Evaluation of Resin-Based Material Containing Copaiba Oleoresin (Copaifera Reticulata Ducke): Biological Effects on the Human Dental Pulp Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Flow Cytometry
2.3. Biomaterials
2.4. Conditioned Media and Experimental Groups
2.5. Cell Viability
2.6. Migration
2.7. Differentiation: Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
2.8. Mineralization: Mineralized Matrix Formation (Alizarin Red Assay)
2.9. Statistical Analysis
3. Results
3.1. The Dental Pulp Cells Presented a Mesenchymal Stem Cell Immunophenotype
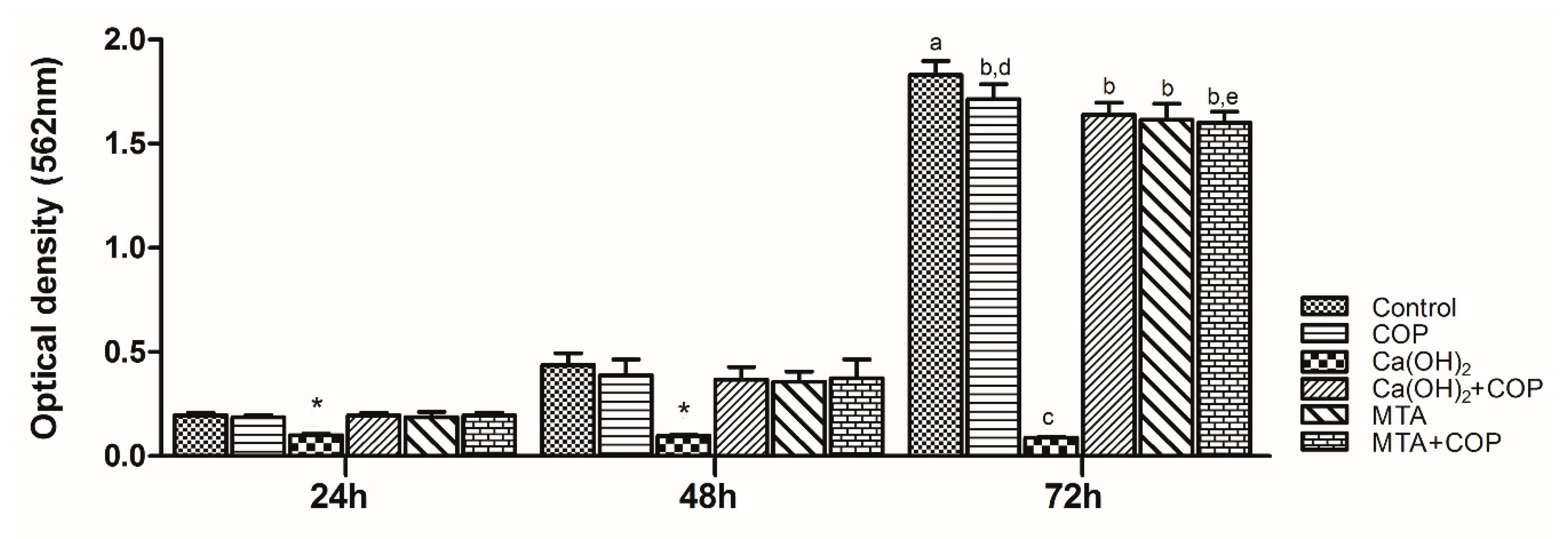
3.2. Copaiba Oleoresin Promoted the Cell Growth per se and When Combined with Other Biomaterials
3.3. The Association of Copaiba Oleoresin and Mineral Trioxide Aggregate (MTA) Promoted Greater Expression of Genes Associated with Biomineralization Process
3.4. The Copaiba Improved the Cell Migration When Compared to the Other Biomaterials
3.5. The Copaiba per se and Combined with Other Biomaterials Promoted the Formation of Mineralization Nodules
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferracane, J.L.; Cooper, P.R.; Smith, A.J. Can interaction of materials with the dentin-pulp complex contribute to dentin regeneration? Odontology 2010, 98, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, P.; Linsuwanont, P. Vital Pulp Therapy in Vital Permanent Teeth with Cariously Exposed Pulp: A Systematic Review. J. Endod. 2011, 37, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Smith, J.; Shelton, R.M.; Cooper, P.R. Harnessing the Natural Regenerative Potential of the Dental Pulp. Dent. Clin. N. Am. 2012, 56, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.; Schmalz, G. Toward a strategic plan for pulp healing: From repair to regeneration. Clin. Oral Investig. 2011, 15, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Accorinte, M.L.R.; Loguercio, A.D.; Reis, A.; Carneiro, E.; Grande, R.H.M.; Murata, S.S.; Holland, R. Response of Human Dental Pulp Capped with MTA and Calcium Hydroxide Powder. Oper. Dent. 2008, 33, 488–495. [Google Scholar] [CrossRef]
- Kanisavaran, Z.M.; Dummer, P.M.H. Properties and applications of calcium hydroxide in endodontics and dental traumatology. Int. Endod. J. 2011, 44, 697–730. [Google Scholar] [CrossRef]
- Dammaschke, T.; Stratmann, U.; Wolff, P.; Sagheri, D.; Schäfer, E. Direct Pulp Capping with Mineral Trioxide Aggregate: An Immunohistologic Comparison with Calcium Hydroxide in Rodents. J. Endod. 2010, 36, 814–819. [Google Scholar] [CrossRef]
- Da Rosa, W.L.O.; Cocco, A.R.; Da Silva, T.M.; Mesquita, L.C.; Galarça, A.D.; Da Silva, A.F.; Piva, E. Current trends and future perspectives of dental pulp capping materials: A systematic review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 106, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Parirokh, M.; Torabinejad, M.; Dummer, P.M.H. Mineral trioxide aggregate and other bioactive endodontic cements: An updated overview-part I: Vital pulp therapy. Int. Endod. J. 2017, 51, 177–205. [Google Scholar] [CrossRef] [PubMed]
- Parirokh, M.; Torabinejad, M. Mineral Trioxide Aggregate: A Comprehensive Literature Review—Part I: Chemical, Physical, and Antibacterial Properties. J. Endod. 2010, 36, 16–27. [Google Scholar] [CrossRef]
- Parirokh, M.; Torabinejad, M. Mineral Trioxide Aggregate: A Comprehensive Literature Review—Part III: Clinical Applications, Drawbacks, and Mechanism of Action. J. Endod. 2010, 36, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Leites, A.; Baldissera, E.; Da Silva, A.F.; Tarquinio, S.; Botero, T.; Piva, E.; Demarco, F.; Demarco, F.F. Histologic Response and Tenascin and Fibronectin Expression After Pulp Capping in Pig Primary Teeth With Mineral Trioxide Aggregate or Calcium Hydroxide. Oper. Dent. 2011, 36, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Veiga Junior, V.F.; Pinto, A.C. The Copaifera L. genus. Química Nova 2002, 25, 273–286. [Google Scholar] [CrossRef]
- Veiga-Junior, V.F.; Rosas, E.; Carvalho, M.; Henriques, M.D.G.; Pinto, A.C. Chemical composition and anti-inflammatory activity of copaiba oils from Copaifera cearensis Huber ex Ducke, Copaifera reticulata Ducke and Copaifera multijuga Hayne—A comparative study. J. Ethnopharmacol. 2007, 112, 248–254. [Google Scholar] [CrossRef]
- Teixeira, F.B.; Silva, R.D.B.; Lameira, O.; Webber, L.P.; Couto, R.; Martins, M.; Lima, R.R. Copaiba oil-resin (Copaifera reticulata Ducke) modulates the inflammation in a model of injury to rats’ tongues. BMC Complement. Altern. Med. 2017, 17, 313. [Google Scholar] [CrossRef] [Green Version]
- Wagner, V.P.; Webber, L.P.; Ortiz, L.; Rados, P.V.; Meurer, L.; Lameira, O.A.; Lima, R.R.; Martins, M.D. Efects of copaiba oil topical administration on oral wound healing. Phytother. Res. 2017, 31, 1283–1288. [Google Scholar] [CrossRef]
- Alvarenga, M.O.P.; Bittencourt, L.O.; Mendes, P.F.S.; Ribeiro, J.T.; Lameira, O.; Monteiro, M.C.; Barboza, C.A.G.; Martins, M.; Lima, R.R. Safety and Effectiveness of Copaiba Oleoresin (C. reticulata Ducke) on Inflammation and Tissue Repair of Oral Wounds in Rats. Int. J. Mol. Sci. 2020, 21, 3568. [Google Scholar] [CrossRef]
- Harada, M.; Kenmotsu, S.-I.; Nakasone, N.; Nakakura-Ohshima, K.; Ohshima, H. Cell dynamics in the pulpal healing process following cavity preparation in rat molars. Histochem. Cell Biol. 2008, 130, 773–783. [Google Scholar] [CrossRef]
- Guimarães-Santos, A.; Santos, D.S.; Santos, I.R.; Lima, R.R.; Pereira, A.; De Moura, L.S.; Carvalho, R.N.; Lameira, O.; Gomes-Leal, W. Copaiba Oil-Resin Treatment Is Neuroprotective and Reduces Neutrophil Recruitment and Microglia Activation after Motor Cortex Excitotoxic Injury. Evid. Based Complement. Altern. Med. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Al-Habib, M.; Huang, G.T.-J. Dental Mesenchymal Stem Cells: Dental Pulp Stem Cells, Periodontal Ligament Stem Cells, Apical Papilla Stem Cells, and Primary Teeth Stem Cells-Isolation, Characterization, and Expansion for Tissue Engineering. Breast Cancer 2019, 1922, 59–76. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and invivo. Proc. Natl. Acad. Sci. USA. 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA. 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeitlin, B.D. Banking on teeth–Stem cells and the dental office. Biomed. J. 2020, 2319. [Google Scholar] [CrossRef]
- Nakamura, S.; Yamada, Y.; Katagiri, W.; Sugito, T.; Ito, K.; Ueda, M. Stem Cell Proliferation Pathways Comparison between Human Exfoliated Deciduous Teeth and Dental Pulp Stem Cells by Gene Expression Profile from Promising Dental Pulp. J. Endod. 2009, 35, 1536–1542. [Google Scholar] [CrossRef]
- Kaukua, N.; Chen, M.; Guarnieri, P.; Dahl, M.; Lim, M.L.; Yucel-Lindberg, T.; Sundstroem, E.; Adameyko, I.; Mao, J.; Fried, K. Molecular differences between stromal cell populations from deciduous and permanent human teeth. Stem Cell Res. Ther. 2015, 6, 59. [Google Scholar] [CrossRef] [Green Version]
- Garcia, L.; Cristiane, S.; Wilson, M.J.; Soraya, M.J.; Lopes, R.A.; Mônica, R.; De Freitas, O. Biocompatibility assessment of pastes containing Copaiba oilresin, propolis, and calcium hydroxide in the subcutaneous tissue of rats. J. Conserv. Dent. 2011, 14, 108–112. [Google Scholar] [CrossRef] [Green Version]
- Lima, R.V.E.; Esmeraldo, M.R.A.; De Carvalho, M.G.F.; De Oliveira, P.T.; De Carvalho, R.A.; Da Silva, F.L.; Costa, E.M.M.D.B. Pulp repair after pulpotomy using different pulp capping agents: A comparative histologic analysis. Pediatr. Dent. 2011, 33, 14–18. [Google Scholar]
- Widbiller, M.; Lindner, S.R.; Buchalla, W.; Eidt, A.; Hiller, K.-A.; Schmalz, G.; Galler, K.M. Three-dimensional culture of dental pulp stem cells in direct contact to tricalcium silicate cements. Clin. Oral Investig. 2015, 20, 237–246. [Google Scholar] [CrossRef]
- Danesh, F.; Vahid, A.; Jahanbani, J.; Mashhadiabbas, F.; Arman, E. Effect of white mineral trioxide aggregate compared with biomimetic carbonated apatite on dentine bridge formation and inflammatory response in a dental pulp model. Int. Endod. J. 2011, 45, 26–34. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Fan, M. BioAggregate and iRoot BP Plus optimize the proliferation and mineralization ability of human dental pulp cells. Int. Endod. J. 2013, 46, 923–929. [Google Scholar] [CrossRef]
- Tran, X.; Gorin, C.; Willig, C.; Baroukh, B.; Pellat, B.; Decup, F.; Vital, S.O.; Chaussain, C.; Boukpessi, T. Effect of a Calcium-silicate-based Restorative Cement on Pulp Repair. J. Dent. Res. 2012, 91, 1166–1171. [Google Scholar] [CrossRef]
- Ji, Y.M.; Jeon, S.H.; Park, J.Y.; Chung, J.H.; Choung, Y.H. Choung PH Dental stem cell therapy with calcium hydroxide in dental pulp capping. Tissue Eng. Part A 2010, 16, 1823–1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Antò, V.; Di Caprio, M.P.; Ametrano, G.; Simeone, M.; Rengo, S.; Spagnuolo, G. Effect of Mineral Trioxide Aggregate on Mesenchymal Stem Cells. J. Endod. 2010, 36, 1839–1843. [Google Scholar] [CrossRef] [PubMed]
- Rickard, D.; Sullivan, T.; Shenker, B.; Leboy, P.; Kazhdan, I. Induction of Rapid Osteoblast Differentiation in Rat Bone Marrow Stromal Cell Cultures by Dexamethasone and BMP-2. Dev. Boil. 1994, 161, 218–228. [Google Scholar] [CrossRef]
- Nakasone, N.; Yoshie, H.; Ohshima, H. The relationship between the termination of cell proliferation and expression of heat-shock protein-25 in the rat developing tooth germ. Eur. J. Oral Sci. 2006, 114, 302–309. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Gao, Y.; Ling, J.; Wei, X.; Xiao, Y. Calcium ions promote osteogenic differentiation and mineralization of human dental pulp cells: Implications for pulp capping materials. J. Mater. Sci. Mater. Electron. 2011, 23, 789–795. [Google Scholar] [CrossRef]





| Materials | Ca(OH)2 | White MTA | COP | Ca(OH)2 + COP | White MTA + COP |
|---|---|---|---|---|---|
| Main Components | Calcium hydroxide | Silicon dioxide, potassium oxide, aluminum oxide, sodium oxide, hematite, sulfur trioxide, calcium oxide, bismuth oxide, magnesium oxide and insoluble residues of crystalline silica, calcium oxide and potassium and sodium sulphates. | δ-elemene; cyclosativene; α-copaene; δ-elemene; cyclosativene; α-copaene; β-elemene; α-gurjunene; β-caryophyllene; trans-α-bergamotene; aromadendrene; epi-β-santalene; α-humulene + (E)-β-farnesene; β-chamigrene; γ-gurjunene; γ-curcumene; β-selinene; α-selinene; (Z)-α-bisabolene; α-bulnesene; β-bisabolene; β-curcumene; β-sesquiphelandrene; (E)-γ-bisabolene; caryophyllene oxide; epi-β-bisabolol and β-bisabolol | Calcium hydroxide, δ-elemene; cyclosativene; α-copaene; δ-elemene; cyclosativene; α-copaene; β-elemene; α-gurjunene; β-caryophyllene; trans-α-bergamotene; aromadendrene; epi-β-santalene; α-humulene + (E)-β-farnesene; β-chamigrene; γ-gurjunene; γ-curcumene; β-selinene; α-selinene; (Z)-α-bisabolene; α-bulnesene; β-bisabolene; β-curcumene; β-sesquiphelandrene; (E)-γ-bisabolene; caryophyllene oxide; epi-β-bisabolol and β-bisabolol | Silicon dioxide, potassium oxide, aluminum oxide, sodium oxide, hematite, sulfur trioxide, calcium oxide, bismuth oxide, magnesium oxide and insoluble residues of crystalline silica, calcium oxide, potassium and sodium sulfates, δ-elemene; cyclosativene; α-copaene; δ-elemene; cyclosativene; α-copaene; β-elemene; α-gurjunene; β-caryophyllene; trans-α-bergamotene; aromadendrene; epi-β-santalene; α-humulene + (E)-β-farnesene; β-chamigrene; γ-gurjunene; γ-curcumene; β-selinene; α-selinene; (Z)-α-bisabolene; α-bulnesene; β-bisabolene; β-curcumene; β-sesquiphelandrene; (E)-γ-bisabolene; caryophyllene oxide; epi-β-bisabolol and β-bisabolol |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Couto, R.S.D.; Rodrigues, M.F.S.D.; Ferreira, L.S.; Diniz, I.M.A.; Silva, F.d.S.; Lopez, T.C.C.; Lima, R.R.; Marques, M.M. Evaluation of Resin-Based Material Containing Copaiba Oleoresin (Copaifera Reticulata Ducke): Biological Effects on the Human Dental Pulp Stem Cells. Biomolecules 2020, 10, 972. https://doi.org/10.3390/biom10070972
Couto RSD, Rodrigues MFSD, Ferreira LS, Diniz IMA, Silva FdS, Lopez TCC, Lima RR, Marques MM. Evaluation of Resin-Based Material Containing Copaiba Oleoresin (Copaifera Reticulata Ducke): Biological Effects on the Human Dental Pulp Stem Cells. Biomolecules. 2020; 10(7):972. https://doi.org/10.3390/biom10070972
Chicago/Turabian StyleCouto, Roberta Souza D’Almeida, Maria Fernanda Setubal Destro Rodrigues, Leila Soares Ferreira, Ivana Márcia Alves Diniz, Fernando de Sá Silva, Talita Christine Camilo Lopez, Rafael Rodrigues Lima, and Márcia Martins Marques. 2020. "Evaluation of Resin-Based Material Containing Copaiba Oleoresin (Copaifera Reticulata Ducke): Biological Effects on the Human Dental Pulp Stem Cells" Biomolecules 10, no. 7: 972. https://doi.org/10.3390/biom10070972
APA StyleCouto, R. S. D., Rodrigues, M. F. S. D., Ferreira, L. S., Diniz, I. M. A., Silva, F. d. S., Lopez, T. C. C., Lima, R. R., & Marques, M. M. (2020). Evaluation of Resin-Based Material Containing Copaiba Oleoresin (Copaifera Reticulata Ducke): Biological Effects on the Human Dental Pulp Stem Cells. Biomolecules, 10(7), 972. https://doi.org/10.3390/biom10070972






