Marine-Derived Surface Active Agents: Health-Promoting Properties and Blue Biotechnology-Based Applications
Abstract
:1. Introduction
2. Health-Promoting Properties of Marine-Derived SAAs
2.1. Anti-Microbial Activity
2.2. Anti-Oxidantl Activity
2.3. Anti-Viral Activity
2.4. Anti-Inflammatory Activity
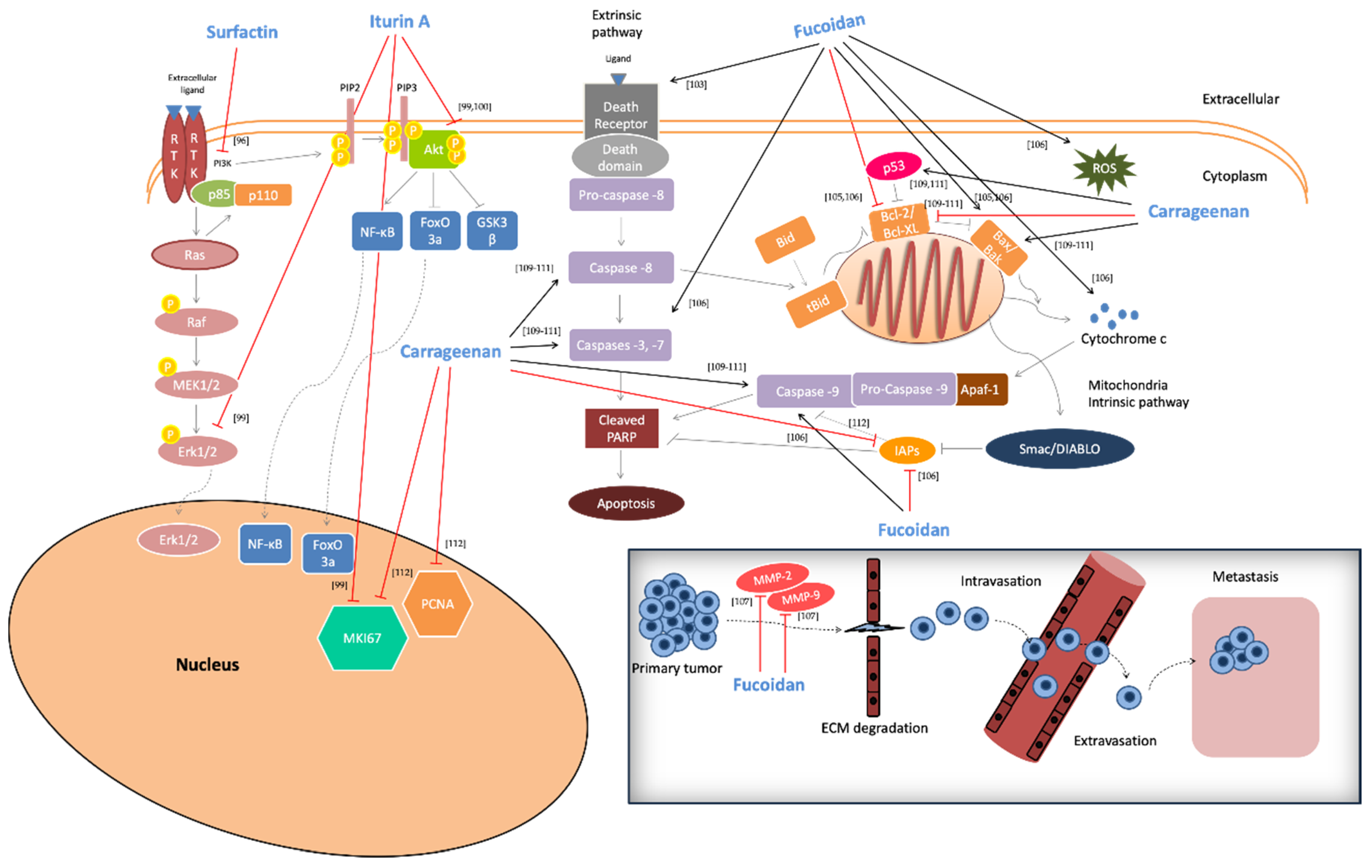
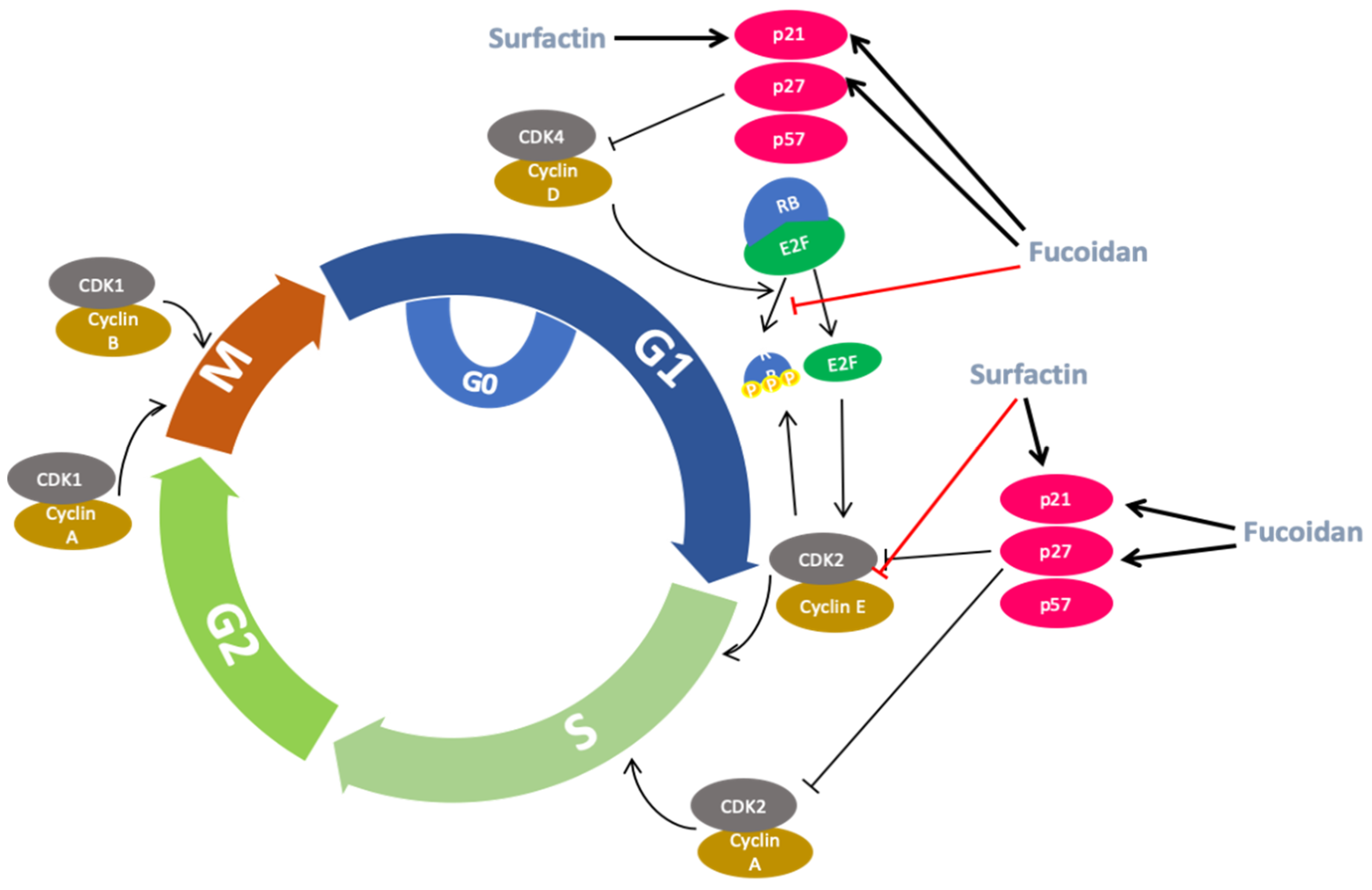
2.5. Anti-Cancer Activity
2.6. Anti-Aging Activity
3. Blue Biotechnology-Based Applications of Marine-Derived SAAs
3.1. Food Applications
3.2. Cosmetic Applications
3.3. Pharmaceutical/Biomedical Applications
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Amaral, P.F.; Coelho, M.A.Z.; Marrucho, I.M.; Coutinho, J.A. Biosurfactants from yeasts: Characteristics, production and application. Adv. Exp. Med. Biol. 2010, 672, 236–249. [Google Scholar]
- Marchant, R.; Banat, I.M. Biosurfactants: A sustainable replacement for chemical surfactants? Biotechnol. Lett. 2012, 34, 1597–1605. [Google Scholar] [CrossRef]
- De, S.; Malik, S.; Ghosh, A.; Saha, R.; Saha, B. A review on natural surfactants. RSC Adv. 2015, 5, 65757–65767. [Google Scholar] [CrossRef]
- Scott, M.J.; Jones, M.N. The biodegradation of surfactants in the environment. Biochim. Biophys. Acta 2000, 1508, 235–251. [Google Scholar] [CrossRef] [Green Version]
- Sriram, M.I.; Gayathiri, S.; Gnanaselvi, U.; Jenifer, P.S.; Raj, S.M.; Gurunathan, S. Novel lipopeptide biosurfactant produced by hydrocarbon degrading and heavy metal tolerant bacterium Escherichia fergusonii KLU01 as a potential tool for bioremediation. Bioresour. Technol. 2011, 102, 9291–9295. [Google Scholar] [CrossRef]
- Mukherjee, S.; Das, P.; Sen, R. Towards commercial production of microbial surfactants. Trends Biotechnol. 2006, 24, 509–515. [Google Scholar] [CrossRef]
- Dhanarajan, G.; Sen, R. Cost Analysis of Biosurfactant Production from a Scientist’s Perspective. In Biosurfactants: Production and Utilization-Processes, Technologies, and Economics; Kosaric, N., Sukan, F.V., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 153–162. [Google Scholar]
- Shekhar, S.; Sundaramanickam, A.; Balasubramanian, T. Biosurfactant producing microbes and their potential applications: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1522–1554. [Google Scholar] [CrossRef]
- Maneerat, S. Biosurfactants from marine microorganisms. Songklanakarin J. Sci. Technol. 2005, 27, 1263–1272. [Google Scholar]
- Naughton, P.; Marchant, R.; Naughton, V.; Banat, I. Microbial biosurfactants: Current trends and applications in agricultural and biomedical industries. J. Appl. Microbiol. 2019, 127, 12–28. [Google Scholar] [CrossRef] [Green Version]
- Banat, I.M.; De Rienzo, M.A.; Quinn, G.A. Microbial biofilms: Biosurfactants as antibiofilm agents. Appl. Microbiol. Biotechnol. 2014, 98, 9915–9929. [Google Scholar] [CrossRef]
- Liu, X.; Ren, B.; Chen, M.; Wang, H.; Kokare, C.R.; Zhou, X.; Wang, J.; Dai, H.; Song, F.; Liu, M.; et al. Production and characterization of a group of bioemulsifiers from the marine Bacillus velezensis strain H3. Appl. Microbiol. Biotechnol. 2010, 87, 1881–1893. [Google Scholar] [CrossRef]
- Liu, X.; Ren, B.; Gao, H.; Liu, M.; Dai, H.; Song, F.; Yu, Z.; Wang, S.; Hu, J.; Kokare, C.R.; et al. Optimization for the production of surfactin with a new synergistic antifungal activity. PLoS ONE 2012, 7, e34430. [Google Scholar] [CrossRef]
- Wu, S.; Liu, G.; Zhou, S.; Sha, Z.; Sun, C. Characterization of antifungal lipopeptide biosurfactants produced by marine bacterium bacillus sp. CS30. Mar. Drugs 2019, 17, 199. [Google Scholar] [CrossRef] [Green Version]
- Kiran, G.S.; Hema, T.A.; Gandhimathi, R.; Selvin, J.; Thomas, T.A.; Ravji, T.R.; Natarajaseenivasan, K. Optimization and production of a biosurfactant from the sponge-associated marine fungus Aspergillus ustus MSF3. Colloids Surf. B. Biointerfaces 2009, 73, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Mukherjee, S.; Sen, R. Antimicrobial potential of a lipopeptide biosurfactant derived from a marine Bacillus circulans. J. Appl. Microbiol. 2008, 104, 1675–1684. [Google Scholar] [CrossRef]
- Das, P.; Mukherjee, S.; Sen, R. Bioresource Technology Substrate dependent production of extracellular biosurfactant by a marine bacterium. Bioresour. Technol. 2009, 100, 1015–1019. [Google Scholar] [CrossRef]
- Sivapathasekaran, C.; Mukherjee, S.; Samanta, R.; Sen, R. High-performance liquid chromatography purification of biosurfactant isoforms produced by a marine bacterium. Annal Bioanal. Chem. 2009, 395, 845–854. [Google Scholar] [CrossRef]
- Kiran, G.S.; Priyadharsini, S.; Sajayan, A.; Priyadharsin, G.B.; Poulose, N.; Selvin, J. Production of lipopeptide biosurfactant by a marine nesterenkonia sp. and its application in food industry. Front. Microbiol. 2017, 8, 8. [Google Scholar] [CrossRef]
- Selvin, J.; Sathiyanarayanan, G.; Lipton, A.N.; Al-Dhabi, N.A.; Valan Arasu, M.; Kiran, G.S. Ketide synthase (KS) domain prediction and analysis of iterative type II PKS gene in marine sponge-associated actinobacteria producing biosurfactants and antimicrobial agents. Front. Microbiol. 2016, 7, 63. [Google Scholar] [CrossRef] [Green Version]
- Balan, S.S.; Kumar, C.G.; Jayalakshmi, S. Aneurinifactin, a new lipopeptide biosurfactant produced by a marine Aneurinib acillusaneurinilyticus SBP-11 isolated from Gulf of Mannar: Purification, characterization and its biological evaluation. Microbiol. Res. 2017, 194, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Balan, S.S.; Kumar, C.G.; Jayalakshmi, S. Pontifactin, a new lipopeptide biosurfactant produced by a marine Pontibacter korlensis strain SBK-47: Purification, characterization and its biological evaluation. Process Biochem. 2016, 51, 2198–2207. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, S.A.; Mou, H.; Ma, Y.; Li, M.; Hu, X. Characterization of lipopeptide biosurfactants produced by Bacillus licheniformis MB01 from marine sediments. Front. Microbiol. 2017, 8, 871. [Google Scholar] [CrossRef] [Green Version]
- Manivasagan, P.; Sivasankar, P.; Venkatesan, J.; Sivakumar, K.; Kim, S.K. Optimization, production and characterization of glycolipid biosurfactant from the marine actinobacterium, streptomyces sp. MAB36. Bioprocess Biosyst. Eng. 2014, 37, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Marzban, A.; Ebrahimipour, G.; Danesh, A. Bioactivity of a novel glycolipid produced by a halophilic buttiauxella sp. and improving submerged fermentation using a response surface method. Molecules 2016, 21, 1256. [Google Scholar] [CrossRef] [Green Version]
- Dusane, D.H.; Pawar, V.S.; Nancharaiah, Y.V.; Venugopalan, V.P.; Kumar, A.R.; Zinjarde, S.S. Anti-biofilm potential of a glycolipid surfactant produced by a tropical marine strain of Serratia marcescens. Biofouling 2011, 27, 645–654. [Google Scholar] [CrossRef]
- Balan, S.S.; Mani, P.; Kumar, C.G.; Jayalakshmi, S. Structural characterization and biological evaluation of Staphylosan (dimannooleate), a new glycolipid surfactant produced by a marine Staphylococcus saprophyticus SBPS-15. Enzym. Microb. Technol. 2019, 120, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hamza, F.; Satpute, S.; Banpurkar, A.; Kumar, A.R.; Zinjarde, S. Biosurfactant from a marine bacterium disrupts biofilms of pathogenic bacteria in a tropical aquaculture system. FEMS Microbiol. Ecol. 2017, 93, 93. [Google Scholar] [CrossRef]
- Saggese, A.; Culurciello, R.; Casillo, A.; Corsaro, M.M.; Ricca, E.; Baccigalupi, L. A marine isolate of bacillus pumilus secretes a pumilacidin active against staphylococcus aureus. Mar. Drugs 2018, 16, 180. [Google Scholar] [CrossRef] [Green Version]
- Ibacache-Quiroga, C.; Ojeda, J.; Espinoza-Vergara, G.; Olivero, P.; Cuellar, M.; Dinamarca, M.A. The hydrocarbon-degrading marine bacterium Cobetia sp. strain MM1IDA2H-1 produces a biosurfactant that interferes with quorum sensing of fish pathogens by signal hijacking. Microb. Biotechnol. 2013, 6, 394–405. [Google Scholar] [CrossRef]
- Khopade, A.; Ren, B.; Liu, X.Y.; Mahadik, K.; Zhang, L.; Kokare, C. Production and characterization of biosurfactant from marine Streptomyces species B3. J. Colloid Interface Sci. 2012, 367, 311–318. [Google Scholar] [CrossRef]
- Nanjundan, J.; Ramasamy, R.; Uthandi, S.; Ponnusamy, M. Antimicrobial activity and spectroscopic characterization of surfactin class of lipopeptides from bacillus amyloliquefaciens SR1. Microb. Pathog. 2019, 128, 374–380. [Google Scholar] [CrossRef]
- Kavita, K.; Singh, V.K.; Mishra, A.; Jha, B. Characterisation and anti-biofilm activity of extracellular polymeric substances from Oceanobacillus iheyensis. Carbohydr. Polym. 2014, 101, 29–35. [Google Scholar] [CrossRef]
- Ruocco, N.; Constantini, S.; Guariniello, S.; Constanti, M. Polysaccharides from the marine environment with pharmacological, cosmeceutical and nutraceutical potential. Molecules 2016, 21, 551. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.E.; Kim, K.H.; Kang, N.J. Beneficial effects of marine algae-derived carbohydrates for skin health. Mar. Drugs 2018, 16, 459. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.M.D.; Chen, C.C.; Huynh, P.; Chang, J.S. Exploring the potential of using algae in cosmetics. Bioresour. Technol. 2015, 184, 355–362. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; de Morais, A.M.; de Morais, R.M. Marine polysaccharides from algae with potential biomedical applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef]
- Hamed, I.; Ozogul, F.; Ozogul, Y.; Regenstein, J.M. Marine bioactive compounds and their health benefits: A Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- Guezennec, J. Deep-sea hydrothermal vents: A new source of innovative bacterial exopolysaccharides of biotechnological interest. J. Ind. Microbiol. Biotechnol. 2002, 29, 204–208. [Google Scholar] [CrossRef]
- Sun, L.; Ling, W.; Jing, L.; Liu, H. Characterization and antioxidant activities of degraded polysaccharides from two marine chrysophyta. Food Chem. 2014, 160, 1–7. [Google Scholar] [CrossRef]
- He, J.; Xu, Y.; Chen, H.; Sun, P. Extraction, structural characterization and potential antioxidant activity of the polysaccharides from four seaweeds. Int. J. Mol. Sci. 2016, 17, 1988. [Google Scholar] [CrossRef]
- Guo, S.; Mao, W.; Han, Y.; Zhang, X.; Yang, C.; Chen, Y.; Chen, Y.; Xu, J.; Li, H.; Qi, X.; et al. Structural characteristics and antioxidant activities of the extracellular polysaccharides produced by marine bacterium Edwardsiella tarda. Bioresour. Technol. 2010, 101, 4729–4732. [Google Scholar] [CrossRef]
- Wu, S.; Liu, G.; Jin, W.; Xiu, P.; Sun, C. Antibiofilm and anti-infection of a marine bacterial exopolysaccharide against pseudomonas aeruginosa. Front. Microbiol. 2016, 7, 102. [Google Scholar] [CrossRef] [Green Version]
- Kalitnik, A.A.; Barabanova, A.O.; Nagorskaya, V.P.; Reunov, A.V.; Glazunov, V.P.; Soloveva, T.F.; Yermak, I.M. Low molecular weight derivatives of different carrageenan types and their antiviral activity. J. Appl. Phycol. 2013, 25, 65–72. [Google Scholar] [CrossRef]
- Gonçalves, A.G.; Ducatti, D.R.; Duarte, M.E.R.; Noseada, M.D. Sulfated and pyruvylated disaccharide alditols obtained from a red seaweed galactan: ESIMS and NMR approaches. Carbohydr. Res. 2002, 337, 2443–2453. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Vet. Med. 2013, 58, 187–205. [Google Scholar] [CrossRef] [Green Version]
- Thevanayagam, H.; Mohamed, S.M.; Chu, W.L. Assessment of UVB-photoprotective and antioxidative activities of carrageenan in keratinocytes. J. Appl. Phycol. 2014, 26, 1813–1821. [Google Scholar] [CrossRef]
- Thomas, N.V.; Manivasagan, P.; Kim, S.K. Potential matrix metalloproteinase inhibitors from edible marine algae: A review. Environ. Toxicol. Pharmacol. 2014, 37, 1090–1100. [Google Scholar] [CrossRef]
- Yuan, H.M.; Song, J.M.; Li, X.G.; Li, N.; Dai, J.C. Immunomodulation and antitumor activity of κ-carrageenan oligosaccharides. Cancer Lett. 2006, 243, 228–234. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.K. Biological activities of carrageenan. Adv. Food Nutr. Res. 2014, 72, 113–124. [Google Scholar]
- Wang, W.; Zhang, P.; Yu, G.L.; Li, C.X.; Hao, C.; Qi, X.; Zhang, L.J.; Guan, H.S. Preparation and anti-influenza A virus activity of κ-carrageenan oligosaccharide and its sulphated derivatives. Food Chem. 2012, 133, 880–888. [Google Scholar] [CrossRef]
- De Azevedo, T.C.; Bezerra, M.E.; Santos Mda, G.; Souza, L.A.; Marques, C.T.; Benevides, N.M.; Leite, E.L. Heparinoids algal and their anticoagulant, hemorrhagic activities and platelet aggregation. Biomed. Pharmacother. 2009, 63, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Seol, K.H.; Lim, D.G.; Jang, A.; Jo, C.; Lee, M. Antimicrobial effect of κ-carrageenan-based edible film containing ovotransferrin in fresh chicken breast stored at 5°C. Meat Sci. 2009, 83, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.B.; Adel, M.; Karimi, P.; Peidayesh, M. Pharmaceutical, cosmeceutical, and traditional applications of marine carbohydrates. Adv. Food Nutr. Res. 2014, 73, 197–220. [Google Scholar] [PubMed]
- Suganya, A.M.; Sanjivkumar, M.; Chandran, M.N.; Palavesam, A.; Immanuel, G. Pharmacological importance of sulphated polysaccharide carrageenan from red seaweed Kappaphycus alvarezii in comparison with commercial carrageenan. Biomed. Pharmacother. 2016, 84, 1300–1312. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Kim, S.K. Anticancer effects of fucoidan. Adv. Food Nutr. Res. 2014, 72, 195–213. [Google Scholar]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [Green Version]
- Wijesekara, I.; Pangestuti, R.; Kim, S.K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohyd. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Saravana, P.S.; Cho, J.; Park, Y.B.; Woo, H.C.; Chun, B.S. Structural, antioxidant and emulsifying activities of fucoidan from saccharina japonica using pressurized liquid extraction. Carbohydr. Polym. 2016, 153, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, S.N.; Shanmugam, S.; Subramanian, B.; Jaganathan, R. Cytotoxic effect of fucoidan extracted from Sargassum cinereum on colon cancer cell line HCT-15. Int. J. Biol. Macromol. 2016, 91, 1215–1223. [Google Scholar] [CrossRef]
- Palanisamy, S.; Vinosha, M.; Marudhupandi, T.; Rajasker, P.; Prabhu, N.M. Isolation of fucoidan from sargassum polycystum brown algae: Structural characterization in vitro antioxidant and anticancer activity. Int. J. Biol. Macromol. 2017, 102, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, S.; Vinosha, M.; Manikandakrishan, M.; Anjali, R.; Rajasekar, P.; Marudhupandi, T.; Manikandan, R.; Vaseeharan, B.; Prabhu, N.M. Investigation of antioxidant and anticancer potential of fucoidan from sargassum polycystum. Int. J. Biol. Macromol. 2018, 116, 151–161. [Google Scholar] [CrossRef]
- Heeba, G.H.; Morsy, M.A. Fucoidan ameliorates steatohepatitis and insulin resistance by suppressing oxidative stress and inflammatory cytokines in experimental non-alcoholic fatty liver disease. Environ. Toxicol. Pharmacol. 2015, 40, 907–914. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, J.H.; Lee, S.J.; Kim, Y. Surfactin exhibits neuroprotective effects by inhibiting amyloid β-mediated microglial activation. Neurotoxicology 2013, 38, 115–123. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, J.H.; Lee, S.J.; Kim, Y. Involvement of PKA and HO-1 signaling in anti-inflammatory effects of surfactin in BV-2 microglial cells. Toxicol. Appl. Pharmacol. 2013, 268, 68–78. [Google Scholar] [CrossRef]
- Ramrajan, K.; Ramakrishnan, N.; Tamizhazhagan, V.; Bhuvaneswari, M. In vitro screening and characterization of biosurfactant from marine streptomyces sp. Eur. J. Pharm. Med. Res. 2017, 4, 531–534. [Google Scholar]
- Martinez, J.P.; Sasse, F.; Brönstrup, M.; Diez, J.; Meyerhans, A. Antiviral drug discovery: Broad-spectrum drugs from nature. Nat. Prod. Rep. 2015, 32, 29–48. [Google Scholar] [CrossRef]
- Carlucci, M.J.; Ciancia, M.; Matulewicz, M.C.; Cerezo, A.S.; Damonte, E.B. Antiherpetic activity and mode of action of natural carrageenans of diverse structural types. Antivir. Res. 1999, 43, 93–102. [Google Scholar] [CrossRef]
- Carlucci, M.J.; Scolaro, L.A.; Damonte, E.B. Herpes simplex virus type 1 variants arising after selection with an antiviral carrageenan: Lack of correlation between drug susceptibility and syn phenotype. J. Med. Virol. 2002, 68, 92–98. [Google Scholar] [CrossRef]
- Shao, Q.; Guo, Q.; Xu, W.; Li, Z.; Zhao, T. Specific inhibitory effect of κ-carrageenan polysaccharide on swine pandemic 2009 H1N1 influenza virus. PLoS ONE 2015, 10, e0126577. [Google Scholar] [CrossRef]
- Roberts, J.N.; Buck, C.B.; Thompson, C.D.; Kines, R.; Bernardo, M.; Choyke, P.L.; Lowy, D.R.; Schiller, J.T. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat. Med. 2007, 13, 857–861. [Google Scholar] [CrossRef] [Green Version]
- Chiu, Y.H.; Chan, Y.L.; Tsai, L.W.; Li, T.L.; Wu, C.J. Prevention of human enterovirus 71 infection by kappa carrageenan. Antivir. Res. 2012, 95, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.X.; Zhang, X.S.; Guan, H.S.; Wang, W. Potential anti-HPV and related cancer agents from marine resources: An overview. Mar. Drugs 2014, 12, 2019–2035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Liu, W.; Wang, W.; Zhang, X.; Zhao, X. The inhibitory effects and mechanisms of 3,6-O-sulfated chitosan against human papillomavirus infection. Carbohydr. Polym. 2018, 198, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; Mateu, C.G.; Chattopadhyay, K.; Pujol, C.A.; Damonte, E.B.; Ray, B. Structural features and antiviral activity of sulphated fucans from the brown seaweed Cystoseira indica. Antivir. Chem. Chemother. 2007, 18, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Thuy, T.T.; Ly, B.M.; Van, T.T.; Quang, N.V.; Tu, H.C.; Zheng, Y.; Seguin-Devaux, C.; Mi, B.; Ai, U. Anti-HIV activity of fucoidans from three brown seaweed species. Carbohydr. Polym. 2015, 115, 122–128. [Google Scholar] [CrossRef]
- Hidari, K.I.; Takahashi, N.; Arihara, M.; Nagaoka, M.; Morita, K.; Suzuki, T. Structure and anti-dengue virus activity of sulfated polysaccharide from a marine alga. Biochem. Biophys. Res. Commun. 2008, 376, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Borsanyiova, M.; Patil, A.; Mukherji, R.; Prabhune, A.; Bopegamage, S. Biological activity of sophorolipids and their possible use as antiviral agents. Folia Microbiol. 2016, 61, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Vollenbroich, D.; Ozel, M.; Vater, J.; Kamp, R.M.; Pauli, G. Mechanism of inactivation of enveloped viruses by the biosurfactant surfactin from bacillus subtilis. Biologicals 1997, 25, 289–297. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, S.; Wang, Y.; Li, Y.; Wang, X.; Yang, Q. Surfactin inhibits membrane fusion during invasion of epithelial cells by enveloped viruses. J. Virol. 2018, 92, e00809. [Google Scholar] [CrossRef] [Green Version]
- Shi, Q.; Wang, A.; Lu, Z.; Qin, C.; Hu, J.; Yin, J. Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds. Carbohydr. Res. 2017, 453, 1–9. [Google Scholar] [CrossRef]
- Elizondo-Gonzalez, R.; Cruz-Suarez, L.E.; Ricque-Marie, D.; Mendoza-Gamboa, E.; Rodriguez-Padilla, C.; Trejo-Avila, L.M. In vitro characterization of the antiviral activity of fucoidan from Cladosiphon okamuranus against Newcastle Disease Virus. Virol. J. 2012, 9, 307. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Briseño, J.A.; Cruz-Suarez, L.E.; Sassi, J.F.; Ricque-Marie, D.; Zapata-Benavides, P.; Mendoza-Gamboa, E.; Rodriguez-Padilla, C.; Trejo-Avila, L.M. Sulphated polysaccharides from Ulva clathrata and Cladosiphono kamuranus seaweeds both inhibit viral attachment/entry and cell-cell fusion in NDV infection. Mar. Drugs 2015, 13, 697–712. [Google Scholar] [CrossRef]
- Araya, N.; Takahashi, K.; Sato, T.; Nakamura, T.; Sawa, C.; Hasegawa, D.; Ando, H.; Aratani, S.; Yagishita, N.; Fujii, R.; et al. Fucoidan therapy decreases the proviral load in patients with human T-lymphotropic virus type-1-associated neurological disease. Antivir. Ther. 2011, 16, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Talarico, L.B.; Noseda, M.D.; Ducatti, D.R.B.; Duarte, M.E.R.; Damonte, E.B. Differential inhibition of denque virus infection in mammalian and mosquito cells by iota-carrageenan. J. Gen. Virol. 2011, 92, 1332–1342. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Dong, B.; Ma, X.; Hou, L.; Cao, X.; Wang, C. Anti-inflammatory activity and mechanism of surfactin in lipopolysaccharide-activated macrophages. Inflammation 2015, 38, 756–764. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, Y. Surfactin inhibits immunostimulatory function of macrophages through blocking NF-kappaB, MAPK and Akt pathway. Int. Immunopharmacol. 2009, 9, 886–893. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.; Fernando, I.P.; Kim, E.A.; Ahn, G.; Jee, Y.; Jeon, Y.J. Anti-inflammatory activity of a sulfated polysaccharide isolated from an enzymatic digest of brown seaweed sargassum horneri in RAW 264.7 cells. Nutr. Res. Pract. 2017, 11, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Park, H.Y.; Han, M.H.; Park, C.; Jin, C.Y.; Kim, G.Y.; Choi, I.W.; Kim, N.D.; Nam, T.J.; Kwon, T.K.; Choi, Y.H. Anti-inflammatory effects of fucoidan through inhibition of NF-κB, MAPK and Akt activation in lipopolysaccharide-induced BV2 microglia cells. Food Chem. Toxicol. 2011, 49, 1745–1752. [Google Scholar] [CrossRef]
- Subash, A.; Veeraraghavan, G.; Sali, V.K.; Bhardwaj, M.; Vasanthi, H.R. Attenuation of inflammation by marine algae turbinariaornata in cotton pellet induced granuloma mediated by fucoidan like sulphated polysaccharide. Carbohydr. Polym. 2016, 151, 1261–1268. [Google Scholar] [CrossRef]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. Consorzio Interuniversitario Nazionale per la Bio-Oncologia, Italy. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef] [Green Version]
- Medeiros, V.P.; Queiroz, K.C.; Cardoso, M.L.; Monteiro, G.R.; Oliveira, F.W.; Chavante, S.F.; Guimaraes, L.A.; Rocha, H.A.; Leite, E.L. Sulfated galactofucan from Lobophora variegata: Anti-coagulant and anti-inflammatory properties. Biochemistry 2008, 73, 1018–1024. [Google Scholar] [CrossRef]
- Senni, K.; Gueniche, F.; Foucault-Bertaud, A.; Igondjo-Tchen, S.; Fioretti, F.; Colliec-Jouault, S.; Durand, P.; Guezennec, J.; Godeau, G.; Letourneur, D. Fucoidan a sulfated polysaccharide from brown algae is a potent modulator of connective tissue proteolysis. Arch. Biochem. Biophys. 2006, 445, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Sarithakumari, C.H.; Kurup, G.M. Alginic acid isolated from Sargassum wightii exhibits anti-inflammatory potential on type II collagen induced arthritis in experimental animals. Int. Immunopharmacol. 2013, 17, 1108–1115. [Google Scholar] [CrossRef]
- Gudiña, E.; Teixeira, J.; Rodrigues, L. Biosurfactants produced by marine microorganisms with therapeutic applications. Mar. Drugs 2016, 14, 38. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Kim, J.Y.; Kim, S.H.; Bae, H.J.; Yi, H.; Yoon, S.H.; Koo, B.S.; Kwon, M.; Cho, J.Y.; Lee, C.E.; et al. Surfactin from Bacillus subtilis displays anti-proliferative effect via apoptosis induction, cell cycle arrest and survival signaling suppression. FEBS Lett. 2007, 581, 865–871. [Google Scholar] [CrossRef] [Green Version]
- Sivapathasekaran, C.; Das, P.; Mukherjee, S.; Saravanakumar, J.; Mandal, M.; Sen, R. Marine bacterium derived lipopeptides: Characterization and cytotoxic activity against cancer cell lines. Int. J. Pept. Res. Ther. 2010, 16, 215–222. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, N.; Hu, J.; Wang, S. Isolation and characterization of a new iturinic lipopeptide, mojavensin A produced by a marine-derived bacterium Bacillus mojavensis B0621A. J. Antibiot. 2012, 65, 317–322. [Google Scholar] [CrossRef] [Green Version]
- Dey, G.; Bharti, R.; Dhanarajan, G.; Das, S.; Dey, K.K.; Kumar, B.N.; Sen, R.; Mandal, M. Marine lipopeptide Iturin A inhibits Akt mediated GSK3β and FoxO3a signaling and triggers apoptosis in breast cancer. Sci. Rep. 2015, 5, 10316. [Google Scholar] [CrossRef] [Green Version]
- Dey, G.; Bharti, R.; Das, A.K.; Sen, R.; Mandal, M. Resensitization of Akt Induced Docetaxel Resistance in Breast Cancer by ‘Iturin A’ a Lipopeptide Molecule from Marine Bacteria Bacillus megaterium. Sci. Rep. 2017, 7, 17324. [Google Scholar] [CrossRef] [Green Version]
- Kristoffersen, V.; Rämä, T.; Isaksson, J.; Andersen, J.; Gerwick, W.; Hansen, E. Characterization of Rhamnolipids Produced by an Arctic Marine Bacterium from the Pseudomonas fluorescence Group. Mar. Drugs 2018, 16, 163. [Google Scholar] [CrossRef] [Green Version]
- Fedorov, S.; Ermakova, S.; Zvyagintseva, T.; Stonik, V. Anticancer and Cancer Preventive Properties of Marine Polysaccharides: Some Results and Prospects. Mar. Drugs 2013, 11, 4876–4901. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.H.; Kwon, M.J.; Nam, T.J. Differences in cell death and cell cycle following fucoidan treatment in high-density HT-29 colon cancer cells. Mol. Med. Rep. 2017, 15, 4116–4122. [Google Scholar] [CrossRef]
- Park, H.; Park, S.H.; Jeong, J.W.; Yoon, D.; Han, M.; Lee, D.S.; Choi, G.; Yim, M.J.; Lee, J.M.; Kim, D.H.; et al. Induction of p53-independent apoptosis and G1 cell cycle arrest by fucoidan in HCT116 human colorectal carcinoma cells. Mar. Drugs 2017, 15, 154. [Google Scholar] [CrossRef] [Green Version]
- Ahn, G.; Lee, W.; Kim, K.N.; Lee, J.H.; Heo, S.J.; Kang, N.; Lee, S.H.; Ahn, C.B.; Jeon, Y.J. A sulfated polysaccharide of Ecklonia cava inhibits the growth of colon cancer cells by inducing apoptosis. EXCLI J. 2015, 14, 294–306. [Google Scholar]
- Yang, L.; Wang, P.; Wang, H.; Li, Q.; Teng, H.; Liu, Z.; Yang, W.; Hou, L.; Zou, X. Fucoidan Derived from Undaria pinnatifida Induces Apoptosis in Human Hepatocellular Carcinoma SMMC-7721 Cells via the ROS-Mediated Mitochondrial Pathway. Mar. Drugs 2013, 11, 1961–1976. [Google Scholar] [CrossRef] [Green Version]
- Delma, C.R.; Somasundaram, S.T.; Srinivasan, G.P.; Khursheed, M.; Bashyam, M.D.; Aravindan, N. Fucoidan from Turbinaria conoides: A multifaceted ‘deliverable’ to combat pancreatic cancer progression. Int. J. Biol. Macromol. 2015, 74, 447–457. [Google Scholar] [CrossRef]
- Souza, R.B.; Frota, A.F.; Silva, J.; Alves, C.; Neugebauer, A.Z.; Pinteus, S.; Rodrigues, J.A.G.; Cordeiro, E.M.S.; de Almeida, R.R.; Pedrosa, R.; et al. In vitro activities of kappa-carrageenan isolated from red marine alga Hypnea musciformis: Antimicrobial, anticancer and neuroprotective potential. Int. J. Biol. Macromol. 2018, 112, 1248–1256. [Google Scholar] [CrossRef]
- Murad, H.; Ghannam, A.; Al-Ktaifani, M.; Abbas, A.; Hawat, M. Algal sulfated carrageenan inhibits proliferation of MDA-MB-231 cells via apoptosis regulatory genes. Mol. Med. Rep. 2015, 11, 2153–2158. [Google Scholar] [CrossRef]
- Jazzara, M.; Ghannam, A.; Soukkarieh, C.; Murad, H. Anti-proliferative activity of λ-carrageenan through the induction of apoptosis in human breast cancer cells. Iran. J. Cancer Prev. 2016, 9, e3836. [Google Scholar] [CrossRef]
- Ghannam, A.; Murad, H.; Jazzara, M.; Odeh, A.; Allaf, A.W. Isolation, Structural characterization, and antiproliferative activity of phycocolloids from the red seaweed Laurencia papillosa on MCF-7 human breast cancer cells. Int. J. Biol. Macromol. 2018, 108, 916–926. [Google Scholar] [CrossRef]
- Ariffin, S.H.; Yeen, W.W.; Abidin, I.Z.; Abdul Wahab, R.M.; Ariffin, Z.Z.; Senafi, S. Cytotoxicity effect of degraded and undegraded kappa and iota carrageenan in human intestine and liver cell lines. BMC Complement. Altern. Med. 2014, 14, 508. [Google Scholar]
- Zhou, G.; Xin, H.; Sheng, W.; Sun, Y.; Li, Z.; Xu, Z. In vivo growth-inhibition of S180 tumor by mixture of 5-Fu and low molecular λ-carrageenan from Chondrus ocellatus. Pharmacol. Res. 2005, 51, 153–157. [Google Scholar] [CrossRef]
- Hu, X.; Jiang, X.; Aubree, E.; Boulenguer, P.; Critchley, A.T. Preparation and in vivo antitumor activity of κ-carrageenan oligosaccharides. Pharm. Biol. 2006, 44, 646–650. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.; Shao, B.; Nie, W.; Wei, X.W.; Li, Y.L.; Wang, B.L.; He, Z.Y.; Liang, X.; Ye, T.H.; Wei, Y.Q. Antitumor and adjuvant activity of λ-carrageenan by stimulating immune response in cancer immunotherapy. Sci. Rep. 2015, 5, 11062. [Google Scholar] [CrossRef]
- Thomas, N.V.; Kim, S.K. Beneficial effects of marine algal compounds in cosmeceuticals. Mar. Drugs 2013, 11, 146–164. [Google Scholar] [CrossRef] [Green Version]
- Pangestuti, R.; Siahaan, E.A.; Kim, S.K. Photoprotective substances derived from marine algae. Mar. Drugs 2018, 16, 399. [Google Scholar] [CrossRef] [Green Version]
- Moon, H.J.; Lee, S.R.; Shim, S.N.; Jeong, S.H.; Stonik, V.A.; Rasskazov, V.A.; Zvyagintseva, T.; Lee, Y.H. Fucoidan inhibits UVB-induced MMP-1 expression in human skin fibroblasts. Biol. Pharm. Bull. 2008, 31, 284–289. [Google Scholar] [CrossRef] [Green Version]
- Moon, H.J.; Lee, S.H.; Ku, M.J.; Yu, B.C.; Jeon, M.J.; Jeong, S.H.; Stonik, V.A.; Zvyagintseva, T.N.; Ermakova, S.P.; Lee, Y.H. Fucoidan inhibits UVB-induced MMP-1 promoter expression and down regulation of type I procollagen synthesis in human skin fibroblasts. Eur. J. Dermatol. 2009, 19, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Moon, H.J.; Park, K.S.; Ku, M.J.; Lee, M.S.; Jeong, S.H.; Imbs, T.I.; Zvyagintseva, T.N.; Ermakova, S.P.; Lee, Y.H. Effect of Costaria costata fucoidan on expression of matrix metalloproteinase-1 promoter, mRNA, and protein. J. Nat. Prod. 2009, 72, 1731–1734. [Google Scholar] [CrossRef]
- Maruyama, H.; Tamauchi, H.; Kawakami, F.; Yoshinaga, K.; Nakano, T. Suppressive effect of dietary fucoidan on proinflammatory response and MMP-1 expression in UVB-irradiated mouse skin. Planta Med. 2015, 81, E4. [Google Scholar]
- Hwang, P.A.; Yan, M.D.; Kuo, K.L.; Nam, N.; Phan, N.N.; Lin, Y.C. A mechanism of low molecular weight fucoidans degraded by enzymatic and acidic hydrolysis for the prevention of UVB damage. J. Appl. Phycol. 2017, 29, 521–529. [Google Scholar] [CrossRef]
- Orthoefer, F.; Kim, D. Applications of emulsifiers in baked foods. In Food Emulsifiers and Their Applications; Hasenhuettl, G.L., Hartel, R.W., Eds.; Springer: New York, NY, USA, 2019; pp. 263–284. [Google Scholar]
- Euston, S.R.; Goff, H.D. Emulsifiers in dairy products and dairy substitutes. In Food Emulsifiers and Their Applications; Hasenhuettl, G.L., Hartel, R.W., Eds.; Springer: New York, NY, USA, 2019; pp. 217–254. [Google Scholar]
- Ahmad, A.; Arshad, N.; Ahmed, Z.; Bhatti, M.S.; Zahoor, T.; Anjum, N.; Ahmad, H.; Afreen, A. Perspective of surface active agents in baking industry: An overview. Crit. Rev. Food Sci. Nutr. 2014, 54, 208–224. [Google Scholar] [CrossRef]
- Young, N.W. Emulsifiers and stabilisers. In Fats in Food Technology; Rajah, K.K., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2014; pp. 253–287. [Google Scholar]
- Dickinson, E. Hydrocolloids as emulsifiers and emulsion stabilizers. Food Hydrocoll. 2009, 23, 1473–1482. [Google Scholar] [CrossRef]
- Hasenhuettl, G.L. Synthesis and commercial preparation of food emulsifiers. In Food Emulsifiers and Their Applications; Hasenhuettl, G.L., Hartel, R.W., Eds.; Springer: New York, NY, USA, 2019; pp. 11–39. [Google Scholar]
- Linder, J.M.; Palkovitz, R.E. The threat of industrial oil palm expansion to primates and their habitats. In Ethnoprimatology: Primate Conservation in the 21st Century; Waller, M.T., Ed.; Springer: Cham, Switzerland, 2016; pp. 21–45. [Google Scholar]
- Gatti, R.C.; Liang, J.; Velichevskaya, A.; Zhou, M. Sustainable palm oil may not be so sustainable. Sci. Total. Environ. 2019, 652, 48–51. [Google Scholar] [CrossRef]
- Hartel, R.W.; Firoozmand, H. Emulsifiers in confectionery. In Food Emulsifiers and Their Applications; Hasenhuettl, G.L., Hartel, R.W., Eds.; Springer: New York, NY, USA, 2019; pp. 323–346. [Google Scholar]
- Campos, J.M.; Montenegro Stamford, T.L.; Sarubbo, L.A.; de Luna, J.M.; Rufino, R.D.; Banat, I.M. Microbial biosurfactants as additives for food industries. Biotechnol. Prog. 2013, 29, 1097–1108. [Google Scholar] [CrossRef]
- Mnif, I.; Ghribi, D. Glycolipid biosurfactants: Main properties and potential applications in agriculture and food industry. J. Sci. Food Agric. 2016, 96, 4310–4320. [Google Scholar] [CrossRef]
- Sharma, D. Biosurfactants in food. In Recent Updates on Biosurfactants in the Food Industry. Microbial Cell Factories; Satpute, S.K., Zinjarde, S.S., Banat, I.M., Eds.; Springer: Cham, Switzerland, 2016; pp. 1–20. [Google Scholar]
- Nitschke, M.; Silva, S.S.E. Recent food applications of microbial surfactants. Crit. Rev. Food Sci. Nutr. 2018, 58, 631–638. [Google Scholar] [CrossRef]
- Carter, H.E.; McCluer, R.H.; Slifer, E.D. Lipids of wheat flour. I. Characterization of galactosylglycerol components1. J. Am. Chem. Soc. 1956, 78, 3735–3738. [Google Scholar] [CrossRef]
- Selmair, P.L.; Koehler, P. Baking performance of synthetic glycolipids in comparison to commercial surfactants. J. Agric. Food Chem. 2008, 56, 6691–6700. [Google Scholar] [CrossRef]
- Selmair, P.L.; Koehler, P. Role of glycolipids in breadmaking. Lipid Technol. 2010, 22, 7–10. [Google Scholar] [CrossRef]
- Kates, M. Glycolipids of higher plants, algae, yeasts, and fungi. In Glycolipids, Phosphoglycolipids, and Sulfoglycolipids; Kates, M., Ed.; Springer: Boston, MA, USA, 1990; pp. 235–320. [Google Scholar]
- van Haesendonck, I.P.H.; Vanzeveren, E.C.A. Rhamnolipids in Bakery Products. Patent WO 2004/040984; International Application Patent, 21 May 2004. [Google Scholar]
- Mnif, I.; Besbes, S.; Ellouze, R.; Ellouze-Chaabouni, S.; Ghribi, D. Improvement of bread quality and bread shelf-life by Bacillus subtilis biosurfactant addition. Food Sci. Biotechnol. 2012, 21, 1105–1112. [Google Scholar] [CrossRef]
- Zouari, R.; Besbes, S.; Ellouze-Chaabouni, S.; Ghribi-Aydi, D. Cookies from composite wheat–sesame peels flours: Dough quality and effect of bacillus subtilis SPB1 biosurfactant addition. Food Chem. 2016, 194, 758–769. [Google Scholar] [CrossRef]
- Stephen, A.M.; Phillips, G.O. Food Polysaccharides and Their Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 1–25. [Google Scholar]
- Jones, A.D.; Boundy-Mills, K.L.; Barla, G.F.; Kumar, S.; Ubanwa, B.; Balan, V. Microbial lipid alternatives to plant lipids. Methods Mol. Biol. 2019, 1995, 1–32. [Google Scholar]
- Colonia, B.S.O.; de Melo Pereira, G.V.; Soccol, C.R. Omega-3 microbial oils from marine thraustochytrids as a sustainable and technological solution: A review and patent landscape. Trends Food Sci. Technol. 2020, 99, 244–256. [Google Scholar] [CrossRef]
- Nitbani, F.O.; Tjitda, P.J.P.; Nurohmah, B.A.; Wogo, H.E. Preparation of Fatty Acid and Monoglyceride from Vegetable Oil. J. Oleo Sci. 2020, 69, 277–295. [Google Scholar] [CrossRef] [Green Version]
- Azizi, M.H.; Rao, G.V. Influence of selected surfactant gels and gums on dough rheogical characteristics and quality. J. Food Qual. 2004, 27, 320–336. [Google Scholar] [CrossRef]
- Kohajdová, Z.; Karovičová, J. Application of hydrocolloids as baking improvers. Chem. Pap. 2009, 63, 26–38. [Google Scholar] [CrossRef]
- Rosell, C.M.; Rojas, J.A.; de Barber, B.C. Influence of hydrocolloids on dough rheology and bread quality. Food Hydrocoll. 2001, 15, 75–81. [Google Scholar] [CrossRef]
- Mandala, I.; Karabela, D.; Kostaropoulos, A. Physical properties of breads containing hydrocolloids stored at low temperature. I. Effect of chilling. Food Hydrocoll. 2007, 21, 1397–1406. [Google Scholar] [CrossRef]
- Guarda, A.; Rosell, C.M.; Benedito, C.; Galotto, M.J. Different hydrocolloids as bread improvers and antistaling agents. Food Hydrocoll. 2004, 18, 241–247. [Google Scholar] [CrossRef]
- León, A.E.; Ribotta, P.D.; Ausar, S.F.; Fernández, C.; Landa, C.A.; Beltramo, D.M. Interactions of different carrageenan isoforms and flour components in breadmaking. J. Agric. Food Chem. 2000, 48, 2634–2638. [Google Scholar] [CrossRef]
- Prakash, S.; Huppertz, T.; Karvchuk, O.; Deeth, H. Ultra-high-temperature processing of chocolate flavoured milk. J. Food Eng. 2010, 96, 179–184. [Google Scholar] [CrossRef]
- Bahramparvar, M.; Mazaheri Tehrani, M. Application and functions of stabilizers in ice cream. Food Rev. Int. 2011, 27, 389–407. [Google Scholar] [CrossRef]
- Casillo, A.; Lanzetta, R.; Parrilli, M.; Corsaro, M.M. Exopolysaccharides from marine and marine extremophilic bacteria: Structures, properties, ecological roles and applications. Mar. Drugs 2018, 16, 69. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, E.; Zuckerberg, A.; Rubinovitz, C.; Gutnick, D.L. Emulsifier of arthrobacter RAG-1: Isolation and emulsifying properties. Appl. Environ. Microbiol. 1979, 37, 402–408. [Google Scholar] [CrossRef] [Green Version]
- Bach, H.; Berdichevsky, Y.; Gutnick, D. An exocellular protein from the oil-degrading microbe Acinetobacter venetianus RAG-1 enhances the emulsifying activity of the polymeric bioemulsifier emulsan. Appl. Environ. Microbiol. 2003, 69, 2608–2615. [Google Scholar] [CrossRef] [Green Version]
- Samain, E.; Miles, M.; Bozzi, L.; Dubreucq, G.; Rinaudo, M. Simultaneous production of two different gel-forming exopolysaccharides by an alteromonas strain originating from deep sea hydrothermal vents. Carbohydr. Polym. 1997, 34, 235–241. [Google Scholar] [CrossRef]
- Bouchotroch, S.; Quesada, E.; Izquierdo, I.; Rodriguez, M.; Béjar, V. Bacterial exopolysaccharides produced by newly discovered bacteria belonging to the genus halomonas, isolated from hypersaline habitats in Morocco. J. Ind. Microbiol. Biotechnol. 2000, 24, 374–378. [Google Scholar] [CrossRef]
- Raguénès, G.; Cambon-Bonavita, M.A.; Lohier, J.F.; Boisset, C.; Guezennec, J. A novel, highly viscous polysaccharide excreted by an alteromonas isolated from a deep-sea hydrothermal vent shrimp. Curr. Microbiol. 2003, 46, 448–452. [Google Scholar] [CrossRef]
- Yim, J.H.; Kim, S.J.; Aan, S.H.; Lee, H.K. Physicochemical and rheological properties of a novel emulsifier, EPS-R, produced by the marine bacterium Hahella chejuensis. Biotechnol. Bioprocess Eng. 2004, 9, 405. [Google Scholar] [CrossRef]
- Kumar, C.G.; Joo, H.S.; Choi, J.W.; Koo, Y.M.; Chang, C.S. Purification and characterization of an extracellular polysaccharide from haloalkalophilic bacillus sp. I-450. Enzym. Microb. Technol. 2004, 34, 673–681. [Google Scholar] [CrossRef]
- Bramhachari, P.V.; Dubey, S.K. Isolation and characterization of exopolysaccharide produced by vibrio harveyi strain VB23. Lett. Appl. Microbiol. 2006, 43, 571–577. [Google Scholar] [CrossRef]
- Iyer, A.; Mody, K.; Jha, B. Emulsifying properties of a marine bacterial exopolysaccharide. Enzym. Microb. Technol. 2006, 38, 220–222. [Google Scholar] [CrossRef]
- Bramhachari, P.V.; Kishor, P.K.; Ramadevi, R.; Rao, B.R.; Dubey, S.K. Isolation and characterization of mucous exopolysaccharide (EPS) produced by vibrio furnissii strain VB0S3. J. Microbiol. Biotechnol. 2007, 17, 44–51. [Google Scholar]
- Gutiérrez, T.; Mulloy, B.; Bavington, C.; Black, K.; Green, D.H. Partial purification and chemical characterization of a glycoprotein (putative hydrocolloid) emulsifier produced by a marine bacterium antarctobacter. Appl. Microbiol. Biotechnol. 2007, 76, 1017. [Google Scholar] [CrossRef]
- Gutierrez, T.; Mulloy, B.; Black, K.; Green, D.H. Glycoprotein emulsifiers from two marine Halomonas species: Chemical and physical characterization. J. Appl. Microbiol. 2007, 103, 1716–1727. [Google Scholar] [CrossRef]
- Martínez-Checa, F.; Toledo, F.L.; El Mabrouki, K.; Quesada, E.; Calvo, C. Characteristics of bioemulsifier V2-7 synthesized in culture media added of hydrocarbons: Chemical composition, emulsifying activity and rheological properties. Bioresour. Technol. 2007, 98, 3130–3135. [Google Scholar] [CrossRef] [PubMed]
- Urai, M.; Yoshizaki, H.; Anzai, H.; Ogihara, J.; Iwabuchi, N.; Harayama, S.; Sunairi, M.; Nakajima, M. Structural analysis of an acidic, fatty acid ester-bonded extracellular polysaccharide produced by a pristane-assimilating marine bacterium, Rhodococcus erythropolis PR4. Carbohydr. Res. 2007, 342, 933–942. [Google Scholar] [CrossRef]
- Gutierrez, T.; Shimmield, T.; Haidon, C.; Black, K.; Green, D.H. Emulsifying and metal ion binding activity of a glycoprotein exopolymer produced by pseudoalteromonas sp. strain TG12. Appl. Environ. Microbiol. 2008, 74, 4867–4876. [Google Scholar] [CrossRef] [Green Version]
- Mata, J.A.; Béjar, V.; Bressollier, P.; Tallon, R.; Urdaci, M.C.; Quesada, E.; Llamas, I. Characterization of exopolysaccharides produced by three moderately halophilic bacteria belonging to the family alteromonadaceae. J. Appl. Microbiol. 2008, 105, 521–528. [Google Scholar] [CrossRef]
- Saravanan, P.; Jayachandran, S. Preliminary characterization of exopolysaccharides produced by a marine biofilm-forming bacterium Pseudoalteromonas ruthenica (SBT 033). Lett. Appl. Microbiol. 2008, 46, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, T.; Morris, G.; Green, D.H. Yield and physicochemical properties of EPS from Halomonas sp. strain TG39 identifies a role for protein and anionic residues (sulfate and phosphate) in emulsification of n-hexadecane. Biotechnol. Bioeng. 2009, 103, 207–216. [Google Scholar] [CrossRef]
- Gutiérrez, T.; Leo, V.V.; Walker, G.M.; Green, D.H. Emulsifying properties of a glycoprotein extract produced by a marine flexibacter species strain TG382. Enzym. Microb. Technol. 2009, 45, 53–57. [Google Scholar] [CrossRef]
- Biswas, J.; Ganguly, J.; Paul, A.K. Partial characterization of an extracellular polysaccharide produced by the moderately halophilic bacterium Halomonas xianhensis SUR308. Biofouling 2015, 31, 735–744. [Google Scholar] [CrossRef]
- Peele, K.A.; Ch, V.R.T.; Kodali, V.P. Emulsifying activity of a biosurfactant produced by a marine bacterium. 3 Biotech 2016, 6, 177. [Google Scholar] [CrossRef] [Green Version]
- Ortega-de la Rosa, N.D.; Vázquez-Vázquez, J.L.; Huerta-Ochoa, S.; Gimeno, M.; Gutiérrez-Rojas, M. Stable bioemulsifiers are produced by Acinetobacter bouvetii UAM25 growing in different carbon sources. Bioprocess Biosyst. Eng. 2018, 41, 859–869. [Google Scholar] [CrossRef]
- Radchenkova, N.; Boyadzhieva, I.; Atanasova, N.; Poli, A.; Finore, I.; Di Donato, P.; Nicolaus, B.; Panchev, I.; Kuncheva, M.; Kambourova, M. Extracellular polymer substance synthesized by a halophilic bacterium chromohalobacter canadensis 28. Appl. Microbiol. Biotechnol. 2018, 102, 4937–4949. [Google Scholar] [CrossRef]
- Vidhyalakshmi, R.; Nachiyar, C.V.; Kumar, G.N.; Sunkar, S.; Badsha, I. Production, characterization and emulsifying property of exopolysaccharide produced by marine isolate of pseudomonas fluorescens. Biocatal. Agric. Biotechnol. 2018, 16, 320–325. [Google Scholar] [CrossRef]
- Sran, K.S.; Sundharam, S.S.; Krishnamurthi, S.; Choudhury, A.R. Production, characterization and bio-emulsifying activity of a novel thermostable exopolysaccharide produced by a marine strain of Rhodobacter johrii CDR-SL 7Cii. Int. J. Biol. Macromol. 2019, 127, 240–249. [Google Scholar] [CrossRef]
- Corinaldesi, C.; Barone, G.; Marcellini, F.; Dell’Anno, A.; Danovaro, R. Marine microbial-derived molecules and their potential use in cosmeceutical and cosmetic products. Mar. Drugs 2017, 15, 118. [Google Scholar] [CrossRef]
- Varvaresou, A.; Iakovou, K. Biosurfactants in cosmetics and biopharmaceuticals. Lett. Appl. Microbiol. 2015, 61, 214–223. [Google Scholar] [CrossRef]
- Lukic, M.; Pantelic, I.; Savic, S. An overview of novel surfactants for formulation of cosmetics with certain emphasis on acidic active substances. Tenside Surf. Deterg. 2016, 53, 7–19. [Google Scholar] [CrossRef]
- Bujak, T.; Wasilewski, T.; Nizioł-Łukaszewska, Z. Role of macromolecules in the safety of use of body wash cosmetics. Colloids Surf. B Biointerfaces 2015, 135, 497–503. [Google Scholar] [CrossRef]
- Lu, G.; Moore, D.J. Study of surfactant-skin interactions by skin impedance measurements. Int. J. Cosmet. Sci. 2012, 34, 74–80. [Google Scholar] [CrossRef]
- Morita, T.; Fukuoka, T.; Imura, T.; Kitamoto, D. Production of mannosylerythritol lipids and their application in cosmetics. Appl. Microbiol. Biotechnol. 2013, 97, 4691–4700. [Google Scholar] [CrossRef]
- Draelos, Z.D. Shampoos, conditioners and camouflage techniques. Dermatol. Clin. 2013, 31, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S. Bacterial Biosurfactant: Characterization, Antimicrobial and Metal Remediation Properties. Ph.D. Thesis, National Institute of Technology, Surat, India, 2012. [Google Scholar]
- Amaral, P.F.F.; da Silva, J.M.; Lehocky, M.; Barros-Timmons, A.M.V.; Coelho, M.A.Z.; Marrucho, I.M.; Coutinho, J.A.P. Production and characterization of a bioemulsifier from Yarrowia lipolytica. Process. Biochem. 2006, 41, 1894–1898. [Google Scholar] [CrossRef]
- Resende, A.H.M.; Farias, J.M.; Silva, D.D.B.; Rufino, R.D.; Luna, J.M.; Stamford, T.C.M.; Sarubbo, L.A. Application of biosurfactants and chitosan in toothpaste formulation. Colloids Surf. B Biointerfaces 2019, 181, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Akanbi, M.H.J.; Post, E.; Meter-Arkema, A.; Rink, R.; Robillard, G.T.; Wang, X.; Wosten, A.A.B.; Scholtmeijer, K. Use of hydrophobins in formulation of water insoluble drugs for oral administration. Colloids Surf. B Biointerfaces 2010, 75, 526–531. [Google Scholar] [CrossRef]
- Pajic, N.Z.B.; Todosijevic, M.N.; Vuleta, G.M.; Cekic, N.D.; Dobricic, V.D.; Vucen, S.R.; Calija, B.R.; Lukic, M.Z.; Ilic, T.M.; Savic, S.D. Alkyl polyglucoside vs. ethoxylated surfactant-based microemulsions as vehicles for two poorly water-soluble drugs: Physicochemical characterization and in vivo skin performance. Acta Pharm. 2017, 67, 415–439. [Google Scholar] [CrossRef] [Green Version]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Shama, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gudiña, E.J.; Rangarajan, V.; Sen, R.; Rodrigues, L.R. Potential therapeutic applications of biosurfactants. Trends Pharmacol. Sci. 2013, 34, 667–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawrence, M.J.; Rees, G.D. Microemulsion-based media as novel drug delivery systems. Adv. Drug Deliv. Rev. 2012, 64, 175–193. [Google Scholar] [CrossRef]
- Płaza, G.A.; Chojniak, J.; Banat, I.M. Biosurfactant mediated biosynthesis of selected metallic nanoparticles. Int. J. Mol. Sci. 2014, 15, 13720–13737. [Google Scholar] [CrossRef] [Green Version]
- Farias, C.B.B.; Silva, A.F.; Rufino, R.D.; Luna, J.M.; Souza, J.E.G.; Sarubbo, L.A. Synthesis of silver nanoparticles using a biosurfactant produced in low-cost medium as stabilizing agent. Electron. J. Biotechnol. 2014, 17, 122–125. [Google Scholar] [CrossRef] [Green Version]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [Green Version]
- Reddy, A.S.; Chen, C.Y.; Chen, C.C.; Jean, J.S.; Chen, H.R.; Tseng, M.J.; Fan, C.W.; Wang, J.C. Biological synthesis of gold and silver nanoparticles mediated by the bacteria bacillus subtilis. J. Nanosci. Nanotechnol. 2010, 10, 6567–6574. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.K.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Biosurfactants: Multifunctional biomolecules of the 21st century. Int. J. Mol. Sci. 2016, 17, 401. [Google Scholar] [CrossRef] [Green Version]
- Kiran, G.S.; Selvin, J.; Manilal, A.; Sujith, S. Biosurfactants as green stabilizers for the biological synthesis of nanoparticles. Crit. Rev. Biotechnol. 2011, 31, 354–364. [Google Scholar] [CrossRef]
- Hayes, D.G. Bioprocessing methods to prepare biobased surfactants for pharmaceutical products. Am. Pharm. Rev. 2011, 14, 8–16. [Google Scholar]
- Nakamura, S.; Ishii, N.; Nakashima, N.; Sakamota, T.; Yuasa, H. Evaluation of sucrose fatty acid esters as lubricants in tablet manufacturing. Chem. Pharm. Bull. 2017, 65, 432–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekhon, B.S. Surfactants: Pharmaceutical and medicinal aspects. J. Pharm. Technol. Res. Manag. 2013, 1, 43–68. [Google Scholar] [CrossRef] [Green Version]
- Kitamoto, D.; Isoda, H.; Nakahara, T. Functions and potential applications of glycolipid biosurfactants--from energy-saving materials to gene delivery carriers. J. Biosci. Bioeng. 2002, 94, 187–201. [Google Scholar] [CrossRef]
- Nakanishi, M.; Inoh, Y.; Kitamoto, D.; Furuno, T. Nano vectors with a biosurfactant for gene transfection and drug delivery. J. Drug Deliv. Sci. Technol. 2009, 19, 165–169. [Google Scholar] [CrossRef]
- Maitani, Y.; Yano, S.; Hattori, Y.; Furuhata, M. Liposome vector containing biosurfactant-complexed DNA as herpes simplex virus thymidine kinase gene delivery. J. Liposome Res. 2006, 16, 359–372. [Google Scholar] [CrossRef]
- Yan, X.; Gu, S.; Cui, X.; Shi, Y.; Wen, S.; Chen, H.; Ge, J. Antimicrobial, antiadhesive and antibiofilm potential of biosurfactants isolated from Pediococcus acidilactici and lactobacillus plantarum against staphylococcus aureus CMCC26003. Microb. Pathog. 2019, 127, 12–20. [Google Scholar] [CrossRef]
- Tally, F.P.; Zeckel, M.; Wasilewski, M.M.; Carini, C.; Berman, C.L.; Drusano, G.L.; Oleson, F.B., Jr. Daptomycin: A novel agent for gram-positive infections. Expert Opin. Investig. Drugs 1999, 8, 1223–1238. [Google Scholar] [CrossRef]
- Senni, K.; Pereira, J.; Gueniche, F.; Delbarre-Ladrat, C.; Sinquin, C.; Ratiscol, J.; Godeau, G.; Fischer, A.M.; Helley, D.; Colliec-Jouault, S. Marine polysaccharides: A source of bioactive molecules for cell therapy and tissue engineering. Mar. Drugs 2011, 9, 1664–1681. [Google Scholar] [CrossRef] [Green Version]

| Techniques for Physicochemical Characterization | Physicochemical Characteristics Analyzed | Strengths | Limitations |
|---|---|---|---|
| Thin Layer Chromatography (TLC) | Qualitative analysis as well as polarity information of the molecules | Low cost and fast procedure | Soluble components of the mixtures could be detected |
| Mass Spectroscopy (MS) | Determination of MW | Accuracy and precision | Expensive equipment |
| Structure elucidation | Accuracy, high sensitivity to detection and fast procedure | Lack of complete databases for identification purposes | |
| Size Exclusion Chromatography (SEC) | Determination of MW and mixture separation | Enables separation and isolation of SAAs. Provides information about MW distribution | Expensive equipment |
| SEC-MALS SEC-Multiple Angle Light Scattering (MALS) | Determination of molecular radius and oligomerization state of high MW surfactants | Relatively accurate determination of absolute MW | Expensive equipment |
| Infrared Spectroscopy (IR) Attenuated Total Reflection—Fourier Transform Infrared (ATR-FTIR) | Provides structural information of surfactants | Fast and inexpensive process | Complicated sample preparation |
| Minimal sample preparation | Interference and strong absorbance of H2O | ||
| Nuclear Magnetic Resonance (NMR) | Determination of size (indirect analysis), structure composition, purity and conformational change(s) | Non-invasive method and minimal sample preparation | Time consuming process. Large amount of sample is required |
| Organism | Emulsifier Structure | Properties | Reference |
|---|---|---|---|
| Alteromonas sp. Strain 1644 | Anionic hetero-polysaccharide (glucose, galactose, mannose, rhamnose, glucuronic acid) | Thickening Gelation | [158] |
| Halomonas strain S30 | Anionic hetero-polysaccharide (glucose, galactose, mannose, glucuronic acid) | Emulsification Thickening | [159] |
| Alteromonas macleodii | Anionic hetero-polysaccharide (glucose, galactose, glucuronic acid, galacturonic acid and pyruvate and acetate substituents) | Thickening | [160] |
| Hahella chejuensis | Heteropolysaccharide (galactose, glucose, xylose, ribose) | Emulsification Thickening | [161] |
| Bacillus sp. I-450 | Anionic hetero-polysaccharide (galactose, fructose, glucose, raffinose, uronic acid, amino-sugars) | Thickening Gelation | [162] |
| Vibrio harveyi VB23 | Heteropolysaccharide (galactose, glucose rhamnose, fucose, ribose, arabinose, xylose and mannose, uronic acids) and protein component | Emulsification | [163] |
| Enterobacter cloacae | Heteropolysacchride (fucose, galactose, glucose, glucuronic acid) | Emulsification | [164] |
| Vibrio furnissii VB0S3 | Heteropolysaccharide (galactose, glucose, rhamnose, fucose, ribose, arabinose, xylose, mannose, uronic acids) and protein component | Emulsification | [165] |
| Antarctobacter sp. TG22 | Anionic hetero-polysaccharide (rhamnose, fucose, galactose, galactosamine, glucose, glucosamine, mannose, muramic acid, galacturonic acid, glucuronic acid) | Emulsification | [166] |
| Halomonas sp, TG39 and TG67 | Two anionic hetero-polysaccharides (rhamnose, fucose, galactose, galactosamine, glucose, glucosamine, mannose, xylose, muramic acid, galacturonic acid, glucuronic acid) | Emulsification | [167] |
| Halomonas eurihalina V2-7 | Anionic hetero-polysaccharide. Protein and uronic acids | Emulsification Thickening | [168] |
| Rhodococcus erythropolis PR4 | Anionic lipo-polysaccharide (galactose, glucose, mannose, glucuronic acid, pyruvic acid, esterified stearic, palmitic acids) | Emulsification | [169] |
| Pseudoalteromonas sp. TG12 | Glycoprotein (rhamnose, fucose, galactose, galactosamine, glucose, glucosamine, mannose, xylose, muramic acid, galacturonic acid, glucuronic acid) | Emulsification | [170] |
| Idiomarina fontislapidosi F32, I.ramblicola R22 | Anionic hetero-polysaccharide (glucose, mannose, galactose) | Emulsification | [171] |
| Alteromonas hispanica F23T | Anionic hetero-polysaccharide (glucose, mannose, xylose) | Emulsification | [170] |
| Pseudoalteromonas ruthenica SBT 033 | Heteropolysaccharide (rhamnose, fructose, ribose, arabinose, xylose, mannose, galactose, glucose) containing uronic acid | Thickening | [172] |
| Halomonas sp. TG39 | Anionic hetero-polysaccharide | Emulsification | [173] |
| Flexibacter sp. TG382 | Glycoprotein | Emulsification Thickening | [174] |
| Halomonas xianhensis SUR308 | Hetero-polysaccharide (glucose, galactose, mannose) | Thickening Heat stable | [175] |
| Acinetobacter sp. | Glyco-lipo-protein | Emulsification Surfactancy | [176] |
| Acinetobacter bouvetii UAM25 | Exopolysaccharide | Emulsification | [177] |
| Chromohalobacter canadensis 28 | Hetropolysaccharide (glucosamine, glucose, rhamnose, xylose), and protein (polyglutamate) complex | Emulsification Foaming Thickening Gelation | [178] |
| Pseudomonas fluorescens | Heteropolysaccharide (galactose, glucose, fructose, mannose, rhamnose) and protein | Emulsification | [179] |
| Rhodobacter johrii CDR-SL 7Cii | Heteropolysaccharide (glucose, glucuronic acid, rhamnose, galactose) | Emulsification Heat stable | [180] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anestopoulos, I.; Kiousi, D.-E.; Klavaris, A.; Maijo, M.; Serpico, A.; Suarez, A.; Sanchez, G.; Salek, K.; Chasapi, S.A.; Zompra, A.A.; et al. Marine-Derived Surface Active Agents: Health-Promoting Properties and Blue Biotechnology-Based Applications. Biomolecules 2020, 10, 885. https://doi.org/10.3390/biom10060885
Anestopoulos I, Kiousi D-E, Klavaris A, Maijo M, Serpico A, Suarez A, Sanchez G, Salek K, Chasapi SA, Zompra AA, et al. Marine-Derived Surface Active Agents: Health-Promoting Properties and Blue Biotechnology-Based Applications. Biomolecules. 2020; 10(6):885. https://doi.org/10.3390/biom10060885
Chicago/Turabian StyleAnestopoulos, Ioannis, Despina-Evgenia Kiousi, Ariel Klavaris, Monica Maijo, Annabel Serpico, Alba Suarez, Guiomar Sanchez, Karina Salek, Stylliani A. Chasapi, Aikaterini A. Zompra, and et al. 2020. "Marine-Derived Surface Active Agents: Health-Promoting Properties and Blue Biotechnology-Based Applications" Biomolecules 10, no. 6: 885. https://doi.org/10.3390/biom10060885
APA StyleAnestopoulos, I., Kiousi, D.-E., Klavaris, A., Maijo, M., Serpico, A., Suarez, A., Sanchez, G., Salek, K., Chasapi, S. A., Zompra, A. A., Galanis, A., Spyroulias, G. A., Gombau, L., Euston, S. R., Pappa, A., & Panayiotidis, M. I. (2020). Marine-Derived Surface Active Agents: Health-Promoting Properties and Blue Biotechnology-Based Applications. Biomolecules, 10(6), 885. https://doi.org/10.3390/biom10060885








