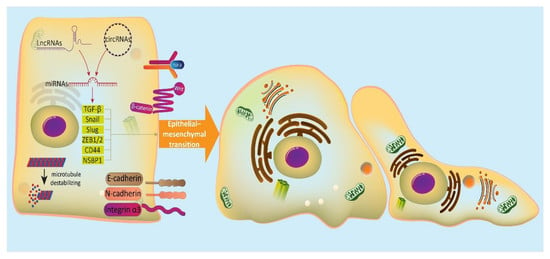
Role of microRNA/Epithelial-to-Mesenchymal Transition Axis in the Metastasis of Bladder Cancer
Abstract
:1. Introduction
2. EMT Mechanism
3. MicroRNAs and Their Role in Cancer Metastasis
4. MicroRNAs Inhibit EMT in Bladder Cancer Cells
4.1. MicroRNAs and PI3K/Akt/EMT Axis
4.2. MicroRNAs and CARMA3/EMT Axis
4.3. MicroRNAs and Wnt/EMT Axis
4.4. MicroRNAs and EMT-Inducing Transcription Factors
4.5. miRNA-200 Family and EMT
4.6. Other microRNAs
5. MicroRNAs can Induce EMT in BC Cells
5.1. MicroRNAs and Wnt/EMT Axis
5.2. MicroRNAs and EMT-Inducing Transcription Factors
5.3. MicroRNAs and EGR1/EMT Axis
5.4. Other microRNAs
6. Regulation of mciroRNA/EMT in Bladder Cancer Cells
6.1. LncRNAs as Main Regulators
6.2. CircRNAs as Main Regulators
6.3. Anti-Tumor Compounds as Main Regulators
7. Conclusions and Future Directions
Funding
Funding
Conflicts of Interest
Abbreviations
| BC | bladder cancer |
| miRNA | microRNA |
| lncRNA | long non-coding RNA |
| EMT | epithelial-to-mesenchymal transition |
| MET | mesenchymal-to-epithelial transition |
| NMIIA | non-muscle myosin IIA |
| 3/-UTR | 3/-untranslated region |
| TGF-β | transforming growth factor-β |
| mTOR | mammalian target of rapamycin |
| EMT-TFs | EMT-promoting transcription factors |
| ITGA3 | integrin α3 |
| ETS1 | E26 transformatin specific-1 |
| CARMA3 | CARD-cotnaining MAGUK 3 |
| NF-βB | nuclear factor-kappaB |
| MMP-2 | matrix metalloproteinase-2 |
| STMN1 | stathmin 1 |
| EGR1 | early growth response gene 1 |
| circRNAs | circular RNAs |
| TAMs | tumor-associated macrophages |
| NaB | sodium butyrate |
References
- Babjuk, M.; Burger, M.; Compérat, E.M.; Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.; Rouprêt, M.; Shariat, S.F.; Sylvester, R. European Association of Urology guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ)—2019 update. Eur. Urol. 2019, 76. [Google Scholar] [CrossRef] [PubMed]
- Alifrangis, C.; McGovern, U.; Freeman, A.; Powles, T.; Linch, M. Molecular and histopathology directed therapy for advanced bladder cancer. Nat. Rev. Urol. 2019, 16, 465–483. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Ervik, M.; Dikshit, R.; Eser, S.; Mathers, C. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012; IARC Publications: Lyon, France, 2013. [Google Scholar]
- Burger, M.; Catto, J.W.; Dalbagni, G.; Grossman, H.B.; Herr, H.; Karakiewicz, P.; Kassouf, W.; Kiemeney, L.A.; La Vecchia, C.; Shariat, S. Epidemiology and risk factors of urothelial bladder cancer. Eur. Urol. 2013, 63, 234–241. [Google Scholar] [CrossRef]
- Chavan, S.; Bray, F.; Lortet-Tieulent, J.; Goodman, M.; Jemal, A. International variations in bladder cancer incidence and mortality. Eur. Urol. 2014, 66, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Zarrabi, A.; Samarghandian, S.; Najafi, M. PTEN: What we know of the function and regulation of this onco-suppressor factor in bladder cancer? Eur. J. Pharmacol. 2020, 881, 173226. [Google Scholar] [CrossRef]
- Van Osch, F.H.; Jochems, S.H.; Van Schooten, F.-J.; Bryan, R.T.; Zeegers, M.P. Quantified relations between exposure to tobacco smoking and bladder cancer risk: A meta-analysis of 89 observational studies. Int. J. Epidemiol. 2016, 45, 857–870. [Google Scholar] [CrossRef] [Green Version]
- Colt, J.S.; Friesen, M.C.; Stewart, P.A.; Donguk, P.; Johnson, A.; Schwenn, M.; Karagas, M.R.; Armenti, K.; Waddell, R.; Verrill, C. A case-control study of occupational exposure to metalworking fluids and bladder cancer risk among men. Occup. Environ. Med. 2014, 71, 667–674. [Google Scholar] [CrossRef] [Green Version]
- Egbers, L.; Grotenhuis, A.J.; Aben, K.K.; Alfred Witjes, J.; Kiemeney, L.A.; Vermeulen, S.H. The prognostic value of family history among patients with urinary bladder cancer. Int. J. Cancer 2015, 136, 1117–1124. [Google Scholar] [CrossRef] [Green Version]
- Al-Zalabani, A.H.; Stewart, K.F.; Wesselius, A.; Schols, A.M.; Zeegers, M.P. Modifiable risk factors for the prevention of bladder cancer: A systematic review of meta-analyses. Eur. J. Epidemiol. 2016, 31, 811–851. [Google Scholar] [CrossRef] [Green Version]
- Steinmaus, C.; Ferreccio, C.; Acevedo, J.; Yuan, Y.; Liaw, J.; Durán, V.; Cuevas, S.; García, J.; Meza, R.; Valdés, R. Increased lung and bladder cancer incidence in adults after in utero and early-life arsenic exposure. Cancer Epidemiol. Prev. Biomark. 2014, 23, 1529–1538. [Google Scholar] [CrossRef] [Green Version]
- Tuccori, M.; Filion, K.B.; Yin, H.; Oriana, H.Y.; Platt, R.W.; Azoulay, L. Pioglitazone use and risk of bladder cancer: Population based cohort study. BMJ 2016, 352, i1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alanee, S.; Alvarado-Cabrero, I.; Murugan, P.; Kumar, R.; Nepple, K.G.; Paner, G.P.; Patel, M.I.; Raspollini, M.R.; Lopez-Beltran, A.; Konety, B.R. Update of the International Consultation on Urological Diseases on bladder cancer 2018: Non-urothelial cancers of the urinary bladder. World J. Urol. 2019, 37, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, P.A.; Moch, H.; Cubilla, A.L.; Ulbright, T.M.; Reuter, V.E. The 2016 WHO classification of tumours of the urinary system and male genital organs—Part B: Prostate and bladder tumours. Eur. Urol. 2016, 70, 106–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, H.; Chen, W.; Mi, L.; Wang, D.; Zhao, Y.; Yu, C.; Zhao, A. Qici Sanling decoction suppresses bladder cancer growth by inhibiting the Wnt/Β-catenin pathway. Pharm. Biol. 2019, 57, 507–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, N.; Liang, Y.; Chen, Y.; Wang, L.; Li, D.; Liang, Z.; Sun, L.; Wang, Y.; Niu, H. Glutamine affects T24 bladder cancer cell proliferation by activating STAT3 through ROS and glutaminolysis. Int. J. Mol. Med. 2019, 44, 2189–2200. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Jiang, D.; Wu, K. p62 promotes bladder cancer cell growth by activating KEAP1/NRF2-dependent antioxidative response. Cancer Sci. 2020, 111, 1156–1164. [Google Scholar] [CrossRef]
- Goan, Y.G.; Wu, W.T.; Liu, C.I.; Neoh, C.A.; Wu, Y.J. Involvement of Mitochondrial Dysfunction, Endoplasmic Reticulum Stress, and the PI3K/AKT/mTOR Pathway in Nobiletin-Induced Apoptosis of Human Bladder Cancer Cells. Molecules 2019, 24. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Wang, D.; Ding, Y.; Zhou, J.; Liu, G.; Ji, Z. lncRNA ZEB1-AS1 promotes migration and metastasis of bladder cancer cells by post-transcriptional activation of ZEB1. Int. J. Mol. Med. 2019, 44, 196–206. [Google Scholar] [CrossRef]
- Bai, J.; Xu, J.; Zhao, J.; Zhang, R. Downregulation of lncRNA AWPPH inhibits colon cancer cell proliferation by downregulating GLUT-1. Oncology Lett. 2019, 18, 2007–2012. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.L.; Zhan, D.M.; Chong, Y.K.; Ding, L.; Yang, Y.G. MiR-652-3p promotes bladder cancer migration and invasion by targeting KCNN3. Eur. Rev. Med. Pharm. Sci. 2019, 23, 8806–8812. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, T.; Feng, H.; Yang, R.; Zhao, X.; Chen, W.; Jiang, B.; Qin, H.; Guo, X.; Liu, M.; et al. Circular RNA circSLC8A1 acts as a sponge of miR-130b/miR-494 in suppressing bladder cancer progression via regulating PTEN. Mol. Cancer 2019, 18, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Yang, C.; Zhang, H.B.; Ma, J.; Jia, J.; Tang, X.; Zeng, J.; Chong, T.; Wang, X.; He, D.; et al. BET inhibitor JQ1 suppresses cell proliferation via inducing autophagy and activating LKB1/AMPK in bladder cancer cells. Cancer Med. 2019, 8, 4792–4805. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Huang, H.; Huang, W.; Xie, Z.; Yang, Y.; Wang, F. LncRNA miR143HG suppresses bladder cancer development through inactivating Wnt/β-catenin pathway by modulating miR-1275/AXIN2 axis. J. Cell. Physiol. 2019, 234, 11156–11164. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhao, P.; Liu, Z.; Li, Z.; Yuan, Y.; Zhang, X.; Yu, Z.; Fang, J.; Xiao, K. MiR-204-3p Inhibited the Proliferation of Bladder Cancer Cells via Modulating Lactate Dehydrogenase-Mediated Glycolysis. Front. Oncol. 2019, 9, 1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Luo, Y.; He, W.; Zhao, Y.; Kong, Y.; Liu, H.; Zhong, G.; Li, Y.; Li, J.; Huang, J.; et al. Exosomal long noncoding RNA LNMAT2 promotes lymphatic metastasis in bladder cancer. J. Clin. Investig. 2020, 130, 404–421. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Tsuda, M.; Matsumoto, R.; Semba, S.; Wang, L.; Sugino, H.; Tanino, M.; Kondo, T.; Tanabe, K.; Tanaka, S. Exosomes containing ErbB2/CRK induce vascular growth in premetastatic niches and promote metastasis of bladder cancer. Cancer Sci. 2019, 110, 2119–2132. [Google Scholar] [CrossRef] [Green Version]
- Sun, P.; Wu, T.; Sun, X.; Cui, Z.; Zhang, H.; Xia, Q.; Zhang, D. KMT2D inhibits the growth and metastasis of bladder Cancer cells by maintaining the tumor suppressor genes. Biomed. Pharm. 2019, 115, 108924. [Google Scholar] [CrossRef]
- Chen, Z.; Du, Y.; Liu, X.; Chen, H.; Weng, X.; Guo, J.; Wang, M.; Wang, X.; Wang, L. EZH2 inhibition suppresses bladder cancer cell growth and metastasis via the JAK2/STAT3 signaling pathway. Oncol. Lett. 2019, 18, 907–915. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.J.; Su, Y.W.; Wang, C.J.; Li, D.F.; Zhou, L. Semaphorin 4D promotes the proliferation and metastasis of bladder cancer by activating the PI3K/AKT pathway. Tumori 2019, 105, 231–242. [Google Scholar] [CrossRef]
- Rodriguez-Aznar, E.; Wiesmüller, L.; Sainz, B., Jr.; Hermann, P.C. EMT and Stemness-Key Players in Pancreatic Cancer Stem Cells. Cancers 2019, 11. [Google Scholar] [CrossRef] [Green Version]
- Teeuwssen, M.; Fodde, R. Wnt Signaling in Ovarian Cancer Stemness, EMT, and Therapy Resistance. J. Clin. Med. 2019, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, G.Y.; Hu, M.; Zhao, L.; Guo, W.S. MiR-451a suppresses cell proliferation, metastasis and EMT via targeting YWHAZ in hepatocellular carcinoma. Eur. Rev. Med. Pharm. Sci. 2019, 23, 5158–5167. [Google Scholar] [CrossRef]
- Li, H.; Song, H.; Yuan, X.; Li, J.; Tang, H. miR-30a reverses TGF-β2-induced migration and EMT in posterior capsular opacification by targeting Smad2. Mol. Biol. Rep. 2019, 46, 3899–3907. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Li, Z.; Yu, Y.; Zeng, Q.; Cheng, Y.; Ji, W.; Xia, W.; Lu, S. Exosomal miR-499a-5p promotes cell proliferation, migration and EMT via mTOR signaling pathway in lung adenocarcinoma. Exp. Cell Res. 2019, 379, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Wang, F.; Zhang, T.; Xu, H.; Zhang, Y.; Shan, Z.; Wu, S.; Fan, Q.; Sun, Y. miR-516a-3p inhibits breast cancer cell growth and EMT by blocking the Pygo2/Wnt signalling pathway. J. Cell. Mol. Med. 2019, 23, 6295–6307. [Google Scholar] [CrossRef]
- Ochoa, A.E.; Choi, W.; Su, X.; Siefker-Radtke, A.; Czerniak, B.; Dinney, C.; McConkey, D.J. Specific micro-RNA expression patterns distinguish the basal and luminal subtypes of muscle-invasive bladder cancer. Oncotarget 2016, 7, 80164–80174. [Google Scholar] [CrossRef] [Green Version]
- Georgakopoulos-Soares, I.; Chartoumpekis, D.V.; Kyriazopoulou, V.; Zaravinos, A. EMT Factors and Metabolic Pathways in Cancer. Front. Oncol. 2020, 10, 499. [Google Scholar] [CrossRef]
- Ko, J.-H.; Yang, M.H.; Baek, S.H.; Nam, D.; Jung, S.H.; Ahn, K.S. Theacrine attenuates epithelial mesenchymal transition in human breast cancer MDA-MB-231 cells. Phytotherapy Research 2019, 33, 1934–1942. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Biagioni, A.; Arunkumar, G.; Shapiro, R.; Chang, K.-C.; Sedeeq, M.; Taiyab, A.; Hashemabadi, M.; Pardakhty, A.; Mandegary, A.; et al. EMT signaling: Potential contribution of CRISPR/Cas gene editing. Cell. Mol. Life Sci. 2020. [Google Scholar] [CrossRef]
- Loh, C.-Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.T.; Wang, L.; Wang, H.; Tang, F.R.; Cai, W.Q.; Sethi, G.; Xin, H.W.; Ma, Z. Insights into Biological Role of LncRNAs in Epithelial-Mesenchymal Transition. Cells 2019, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashrafizadeh, M.; Zarrabi, A.; Hushmandi, K.; Kalantari, M.; Mohammadinejad, R.; Javaheri, T.; Sethi, G. Association of the Epithelial-Mesenchymal Transition (EMT) with Cisplatin Resistance. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, M.; Neilson, E.G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Investig. 2009, 119, 1429–1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef] [Green Version]
- Morel, A.P.; Lièvre, M.; Thomas, C.; Hinkal, G.; Ansieau, S.; Puisieux, A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS ONE 2008, 3, e2888. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [Green Version]
- Tentler, D.; Lomert, E.; Novitskaya, K.; Barlev, N.A. Role of ACTN4 in Tumorigenesis, Metastasis, and EMT. Cells 2019, 8. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Lee, S.; Shin, E.; Seong, K.M.; Jin, Y.W.; Youn, H.; Youn, B. The Emerging Roles of Exosomes as EMT Regulators in Cancer. Cells 2020, 9. [Google Scholar] [CrossRef]
- Pattabiraman, D.R.; Bierie, B.; Kober, K.I.; Thiru, P.; Krall, J.A.; Zill, C.; Reinhardt, F.; Tam, W.L.; Weinberg, R.A. Activation of PKA leads to mesenchymal-to-epithelial transition and loss of tumor-initiating ability. Science 2016, 351, aad3680. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Chen, L.; Liu, L.; Niu, X. EMT-Mediated Acquired EGFR-TKI Resistance in NSCLC: Mechanisms and Strategies. Front. Oncol. 2019, 9, 1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briem, E.; Ingthorsson, S.; Traustadottir, G.A.; Hilmarsdottir, B.; Gudjonsson, T. Application of the D492 Cell Lines to Explore Breast Morphogenesis, EMT and Cancer Progression in 3D Culture. J. Mammary Gland Biol. Neoplasia 2019, 24, 139–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.T.; Zhong, H.T.; Li, G.W.; Shen, J.X.; Ye, Q.Q.; Zhang, M.L.; Liu, J. Oncogenic functions of the EMT-related transcription factor ZEB1 in breast cancer. J. Trans. Med. 2020, 18, 51. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Chinnathambi, A.; Alharbi, S.A.; Shair, O.H.M.; Sethi, G.; Ahn, K.S. Farnesol abrogates epithelial to mesenchymal transition process through regulating Akt/mTOR pathway. Pharm. Res. 2019, 150, 104504. [Google Scholar] [CrossRef] [PubMed]
- Prasannan, R.; Kalesh, K.A.; Shanmugam, M.K.; Nachiyappan, A.; Ramachandran, L.; Nguyen, A.H.; Kumar, A.P.; Lakshmanan, M.; Ahn, K.S.; Sethi, G. Key cell signaling pathways modulated by zerumbone: Role in the prevention and treatment of cancer. Biochem. Pharmacol. 2012, 84, 1268–1276. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Zheng, Y.; Zhang, H.; Liu, Y.; Sun, H.; Zhang, P. Galectin-1 induces metastasis and epithelial-mesenchymal transition (EMT) in human ovarian cancer cells via activation of the MAPK JNK/p38 signalling pathway. Am. J. Trans. Res. 2019, 11, 3862–3878. [Google Scholar]
- Baek, S.H.; Ko, J.H.; Lee, J.H.; Kim, C.; Lee, H.; Nam, D.; Lee, J.; Lee, S.G.; Yang, W.M.; Um, J.Y.; et al. Ginkgolic Acid Inhibits Invasion and Migration and TGF-β-Induced EMT of Lung Cancer Cells Through PI3K/Akt/mTOR Inactivation. J. Cell. Physiol. 2017, 232, 346–354. [Google Scholar] [CrossRef]
- Ko, J.-H.; Nam, D.; Um, J.-Y.; Jung, S.H.; Sethi, G.; Ahn, K.S. Bergamottin Suppresses Metastasis of Lung Cancer Cells through Abrogation of Diverse Oncogenic Signaling Cascades and Epithelial-to-Mesenchymal Transition. Molecules 2018, 23, 1601. [Google Scholar] [CrossRef] [Green Version]
- Steinbichler, T.B.; Dudás, J.; Riechelmann, H.; Skvortsova, I.-I. The role of exosomes in cancer metastasis. Semin. Cancer Biol. 2017, 44, 170–181. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Chen, L.; Qi, S.; Yu, S.; Weng, Z.; Hu, Z.; Zhou, Q.; Xin, Z.; Shi, L.; et al. HERC3-Mediated SMAD7 Ubiquitination Degradation Promotes Autophagy-Induced EMT and Chemoresistance in Glioblastoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 3602–3616. [Google Scholar] [CrossRef] [Green Version]
- Thege, F.I.; Gruber, C.N.; Cardle, I.I.; Cong, S.H.; Lannin, T.B.; Kirby, B.J. anti-EGFR capture mitigates EMT- and chemoresistance-associated heterogeneity in a resistance-profiling CTC platform. Anal. Biochem. 2019, 577, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Jordan, N.V.; Johnson, G.L.; Abell, A.N. Tracking the intermediate stages of epithelial-mesenchymal transition in epithelial stem cells and cancer. Cell Cycle 2011, 10, 2865–2873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, T.; Watanabe, K.; Ta, C.H.; Villarreal-Ponce, A.; Nie, Q.; Dai, X. An Ovol2-Zeb1 Mutual Inhibitory Circuit Governs Bidirectional and Multi-step Transition between Epithelial and Mesenchymal States. PLoS Comput. Biol. 2015, 11, e1004569. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef] [Green Version]
- Cook, D.P.; Vanderhyden, B.C. Ovarian cancer and the evolution of subtype classifications using transcriptional profiling. Biol. Reprod. 2019, 101, 645–658. [Google Scholar] [CrossRef]
- Kröger, C.; Afeyan, A.; Mraz, J.; Eaton, E.N.; Reinhardt, F.; Khodor, Y.L.; Thiru, P.; Bierie, B.; Ye, X.; Burge, C.B.; et al. Acquisition of a hybrid E/M state is essential for tumorigenicity of basal breast cancer cells. Proc. Natl. Acad. Sci. USA 2019, 116, 7353–7362. [Google Scholar] [CrossRef] [Green Version]
- Jolly, M.K.; Boareto, M.; Huang, B.; Jia, D.; Lu, M.; Ben-Jacob, E.; Onuchic, J.N.; Levine, H. Implications of the Hybrid Epithelial/Mesenchymal Phenotype in Metastasis. Front. Oncol. 2015, 5, 155. [Google Scholar] [CrossRef] [Green Version]
- Pastushenko, I.; Brisebarre, A.; Sifrim, A.; Fioramonti, M.; Revenco, T.; Boumahdi, S.; Van Keymeulen, A.; Brown, D.; Moers, V.; Lemaire, S.; et al. Identification of the tumour transition states occurring during EMT. Nature 2018, 556, 463–468. [Google Scholar] [CrossRef]
- Lazar, M.A.; Birnbaum, M.J. Physiology. De-meaning of metabolism. Science 2012, 336, 1651–1652. [Google Scholar] [CrossRef]
- Baenke, F.; Peck, B.; Miess, H.; Schulze, A. Hooked on fat: The role of lipid synthesis in cancer metabolism and tumour development. Dis. Models Mech. 2013, 6, 1353–1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaupel, P.; Schmidberger, H.; Mayer, A. The Warburg effect: Essential part of metabolic reprogramming and central contributor to cancer progression. Int. J. Radiat. Biol. 2019, 95, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, W. Epithelial-mesenchymal transition in human cancer: Comprehensive reprogramming of metabolism, epigenetics, and differentiation. Pharm. Ther. 2015, 150, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, S.K.; Ramirez-Peña, E.; Arnold, J.M.; Putluri, V.; Sphyris, N.; Michailidis, G.; Putluri, N.; Ambs, S.; Sreekumar, A.; Mani, S.A. EMT-induced metabolite signature identifies poor clinical outcome. Oncotarget 2015, 6, 42651–42660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Y.; Chen, Q.; Li, L. Endostar regulates EMT, migration and invasion of lung cancer cells through the HGF-Met pathway. Mol. Cell. Probes 2019, 45, 57–64. [Google Scholar] [CrossRef]
- Tan, S.M.; Li, F.; Rajendran, P.; Kumar, A.P.; Hui, K.M.; Sethi, G. Identification of β-escin as a novel inhibitor of signal transducer and activator of transcription 3/Janus-activated kinase 2 signaling pathway that suppresses proliferation and induces apoptosis in human hepatocellular carcinoma cells. J. Pharmacol. Exp. Ther. 2010, 334, 285–293. [Google Scholar] [CrossRef]
- Kim, C.; Cho, S.K.; Kapoor, S.; Kumar, A.; Vali, S.; Abbasi, T.; Kim, S.H.; Sethi, G.; Ahn, K.S. β-Caryophyllene oxide inhibits constitutive and inducible STAT3 signaling pathway through induction of the SHP-1 protein tyrosine phosphatase. Mol. Carcinog. 2014, 53, 793–806. [Google Scholar] [CrossRef]
- Liang, F.; Ren, C.; Wang, J.; Wang, S.; Yang, L.; Han, X.; Chen, Y.; Tong, G.; Yang, G. The crosstalk between STAT3 and p53/RAS signaling controls cancer cell metastasis and cisplatin resistance via the Slug/MAPK/PI3K/AKT-mediated regulation of EMT and autophagy. Oncogenesis 2019, 8, 59. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P.; Li, Y.; Li, B.; Zhang, M.; Liu, Y.; Yao, Y.; Li, D. NMIIA promotes tumor growth and metastasis by activating the Wnt/β-catenin signaling pathway and EMT in pancreatic cancer. Oncogene 2019, 38, 5500–5515. [Google Scholar] [CrossRef]
- Meng, H.; Wu, J.; Huang, Q.; Yang, X.; Yang, K.; Qiu, Y.; Ren, J.; Shen, R.; Qi, H. NEDD9 promotes invasion and migration of colorectal cancer cell line HCT116 via JNK/EMT. Oncol. Lett. 2019, 18, 4022–4029. [Google Scholar] [CrossRef]
- Yan, H.; Li, H.; Silva, M.A.; Guan, Y.; Yang, L.; Zhu, L.; Zhang, Z.; Li, G.; Ren, C. LncRNA FLVCR1-AS1 mediates miR-513/YAP1 signaling to promote cell progression, migration, invasion and EMT process in ovarian cancer. J. Exp. Clin. Cancer Res. 2019, 38, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Chu, D.; Kawamura, T.; Tanaka, K.; He, S. GRIM-19 repressed hypoxia-induced invasion and EMT of colorectal cancer by repressing autophagy through inactivation of STAT3/HIF-1α signaling axis. J. Cell. Physiol. 2019, 234, 12800–12808. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Du, Y.; Lu, Y.; Luan, B.; Xu, C.; Yu, Y.; Zhao, H. CD44 Expression Predicts Prognosis of Ovarian Cancer Patients Through Promoting Epithelial-Mesenchymal Transition (EMT) by Regulating Snail, ZEB1, and Caveolin-1. Front. Oncol. 2019, 9, 802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, S.; Peng, P.; Li, J.; Deng, H.; Zhan, N.; Zeng, Z.; Dong, W. SERPINH1 regulates EMT and gastric cancer metastasis via the Wnt/β-catenin signaling pathway. Aging 2020, 12, 3574–3593. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.R.; Liu, B.; Zhou, L.; Huang, Y.X. MicroRNA-124-3p suppresses cell migration and invasion by targeting ITGA3 signaling in bladder cancer. Cancer Biomark. Sect. A Dis. Mark. 2019, 24, 159–172. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, G.; Wu, R.; Gong, N. High expression of miR-135b predicts malignant transformation and poor prognosis of gastric cancer. Life Sci. 2020, 257, 118133. [Google Scholar] [CrossRef]
- Ravegnini, G.; Cargnin, S.; Sammarini, G.; Zanotti, F.; Bermejo, J.L.; Hrelia, P.; Terrazzino, S.; Angelini, S. Prognostic Role of miR-221 and miR-222 Expression in Cancer Patients: A Systematic Review and Meta-Analysis. Cancers 2019, 11, 970. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Humphries, B.; Yang, C.; Wang, Z. MiR-205 Dysregulations in Breast Cancer: The Complexity and Opportunities. Noncoding RNA 2019, 5, 53. [Google Scholar] [CrossRef] [Green Version]
- Gulei, D.; Raduly, L.; Broseghini, E.; Ferracin, M.; Berindan-Neagoe, I. The extensive role of miR-155 in malignant and non-malignant diseases. Mol. Asp. Med. 2019, 70, 33–56. [Google Scholar] [CrossRef]
- Taddei, M.L.; Cavallini, L.; Ramazzotti, M.; Comito, G.; Pietrovito, L.; Morandi, A.; Giannoni, E.; Raugei, G.; Chiarugi, P. Stromal-induced downregulation of miR-1247 promotes prostate cancer malignancy. J. Cell. Physiol. 2019, 234, 8274–8285. [Google Scholar] [CrossRef]
- Pan, Y.J.; Wan, J.; Wang, C.B. MiR-326: Promising Biomarker for Cancer. Cancer Manag. Res. 2019, 11, 10411–10418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Song, J.; Bian, H.; Yang, X.; Xie, X.; Zhu, Q.; Qin, C.; Qi, J. The functions and targets of miR-212 as a potential biomarker of cancer diagnosis and therapy. J. Cell. Mol. Med. 2020, 24, 2392–2401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimoto, S.; Tanaka, F.; Morita, H.; Hiraki, A.; Hashimoto, S. Hypoxia-induced HIF-1α and ZEB1 are critical for the malignant transformation of ameloblastoma via TGF-β-dependent EMT. Cancer Med. 2019, 8, 7822–7832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, Y.; Gao, T.; Huang, W.; Yang, Y.; Qiu, R.; Hou, Y.; Yu, W.; Leng, S.; Feng, D.; Liu, W.; et al. MicroRNA-455-3p mediates GATA3 tumor suppression in mammary epithelial cells by inhibiting TGF-β signaling. J. Biol. Chem. 2019, 294, 15808–15825. [Google Scholar] [CrossRef]
- Yi, J.; Fan, Y.; Zhang, L.; Wang, H.; Mu, T.; Xie, H.; Gao, H.; Liu, M.; Li, S.; Tang, H. MiR-HCC2 Up-regulates BAMBI and ELMO1 Expression to Facilitate the Proliferation and EMT of Hepatocellular Carcinoma Cells. J. Cancer 2019, 10, 3407–3419. [Google Scholar] [CrossRef] [Green Version]
- Koshizuka, K.; Hanazawa, T.; Kikkawa, N.; Arai, T.; Okato, A.; Kurozumi, A.; Kato, M.; Katada, K.; Okamoto, Y.; Seki, N. Regulation of ITGA3 by the anti-tumor miR-199 family inhibits cancer cell migration and invasion in head and neck cancer. Cancer Sci. 2017, 108, 1681–1692. [Google Scholar] [CrossRef] [Green Version]
- Sa, K.D.; Zhang, X.; Li, X.F.; Gu, Z.P.; Yang, A.G.; Zhang, R.; Li, J.P.; Sun, J.Y. A miR-124/ITGA3 axis contributes to colorectal cancer metastasis by regulating anoikis susceptibility. Biochem. Biophys. Res. Commun. 2018, 501, 758–764. [Google Scholar] [CrossRef]
- Yan, T.; Ye, X.X. MicroRNA-328-3p inhibits the tumorigenesis of bladder cancer through targeting ITGA5 and inactivating PI3K/AKT pathway. Eur. Rev. Med. Pharm. Sci. 2019, 23, 5139–5148. [Google Scholar] [CrossRef]
- Mohan, C.D.; Srinivasa, V.; Rangappa, S.; Mervin, L.; Mohan, S.; Paricharak, S.; Baday, S.; Li, F.; Shanmugam, M.K.; Chinnathambi, A.; et al. Trisubstituted-imidazoles induce apoptosis in human breast cancer cells by targeting the oncogenic PI3K/Akt/mTOR signaling pathway. PLoS ONE 2016, 11, e0153155. [Google Scholar] [CrossRef] [Green Version]
- Ong, P.S.; Wang, L.Z.; Dai, X.; Tseng, S.H.; Loo, S.J.; Sethi, G. Judicious Toggling of mTOR Activity to Combat Insulin Resistance and Cancer: Current Evidence and Perspectives. Front. Pharmacol. 2016, 7, 395. [Google Scholar] [CrossRef]
- Siveen, K.S.; Ahn, K.S.; Ong, T.H.; Shanmugam, M.K.; Li, F.; Yap, W.N.; Kumar, A.P.; Fong, C.W.; Tergaonkar, V.; Hui, K.M.; et al. Y-tocotrienol inhibits angiogenesis-dependent growth of human hepatocellular carcinoma through abrogation of AKT/mTOR pathway in an orthotopic mouse model. Oncotarget 2014, 5, 1897–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, T.; Zhou, L.; Wang, L.; Kazobinka, G.; Chen, Y.; Zhang, X.; Chen, Z. Leupaxin Promotes Bladder Cancer Proliferation, Metastasis, and Angiogenesis Through the PI3K/AKT Pathway. Cell. Physiol. Biochem. 2018, 47, 2250–2260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qiang, J.; Yang, X.; Wang, D.; Rehman, A.U.; He, X.; Chen, W.; Sheng, D.; Zhou, L.; Jiang, Y.Z.; et al. IL1R2 Blockade Suppresses Breast Tumorigenesis and Progression by Impairing USP15-Dependent BMI1 Stability. Adv. Sci. 2020, 7, 1901728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Ma, Y.B.; Tian, Y.H.; Xu, X.L.; Gao, Y.; He, Y.Q.; Pan, W.T.; Zhang, J.W.; He, C.J.; Wei, L. Silencing lncRNA SNHG6 suppresses proliferation and invasion of breast cancer cells through miR-26a/VASP axis. Pathol. Res. Pract. 2019, 215, 152575. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, C.Z.; Ma, M.; Shao, G.F. MiR-15 suppressed the progression of bladder cancer by targeting BMI1 oncogene via PI3K/AKT signaling pathway. Eur. Rev. Med. Pharm. Sci. 2019, 23, 8813–8822. [Google Scholar] [CrossRef]
- Guo, C.C.; Majewski, T.; Zhang, L.; Yao, H.; Bondaruk, J.; Wang, Y.; Zhang, S.; Wang, Z.; Lee, J.G.; Lee, S.; et al. Dysregulation of EMT Drives the Progression to Clinically Aggressive Sarcomatoid Bladder Cancer. Cell Rep. 2019, 27, 1781–1793. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Xu, X.; Deng, W.; Huang, M.; Wu, Y.; Zhou, Z.; Zhu, K.; Wang, Y.; Cheng, X.; Zhou, X.; et al. CCL18 enhances migration, invasion and EMT by binding CCR8 in bladder cancer cells. Mol. Med. Rep. 2019, 19, 1678–1686. [Google Scholar] [CrossRef]
- Banan, A.; Zhang, L.; Farhadi, A.; Fields, J.; Shaikh, M.; Keshavarzian, A. PKC-β1 isoform activation is required for EGF-induced NF-κB inactivation and IκBα stabilization and protection of F-actin assembly and barrier function in enterocyte monolayers. Am. J. Physiol. Cell Physiol. 2004, 286, C723–C738. [Google Scholar] [CrossRef]
- McAllister-Lucas, L.M.; Ruland, J.; Siu, K.; Jin, X.; Gu, S.; Kim, D.S.; Kuffa, P.; Kohrt, D.; Mak, T.W.; Nuñez, G. CARMA3/Bcl10/MALT1-dependent NF-κB activation mediates angiotensin II-responsive inflammatory signaling in nonimmune cells. Proc. Natl. Acad. Sci. USA 2007, 104, 139–144. [Google Scholar] [CrossRef] [Green Version]
- Grabiner, B.C.; Blonska, M.; Lin, P.-C.; You, Y.; Wang, D.; Sun, J.; Darnay, B.G.; Dong, C.; Lin, X. CARMA3 deficiency abrogates G protein-coupled receptor-induced NF-κB activation. Genes Dev. 2007, 21, 984–996. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; You, Y.; Lin, P.-C.; Xue, L.; Morris, S.W.; Zeng, H.; Wen, R.; Lin, X. Bcl10 plays a critical role in NF-κB activation induced by G protein-coupled receptors. Proc. Natl. Acad. Sci. USA 2007, 104, 145–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Man, X.; Liu, T.; Jiang, Y.; Zhang, Z.; Zhu, Y.; Li, Z.; Kong, C.; He, J. Silencing of CARMA3 inhibits bladder cancer cell migration and invasion via deactivating β-catenin signaling pathway. Oncotarget. Ther. 2019, 12, 6309–6322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Zhang, C.; Liu, W.; Zheng, W.; Zhang, Y.; Wang, S.; Huang, D.; Liu, X.; Bai, Z. MicroRNA-24 upregulation inhibits proliferation, metastasis and induces apoptosis in bladder cancer cells by targeting CARMA3. Int. J. Oncol. 2015, 47, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhu, H.; Gao, Z.; Li, J.; Zhuang, J.; Dong, Y.; Shen, B.; Li, M.; Zhou, H.; Guo, H.; et al. Wnt7a activates canonical Wnt signaling, promotes bladder cancer cell invasion, and is suppressed by miR-370-3p. J. Biol. Chem. 2018, 293, 6693–6706. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Zhou, L.; Chen, L.; Xiong, M.; Kazobinka, G.; Pang, Z.; Hou, T. RSPO3 promotes the aggressiveness of bladder cancer via Wnt/β-catenin and Hedgehog signaling pathways. Carcinogenesis 2019, 40, 360–369. [Google Scholar] [CrossRef]
- Bhuvanalakshmi, G.; Gamit, N.; Patil, M.; Arfuso, F.; Sethi, G.; Dharmarajan, A.; Kumar, A.P.; Warrier, S. Stemness, Pluripotentiality, and Wnt Antagonism: sFRP4, a Wnt antagonist Mediates Pluripotency and Stemness in Glioblastoma. Cancers 2018, 11. [Google Scholar] [CrossRef] [Green Version]
- Bhuvanalakshmi, G.; Basappa; Rangappa, K.S.; Dharmarajan, A.; Sethi, G.; Kumar, A.P.; Warrier, S. Breast Cancer Stem-Like Cells Are Inhibited by Diosgenin, a Steroidal Saponin, by the Attenuation of the Wnt β-Catenin Signaling via the Wnt Antagonist Secreted Frizzled Related Protein-4. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Ong, M.S.; Cai, W.; Yuan, Y.; Leong, H.C.; Tan, T.Z.; Mohammad, A.; You, M.L.; Arfuso, F.; Goh, B.C.; Warrier, S.; et al. ’Lnc’-ing Wnt in female reproductive cancers: Therapeutic potential of long non-coding RNAs in Wnt signalling. Br. J. Pharmacol. 2017, 174, 4684–4700. [Google Scholar] [CrossRef] [Green Version]
- Pang, G.; Xie, Q.; Yao, J. Mitofusin 2 inhibits bladder cancer cell proliferation and invasion via the Wnt/β-catenin pathway. Oncol. Lett. 2019, 18, 2434–2442. [Google Scholar] [CrossRef]
- Garg, M.; Maurya, N. WNT/β-catenin signaling in urothelial carcinoma of bladder. World J. Nephrol. 2019, 8, 83–94. [Google Scholar] [CrossRef]
- Wang, R.; Song, Y.; Liu, X.; Wang, Q.; Wang, Y.; Li, L.; Kang, C.; Zhang, Q. UBE2C induces EMT through Wnt/β-catenin and PI3K/Akt signaling pathways by regulating phosphorylation levels of Aurora-A. Int. J. Oncol. 2017, 50, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, Z.; Zhang, S.; Lu, X.; Wu, J.; Yu, K.; Ji, A.; Lu, W.; Wang, Z.; Wu, J.; et al. IQGAP1 promotes pancreatic cancer progression and epithelial-mesenchymal transition (EMT) through Wnt/β-catenin signaling. Sci. Rep. 2019, 9, 7539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Cai, H.; Sun, L.; Zhan, P.; Chen, M.; Zhang, F.; Ran, Y.; Wan, J. LGR5, a novel functional glioma stem cell marker, promotes EMT by activating the Wnt/β-catenin pathway and predicts poor survival of glioma patients. J. Exp. Clin. Cancer Res. CR 2018, 37, 225. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.W.; Yang, Z.M.; Deng, P.; Chen, Y.R.; He, Z.J.; Yang, X.; Zhang, S.; Wu, H.J.; Ren, Z.H. HOXC10 promotes migration and invasion via the WNT-EMT signaling pathway in oral squamous cell carcinoma. J. Cancer 2019, 10, 4540–4551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, C.Y.; Zhu, M.X.; Yang, Y.W.; Zhang, P.F.; Yang, X.; Peng, R.; Gao, C.; Lu, J.C.; Wang, L.; Deng, X.Y.; et al. Downregulation of RNF128 activates Wnt/β-catenin signaling to induce cellular EMT and stemness via CD44 and CTTN ubiquitination in melanoma. J. Hematol. Oncol. 2019, 12, 21. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Miao, S.; Han, X.; Li, C.; Zhang, M.; Cui, K.; Xiong, T.; Chen, Z.; Wang, C.; Xu, H. MicroRNA-3619-5p suppresses bladder carcinoma progression by directly targeting β-catenin and CDK2 and activating p21. Cell Death Dis. 2018, 9, 960. [Google Scholar] [CrossRef]
- Hao, Y.; Baker, D.; Ten Dijke, P. TGF-β-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, M.A.; Freitas, J.P.; Mazher Hussain, S.; Glazer, E.S. TGF-β Inhibitors in Metastatic Pancreatic Ductal Adenocarcinoma. J. Gastrointest. Cancer 2019, 50, 207–213. [Google Scholar] [CrossRef]
- Howley, B.V.; Howe, P.H. TGF-beta signaling in cancer: Post-transcriptional regulation of EMT via hnRNP E1. Cytokine 2019, 118, 19–26. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Z.; Song, C.; Deng, H.; Yang, R.; Zhou, L.; Sun, Y.; Zhang, Q. Glaucocalyxin A reverses EMT and TGF-β1-induced EMT by inhibiting TGF-β1/Smad2/3 signaling pathway in osteosarcoma. Chem. Biol. Interact. 2019, 307, 158–166. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, R.; Wang, G.; Zhang, Y.; Liu, F. Exosomes derived from mesenchymal stem cells reverse EMT via TGF-β1/Smad pathway and promote repair of damaged endometrium. Stem Cell Res. Ther. 2019, 10, 225. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.C.; Lv, Z.H. MicroRNA-132 inhibits migration, invasion and epithelial-mesenchymal transition via TGFβ1/Smad2 signaling pathway in human bladder cancer. Oncotarget. Ther. 2019, 12, 5937–5945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.; Hong, L.; Yu, D.; Cao, T.; Zhou, Z.; He, S. LncRNA XIST promotes pancreatic cancer migration, invasion and EMT by sponging miR-429 to modulate ZEB1 expression. Int. J. Biochem. Cell Biol. 2019, 113, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.Y.; Lin, L.; Li, Y.H.; Jiang, H.P.; Zhu, L.T.; Deng, Y.R.; Lin, D.; Chen, W.; Zeng, C.Y.; Wang, L.J.; et al. ZEB1 promotes invasion and metastasis of endometrial cancer by interacting with HDGF and inducing its transcription. Am. J. Cancer Res. 2019, 9, 2314–2330. [Google Scholar] [PubMed]
- Majid, S.; Dar, A.A.; Saini, S.; Deng, G.; Chang, I.; Greene, K.; Tanaka, Y.; Dahiya, R.; Yamamura, S. MicroRNA-23b functions as a tumor suppressor by regulating Zeb1 in bladder cancer. PLoS ONE 2013, 8, e67686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, D.; Wu, F.; Mu, S.; Hu, B.; Zhong, B.; Gao, F.; Qing, X.; Liu, J.; Zhang, Z.; Shao, Z. LncRNA AFAP1-AS1 promotes tumorigenesis and epithelial-mesenchymal transition of osteosarcoma through RhoC/ROCK1/p38MAPK/Twist1 signaling pathway. J. Exp. Clin. Cancer Res. 2019, 38, 375. [Google Scholar] [CrossRef] [Green Version]
- Yao, N.; Fu, Y.; Chen, L.; Liu, Z.; He, J.; Zhu, Y.; Xia, T.; Wang, S. Long non-coding RNA NONHSAT101069 promotes epirubicin resistance, migration, and invasion of breast cancer cells through NONHSAT101069/miR-129-5p/Twist1 axis. Oncogene 2019, 38, 7216–7233. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, J.; Xiao, M.; Yang, J.; Zhang, N. miR-203 Suppresses Bladder Cancer Cell Growth and Targets Twist1. Oncol. Res. 2018, 26, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.H.; Lin, Y.W.; Wen, Y.C.; Yang, Y.C.; Hsiao, M.; Chang, J.L.; Huang, H.C.; Lee, W.J. Targeting the SPOCK1-snail/slug axis-mediated epithelial-to-mesenchymal transition by apigenin contributes to repression of prostate cancer metastasis. J. Exp. Clin. Cancer Res. 2019, 38, 246. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Dong, Z.Y.; Wang, J.Y. LncRNA NNT-AS1 is a major mediator of cisplatin chemoresistance in non-small cell lung cancer through MAPK/Slug pathway. Eur. Rev. Med. Pharm. Sci. 2018, 22, 4879–4887. [Google Scholar] [CrossRef]
- Li, W.; He, Q.Z.; Wu, C.Q.; Pan, X.Y.; Wang, J.; Tan, Y.; Shan, X.Y.; Zeng, H.C. PFOS Disturbs BDNF-ERK-CREB Signalling in Association with Increased MicroRNA-22 in SH-SY5Y Cells. Biomed. Res. Int. 2015, 2015, 302653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Fan, Z.; Yang, J.; Ding, J.; Yang, C.; Chen, L. microRNA-22 attenuates myocardial ischemia-reperfusion injury via an anti-inflammatory mechanism in rats. Exp. Ther. Med. 2016, 12, 3249–3255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.; Li, J.; Wang, X.; Meng, S.; Shen, J.; Wang, S.; Xu, X.; Xie, B.; Liu, B.; Xie, L. MiR-22 suppresses epithelial-mesenchymal transition in bladder cancer by inhibiting Snail and MAPK1/Slug/vimentin feedback loop. Cell Death Dis. 2018, 9, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Liang, Z.; Xu, X.; Li, J.; Zhu, Y.; Meng, S.; Li, S.; Wang, S.; Xie, B.; Ji, A.; et al. miR-148a-3p represses proliferation and EMT by establishing regulatory circuits between ERBB3/AKT2/c-myc and DNMT1 in bladder cancer. Cell Death Dis. 2016, 7, e2503. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Zhu, Y.; Liang, Z.; Li, S.; Xu, X.; Wang, X.; Wu, J.; Hu, Z.; Meng, S.; Liu, B.; et al. c-Met and CREB1 are involved in miR-433-mediated inhibition of the epithelial-mesenchymal transition in bladder cancer by regulating Akt/GSK-3β/Snail signaling. Cell Death Dis. 2016, 7, e2088. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Xu, X.; Meng, S.; Liang, Z.; Wang, X.; Xu, M.; Wang, S.; Li, S.; Zhu, Y.; Xie, B.; et al. MET/SMAD3/SNAIL circuit mediated by miR-323a-3p is involved in regulating epithelial-mesenchymal transition progression in bladder cancer. Cell Death Dis. 2017, 8, e3010. [Google Scholar] [CrossRef]
- Chen, M.F.; Zeng, F.; Qi, L.; Zu, X.B.; Wang, J.; Liu, L.F.; Li, Y. Transforming growth factor-β1 induces epithelial-mesenchymal transition and increased expression of matrix metalloproteinase-16 via miR-200b downregulation in bladder cancer cells. Mol. Med. Rep. 2014, 10, 1549–1554. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ma, D.L.; Yu, C.H.; Sha, K.F.; Zhao, M.J.; Liu, T.J. MicroRNA-370 suppresses SOX12 transcription and acts as a tumor suppressor in bladder cancer. Eur. Rev. Med. Pharm. Sci. 2020, 24, 2303–2312. [Google Scholar] [CrossRef]
- Yu, G.; Yao, W.; Xiao, W.; Li, H.; Xu, H.; Lang, B. MicroRNA-34a functions as an anti-metastatic microRNA and suppresses angiogenesis in bladder cancer by directly targeting CD44. J. Exp. Clin. Cancer Res. 2014, 33, 779. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Duan, P.; Zhu, H.; Rao, D. miR-613 inhibits bladder cancer proliferation and migration through targeting SphK1. Am. J. Trans. Res. 2017, 9, 1213–1221. [Google Scholar]
- Yao, K.; He, L.; Gan, Y.; Zeng, Q.; Dai, Y.; Tan, J. MiR-186 suppresses the growth and metastasis of bladder cancer by targeting NSBP1. Diagn. Pathol. 2015, 10, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhang, D.; Lv, J.; Wang, S.; Zhang, Q. MiR-125a-5p suppresses bladder cancer progression through targeting FUT4. Biomed. Pharm. 2018, 108, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Na, X.Y.; Shang, X.S.; Zhao, Y.; Ren, P.P.; Hu, X.Q. MiR-203a functions as a tumor suppressor in bladder cancer by targeting SIX4. Neoplasma 2019, 66, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhang, J.; Yang, T.; Zhang, W.; Liu, M. MiR-22 suppresses the growth and metastasis of bladder cancer cells by targeting E2F3. Int. J. Clin. Exp. Pathol. 2020, 13, 587–596. [Google Scholar]
- Wang, S.; Zhang, G.; Zheng, W.; Xue, Q.; Wei, D.; Zheng, Y.; Yuan, J. MiR-454-3p and miR-374b-5p suppress migration and invasion of bladder cancer cells through targetting ZEB2. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [Green Version]
- Zeng, T.; Peng, L.; Chao, C.; Fu, B.; Wang, G.; Wang, Y.; Zhu, X. miR-451 inhibits invasion and proliferation of bladder cancer by regulating EMT. Int. J. Clin. Exp. Pathol. 2014, 7, 7653–7662. [Google Scholar]
- Zhou, M.; Wang, S.; Hu, L.; Liu, F.; Zhang, Q.; Zhang, D. miR-199a-5p suppresses human bladder cancer cell metastasis by targeting CCR7. BMC Urol. 2016, 16, 64. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Qiu, M.; An, Y.; Huang, J.; Gong, C. miR-7-5p acts as a tumor suppressor in bladder cancer by regulating the hedgehog pathway factor Gli3. Biochem. Biophys. Res. Commun. 2018, 503, 2101–2107. [Google Scholar] [CrossRef]
- Adam, L.; Zhong, M.; Choi, W.; Qi, W.; Nicoloso, M.; Arora, A.; Calin, G.; Wang, H.; Siefker-Radtke, A.; McConkey, D.; et al. miR-200 expression regulates epithelial-to-mesenchymal transition in bladder cancer cells and reverses resistance to epidermal growth factor receptor therapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 5060–5072. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Qiu, M.; Tan, G.; Liang, Z.; Qin, Y.; Chen, L.; Chen, H.; Liu, J. miR-200c inhibits invasion, migration and proliferation of bladder cancer cells through down-regulation of BMI-1 and E2F3. J. Trans. Med. 2014, 12, 305. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Li, Q.; Wang, S.; Zhang, J. miR-485-5p inhibits bladder cancer metastasis by targeting HMGA2. Int. J. Mol. Med. 2015, 36, 1136–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.L.; Ho, J.Y.; Chou, S.C.; Yu, D.S. MiR-429 reverses epithelial-mesenchymal transition by restoring E-cadherin expression in bladder cancer. Oncotarget 2016, 7, 26593–26603. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ying, Y.; Xie, H.; Jin, K.; Yan, H.; Wang, S.; Xu, M.; Xu, X.; Wang, X.; Yang, K.; et al. Dual regulatory role of CCNA2 in modulating CDK6 and MET-mediated cell-cycle pathway and EMT progression is blocked by miR-381-3p in bladder cancer. FASEB J. 2019, 33, 1374–1388. [Google Scholar] [CrossRef]
- Guttilla, I.K.; Adams, B.D.; White, B.A. ERα, microRNAs, and the epithelial-mesenchymal transition in breast cancer. Trends Endocrinol. Metab. 2012, 23, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Xu, L.Y.; Li, E.M. A family of pleiotropically acting microRNAs in cancer progression, miR-200: Potential cancer therapeutic targets. Curr. Pharm. Des. 2014, 20, 1896–1903. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, W.; Liu, C.; Li, G. miR-200 affects tamoxifen resistance in breast cancer cells through regulation of MYB. Sci. Rep. 2019, 9, 18844. [Google Scholar] [CrossRef]
- Gregory, P.A.; Bert, A.G.; Paterson, E.L.; Barry, S.C.; Tsykin, A.; Farshid, G.; Vadas, M.A.; Khew-Goodall, Y.; Goodall, G.J. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 2008, 10, 593–601. [Google Scholar] [CrossRef]
- Yang, K.; Tang, H.; Ding, M.; Guo, Y.; Kai, K.; Xiao, J.; Shen, Y.; Miao, S.; Zhou, R. Expression of miR-195 and MEK1 in patients with bladder cancer and their relationship to prognosis. Int. J. Clin. Exp. Pathol. 2019, 12, 843–850. [Google Scholar]
- Zhao, C.; Qi, L.; Chen, M.; Liu, L.; Yan, W.; Tong, S.; Zu, X. microRNA-195 inhibits cell proliferation in bladder cancer via inhibition of cell division control protein 42 homolog/signal transducer and activator of transcription-3 signaling. Exp. Ther. Med. 2015, 10, 1103–1108. [Google Scholar] [CrossRef] [Green Version]
- Li, H.J.; Sun, X.M.; Li, Z.K.; Yin, Q.W.; Pang, H.; Pan, J.J.; Li, X.; Chen, W. LncRNA UCA1 Promotes Mitochondrial Function of Bladder Cancer via the MiR-195/ARL2 Signaling Pathway. Cell. Physiol. Biochem. 2017, 43, 2548–2561. [Google Scholar] [CrossRef]
- Chen, S.; Wang, W.; Lin, G.; Zhong, S. MicroRNA-195 inhibits epithelial-mesenchymal transition via downregulating CDK4 in bladder cancer. Int. J. Clin. Exp. Pathol. 2018, 11, 3891–3902. [Google Scholar] [PubMed]
- Liu, L.; Liu, Y.; Zhang, X.; Chen, M.; Wu, H.; Lin, M.; Zhan, Y.; Zhuang, C.; Lin, J.; Li, J.; et al. Inhibiting cell migration and cell invasion by silencing the transcription factor ETS-1 in human bladder cancer. Oncotarget 2016, 7, 25125–25134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, K.; Wu, S.; Yue, Y.; Gu, Y.; Guan, B.; Wang, X.; Hsieh, J.T.; Chang, L.S.; He, D.; Wu, K. RASAL2 inhibits tumor angiogenesis via p-AKT/ETS1 signaling in bladder cancer. Cell. Signal. 2018, 48, 38–44. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, R.; Zhang, S.N.; Zhang, H.Z.; Ruan, X.J.; Cao, Z.; Gu, X.Z. MicroRNA-338-3p inhibits the progression of bladder cancer through regulating ETS1 expression. Eur. Rev. Med. Pharm. Sci. 2019, 23, 1986–1995. [Google Scholar] [CrossRef]
- Mao, X.W.; Xiao, J.Q.; Li, Z.Y.; Zheng, Y.C.; Zhang, N. Effects of microRNA-135a on the epithelial-mesenchymal transition, migration and invasion of bladder cancer cells by targeting GSK3β through the Wnt/β-catenin signaling pathway. Exp. Mol. Med. 2018, 50, e429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, C.; Zhang, Q.; Gu, R.; Lou, Y.; Liu, W. miR-96 regulates migration and invasion of bladder cancer through epithelial-mesenchymal transition in response to transforming growth factor-β1. J. Cell. Biochem. 2018, 119, 7807–7817. [Google Scholar] [CrossRef]
- Baldassarre, G.; Belletti, B.; Nicoloso, M.S.; Schiappacassi, M.; Vecchione, A.; Spessotto, P.; Morrione, A.; Canzonieri, V.; Colombatti, A. p27(Kip1)-stathmin interaction influences sarcoma cell migration and invasion. Cancer Cell 2005, 7, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Rana, S.; Maples, P.B.; Senzer, N.; Nemunaitis, J. Stathmin 1: A novel therapeutic target for anticancer activity. Exp. Rev. Anticancer Ther. 2008, 8, 1461–1470. [Google Scholar] [CrossRef]
- Suzuki, K.; Watanabe, A.; Araki, K.; Yokobori, T.; Harimoto, N.; Gantumur, D.; Hagiwara, K.; Yamanaka, T.; Ishii, N.; Tsukagoshi, M.; et al. High STMN1 Expression Is Associated with Tumor Differentiation and Metastasis in Clinical Patients with Pancreatic Cancer. Anticancer Res. 2018, 38, 939–944. [Google Scholar] [CrossRef]
- Osone, K.; Yokobori, T.; Katayama, C.; Takahashi, R.; Kato, R.; Tatsuski, H.; Takada, T.; Yajima, R.; Motegi, Y.; Ogawa, H.; et al. STMN1 accumulation is associated with dysplastic and neoplastic lesions in patients with ulcerative colitis. Oncol. Lett. 2019, 18, 4712–4718. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Cao, J.; Zhao, X. miR-221 facilitates the TGFbeta1-induced epithelial-mesenchymal transition in human bladder cancer cells by targeting STMN1. BMC Urol. 2015, 15, 36. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Wu, F.; Zhang, J.; Sun, R.; Li, F.; Li, Y.; Chang, S.; Wang, L.; Wang, X.; Liu, L.; et al. EGR1 interacts with DNMT3L to inhibit the transcription of miR-195 and plays an anti-apoptotic role in the development of gastric cancer. J. Cell. Mol. Med. 2019, 23, 7372–7381. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ameri, A.H.; Wang, S.; Jansson, K.H.; Casey, O.M.; Yang, Q.; Beshiri, M.L.; Fang, L.; Lake, R.G.; Agarwal, S.; et al. EGR1 regulates angiogenic and osteoclastogenic factors in prostate cancer and promotes metastasis. Oncogene 2019, 38, 6241–6255. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Long, J.L.; Yin, Y.T.; Guo, H.N.; Jiang, E.P.; Li, Y.L.; He, Q.L.; Zeng, C.; Sun, Y.Q. MicroRNA-34a suppresses the invasion and migration of colorectal cancer cells by enhancing EGR1 and inhibiting vimentin. Exp. Ther. Med. 2019, 18, 2459–2466. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Wang, Y.; Liang, J.; Liu, Z.; Sun, X.; Cai, K. MiR-301b promotes the proliferation, mobility, and epithelial-to-mesenchymal transition of bladder cancer cells by targeting EGR1. Biochem. Cell Biol. 2017, 95, 571–577. [Google Scholar] [CrossRef]
- Huang, M.; Prendergast, G. RhoB in cancer suppression. Histol. Histopathol. 2006, 21, 6. [Google Scholar]
- Zhou, J.; Zhu, Y.; Zhang, G.; Liu, N.; Sun, L.; Liu, M.; Qiu, M.; Luo, D.; Tang, Q.; Liao, Z. A distinct role of RhoB in gastric cancer suppression. Int. J. Cancer 2011, 128, 1057–1068. [Google Scholar] [CrossRef]
- Bousquet, E.; Calvayrac, O.; Mazieres, J.; Lajoie-Mazenc, I.; Boubekeur, N.; Favre, G.; Pradines, A. RhoB loss induces Rac1-dependent mesenchymal cell invasion in lung cells through PP2A inhibition. Oncogene 2016, 35, 1760–1769. [Google Scholar] [CrossRef]
- Bi, J.; Liu, H.; Dong, W.; Xie, W.; He, Q.; Cai, Z.; Huang, J.; Lin, T. Circular RNA circ-ZKSCAN1 inhibits bladder cancer progression through miR-1178-3p/p21 axis and acts as a prognostic factor of recurrence. Mol. Cancer 2019, 18, 133. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Toyooka, S.; Gazdar, A.F.; Hsieh, J.-T. Epigenetic regulation of a novel tumor suppressor gene (hDAB2IP) in prostate cancer cell lines. J. Biol. Chem. 2003, 278, 3121–3130. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.J.; Kong, Z.L.; Wan, F.N.; Wang, H.K.; Bian, X.J.; Gan, H.L.; Wang, C.F.; Ye, D.W. Downregulation of DAB2IP results in cell proliferation and invasion and contributes to unfavorable outcomes in bladder cancer. Cancer Sci. 2014, 105, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Wang, B.; Chen, Y.; Zhou, J.; Huang, J.; Hui, K.; Zeng, J.; Zhu, J.; Zhang, K.; Li, L.; et al. DAB2IP regulates the chemoresistance to pirarubicin and tumor recurrence of non-muscle invasive bladder cancer through STAT3/Twist1/P-glycoprotein signaling. Cell. Signal. 2015, 27, 2515–2523. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, B.; Hui, K.; Zeng, J.; Fan, J.; Wang, X.; Hsieh, J.T.; He, D.; Wu, K. miR-92b targets DAB2IP to promote EMT in bladder cancer migration and invasion. Oncol. Rep. 2016, 36, 1693–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, M.C.; Ni, J.J.; Cui, W.Y.; Wang, B.Y.; Zhuo, W. Emerging roles of lncRNA in cancer and therapeutic opportunities. Am. J. Cancer Res. 2019, 9, 1354–1366. [Google Scholar]
- Liang, Z.X.; Liu, H.S.; Wang, F.W.; Xiong, L.; Zhou, C.; Hu, T.; He, X.W.; Wu, X.J.; Xie, D.; Wu, X.R.; et al. LncRNA RPPH1 promotes colorectal cancer metastasis by interacting with TUBB3 and by promoting exosomes-mediated macrophage M2 polarization. Cell Death Dis. 2019, 10, 829. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Xu, X.; Hong, L.; Wang, Q.; Huang, J.; Jiang, L. Upregulation of lncRNA GAS5 inhibits the growth and metastasis of cervical cancer cells. J. Cell. Physiol. 2019, 234, 23571–23580. [Google Scholar] [CrossRef]
- López-Urrutia, E.; Montes, L.P.B.; de Guevara Cervantes, D.L.; Pérez-Plasencia, C.; Campos-Parra, A.D. Crosstalk between long non-coding RNAs, micro-RNAs and mRNAs: Deciphering molecular mechanisms of master regulators in cancer. Front. Oncol. 2019, 9. [Google Scholar] [CrossRef]
- Wilusz, J.E.; Sunwoo, H.; Spector, D.L. Long noncoding RNAs: Functional surprises from the RNA world. Genes Dev. 2009, 23, 1494–1504. [Google Scholar] [CrossRef] [Green Version]
- Mercer, T.R.; Dinger, M.E.; Mattick, J.S. Long non-coding RNAs: Insights into functions. Nat. Rev. Genet. 2009, 10, 155–159. [Google Scholar] [CrossRef]
- Feng, C.; Zhao, Y.; Li, Y.; Zhang, T.; Ma, Y.; Liu, Y. LncRNA MALAT1 Promotes Lung Cancer Proliferation and Gefitinib Resistance by Acting as a miR-200a Sponge. Archivos de Bronconeumologia 2019, 55, 627–633. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, C.; Li, Y.; Ma, Y.; Cai, R. LncRNA H19 promotes lung cancer proliferation and metastasis by inhibiting miR-200a function. Mol. Cell. Biochem. 2019, 460, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lai, Q.; He, J.; Li, Q.; Ding, J.; Lan, Z.; Gu, C.; Yan, Q.; Fang, Y.; Zhao, X.; et al. LncRNA SNHG6 promotes proliferation, invasion and migration in colorectal cancer cells by activating TGF-β/Smad signaling pathway via targeting UPF1 and inducing EMT via regulation of ZEB1. Int. J. Med. Sci. 2019, 16, 51–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Tao, W.; Ni, S.; Chen, Q. Upregulation of lncRNA snoRNA host gene 6 regulates NUAK family SnF1-like kinase-1 expression by competitively binding microRNA-125b and interacting with Snail1/2 in bladder cancer. J. Cell. Biochem. 2019, 120, 357–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, M.; Li, Z.; Wang, W.; Zeng, Y.; Liu, Z.; Qiu, J. Long non-coding RNA H19 increases bladder cancer metastasis by associating with EZH2 and inhibiting E-cadherin expression. Cancer Lett. 2013, 333, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Xu, L.; Wan, Y.; Zhou, J.; Fu, D.; Chao, H.; Bao, K.; Zeng, T. Inhibition of E-cadherin expression by lnc-RNA H19 to facilitate bladder cancer metastasis. Cancer Biomark. Sect. A Dis. Mark. 2018, 22, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Zhong, Z.; Huang, M.; Tian, Q.; Jiang, R.; Chen, J. lncRNA H19 regulates epithelial-mesenchymal transition and metastasis of bladder cancer by miR-29b-3p as competing endogenous RNA. Biochimica et Biophysica Acta Mol. Cell Res. 2017, 1864, 1887–1899. [Google Scholar] [CrossRef]
- Miao, L.; Liu, H.Y.; Zhou, C.; He, X. LINC00612 enhances the proliferation and invasion ability of bladder cancer cells as ceRNA by sponging miR-590 to elevate expression of PHF14. J. Exp. Clin. Cancer Res. 2019, 38, 143. [Google Scholar] [CrossRef]
- Mao, W.; Huang, X.; Wang, L.; Zhang, Z.; Liu, M.; Li, Y.; Luo, M.; Yao, X.; Fan, J.; Geng, J. Circular RNA hsa_circ_0068871 regulates FGFR3 expression and activates STAT3 by targeting miR-181a-5p to promote bladder cancer progression. J. Exp. Clin. Cancer Res. 2019, 38, 169. [Google Scholar] [CrossRef]
- Bi, J.; Liu, H.; Cai, Z.; Dong, W.; Jiang, N.; Yang, M.; Huang, J.; Lin, T. Circ-BPTF promotes bladder cancer progression and recurrence through the miR-31-5p/RAB27A axis. Aging 2018, 10, 1964–1976. [Google Scholar] [CrossRef]
- Yang, C.; Yuan, W.; Yang, X.; Li, P.; Wang, J.; Han, J.; Tao, J.; Li, P.; Yang, H.; Lv, Q.; et al. Circular RNA circ-ITCH inhibits bladder cancer progression by sponging miR-17/miR-224 and regulating p21, PTEN expression. Mol. Cancer 2018, 17, 19. [Google Scholar] [CrossRef] [Green Version]
- Yan, D.; Dong, W.; He, Q.; Yang, M.; Huang, L.; Kong, J.; Qin, H.; Lin, T.; Huang, J. Circular RNA circPICALM sponges miR-1265 to inhibit bladder cancer metastasis and influence FAK phosphorylation. EBioMedicine 2019, 48, 316–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Chang, J.K.; Hou, J.Q.; Zhao, Z.H.; Zhang, L.D. Inhibition of miR-221 influences bladder cancer cell proliferation and apoptosis. Eur. Rev. Med. Pharm. Sci. 2017, 21, 3193–3199. [Google Scholar]
- Fu, B.; Wang, Y.; Zhang, X.; Lang, B.; Zhou, X.; Xu, X.; Zeng, T.; Liu, W.; Zhang, X.; Guo, J.; et al. MiR-221-induced PUMA silencing mediates immune evasion of bladder cancer cells. Int. J. Oncol. 2015, 46, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Feng, W.; Shi, J.; Chen, L.; Huang, J.; Lin, T. circRIP2 accelerates bladder cancer progression via miR-1305/Tgf-β2/smad3 pathway. Mol. Cancer 2020, 19, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, T.-T.; Liu, X.-D.; Zhan, Z.-P.; Wu, Q.-J. Sulforaphane enhances the cisplatin sensitivity through regulating DNA repair and accumulation of intracellular cisplatin in ovarian cancer cells. Exp. Cell Res. 2020, 393, 112061. [Google Scholar] [CrossRef] [PubMed]
- Calcabrini, C.; Maffei, F.; Turrini, E.; Fimognari, C. Sulforaphane Potentiates Anticancer Effects of Doxorubicin and Cisplatin and Mitigates Their Toxic Effects. Front. Pharmacol. 2020, 11, 567. [Google Scholar] [CrossRef]
- Shan, Y.; Zhang, L.; Bao, Y.; Li, B.; He, C.; Gao, M.; Feng, X.; Xu, W.; Zhang, X.; Wang, S. Epithelial-mesenchymal transition, a novel target of sulforaphane via COX-2/MMP2, 9/Snail, ZEB1 and miR-200c/ZEB1 pathways in human bladder cancer cells. J. Nutr. Biochem. 2013, 24, 1062–1069. [Google Scholar] [CrossRef]
- Gakis, G. The role of inflammation in bladder cancer. In Inflammation and Cancer; Springer: New York, NY, USA, 2014; pp. 183–196. [Google Scholar]
- Masson-Lecomte, A.; Rava, M.; Real, F.X.; Hartmann, A.; Allory, Y.; Malats, N. Inflammatory biomarkers and bladder cancer prognosis: A systematic review. Eur. Urol. 2014, 66, 1078–1091. [Google Scholar] [CrossRef]
- Patel, R.; Baker, S.S.; Liu, W.; Desai, S.; Alkhouri, R.; Kozielski, R.; Mastrandrea, L.; Sarfraz, A.; Cai, W.; Vlassara, H. Effect of dietary advanced glycation end products on mouse liver. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Galdiero, M.R.; Garlanda, C.; Jaillon, S.; Marone, G.; Mantovani, A. Tumor associated macrophages and neutrophils in tumor progression. J. Cell. Physiol. 2013, 228, 1404–1412. [Google Scholar] [CrossRef]
- Li, F.; Shanmugam, M.K.; Chen, L.; Chatterjee, S.; Basha, J.; Kumar, A.P.; Kundu, T.K.; Sethi, G. Garcinol, a polyisoprenylated benzophenone modulates multiple proinflammatory signaling cascades leading to the suppression of growth and survival of head and neck carcinoma. Cancer Prev. Res. 2013, 6, 843–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawhney, M.; Rohatgi, N.; Kaur, J.; Shishodia, S.; Sethi, G.; Gupta, S.D.; Deo, S.V.; Shukla, N.K.; Aggarwal, B.B.; Ralhan, R. Expression of NF-kappaB parallels COX-2 expression in oral precancer and cancer: Association with smokeless tobacco. Int. J. Cancer 2007, 120, 2545–2556. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.S.; Sethi, G.; Jain, A.K.; Jaiswal, A.K.; Aggarwal, B.B. Genetic deletion of NAD(P)H:Quinone oxidoreductase 1 abrogates activation of nuclear factor-κB, IκBα kinase, c-Jun N-terminal kinase, Akt, p38, and p44/42 mitogen-activated protein kinases and potentiates apoptosis. J. Biol. Chem. 2006, 281, 19798–19808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siveen, K.S.; Mustafa, N.; Li, F.; Kannaiyan, R.; Ahn, K.S.; Kumar, A.P.; Chng, W.J.; Sethi, G. Thymoquinone overcomes chemoresistance and enhances the anticancer effects of bortezomib through abrogation of NF-kappaB regulated gene products in multiple myeloma xenograft mouse model. Oncotarget 2014, 5, 634–648. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Mao, Z.; Sun, J. NF-κB inhibitor, BAY11-7082, suppresses M2 tumor-associated macrophage induced EMT potential via miR-30a/NF-κB/Snail signaling in bladder cancer cells. Gene 2019, 710, 91–97. [Google Scholar] [CrossRef]
- Tailor, D.; Hahm, E.-R.; Kale, R.K.; Singh, S.V.; Singh, R.P. Sodium butyrate induces DRP1-mediated mitochondrial fusion and apoptosis in human colorectal cancer cells. Mitochondrion 2014, 16, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Salimi, V.; Shahsavari, Z.; Safizadeh, B.; Hosseini, A.; Khademian, N.; Tavakoli-Yaraki, M. Sodium butyrate promotes apoptosis in breast cancer cells through reactive oxygen species (ROS) formation and mitochondrial impairment. Lipids Health Dis. 2017, 16, 208. [Google Scholar] [CrossRef] [Green Version]
- Kuefer, R.; Hofer, M.; Altug, V.; Zorn, C.; Genze, F.; Kunzi-Rapp, K.; Hautmann, R.; Gschwend, J. Sodium butyrate and tributyrin induce in vivo growth inhibition and apoptosis in human prostate cancer. Br. J. Cancer 2004, 90, 535–541. [Google Scholar] [CrossRef]
- Maruyama, T.; Yamamoto, S.; Qiu, J.; Ueda, Y.; Suzuki, T.; Nojima, M.; Shima, H. Apoptosis of bladder cancer by sodium butyrate and cisplatin. J. Infect. Chemother. 2012, 18, 288–295. [Google Scholar] [CrossRef]
- Wang, F.; Wu, H.; Fan, M.; Yu, R.; Zhang, Y.; Liu, J.; Zhou, X.; Cai, Y.; Huang, S.; Hu, Z.; et al. Sodium butyrate inhibits migration and induces AMPK-mTOR pathway-dependent autophagy and ROS-mediated apoptosis via the miR-139-5p/Bmi-1 axis in human bladder cancer cells. FASEB J. 2020, 34, 4266–4282. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Chen, P.; Zhang, Y.; Lu, W.; Ding, W.; Luo, Y.; Wen, S.; Xu, R.; Liu, P.; Huang, P. Synergy between Auranofin and Celecoxib against Colon Cancer In Vitro and In Vivo through a Novel Redox-Mediated Mechanism. Cancers 2019, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velmurugan, B.K.; Hua, C.H.; Tsai, M.H.; Lee, C.P.; Chung, C.M.; Ko, Y.C. Combination of celecoxib and calyculin-A inhibits epithelial-mesenchymal transition in human oral cancer cells. Biotech. Histochem. 2020, 95, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, Y.; Zhou, Z.; Huang, M.; Deng, W.; Wang, Y.; Zhou, X.; Chen, L.; Li, Y.; Zeng, T.; et al. Celecoxib inhibits the epithelial-to-mesenchymal transition in bladder cancer via the miRNA-145/TGFBR2/Smad3 axis. Int. J. Mol. Med. 2019, 44, 683–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, M.N.; Choi, W.; Wszolek, M.F.; Navai, N.; Lee, I.L.; Nitti, G.; Wen, S.; Flores, E.R.; Siefker-Radtke, A.; Czerniak, B.; et al. The p63 protein isoform ΔNp63α inhibits epithelial-mesenchymal transition in human bladder cancer cells: Role of MIR-205. J. Biol. Chem. 2013, 288, 3275–3288. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhang, H.; Situ, J.; Li, M.; Sun, H. KCNQ1OT1 aggravates cell proliferation and migration in bladder cancer through modulating miR-145-5p/PCBP2 axis. Cancer Cell Int. 2019, 19, 325. [Google Scholar] [CrossRef] [Green Version]
- Zhan, Y.; Chen, Z.; Li, Y.; He, A.; He, S.; Gong, Y.; Li, X.; Zhou, L. Long non-coding RNA DANCR promotes malignant phenotypes of bladder cancer cells by modulating the miR-149/MSI2 axis as a ceRNA. J. Exp. Clin. Cancer Res. 2018, 37, 273. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.; Long, Z.; Zhang, X.; Cheng, J.; Qi, F.; Wu, S.; Huang, T. LncARSR sponges miR-129-5p to promote proliferation and metastasis of bladder cancer cells through increasing SOX4 expression. Int. J. Biol. Sci. 2020, 16, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Zhu, X.; Chen, F.; Huang, C.; Ai, K.; Wu, H.; Zhang, L.; Zhao, X. LncRNA XIST/miR-200c regulates the stemness properties and tumourigenicity of human bladder cancer stem cell-like cells. Cancer Cell Int. 2018, 18, 41. [Google Scholar] [CrossRef]
- Luo, J.; Chen, J.; Li, H.; Yang, Y.; Yun, H.; Yang, S.; Mao, X. LncRNA UCA1 promotes the invasion and EMT of bladder cancer cells by regulating the miR-143/HMGB1 pathway. Oncol. Lett. 2017, 14, 5556–5562. [Google Scholar] [CrossRef] [Green Version]
- Xue, M.; Pang, H.; Li, X.; Li, H.; Pan, J.; Chen, W. Long non-coding RNA urothelial cancer-associated 1 promotes bladder cancer cell migration and invasion by way of the hsa-miR-145-ZEB1/2-FSCN1 pathway. Cancer Sci. 2016, 107, 18–27. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Ma, W.; Zhou, J.; Sun, Z.; Yan, X. Long noncoding RNA AC114812.8 promotes the progression of bladder cancer through miR-371b-5p/FUT4 axis. Biomed. Pharm. 2020, 121, 109605. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Qiu, K.; Li, M.; Liang, Y. Double-negative feedback loop between long non-coding RNA TUG1 and miR-145 promotes epithelial to mesenchymal transition and radioresistance in human bladder cancer cells. FEBS Lett. 2015, 589, 3175–3181. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hong, S.; Liu, Z. LncRNA LINC00641 predicts prognosis and inhibits bladder cancer progression through miR-197-3p/KLF10/PTEN/PI3K/AKT cascade. Biochem. Biophys. Res. Commun. 2018, 503, 1825–1829. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, B.; Shi, H.; Zhou, J.; Zhou, F.; Cao, J.; Sun, X. miR-758-3p suppresses human bladder cancer cell proliferation, migration and invasion by targeting NOTCH2. Exp. Ther. Med. 2019, 17, 4273–4278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qin, H.; Jiang, B.; Chen, W.; Cao, W.; Zhao, X.; Yuan, H.; Qi, W.; Zhuo, D.; Guo, H. miR-30e-5p suppresses cell proliferation and migration in bladder cancer through regulating metadherin. J. Cell. Biochem. 2019, 120, 15924–15932. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, T.; Jin, H.; Yin, L.; Yu, H.; Bi, J. MiR-411 suppresses the development of bladder cancer by regulating ZnT1. Oncotarget. Ther. 2018, 11, 8695–8704. [Google Scholar] [CrossRef] [Green Version]



| MicroRNA | Downstream Target | Cell Line | Major Outcomes | Refs |
|---|---|---|---|---|
| miRNA-370 | SOX12 | Immortalized bladder cell line SV-HUC-1 (ATCC® CRL-9520™) and the human BC cell lines 5637 (ATCC® HTB-9™) and J82 (ATCC® HTB-1™) | miRNA-370 inhibits EMT via SOX12 downregulation, leading to a decreased metastasis of cancer cells | [149] |
| miRNA-34a | CD44 | Human bladder cancer cell lines (5637, T24, HT-1376, J82, SCABER and EJ) | Suppressing EMT via CD44 inhibition | [150] |
| miRNA-613 | SphK1 | Bladder cancer cell lines (J82, T24, UMUC3 and 5637) and a normal bladder cell line (SV-HUC-1) | Inhibition of SphK1 and reduced metastasis and EMT | [151] |
| miRNA-186 | NSBP1 | Human bladder cancer cell lines (J82, HT1376, RT4, T24 and TCCSUP) and immortalized human bladder epithelium (HCV29) cells | downregulation of NSBP1 and suppressed metastasis of cancer cells | [152] |
| miRNA-125a-5p | FUT4 | Bladder cancer cell lines (J82, T24, 5637 and BIU-87) and im-mortalized bladder cell line (SV-HUC-1) | miRNA-125a-5p inhibits invasion and EMT in BC cells via FUT4 downregulation | [153] |
| miRNA-203a | SIX4 | SV-HUC-1 human uro-epithelial cells and the bladder cancer cell lines T24, EJ, J8 and 5637 | There is a negative relationship between miRNA-203a and SIX4. This miRNA inhibits EMT via SIX4 downregulation | [154] |
| miRNA-22 | E2F3 | Human bladder cancer cell lines (5637 and T24) | Preventing expression of E2F3 and suppressed BC metastasis and EMT | [155] |
| miRNA-454-3p miRNA-374b-5p | ZEB2 | SV-HUC, TCC, 253J, 5637, J82, T24, EJ, HEK-293T cells | Suppressing EMT via ZEB2 downregulation | [156] |
| miRNA-451 | - | T24, 5637 and J28 bladder cancer cell lines | Inhibiting EMT via E-cadherin upregulation, and N-cadherin and vimentin downregulation | [157] |
| miRNA-199a-5p | CCR7 | Human bladder cancer T24 cell line and human normal bladder epithelial cell line SV-HUC-1 | Interfering with metastasis of cancer cells by downregulation of CCR7, and subsequent inhibition of EMT | [158] |
| miRNA-7-5p | Gli3 | TCC, 253J, 5637, T24, EJ, J82 (BCa cell lines) and SV-HUC (human bladder epithelium immortalized cell) | miRNA-7-5p reduces expression of Gli3 as a member of Hedgehog signaling pathway to suppress EMT | [159] |
| miRNA-200 | - | UMUC series of urothelial carcinomas and 253J BV cells | miRNA-200 enhances E-cadherin levels, and reduces ZEB1 and ZEB2 levels to inhibit EMT, leading to a diminution in metastasis and enhanced sensitivity into chemotherapy | [160] |
| miRNA-200c | BMI1 E2F3 | Human bladder cancer cell lines (UMUC-3 and T24) | Suppressing EMT and increasing E-cadherin levels via inhibition of BMI1 and E2F3 | [161] |
| miRNA-485-5p | HMGA2 | Human bladder cancer cell lines (SW780, T24, HT1376 and HT5637) and human bladder epithelial cell lines HU609 and HEK293 cell | Disrupting invasion of cancer cells by EMT inhibition via HMGA2 downregulation | [162] |
| miRNA-429 | ZEB1/2 β-catenin | Human UCC cell lines, T24 | Enhancing E-cadherin level via ZEB1/2 downregulation and subsequent inhibition of EMT Suppressing nuclear translocation of β-catenin and its interaction with TCF/LEF1, resulting in EMT inhibition | [163] |
| miRNA-381-3p | CCNA2 | T24, UM-UC3, and 5637 human BCa cell lines | Inhibition of EMT via CCNA2 downregulation | [164] |
| Upstream Regulator | MicroRNA | Downstream Target | Cell Line | Major Outcomes | Refs |
|---|---|---|---|---|---|
| LncRNA DANCR | miRNA-149 | MSI2 | Bladder cancer 5637, SW780, UM-UC-3, T24 and SV-HUC-1 cells | DANCR enhanced metastasis and invasion of cancer cells by downregulation of miRNA-149, and subsequent activation of MSI2, resulting in EMT | [238] |
| LncRNA ARSR | miRNA-129-5p | SOX4 | RT4 and 5637 cells | ARSR reduces expression of miRNA-129-5p via sponging to upregulate SOX4, leading to EMT | [239] |
| LncRNA XIST | miRNA-200c | - | Human bladder cancer cell lines 5637 and T24 | Stimulation of EMT by downregulation of miRNA-200c | [240] |
| LncRNA UCA1 | miRNA-143 | HMGB1 | Human bladder cancer cell lines (T24, 5637, J82, RT4 and HT1376) | UCA1 induces expression of HMGB1 via miRNA-143 downregulation, leading to EMT | [241] |
| LncRNA UCA1 | miRNA-145 | ZEB1/2 | Human bladder cancer cells 5637, T24, and UMUC2 | Stimulation of EMT by downregulation of miRNA-145 and subsequent activation of ZEB1/2 | [242] |
| LncRNA AC114812.8 | miRNA-371b-5p | FUT4 | Human BC cell lines T24, UM-UC-3, J82, and 5637, and the human immortalized normal urinary epithelial cell line SV-HUC-1 | Sponging of miiR-371b-5p by AC114812.8 leads to induction of FUT4, and EMT | [243] |
| LncRNA TUG1 | miRNA-145 | - | SV-HUC-1 cells | TUG1 induces EMT via downregulation of miRNA-145 | [244] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashrafizadeh, M.; Hushmandi, K.; Hashemi, M.; Akbari, M.E.; Kubatka, P.; Raei, M.; Koklesova, L.; Shahinozzaman, M.; Mohammadinejad, R.; Najafi, M.; et al. Role of microRNA/Epithelial-to-Mesenchymal Transition Axis in the Metastasis of Bladder Cancer. Biomolecules 2020, 10, 1159. https://doi.org/10.3390/biom10081159
Ashrafizadeh M, Hushmandi K, Hashemi M, Akbari ME, Kubatka P, Raei M, Koklesova L, Shahinozzaman M, Mohammadinejad R, Najafi M, et al. Role of microRNA/Epithelial-to-Mesenchymal Transition Axis in the Metastasis of Bladder Cancer. Biomolecules. 2020; 10(8):1159. https://doi.org/10.3390/biom10081159
Chicago/Turabian StyleAshrafizadeh, Milad, Kiavash Hushmandi, Mehrdad Hashemi, Mohammad Esmaeil Akbari, Peter Kubatka, Mehdi Raei, Lenka Koklesova, Md Shahinozzaman, Reza Mohammadinejad, Masoud Najafi, and et al. 2020. "Role of microRNA/Epithelial-to-Mesenchymal Transition Axis in the Metastasis of Bladder Cancer" Biomolecules 10, no. 8: 1159. https://doi.org/10.3390/biom10081159