The Alcohol–High-Density Lipoprotein Athero-Protective Axis
Abstract
:1. Introduction
2. Correlating HDL, Alcohol Ingestion, and ASCVD
3. Alcohol Metabolism
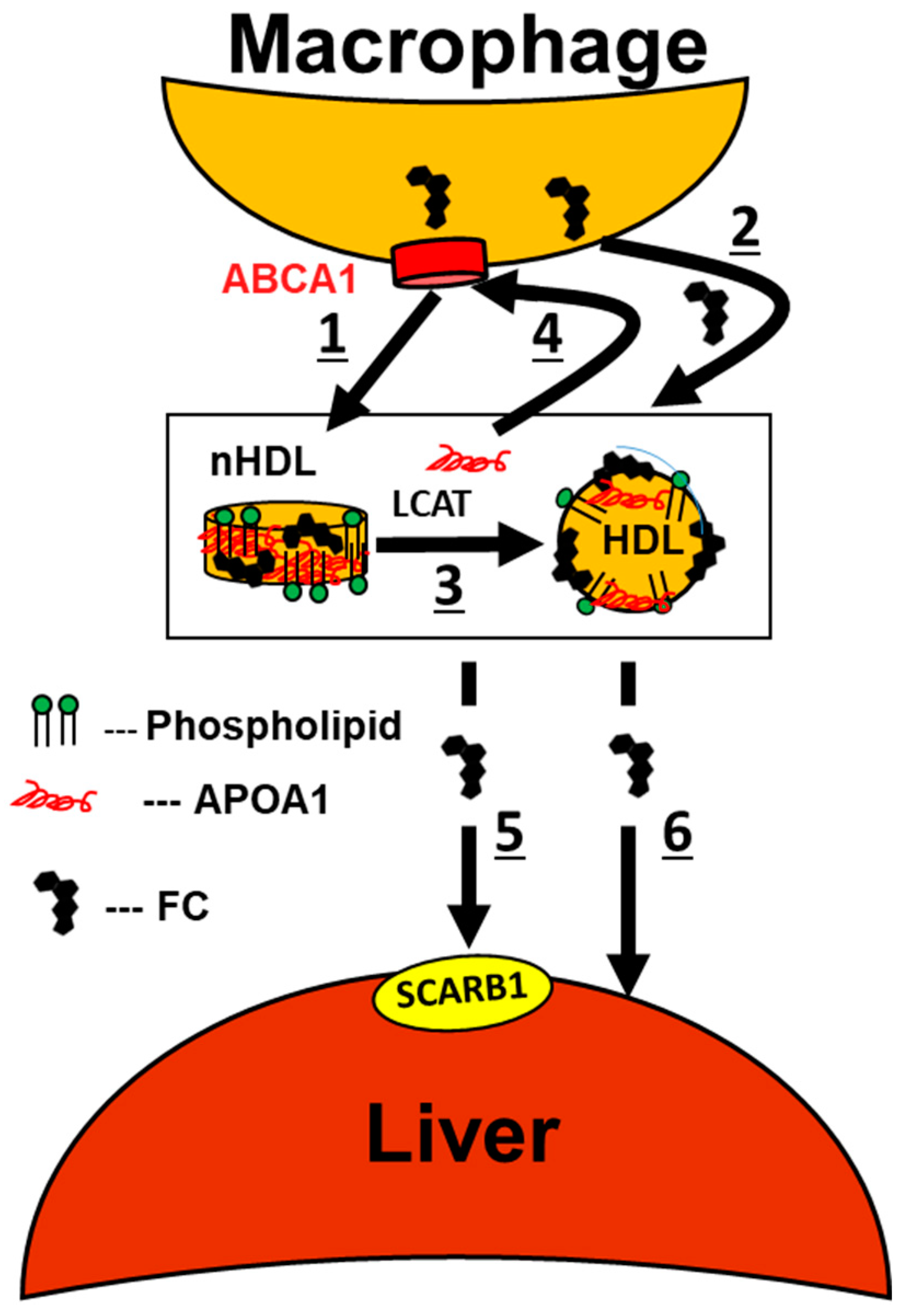
4. Chronic Alcohol Consumption Enhances MCE
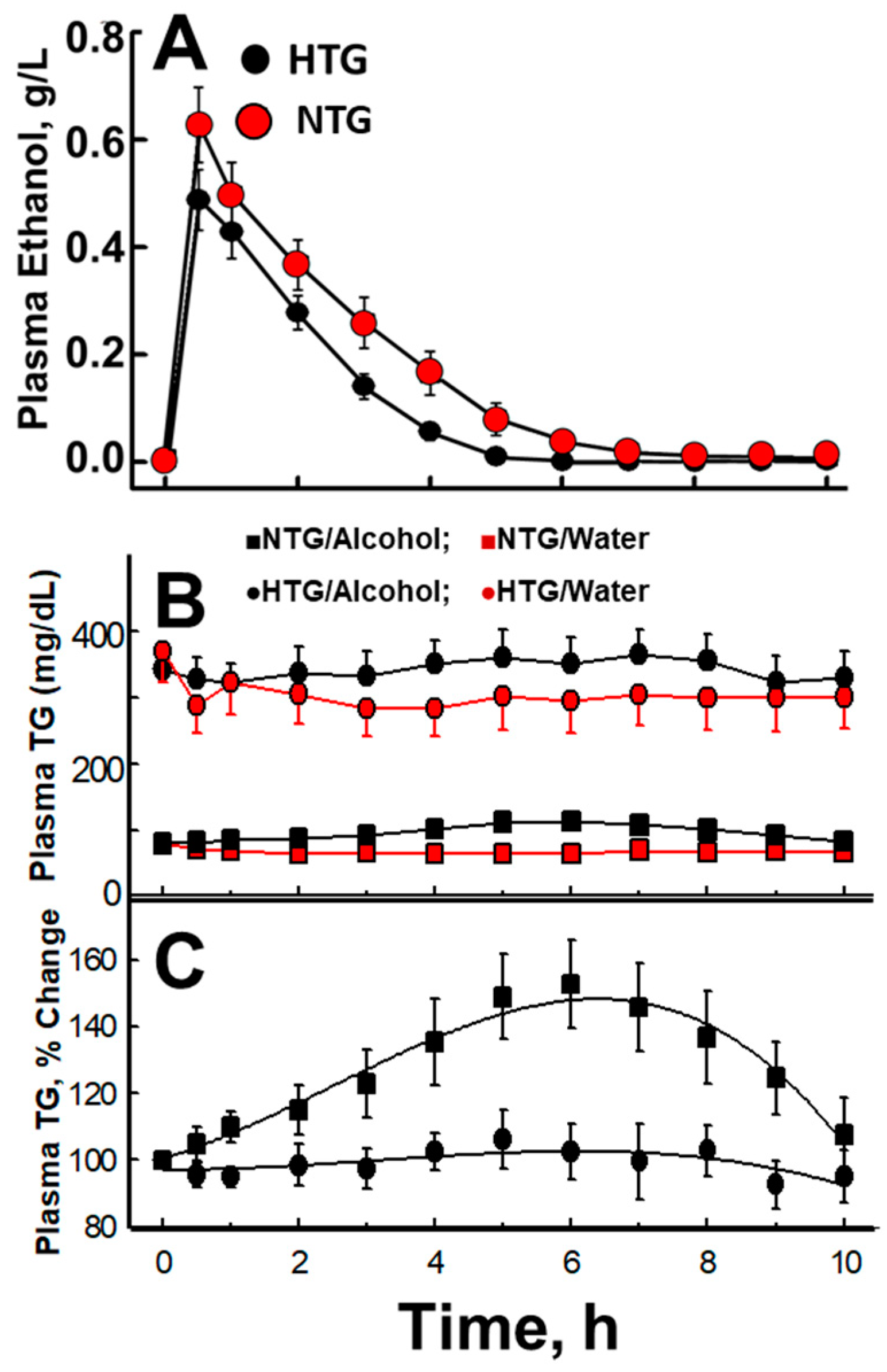
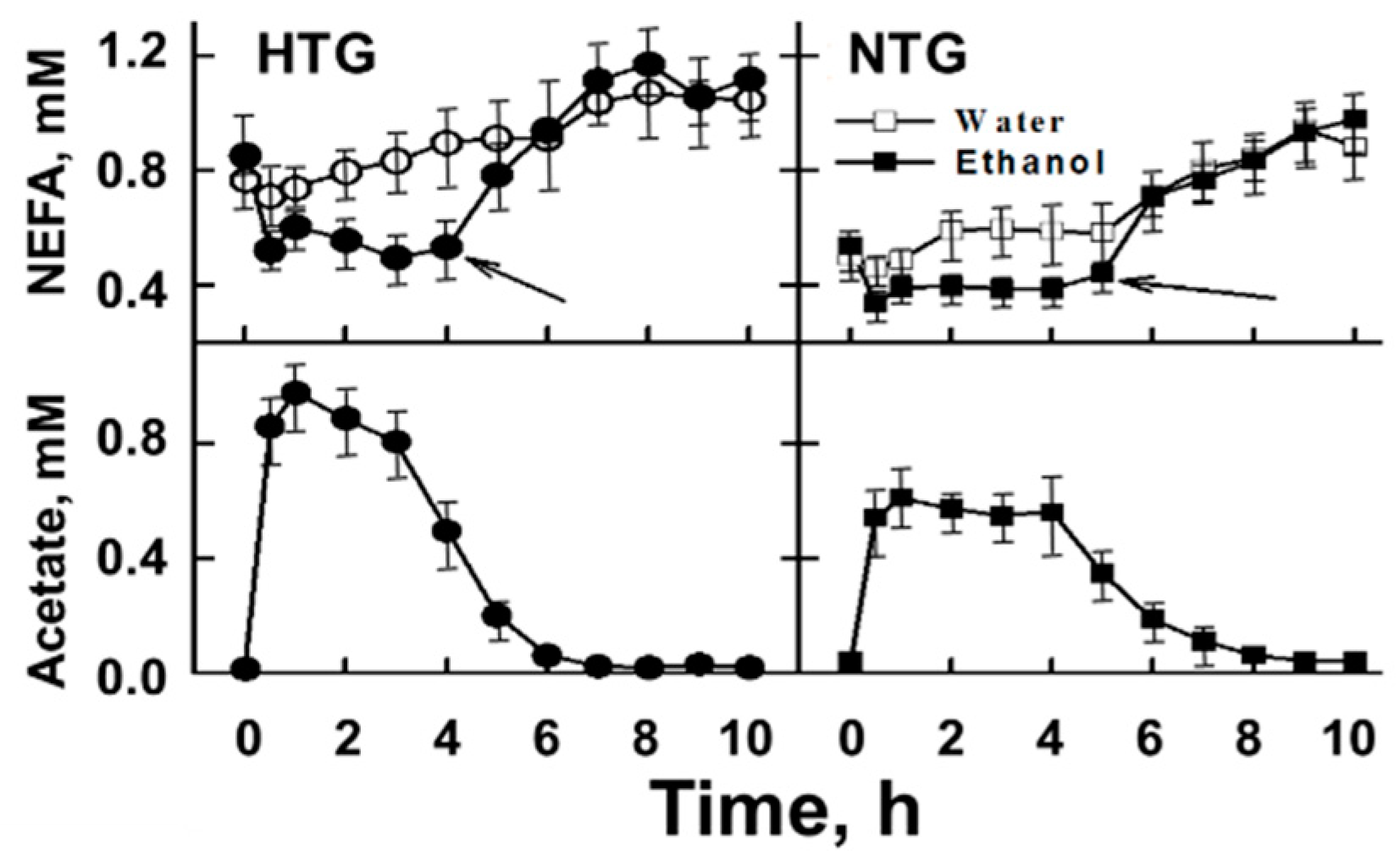
5. Acute Effects of Alcohol Consumption
6. The Role of Acetate in Alcohol-Mediated Effects on Plasma Lipid Metabolism
7. Mechanisms Underlying the Alcohol–HDL Athero-Protective Axis
8. A Mechanistic Model Links Alcohol Ingestion to a Cardio-Protective Plasma Lipoprotein Profile
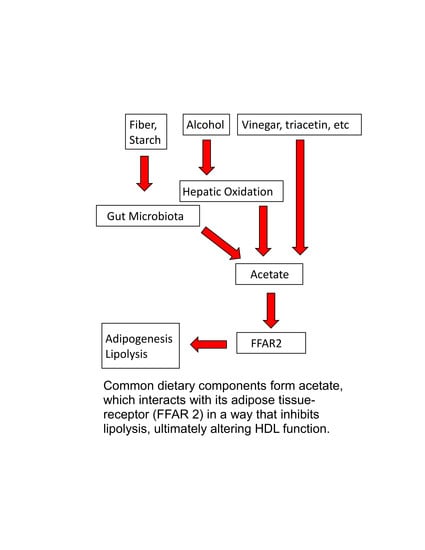
9. Acetate in Foods
10. Acetate as ASCVD Therapy
11. Open Questions
- Does acetate ingestion molar-equivalently enhance the postprandial lipemia seen with alcohol only?
- Whereas the lipemia induced by both alcohol and fat consumed separately increases with the magnitude of fasting plasma TG levels, is a similar relationship seen when acetate is consumed with fat-containing food and, if so, are the effects of acetate and fat co-ingestion synergistic or additive?
- Is the occurrence of obesity-linked diabetes, pancreatitis, and ASCVD among persons with mutation-associated deficiencies in alcohol-metabolizing enzymes different from that of those carrying the metabolically competent alleles?
- What if any role does the FFAR2 have in the etiology of ASCVD in consumers vs. non-consumers of alcohol and acetate or its precursors?
- Do molar-equivalent amounts of acetate and alcohol provide similar cardio-protective effects?
Author Contributions
Funding
Conflicts of Interest
References
- Gofman, J.W.; Young, W.; Tandy, R. Ischemic Heart Disease, Atherosclerosis, and Longevity. Circulation 1966, 34, 679–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, P.T.; Feldman, D.E. Prospective study of coronary heart disease vs. HDL2, HDL3, and other lipoproteins in Gofman’s Livermore Cohort. Atherosclerosis 2011, 214, 196–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, P.W.; Abbott, R.D.; Castelli, W.P. High density lipoprotein cholesterol and mortality. The Framingham Heart Study. Arteriosclerosis 1988, 8, 737–741. [Google Scholar] [CrossRef] [Green Version]
- Frick, M.H.; Elo, O.; Haapa, K.; Heinonen, O.P.; Heinsalmi, P.; Helo, P.; Huttunen, J.K.; Kaitaniemi, P.; Koskinen, P.; Manninen, V.; et al. Helsinki Heart Study: Primary-Prevention Trial with Gemfibrozil in Middle-Aged Men with Dyslipidemia. New Engl. J. Med. 1987, 317, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Manninen, V.; Elo, M.O.; Frick, M.H.; Haapa, K.; Heinonen, O.P.; Heinsalmi, P.; Helo, P.; Huttunen, J.K.; Kaitaniemi, P.; Koskinen, P.; et al. Lipid Alterations and Decline in the Incidence of Coronary Heart Disease in the Helsinki Heart Study. JAMA 1988, 260, 641. [Google Scholar] [CrossRef] [PubMed]
- Robins, S.; Collins, D.; Wittes, J. Relation of gemfibrozil treatment and lipid levels with major coronary events. VA-HIT: A randomized controlled trial. ACC Curr. J. Rev. 2001, 10, 23–24. [Google Scholar] [CrossRef]
- Cuchel, M.; Rader, D. Macrophage Reverse Cholesterol Transport. Circulation 2006, 113, 2548–2555. [Google Scholar] [CrossRef]
- Zhong, S.; Sharp, D.S.; Grove, J.S.; Bruce, C.; Yano, K.; Curb, J.D.; Tall, A.R. Increased coronary heart disease in Japanese-American men with mutation in the cholesteryl ester transfer protein gene despite increased HDL levels. J. Clin. Investig. 1996, 97, 2917–2923. [Google Scholar] [CrossRef]
- Haase, C.L.; Tybjaerg-Hansen, A.; Grande, P.; Frikke-Schmidt, R. Genetically Elevated Apolipoprotein A-I, High-Density Lipoprotein Cholesterol Levels, and Risk of Ischemic Heart Disease. J. Clin. Endocrinol. Metab. 2010, 95, 500–510. [Google Scholar] [CrossRef] [Green Version]
- Voight, B.F.; Peloso, G.M.; Orho-Melander, M.; Frikke-Schmidt, R.; Barbalić, M.; Jensen, M.K.; Hindy, G.; Holm, H.; Ding, E.L.; Johnson, T.; et al. Plasma HDL cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet 2012, 380, 572–580. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, G.G.; Olsson, A.G.; Abt, M.; Ballantyne, C.M.; Barter, P.J.; Brumm, J.; Chaitman, B.R.; Holme, I.; Kallend, D.; Leiter, L.A.; et al. Effects of Dalcetrapib in Patients with a Recent Acute Coronary Syndrome. New Engl. J. Med. 2012, 367, 2089–2099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barter, P.J.; Caulfield, M.J.; Eriksson, M.; Grundy, S.M.; Kastelein, J.J.; Komajda, M.; López-Sendón, J.; Mosca, L.; Tardif, J.-C.; Waters, D.D.; et al. Effects of Torcetrapib in Patients at High Risk for Coronary Events. New Engl. J. Med. 2007, 357, 2109–2122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boden, W.E.; Probstfield, J.L.; Anderson, T.; Chaitman, B.R.; Desvignes-Nickens, P.; Koprowicz, K.; McBride, R.; Teo, K.; Weintraub, W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 2011, 365, 2255–2267. [Google Scholar]
- Santander, N.G.; Contreras-Duarte, S.; Awad, M.F.; Lizama, C.; Passalacqua, I.; Rigotti, A.; Busso, D. Developmental abnormalities in mouse embryos lacking the HDL receptor SR-BI. Hum. Mol. Genet. 2013, 22, 2551. [Google Scholar] [CrossRef] [Green Version]
- Anonymous. The coronary drug project. Findings leading to further modifications of its protocol with respect to dextrothyroxine. The coronary drug project research group. JAMA 1972, 220, 996–1008. [Google Scholar]
- Maclure, M. Demonstration of Deductive Meta-Analysis: Ethanol Intake and Risk of Myocardial Infarction. Epidemiol. Rev. 1993, 15, 328–351. [Google Scholar] [CrossRef]
- Gaziano, J.; Buring, J.E. Alcohol intake, lipids and risks of myocardial infarction. Ciba Foundation Symposium—Bilharziasis 1998, 216, 86–110. [Google Scholar] [CrossRef]
- Toth, P.P.; Barter, P.J.; Rosenson, R.S.; Boden, W.E.; Chapman, M.J.; Cuchel, M.; D’Agostino, R.B.; Davidson, M.H.; Davidson, W.S.; Heinecke, J.W.; et al. High-density lipoproteins: A consensus statement from the National Lipid Association. J. Clin. Lipidol. 2013, 7, 484–525. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Brewer, H.B.; Davidson, W.S.; Fayad, Z.A.; Fuster, V.; Goldstein, J.; Hellerstein, M.; Jiang, X.-C.; Phillips, M.; Rader, D.J.; et al. Cholesterol efflux and atheroprotection: Advancing the concept of reverse cholesterol transport. Circulation 2012, 125, 1905–1919. [Google Scholar] [CrossRef] [Green Version]
- Rosenson, R.S.; Brewer, H.B.; Ansell, B.J.; Barter, P.; Chapman, M.J.; Heinecke, J.W.; Kontush, A.; Tall, A.R.; Webb, N.R. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat. Rev. Cardiol. 2015, 13, 48–60. [Google Scholar] [CrossRef]
- Rohatgi, A.; Khera, A.; Berry, J.D.; Givens, E.G.; Ayers, C.R.; Wedin, K.E.; Neeland, I.J.; Yuhanna, I.S.; Rader, D.R.; De Lemos, J.A.; et al. HDL cholesterol efflux capacity and incident cardiovascular events. New Engl. J. Med. 2014, 371, 2383–2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khera, A.V.; Cuchel, M.; De La Llera-Moya, M.; Rodrigues, A.; Burke, M.F.; Jafri, K.; French, B.C.; Phillips, J.A.; Mucksavage, M.L.; Wilensky, R.L.; et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. New Engl. J. Med. 2011, 364, 127–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleheen, D.; Scott, R.; Javad, S.; Zhao, W.; Rodrigues, A.; Picataggi, A.; Lukmanova, D.; Mucksavage, M.L.; Luben, R.N.; Billheimer, J.; et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: A prospective case-control study. Lancet Diabetes Endocrinol. 2015, 3, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Soria-Florido, M.T.; Castañer, O.; Lassale, C.; Estruch, R.; Salas-Salvadó, J.; Martínez-González, M.Á.; Corella, D.; Ros, E.; Arós, F.; Elosua, R.; et al. Dysfunctional High-Density Lipoproteins Are Associated With a Greater Incidence of Acute Coronary Syndrome in a Population at High Cardiovascular Risk. Circulation 2020, 141, 444–453. [Google Scholar] [CrossRef]
- Swertfeger, D.; Rebholz, S.; Li, H.; Shah, A.S.; Davidson, W.S.; Lu, L.J. Feasibility of a plasma bioassay to assess oxidative protection of low-density lipoproteins by high-density lipoproteins. J. Clin. Lipidol. 2018, 12, 1539–1548. [Google Scholar] [CrossRef]
- Yvan-Charvet, L.; Wang, N.; Tall, A.R. Role of HDL, ABCA1, and ABCG1 Transporters in Cholesterol Efflux and Immune Responses. Arter. Thromb. Vasc. Boil. 2010, 30, 139–143. [Google Scholar] [CrossRef] [Green Version]
- Zimmer, S.; Grebe, A.; Bakke, S.S.; Bode, N.; Halvorsen, B.; Ulas, T.; Skjelland, M.; De Nardo, D.; Labzin, L.; Kerksiek, A.; et al. Cyclodextrin promotes atherosclerosis regression via macrophage reprogramming. Sci. Transl. Med. 2016, 8, 333ra50. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Schwabe, R.F.; DeVries-Seimon, T.; Yao, P.M.; Gerbod-Giannone, M.-C.; Tall, A.R.; Davis, R.J.; Flavell, R.; Brenner, D.A.; Tabas, I. Free Cholesterol-loaded Macrophages Are an Abundant Source of Tumor Necrosis Factor-α and Interleukin-6. J. Boil. Chem. 2005, 280, 21763–21772. [Google Scholar] [CrossRef] [Green Version]
- Fotakis, P.; Kothari, V.; Thomas, D.G.; Westerterp, M.; Molusky, M.M.; Altin, E.; Abramowicz, S.; Wang, N.; He, Y.; Heinecke, J.W.; et al. Anti-Inflammatory Effects of HDL (High-Density Lipoprotein) in Macrophages Predominate Over Proinflammatory Effects in Atherosclerotic Plaques. Arter. Thromb. Vasc. Boil. 2019, 39, 253. [Google Scholar] [CrossRef]
- Tchoua, U.; Rosales, C.; Tang, D.; Gillard, B.K.; Vaughan, A.; Lin, H.Y.; Courtney, H.S.; Pownall, H.J. Serum Opacity Factor Enhances HDL-Mediated Cholesterol Efflux, Esterification and Anti Inflammatory Effects. Lipids 2010, 45, 1117–1126. [Google Scholar] [CrossRef] [Green Version]
- Ross, R.; A Glomset, J. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science 1973, 180, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Gillard, B.K.; Bassett, G.R.; Gotto, A.M.; Rosales, C.; Pownall, H.J. Scavenger receptor B1 (SR-B1) profoundly excludes high density lipoprotein (HDL) apolipoprotein AII as it nibbles HDL-cholesteryl ester. J. Boil. Chem. 2017, 292, 8864–8873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glass, C.; Pittman, R.C.; Weinstein, D.B.; Steinberg, D. Dissociation of tissue uptake of cholesterol ester from that of apoprotein A-I of rat plasma high density lipoprotein: Selective delivery of cholesterol ester to liver, adrenal, and gonad. Proc. Natl. Acad. Sci. USA 1983, 80, 5435–5439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acton, S.; Rigotti, A.; Landschulz, K.T.; Xu, S.; Hobbs, H.H.; Krieger, M. Identification of Scavenger Receptor SR-BI as a High Density Lipoprotein Receptor. Science 1996, 271, 518–520. [Google Scholar] [CrossRef]
- Smoak, K.A.; Aloor, J.J.; Madenspacher, J.; Merrick, B.A.; Collins, J.B.; Zhu, X.; Cavigiolio, G.; Oda, M.N.; Parks, J.S.; Fessler, M.B. Myeloid Differentiation Primary Response Protein 88 Couples Reverse Cholesterol Transport to Inflammation. Cell Metab. 2010, 11, 493–502. [Google Scholar] [CrossRef] [Green Version]
- Van der Vorst, E.P.C.; Theodorou, K.; Wu, Y.; Hoeksema, M.A.; Goossens, P.; Bursill, C.A.; Aliyev, T.; Huitema, L.F.A.; Tas, S.W.; Wolfs, I.M.J.; et al. High-Density Lipoproteins Exert Pro-inflammatory Effects on Macrophages via Passive Cholesterol Depletion and PKC-NF-κB/STAT1-IRF1 Signaling. Cell Metab. 2017, 25, 197–207. [Google Scholar] [CrossRef] [Green Version]
- Li, X.-M.; Tang, W.H.W.; Mosior, M.K.; Huang, Y.; Wu, Y.; Matter, W.; Gao, V.; Schmitt, D.; DiDonato, J.A.; Fisher, E.A.; et al. Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arter. Thromb. Vasc. Boil. 2013, 33, 1696–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mutharasan, R.K.; Thaxton, C.S.; Berry, J.; Daviglus, M.L.; Yuan, C.; Sun, J.; Ayers, C.; Lloyd-Jones, D.; Wilkins, J.T. HDL efflux capacity, HDL particle size, and high-risk carotid atherosclerosis in a cohort of asymptomatic older adults: The Chicago Healthy Aging Study. J. Lipid Res. 2017, 58, 600–606. [Google Scholar] [CrossRef] [Green Version]
- Aeddula, N.R.; Trivedi, N.; Pathireddy, S.; De Vries, R.; Groen, A.K.; Dullaart, R.P.F.; Asleh, R.; Levy, A.P.; Blum, S.; Frohlich, J.; et al. Cholesterol Efflux Capacity and Atherosclerosis. New Engl. J. Med. 2011, 364, 1472–1475. [Google Scholar] [CrossRef]
- Josefs, T.; Wouters, K.; Tietge, U.J.; Annema, W.; Dullaart, R.P.; Vaisar, T.; Arts, I.C.; Van Der Kallen, C.J.; Stehouwer, C.D.; Schalkwijk, C.G.; et al. High-density lipoprotein cholesterol efflux capacity is not associated with atherosclerosis and prevalence of cardiovascular outcome: The CODAM study. J. Clin. Lipidol. 2020, 14, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Brenn, T. The Tromso heart study: Alcoholic beverages and coronary risk factors. J. Epidemiol. Community Heal. 1986, 40, 249–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuvelier, I.; Steinmetz, J.; Mikstacki, T.; Siest, G. Variations in total phospholipids and high-density lipoprotein phospholipids in plasma from a general population: Reference intervals and influence of xenobiotics. Clin. Chem. 1985, 31, 763–766. [Google Scholar] [CrossRef]
- Clevidence, B.A.; Reichman, M.E.; Judd, J.T.; Muesing, R.A.; Schatzkin, A.; Schaefer, E.J.; Li, Z.; Jenner, J.; Brown, C.C.; Sunkin, M. Effects of alcohol consumption on lipoproteins of premenopausal women. A controlled diet study. Arter. Thromb. Vasc. Boil. 1995, 15, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, H.F.J.; Veenstra, J.; Van Tol, A.; Groener, J.E.M.; Schaafsma, G. Modearte Doses of Alcoholic Beverages with Dinner and Postprandial High Density Lipoprotein Composition. Alcohol Alcohol. 1998, 33, 403–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baer, D.J.; Judd, J.T.; Clevidence, B.A.; Muesing, R.A.; Campbell, W.S.; Brown, E.D.; Taylor, P.R. Moderate alcohol consumption lowers risk factors for cardiovascular disease in postmenopausal women fed a controlled diet. Am. J. Clin. Nutr. 2002, 75, 593–599. [Google Scholar] [CrossRef]
- Hartung, G.H.; Lawrence, S.J.; Reeves, R.S.; Foreyt, J.P. Effect of alcohol and exercise on postprandial lipemia and triglyceride clearance in men. Atherosclerosis 1993, 100, 33–40. [Google Scholar] [CrossRef]
- Hartung, G.H.; Foreyt, J.P.; Mitchell, R.E.; Reeves, R.S.; Gotto, A.M. Effect of Alcohol Intake on High-Density Lipoprotein Cholesterol Levels in Runners and Inactive Men. JAMA 1983, 249, 747. [Google Scholar] [CrossRef]
- Kabagambe, E.K.; Baylin, A.; Ruiz-Narvaez, E.; Rimm, E.B.; Campos, H. Alcohol intake, drinking patterns, and risk of nonfatal acute myocardial infarction in Costa Rica. Am. J. Clin. Nutr. 2005, 82, 1336–1345. [Google Scholar] [CrossRef] [Green Version]
- Mukamal, K.J.; Jensen, M.K.; Grønbæk, M.; Stampfer, M.J.; Manson, J.E.; Pischon, T.; Rimm, E.B. Drinking Frequency, Mediating Biomarkers, and Risk of Myocardial Infarction in Women and Men. Circulation 2005, 112, 1406–1413. [Google Scholar] [CrossRef] [Green Version]
- Beulens, J.W.J.; Rimm, E.B.; Ascherio, A.; Spiegelman, N.; Hendriks, H.F.J.; Mukamal, K.J. Alcohol Consumption and Risk for Coronary Heart Disease among Men with Hypertension. Ann. Intern. Med. 2007, 146, 10. [Google Scholar] [CrossRef]
- Mukamal, K.J.; Chiuve, S.E.; Rimm, E.B. Alcohol Consumption and Risk for Coronary Heart Disease in Men with Healthy Lifestyles. Arch. Intern. Med. 2006, 166, 2145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Goldberg, I.J.; Mosca, L.; Piano, M.R.; Fisher, E.A. Wine and Your Heart. A Science Advisory for Healthcare Professionals from the Nutrition Committee, Council on Epidemiology and Prevention, and Council on Cardiovascular Nursing of the American Heart Association. Circulation 2001, 103, 472–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linn, S.; Carroll, M.; Johnson, C.; Fulwood, R.; Kalsbeek, W.; Briefel, R. High-density lipoprotein cholesterol and alcohol consumption in US white and black adults: Data from NHANES II. Am. J. Public Heal. 1993, 83, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.R.D.O.E.; Foster, D.; Harper, M.M.; Seidman, C.E.; Smith, J.D.; Breslow, J.L.; Brinton, E.A. Alcohol consumption raises HDL cholesterol levels by increasing the transport rate of apolipoproteins A-I and A-II. Circulation 2000, 102, 2347–2352. [Google Scholar] [CrossRef]
- Thun, M.J.; Monaco, J.H.; Peto, R.; Heath, C.W.; Doll, R.; Lopez, A.; Henley, S.J. Alcohol Consumption and Mortality among Middle-Aged and Elderly U.S. Adults. New Engl. J. Med. 1997, 337, 1705–1714. [Google Scholar] [CrossRef]
- Gordon, T.; Kannel, W.B. Drinking and Mortality: The Framingham Study. Am. J. Epidemiology 1984, 120, 97–107. [Google Scholar] [CrossRef]
- Magnus, P.; Bakke, E.; Hoff, D.A.; Høiseth, G.; Graff-Iversen, S.; Knudsen, G.P.S.; Myhre, R.; Normann, P.T.; Næss, Ø.; Tambs, K.; et al. Controlling for High-Density Lipoprotein Cholesterol Does Not Affect the Magnitude of the Relationship Between Alcohol and Coronary Heart Disease. Circulation 2011, 124, 2296–2302. [Google Scholar] [CrossRef] [Green Version]
- Marques-Vidal, P.; Bochud, M.; Paccaud, F.; Waterworth, D.; Bergmann, S.; Preisig, M.; Waeber, G.; Vollenweider, P. No interaction between alcohol consumption and HDL-related genes on HDL cholesterol levels. Atherosclerosis 2010, 211, 551–557. [Google Scholar] [CrossRef]
- Baraona, E.; Lieber, C.S. Alcohol and lipids. Recent Dev. Alcohol. 1998, 14, 97–134. [Google Scholar]
- World Health Organization. United States of America, Alcohol Consumption. 2016. Available online: https://www.who.int/substance_abuse/publications/global_alcohol_report/profiles/usa.pdf (accessed on 26 June 2018).
- Eng, M.Y.; Luczak, S.E.; Wall, T.L. ALDH2, ADH1B, and ADH1C Genotypes in Asians: A Literature Review. Alcohol Res. Heal. J. Natl. Inst. Alcohol Abus. Alcohol. 2007, 30, 22–27. [Google Scholar]
- Ebtehaj, S.; Gruppen, E.G.; Bakker, S.J.; Dullaart, R.P.; Tietge, U.J. HDL (High-Density Lipoprotein) Cholesterol Efflux Capacity Is Associated With Incident Cardiovascular Disease in the General Population. Arter. Thromb. Vasc. Boil. 2019, 39, 1874–1883. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.V.; Demler, O.V.; Adelman, S.J.; Collins, H.L.; Glynn, R.J.; Ridker, P.M.; Rader, D.J.; Mora, S. Cholesterol Efflux Capacity, High-Density Lipoprotein Particle Number, and Incident Cardiovascular Events: An Analysis From the JUPITER Trial (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin). Circulation 2017, 135, 2494–2504. [Google Scholar] [CrossRef] [PubMed]
- Sierksma, A.; Vermunt, S.H.F.; Lankhuizen, I.M.; Van Der Gaag, M.S.; Scheek, L.M.; Grobbee, D.E.; Van Tol, A.; Hendriks, H.F. Effect of Moderate Alcohol Consumption on Parameters of Reverse Cholesterol Transport in Postmenopausal Women. Alcohol. Clin. Exp. Res. 2004, 28, 662–666. [Google Scholar] [CrossRef] [Green Version]
- Lesná, I.K.; Suchánek, P.; Stávek, P.; Poledne, R. May alcohol-induced increase of HDL be considered as atheroprotective? Physiol. Res. 2009, 59, 407–413. [Google Scholar]
- Van Der Gaag, M.S.; Van Tol, A.; Vermunt, S.H.; Scheek, L.M.; Schaafsma, G.; Hendriks, H.F. Alcohol consumption stimulates early steps in reverse cholesterol transport. J. Lipid Res. 2001, 42, 2077–2083. [Google Scholar]
- Perret, B.; Ruidavets, J.-B.; Vieu, C.; Jaspard, B.; Cambou, J.-P.; Tercé, F.; Collet, X. Alcohol consumption is associated with enrichment of high-density lipoprotein particles in polyunsaturated lipids and increased cholesterol esterification rate. Alcohol. Clin. Exp. Res. 2002, 26, 1134–1140. [Google Scholar] [CrossRef]
- Senault, C.; Betoulle, D.; Luc, G.; Hauw, P.; Rigaud, D.; Fumeron, F. Beneficial effects of a moderate consumption of red wine on cellular cholesterol efflux in young men. Nutr. Metab. Cardiovasc. Dis. 2000, 10, 63–69. [Google Scholar]
- Davidson, W.S.; Gillotte, K.L.; Lund-Katz, S.; Johnson, W.J.; Rothblat, G.H.; Phillips, M. The Effect of High Density Lipoprotein Phospholipid Acyl Chain Composition on the Efflux of Cellular Free Cholesterol. J. Boil. Chem. 1995, 270, 5882–5890. [Google Scholar] [CrossRef] [Green Version]
- Akopian, D.; Kawashima, R.L.; Medh, J. Phosphatidylcholine-Mediated Aqueous Diffusion of Cellular Cholesterol Down-Regulates the ABCA1 Transporter in Human Skin Fibroblasts. Int. J. Biochem. Res. Rev. 2015, 5, 214–224. [Google Scholar] [CrossRef] [Green Version]
- Tchoua, U.; Gillard, B.K.; Pownall, H.J. HDL superphospholipidation enhances key steps in reverse cholesterol transport. Atherosclerosis 2009, 209, 430–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillotte, K.L.; Davidson, W.S.; Lund-Katz, S.; Rothblat, G.H.; Phillips, M.C. Removal of cellular cholesterol by pre-beta-HDL involves plasma membrane microsolubilization. J. Lipid Res. 1998, 39, 1918–1928. [Google Scholar] [PubMed]
- Beulens, J.W.; Sierksma, A.; Van Tol, A.; Van Gent, T.; Fournier, N.; Paul, J.-L.; Hendriks, H.F. Moderate Alcohol Consumption Increases Cholesterol Reflux mediated by ABCA1. Alcohol. Clin. Exp. Res. 2004, 28, 7. [Google Scholar] [CrossRef] [Green Version]
- Chung, B.-H.; Franklin, F.; Cho, B.H.S.; Segrest, J.P.; Hart, K.; Darnell, B.E. Potencies of Lipoproteins in Fasting and Postprandial Plasma to Accept Additional Cholesterol Molecules Released From Cell Membranes. Arter. Thromb. Vasc. Boil. 1998, 18, 1217–1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, D.E.; Schreibman, P.H.; Brewster, A.C.; Arky, R.A. The enhancement of alimentary lipemia by ethanol in man. J. Lab. Clin. Med. 1970, 75, 264–274. [Google Scholar] [PubMed]
- Pownall, H.J. Dietary ethanol is associated with reduced lipolysis of intestinally derived lipoproteins. J. Lipid Res. 1994, 35, 2105–2113. [Google Scholar] [PubMed]
- Julia, Z.; Duchene, E.; Fournier, N.; Bellanger, N.; Chapman, M.J.; Le Goff, W.; Guerin, M. Postprandial lipemia enhances the capacity of large HDL2 particles to mediate free cholesterol efflux via SR-BI and ABCG1 pathways in type IIB hyperlipidemia. J. Lipid Res. 2010, 51, 3350–3358. [Google Scholar] [CrossRef] [Green Version]
- Berryman, C.E.; Grieger, J.A.; West, S.G.; Chen, C.-Y.O.; Blumberg, J.B.; Rothblat, G.H.; Sankaranarayanan, S.; Kris-Etherton, P.M. Acute consumption of walnuts and walnut components differentially affect postprandial lipemia, endothelial function, oxidative stress, and cholesterol efflux in humans with mild hypercholesterolemia. J. Nutr. 2013, 143, 788–794. [Google Scholar] [CrossRef] [Green Version]
- Vasudevan, M.; Tchoua, U.; Gillard, B.K.; Jones, P.H.; Ballantyne, C.M.; Pownall, H.J. Modest diet-induced weight loss reduces macrophage cholesterol efflux to plasma of patients with metabolic syndrome. J. Clin. Lipidol. 2013, 7, 661–670. [Google Scholar] [CrossRef] [Green Version]
- Van Tol, A.; Groener, J.E.M.; Scheek, L.M.; Van Gent, T.; Veenstra, J.; Van De Pol, H.; Hendriks, H.F.J.; Schaafsma, G. Induction of net mass lipid transfer reactions in plasma by wine consumption with dinner. Eur. J. Clin. Investig. 1995, 25, 390–395. [Google Scholar] [CrossRef]
- Gaubatz, J.W.; Gillard, B.K.; Rosales, C.; Pownall, H.J. Dietary Alcohol and Fat Differentially Affect Plasma Cholesteryl Ester Transfer Activity and Triglycerides in Normo- and Hypertriglyceridemic Subjects. Lipids 2020. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Watanabe, J.; Kawajiri, K. Genetic polymorphisms in the 5′-flanking region change transcriptional regulation of the human cytochrome P450IIE1 gene. J. Biochem. 1991, 110, 559–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rimm, E.B.; Klatsky, A.; Grobbee, D.; Stampfer, M.J. Review of moderate alcohol consumption and reduced risk of coronary heart disease: Is the effect due to beer, wine, or spirits? BMJ 1996, 312, 731–736. [Google Scholar] [CrossRef] [Green Version]
- Barefoot, J.C.; Grønbæk, M.; Feaganes, J.R.; McPherson, R.S.; Williams, R.; Siegler, I.C. Alcoholic beverage preference, diet, and health habits in the UNC Alumni Heart Study. Am. J. Clin. Nutr. 2002, 76, 466–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pownall, H.J.; Ballantyne, C.M.; Kimball, K.T.; Simpson, S.L.; Yeshurun, D.; Gotto, A.M. Effect of moderate alcohol consumption on hypertriglyceridemia: A study in the fasting state. Arch. Intern. Med. 1999, 159, 981–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugiyama, S.; Fushimi, T.; Kishi, M.; Irie, S.; Tsuji, S.; Hosokawa, N.; Kaga, T. Bioavailability of acetate from two vinegar supplements: Capsule and drink. J. Nutr. Sci. Vitaminol. 2010, 56, 266–269. [Google Scholar] [CrossRef]
- Nilsson, N.O.; Belfrage, P. Effects of acetate, acetaldehyde, and ethanol on lipolysis in isolated rat adipocytes. J. Lipid Res. 1978, 19, 737–741. [Google Scholar]
- Nilsson, N.E.; Kotarsky, K.; Owman, C.; Olde, B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem. Biophys. Res. Commun. 2003, 303, 1047–1052. [Google Scholar] [CrossRef]
- Hong, Y.-H.; Nishimura, Y.; Hishikawa, D.; Tsuzuki, H.; Miyahara, H.; Gotoh, C.; Choi, K.-C.; Feng, D.D.; Chen, C.; Lee, H.-G.; et al. Acetate and Propionate Short Chain Fatty Acids Stimulate Adipogenesis via GPCR43. Endocrinology 2005, 146, 5092–5099. [Google Scholar] [CrossRef] [Green Version]
- Ge, H.; Li, X.; Weiszmann, J.; Wang, P.; Baribault, H.; Chen, J.-L.; Tian, H.; Li, Y. Activation of G Protein-Coupled Receptor 43 in Adipocytes Leads to Inhibition of Lipolysis and Suppression of Plasma Free Fatty Acids. Endocrinology 2008, 149, 4519–4526. [Google Scholar] [CrossRef] [Green Version]
- Abramson, E.A.; Arky, R.A. Acute antilipolytic effects of ethyl alcohol and acetate in man. J. Lab. Clin. Med. 1968, 72, 105–117. [Google Scholar] [PubMed]
- Pownall, H.J.; Brauchi, D.; Kilinç, C.; Osmundsen, K.; Pao, Q.; Payton-Ross, C.; Gotto, A.M.; Ballantyne, C.M. Correlation of serum triglyceride and its reduction by omega-3 fatty acids with lipid transfer activity and the neutral lipid compositions of high-density and low-density lipoproteins. Atherosclerosis 1999, 143, 285–297. [Google Scholar] [CrossRef]
- Balasubramanyam, A.; Sekhar, R.V.; Jahoor, F.; Jones, P.H.; Pownall, H.J. Pathophysiology of dyslipidemia and increased cardiovascular risk in HIV lipodystrophy: A model of ‘systemic steatosis’. Curr. Opin. Lipidol. 2004, 15, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Hlebowicz, J.; Darwich, G.; Björgell, O.; Almér, L.-O. Effect of apple cider vinegar on delayed gastric emptying in patients with type 1 diabetes mellitus: A pilot study. BMC Gastroenterol. 2007, 7, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, C.S.; Kim, C.M.; Buller, A.J. Vinegar improves insulin sensitivity to a high-carbohydrate meal in subjects with insulin resistance or type 2 diabetes. Diabetes Care 2004, 27, 281–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, C.S.; White, A.M.; Kent, S.M. Preliminary evidence that regular vinegar ingestion favorably influences hemoglobin A1c values in individuals with type 2 diabetes mellitus. Diabetes Res. Clin. Pr. 2009, 84, e15–e17. [Google Scholar] [CrossRef]
- Liatis, S.; Grammatikou, S.; Poulia, K.A.; Perrea, D.; Makrilakis, K.; Diakoumopoulou, E.; Katsilambros, N. Vinegar reduces postprandial hyperglycaemia in patients with type II diabetes when added to a high, but not to a low, glycaemic index meal. Eur. J. Clin. Nutr. 2010, 64, 727–732. [Google Scholar] [CrossRef]
- Balliett, M.; Burke, J.R. Changes in anthropometric measurements, body composition, blood pressure, lipid profile, and testosterone in patients participating in a low-energy dietary intervention. J. Chiropr. Med. 2013, 12, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Shishehbor, F.; Mansoori, A.; Sarkaki, A.R.; Jalali, M.T.; Latifi, S.M. Apple Cider Vinegar Attenuates Lipid Profile in Normal and Diabetic Rats. Pak. J. Boil. Sci. 2008, 11, 2634–2638. [Google Scholar] [CrossRef]
- Jasbi, P.; Baker, O.; Shi, X.; Gonzalez, L.A.; Wang, S.; Anderson, S.; Xi, B.; Gu, H.; Johnston, C.S. Daily red wine vinegar ingestion for eight weeks improves glucose homeostasis and affects the metabolome but does not reduce adiposity in adults. Food Funct. 2019, 10, 7343–7355. [Google Scholar] [CrossRef]
- Mitrou, P.; Petsiou, E.; Papakonstantinou, E.; Maratou, E.; Lambadiari, V.; Dimitriadis, P.; Spanoudi, F.; A Raptis, S.; Dimitriadis, G. The role of acetic acid on glucose uptake and blood flow rates in the skeletal muscle in humans with impaired glucose tolerance. Eur. J. Clin. Nutr. 2015, 69, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.S.; Steplewska, I.; Long, C.A.; Harris, L.N.; Ryals, R.H. Examination of the Antiglycemic Properties of Vinegar in Healthy Adults. Ann. Nutr. Metab. 2010, 56, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Bouderbala, H.; Kaddouri, H.; Maharrar, M.; Kheroua, O.; Saidi, D. P2-11: Anti obesogenic effects of apple cider vinegar in rats subjected to a high fat diet. Annales de Cardiologie et d’Angéiologie 2015, 64, S27. [Google Scholar] [CrossRef]
- Fushimi, T.; Suruga, K.; Oshima, Y.; Fukiharu, M.; Tsukamoto, Y.; Goda, T. Dietary acetic acid reduces serum cholesterol and triacylglycerols in rats fed a cholesterol-rich diet. Br. J. Nutr. 2006, 95, 916–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, C.S.; Gaas, C.A. Vinegar: Medicinal Uses and Antiglycemic Effect. MedGenMed Medscape Gen. Med. 2006, 8, 61. [Google Scholar]
- Halima, B.H.; Sonia, G.; Sarra, K.; Houda, B.J.; Fethi, B.S.; Abdallah, A. Apple Cider Vinegar Attenuates Oxidative Stress and Reduces the Risk of Obesity in High-Fat-Fed Male Wistar Rats. J. Med. Food 2018, 21, 70–80. [Google Scholar] [CrossRef]
- Pingitore, A.; Gonzalez-Abuin, N.; Ruz-Maldonado, I.; Huang, G.C.; Frost, G.; Persaud, S.J. Short chain fatty acids stimulate insulin secretion and reduce apoptosis in mouse and human islets in vitro: Role of free fatty acid receptor 2. Diabetes, Obes. Metab. 2018, 21, 330–339. [Google Scholar] [CrossRef] [Green Version]
- Pomare, E.W.; Branch, W.J.; Cummings, J.H. Carbohydrate fermentation in the human colon and its relation to acetate concentrations in venous blood. J. Clin. Investig. 1985, 75, 1448–1454. [Google Scholar] [CrossRef] [Green Version]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef] [Green Version]
- Lundquist, F.; Tygstrup, N.; Winkler, K.; Mellemgaard, K.; Munck-Petersen, S. Etanol Metabolism and Production of Free Acetate in the Human Liver. J. Clin. Investig. 1962, 41, 955–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siler, S.Q.; Neese, R.A.; Hellerstein, M.K. De novo lipogenesis, lipid kinetics, and whole-body lipid balances in humans after acute alcohol consumption. Am. J. Clin. Nutr. 1999, 70, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Liljeberg, H.; Björck, I. Delayed gastric emptying rate may explain improved glycaemia in healthy subjects to a starchy meal with added vinegar. Eur. J. Clin. Nutr. 1998, 52, 368–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leeman, M.; Ostman, E.; Björck, I. Vinegar dressing and cold storage of potatoes lowers postprandial glycaemic and insulinaemic responses in healthy subjects. Eur. J. Clin. Nutr. 2005, 59, 1266–1271. [Google Scholar] [CrossRef]
- Sugiyama, M.; Tang, A.C.; Wakaki, Y.; Koyama, W. Glycemic index of single and mixed meal foods among common Japanese foods with white rice as a reference food. Eur. J. Clin. Nutr. 2003, 57, 743–752. [Google Scholar] [CrossRef] [Green Version]
- Ostman, E.; Elmståhl, H.G.L.; Björck, I.M.E. Inconsistency between glycemic and insulinemic responses to regular and fermented milk products. Am. J. Clin. Nutr. 2001, 74, 96–100. [Google Scholar] [CrossRef]
- Vu, C.N.; Ruiz-Esponda, R.; Yang, E.Y.; Chang, E.; Gillard, B.K.; Pownall, H.J.; Hoogeveen, R.; Coraza, I.; Balasubramanyam, A. Altered relationship of plasma triglycerides to HDL cholesterol in patients with HIV/HAART-associated dyslipidemia: Further evidence for a unique form of Metabolic Syndrome in HIV patients. Metabolism 2013, 62, 1014–1020. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosales, C.; Gillard, B.K.; Gotto, A.M., Jr.; Pownall, H.J. The Alcohol–High-Density Lipoprotein Athero-Protective Axis. Biomolecules 2020, 10, 987. https://doi.org/10.3390/biom10070987
Rosales C, Gillard BK, Gotto AM Jr., Pownall HJ. The Alcohol–High-Density Lipoprotein Athero-Protective Axis. Biomolecules. 2020; 10(7):987. https://doi.org/10.3390/biom10070987
Chicago/Turabian StyleRosales, Corina, Baiba K. Gillard, Antonio M. Gotto, Jr., and Henry J. Pownall. 2020. "The Alcohol–High-Density Lipoprotein Athero-Protective Axis" Biomolecules 10, no. 7: 987. https://doi.org/10.3390/biom10070987
APA StyleRosales, C., Gillard, B. K., Gotto, A. M., Jr., & Pownall, H. J. (2020). The Alcohol–High-Density Lipoprotein Athero-Protective Axis. Biomolecules, 10(7), 987. https://doi.org/10.3390/biom10070987