, R.; Willecke, K. Klaus Connexin-Caused Genetic Diseases and Corresponding Mouse Models. Antioxid. Redox Signal. 2009, 11, 283–296. [Google Scholar] [CrossRef]
- Loewenstein, W.; Kanno, Y. Intercellular Communication and Control of Tissue Growth-Lack of Communication Between Cancer Cells. Nature 1966, 209, 1248–1249. [Google Scholar] [CrossRef]
- Yamasaki, H. Aberrant expression and function of gap junctions during carcinogenesis. Environ. Health Perspect. 1991, 93, 191–197. [Google Scholar] [CrossRef]
- Hitomi, M.; Deleyrolle, L.P.; Mulkearns-Hubert, E.E.; Jarrar, A.; Li, M.; Sinyuk, M.; Otvos, B.; Brunet, S.; Flavahan, W.A.; Hubert, C.G.; et al. Differential Connexin Function Enhances Self-Renewal in Glioblastoma. Cell Rep. 2015, 11, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Gakhar, G.; Madgwick, D.; Hurt, A.; Takemoto, D.; Nguyen, T.A. A novel role of gap junction connexin46 protein to protect breast tumors from hypoxia. Int. J. Cancer 2010, 127, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D. Connexin’s connection in breast cancer growth and progression. Int. J. Cell Biol. 2016, 2016, 9025905. [Google Scholar] [CrossRef] [PubMed]
- Teleki, I.; Szasz, A.M.; Maros, M.E.; Gyorffy, B.; Kulka, J.; Meggyeshazi, N.; Kiszner, G.; Balla, P.; Samu, A.; Krenacs, T. Correlations of Differentially Expressed Gap Junction Connexins Cx26, Cx30, Cx32, Cx43 and Cx46 with Breast Cancer Progression and Prognosis. PLoS ONE 2014, 9, e112541. [Google Scholar] [CrossRef]
- Teleki, I.; Krenacs, T.; Szasz, M.A.; Kulka, J.; Wichmann, B.; Leo, C. The potential prognostic value of connexin 26 and 46 expression in neoadjuvant-treated breast cancer. BMC Cancer 2013, 13, 50. [Google Scholar] [CrossRef]
- Mesnil, M. Connexins and cancer. Biol. Cell 2002, 94, 493–500. [Google Scholar] [CrossRef]
- Naus, C.C.; Laird, D.W. Implications and challenges of connexin connections to cancer. Nat. Rev. Cancer 2010, 10, 435–441. [Google Scholar] [CrossRef]
- Grek, C.L.; Rhett, J.M.; Bruce, J.S.; Ghatnekar, G.S.; Yeh, E.S. Connexin 43, breast cancer tumor suppressor: Missed connections? Cancer Lett. 2016, 374, 117–126. [Google Scholar] [CrossRef]
- Tittarelli, A.; Guerrero, I.; Tempio, F.; Gleisner, M.A.; Avalos, I.; Sabanegh, S.; Ortı, C. Overexpression of connexin 43 reduces melanoma proliferative and metastatic capacity. Br. J. Cancer 2015, 113, 259–267. [Google Scholar] [CrossRef]
- Soares, A.R.; Martins-marques, T.; Ribeiro-rodrigues, T.; Ferreira, J.V.; Catarino, S.; Pinho, M.J.; Zuzarte, M.; Anjo, S.I.; Manadas, B.; Sluijter, J.P.G.; et al. Gap junctional protein Cx43 is involved in the communication between extracellular vesicles and mammalian cells. Sci. Rep. 2015, 5, 1–13. [Google Scholar]
- Raposo, G.; Stoorvogel, W.; Tong, W.; Tong, G.; Liu, Y.; Mesnil, M.; Aasen, T.; Boucher, J.; Chépied, A.; Cronier, L.; et al. Extracellular vesicles: Exosomes, microvesicles, and friends. BBA Biomembr. 2018, 1860, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 41–68. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; el Andaloussi, S.; Wood, M.J.a. Exosomes and microvesicles: Extracellular vesicles for genetic information transfer and gene therapy. Hum. Mol. Genet. 2012, 21, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Pegtel, D.M.; Cosmopoulos, K.; Thorley-Lawson, D.a.; van Eijndhoven, M.a.J.; Hopmans, E.S.; Lindenberg, J.L.; de Gruijl, T.D.; Würdinger, T.; Middeldorp, J.M. Functional delivery of viral miRNAs via exosomes. Proc. Natl. Acad. Sci. USA 2010, 107, 6328–6333. [Google Scholar] [CrossRef] [PubMed]
- Couto, N.; Galán, S.C.; Maia, J.; Moraes, M.C.S.; Costa-Silva, B. Exosomes as emerging players in cancer biology. Biochimie 2018, 155, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.; Wildman, D.E. Extracellular Vesicles and the Promise of Continuous Liquid Biopsies. J. Pathol. Trans. Med. 2018, 52, 1–8. [Google Scholar] [CrossRef]
- Bebelman, M.P.; Smit, M.J.; Pegtel, D.M.; Baglio, S.R. Biogenesis and function of extracellular vesicles in cancer. Pharmacol. Ther. 2018, 188, 1–11. [Google Scholar] [CrossRef]
- Yousef, M.H.; Abdelnaser, A. Exosomes: Biological Couriers with Transformative Messages. J. Biomed. 2019, 4, 14–34. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Raul, D.; Carter, F. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 1, 1–14. [Google Scholar] [CrossRef]
- Kahlert, C.; Kalluri, R. Exosomes in Tumor Microenvironment Influence Cancer Progression and Metastasis. J. Mol. Med. 2014, 91, 431–437. [Google Scholar] [CrossRef]
- Azmi1, F.H.S.A.S.; Bao, B. Exosomes in Cancer Development, Metastasis and Drug Resistance: A Comprehensive Review. Cancer Metastasis Rev. 2014, 32, 623–642. [Google Scholar] [CrossRef] [PubMed]
- Maia, J.; Caja, S.; Moraes, M.C.S.; Couto, N.; Costa-Silva, B. Exosome-Based Cell-Cell Communication in the Tumor Microenvironment. Front. Cell Dev. Biol. 2018, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Cappello, F.; Logozzi, M.; Campanella, C.; Bavisotto, C.C.; Marcilla, A.; Properzi, F.; Fais, S. Exosome levels in human body fluids: A tumor marker by themselves? Eur. J. Pharm. Sci. 2016, 96, 93–98. [Google Scholar] [CrossRef]
- Valencia, K.; Luis-Ravelo, D.; Bovy, N.; Antón, I.; Martínez-Canarias, S.; Zandueta, C.; Ormazábal, C.; Struman, I.; Tabruyn, S.; Rebmann, V.; et al. MiRNA cargo within exosome-like vesicle transfer influences metastatic bone colonization. Mol. Oncol. 2014, 8, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Donnarumma, E.; Fiore, D.; Nappa, M.; Roscigno, G.; Adamo, A.; Iaboni, M.; Russo, V.; Affinito, A.; Puoti, I.; Quintavalle, C.; et al. Cancer-associated fibroblasts release exosomal microRNAs that dictate an aggressive phenotype in breast cancer. Oncotarget 2017, 8, 19592–19608. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Fedele, C.; Lu, H.; Nevalainen, M.; Keen, J.; Languino, L.R. Exosome-mediated Transfer of αv β3 Integrin from Tumorigenic to Non-Tumorigenic Cells Promotes a Migratory Phenotype. Mol. Cancer Res. 2017, 14, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Fornaro, M.; Manes, T.; Languino, L.R. Integrins and prostate cancer metastases. Cancer Metastasis Rev. 2001, 20, 321–331. [Google Scholar] [CrossRef]
- Mathias, R.T.; White, T.W.; Gong, X. Lens Gap Junctions in Growth, Differentiation, and Homeostasis. Physiol. Rev. 2010, 90, 179–206. [Google Scholar] [CrossRef]
- Koval, M.; Harley, J.E.; Hick, E.; Steinberg, T.H. Connexin46 Is Retained as Monomers in a trans-Golgi Compartment of Osteoblastic Cells. J. Cell Biol. 1997, 137, 847–857. [Google Scholar] [CrossRef]
- Abraham, V.; Chou, M.L.; George, P.; Pooler, P.; Zaman, A.; Savani, R.C.; Koval, M.; Chou, M.L.; Pooler, P.; Zaman, A.; et al. Heterocellular gap junctional communication between alveolar epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2001, 280, 1085–1093. [Google Scholar] [CrossRef]
- Chi, N.C.; Bussen, M.; Brand-arzamendi, K.; Ding, C.; Olgin, J.E. Cardiac conduction is required to preserve cardiac chamber morphology. Proc. Natl. Acad. Sci. USA 2010, 107, 14662–14667. [Google Scholar] [CrossRef] [PubMed]
- Dermietzel, R.; Gao, Y.; Scemes, E.; Vieira, D.; Urban, M.; Kremer, M.; Bennett, M.V.L.; Spray, D.C. Connexin43 null mice reveal that astrocytes express multiple connexins. Brain Res. Rev. 2000, 32, 45–56. [Google Scholar] [CrossRef]
- Mackay, D.; Ionides, A.; Kibar, Z.; Rouleau, G.; Berry, V.; Moore, A.; Shiels, A.; Bhattacharya, S. Connexin46 mutations in autosomal dominant congenital cataract. Am. J. Hum. Genet. 1999, 64, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Pal, J.A.Y.D.; Liu, X.; Mackay, D.; Shiels, A.; Berthoud, V.M.; Beyer, E.C.; Ebihara, L.; Jay, D.; Liu, X.; Mackay, D.; et al. Connexin46 mutations linked to congenital cataract show loss of gap junction channel function. Am. J. Physiol. Cell Physiol. 2000, 279, 596–602. [Google Scholar] [CrossRef]
- Ebihara, L.; Tong, J.; Vertel, B.; White, T.W.; Chen, T. Properties of Connexin 46 Hemichannels in Dissociated Lens Fiber Cells. Investig. Ophthalmol. Visual. Sci. 2011, 52, 882–889. [Google Scholar] [CrossRef]
- Mulkearns-hubert, E.E.; Torre-healy, L.A.; Silver, D.J.; Eurich, J.T.; Bayik, D.; Serbinowski, E.; Hitomi, M.; Zhou, J.; Zhang, R.; Sprowls, S.A.; et al. Development of a Cx46 Targeting Strategy for Cancer Stem Cells. Cell Rep. 2019, 27, 1062–1072. [Google Scholar] [CrossRef]
- Beyer, E.C.; Berthoud, V.M. Connexin hemichannels in the lens. Front. Physiol. 2014, 5, 1–11. [Google Scholar] [CrossRef]
- Burr, D.B.; Molina, S.A.; Banerjee, D.; Low, D.M.; Takemoto, D.J. Treatment with connexin 46 siRNA suppresses the growth of human Y79 retinoblastoma cell xenografts in vivo. Exp. Eye Res. 2011, 92, 251–259. [Google Scholar] [CrossRef]
- Schneider, C.a.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Bonacquisti, E.E.; Nguyen, J. Connexin 43 (Cx43) in cancer: Implications for therapeutic approaches via gap junctions. Cancer Lett. 2018, 442, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Martins-Marques, T.; Pinho, M.J.; Zuzarte, M.; Oliveira, C.; Pereira, P.; Sluijter, J.P.G.; Gomes, C.; Girao, H. Presence of Cx43 in extracellular vesicles reduces the cardiotoxicity of the anti-tumour therapeutic approach with doxorubicin. J. Extracell. Vesicles 2016, 5, 32538. [Google Scholar] [CrossRef] [PubMed]
- Thierry; Théxry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and Characterization of Exosomes from Cell Culture Supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 3, 1–29. [Google Scholar]
- Mathivanan, S.; Fahner, C.J.; Reid, G.E.; Simpson, R.J. ExoCarta 2012: Database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012, 40, 1241–1244. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA. 2016, 113, E968–E977. [Google Scholar] [CrossRef]
- Retamal, M.A.; Evangelista-Martínez, F.; León-Paravic, C.G.; Altenberg, G.A.; Reuss, L. Biphasic effect of linoleic acid on connexin 46 hemichannels. Pflügers Arch. Eur. J. Physiol. 2011, 461, 635–643. [Google Scholar] [CrossRef][Green Version]
- Pijuan, J.; Barceló, C.; Moreno, D.F.; Maiques, O.; Sisó, P.; Marti, R.M.; Macià, A.; Panosa, A. In vitro cell migration, invasion, and adhesion assays: From cell imaging to data analysis. Front. Cell Dev. Biol. 2019, 7, 1–16. [Google Scholar] [CrossRef]
- Rodríguez, M.; Silva, J.; Herrera, A.; Herrera, M.; Peña, C.; Martín, P.; Gil-Calderón, B.; Larriba, M.J.; Coronado, M.J.; Soldevilla, B.; et al. Exosomes enriched in stemness/metastatic-related mRNAS promote oncogenic potential in breast cancer. Oncotarget 2015, 6, 40575–40587. [Google Scholar] [CrossRef]
- Fais, S.; Driscoll, L.O.; Borras, F.E.; Buzas, E.; Camussi, G.; Cappello, F.; Carvalho, J.; Cordeiro, A.; del Portillo, H.; el Andaloussi, S.; et al. Evidence-Based Clinical Use of Nanoscale Extracellular Vesicles in Nanomedicine. ACS Nano 2016, 10, 3886–3899. [Google Scholar] [CrossRef]
- van Niel, G.; Angelo, G.D.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213. [Google Scholar] [CrossRef]
- Boulanger, C.; Editor, G.; Coumans, F.A.W.; Brisson, A.R.; Buzas, E.I.; Dignat-george, F.; Drees, E.E.E.; El-andaloussi, S.; Emanueli, C.; Gasecka, A.; et al. Methodological Guidelines to Study Extracellular Vesicles. Circ. Res. 2007, 1632–1648. [Google Scholar]
- Shao, C.; Yang, F.; Miao, S.; Liu, W.; Wang, C.; Shu, Y. Role of hypoxia-induced exosomes in tumor biology. Mol. Cancer 2018, 19, 213. [Google Scholar] [CrossRef]
- King, H.W.; Michael, M.Z.; Gleadle, J.M. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer 2012, 12, 421. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, X.; Yao, Q.; Liu, Y.; Zhang, H.; Dong, Z. HIF-1-mediated production of exosomes during hypoxia is protective in renal tubular cells. Am. J. Physiol. Ren. Physiol. 2017, 313, F906–F913. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Zhang, T.; Zheng, M.; Liu, Y.; Chen, Z. Exosomal proteins as potential markers of tumor diagnosis. J. Hematol. Oncol. 2017, 10, 175. [Google Scholar] [CrossRef] [PubMed]
- Gemel, J.; Kilkus, J.; Dawson, G.; Beyer, E.C. Connecting exosomes and connexins. Cancers 2019, 11, 476. [Google Scholar] [CrossRef]
- Peter, J.T.; Matthew, J.M.; Ebihara, L.; Cx, H.Á. The Connexin46 Mutant, Cx46T19M, Causes Loss of Gap Junction Function and Alters Hemi-channel Gating. J. Membr. Biol. 2015, 248, 145–155. [Google Scholar]
- Varela-eirin, M.; Varela-vazquez, A.; Mateos, M.R.; Vila-sanjurjo, A.; Fonseca, E.; Mascareñas, J.L.; Vázquez, M.E.; Mayan, M.D. Recruitment of RNA molecules by connexin RNA-binding motifs: Implication in RNA and DNA transport through microvesicles and exosomes. BBA Mol. Cell Res. 2017, 1864, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Cascio, M. Connexins and their environment: Effects of lipids composition on ion channels. Biochim. Biophys. Acta Biomembr. 2005, 1711, 142–153. [Google Scholar] [CrossRef]
- Singh, R.; Pochampally, R.; Watabe, K.; Lu, Z.; Mo, Y.Y. Exosome-mediated transfer of miR-10b promotes cell invasion in breast cancer. Mol. Cancer 2014, 13, 1–11. [Google Scholar] [CrossRef]
- O’Brien, K.; Rani, S.; Corcoran, C.; Wallace, R.; Hughes, L.; Friel, A.M.; McDonnell, S.; Crown, J.; Radomski, M.W.; O’Driscoll, L. Exosomes from triple-negative breast cancer cells can transfer phenotypic traits representing their cells of origin to secondary cells. Eur. J. Cancer 2013, 49, 1845–1859. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.A.; Patel, S.H.; Gucek, M.; Hendrix, A.; Westbroek, W.; Taraska, J.W. Exosomes released from breast cancer carcinomas stimulate cell movement. PLoS ONE 2015, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acuña, R.A.; Varas-Godoy, M.; Berthoud, V.M.; Alfaro, I.E.; Retamal, M.A. Connexin-46 Contained in Extracellular Vesicles Enhance Malignancy Features in Breast Cancer Cells. Biomolecules 2020, 10, 676. https://doi.org/10.3390/biom10050676
Acuña RA, Varas-Godoy M, Berthoud VM, Alfaro IE, Retamal MA. Connexin-46 Contained in Extracellular Vesicles Enhance Malignancy Features in Breast Cancer Cells. Biomolecules. 2020; 10(5):676. https://doi.org/10.3390/biom10050676
Chicago/Turabian StyleAcuña, Rodrigo A., Manuel Varas-Godoy, Viviana M. Berthoud, Ivan E. Alfaro, and Mauricio A. Retamal. 2020. "Connexin-46 Contained in Extracellular Vesicles Enhance Malignancy Features in Breast Cancer Cells" Biomolecules 10, no. 5: 676. https://doi.org/10.3390/biom10050676
APA StyleAcuña, R. A., Varas-Godoy, M., Berthoud, V. M., Alfaro, I. E., & Retamal, M. A. (2020). Connexin-46 Contained in Extracellular Vesicles Enhance Malignancy Features in Breast Cancer Cells. Biomolecules, 10(5), 676. https://doi.org/10.3390/biom10050676





