MicroRNAs and Their Influence on the ZEB Family: Mechanistic Aspects and Therapeutic Applications in Cancer Therapy
Abstract
:1. Introduction
2. ZEB Family
2.1. ZEB1
2.2. ZEB2
3. MicroRNAs
MicroRNAs in Cancer Metastasis
4. MicroRNA, ncRNA, and ZEB: Role in EMT and Cancer Metastasis
4.1. ZEB1
4.1.1. MiRs as Modulators of ZEB1
4.1.2. LncRNAs as Modulators of miR/ZEB1 Axis
4.1.3. CircRNAs as Modulators of miR/ZEB1 Axis
4.2. ZEB2
4.2.1. MiRs as Modulators of ZEB2
4.2.2. LncRNAs as Modulators of miR/ZEB2 Axis
4.2.3. CircRNAs as Modulators of miR/ZEB2 Axis
5. MicroRNAs, ZEB, and Their Role in Tumor Resistance
5.1. ZEB1
5.1.1. Paclitaxel Resistance
5.1.2. Gemcitabine Resistance
5.1.3. Cisplatin Resistance
5.1.4. 5-Fluorouracil
5.2. ZEB2
6. MicroRNAs Target ZEB Family in Immune Cells
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Wu, X.; Xin, Z.; Zou, Z.; Lu, C.; Yu, Z.; Feng, S.; Pan, P.; Hao, G.; Dong, Y.; Yang, Y. SRY-related high-mobility-group box 4: Crucial regulators of the EMT in cancer. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Wang, Y.; Zhou, B.P. Epithelial-mesenchymal transition—a hallmark of breast cancer metastasis. Cancer Hallm. 2013, 1, 38–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz, V.M.; de Herreros, A.G. F-box proteins: Keeping the epithelial-to-mesenchymal transition (EMT) in check. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 71–79. [Google Scholar]
- Kar, R.; Jha, N.K.; Jha, S.K.; Sharma, A.; Dholpuria, S.; Asthana, N.; Chaurasiya, K.; Singh, V.K.; Burgee, S.; Nand, P. A “NOTCH” Deeper into the Epithelial-To-Mesenchymal Transition (EMT) Program in Breast Cancer. Genes 2019, 10, 961. [Google Scholar] [CrossRef] [Green Version]
- Seccia, T.; Caroccia, B.; Piazza, M.; Rossi, G.P. The Key Role of Epithelial to Mesenchymal Transition (EMT) in Hypertensive Kidney Disease. Int. J. Mol. Sci. 2019, 20, 3567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty, S.; Mir, K.B.; Seligson, N.D.; Nayak, D.; Kumar, R.; Goswami, A. Integration of EMT and cellular survival instincts in reprogramming of programmed cell death to anastasis. Cancer Metastasis Rev. 2020, 39, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, X.; Han, G.; Dai, S.; Ni, Q.; Xiao, S.; Huang, J. Downregulated lncRNA UCA1 acts as ceRNA to adsorb microRNA-498 to repress proliferation, invasion and epithelial mesenchymal transition of esophageal cancer cells by decreasing ZEB2 expression. Cell Cycle 2019, 18, 2359–2376. [Google Scholar] [CrossRef]
- Seton-Rogers, S. Epithelial–mesenchymal transition: Untangling EMT’s functions. Nat. Rev. Cancer 2015, 16, 1. [Google Scholar] [CrossRef]
- Yilmaz, M.; Christofori, G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009, 28, 15–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez-Martinez, R.; Ramkumar, N.; Anderson, K.V. p120-catenin regulates WNT signaling and EMT in the mouse embryo. Proc. Natl. Acad. Sci. USA 2019, 116, 16872–16881. [Google Scholar] [CrossRef] [Green Version]
- Moly, P.K.; Cooley, J.R.; Zeltzer, S.L.; Yatskievych, T.A.; Antin, P.B. Gastrulation EMT is independent of P-cadherin downregulation. PLoS ONE 2016, 11, e0153591. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Zheng, Y.; Zhang, H.; Liu, Y.; Sun, H.; Zhang, P. Galectin-1 induces metastasis and epithelial-mesenchymal transition (EMT) in human ovarian cancer cells via activation of the MAPK JNK/p38 signalling pathway. Am. J. Transl. Res. 2019, 11, 3862–3878. [Google Scholar]
- Chi, Y.; Wang, F.; Zhang, T.; Xu, H.; Zhang, Y.; Shan, Z.; Wu, S.; Fan, Q.; Sun, Y. miR-516a-3p inhibits breast cancer cell growth and EMT by blocking the Pygo2/Wnt signalling pathway. J. Cell. Mol. Med. 2019, 23, 6295–6307. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Li, Y.; Li, B.; Zhang, M.; Liu, Y.; Yao, Y.; Li, D. NMIIA promotes tumor growth and metastasis by activating the Wnt/β-catenin signaling pathway and EMT in pancreatic cancer. Oncogene 2019, 38, 5500–5515. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yang, Y.J.; An, Q. LINC00963 Promotes Ovarian Cancer Proliferation, Migration and EMT via the miR-378g/CHI3L1 Axis. Cancer Manag. Res. 2020, 12, 463–473. [Google Scholar] [CrossRef] [Green Version]
- Schulz, A.; Gorodetska, I.; Behrendt, R.; Fuessel, S.; Erdmann, K.; Foerster, S.; Datta, K.; Mayr, T.; Dubrovska, A.; Muders, M.H. Linking NRP2 With EMT and Chemoradioresistance in Bladder Cancer. Front. Oncol. 2019, 9, 1461. [Google Scholar] [CrossRef] [PubMed]
- Afzal Ashaie, M.; Hoque Chowdhury, E. Cadherins: The superfamily critically involved in breast cancer. Curr. Pharm. Des. 2016, 22, 616–638. [Google Scholar] [CrossRef] [PubMed]
- Carpinelli, M.R.; de Vries, M.E.; Auden, A.; Butt, T.; Deng, Z.; Partridge, D.D.; Miles, L.B.; Georgy, S.R.; Haigh, J.J.; Darido, C.; et al. Inactivation of Zeb1 in GRHL2-deficient mouse embryos rescues mid-gestation viability and secondary palate closure. Dis. Models Mech. 2020. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.J.; Oh, N.; Park, J.H.; Kim, K.S.; Kim, H.K.; Lee, E.; Hwang, S.; Kim, S.J.; Park, K.S. ZEB1 Collaborates with ELK3 to Repress E-Cadherin Expression in Triple-Negative Breast Cancer Cells. Mol. Cancer Res. MCR 2019, 17, 2257–2266. [Google Scholar] [CrossRef] [Green Version]
- Yoshimoto, S.; Tanaka, F.; Morita, H.; Hiraki, A.; Hashimoto, S. Hypoxia-induced HIF-1alpha and ZEB1 are critical for the malignant transformation of ameloblastoma via TGF-beta-dependent EMT. Cancer Med. 2019, 8, 7822–7832. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, W.; Li, Z.; Dong, X.; Zhao, N.; Liu, Y.; Wang, C.; Chen, J. Schisandrin B inhibits TGF-beta1-induced epithelial-mesenchymal transition in human A549 cells through epigenetic silencing of ZEB1. Exp. Lung Res. 2019, 45, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Soen, B.; Vandamme, N.; Berx, G.; Schwaller, J.; Van Vlierberghe, P.; Goossens, S. ZEB Proteins in Leukemia: Friends, Foes, or Friendly Foes? HemaSphere 2018, 2, e43. [Google Scholar] [CrossRef]
- Comijn, J.; Berx, G.; Vermassen, P.; Verschueren, K.; van Grunsven, L.; Bruyneel, E.; Mareel, M.; Huylebroeck, D.; van Roy, F. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol. Cell 2001, 7, 1267–1278. [Google Scholar] [CrossRef] [Green Version]
- Verschueren, K.; Remacle, J.E.; Collart, C.; Kraft, H.; Baker, B.S.; Tylzanowski, P.; Nelles, L.; Wuytens, G.; Su, M.T.; Bodmer, R.; et al. SIP1, a novel zinc finger/homeodomain repressor, interacts with Smad proteins and binds to 5’-CACCT sequences in candidate target genes. J. Biol. Chem. 1999, 274, 20489–20498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remacle, J.E.; Kraft, H.; Lerchner, W.; Wuytens, G.; Collart, C.; Verschueren, K.; Smith, J.C.; Huylebroeck, D. New mode of DNA binding of multi-zinc finger transcription factors: deltaEF1 family members bind with two hands to two target sites. EMBO J. 1999, 18, 5073–5084. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Sawada, J.-i.; Sui, G.; Affar, E.B.; Whetstine, J.R.; Lan, F.; Ogawa, H.; Luke, M.P.-S.; Nakatani, Y.; Shi, Y. Coordinated histone modifications mediated by a CtBP co-repressor complex. Nature 2003, 422, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, Y.; Ma, L. ZEB1: At the crossroads of epithelial-mesenchymal transition, metastasis and therapy resistance. Cell Cycle 2015, 14, 481–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shargh, S.A.; Sakizli, M.; Khalaj, V.; Movafagh, A.; Yazdi, H.; Hagigatjou, E.; Sayad, A.; Mansouri, N.; Mortazavi-Tabatabaei, S.A.; Khorshid, H.R.K. Downregulation of E-cadherin expression in breast cancer by promoter hypermethylation and its relation with progression and prognosis of tumor. Med. Oncol. 2014, 31, 250. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, D.S.; Wyatt, D.; Zlobin, A.; Piracha, A.; Ng, J.; Dingwall, A.K.; Albain, K.S.; Osipo, C. DAXX Suppresses Tumor-Initiating Cells in Estrogen Receptor–Positive Breast Cancer Following Endocrine Therapy. Cancer Res. 2019, 79, 4965–4977. [Google Scholar] [CrossRef] [Green Version]
- Liang, F.; Ren, C.; Wang, J.; Wang, S.; Yang, L.; Han, X.; Chen, Y.; Tong, G.; Yang, G. The crosstalk between STAT3 and p53/RAS signaling controls cancer cell metastasis and cisplatin resistance via the Slug/MAPK/PI3K/AKT-mediated regulation of EMT and autophagy. Oncogenesis 2019, 8, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Zhou, Y.; He, J.; Sun, H.; Jin, Z. Long non-coding RNA H19 mediates ovarian cancer cell cisplatin-resistance and migration during EMT. Int. J. Clin. Exp. Pathol. 2019, 12, 2506. [Google Scholar] [PubMed]
- Zhou, Y.; Zhou, Y.; Wang, K.; Li, T.; Zhang, M.; Yang, Y.; Wang, R.; Hu, R. ROCK2 Confers Acquired Gemcitabine Resistance in Pancreatic Cancer Cells by Upregulating Transcription Factor ZEB1. Cancers 2019, 11, 1881. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Yang, X.; Qin, X.; Zhao, Y. TCF4 promotes colorectal cancer drug resistance and stemness via regulating ZEB1/ZEB2 expression. Protoplasma 2020. [Google Scholar] [CrossRef] [PubMed]
- Orellana-Serradell, O.; Herrera, D.; Castellon, E.A.; Contreras, H.R. The transcription factor ZEB1 promotes chemoresistance in prostate cancer cell lines. Asian J. Androl. 2019, 21, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Han, H.; Wu, L.; Xiong, Y.; Zhang, J.; Dong, B.; Yang, Y.; Chen, J. MTBP promotes migration and invasion by regulation of ZeB2-mediated epithelial–mesenchymal transition in lung cancer cells. OncoTargets Ther. 2018, 11, 6741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, Y.-S.; Jou, Y.-C.; Tsai, H.-T.; Cheong, I.-S.; Tzai, T.-S. Indoleamine-2, 3-dioxygenase-1 expression predicts poorer survival and up-regulates ZEB2 expression in human early stage bladder cancer. In Urologic Oncology: Seminars and Original Investigations; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Yalim-Camci, I.; Balcik-Ercin, P.; Cetin, M.; Odabas, G.; Tokay, N.; Sayan, A.E.; Yagci, T. ETS1 is coexpressed with ZEB2 and mediates ZEB2-induced epithelial-mesenchymal transition in human tumors. Mol. Carcinog. 2019, 58, 1068–1081. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.M.; Zhang, T.; Liu, Y.B.; Deng, S.H.; Han, R.; Liu, T.; Li, J.; Xu, Y. The PAX6-ZEB2 axis promotes metastasis and cisplatin resistance in non-small cell lung cancer through PI3K/AKT signaling. Cell Death Dis. 2019, 10, 349. [Google Scholar] [CrossRef]
- ElKhouly, A.M.; Youness, R.; Gad, M. MicroRNA-486-5p and MicroRNA-486-3p: Multifaceted Pleiotropic Mediators in Oncological and Non-Oncological Conditions. Non-coding RNA Res. 2020, 5, 11–21. [Google Scholar] [CrossRef]
- Delihas, N. Discovery and characterization of the first non-coding RNA that regulates gene expression, micF RNA: A historical perspective. World J. Biol. Chem. 2015, 6, 272. [Google Scholar] [CrossRef]
- Anastasiadou, E.; Jacob, L.S.; Slack, F.J. Non-coding RNA networks in cancer. Nat. Rev. Cancer 2018, 18, 5. [Google Scholar] [CrossRef]
- Chen, X.; Tang, F.R.; Arfuso, F.; Cai, W.Q.; Ma, Z.; Yang, J.; Sethi, G. The Emerging Role of Long Non-Coding RNAs in the Metastasis of Hepatocellular Carcinoma. Biomolecules 2019, 10, 66. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Wang, Y.Y.; Xin, H.W.; Wang, L.; Arfuso, F.; Dharmarajan, A.; Kumar, A.P.; Wang, H.; Tang, F.R.; Warrier, S.; et al. The expanding roles of long non-coding RNAs in the regulation of cancer stem cells. Int. J. Biochem. Cell Biol. 2019, 108, 17–20. [Google Scholar] [CrossRef]
- Cheng, J.T.; Wang, L.; Wang, H.; Tang, F.R.; Cai, W.Q.; Sethi, G.; Xin, H.W.; Ma, Z. Insights into Biological Role of LncRNAs in Epithelial-Mesenchymal Transition. Cells 2019, 8, 1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourhanifeh, M.H.; Mahjoubin-Tehran, M.; Karimzadeh, M.R.; Mirzaei, H.R.; Razavi, Z.S.; Sahebkar, A.; Hosseini, N.; Mirzaei, H.; Hamblin, M.R. Autophagy in cancers including brain tumors: Role of MicroRNAs. Cell Commun. Signal. 2020, 18, 88. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, F.; Shabaninejad, Z.; Vakili, S.; Derakhshan, M.; Movahedpour, A.; Dabiri, H.; Ghasemi, Y.; Mahjoubin-Tehran, M.; Nikoozadeh, A.; Savardashtaki, A.; et al. TGF-β and WNT signaling pathways in cardiac fibrosis: Non-coding RNAs come into focus. Cell Commun. Signal. 2020, 18, 87. [Google Scholar] [CrossRef]
- Jamali, Z.; Taheri-Anganeh, M.; Shabaninejad, Z.; Keshavarzi, A.; Taghizadeh, H.; Razavi, Z.S.; Mottaghi, R.; Abolhassan, M.; Movahedpour, A.; Mirzaei, H. Autophagy regulation by microRNAs: Novel insights into osteosarcoma therapy. IUBMB Life 2020, 72, 1306–1321. [Google Scholar] [CrossRef] [PubMed]
- Naeli, P.; Yousefi, F.; Ghasemi, Y.; Savardashtaki, A.; Mirzaei, H. The Role of MicroRNAs in Lung Cancer: Implications for Diagnosis and Therapy. Curr. Mol. Med. 2020, 20, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Losko, M.; Kotlinowski, J.; Jura, J. Long noncoding RNAs in metabolic syndrome related disorders. Mediat. Inflamm. 2016, 2016, 5365209. [Google Scholar] [CrossRef] [Green Version]
- Goh, J.N.; Loo, S.Y.; Datta, A.; Siveen, K.S.; Yap, W.N.; Cai, W.; Shin, E.M.; Wang, C.; Kim, J.E.; Chan, M.; et al. microRNAs in breast cancer: Regulatory roles governing the hallmarks of cancer. Biol. Rev. Camb. Philos. Soc. 2016, 91, 409–428. [Google Scholar] [CrossRef]
- Varghese, E.; Liskova, A.; Kubatka, P.; Samuel, S.M.; Büsselberg, D. Anti-Angiogenic Effects of Phytochemicals on miRNA Regulating Breast Cancer Progression. Biomolecules 2020, 10, 191. [Google Scholar] [CrossRef] [Green Version]
- Magalhaes, M.; Jorge, J.; Goncalves, A.C.; Sarmento-Ribeiro, A.B.; Carvalho, R.; Figueiras, A.; Santos, A.C.; Veiga, F. miR-29b and retinoic acid co-delivery: A promising tool to induce a synergistic antitumoral effect in non-small cell lung cancer cells. Drug Deliv. Transl. Res. 2020. [Google Scholar] [CrossRef]
- Ragheb, M.A.; Soliman, M.H.; Elzayat, E.M.; Mohamed, M.S.; El-Ekiaby, N.; Abdelaziz, A.I.; Abdelwahab, A.A. MiR-520c-3p Modulates Doxorubicin-chemosensitivity in HepG2 cells. Anti-Cancer Agents Med. Chem. 2020. [Google Scholar] [CrossRef]
- Taherdangkoo, K.; Kazemi Nezhad, S.R.; Hajjari, M.R.; Tahmasebi Birgani, M. miR-485-3p suppresses colorectal cancer via targeting TPX2. Bratisl. Lek. Listy 2020, 121, 302–307. [Google Scholar] [CrossRef]
- Park, J.H.; Jeong, G.H.; Lee, K.S.; Lee, K.H.; Suh, J.S.; Eisenhut, M.; van der Vliet, H.J.; Kronbichler, A.; Stubbs, B.; Solmi, M.; et al. Genetic variations in MicroRNA genes and cancer risk: A field synopsis and meta-analysis. Eur. J. Clin. Investig. 2020. [Google Scholar] [CrossRef] [PubMed]
- Salomão, K.B.; Pezuk, J.A.; de Souza, G.R.; Chagas, P.; Pereira, T.C.; Valera, E.T.; Brassesco, M.S. MicroRNA dysregulation interplay with childhood abdominal tumors. Cancer Metastasis Rev. 2019, 38, 783–811. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.A.; Moeng, S.; Sim, S.; Kuh, H.J.; Choi, S.Y.; Park, J.K. MicroRNA-Based Combinatorial Cancer Therapy: Effects of MicroRNAs on the Efficacy of Anti-Cancer Therapies. Cells 2020, 9, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, X.; Qian, X.; Wu, L.; Li, B.; Wang, Y.; Kong, X.; Xiong, L. microRNA: The Impact on Cancer Stemness and Therapeutic Resistance. Cells 2020, 9, 8. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Bao, J.; Kim, M.; Yuan, S.; Tang, C.; Zheng, H.; Mastick, G.S.; Xu, C.; Yan, W. Two miRNA clusters, miR-34b/c and miR-449, are essential for normal brain development, motile ciliogenesis, and spermatogenesis. Proc. Natl. Acad. Sci. USA 2014, 111, E2851–E2857. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.Z.; Schaffert, S.; Fragoso, R.; Loh, C. Regulation of immune responses and tolerance: The micro RNA perspective. Immunol. Rev. 2013, 253, 112–128. [Google Scholar] [CrossRef] [Green Version]
- Follert, P.; Cremer, H.; Béclin, C. MicroRNAs in brain development and function: A matter of flexibility and stability. Front. Mol. Neurosci. 2014, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, R.-u.; Miyazaki, H.; Ochiya, T. The role of microRNAs in the regulation of cancer stem cells. Front. Genet. 2014, 4, 295. [Google Scholar] [CrossRef] [Green Version]
- Raza, U.; Zhang, J.D.; Şahin, Ö. MicroRNAs: Master regulators of drug resistance, stemness, and metastasis. J. Mol. Med. 2014, 92, 321–336. [Google Scholar] [CrossRef] [Green Version]
- Filipowicz, W.; Bhattacharyya, S.N.; Sonenberg, N. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat. Rev. Genet. 2008, 9, 102–114. [Google Scholar] [CrossRef]
- Eulalio, A.; Huntzinger, E.; Izaurralde, E. Getting to the root of miRNA-mediated gene silencing. Cell 2008, 132, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sartorius, K.; Makarova, J.; Sartorius, B.; An, P.; Winkler, C.; Chuturgoon, A.; Kramvis, A. The Regulatory Role of MicroRNA in Hepatitis-B Virus-Associated Hepatocellular Carcinoma (HBV-HCC) Pathogenesis. Cells 2019, 8, 1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syed, S.N.; Frank, A.C.; Raue, R.; Brune, B. MicroRNA-A Tumor Trojan Horse for Tumor-Associated Macrophages. Cells 2019, 8, 1482. [Google Scholar] [CrossRef] [Green Version]
- Handa, H.; Murakami, Y.; Ishihara, R.; Kimura-Masuda, K.; Masuda, Y. The Role and Function of microRNA in the Pathogenesis of Multiple Myeloma. Cancers 2019, 11, 1738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosentino, G.; Plantamura, I.; Cataldo, A.; Iorio, M.V. MicroRNA and Oxidative Stress Interplay in the Context of Breast Cancer Pathogenesis. Int. J. Mol. Sci. 2019, 20, 5143. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Jia, Y.; Wang, S.; Liu, N.; Gao, D.; Zhang, L.; Lin, Z.; Wang, S.; Kong, F.; Peng, C. Downregulation of MUTYH contributes to cisplatin-resistance of esophageal squamous cell carcinoma cells by promoting Twist-mediated EMT. Oncol. Rep. 2019, 42, 2716–2727. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.M.; Zhang, G.M.; Qian, H.N.; Cheng, S.J.; Wang, M.; Liu, M.; Li, D. MiR-1266 suppresses the growth and metastasis of prostate cancer via targeting PRMT5. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6436–6444. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.Y.; Yu, Y.J.; Ye, F.; Peng, G.Y.; Li, Y.J.; Zhou, X.M. MicroRNA-506-3p inhibits proliferation and promotes apoptosis in ovarian cancer cell via targeting SIRT1/AKT/FOXO3a signaling pathway. Neoplasma 2020, 67, 344–353. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Zhou, X.; Yao, X.; Zhang, Z.; Cui, M.; Lin, Y. MicroRNA-612 inhibits cervical cancer progression by targeting NOB1. J. Cell. Mol. Med. 2020, 24, 3149–3156. [Google Scholar] [CrossRef] [PubMed]
- Nong, K.; Zhang, D.; Chen, C.; Yang, Y.; Yang, Y.; Liu, S.; Cai, H. MicroRNA-519 inhibits hypoxia-induced tumorigenesis of pancreatic cancer by regulating immune checkpoint PD-L1. Oncol. Lett. 2020, 19, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- ZHAO, L.; YU, A.; ZHANG, Y.; WANG, X.; HAN, B.; WANG, X. MicroRNA-149 suppresses the malignant phenotypes of ovarian cancer via downregulation of MSI2 and inhibition of PI3K/AKT pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 55–64. [Google Scholar] [PubMed]
- Allahverdi, A.; Arefian, E.; Soleimani, M.; Ai, J.; Nahanmoghaddam, N.; Yousefi-Ahmadipour, A.; Ebrahimi-Barough, S. MicroRNA-4731-5p delivered by AD-mesenchymal stem cells induces cell cycle arrest and apoptosis in glioblastoma. J. Cell. Physiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Piao, H.; Zhang, Y.; Sun, P.; Yao, B. Overexpression of microRNA-129-5p in glioblastoma inhibits cell proliferation, migration, and colony-forming ability by targeting ZFP36L1. Bosn. J. Basic Med. Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; He, B. MiR-760 inhibits the progression of non-small cell lung cancer through blocking ROS1/Ras/Raf/MEK/ERK pathway. Biosci. Rep. 2020, BSR20182483. [Google Scholar] [CrossRef]
- Huang, C.; Liu, J.; Pan, X.; Peng, C.; Xiong, B.; Feng, M.; Yang, X. miR-454 promotes survival and induces oxaliplatin resistance in gastric carcinoma cells by targeting CYLD. Exp. Ther. Med. 2020, 19, 3604–3610. [Google Scholar] [CrossRef]
- Qin, X.; Wang, X.Y.; Fei, J.W.; Li, F.H.; Han, J.; Wang, H.X. MiR-20a promotes lung tumorigenesis by targeting RUNX3 via TGF-beta signaling pathway. J. Biol. Regul. Homeost. Agents 2020, 34. [Google Scholar] [CrossRef]
- Liu, X.; Fu, Y.; Zhang, G.; Zhang, D.; Liang, N.; Li, F.; Li, C.; Sui, C.; Jiang, J.; Lu, H.; et al. miR-424-5p Promotes Anoikis Resistance and Lung Metastasis by Inactivating Hippo Signaling in Thyroid Cancer. Mol. Ther. Oncolytics 2019, 15, 248–260. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Zou, C.; Tang, Y.; Wa, Q.; Peng, X.; Chen, X.; Yang, C.; Ren, D.; Huang, Y.; Liao, Z.; et al. miR-582-3p and miR-582-5p Suppress Prostate Cancer Metastasis to Bone by Repressing TGF-β Signaling. Mol. Ther. Nucleic Acids 2019, 16, 91–104. [Google Scholar] [CrossRef] [Green Version]
- Vescarelli, E.; Gerini, G.; Megiorni, F.; Anastasiadou, E.; Pontecorvi, P.; Solito, L.; De Vitis, C.; Camero, S.; Marchetti, C.; Mancini, R.; et al. MiR-200c sensitizes Olaparib-resistant ovarian cancer cells by targeting Neuropilin 1. J. Exp. Clin. Cancer Res. CR 2020, 39, 3. [Google Scholar] [CrossRef]
- Meng, Z.; Zhang, R.; Wang, Y.; Zhu, G.; Jin, T.; Li, C.; Zhang, S. miR-200c/PAI-2 promotes the progression of triple negative breast cancer via M1/M2 polarization induction of macrophage. Int. Immunopharmacol. 2019, 106028. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Chaudhary, A.; Chowdhury, P.; Karmakar, D.; Basu, K.; Karmakar, D.; Chatterjee, J.; Sengupta, S. Evaluating the role of hsa-miR-200c in reversing the epithelial to mesenchymal transition in prostate cancer. Gene 2020, 730, 144264. [Google Scholar] [CrossRef]
- Tang, H.; Song, C.; Ye, F.; Gao, G.; Ou, X.; Zhang, L.; Xie, X.; Xie, X. miR-200c suppresses stemness and increases cellular sensitivity to trastuzumab in HER2+ breast cancer. J. Cell. Mol. Med. 2019, 23, 8114–8127. [Google Scholar] [CrossRef]
- Lin, H.; Yang, L.; Tian, F.; Nie, S.; Zhou, H.; Liu, J.; Chen, W. Up-regulated LncRNA-ATB regulates the growth and metastasis of cholangiocarcinoma via miR-200c signals. OncoTargets Ther. 2019, 12, 7561–7571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Breast cancer statistics, 2019. CA A Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef]
- Abolghasemi, M.; Tehrani, S.S.; Yousefi, T.; Karimian, A.; Mahmoodpoor, A.; Ghamari, A.; Jadidi-Niaragh, F.; Yousefi, M.; Kafil, H.S.; Bastami, M.; et al. MicroRNAs in breast cancer: Roles, functions, and mechanism of actions. J. Cell. Physiol. 2020, 235, 5008–5029. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Zhou, E.; Xu, F.; Zhang, D.; Yi, W.; Yao, J. A TP73-AS1/miR-200a/ZEB1 regulating loop promotes breast cancer cell invasion and migration. J. Cell. Biochem. 2018, 119, 2189–2199. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, Z.; Luo, X.H.; Xu, P. Long noncoding RNA TTN-AS1 promotes the proliferation and migration of prostate cancer by inhibiting miR-1271 level. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 10678–10684. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Chen, Y.Y.; Han, A.L. MiR-1271 inhibits cell proliferation and metastasis by targeting LDHA in endometrial cancer. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5648–5656. [Google Scholar] [CrossRef]
- Yao, H.; Sun, Q.; Zhu, J. miR-1271 enhances the sensitivity of colorectal cancer cells to cisplatin. Exp. Ther. Med. 2019, 17, 4363–4370. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhu, G.; Yu, J.; Li, Y.; Wu, M.; Zhao, J.; Tian, X. miR-1271 inhibits growth, invasion and epithelial–mesenchymal transition by targeting ZEB1 in ovarian cancer cells. OncoTargets Ther. 2019, 12, 6973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Jin, S.; Tan, S.; Shen, Q.; Xue, Y. MiR-203 acts as a radiosensitizer of gastric cancer cells by directly targeting ZEB1. OncoTargets Ther. 2019, 12, 6093–6104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Xia, L.; Zhou, Z.; Zuo, Z.; Xu, C.; Song, H.; Cai, J. MiR-186-5p upregulation inhibits proliferation, metastasis and epithelial-to-mesenchymal transition of colorectal cancer cell by targeting ZEB1. Arch. Biochem. Biophys. 2018, 640, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cai, Q.Y.; Liu, J.; Peng, J.; Chen, Y.Q.; Sferra, T.J.; Lin, J.M. Ursolic acid suppresses the invasive potential of colorectal cancer cells by regulating the TGF-beta1/ZEB1/miR-200c signaling pathway. Oncol. Lett. 2019, 18, 3274–3282. [Google Scholar] [CrossRef] [Green Version]
- Fan, M.J.; Zou, Y.H.; He, P.J.; Zhang, S.; Sun, X.M.; Li, C.Z. Long non-coding RNA SPRY4-IT1 promotes epithelial-mesenchymal transition of cervical cancer by regulating the miR-101-3p/ZEB1 axis. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Y.; Wu, Z.; Lang, C.; Zhang, X.; He, S.; Yang, Q.; Guo, W.; Lai, Y.; Du, H.; Peng, X.; et al. Copy number gain of ZEB1 mediates a double-negative feedback loop with miR-33a-5p that regulates EMT and bone metastasis of prostate cancer dependent on TGF-β signaling. Theranostics 2019, 9, 6063–6079. [Google Scholar] [CrossRef]
- Sharma, N.; Nanta, R.; Sharma, J.; Gunewardena, S.; Singh, K.P.; Shankar, S.; Srivastava, R.K. PI3K/AKT/mTOR and sonic hedgehog pathways cooperate together to inhibit human pancreatic cancer stem cell characteristics and tumor growth. Oncotarget 2015, 6, 32039. [Google Scholar] [CrossRef]
- Ong, P.S.; Wang, L.Z.; Dai, X.; Tseng, S.H.; Loo, S.J.; Sethi, G. Judicious Toggling of mTOR Activity to Combat Insulin Resistance and Cancer: Current Evidence and Perspectives. Front. Pharmacol. 2016, 7, 395. [Google Scholar] [CrossRef]
- Singh, S.S.; Yap, W.N.; Arfuso, F.; Kar, S.; Wang, C.; Cai, W.; Dharmarajan, A.M.; Sethi, G.; Kumar, A.P. Targeting the PI3K/Akt signaling pathway in gastric carcinoma: A reality for personalized medicine? World J. Gastroenterol. 2015, 21, 12261–12273. [Google Scholar] [CrossRef]
- Huang, J.; Wang, X.; Wen, G.; Ren, Y. miRNA2055p functions as a tumor suppressor by negatively regulating VEGFA and PI3K/Akt/mTOR signaling in renal carcinoma cells. Oncol. Rep. 2019, 42, 1677–1688. [Google Scholar] [CrossRef]
- Liu, Z.; Luo, S.; Wu, M.; Huang, C.; Shi, H.; Song, X. LncRNA GHET1 promotes cervical cancer progression through regulating AKT/mTOR and Wnt/beta-catenin signaling pathways. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef]
- Yin, C.; Lin, X.; Wang, Y.; Liu, X.; Xiao, Y.; Liu, J.; Snijders, A.M.; Wei, G.; Mao, J.H.; Zhang, P. FAM83D promotes epithelial-mesenchymal transition, invasion and cisplatin resistance through regulating the AKT/mTOR pathway in non-small-cell lung cancer. Cell. Oncol. (Dordrecht) 2020. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Dang, W.; Shilei, L.; Huang, L.; Li, Y.; Li, G.; Yan, S.; Jiang, C.; Song, X.; Hu, Y.; et al. ANTXR1/TEM8 promotes gastric cancer progression through activation of the PI3K/AKT/mTOR signaling pathway. Cancer Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Han, B.; Wang, X.; Li, Z.; Wang, J.; Lv, Y. Long Non-Coding RNA TTN-AS1 Promotes the Proliferation and Invasion of Colorectal Cancer Cells by Activating miR-497-Mediated PI3K/Akt/mTOR Signaling. OncoTargets Ther. 2019, 12, 11531–11539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, S.; Liu, J.; Feng, Y.; Li, Z.; He, L.; Li, L.; Cao, X.; Wang, Z.; Zhang, Y. Knockdown of circDENND4C inhibits glycolysis, migration and invasion by up-regulating miR-200b/c in breast cancer under hypoxia. J. Exp. Clin. Cancer Res. CR 2019, 38, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Kong, K.-K.; Xu, X.-K.; Chen, C.; Li, H.; Wang, F.-Y.; Peng, X.-F.; Zhang, Z.; Li, P.; Li, J.-L. Downregulation of miR-205 is associated with glioblastoma cell migration, invasion, and the epithelial-mesenchymal transition, by targeting ZEB1 via the Akt/mTOR signaling pathway. Int. J. Oncol. 2018, 52, 485–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.; Hang, T.; Zhang, B.; Zhu, L.; Wu, Y.; Lv, X.; Huang, Q.; Yao, H. miRNA-708 functions as a tumor suppressor in colorectal cancer by targeting ZEB1 through Akt/mTOR signaling pathway. Am. J. Transl. Res. 2019, 11, 5338–5356. [Google Scholar]
- Xie, R.; Wang, M.; Zhou, W.; Wang, D.; Yuan, Y.; Shi, H.; Wu, L. Long Non-Coding RNA (LncRNA) UFC1/miR-34a Contributes to Proliferation and Migration in Breast Cancer. Med. Sci. Monit. 2019, 25, 7149–7157. [Google Scholar] [CrossRef]
- Du, H.; Wang, X.; Dong, R.; Hu, D.; Xiong, Y. miR-601 inhibits proliferation, migration and invasion of prostate cancer stem cells by targeting KRT5 to inactivate the Wnt signaling pathway. Int. J. Clin. Exp. Pathol. 2019, 12, 4361–4379. [Google Scholar]
- Kong, B.; Lv, Z.D.; Xia, J.; Jin, L.Y.; Yang, Z.C. DLC-3 suppresses cellular proliferation, migration, and invasion in triple-negative breast cancer by the Wnt/beta-catenin pathway. Int. J. Clin. Exp. Pathol. 2019, 12, 1224–1232. [Google Scholar]
- Guo, Y.; Li, B.; Yan, X.; Shen, X.; Ma, J.; Liu, S.; Zhang, D. Bisphenol A and polychlorinated biphenyls enhance the cancer stem cell properties of human ovarian cancer cells by activating the WNT signaling pathway. Chemosphere 2019, 246, 125775. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.H.; Zhang, Y.L.; Zhang, Z.Y.; Guo, S.S.; Chen, X.B.; Guo, Y.Z. MicroRNA-96-5p represses breast cancer proliferation and invasion through Wnt/beta-catenin signaling via targeting CTNND1. Sci. Rep. 2020, 10, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pohl, S.G.; Brook, N.; Agostino, M.; Arfuso, F.; Kumar, A.P.; Dharmarajan, A. Wnt signaling in triple-negative breast cancer. Oncogenesis 2017, 6, e310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surana, R.; Sikka, S.; Cai, W.; Shin, E.M.; Warrier, S.R.; Tan, H.J.; Arfuso, F.; Fox, S.A.; Dharmarajan, A.M.; Kumar, A.P. Secreted frizzled related proteins: Implications in cancers. Biochim. Biophys. Acta 2014, 1845, 53–65. [Google Scholar] [CrossRef]
- Qu, J.; Li, M.; An, J.; Zhao, B.; Zhong, W.; Gu, Q.; Cao, L.; Yang, H.; Hu, C. MicroRNA-33b inhibits lung adenocarcinoma cell growth, invasion, and epithelial-mesenchymal transition by suppressing Wnt/beta-catenin/ZEB1 signaling. Int. J. Oncol. 2015, 47, 2141–2152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cong, N.; Du, P.; Zhang, A.; Shen, F.; Su, J.; Pu, P.; Wang, T.; Zjang, J.; Kang, C.; Zhang, Q. Downregulated microRNA-200a promotes EMT and tumor growth through the wnt/beta-catenin pathway by targeting the E-cadherin repressors ZEB1/ZEB2 in gastric adenocarcinoma. Oncol. Rep. 2013, 29, 1579–1587. [Google Scholar] [CrossRef] [Green Version]
- Shang, A.; Wang, W.; Gu, C.; Chen, C.; Zeng, B.; Yang, Y.; Ji, P.; Sun, J.; Wu, J.; Lu, W.; et al. Long non-coding RNA HOTTIP enhances IL-6 expression to potentiate immune escape of ovarian cancer cells by upregulating the expression of PD-L1 in neutrophils. J. Exp. Clin. Cancer Res. 2019, 38, 411. [Google Scholar] [CrossRef]
- Abolghasemi, M.; Tehrani, S.S.; Yousefi, T.; Karimian, A.; Mahmoodpoor, A.; Ghamari, A.; Jadidi-Niaragh, F.; Yousefi, M.; Kafil, H.S.; Bastami, M.; et al. Critical roles of long noncoding RNAs in breast cancer. J. Cell. Physiol. 2020, 235, 5059–5071. [Google Scholar] [CrossRef]
- Vafadar, A.; Shabaninejad, Z.; Movahedpour, A.; Mohammadi, S.; Fathullahzadeh, S.; Mirzaei, H.R.; Namdar, A.; Savardashtaki, A.; Mirzaei, H. Long Non-Coding RNAs As Epigenetic Regulators in Cancer. Curr. Pharm. Des. 2019, 25, 3563–3577. [Google Scholar] [CrossRef]
- Yang, C.; Di Wu, L.G.; Liu, X.; Jin, Y.; Wang, D.; Wang, T.; Li, X. Competing endogenous RNA networks in human cancer: Hypothesis, validation, and perspectives. Oncotarget 2016, 7, 13479. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Hong, L.; Yu, D.; Cao, T.; Zhou, Z.; He, S. LncRNA XIST promotes pancreatic cancer migration, invasion and EMT by sponging miR-429 to modulate ZEB1 expression. Int. J. Biochem. Cell Biol. 2019, 113, 17–26. [Google Scholar] [CrossRef]
- Li, D.; Wang, J.; Zhang, M.; Hu, X.; She, J.; Qiu, X.; Zhang, X.; Xu, L.; Liu, Y.; Qin, S. LncRNA MAGI2-AS3 Is Regulated by BRD4 and Promotes Gastric Cancer Progression via Maintaining ZEB1 Overexpression by Sponging miR-141/200a. Mol. Ther. Nucleic Acids 2020, 19, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, T.; Li, T.; Yu, J. LncRNA IUR downregulates ZEB1 by upregulating miR-200 to inhibit prostate carcinoma. Physiol. Genom. 2019, 51, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Jiang, Y.; Zhang, T.; Mo, K.; Su, S.; Wang, A.; Zhu, Y.; Huang, G.; Zhou, R. The lncRNA TDRG1 promotes cell proliferation, migration and invasion by targeting miR-326 to regulate MAPK1 expression in cervical cancer. Cancer Cell Int. 2019, 19, 152. [Google Scholar] [CrossRef] [Green Version]
- Deng, S.-j.; Chen, H.-y.; Ye, Z.; Deng, S.-c.; Zhu, S.; Zeng, Z.; He, C.; Liu, M.-l.; Huang, K.; Zhong, J.-x. Hypoxia-induced LncRNA-BX111 promotes metastasis and progression of pancreatic cancer through regulating ZEB1 transcription. Oncogene 2018, 37, 5811–5828. [Google Scholar] [CrossRef]
- Hu, X.; Mu, Y.; Wang, J.; Zhao, Y. LncRNA TDRG1 promotes the metastasis of NSCLC cell through regulating miR-873-5p/ZEB1 axis. J. Cell. Biochem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Wang, J.; Lv, W.; Xu, J.; Mei, S.; Shan, A. LncRNA TTN-AS1 drives invasion and migration of lung adenocarcinoma cells via modulation of miR-4677-3p/ZEB1 axis. J. Cell. Biochem. 2019, 120, 17131–17141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, W.; Wang, Z.; Wang, X. lncRNA NEAT1 is closely related with progression of breast cancer via promoting proliferation and EMT. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1020–1026. [Google Scholar]
- Tan, X.; Wang, P.; Lou, J.; Zhao, J. Knockdown of lncRNA NEAT1 suppresses hypoxia-induced migration, invasion and glycolysis in anaplastic thyroid carcinoma cells through regulation of miR-206 and miR-599. Cancer Cell Int. 2020, 20, 132. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Zhou, Y.; Sun, A.J.; Xue, J.L. NEAT1 contributes to breast cancer progression through modulating miR-448 and ZEB1. J. Cell. Physiol. 2018, 233, 8558–8566. [Google Scholar] [CrossRef]
- Shao, Q.; Wang, Q.; Wang, J. LncRNA SCAMP1 regulates ZEB1/JUN and autophagy to promote pediatric renal cell carcinoma under oxidative stress via miR-429. Biomed. Pharmacother. 2019, 120, 109460. [Google Scholar] [CrossRef]
- Kirtonia, A.; Sethi, G.; Garg, M. The multifaceted role of reactive oxygen species in tumorigenesis. Cell. Mol. Life Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, M.A.; Sethi, G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Sun, J.; Liu, W.; Yang, Y.; Chu, Z.; Yang, T.; Gui, Y.; Wang, D. Long noncoding RNA HCP5 contributes to epithelial-mesenchymal transition in colorectal cancer through ZEB1 activation and interacting with miR-139-5p. Am. J. Transl. Res. 2019, 11, 953–963. [Google Scholar] [PubMed]
- Pu, J.; Wang, J.; Wei, H.; Lu, T.; Wu, X.; Wu, Y.; Shao, Z.; Luo, C.; Lu, Y. lncRNA MAGI2-AS3 Prevents the Development of HCC via Recruiting KDM1A and Promoting H3K4me2 Demethylation of the RACGAP1 Promoter. Mol. Ther. Nucleic Acids 2019, 18, 351–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Yang, H.; Xu, M.; Zhang, H.; Sun, M.; Mu, P.; Dong, T.; Du, S.; Liu, K. Long non-coding RNA (lncRNA) MAGI2-AS3 inhibits breast cancer cell growth by targeting the Fas/FasL signalling pathway. Human Cell 2018, 31, 232–241. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y.; Li, Z.; Zheng, S.; Wang, Z.; Li, W.; Bi, Z.; Li, L.; Jiang, Y.; Luo, Y. Linc00511 acts as a competing endogenous RNA to regulate VEGFA expression through sponging hsa-miR-29b-3p in pancreatic ductal adenocarcinoma. J. Cell. Mol. Med. 2018, 22, 655–667. [Google Scholar] [CrossRef]
- Du, X.; Tu, Y.; Liu, S.; Zhao, P.; Bao, Z.; Li, C.; Li, J.; Pan, M.; Ji, J. LINC00511 contributes to glioblastoma tumorigenesis and epithelial-mesenchymal transition via LINC00511/miR-524-5p/YB1/ZEB1 positive feedback loop. J. Cell. Mol. Med. 2019, 24, 1474–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, T.; Wang, Z.; Yang, J.; Zhang, Y.; Shang, Y.; Sun, J. MALAT1 knockdown inhibits prostate cancer progression by regulating miR-140/BIRC6 axis. Biomed. Pharmacother. 2020, 123, 109666. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Liu, Q. Long noncoding RNA MALAT1 modulates sepsis-induced cardiac inflammation through the miR-150-5p/NF-kappaB axis. Int. J. Clin. Exp. Pathol. 2019, 12, 3311–3319. [Google Scholar]
- Chen, L.; Yao, H.; Wang, K.; Liu, X. Long non-coding RNA MALAT1 regulates ZEB1 expression by sponging miR-143-3p and promotes hepatocellular carcinoma progression. J. Cell. Biochem. 2017, 118, 4836–4843. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, X.J.; Zhou, W.; Chu, Y.X. MicroRNA-429 inhibits the proliferation and migration of esophageal squamous cell carcinoma cells by targeting RAB23 through the NF-kappaB pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Zheng, H.; Zhou, L.; Li, W.; Xu, X. Overexpression of MALAT1 contributes to cervical cancer progression by acting as a sponge of miR-429. J. Cell Physiol. 2019, 234, 11219–11226. [Google Scholar] [CrossRef] [PubMed]
- Szigeti, T.; Dunster, C.; Cattaneo, A.; Cavallo, D.; Spinazze, A.; Saraga, D.E.; Sakellaris, I.A.; de Kluizenaar, Y.; Cornelissen, E.J.; Hanninen, O.; et al. Oxidative potential and chemical composition of PM2.5 in office buildings across Europe—The OFFICAIR study. Environ. Int. 2016, 92–93, 324–333. [Google Scholar] [CrossRef] [Green Version]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K.; Loridas, S. Pulmonary oxidative stress, inflammation and cancer: Respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int. J. Environ. Res. Public Health 2013, 10, 3886–3907. [Google Scholar] [CrossRef]
- Luo, F.; Wei, H.; Guo, H.; Li, Y.; Feng, Y.; Bian, Q.; Wang, Y. LncRNA MALAT1, an lncRNA acting via the miR-204/ZEB1 pathway, mediates the EMT induced by organic extract of PM2.5 in lung bronchial epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 317, L87–Ll98. [Google Scholar] [CrossRef]
- Wang, K.C.; Yang, Y.W.; Liu, B.; Sanyal, A.; Corces-Zimmerman, R.; Chen, Y.; Lajoie, B.R.; Protacio, A.; Flynn, R.A.; Gupta, R.A. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature 2011, 472, 120–124. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Wang, W.; Liu, G.; Xie, S.; Li, Q.; Li, Y.; Lin, Z. Long non-coding RNA HOTTIP promotes hypoxia-induced epithelial-mesenchymal transition of malignant glioma by regulating the miR-101/ZEB1 axis. Biomed. Pharmacother. 2017, 95, 711–720. [Google Scholar] [CrossRef]
- Dai, B.; Zhou, G.; Hu, Z.; Zhu, G.; Mao, B.; Su, H.; Jia, Q. MiR-205 suppresses epithelial-mesenchymal transition and inhibits tumor growth of human glioma through down-regulation of HOXD9. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [Green Version]
- Dong, N.; Guo, J.; Han, S.; Bao, L.; Diao, Y.; Lin, Z. Positive feedback loop of lncRNA HOXC-AS2/miR-876-5p/ZEB1 to regulate EMT in glioma. OncoTargets Ther. 2019, 12, 7601–7609. [Google Scholar] [CrossRef] [Green Version]
- Kulcheski, F.R.; Christoff, A.P.; Margis, R. Circular RNAs are miRNA sponges and can be used as a new class of biomarker. J. Biotechnol. 2016, 238, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Zhou, H.; Feng, Z.; Xu, Z.; Tang, Y.; Li, P.; Wu, M. CircRNA: Functions and properties of a novel potential biomarker for cancer. Mol. Cancer 2017, 16, 94. [Google Scholar] [CrossRef]
- Conn, S.J.; Pillman, K.A.; Toubia, J.; Conn, V.M.; Salmanidis, M.; Phillips, C.A.; Roslan, S.; Schreiber, A.W.; Gregory, P.A.; Goodall, G.J. The RNA binding protein quaking regulates formation of circRNAs. Cell 2015, 160, 1125–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Zhang, Z.; Qi, D. Circular RNA hsa_circ_0023404 promotes proliferation, migration and invasion in non-small cell lung cancer by regulating miR-217/ZEB1 axis. OncoTargets Ther. 2019, 12, 6181–6189. [Google Scholar] [CrossRef] [PubMed]
- Re, M.; Magliulo, G.; Gioacchini, F.M.; Bajraktari, A.; Bertini, A.; Çeka, A.; Rubini, C.; Ferrante, L.; Procopio, A.D.; Olivieri, F. Expression levels and clinical significance of miR-21-5p, miR-let-7a, and miR-34c-5p in laryngeal squamous cell carcinoma. BioMed. Res. Int. 2017, 2017, 3921258. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.; Yu, D.; Liu, M.; Wang, M.; Zhao, M.; Liu, M.; Jia, W.; Ma, H.; Fang, J.; Xu, W. Genome-wide association study identifies three susceptibility loci for laryngeal squamous cell carcinoma in the Chinese population. Nat. Genet. 2014, 46, 1110. [Google Scholar] [CrossRef]
- Fu, D.; Huang, Y.; Gao, M. Hsa_circ_0057481 promotes laryngeal cancer proliferation and migration by modulating the miR-200c/ZEB1 axis. Int. J. Clin. Exp. Pathol. 2019, 12, 4066. [Google Scholar]
- Ying, X.; Zhu, J.; Zhang, Y. Circular RNA circ-TSPAN4 promotes lung adenocarcinoma metastasis by upregulating ZEB1 via sponging miR-665. Mol. Genet. Genom. Med. 2019, 7, e991. [Google Scholar] [CrossRef] [Green Version]
- Conte, R.; Valentino, A.; Di Cristo, F.; Peluso, G.; Cerruti, P.; Di Salle, A.; Calarco, A. Cationic Polymer Nanoparticles-Mediated Delivery of miR-124 Impairs Tumorigenicity of Prostate Cancer Cells. Int. J. Mol. Sci. 2020, 21, 869. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhong, Y.; Li, L. miR-124 and miR-203 synergistically inactivate EMT pathway via coregulation of ZEB2 in clear cell renal cell carcinoma (ccRCC). J. Transl. Med. 2020, 18, 69. [Google Scholar] [CrossRef]
- Chen, L.; Hu, N.; Wang, C.; Zhao, H. HOTAIRM1 knockdown enhances cytarabine-induced cytotoxicity by suppression of glycolysis through the Wnt/beta-catenin/PFKP pathway in acute myeloid leukemia cells. Arch. Biochem. Biophys. 2020, 680, 108244. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, S.; Wu, L.; Pei, M. Interaction between LncRNA-ROR and miR-145 contributes to epithelial-mesenchymal transition of ovarian cancer cells. Gen. Physiol. Biophys. 2019, 38, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, J.; Wang, B.; Zheng, Y.; Wan, Y.; Wang, Y.; Zhou, L.; Liu, S.; Li, G.; Yan, Y. miR-145 modulates epithelial-mesenchymal transition and invasion by targeting ZEB2 in non-small cell lung cancer cell lines. J. Cell. Biochem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Wang, L.; Zhang, X.; Xing, R.-G.; Zhou, W.-W.; Liu, C.-R.; Zhang, X.-L.; Tian, L. miR-30a inhibits breast cancer progression through the Wnt/β-catenin pathway. Int. J. Clin. Exp. Pathol. 2019, 12, 241. [Google Scholar]
- Wang, T.; Chen, G.; Ma, X.; Yang, Y.; Chen, Y.; Peng, Y.; Bai, Z.; Zhang, Z.; Pei, H.; Guo, W. MiR-30a regulates cancer cell response to chemotherapy through SNAI1/IRS1/AKT pathway. Cell Death Dis. 2019, 10, 153. [Google Scholar] [CrossRef]
- Di Gennaro, A.; Damiano, V.; Brisotto, G.; Armellin, M.; Perin, T.; Zucchetto, A.; Guardascione, M.; Spaink, H.P.; Doglioni, C.; Snaar-Jagalska, B.E. A p53/miR-30a/ZEB2 axis controls triple negative breast cancer aggressiveness. Cell Death Differ. 2018, 25, 2165–2180. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, T.; Deng, Z.; Sun, L. MicroRNA3653 inhibits the growth and metastasis of hepatocellular carcinoma by inhibiting ITGB1. Oncol. Rep. 2019, 41, 1669–1677. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Luo, X.; Fu, H.; Liu, L.; Sun, P.; Wang, Z. miR-3653 inhibits the metastasis and epithelial-mesenchymal transition of colon cancer by targeting Zeb2. Pathol. Res. Pract. 2019, 215, 152577. [Google Scholar] [CrossRef]
- Zhu, D.; Gu, L.; Li, Z.; Jin, W.; Lu, Q.; Ren, T. MiR-138-5p suppresses lung adenocarcinoma cell epithelial-mesenchymal transition, proliferation and metastasis by targeting ZEB2. Pathol. Res. Pract. 2019, 215, 861–872. [Google Scholar] [CrossRef]
- Eleutério, S.J.P.; Senerchia, A.A.; Almeida, M.T.; Costa, C.M.D.; Lustosa, D.; Calheiros, L.M.; Barreto, J.H.S.; Brunetto, A.L.; Macedo, C.R.P.D.; Petrilli, A.S. Osteosarcoma in patients younger than 12 years old without metastases have similar prognosis as adolescent and young adults. Pediatric Blood Cancer 2015, 62, 1209–1213. [Google Scholar] [CrossRef]
- Wang, S.-N.; Luo, S.; Liu, C.; Piao, Z.; Gou, W.; Wang, Y.; Guan, W.; Li, Q.; Zou, H.; Yang, Z.-Z. miR-491 inhibits osteosarcoma lung metastasis and chemoresistance by targeting αB-crystallin. Mol. Ther. 2017, 25, 2140–2149. [Google Scholar] [CrossRef]
- Lin, H.; Zheng, X.; Lu, T.; Gu, Y.; Zheng, C.; Yan, H. The proliferation and invasion of osteosarcoma are inhibited by miR-101 via targetting ZEB2. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Feng, S.; Li, H.; Chen, X.; Bai, S.; Liu, Y. Long non-coding RNA MALAT1 facilitates the tumorigenesis, invasion and glycolysis of multiple myeloma via miR-1271-5p/SOX13 axis. J. Cancer Res. Clin. Oncol. 2020, 146, 367–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, H.; Zhang, M.; Hou, H.; Zhang, Z.; Li, N. HOTAIRM1 suppresses cell proliferation and invasion in ovarian cancer through facilitating ARHGAP24 expression by sponging miR-106a-5p. Life Sci. 2020, 117296. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Guo, D.; Xu, H.; Su, G.; Chen, J.; Zhao, Z.; Shi, J.; Wedemeyer, M.; Attenello, F.; Zhang, L.; et al. HOTAIRM1, an enhancer lncRNA, promotes glioma proliferation by regulating long-range chromatin interactions within HOXA cluster genes. Mol. Biol. Rep. 2020, 47, 2723–2733. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Guo, L.; Yan, F.; Dou, Z.Q.; Yu, Q.; Chen, G. Long non-coding RNA HOTAIRM1 promotes proliferation and inhibits apoptosis of glioma cells by regulating the miR-873-5p/ZEB2 axis. Chin. Med. J. (Engl.) 2020, 133, 174–182. [Google Scholar] [CrossRef]
- Ren, L.; Yao, Y.; Wang, Y.; Wang, S. MiR-505 suppressed the growth of hepatocellular carcinoma cells via targeting IGF-1R. Biosci. Rep. 2019, 39, BSR20182442. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.; Wang, Z.Y.; Hao, J.; Zhang, X.Y. miR-505 acts as a tumor suppressor in gastric cancer progression through targeting HMGB1. J. Cell. Biochem. 2019, 120, 8044–8052. [Google Scholar] [CrossRef]
- Shi, H.; Yang, H.; Xu, S.; Zhao, Y.; Liu, J. miR-505 functions as a tumor suppressor in glioma by targeting insulin like growth factor 1 receptor expression. Int. J. Clin. Exp. Pathol. 2018, 11, 4405. [Google Scholar]
- Gong, X.; Huang, A. LEF-AS1 participates in occurrence of colorectal cancer through adsorbing miR-505 and promoting KIF3B expression. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9362–9370. [Google Scholar]
- Wang, Z.; Wang, Q.; Xu, G.; Meng, N.; Huang, X.; Jiang, Z.; Chen, C.; Zhang, Y.; Chen, J.; Li, A.; et al. The long noncoding RNA CRAL reverses cisplatin resistance via the miR-505/CYLD/AKT axis in human gastric cancer cells. RNA Biol. 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Guan, H.; Dai, Z.; Ma, Y.; Zhao, Y.; Liu, D. Long noncoding RNA DLX6-AS1 promotes breast cancer progression via miR-505-3p/RUNX2 axis. Eur. J. Pharmacol. 2019, 865, 172778. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Liu, W.; Bai, X.; Pan, W.; Jia, Z.; Zhang, S.; Zhu, Y.; Tan, W. LncRNA-CTS promotes metastasis and epithelial-to-mesenchymal transition through regulating miR-505/ZEB2 axis in cervical cancer. Cancer Lett. 2019, 465, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Qiao, F.; Wu, H.; Cui, H.; Li, Y.; Zheng, Y.; Zhou, M.; Fan, H. LncRNA UCA1 promotes tumor metastasis by inducing miR-203/ZEB2 axis in gastric cancer. Cell Death Dis. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, S.; Yu, Y.; Shi, Y.; Zheng, H. Knockdown of SNHG12 suppresses tumor metastasis and epithelial-mesenchymal transition via the Slug/ZEB2 signaling pathway by targeting miR-218 in NSCLC. Oncol. Lett. 2019, 17, 2356–2364. [Google Scholar] [CrossRef] [Green Version]
- Weller, M.; Wick, W.; Aldape, K.; Brada, M.; Berger, M.; Pfister, S.M.; Nishikawa, R.; Rosenthal, M.; Wen, P.Y.; Stupp, R. Glioma. Nat. Rev. Dis. Primers 2015, 1, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Bauchet, L.; Davis, F.G.; Deltour, I.; Fisher, J.L.; Langer, C.E.; Pekmezci, M.; Schwartzbaum, J.A.; Turner, M.C.; Walsh, K.M. Response to “the epidemiology of glioma in adults: A ‘state of the science’review”. Neuro-Oncology 2015, 17, 624–626. [Google Scholar] [CrossRef] [Green Version]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma multiforme: A review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac. J. Cancer Prev. APJCP 2017, 18, 3. [Google Scholar]
- Rodriguez, A.; Tatter, S.B. Laser ablation of recurrent malignant gliomas: Current status and future perspective. Neurosurgery 2016, 79, S35–S39. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Wang, Q.; Wang, Y.A.; Hua, S.; Sauvé, C.-E.G.; Ong, D.; Lan, Z.D.; Chang, Q.; Ho, Y.W.; Monasterio, M.M. Epigenetic activation of WNT5A drives glioblastoma stem cell differentiation and invasive growth. Cell 2016, 167, 1281–1295.e18. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Deng, Y.; Lv, Z.; Liu, C.; Guo, Z.; Li, Y.; Liu, H.; Xie, B.; Jin, Z.; Lin, F. LncRNA SNHG5 Promotes Proliferation of Glioma by Regulating miR-205-5p/ZEB2 Axis. OncoTargets Ther. 2019, 12, 11487. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Liu, H.Y.; Liu, W.J.; Shi, Y.L.; Bao, D. miR-377-5p inhibits lung cancer cell proliferation, invasion, and cell cycle progression by targeting AKT1 signaling. J. Cell. Biochem. 2019, 120, 8120–8128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Liu, Q.H.; Wang, T.J.; Zhao, J.; Cheng, X.H.; Wang, J.S. CircZFR serves as a prognostic marker to promote bladder cancer progression by regulating miR-377/ZEB2 signaling. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Tang, J.; Zhu, X.; Jiang, H. Silencing of hsa_circ_0004771 inhibits proliferation and induces apoptosis in breast cancer through activation of miR-653 by targeting ZEB2 signaling pathway. Biosci. Rep. 2019, 39, BSR20181919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin. 2012, 62, 10–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ljungberg, B.; Campbell, S.C.; Cho, H.Y.; Jacqmin, D.; Lee, J.E.; Weikert, S.; Kiemeney, L.A. The epidemiology of renal cell carcinoma. Eur. Urol. 2011, 60, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Znaor, A.; Lortet-Tieulent, J.; Laversanne, M.; Jemal, A.; Bray, F. International variations and trends in renal cell carcinoma incidence and mortality. Eur. Urol. 2015, 67, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zheng, P.; Li, Z.; Li, H.; Wang, X.; Shi, Z.; Han, Q. CircPCNXL2 sponges miR-153 to promote the proliferation and invasion of renal cancer cells through upregulating ZEB2. Cell Cycle 2018, 17, 2644–2654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riganti, C.; Contino, M. New Strategies to Overcome Resistance to Chemotherapy and Immune System in Cancer. Int. J. Mol. Sci. 2019, 20, 4783. [Google Scholar] [CrossRef] [Green Version]
- Nedeljković, M.; Damjanović, A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer-How We Can Rise to the Challenge. Cells 2019, 8, 957. [Google Scholar] [CrossRef] [Green Version]
- Sajid, A.; Raju, N.; Lusvarghi, S.; Vahedi, S.; Swenson, R.E.; Ambudkar, S.V. Synthesis and Characterization of Bodipy-FL-Cyclosporine A as a Substrate for Multidrug Resistance-Linked P-Glycoprotein (ABCB1). Drug Metab. Dispos. Biol. Fate Chem. 2019, 47, 1013–1023. [Google Scholar] [CrossRef]
- van Hoppe, S.; Jamalpoor, A.; Rood, J.J.M.; Wagenaar, E.; Sparidans, R.W.; Beijnen, J.H.; Schinkel, A.H. Brain accumulation of osimertinib and its active metabolite AZ5104 is restricted by ABCB1 (P-glycoprotein) and ABCG2 (breast cancer resistance protein). Pharmacol. Res. 2019, 146, 104297. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Wang, Y.; Xi, L.; Su, F.; Li, S. Biocompatibility and paclitaxel/cisplatin dual-loading of nanotubes prepared from poly(ethylene glycol)-polylactide-poly(ethylene glycol) triblock copolymers for combination cancer therapy. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2019, 27, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Du, J.; Li, X.; Zhi, Z.; Jiang, S. microRNA-137 downregulates MCL1 in ovarian cancer cells and mediates cisplatin-induced apoptosis. Pharmacogenomics 2020, 21, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Abu Samaan, T.M.; Samec, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Paclitaxel’s Mechanistic and Clinical Effects on Breast Cancer. Biomolecules 2019, 9, 789. [Google Scholar] [CrossRef] [Green Version]
- Khodaverdi, S.; Jafari, A.; Movahedzadeh, F.; Madani, F.; Yousefi Avarvand, A.; Falahatkar, S. Evaluating Inhibitory Effects of Paclitaxel and Vitamin D3 Loaded Poly Lactic Glycolic Acid Co-Delivery Nanoparticles on the Breast Cancer Cell Line. Adv. Pharm. Bull. 2020, 10, 30–38. [Google Scholar] [CrossRef]
- Miyagawa, Y.; Yanai, A.; Yanagawa, T.; Inatome, J.; Egawa, C.; Nishimukai, A.; Takamoto, K.; Morimoto, T.; Kikawa, Y.; Suwa, H.; et al. Baseline neutrophil-to-lymphocyte ratio and c-reactive protein predict efficacy of treatment with bevacizumab plus paclitaxel for locally advanced or metastatic breast cancer. Oncotarget 2020, 11, 86–98. [Google Scholar] [CrossRef]
- Gao, L.; Zhao, P.; Li, Y.; Yang, D.; Hu, P.; Li, L.; Cheng, Y.; Yao, H. Reversal of PGlycoprotein-Mediated Multidrug Resistance by Novel Curcumin Analogues in Paclitaxel-resistant Human Breast Cancer Cells. Biochem. Cell Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bashmail, H.A.; Alamoudi, A.A.; Noorwali, A.; Hegazy, G.A.; Ajabnoor, G.M.; Al-Abd, A.M. Thymoquinone Enhances Paclitaxel Anti-Breast Cancer Activity via Inhibiting Tumor-Associated Stem Cells Despite Apparent Mathematical Antagonism. Molecules 2020, 25, 426. [Google Scholar] [CrossRef] [Green Version]
- Sakata, J.; Utsumi, F.; Suzuki, S.; Niimi, K.; Yamamoto, E.; Shibata, K.; Senga, T.; Kikkawa, F.; Kajiyama, H. Inhibition of ZEB1 leads to inversion of metastatic characteristics and restoration of paclitaxel sensitivity of chronic chemoresistant ovarian carcinoma cells. Oncotarget 2017, 8, 99482–99494. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Zhang, L.Y.; Du, W.Z. Circular RNA circ-PVT1 contributes to paclitaxel resistance of gastric cancer cells through the regulation of ZEB1 expression by sponging miR-124-3p. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Jiang, G.; Wen, L.; Deng, W.; Jian, Z.; Zheng, H. Regulatory role of miR-211-5p in hepatocellular carcinoma metastasis by targeting ZEB2. Biomed. Pharmacother. 2017, 90, 806–812. [Google Scholar] [CrossRef]
- Papa, A.-L.; Sidiqui, A.; Balasubramanian, S.U.A.; Sarangi, S.; Luchette, M.; Sengupta, S.; Harfouche, R. PEGylated liposomal Gemcitabine: Insights into a potential breast cancer therapeutic. Cell. Oncol. 2013, 36, 449–457. [Google Scholar] [CrossRef]
- Samanta, K.; Setua, S.; Kumari, S.; Jaggi, M.; Yallapu, M.M.; Chauhan, S.C. Gemcitabine Combination Nano Therapies for Pancreatic Cancer. Pharmaceutics 2019, 11, 574. [Google Scholar] [CrossRef] [Green Version]
- Goel, S.; Sinha, R.J.; Bhaskar, V.; Aeron, R.; Sharma, A.; Singh, V. Role of gemcitabine and cisplatin as neoadjuvant chemotherapy in muscle invasive bladder cancer: Experience over the last decade. Asian J. Urol. 2019, 6, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, K.; Chi, Z.; Zhou, X.; Ren, G.; Zhou, R.; Li, Y.; Tang, X.; Wang, X.J. Interplay of MKP-1 and Nrf2 drives tumor growth and drug resistance in non-small cell lung cancer. Aging 2019, 11, 11329–11346. [Google Scholar] [CrossRef]
- Yuan, Q.; Chu, H.; Ge, Y.; Ma, G.; Du, M.; Wang, M.; Zhang, Z.; Zhang, W. LncRNA PCAT1 and its genetic variant rs1902432 are associated with prostate cancer risk. J. Cancer 2018, 9, 1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, X.; Guo, H.; Yin, S.; Du, H. miR-139-5p functions as a tumor suppressor in cervical cancer by targeting TCF4 and inhibiting Wnt/beta-catenin signaling. OncoTargets Ther. 2019, 12, 7739–7748. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.S.; Wang, Y.; Fan, Q.X. Raddeanin A promotes apoptosis and ameliorates 5-fluorouracil resistance in cholangiocarcinoma cells. World J. Gastroenterol. 2019, 25, 3380–3391. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Kuranaga, Y.; Tahara, T.; Yamashita, H.; Shibata, T.; Nagasaka, M.; Funasaka, K.; Ohmiya, N.; Akao, Y. Induced miR-31 by 5-fluorouracil exposure contributes to the resistance in colorectal tumors. Cancer Sci. 2019, 110, 2540–2548. [Google Scholar] [CrossRef]
- Zhao, P.; Ma, Y.G.; Zhao, Y.; Liu, D.; Dai, Z.J.; Yan, C.Y.; Guan, H.T. MicroRNA-552 deficiency mediates 5-fluorouracil resistance by targeting SMAD2 signaling in DNA-mismatch-repair-deficient colorectal cancer. Cancer Chemother. Pharmacol. 2019, 84, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Shan, G.; Tang, T.; Xia, Y.; Qian, H.-J. Long non-coding RNA NEAT1 promotes bladder progression through regulating miR-410 mediated HMGB1. Biomed. Pharmacother. 2020, 121, 109248. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ge, L.; Xu, X.J.; Yang, T.; Yuan, Y.; Ma, X.L.; Zhang, X.H. LncRNA NEAT1 promotes endometrial cancer cell proliferation, migration and invasion by regulating the miR-144-3p/EZH2 axis. Radiol. Oncol. 2019, 53, 434–442. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Ai, F.Y.; Zhang, D.C.; Tian, L.; Yang, Z.Y.; Liu, S.J. LncRNA NEAT1 knockdown attenuates autophagy to elevate 5-FU sensitivity in colorectal cancer via targeting miR-34a. Cancer Med. 2020, 9, 1079–1091. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Kong, D.; Sun, D.; Li, J. Long non-coding RNA CCAT2 acts as an oncogene in osteosarcoma through regulation of miR-200b/VEGF. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2994–3003. [Google Scholar] [CrossRef] [Green Version]
- Soung, Y.H.; Chung, H.; Yan, C.; Ju, J.; Chung, J. Arrestin Domain Containing 3 Reverses Epithelial to Mesenchymal Transition and Chemo-Resistance of TNBC Cells by Up-Regulating Expression of miR-200b. Cells 2019, 8, 692. [Google Scholar] [CrossRef] [Green Version]
- Fang, S.; Zeng, X.; Zhu, W.; Tang, R.; Chao, Y.; Guo, L. Zinc finger E-box-binding homeobox 2 (ZEB2) regulated by miR-200b contributes to multi-drug resistance of small cell lung cancer. Exp. Mol. Pathol. 2014, 96, 438–444. [Google Scholar] [CrossRef]
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.S. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2015; pp. S185–S198. [Google Scholar]
- Spranger, S. Mechanisms of tumor escape in the context of the T-cell-inflamed and the non-T-cell-inflamed tumor microenvironment. Int. Immunol. 2016, 28, 383–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, R.J.; Van Waes, C.; Allen, C.T. Overcoming barriers to effective immunotherapy: MDSCs, TAMs, and Tregs as mediators of the immunosuppressive microenvironment in head and neck cancer. Oral Oncol. 2016, 58, 59–70. [Google Scholar] [CrossRef] [Green Version]
- Restifo, N.P.; Dudley, M.E.; Rosenberg, S.A. Adoptive immunotherapy for cancer: Harnessing the T cell response. Nat. Rev. Immunol. 2012, 12, 269–281. [Google Scholar] [CrossRef]
- Wherry, E.J. T cell exhaustion. Nat. Immunol. 2011, 12, 492–499. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.-J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Flies, D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.-J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.W.; Weber, J.S. Safety and tumor responses with lambrolizumab (anti–PD-1) in melanoma. N. Engl. J. Med. 2013, 369, 134–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Youngblood, B.; Wherry, E.J.; Ahmed, R. Acquired transcriptional programming in functional and exhausted virus-specific CD8 T cells. Curr. Opin. HIV AIDS 2012, 7, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Freeman, G.J.; Sharpe, A.H.; Kuchroo, V.K. Protect the killer: CTLs need defenses against the tumor. Nat. Med. 2002, 8, 787–789. [Google Scholar] [CrossRef]
- Hobo, W.; Maas, F.; Adisty, N.; de Witte, T.; Schaap, N.; van der Voort, R.; Dolstra, H. siRNA silencing of PD-L1 and PD-L2 on dendritic cells augments expansion and function of minor histocompatibility antigen–specific CD8+ T cells. Blood J. Am. Soc. Hematol. 2010, 116, 4501–4511. [Google Scholar] [CrossRef] [Green Version]
- Zha, H.; Han, X.; Zhu, Y.; Yang, F.; Li, Y.; Li, Q.; Guo, B.; Zhu, B. Blocking C5aR signaling promotes the anti-tumor efficacy of PD-1/PD-L1 blockade. Oncoimmunology 2017, 6, e1349587. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, Y.; Zhang, J.; Liu, Y.; Qi, Q. LncRNA SNHG14/miR-5590-3p/ZEB1 positive feedback loop promoted diffuse large B cell lymphoma progression and immune evasion through regulating PD-1/PD-L1 checkpoint. Cell Death Dis. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Chen, L.; Gibbons, D.L.; Goswami, S.; Cortez, M.A.; Ahn, Y.H.; Byers, L.A.; Zhang, X.; Yi, X.; Dwyer, D.; Lin, W.; et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat. Commun. 2014, 5, 5241. [Google Scholar] [CrossRef]
- Noman, M.Z.; Janji, B.; Abdou, A.; Hasmim, M.; Terry, S.; Tan, T.Z.; Mami-Chouaib, F.; Thiery, J.P.; Chouaib, S. The immune checkpoint ligand PD-L1 is upregulated in EMT-activated human breast cancer cells by a mechanism involving ZEB-1 and miR-200. Oncoimmunology 2017, 6, e1263412. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Y.; Guan, W.; Zhao, H. Role of miR-23a/Zeb1 negative feedback loop in regulating epithelial-mesenchymal transition and tumorigenicity of intraocular tumors. Oncol. Lett. 2018, 16, 2462–2470. [Google Scholar] [CrossRef]
- Majid, S.; Dar, A.A.; Saini, S.; Deng, G.; Chang, I.; Greene, K.; Tanaka, Y.; Dahiya, R.; Yamamura, S. MicroRNA-23b functions as a tumor suppressor by regulating Zeb1 in bladder cancer. PLoS ONE 2013, 8, e67686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Huang, C.; Fu, C.; Tian, Y.; Hu, Y.; Wang, B.; Strasner, A.; Song, Y.; Song, E. Cordycepin (3’-deoxyadenosine) suppressed HMGA2, Twist1 and ZEB1-dependent melanoma invasion and metastasis by targeting miR-33b. Oncotarget 2015, 6, 9834–9853. [Google Scholar] [CrossRef] [Green Version]
- Jiang, R.; Zhang, C.; Liu, G.; Gu, R.; Wu, H. MicroRNA-126 Inhibits Proliferation, Migration, Invasion, and EMT in Osteosarcoma by Targeting ZEB1. J. Cell. Biochem. 2017, 118, 3765–3774. [Google Scholar] [CrossRef]
- Sun, X.; Li, Y.; Yu, J.; Pei, H.; Luo, P.; Zhang, J. miR-128 modulates chemosensitivity and invasion of prostate cancer cells through targeting ZEB1. Jpn. J. Clin. Oncol. 2015, 45, 474–482. [Google Scholar] [CrossRef] [Green Version]
- Dong, P.; Karaayvaz, M.; Jia, N.; Kaneuchi, M.; Hamada, J.; Watari, H.; Sudo, S.; Ju, J.; Sakuragi, N. Mutant p53 gain-of-function induces epithelial-mesenchymal transition through modulation of the miR-130b-ZEB1 axis. Oncogene 2013, 32, 3286–3295. [Google Scholar] [CrossRef] [Green Version]
- Qiu, G.; Lin, Y.; Zhang, H.; Wu, D. miR-139-5p inhibits epithelial-mesenchymal transition, migration and invasion of hepatocellular carcinoma cells by targeting ZEB1 and ZEB2. Biochem. Biophys. Res. Commun. 2015, 463, 315–321. [Google Scholar] [CrossRef]
- Yue, S.; Wang, L.; Zhang, H.; Min, Y.; Lou, Y.; Sun, H.; Jiang, Y.; Zhang, W.; Liang, A.; Guo, Y.; et al. miR-139-5p suppresses cancer cell migration and invasion through targeting ZEB1 and ZEB2 in GBM. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2015, 36, 6741–6749. [Google Scholar] [CrossRef]
- Al-Khalaf, H.H.; Aboussekhra, A. MicroRNA-141 and microRNA-146b-5p inhibit the prometastatic mesenchymal characteristics through the RNA-binding protein AUF1 targeting the transcription factor ZEB1 and the protein kinase AKT. J. Biol. Chem. 2014, 289, 31433–31447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Y.; Zhang, J.; Fu, H.; Shen, L. miR-144 functions as a tumor suppressor in breast cancer through inhibiting ZEB1/2-mediated epithelial mesenchymal transition process. OncoTargets Ther. 2016, 9, 6247–6255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, H.; Liang, W.; Xie, Z.; Li, H.; Liu, J.; Liu, L.; Xiu, L.; Li, Y. Down-regulation of miR-144 promotes thyroid cancer cell invasion by targeting ZEB1 and ZEB2. Endocrine 2015, 48, 566–574. [Google Scholar] [CrossRef]
- Yokobori, T.; Suzuki, S.; Tanaka, N.; Inose, T.; Sohda, M.; Sano, A.; Sakai, M.; Nakajima, M.; Miyazaki, T.; Kato, H.; et al. MiR-150 is associated with poor prognosis in esophageal squamous cell carcinoma via targeting the EMT inducer ZEB1. Cancer Sci. 2013, 104, 48–54. [Google Scholar] [CrossRef]
- Jin, M.; Yang, Z.; Ye, W.; Xu, H.; Hua, X. MicroRNA-150 predicts a favorable prognosis in patients with epithelial ovarian cancer, and inhibits cell invasion and metastasis by suppressing transcriptional repressor ZEB1. PLoS ONE 2014, 9, e103965. [Google Scholar] [CrossRef] [Green Version]
- Liang, L.; Zhang, Z.; Qin, X.; Gao, Y.; Zhao, P.; Liu, J.; Zeng, W. Gambogic Acid Inhibits Melanoma through Regulation of miR-199a-3p/ZEB1 Signalling. Basic Clin. Pharmacol. Toxicol. 2018, 123, 692–703. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhou, F.; Yin, L.; Zhao, L.; Zhang, Y.; Wang, J. MicroRNA-199b targets the regulation of ZEB1 expression to inhibit cell proliferation, migration and invasion in nonsmall cell lung cancer. Mol. Med. Rep. 2017, 16, 5007–5014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ungewiss, C.; Rizvi, Z.H.; Roybal, J.D.; Peng, D.H.; Gold, K.A.; Shin, D.H.; Creighton, C.J.; Gibbons, D.L. The microRNA-200/Zeb1 axis regulates ECM-dependent beta1-integrin/FAK signaling, cancer cell invasion and metastasis through CRKL. Sci. Rep. 2016, 6, 18652. [Google Scholar] [CrossRef]
- Asakura, T.; Yamaguchi, N.; Ohkawa, K.; Yoshida, K. Proteasome inhibitor-resistant cells cause EMT-induction via suppression of E-cadherin by miR-200 and ZEB1. Int. J. Oncol. 2015, 46, 2251–2260. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.T.; Kuo, S.H.; Cheng, A.L.; Lin, C.W. Inhibition of ZEB1 by miR-200 characterizes Helicobacter pylori-positive gastric diffuse large B-cell lymphoma with a less aggressive behavior. Mod. Pathol. Off. J. USA Can. Acad. Pathol. Inc. 2014, 27, 1116–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Title, A.C.; Hong, S.J.; Pires, N.D.; Hasenohrl, L.; Godbersen, S.; Stokar-Regenscheit, N.; Bartel, D.P.; Stoffel, M. Genetic dissection of the miR-200-Zeb1 axis reveals its importance in tumor differentiation and invasion. Nat. Commun. 2018, 9, 4671. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zeng, C.; Tu, M.; Jiang, W.; Dai, Z.; Hu, Y.; Deng, Z.; Xiao, W. MicroRNA-200b acts as a tumor suppressor in osteosarcoma via targeting ZEB1. OncoTargets Ther. 2016, 9, 3101–3111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, S.C.; Lin, C.C.; Shih, T.C.; Tseng, R.J.; Yu, M.C.; Lin, Y.J.; Hsieh, S.Y. The miR-200b-ZEB1 circuit regulates diverse stemness of human hepatocellular carcinoma. Mol. Carcinog. 2017, 56, 2035–2047. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, N.; Seike, M.; Soeno, C.; Chiba, M.; Miyanaga, A.; Noro, R.; Sugano, T.; Matsumoto, M.; Kubota, K.; Gemma, A. miR-200/ZEB axis regulates sensitivity to nintedanib in non-small cell lung cancer cells. Int. J. Oncol. 2016, 48, 937–944. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.L.; Liang, L.S.; Wang, X.K. miR-200c inhibits invasion and migration in human colon cancer cells SW480/620 by targeting ZEB1. Clin. Exp. Metastasis 2012, 29, 457–469. [Google Scholar] [CrossRef]
- Kurata, A.; Yamada, M.; Ohno, S.I.; Inoue, S.; Hashimoto, H.; Fujita, K.; Takanashi, M.; Kuroda, M. Expression level of microRNA-200c is associated with cell morphology in vitro and histological differentiation through regulation of ZEB1/2 and E-cadherin in gastric carcinoma. Oncol. Rep. 2018, 39, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Shan, Y.; Zhang, L.; Bao, Y.; Li, B.; He, C.; Gao, M.; Feng, X.; Xu, W.; Zhang, X.; Wang, S. Epithelial-mesenchymal transition, a novel target of sulforaphane via COX-2/MMP2, 9/Snail, ZEB1 and miR-200c/ZEB1 pathways in human bladder cancer cells. J. Nutr. Biochem. 2013, 24, 1062–1069. [Google Scholar] [CrossRef]
- Karagur, E.R.; Ozay, C.; Mammadov, R.; Akca, H. Anti-invasive effect of Cyclamen pseudibericum extract on A549 non-small cell lung carcinoma cells via inhibition of ZEB1 mediated by miR-200c. J. Nat. Med. 2018, 72, 686–693. [Google Scholar] [CrossRef]
- Zhou, G.; Zhang, F.; Guo, Y.; Huang, J.; Xie, Y.; Yue, S.; Chen, M.; Jiang, H.; Li, M. miR-200c enhances sensitivity of drug-resistant non-small cell lung cancer to gefitinib by suppression of PI3K/Akt signaling pathway and inhibites cell migration via targeting ZEB1. Biomed. Pharmacother. 2017, 85, 113–119. [Google Scholar] [CrossRef]
- Gao, H.X.; Yan, L.; Li, C.; Zhao, L.M.; Liu, W. miR-200c regulates crizotinib-resistant ALK-positive lung cancer cells by reversing epithelial-mesenchymal transition via targeting ZEB1. Mol. Med. Rep. 2016, 14, 4135–4143. [Google Scholar] [CrossRef] [Green Version]
- Guo, E.; Wang, Z.; Wang, S. MiR-200c and miR-141 inhibit ZEB1 synergistically and suppress glioma cell growth and migration. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3385–3391. [Google Scholar] [PubMed]
- Zhou, X.; Wang, Y.; Shan, B.; Han, J.; Zhu, H.; Lv, Y.; Fan, X.; Sang, M.; Liu, X.D.; Liu, W. The downregulation of miR-200c/141 promotes ZEB1/2 expression and gastric cancer progression. Med. Oncol. (Northwood Lond. Engl.) 2015, 32, 428. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Prasad, R.; Katiyar, S.K. Therapeutic intervention of silymarin on the migration of non-small cell lung cancer cells is associated with the axis of multiple molecular targets including class 1 HDACs, ZEB1 expression, and restoration of miR-203 and E-cadherin expression. Am. J. Cancer Res. 2016, 6, 1287–1301. [Google Scholar]
- Wu, G.; Wang, J.; Chen, G.; Zhao, X. microRNA-204 modulates chemosensitivity and apoptosis of prostate cancer cells by targeting zinc-finger E-box-binding homeobox 1 (ZEB1). Am. J. Transl. Res. 2017, 9, 3599–3610. [Google Scholar]
- Niu, K.; Shen, W.; Zhang, Y.; Zhao, Y.; Lu, Y. MiR-205 promotes motility of ovarian cancer cells via targeting ZEB1. Gene 2015, 574, 330–336. [Google Scholar] [CrossRef]
- El Bezawy, R.; Tinelli, S.; Tortoreto, M.; Doldi, V.; Zuco, V.; Folini, M.; Stucchi, C.; Rancati, T.; Valdagni, R.; Gandellini, P.; et al. miR-205 enhances radiation sensitivity of prostate cancer cells by impairing DNA damage repair through PKCepsilon and ZEB1 inhibition. J. Exp. Clin. Cancer Res. CR 2019, 38, 51. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, L.; Rodriguez-Aguayo, C.; Yuan, Y.; Debeb, B.G.; Chen, D.; Sun, Y.; You, M.J.; Liu, Y.; Dean, D.C.; et al. miR-205 acts as a tumour radiosensitizer by targeting ZEB1 and Ubc13. Nat. Commun. 2014, 5, 5671. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Li, S. miR-205-5p inhibits cell migration and invasion in prostatic carcinoma by targeting ZEB1. Oncol. Lett. 2018, 16, 1715–1721. [Google Scholar] [CrossRef] [Green Version]
- Hou, L.K.; Yu, Y.; Xie, Y.G.; Wang, J.; Mao, J.F.; Zhang, B.; Wang, X.; Cao, X.C. miR-340 and ZEB1 negative feedback loop regulates TGF-beta- mediated breast cancer progression. Oncotarget 2016, 7, 26016–26026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, H.; Zhang, B.; Fang, C.; Chen, L. miR-340 alleviates chemoresistance of osteosarcoma cells by targeting ZEB1. Anti-Cancer Drugs 2018, 29, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Barbachano, A.; Fernandez-Barral, A.; Pereira, F.; Segura, M.F.; Ordonez-Moran, P.; Carrillo-de Santa Pau, E.; Gonzalez-Sancho, J.M.; Hanniford, D.; Martinez, N.; Costales-Carrera, A.; et al. SPROUTY-2 represses the epithelial phenotype of colon carcinoma cells via upregulation of ZEB1 mediated by ETS1 and miR-200/miR-150. Oncogene 2016, 35, 2991–3003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, J.; Liu, L.; Wang, Q.; Yin, F.; Yang, Z.; Zhang, W.; Li, L. Downregulation of miR-429 contributes to the development of drug resistance in epithelial ovarian cancer by targeting ZEB1. Am. J. Transl. Res. 2017, 9, 1357–1368. [Google Scholar] [PubMed]
- Lei, W.; Liu, Y.E.; Zheng, Y.; Qu, L. MiR-429 inhibits oral squamous cell carcinoma growth by targeting ZEB1. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 383–389. [Google Scholar] [CrossRef]
- Sun, K.; Zeng, T.; Huang, D.; Liu, Z.; Huang, S.; Liu, J.; Qu, Z. MicroRNA-431 inhibits migration and invasion of hepatocellular carcinoma cells by targeting the ZEB1-mediated epithelial-mensenchymal transition. FEBS Open Bio 2015, 5, 900–907. [Google Scholar] [CrossRef] [Green Version]
- Ma, P.; Ni, K.; Ke, J.; Zhang, W.; Feng, Y.; Mao, Q. miR-448 inhibits the epithelial-mesenchymal transition in breast cancer cells by directly targeting the E-cadherin repressor ZEB1/2. Exp. Biol. Med. (Maywood, NJ, USA) 2018, 243, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Ping, C.; Tang, J.; Zhang, W. MicroRNA-455 suppresses non-small cell lung cancer through targeting ZEB1. Cell Biol. Int. 2016, 40, 621–628. [Google Scholar] [CrossRef]
- Hu, Y.; Xie, H.; Liu, Y.; Liu, W.; Liu, M.; Tang, H. miR-484 suppresses proliferation and epithelial-mesenchymal transition by targeting ZEB1 and SMAD2 in cervical cancer cells. Cancer Cell Int. 2017, 17, 36. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Li, X.; Zhan, G.; Zhu, Y.; Yu, J.; Wang, J.; Li, L.; Wu, W.; Liu, N.; Guo, X. Long noncoding RNA MAGI1-IT1 promoted invasion and metastasis of epithelial ovarian cancer via the miR-200a/ZEB axis. Cell Cycle 2019, 18, 1393–1406. [Google Scholar] [CrossRef]
- Guo, S.J.; Zeng, H.X.; Huang, P.; Wang, S.; Xie, C.H.; Li, S.J. MiR-508-3p inhibits cell invasion and epithelial-mesenchymal transition by targeting ZEB1 in triple-negative breast cancer. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6379–6385. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, R.; Zhang, S.; Xu, R.; Yang, Q. MicroRNA-574-3p regulates epithelial mesenchymal transition and cisplatin resistance via targeting ZEB1 in human gastric carcinoma cells. Gene 2019, 700, 110–119. [Google Scholar] [CrossRef]
- Pang, H.; Zheng, Y.; Zhao, Y.; Xiu, X.; Wang, J. miR-590-3p suppresses cancer cell migration, invasion and epithelial-mesenchymal transition in glioblastoma multiforme by targeting ZEB1 and ZEB2. Biochem. Biophys. Res. Commun. 2015, 468, 739–745. [Google Scholar] [CrossRef]
- Yao, R.; Zheng, H.; Wu, L.; Cai, P. miRNA-641 inhibits the proliferation, migration, and invasion and induces apoptosis of cervical cancer cells by directly targeting ZEB1. OncoTargets Ther. 2018, 11, 8965–8976. [Google Scholar] [CrossRef] [Green Version]
- Deng, S.; Li, X.; Niu, Y.; Zhu, S.; Jin, Y.; Deng, S.; Chen, J.; Liu, Y.; He, C.; Yin, T.; et al. MiR-652 inhibits acidic microenvironment-induced epithelial-mesenchymal transition of pancreatic cancer cells by targeting ZEB1. Oncotarget 2015, 6, 39661–39675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harazono, Y.; Muramatsu, T.; Endo, H.; Uzawa, N.; Kawano, T.; Harada, K.; Inazawa, J.; Kozaki, K. miR-655 Is an EMT-suppressive microRNA targeting ZEB1 and TGFBR2. PLoS ONE 2013, 8, e62757. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhang, Y.; Wei, H.; Zhang, X.; Wu, Y.; Gong, A.; Xia, Y.; Wang, W.; Xu, M. The mir-675-5p regulates the progression and development of pancreatic cancer via the UBQLN1-ZEB1-mir200 axis. Oncotarget 2017, 8, 24978–24987. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Xu, Y.; Wang, S.; Yan, W.; Zhao, Q.; Guo, J. MiR-873-5p inhibits cell migration, invasion and epithelial-mesenchymal transition in colorectal cancer via targeting ZEB1. Pathol. Res. Pract. 2019, 215, 34–39. [Google Scholar] [CrossRef]
- El Bezawy, R.; Cominetti, D.; Fenderico, N.; Zuco, V.; Beretta, G.L.; Dugo, M.; Arrighetti, N.; Stucchi, C.; Rancati, T.; Valdagni, R.; et al. miR-875-5p counteracts epithelial-to-mesenchymal transition and enhances radiation response in prostate cancer through repression of the EGFR-ZEB1 axis. Cancer Lett. 2017, 395, 53–62. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Liu, X.; Yu, T. miR-1271 inhibits migration, invasion and epithelial-mesenchymal transition by targeting ZEB1 and TWIST1 in pancreatic cancer cells. Biochem. Biophys. Res. Commun. 2016, 472, 346–352. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, S.; Liu, X.; Zhang, W.; Li, Y.; Dong, R.; Zhang, Q.; Yang, Q.; Yuan, C.; Shen, K.; et al. miR-1236-3p represses the cell migration and invasion abilities by targeting ZEB1 in high-grade serous ovarian carcinoma. Oncol. Rep. 2014, 31, 1905–1910. [Google Scholar] [CrossRef] [Green Version]
- Liang, T.C.; Fu, W.G.; Zhong, Y.S. MicroRNA-1236-3p inhibits proliferation and invasion of breast cancer cells by targeting ZEB1. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9988–9995. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, Z.; Dong, R.; Wang, X.; Zhang, M.; Guo, X.; Yu, N.; Zeng, A. MicroRNA-3662 targets ZEB1 and attenuates the invasion of the highly aggressive melanoma cell line A375. Cancer Manag. Res. 2019, 11, 5845–5856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Wang, L.; Yang, J.; Fu, Y.; Li, H.; Xie, L.; Cui, Y. MicroRNA-33a-5p suppresses esophageal squamous cell carcinoma progression via regulation of lncRNA DANCR and ZEB1. Eur. J. Pharmacol. 2019, 861, 172590. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Yuan, Y.; Li, C.; Guo, T.; Qi, H.; Xiao, Y.; Dong, X.; Liu, Z.; Liu, Q. Upregulation of SNHG6 regulates ZEB1 expression by competitively binding miR-101-3p and interacting with UPF1 in hepatocellular carcinoma. Cancer Lett. 2016, 383, 183–194. [Google Scholar] [CrossRef]
- Liang, H.; Yu, T.; Han, Y.; Jiang, H.; Wang, C.; You, T.; Zhao, X.; Shan, H.; Yang, R.; Yang, L.; et al. LncRNA PTAR promotes EMT and invasion-metastasis in serous ovarian cancer by competitively binding miR-101-3p to regulate ZEB1 expression. Mol. Cancer 2018, 17, 119. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lv, M.; Song, Z.; Lou, Z.; Wang, R.; Zhuang, M. Long non-coding RNA NNT-AS1 affects progression of breast cancer through miR-142-3p/ZEB1 axis. Biomed. Pharmacother. 2018, 103, 939–946. [Google Scholar] [CrossRef]
- He, C.; Liu, Z.; Jin, L.; Zhang, F.; Peng, X.; Xiao, Y.; Wang, X.; Lyu, Q.; Cai, X. lncRNA TUG1-Mediated Mir-142-3p Downregulation Contributes to Metastasis and the Epithelial-to-Mesenchymal Transition of Hepatocellular Carcinoma by Targeting ZEB1. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 48, 1928–1941. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, J.; Song, H.; Chen, L.B. SNHG16/miR-140-5p axis promotes esophagus cancer cell proliferation, migration and EMT formation through regulating ZEB1. Oncotarget 2018, 9, 1028–1040. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Cheng, D.; Qiu, X.; Zhuang, M.; Liu, Z. Long Noncoding RNA SNHG16 Promotes Cell Proliferation by Sponging MicroRNA-205 and Upregulating ZEB1 Expression in Osteosarcoma. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 51, 429–440. [Google Scholar] [CrossRef]
- Wang, B.; Qu, X.L.; Liu, J.; Lu, J.; Zhou, Z.Y. HOTAIR promotes osteosarcoma development by sponging miR-217 and targeting ZEB1. J. Cell. Physiol. 2019, 234, 6173–6181. [Google Scholar] [CrossRef]
- Yang, T.; He, X.; Chen, A.; Tan, K.; Du, X. LncRNA HOTAIR contributes to the malignancy of hepatocellular carcinoma by enhancing epithelial-mesenchymal transition via sponging miR-23b-3p from ZEB1. Gene 2018, 670, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Pang, H.; Li, X.; Li, H.; Pan, J.; Chen, W. Long non-coding RNA urothelial cancer-associated 1 promotes bladder cancer cell migration and invasion by way of the hsa-miR-145-ZEB1/2-FSCN1 pathway. Cancer Sci. 2016, 107, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Yang, Y.; Guan, J.; Lv, T.; Qu, S.; Fu, Q.; Zhao, H. LncRNA UCA1 sponges miR-204-5p to promote migration, invasion and epithelial-mesenchymal transition of glioma cells via upregulation of ZEB1. Pathol. Res. Pract. 2018, 214, 1474–1481. [Google Scholar] [CrossRef]
- Meng, L.; Ma, P.; Cai, R.; Guan, Q.; Wang, M.; Jin, B. Long noncoding RNA ZEB1-AS1 promotes the tumorigenesis of glioma cancer cells by modulating the miR-200c/141-ZEB1 axis. Am. J. Transl. Res. 2018, 10, 3395–3412. [Google Scholar] [PubMed]
- Qu, R.; Chen, X.; Zhang, C. LncRNA ZEB1-AS1/miR-409-3p/ZEB1 feedback loop is involved in the progression of non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2018, 507, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.C.; Han, N.; Wu, N.; Zhao, K.L.; Han, C.; Wang, H.X.; Ping, G.F.; Zheng, P.F.; Feng, H.; Qin, L.; et al. Interplay between long noncoding RNA ZEB1-AS1 and miR-101/ZEB1 axis regulates proliferation and migration of colorectal cancer cells. Am. J. Transl. Res. 2018, 10, 605–617. [Google Scholar]
- Jin, H.; Jin, X.; Chai, W.; Yin, Z.; Li, Y.; Dong, F.; Wang, W. Long non-coding RNA MIAT competitively binds miR-150-5p to regulate ZEB1 expression in osteosarcoma. Oncol. Lett. 2019, 17, 1229–1236. [Google Scholar] [CrossRef]
- Gao, H.; Li, S.P.; Xu, H.X.; Yu, Y.; He, J.D.; Wang, Z.; Xu, Y.J.; Wang, C.Y.; Zhang, H.M.; Zhang, R.X.; et al. LncRNA HULC enhances epithelial-mesenchymal transition to promote tumorigenesis and metastasis of hepatocellular carcinoma via the miR-200a-3p/ZEB1 signaling pathway. Oncotarget 2016, 7, 42431–42446. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Li, T.; Wei, G.; Liu, L.; Chen, Q.; Xu, L.; Zhang, K.; Zeng, D.; Liao, R. The long non-coding RNA NEAT1 regulates epithelial to mesenchymal transition and radioresistance in through miR-204/ZEB1 axis in nasopharyngeal carcinoma. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2016, 37, 11733–11741. [Google Scholar] [CrossRef]
- Zhong, Q.; Chen, Y.; Chen, Z. LncRNA MINCR regulates irradiation resistance in nasopharyngeal carcinoma cells via the microRNA-223/ZEB1 axis. Cell Cycle (Georgetown, Tex.) 2020, 19, 53–66. [Google Scholar] [CrossRef]
- Yuan, G.; Quan, J.; Dong, D.; Wang, Q. Long Noncoding RNA CAT104 Promotes Cell Viability, Migration, and Invasion in Gastric Carcinoma Cells Through Activation of MicroRNA-381-Inhibiting Zinc Finger E-box-Binding Homeobox 1 (ZEB1) Expression. Oncol. Res. 2018, 26, 1037–1046. [Google Scholar] [CrossRef]
- Wang, P.S.; Chou, C.H.; Lin, C.H.; Yao, Y.C.; Cheng, H.C.; Li, H.Y.; Chuang, Y.C.; Yang, C.N.; Ger, L.P.; Chen, Y.C.; et al. A novel long non-coding RNA linc-ZNF469-3 promotes lung metastasis through miR-574-5p-ZEB1 axis in triple negative breast cancer. Oncogene 2018, 37, 4662–4678. [Google Scholar] [CrossRef]
- Zheng, L.; Xu, M.; Xu, J.; Wu, K.; Fang, Q.; Liang, Y.; Zhou, S.; Cen, D.; Ji, L.; Han, W.; et al. ELF3 promotes epithelial-mesenchymal transition by protecting ZEB1 from miR-141-3p-mediated silencing in hepatocellular carcinoma. Cell Death Dis. 2018, 9, 387. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Chen, C.; Shi, M.; Wang, F.; Chen, X.; Diao, D.; Hu, M.; Yu, M.; Qian, L.; Guo, N. Stat3-coordinated Lin-28-let-7-HMGA2 and miR-200-ZEB1 circuits initiate and maintain oncostatin M-driven epithelial-mesenchymal transition. Oncogene 2013, 32, 5272–5282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Liu, Y.; Wang, Y.; Zhao, M.; Tu, L.; Luo, F. Decitabine reverses TGF-beta1-induced epithelial-mesenchymal transition in non-small-cell lung cancer by regulating miR-200/ZEB axis. Drug Des. Dev. Ther. 2017, 11, 969–983. [Google Scholar] [CrossRef] [Green Version]
- Chung, V.Y.; Tan, T.Z.; Tan, M.; Wong, M.K.; Kuay, K.T.; Yang, Z.; Ye, J.; Muller, J.; Koh, C.M.; Guccione, E.; et al. GRHL2-miR-200-ZEB1 maintains the epithelial status of ovarian cancer through transcriptional regulation and histone modification. Sci. Rep. 2016, 6, 19943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, X.; Ding, X.; Li, X.; Gao, S.; Yang, Q. 53BP1 suppresses epithelial-mesenchymal transition by downregulating ZEB1 through microRNA-200b/429 in breast cancer. Cancer Sci. 2015, 106, 982–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.Y.; Park, M.K.; Park, J.H.; Lee, H.J.; Shin, D.H.; Kang, Y.; Lee, C.H.; Kong, G. Loss of the polycomb protein Mel-18 enhances the epithelial-mesenchymal transition by ZEB1 and ZEB2 expression through the downregulation of miR-205 in breast cancer. Oncogene 2014, 33, 1325–1335. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Wang, H.; Han, X.; Ma, J.; Zhou, Y.; Chen, Z.; Zhou, H.; Xu, H.; Sun, Z.; Kong, B.; et al. DeltaNp63alpha attenuates tumor aggressiveness by suppressing miR-205/ZEB1-mediated epithelial-mesenchymal transition in cervical squamous cell carcinoma. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2016, 37, 10621–10632. [Google Scholar] [CrossRef]
- Bian, Y.; Gao, G.; Zhang, Q.; Qian, H.; Yu, L.; Yao, N.; Qian, J.; Liu, B.; Qian, X. KCNQ1OT1/miR-217/ZEB1 feedback loop facilitates cell migration and epithelial-mesenchymal transition in colorectal cancer. Cancer Biol. Ther. 2019, 20, 886–896. [Google Scholar] [CrossRef]
- Xiao, T.; Xue, J.; Shi, M.; Chen, C.; Luo, F.; Xu, H.; Chen, X.; Sun, B.; Sun, Q.; Yang, Q.; et al. Circ008913, via miR-889 regulation of DAB2IP/ZEB1, is involved in the arsenite-induced acquisition of CSC-like properties by human keratinocytes in carcinogenesis. Met. Integr. Biometal Sci. 2018, 10, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; An, X.; Yu, H.; Zhang, S.; Tang, B.; Zhang, X.; Li, Z. MiR-29b/TET1/ZEB2 signaling axis regulates metastatic properties and epithelial-mesenchymal transition in breast cancer cells. Oncotarget 2017, 8, 102119–102133. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, J.; Zhang, Z.; Feng, Z.; Wei, J.; Lu, J.; Fang, Y.; Liang, Y.; Cen, J.; Pan, Y.; et al. The putative tumor suppressor microRNA-30a-5p modulates clear cell renal cell carcinoma aggressiveness through repression of ZEB2. Cell Death Dis. 2017, 8, e2859. [Google Scholar] [CrossRef]
- Ji, H.; Sang, M.; Liu, F.; Ai, N.; Geng, C. miR-124 regulates EMT based on ZEB2 target to inhibit invasion and metastasis in triple-negative breast cancer. Pathol. Res. Pract. 2019, 215, 697–704. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Bi, H.; Bai, S.; Zhao, L.; Zhang, J.; Qi, C. Targeting ZEB2 By microRNA-129 In Non-Small Cell Lung Cancer Suppresses Cell Proliferation, Invasion And Migration Via Regulating Wnt/beta-Catenin Signaling Pathway And Epithelial-Mesenchymal Transition. OncoTargets Ther. 2019, 12, 9165–9175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.B.; Luo, H.P.; Shi, Q.; Hao, Z.N.; Ding, Y.; Wang, Q.S.; Li, S.B.; Xiao, G.C.; Tong, S.L. miR-132 inhibits colorectal cancer invasion and metastasis via directly targeting ZEB2. World J. Gastroenterol. 2014, 20, 6515–6522. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Li, Y.; Fang, N.; Liu, B.; Zu, L.; Chang, R.; Li, X.; Zhou, Q. MiR-132 suppresses the migration and invasion of lung cancer cells via targeting the EMT regulator ZEB2. PLoS ONE 2014, 9, e91827. [Google Scholar] [CrossRef]
- Wu, S.-M.; Ai, H.-W.; Zhang, D.-Y.; Han, X.-Q.; Pan, Q.; Luo, F.-L.; Zhang, X.-L. MiR-141 targets ZEB2 to suppress HCC progression. Tumor Biol. 2014, 35, 9993–9997. [Google Scholar] [CrossRef]
- Li, W.; Wang, Q.; Su, Q.; Ma, D.; An, C.; Ma, L.; Liang, H. Honokiol suppresses renal cancer cells’ metastasis via dual-blocking epithelial-mesenchymal transition and cancer stem cell properties through modulating miR-141/ZEB2 signaling. Mol. Cells 2014, 37, 383–388. [Google Scholar] [CrossRef] [Green Version]
- Ren, D.; Wang, M.; Guo, W.; Huang, S.; Wang, Z.; Zhao, X.; Du, H.; Song, L.; Peng, X. Double-negative feedback loop between ZEB2 and miR-145 regulates epithelial-mesenchymal transition and stem cell properties in prostate cancer cells. Cell Tissue Res. 2014, 358, 763–778. [Google Scholar] [CrossRef]
- Jiang, S.B.; He, X.J.; Xia, Y.J.; Hu, W.J.; Luo, J.G.; Zhang, J.; Tao, H.Q. MicroRNA-145-5p inhibits gastric cancer invasiveness through targeting N-cadherin and ZEB2 to suppress epithelial-mesenchymal transition. OncoTargets Ther. 2016, 9, 2305–2315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Xie, M.; Shi, Y.; Luo, B.; Gong, G.; Li, J.; Wang, J.; Zhao, W.; Zi, Y.; Wu, X.; et al. MicroRNA-153 functions as a tumor suppressor by targeting SET7 and ZEB2 in ovarian cancer cells. Oncol. Rep. 2015, 34, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Yang, Z.; Zhang, P.; Liu, Y.; Shao, G. miR-154 inhibits migration and invasion of human non-small cell lung cancer by targeting ZEB2. Oncol. Lett. 2016, 12, 301–306. [Google Scholar] [CrossRef] [Green Version]
- Pang, X.; Huang, K.; Zhang, Q.; Zhang, Y.; Niu, J. miR-154 targeting ZEB2 in hepatocellular carcinoma functions as a potential tumor suppressor. Oncol. Rep. 2015, 34, 3272–3279. [Google Scholar] [CrossRef]
- Fei, D.; Zhao, K.; Yuan, H.; Xing, J.; Zhao, D. MicroRNA-187 exerts tumor-suppressing functions in osteosarcoma by targeting ZEB2. Am. J. Cancer Res. 2016, 6, 2859–2868. [Google Scholar] [PubMed]
- Bendoraite, A.; Knouf, E.C.; Garg, K.S.; Parkin, R.K.; Kroh, E.M.; O’Briant, K.C.; Ventura, A.P.; Godwin, A.K.; Karlan, B.Y.; Drescher, C.W.; et al. Regulation of miR-200 family microRNAs and ZEB transcription factors in ovarian cancer: Evidence supporting a mesothelial-to-epithelial transition. Gynecol. Oncol. 2010, 116, 117–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdigao-Henriques, R.; Petrocca, F.; Altschuler, G.; Thomas, M.; Le, M.; Tan, S.; Hide, W.; Lieberman, J. miR-200 promotes the mesenchymal to epithelial transition by suppressing multiple members of the Zeb2 and Snail1 transcriptional repressor complexes. Oncogene 2016, 35, 158–172. [Google Scholar] [CrossRef]
- Xia, H.; Ng, S.S.; Jiang, S.; Cheung, W.K.; Sze, J.; Bian, X.-W.; Kung, H.-f.; Lin, M.C. miR-200a-mediated downregulation of ZEB2 and CTNNB1 differentially inhibits nasopharyngeal carcinoma cell growth, migration and invasion. Biochem. Biophys. Res. Commun. 2010, 391, 535–541. [Google Scholar] [CrossRef]
- Yang, X.; Wang, J.; Qu, S.; Zhang, H.; Ruan, B.; Gao, Y.; Ma, B.; Wang, X.; Wu, N.; Li, X.; et al. MicroRNA-200a suppresses metastatic potential of side population cells in human hepatocellular carcinoma by decreasing ZEB2. Oncotarget 2015, 6, 7918–7929. [Google Scholar] [CrossRef]
- Wu, Q.; Guo, R.; Lin, M.; Zhou, B.; Wang, Y. MicroRNA-200a inhibits CD133/1+ ovarian cancer stem cells migration and invasion by targeting E-cadherin repressor ZEB2. Gynecol. Oncol. 2011, 122, 149–154. [Google Scholar] [CrossRef]
- Kurashige, J.; Kamohara, H.; Watanabe, M.; Hiyoshi, Y.; Iwatsuki, M.; Tanaka, Y.; Kinoshita, K.; Saito, S.; Baba, Y.; Baba, H. MicroRNA-200b regulates cell proliferation, invasion, and migration by directly targeting ZEB2 in gastric carcinoma. Ann. Surg. Oncol. 2012, 19 (Suppl. 3), S656–S664. [Google Scholar] [CrossRef]
- Li, J.; Yuan, J.; Yuan, X.; Zhao, J.; Zhang, Z.; Weng, L.; Liu, J. MicroRNA-200b inhibits the growth and metastasis of glioma cells via targeting ZEB2. Int. J. Oncol. 2016, 48, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-m.; Shang, C.; Ou, Y.-l.; Yin, D.; Li, Y.-N.; Li, X.; Wang, N.; Zhang, S.-l. miR-200c modulates ovarian cancer cell metastasis potential by targeting zinc finger E-box-binding homeobox 2 (ZEB2) expression. Med. Oncol. 2014, 31, 134. [Google Scholar] [CrossRef]
- Jiao, A.; Sui, M.; Zhang, L.; Sun, P.; Geng, D.; Zhang, W.; Wang, X.; Li, J. MicroRNA-200c inhibits the metastasis of non-small cell lung cancer cells by targeting ZEB2, an epithelial-mesenchymal transition regulator. Mol. Med. Rep. 2016, 13, 3349–3355. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Qin, Y.; Chen, C.; Yang, J.; Song, N.; Gu, M. MicroRNA-200c-3p/ZEB2 loop plays a crucial role in the tumor progression of prostate carcinoma. Ann. Transl. Med. 2019, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Fu, Z.; Gao, L.; Zhou, J.; Deng, X.; Luo, X.; Fang, W.; Luo, R. Direct interaction between miR-203 and ZEB2 suppresses epithelial–mesenchymal transition signaling and reduces lung adenocarcinoma chemoresistance. Acta Biochim. Biophys. Sin. 2016, 48, 1042–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Q.; Zhou, Y.; Yang, H.; Li, L.; Deng, X.; Cheng, C.; Xie, Y.; Luo, X.; Fang, W.; Liu, Z. A directly negative interaction of miR-203 and ZEB2 modulates tumor stemness and chemotherapy resistance in nasopharyngeal carcinoma. Oncotarget 2016, 7, 67288. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tang, Z.Y.; He, Y.; Liu, L.F.; Li, D.J.; Chen, X. miRNA-205 is a candidate tumor suppressor that targets ZEB2 in renal cell carcinoma. Oncology Res. Treat. 2014, 37, 658–664. [Google Scholar] [CrossRef]
- Chen, X.F.; Guo, J.F.; Xu, J.F.; Yin, S.H.; Cao, W.L. MiRNA-206 inhibits proliferation of renal clear cell carcinoma by targeting ZEB2. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7826–7834. [Google Scholar] [CrossRef]
- Hou, Y.; Zhen, J.; Xu, X.; Zhen, K.; Zhu, B.; Pan, R.; Zhao, C. miR-215 functions as a tumor suppressor and directly targets ZEB2 in human non-small cell lung cancer. Oncol. Lett. 2015, 10, 1985–1992. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Zhang, Z.; Liu, Z.; Qiu, B.; Liu, K.; Dong, G. MicroRNA-335 inhibits invasion and metastasis of colorectal cancer by targeting ZEB2. Med. Oncol. (Northwood Lond. Engl.) 2014, 31, 982. [Google Scholar] [CrossRef] [PubMed]
- Kan, Q.; Su, Y.; Yang, H. MicroRNA-335 is downregulated in papillary thyroid cancer and suppresses cancer cell growth, migration and invasion by directly targeting ZEB2. Oncol. Lett. 2017, 14, 7622–7628. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Wu, Z.; Lin, L.; Zhou, M.; Wang, L.; Ma, H.; Xia, J.; Bin, J.; Liao, Y.; Liao, W. MiR-338-3p inhibits epithelial-mesenchymal transition in gastric cancer cells by targeting ZEB2 and MACC1/Met/Akt signaling. Oncotarget 2015, 6, 15222. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Pan, G.; He, Q.; Yin, L.; Guo, R.; Bi, H. MicroRNA-545 targets ZEB2 to inhibit the development of non-small cell lung cancer by inactivating Wnt/beta-catenin pathway. Oncol. Lett. 2019, 18, 2931–2938. [Google Scholar] [CrossRef] [Green Version]
- Tong, X.; Su, P.; Yang, H.; Chi, F.; Shen, L.; Feng, X.; Jiang, H.; Zhang, X.; Wang, Z. MicroRNA-598 inhibits the proliferation and invasion of non-small cell lung cancer cells by directly targeting ZEB2. Exp. Ther. Med. 2018, 16, 5417–5423. [Google Scholar] [CrossRef]
- Song, Q.; Pang, H.; Qi, L.; Liang, C.; Wang, T.; Wang, W.; Li, R. Low microRNA-622 expression predicts poor prognosis and is associated with ZEB2 in glioma. OncoTargets Ther. 2019, 12, 7387–7397. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Yang, S.; Gao, Y.; Zhang, C.; Sui, Q. MicroRNA-769-3p inhibits tumor progression in glioma by suppressing ZEB2 and inhibiting the Wnt/beta-catenin signaling pathway. Oncol. Lett. 2020, 19, 992–1000. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Zhou, F.; Yu, T.; Xu, G.; Zhang, J.; Wang, Y.; Zhao, L.; Liu, N. MicroRNA-940 inhibits epithelial-mesenchymal transition of glioma cells via targeting ZEB2. Am. J. Transl. Res. 2019, 11, 7351–7363. [Google Scholar] [PubMed]
- Gao, H.B.; Gao, F.Z.; Chen, X.F. MiRNA-1179 suppresses the metastasis of hepatocellular carcinoma by interacting with ZEB2. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5149–5157. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, H.; Cheng, H.; Li, Y.; Li, X.; Zhu, C. Downregulation of long noncoding RNA TUG1 inhibits proliferation and induces apoptosis through the TUG1/miR-142/ZEB2 axis in bladder cancer cells. OncoTargets Ther. 2017, 10, 2461–2471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Lu, L.; Feng, B.; Zhang, K.; Han, S.; Hou, D.; Chen, L.; Chu, X.; Wang, R. The lincRNA-ROR/miR-145 axis promotes invasion and metastasis in hepatocellular carcinoma via induction of epithelial-mesenchymal transition by targeting ZEB2. Sci. Rep. 2017, 7, 4637. [Google Scholar] [CrossRef]
- Xiao, H.; Tang, K.; Liu, P.; Chen, K.; Hu, J.; Zeng, J.; Xiao, W.; Yu, G.; Yao, W.; Zhou, H. LncRNA MALAT1 functions as a competing endogenous RNA to regulate ZEB2 expression by sponging miR-200s in clear cell kidney carcinoma. Oncotarget 2015, 6, 38005. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, Y.; Yang, Z.; Chen, B.; Huang, W.; Liu, Y.; Zhang, Y. MiR-204/ZEB2 axis functions as key mediator for MALAT1-induced epithelial-mesenchymal transition in breast cancer. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2017, 39, 1010428317690998. [Google Scholar] [CrossRef]
- Chen, D.L.; Lu, Y.X.; Zhang, J.X.; Wei, X.L.; Wang, F.; Zeng, Z.L.; Pan, Z.Z.; Yuan, Y.F.; Wang, F.H.; Pelicano, H.; et al. Long non-coding RNA UICLM promotes colorectal cancer liver metastasis by acting as a ceRNA for microRNA-215 to regulate ZEB2 expression. Theranostics 2017, 7, 4836–4849. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wan, L.; Liu, Z.; Xu, G.; Wang, S.; Su, Z.; Zhang, Y.; Zhang, C.; Liu, X.; Lei, Z. Long non-coding RNA XIST promotes TGF-β-induced epithelial-mesenchymal transition by regulating miR-367/141-ZEB2 axis in non-small-cell lung cancer. Cancer Lett. 2018, 418, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, Q.; Wen, X.; Hong, X.; Yang, X.; Tang, X.; Zhang, P.; Lei, Y.; Sun, Y.; Zhang, J.; et al. EZH2-DNMT1-mediated epigenetic silencing of miR-142-3p promotes metastasis through targeting ZEB2 in nasopharyngeal carcinoma. Cell Death Differ. 2019, 26, 1089–1106. [Google Scholar] [CrossRef]
- Li, X.; Tian, Y.; Hu, Y.; Yang, Z.; Zhang, L.; Luo, J. CircNUP214 sponges miR-145 to promote the expression of ZEB2 in thyroid cancer cells. Biochem. Biophys. Res. Commun. 2018, 507, 168–172. [Google Scholar] [CrossRef]
- Brown, C.Y.; Dayan, S.; Wong, S.W.; Kaczmarek, A.; Hope, C.M.; Pederson, S.M.; Arnet, V.; Goodall, G.J.; Russell, D.; Sadlon, T.J. FOXP3 and miR-155 cooperate to control the invasive potential of human breast cancer cells by down regulating ZEB2 independently of ZEB1. Oncotarget 2018, 9, 27708. [Google Scholar] [CrossRef]
- Li, H.; Xu, L.; Li, C.; Zhao, L.; Ma, Y.; Zheng, H.; Li, Z.; Zhang, Y.; Wang, R.; Liu, Y.; et al. Ubiquitin ligase Cbl-b represses IGF-I-induced epithelial mesenchymal transition via ZEB2 and microRNA-200c regulation in gastric cancer cells. Mol. Cancer 2014, 13, 136. [Google Scholar] [CrossRef] [Green Version]
- Truong, H.H.; Xiong, J.; Ghotra, V.P.; Nirmala, E.; Haazen, L.; Le Devedec, S.E.; Balcioglu, H.E.; He, S.; Snaar-Jagalska, B.E.; Vreugdenhil, E.; et al. beta1 integrin inhibition elicits a prometastatic switch through the TGFbeta-miR-200-ZEB network in E-cadherin-positive triple-negative breast cancer. Sci. Signal. 2014, 7, ra15. [Google Scholar] [CrossRef]
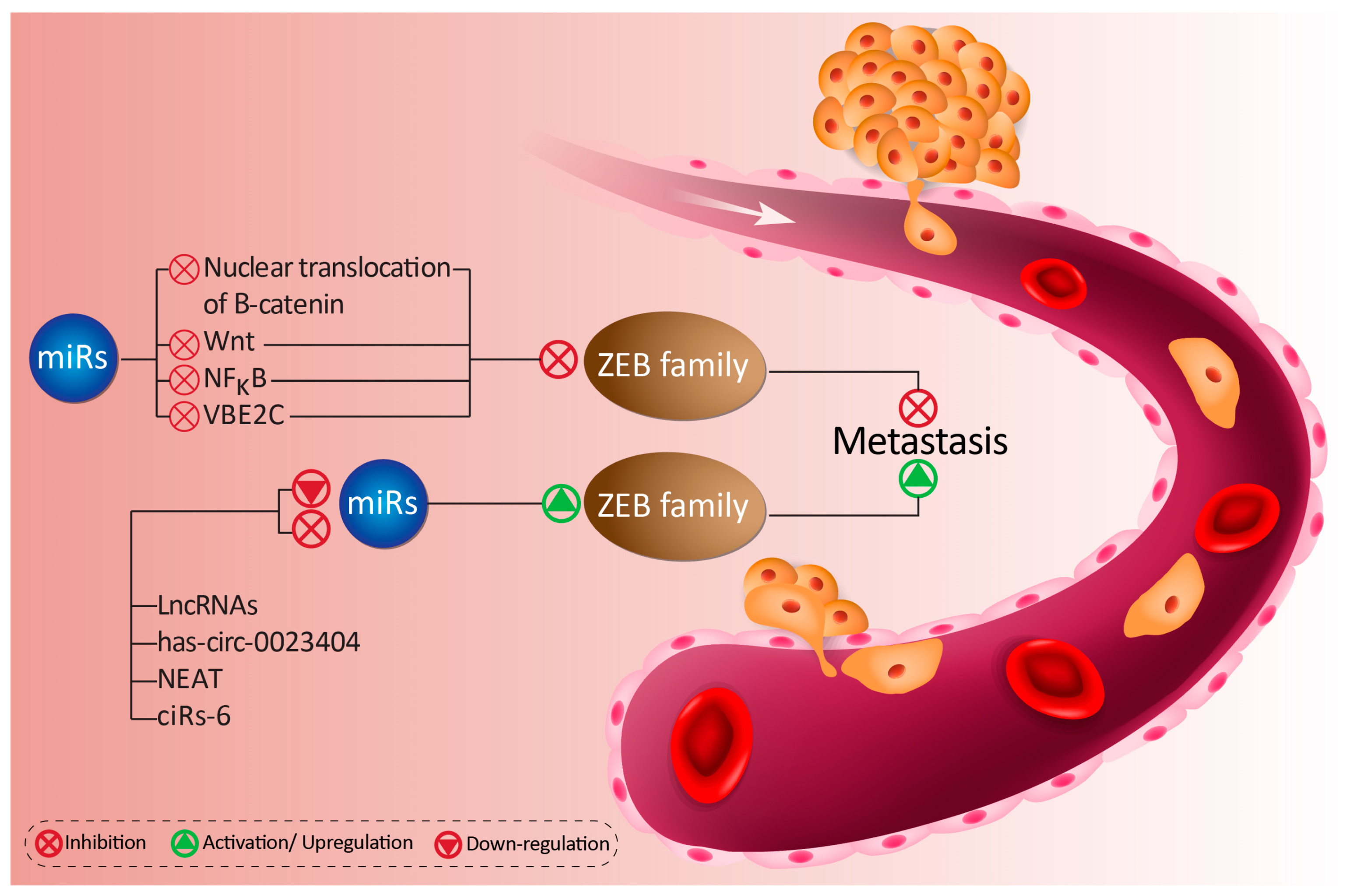

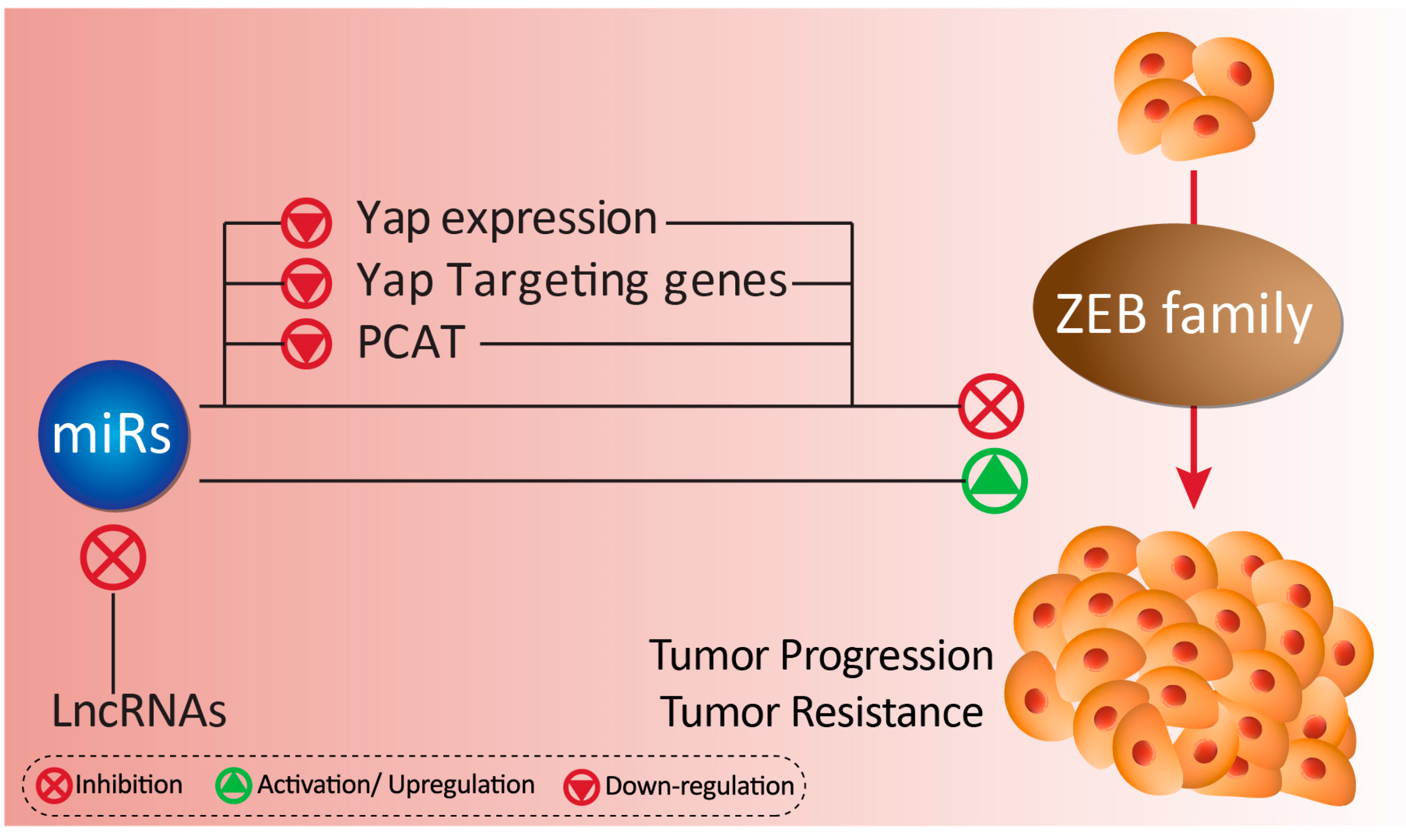
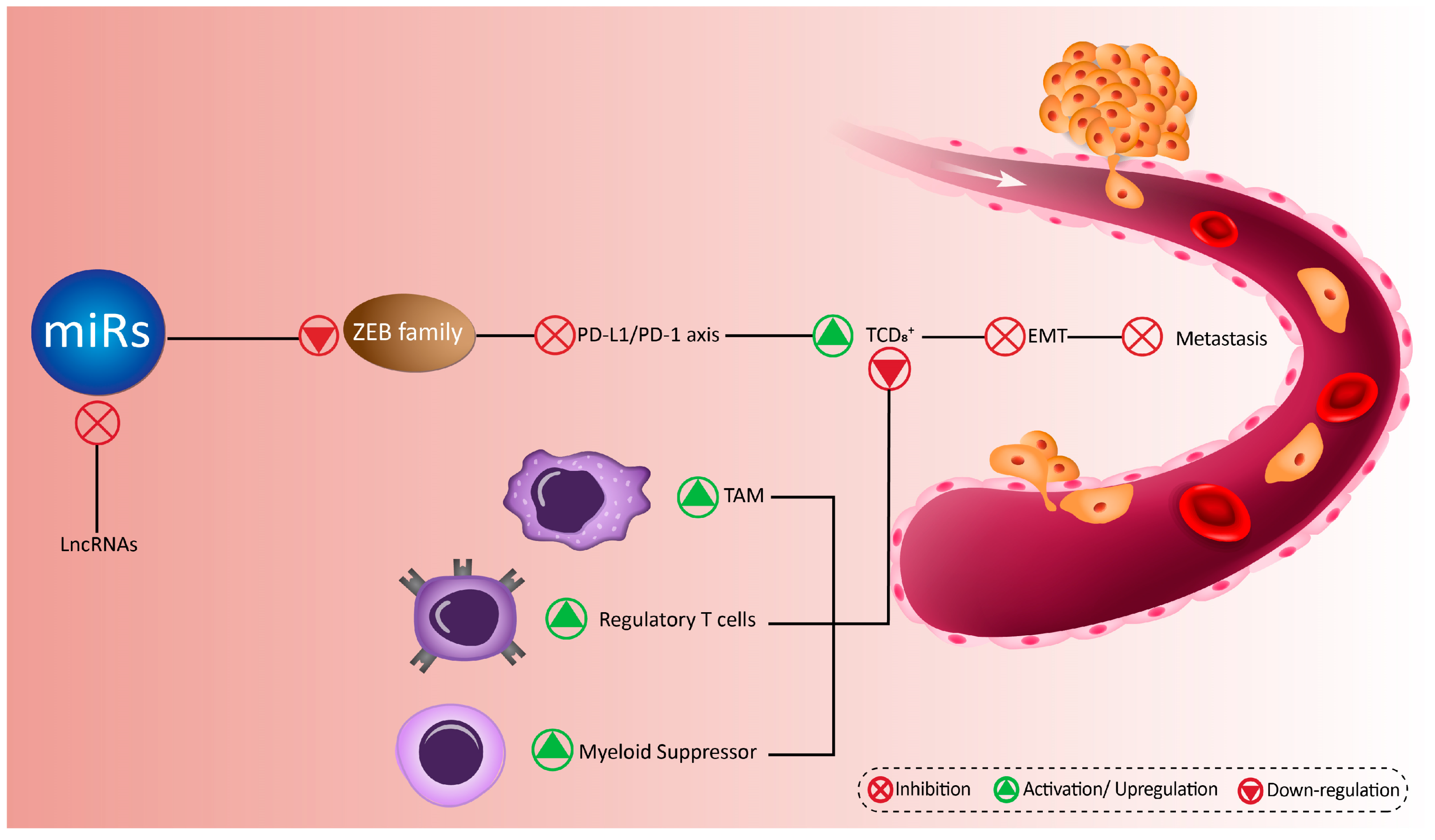
| MiR | Down-Stream Target | Cancer Type | Major Outcomes | Refs |
|---|---|---|---|---|
| MiR-23a | ZEB1 | Intraocular tumor | A negative feedback loop between miR-23a and ZEB1 regulates EMT and overexpression of miR-23a inhibits EMT by ZEB1 down-regulation | [248] |
| MiR-23b | ZEB1 | Bladder cancer | MiR-23b induces apoptosis and cell cycle arrest, and decreases the invasion and EMT through ZEB1 inhibition | [249] |
| MiR-33b | ZEB1 | Melanoma | Cordycepin enhances the expression of miR-33b to inhibit ZEB1 and induces mesenchymal-epithelial transition in cancer cells, resulting in decreased invasion and migration of cancer cells | [250] |
| MiR-126 | ZEB1 | Osteosarcoma | Inhibition of EMT, migration, and metastasis of cancer cells through ZEB1 down-regulation | [251] |
| MiR-128 | ZEB1 | Prostate cancer | MiR-128 sensitizes cancer cells into cisplatin chemotherapy by ZEB1 down-regulation and decreasing the malignancy and invasion of cancer cells | [252] |
| MiR-130b | ZEB1 | Endometrial cancer | The miR-130b down-regulates the expression of ZEB1 to inhibit the malignancy and invasion of cancer cells | [253] |
| MiR-139-5p | ZEB1/2 | Hepatocellular carcinoma | Reduced invasion, migration, metastasis, and EMT by ZEB1/2 down-regulation through miR-139-5p | [254] |
| Glioblastoma multiforme | Suppressing the invasion and migration of cancer cells through ZEB1/2 inhibition | [255] | ||
| MiR-141 and miR-146b-5p | AUF1/ZEB1 | Osteosarcoma | These miRs are able to down-regulate the expression of AUF1 to repress ZEB1, resulting in an increase in epithelial markers (E-cadherin and Epcam) and a decrease in mesenchymal markers (N-cadherin and Vimentin) | [256] |
| MiR-144 MiR-144 | ZEB1/2 | Breast cancer | MiR-144 is an onco-suppressor that inhibits EMT and migration invasion through ZEB1/2 down-regulation | [257] |
| Thyroid cancer | MiR-144 down-regulates the expression of ZEB1/2 to prevent cancer progression and proliferation | [258] | ||
| MiR-150 | ZEB1 | Esophageal squamous cell carcinoma | MiR-150 degrades ZEB1 to induce mesenchymal-epithelial transition (MET), resulting in a decrease in tumor depth, lymph node metastasis, and lymphatic invasion | [259] |
| Ovarian cancer | Suppressing the malignancy and invasion of cancer cells through ZEB1 inhibition | [260] | ||
| MiR-199a-3p | ZEB1 | Melanoma | The administration of gambogic acid is associated with up-regulation of miR-199a-3p and subsequent inhibition of ZEB1 to suppress cancer progression both in vitro and in vivo | [261] |
| MiR-199b | ZEB1 | Non-small cell lung cancer | Suppressing the proliferation, migration, and invasion of cancer cells through ZEB1 down-regulation | [262] |
| MiR-200 | ZEB1-FAK/Src | Human lung cancer | The miR-200 up-regulation decreases the invasion and malignancy of cancer cells through enhancing ZEB1 expression and subsequent activation of FAK/Src | [263] |
| ZEB1 | Endometrial carcinoma | The expression of miR-200 undergoes down-regulation in endometrial carcinoma cells to induce ZEB1 and subsequently, EMT mechanism to elevate the invasion and malignancy of cancer cells | [264] | |
| Lymphoma | Generation of a less aggressive behavior by ZEB1 inhibition through miR-200 | [265] | ||
| Insulinoma mouse model | Overexpression of miR-200 is associated with ZEB1 inhibition and decreased migration and proliferation of cancer cells | [266] | ||
| MiR-200b | ZEB1 | Osteosarcoma | Overexpression of miR-200b is associated with down-regulation of ZEB1 and decreased invasion and malignancy of cancer cells | [267] |
| Human hepatocellular carcinoma | By down-regulation of ZEB1, miR-200b reduces the stemness of cancer cells | [268] | ||
| MiR-200b and miR-141 | ZEB1 | Non-small cell lung cancer | The overexpression of miR-200b and miR-141 is related to the inhibition of ZEB1 and sensitizing cancer cells into nintedanib | [269] |
| MiR-200c | ZEB1 | Human colon cancer | Suppressing the invasion and migration of cancer cells through ZEB1 down-regulation | [270] |
| Gastric carcinoma | Overexpression of miR-200c is related to the ZEB1 down-regulation and enhanced levels of E-cadherin protein | [271] | ||
| Human bladder cancer | Administration of sulforaphane is associated with miR-200c induction and subsequently, inhibition of ZEB1 and malignancy of cancer cells | [272] | ||
| Non-small cell lung carcinoma | The cyclamen pseudibericum extract up-regulates miR-200c to induce ZEB1 down-regulation, resulting in suppressing cancer progression and proliferation | [273] | ||
| Non-small cell lung cancer | MiR-200c sensitizes cancer cells to the gefitinib-mediated apoptosis by down-regulation of ZEB1 | [274] | ||
| Lung cancer | MiR-200c sensitizes lung cancer cells into crizotinib chemotherapy by inhibition of ZEB1, and subsequently, EMT inhibition | [275] | ||
| MiR-200c and miR-141 | ZEB1 | Glioma cell | The miR-200c and -141 synergistically inhibit ZEB1 to prevent the malignancy and invasion of cancer cells | [276] |
| ZEB1/2 | Gastric cancer | MiR-200c/141 significantly decreases ZEB1/2 expression to suppress cancer malignancy | [277] | |
| MiR-203 | ZEB1 | Non-small cell lung cancer | The administration of silymarin enhances the expression of miR-203 to inhibit ZEB1 and elevate the levels of E-cadherin, resulting in suppressing cancer | [278] |
| MiR-204 | ZEB1 | Prostate cancer | MiR-204 up-regulation sensitizes cancer cells into docetaxel-mediated apoptosis through ZEB1 down-regulation | [279] |
| MiR-205 | ZEB1 | Ovarian cancer | MiR-205 enhances the invasion and migration of cancer cells via ZEB1 up-regulation. Reducing the expression of miR-205 is of interest in suppressing the malignancy of cancer cells | [280] |
| Prostate cancer | By inhibition of ZEB1, miR-205 sensitizes cancer cells into radiotherapy and induces DNA damage | [281] | ||
| Breast cancer | MiR-205 sensitizes cancer cells into radiotherapy and prevents DNA repair by ZEB1 down-regulation | [282] | ||
| MiR-205-5p | ZEB1 | Prostatic carcinoma | Suppressing the migration and invasion of cancer cells by ZEB1 down-regulation | [283] |
| MiR-340 | ZEB1/TGF-β | Breast cancer | MiR-340 inhibits ZEB1 to suppress TGF-β-mediated cancer progression | [284] |
| ZEB1 | Osteosarcoma | MiR-340 down-regulates the expression of ZEB1 to sensitize cancer cells into cisplatin-mediated apoptotic cell death | [285] | |
| MiR-409-3p | ZEB1 | Breast cancer | MiR-409-3p binds to the 3′-UTR of ZEB1 to inhibit the progression and metastasis of cancer cells | [286] |
| MiR-429 | ZEB1 | Ovarian cancer | Down-regulation of miR-429 is related to the resistance of cancer cells into cisplatin chemotherapy. Up-regulation of miR-429 suppresses ZEB1 to sensitize cancer cells into apoptosis | [287] |
| Oral squamous cell carcinoma | MiR-429 suppresses the viability and progression of cancer cells via ZEB1 down-regulation | [288] | ||
| Human thyroid cancer | MiR-429 binds to the 3′-UTR to inhibit ZEB1, resulting in suppressing invasion of cancer cells | [110] | ||
| MiR-431 | ZEB1 | Hepatocellular carcinoma | MiR-431 suppresses the migration and invasion capabilities of cancer cells through inhibition of ZEB1-mediated EMT | [289] |
| MiR-448 | ZEB1/2 | Breast cancer | The miR-448 significantly reduces the expressions of ZEB1/2 to inhibit the malignancy and invasion of cancer cells via EMT down-regulation | [290] |
| MiR-455 | ZEB1 | Non-small cell lung cancer | The miR-455 reduces the expression of ZEB1 to inhibit the malignancy of cancer cells | [291] |
| MiR-484 | Smad2/ZEB1 | Cervical cancer | Overexpression of miR-484 inhibits Smad2/ZEB1 to suppress cancer malignancy and miR-484 expression can be considered as a biomarker | [292] |
| MiR-508 | ZEB1 | Renal cell carcinoma | Up-regulation of miR-508 significantly reduces the expression of ZEB1 to inhibit EMT, leading to a decrease in cancer migration and metastasis | [293] |
| MiR-508-3p | ZEB1 | Triple negative breast cancer | Suppressing the invasion and EMT of cancer cells by down-regulation of ZEB1 | [294] |
| MiR-574-3p | ZEB1 | Human gastric carcinoma | MiR-574-3p reduces the expression of ZEB1 by binding into 3′-UTR to decrease the malignancy of cancer cells, and simultaneously, sensitize cancer cells into cisplatin therapy | [295] |
| MiR-590-3p | ZEB1/2 | Glioblastoma multiforme | Decreased invasion and migration of cancer cells by ZEB1/2 down-regulation | [296] |
| MiR-641 | ZEB1 | Cervical cancer | Negatively affecting the proliferation, migration, and invasion of cancer cells through ZEB1 down-regulation | [297] |
| MiR-652 | ZEB1 | Pancreatic cancer | Acidic microenvironment of tumor cells induces EMT through ZEB1 up-regulation. Enhancing the expression of miR-652 inhibits acidic-mediated EMT and ZEB1 induction | [298] |
| MiR-655 | TGF-β/ZEB1 | Pancreatic cancer | MiR-655 inhibits TGF-β/ZEB1 axis to suppress EMT in cancer cells | [299] |
| MiR-675-5p | UBQLN1/ZEB1/miR200 | Pancreatic cancer | The miR-675-5p reduces the malignancy of cancer cells and ZEB1 protein by up-regulation of UBQLN1 and down-regulation of miR-200 | [300] |
| MiR-873-5p | ZEB1 | Colorectal cancer | The inhibitory effect of miR-873-5p on the migration, EMT formation, and invasion of cancer cells is mediated through ZEB1 down-regulation | [301] |
| MiR-875-5p | EGFR/ZEB1 | Prostate cancer | By suppressing EGFR/ZEB1 axis, miR-875-5p inhibits EMT mechanism and sensitizes cancer cells to radiotherapy | [302] |
| MiR-1271 | ZEB1 | Pancreatic cancer | Suppressing the invasion, progression, and EMT in cancer cells by ZEB1 down-regulation | [303] |
| MiR-1236-3p | ZEB1 | High-grade serous ovarian carcinoma | There is a negative relationship between miR-1236-3p and ZEB1 to suppress the migration and invasion of cancer cells | [304] |
| MiR-1236-3p | ZEB1 | Breast cancer | ZEB1 inhibition by miR-1236-3p contributes to the inhibitory effect of this miR on the migration and invasion of cancer cells | [305] |
| MiR-3662 | ZEB1 | Melanoma | Amelioration of invasiveness and malignancy of cancer cells by ZEB1 down-regulation | [306] |
| LncRNA | MiR | Down-Stream Target | Cancer Type | Major Outcomes | Refs |
|---|---|---|---|---|---|
| LncRNA DANCR | MiR-33a-5p | ZEB1 | Esophageal squamous cell carcinoma | MiR-33a-5p suppresses cancer malignancy via reducing ZEB1 expression. LncRNA DANCR sponges miR-33a-5p to enhances the invasion via ZEB1 induction | [307] |
| LncRNA SNHG6 | MiR-101-3p | ZEB1 | Hepatocellular carcinoma | LncRNA SNHG6 down-regulates the expression of miR-101-3p to induce ZEB1 and enhance the malignancy of cancer cells | [308] |
| LncRNA PTAR | MiR-101-3p | ZEB1 | Serous ovarian cancer | LncRNA PTAR decreases the expression of miR-101-3p to induce ZEB1 and EMT mechanism, leading to the invasion and metastasis of cancer cells | [309] |
| LncRNA NNT-AS1 | MiR-142-3p | ZEB1 | Breast cancer | Enhancing the progression of cancer cells by sponging miR-142-3p and induction of ZEB1 | [310] |
| LncRNA TUG1 | MiR-142-3p | ZEB1 | Hepatocellular carcinoma | By down-regulation of miR-142-3p, lncRNA TUG1 enhances the expression of ZEB1 to ensure the proliferation and malignancy of cancer cells | [311] |
| LncRNA SNHG16 | MiR-140-5p | ZEB1 | Esophageal squamous cell carcinoma | The lncRNA SNHG16 functions as an oncogenic factor and neutralizes the inhibitory effect of miR-140-5p on ZEB1 to induce EMT and enhance the migration and invasion of cancer cells | [312] |
| MiR-205 | ZEB1 | Osteosarcoma | SNHG16 reduces the expression of miR-205 to elevate the expression of ZEB1, resulting in an increase in the viability, proliferation, and migration of cancer cells | [313] | |
| LncRNA HOTAIR | MiR-217 | ZEB1 | Osteosarcoma | By reducing the expression of miR-217, lncRNA HOTAIR enhances the expression of ZEB1 and improves their malignancy | [314] |
| MiR-23b-3p | ZEB1 | Hepatocellular carcinoma | The miR-23b-3p inhibits ZEB1 and lncRNA HOTAIR prevents the inhibitory effect of miR-23b-3p on ZEB1 to induce EMT | [315] | |
| lncRNA UCA1 | Has-miR-145 | ZEB1/2-FSCN1 | Bladder cancer | There is a reverse relationship between lncRNA UCA1 and has-miR-145. Decreased expression of has-miR-145 enhances the expression of ZEB1/2 and FSCN1 to elevate the migration and invasion of cancer cells | [316] |
| MiR-204-5p | ZEB1 | Glioma cells | By sponging miR-204-5p, lncRNA UCA1 stimulates ZEB1 and activates EMT mechanism | [317] | |
| LncRNA ZEB1-AS1 | MiR-200c/141 | ZEB1 | Glioma cancer | LncRNA ZEB1-AS1 down-regulates the expression of miR-200c/141 to induce ZEB1 and enhance the malignancy and invasion of cancer cells | [318] |
| MiR-409-3p | ZEB1 | Non-small cell lung cancer | A feedback loop is involved, so that lncRNA ZEB1-AS1 induces ZEB1 through miR-409-3p down-regulation, leading to the metastasis and survival of cancer cells | [319] | |
| MiR-101 | ZEB1 | Colorectal cancer | Elevating the proliferation and migration of cancer cells via down-regulation of MiR-101 and up-regulation of ZEB1 by lncRNA ZEB1-AS1 | [320] | |
| LncRNA MIAT | MiR-150-5p | ZEB1 | Osteosarcoma | The miR-150-5p is down-regulated by MIAT to induce ZEB1 and enhance the malignancy of cancer cells | [321] |
| LncRNA MAGI1-IT1 | MiR-200a | ZEB1/2 | Ovarian cancer | Via competitively binding into miR-200a, lncRNA MAGI1-IT1 enhances the expression of ZEB1/2 to ensure the invasion and metastasis of cancer cells | [293] |
| LncRNA HULC | MiR-200a-3p | ZEB1 | Hepatocellular carcinoma | By sequestering miR-200a-3p, lncRNA HULC stimulates ZEB1 to enhance the malignancy and progression of tumor cells | [322] |
| LncRNA NEAT1 | MiR-204 | ZEB1 | Nasopharyngeal carcinoma | MiR-204 inhibits EMT through ZEB1 down-regulation, and lncRNA NEAT1 reverse this axis to enhance the proliferation and viability of cancer cells | [323] |
| LncRNA MINCR | MiR-223 | ZEB1-Akt/PI3K | Nasopharyngeal carcinoma | MINCR induces ZEB1 by sponging miR-223, resulting in activation of Akt/PI3K and resistance of cancer cells into radiotherapy | [324] |
| LncRNA CAT104 | MiR-381 | ZEB1 | Gastric carcinoma | LncRNA CAT104 down-regulates the expression of miR-381 to enhances ZEB1 levels, resulting in enhanced invasion of cancer cells. Additionally, there is a negative feedback loop between ZEB1 and miR-381. | [325] |
| LncRNA ZNF469-3 | MiR-574-5p | ZEB1 | Triple negative breast cancer | The reverse relationship between ZNF469-3 and miR-574-5p paves the road for up-regulation of ZEB1 and subsequent activation of EMT, leading to the cancer progression and malignancy | [326] |
| Upstream Mediator | MiR | Down-Stream Target | Cancer Type | Major Outcomes | Refs |
|---|---|---|---|---|---|
| ELF3 | MiR-141-3p | ZEB1 | Hepatocellular carcinoma | Overexpression of miR-141-3p down-regulates ZEB1. The ELF3 reduces the expression of miR-141-3p to induce ZEB1 and EMT mechanism | [327] |
| SPROUTY-2 | MiR-200/miR-150 | ZEB1 | Colon cancer | By reducing the expression of miR-200/miR-150, SPROUTY-2 induces ZEB1 to facilitate the mesenchymal phenotype acquisition of cancer cells | [286] |
| STAT3 | MiR-200 | ZEB1 | Invasive breast carcinoma | ZEB1 stimulation by miR-200 down-regulation via STAT3-dependent manner enhances the EMT acquisition in cancer cells | [328] |
| TGF-β1 | MiR-200 | ZEB1/2 | Non-small cell lung cancer | The administration of decitabine induces miR-200 expression through TGF-β1 inhibition to down-regulate ZEB1/2, leading to the suppressing EMT and migration of cancer cells | [329] |
| GRHL2 | MiR-200b/a | ZEB1 | Ovarian cancer | GRHL2 down-regulates the expression of ZEB1 by miR-200a/b overexpression to preserve the epithelial phenotype | [330] |
| 53BP1 | MiR-200b and miR-429 | ZEB1 | Breast cancer | The 53BP1 enhances the expression of miR-200b and miR-429 to elevate E-cadherin levels and suppress EMT mechanism through ZEB1 down-regulation | [331] |
| Mel-18 | MiR-205 | ZEB1/2 | Breast cancer | Mel-18 enhances the expression of miR-205 to inhibit ZEB1/2, resulting in decreased progression and invasion of cancer cells | [332] |
| ΔNp63α | MiR-205 | ZEB1 | Cervical squamous cell carcinoma | ΔNp63α alleviates cancer progression and malignancy by enhancing the expression of miR-205, subsequently down-regulating of ZEB1, and consequently, inhibition of EMT, and enhancing E-cadherin levels | [333] |
| KCNQ1OT1 | MiR-217 | ZEB1 | Colorectal cancer | KCNQ1OT1 inhibits miR-217 to stimulate ZEB1 and EMT mechanism in cancer cells. There is a feedback loop, so that ZEB1 also enhances the expression of KCNQ1OT1 to elevate its inhibitory effect on miR-217 | [334] |
| Circ008913 | MiR-889 | DAB2IP/ZEB1 | Skin carcinogenesis | Arsenite down-regulates the expression of circ008913 to up-regulate miR-889. Then, a decrease occurs in DAB2IP to induce ZEB1 and carcinogenesis | [335] |
| Pituitary tumor-transforming gene 1 | MiR-3666 | ZEB1 | Cervical cancer | The expression of miR-3666 reduces to neutralize its inhibitory impact of ZEB1, and consequently, elevate the metastasis and progression of cancer cells | [289] |
| MiR | Down-Stream Target | Cancer Type | Major Outcomes | Refs |
|---|---|---|---|---|
| MiR-29b | TET1/ZEB2 | Breast cancer | The miR-29b is an oncogene miR that inhibits TET1 to induce ZEB2 expression, leading to the EMT and colony formation of cancer cells | [336] |
| MiR-30a-5p | ZEB2 | Renal cancer | The miR-30a-5p reduces the expression of ZEB2 to be related with desirable prognosis of cancer cells | [337] |
| MiR-101 | ZEB2 | Osteosarcoma | Suppressing the invasion and proliferation of cancer cells through ZEB2 down-regulation | [175] |
| MiR-124 | ZEB2 | Triple negative breast cancer | MiR-124 diminishes the expression of ZEB2 to inhibit the EMT and invasion of cancer cells | [338] |
| MiR-129 | Wnt-β-catenin/ZEB2 | Non-small cell lung cancer | The miR-129 disrupts Wnt/ZEB2 axis to inhibit EMT | [339] |
| MiR-132 | ZEB2 | Colorectal cancer | Reducing the invasion and metastasis of cancer cells through ZEB2 down-regulation | [340] |
| Lung cancer | Diminishing the migration and invasion of cancer cells through ZEB2 inhibition | [341] | ||
| MiR-138 | ZEB2 | Bladder cancer | The miR-138 binds to the 3′-UTR of ZEB2 to inhibit the metastasis and invasion of cancer cells | [289] |
| MiR-141 | ZEB2 | Hepatocellular carcinoma | The miR-141 decreases the expression of ZEB2 to induce apoptosis and diminish viability and proliferation of cancer cells | [342] |
| Renal cancer | The administration of honokiol is associated with miR-141 induction and subsequent downregulation of ZEB2 to inhibit the malignancy of cancer cells | [343] | ||
| MiR-145 | ZEB2 | Non-small cell lung cancer | MiR-145 acts as an onco-suppressor miR that negatively affects the expression of ZEB2 to inhibit the progression and malignancy of cancer cells | [166] |
| Prostate cancer | There is a negative feedback loop between miR-145 and ZEB2, so that overexpression of miR-145 down-regulates the expression of ZEB2 to ensure the reduced viability and proliferation of cancer cells | [344] | ||
| MiR-145-5p | ZEB2 | Gastric cancer | The miR-145-5p decreases the levels of N-cadherin by ZEB2 down-regulation | [345] |
| MiR-153 | ZEB2 | Ovarian cancer | Acting as an onco-suppressor miR and reduces ZEB2 expression to EMT inhibition | [346] |
| MiR-154 | ZEB2 | Non-small cell lung cancer | The miR-154 exerts an anti-tumor impact by ZEB2 down-regulation | [347] |
| Hepatocellular carcinoma | The miR-154 functions as an onco-suppressor miR by inhibition ZEB2 expression and reducing cancer malignancy and proliferation | [348] | ||
| MiR-155 and FOXP3 | ZEB2 | Colorectal cancer | The miR-155 and FOXP3 inhibit ZEB2 expression to suppress EMT via E-cadherin level up-regulation and Vimentin level downregulation | [307] |
| MiR-187 | ZEB2 | Osteosarcoma | The miR-187 decreases the expression of ZEB2 to inhibit the malignancy and migration of tumor cells | [349] |
| MiR-200 | ZEB1/2 | Ovarian cancer | The cancer cells acquire an epithelial phenotype by enhancing the expression of miR-200 and subsequent inhibition of ZEB1 and ZEB2 proteins | [350] |
| ZEB2 | Breast cancer | As an onco-suppressor miR, miR-200 decreases the expression of ZEB2 and its targets gene Snail1 to induce mesenchymal to epithelial transition | [351] | |
| MiR-200a | ZEB2 | Nasopharyngeal carcinoma | Suppressing the growth and invasion of cancer cells through ZEB2 down-regulation | [352] |
| Hepatocellular carcinoma | The miR-200a diminishes the expression of ZEB2 to suppress EMT and invasion of cancer cells | [353] | ||
| Ovarian cancer | The miR-200a increases the levels of E-cadherin by EMT inhibition and ZEB2 down-regulation | [354] | ||
| MiR-200b | ZEB2 | Gastric carcinoma | Inhibition of ZEB2 by miR-200b suppresses invasion, metastasis, and migration of cancer cells | [355] |
| Glioma | Reducing the growth and metastasis of ZEB2 inhibition | [356] | ||
| MiR-200c | ZEB2 | Ovarian cancer | MiR-200c reduces the expression of ZEB2 to inhibit EMT by enhancing E-cadherin levels and reducing Vimentin levels | [357] |
| Non-small cell lung cancer | The miR-200c inhibits EMT mechanism by ZEB2 down-regulation | [358] | ||
| MiR-200c-3p | ZEB2 | Prostate carcinoma | The miR-200c-3p functions as an anti-tumor miR that inhibits the progression and invasion of cancer cells through ZEB2 down-regulation | [359] |
| MiR-203 | ZEB2 | Lung adenocarcinoma and nasopharyngeal carcinoma | MiR-203 enhances the efficacy of cisplatin in chemotherapy and eradication of cancer cells, and also inhibits their invasion by EMT down-regulation through ZEB2 inhibition | [360,361] |
| MiR-205 | ZEB2 | Renal cell carcinoma | The miR-205 is related to the favorable prognosis and reduced invasion of cancer cells through ZEB2 down-regulation | [362] |
| MiR-206 | ZEB2 | Renal cancer | Decreasing the proliferation of tumor cells through ZEB2 down-regulation | [363] |
| MiR-211-5p | ZEB2 | Hepatocellular carcinoma | The miR-211-5p suppresses the metastasis of cancer cells via ZEB2 down-regulation | [215] |
| MiR-215 | ZEB2 | Non-small cell lung cancer | The in vitro and in vivo experiments demonstrate the potential of miR-215 in down-regulation of ZEB2 and suppressing the invasion, progression, and malignancy of cancer cells, and induction of apoptotic cell death | [364] |
| MiR-335 | ZEB2 | Colorectal cancer | The inhibition of metastasis and invasion of cancer cells through ZEB2 down-regulation | [365] |
| Papillary thyroid cancer | Through reducing the expression of ZEB2, miR-335 suppresses the growth and metastasis of cancer cells | [366] | ||
| MiR-338-3p | ZEB2 | Gastric cancer | MiR-338-3p diminishes the expression of ZEB2 to inhibit EMT in cancer cells | [367] |
| MiR-454-3p and miR-374b-5p | ZEB2 | Bladder cancer | Reducing the expression of ZEB2 significantly decreases the migration and invasion of cancer cells | [325] |
| MiR-506 | ZEB2 | Gastric carcinoma | The miR-506 suppresses metastasis through ZEB2 down-regulation | [130] |
| MiR-545 | Wnt-β-catenin/ZEB2 | Non-small cell lung cancer | The miR-545 reduces the expression of Wnt/β−catenin to down-regulate the expression of ZEB2, leading to the decreased migration and invasion of cancer cells | [368] |
| MiR-598 | ZEB2 | Non-small cell lung cancer | The in vitro experiment demonstrated that miR-598 decreases the expression of ZEB2 to inhibit the migration and metastasis of cancer cells | [369] |
| MiR-622 | ZEB2 | Glioma | The increased expression of miR-622 is related to the desirable prognosis via ZEB2 down-regulation | [370] |
| MiR-769-3p | Wnt-β-catenin/ZEB2 | Glioma | The miR-769-3p down-regulates the expression of Wnt and inhibits nuclear translocation of β−catenin to suppress ZEB2, leading to the decreased viability, proliferation and invasion of cancer cells | [371] |
| MiR-940 | ZEB2 | Glioma | Inhibition of cancer progression and EMT through ZEB2 down-regulation | [372] |
| MiR-1179 | ZEB2 | Hepatocellular carcinoma | The miR-1179 reduces the expression of ZEB2 to inhibit cancer progression and malignancy | [373] |
| MiR-3653 | ZEB2 | Colon cancer | Suppressing metastasis and EMT by inhibition of ZEB2 | [171] |
| LncRNA | MiR | Down-Stream Target | Cancer Type | Major Outcomes | Refs |
|---|---|---|---|---|---|
| LncRNA TUG1 | MiR-142 | ZEB2 | Bladder cancer | The lncRNA TUG1 stimulates ZEB2 through miR-142 down-regulation to inhibit apoptosis and enhance the proliferation of cancer cells | [374] |
| LncRNA ROR | MiR-145 | ZEB2 | Hepatocellular carcinoma | The lncRNA ROR elevates the expression of ZEB2 through miR-145 sponging to inhibit the EMT and malignancy of cancer cells | [375] |
| LncRNA MALAT1 | MiR-200s | ZEB2 | Kidney carcinoma | The lncRNA MALAT1 induces ZEB2 via miR-200s sponging, predisposing cancer cells into growth and proliferation | [376] |
| MiR-204 | ZEB2 | Breast cancer | The negative relationship between MALAT1 and miR-204 results in ZEB2 induction to enhance the migration and invasion of cancer cells | [377] | |
| LncRNA UCA1 | MiR-203 | ZEB2 | Gastric cancer | This lncRNA sponges miR-203 to induce ZEB2, leading to the enhanced malignancy, invasion, and proliferation of tumor cells | [187] |
| LncRNA SNHG5 | MiR-205-5p | ZEB2 | Glioma | LncRNA SNHG5 stimulates ZEB2 by sponging miR-205-5p to elevate the proliferation of cancer cells | [194] |
| LncRNA UICLM | MiR-215 | ZEB2 | Colorectal cancer | The in vivo and in vitro experiments demonstrated that lncRNA induces ZEB2 via miR-215 down-regulation to enhance the migration and malignancy of cancer cells | [378] |
| LncRNA SNHG12 | MiR-218 | Slug/ZEB2 | Non-small cell lung cancer | MiR-218 inhibits Slug/ZEB2 axis to suppress EMT in cancer cells. LncRNA SNHG16 activates Slug/ZEB2 axis through miR-218 sponging | [188] |
| LncRNA XIST | MiR-367 and miR-141 | ZEB2 | Non-small cell lung cancer | The lncRNA XIST up-regulates the expression of ZEB2 by inhibition of miR-367 and miR-141, leading to the TGF- β-induced EMT | [379] |
| LncRNA UCA1 | MiR-498 | ZEB2 | Esophageal cancer | The lncRNA UCA1 inhibits ZEB2 via miR-498 down-regulation to suppress the migration, proliferation, invasion, and EMT | [7] |
| LncRNA CTS | MiR-505 | ZEB2 | Cervical cancer | Down-regulation of miR-505 by CTS is associated with increased malignancy of cancer cells through ZEB2 induction | [186] |
| LncRNA HOTAIRM1 | MiR-873-5p | ZEB2 | Glioma | LncRNA HOTAIRM1 decreases the expression of miR-873-5p by sponging to up-regulate the expression of ZEB2, leading to an increase in progression of glioma cells and a decrease in apoptotic cell death | [179] |
| Upstream Mediator | MiR | Down-Stream Target | Cancer Type | Major Outcomes | Refs |
|---|---|---|---|---|---|
| P53 | MiR-30a | ZEB2 | Breast cancer | P53 stimulates the expression of miR-30a to upregulate ZEB2, resulting in reduced viability, proliferation, and invasion of cancer cells | [169] |
| EZH2-DNMT1 | MiR-142-3p | ZEB2 | Nasopharyngeal carcinoma | The EZH2-DNMT1 induces ZEB2 through miR-142-3p sponging, resulting in an increase in cancer progression | [380] |
| CircNUP214 | MiR-145 | ZEB2 | Thyroid cancer | CircNUP214 induces ZEB2 through miR-145 down-regulation to enhance the malignancy and progression of cancer cells | [381] |
| CircPCNXL2 | MiR-153 | ZEB2 | Renal cancer | The circPCNXL2 stimulates the expression of ZEB2 through miR-153 down-regulation to suppress the malignancy and invasion of cancer cells | [201] |
| FOXP3 | MiR-155 | ZEB2 | Human breast cancer | FOXP3 and miR-155 synergistically down-regulate the expression of ZEB2 to diminish the invasiveness of cancer cells | [382] |
| Akt/ERK | MiR-200c | ZEB2 | Gastric cancer | The inhibition of Akt/ERK enhances the expression of miR-200c to suppress IGF-I-mediated ZEB2, leading to the reduced invasion and EMT of cancer cells | [383] |
| β1 integrin | TGF-β/miR-200 | ZEB2 | Triple negative breast cancer | Enhancing the expression of β1 integrin reduces the metastasis of cancer cells into lung. This is followed by disrupting TFG−β/miR-200/ZEB2, elevating the E-cadherin levels, and restoring the cohesion of cells | [384] |
| CircZFR | MiR-377 | ZEB2 | Bladder cancer | Enhanced progression and malignancy of cancer cells result from down-regulation of miR-377 by circZFR and subsequent induction of ZEB2 | [196] |
| Hsa-circ-0004771 | MiR-653 | ZEB2 | Breast cancer | MiR-653 reduces the expression of ZEB2 and is associated with desirable prognosis. Hsa-circ-0004771 diminishes miR-653 expression to induce ZEB2, leading to the inhibition of apoptosis and enhanced migration and invasion of cancer cells | [197] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashrafizadeh, M.; Ang, H.L.; Moghadam, E.R.; Mohammadi, S.; Zarrin, V.; Hushmandi, K.; Samarghandian, S.; Zarrabi, A.; Najafi, M.; Mohammadinejad, R.; et al. MicroRNAs and Their Influence on the ZEB Family: Mechanistic Aspects and Therapeutic Applications in Cancer Therapy. Biomolecules 2020, 10, 1040. https://doi.org/10.3390/biom10071040
Ashrafizadeh M, Ang HL, Moghadam ER, Mohammadi S, Zarrin V, Hushmandi K, Samarghandian S, Zarrabi A, Najafi M, Mohammadinejad R, et al. MicroRNAs and Their Influence on the ZEB Family: Mechanistic Aspects and Therapeutic Applications in Cancer Therapy. Biomolecules. 2020; 10(7):1040. https://doi.org/10.3390/biom10071040
Chicago/Turabian StyleAshrafizadeh, Milad, Hui Li Ang, Ebrahim Rahmani Moghadam, Shima Mohammadi, Vahideh Zarrin, Kiavash Hushmandi, Saeed Samarghandian, Ali Zarrabi, Masoud Najafi, Reza Mohammadinejad, and et al. 2020. "MicroRNAs and Their Influence on the ZEB Family: Mechanistic Aspects and Therapeutic Applications in Cancer Therapy" Biomolecules 10, no. 7: 1040. https://doi.org/10.3390/biom10071040
APA StyleAshrafizadeh, M., Ang, H. L., Moghadam, E. R., Mohammadi, S., Zarrin, V., Hushmandi, K., Samarghandian, S., Zarrabi, A., Najafi, M., Mohammadinejad, R., & Kumar, A. P. (2020). MicroRNAs and Their Influence on the ZEB Family: Mechanistic Aspects and Therapeutic Applications in Cancer Therapy. Biomolecules, 10(7), 1040. https://doi.org/10.3390/biom10071040







