Alanyl-Glutamine Restores Tight Junction Organization after Disruption by a Conventional Peritoneal Dialysis Fluid
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Transendothelial Resistance Measurements
2.3. Paracellular Dextran Transport Assessment
2.4. Western Blot
2.5. Mouse Model
2.6. Immunohistochemistry
2.7. Digital Image Analysis
2.8. Immunofluorescence Staining
2.9. Single Molecule Localization Microscopy (SMLM)
2.10. SMLM Data Analysis
2.11. Statistics
3. Results
3.1. AlaGln Increases Transepithelial Resistance and Reduces 10 kDa and 70 kDa Transport In Vitro
3.2. AlaGln Preserves ZO-1 and CLDN5 Abundance in CPDF Treated HUVEC
3.3. Junction Complex Organization is Altered after Alagln Incubation
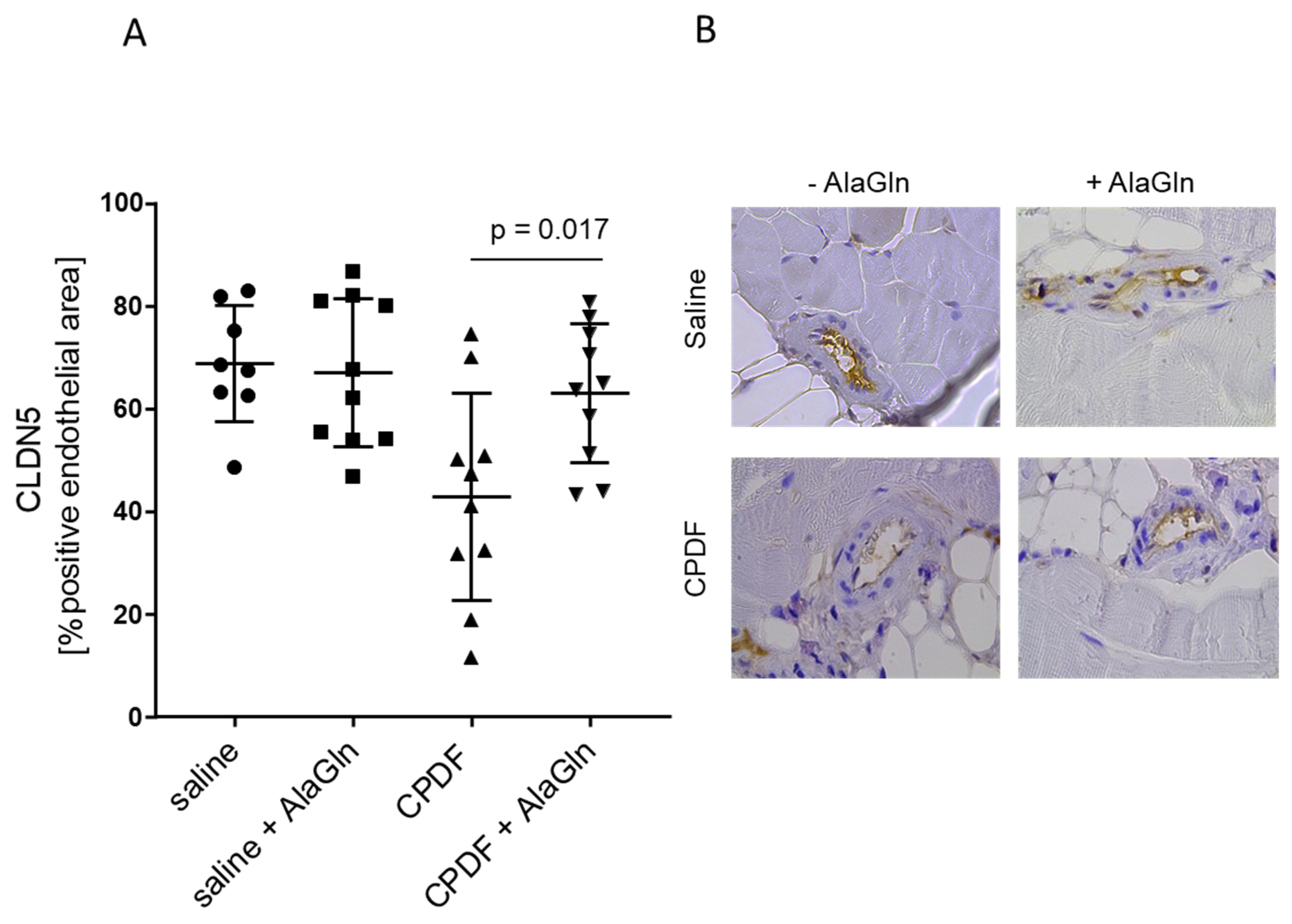
3.4. In Vivo Effect of AlaGln Supplementation to CPDF
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McDonald, S.P.; Marshall, M.R.; Johnson, D.W.; Polkinghorne, K.R. Relationship between dialysis modality and mortality. J. Am. Soc. Nephrol. 2009, 20, 155–163. [Google Scholar] [CrossRef]
- Waldum-Grevbo, B.; Leivestad, T.; Reisaeter, A.V.; Os, I. Impact of initial dialysis modality on mortality: A propensity-matched study. BMC Nephrol. 2015, 16, 179. [Google Scholar] [CrossRef] [PubMed]
- van de Luijtgaarden, M.W.; Jager, K.J.; Segelmark, M.; Pascual, J.; Collart, F.; Hemke, A.C.; Remon, C.; Metcalfe, W.; Miguel, A.; Kramar, R.; et al. Trends in dialysis modality choice and related patient survival in the ERA-EDTA Registry over a 20-year period. Nephrol. Dial. Transpl. 2016, 31, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Guedes, A.M. Peritoneal Protein Loss, Leakage or Clearance in Peritoneal Dialysis, Where Do We Stand? Perit. Dial. Int. 2019, 39, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Ortega, L.M.; Materson, B.J. Hypertension in peritoneal dialysis patients: Epidemiology, pathogenesis, and treatment. J. Am. Soc. Hypertens. 2011, 5, 128–136. [Google Scholar] [CrossRef]
- Mehrotra, R.; Devuyst, O.; Davies, S.J.; Johnson, D.W. The Current State of Peritoneal Dialysis. J. Am. Soc. Nephrol. 2016, 27, 3238–3252. [Google Scholar] [CrossRef]
- Li, P.K.; Chow, K.M.; Van de Luijtgaarden, M.W.; Johnson, D.W.; Jager, K.J.; Mehrotra, R.; Naicker, S.; Pecoits-Filho, R.; Yu, X.Q.; Lameire, N. Changes in the worldwide epidemiology of peritoneal dialysis. Nat. Rev. Nephrol. 2017, 13, 90–103. [Google Scholar] [CrossRef]
- Bartosova, M.; Schmitt, C.P. Biocompatible Peritoneal Dialysis: The Target Is Still Way Off. Front. Physiol. 2018, 9, 1853. [Google Scholar] [CrossRef]
- Chang, T.I.; Kang, E.W.; Lee, Y.K.; Shin, S.K. Higher peritoneal protein clearance as a risk factor for cardiovascular disease in peritoneal dialysis patient. PLoS ONE 2013, 8, e56223. [Google Scholar] [CrossRef]
- Dong, J.; Chen, Y.; Luo, S.; Xu, R.; Xu, Y. Peritoneal protein leakage, systemic inflammation, and peritonitis risk in patients on peritoneal dialysis. Perit. Dial. Int. 2013, 33, 273–279. [Google Scholar] [CrossRef]
- Rajakaruna, G.; Caplin, B.; Davenport, A. Peritoneal protein clearance rather than faster transport status determines outcomes in peritoneal dialysis patients. Perit. Dial. Int. 2015, 35, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Perl, J.; Huckvale, K.; Chellar, M.; John, B.; Davies, S.J. Peritoneal protein clearance and not peritoneal membrane transport status predicts survival in a contemporary cohort of peritoneal dialysis patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Balafa, O.; Halbesma, N.; Struijk, D.G.; Dekker, F.W.; Krediet, R.T. Peritoneal albumin and protein losses do not predict outcome in peritoneal dialysis patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.D.; Craig, K.J.; Topley, N.; Von Ruhland, C.; Fallon, M.; Newman, G.R.; Mackenzie, R.K.; Williams, G.T. Morphologic changes in the peritoneal membrane of patients with renal disease. J. Am. Soc. Nephrol. 2002, 13, 470–479. [Google Scholar] [PubMed]
- Zeier, M.; Schwenger, V.; Deppisch, R.; Haug, U.; Weigel, K.; Bahner, U.; Wanner, C.; Schneider, H.; Henle, T.; Ritz, E. Glucose degradation products in PD fluids: Do they disappear from the peritoneal cavity and enter the systemic circulation? Kidney Int. 2003, 63, 298–305. [Google Scholar] [CrossRef]
- Schmitt, C.P.; von Heyl, D.; Rieger, S.; Arbeiter, K.; Bonzel, K.E.; Fischbach, M.; Misselwitz, J.; Pieper, A.K.; Schaefer, F. Reduced systemic advanced glycation end products in children receiving peritoneal dialysis with low glucose degradation product content. Nephrol. Dial. Transpl. 2007, 22, 2038–2044. [Google Scholar] [CrossRef]
- Schaefer, B.; Bartosova, M.; Macher-Goeppinger, S.; Sallay, P.; Voros, P.; Ranchin, B.; Vondrak, K.; Ariceta, G.; Zaloszyc, A.; Bayazit, A.K.; et al. Neutral pH and low-glucose degradation product dialysis fluids induce major early alterations of the peritoneal membrane in children on peritoneal dialysis. Kidney Int. 2018, 94, 419–429. [Google Scholar] [CrossRef]
- Johnson, D.W.; Brown, F.G.; Clarke, M.; Boudville, N.; Elias, T.J.; Foo, M.W.; Jones, B.; Kulkarni, H.; Langham, R.; Ranganathan, D.; et al. Effects of biocompatible versus standard fluid on peritoneal dialysis outcomes. J. Am. Soc. Nephrol. 2012, 23, 1097–1107. [Google Scholar] [CrossRef]
- Johnson, D.W.; Brown, F.G.; Clarke, M.; Boudville, N.; Elias, T.J.; Foo, M.W.; Jones, B.; Kulkarni, H.; Langham, R.; Ranganathan, D.; et al. The effect of low glucose degradation product, neutral pH versus standard peritoneal dialysis solutions on peritoneal membrane function: The balANZ trial. Nephrol. Dial. Transpl. 2012, 27, 4445–4453. [Google Scholar] [CrossRef]
- Cho, Y.; Johnson, D.W.; Craig, J.C.; Strippoli, G.F.; Badve, S.V.; Wiggins, K.J. Biocompatible dialysis fluids for peritoneal dialysis. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef]
- Htay, H.; Johnson, D.W.; Wiggins, K.J.; Badve, S.V.; Craig, J.C.; Strippoli, G.F.; Cho, Y. Biocompatible dialysis fluids for peritoneal dialysis. Cochrane Database Syst. Rev. 2018, 10, Cd007554. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.P.; Aufricht, C. Is there such a thing as biocompatible peritoneal dialysis fluid? Pediatr. Nephrol. 2017, 32, 1835–1843. [Google Scholar] [CrossRef] [PubMed]
- Kratochwill, K.; Boehm, M.; Herzog, R.; Lichtenauer, A.M.; Salzer, E.; Lechner, M.; Kuster, L.; Bergmeister, K.; Rizzi, A.; Mayer, B.; et al. Alanyl-glutamine dipeptide restores the cytoprotective stress proteome of mesothelial cells exposed to peritoneal dialysis fluids. Nephrol. Dial. Transpl. 2012, 27, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Wiesenhofer, F.M.; Herzog, R.; Boehm, M.; Wagner, A.; Unterwurzacher, M.; Kasper, D.C.; Alper, S.L.; Vychytil, A.; Aufricht, C.; Kratochwill, K. Targeted Metabolomic Profiling of Peritoneal Dialysis Effluents Shows Anti-oxidative Capacity of Alanyl-Glutamine. Front. Physiol. 2018, 9, 1961. [Google Scholar] [CrossRef]
- Ferrantelli, E.; Liappas, G.; Vila Cuenca, M.; Keuning, E.D.; Foster, T.L.; Vervloet, M.G.; Lopez-Cabrera, M.; Beelen, R.H. The dipeptide alanyl-glutamine ameliorates peritoneal fibrosis and attenuates IL-17 dependent pathways during peritoneal dialysis. Kidney Int. 2016, 89, 625–635. [Google Scholar] [CrossRef]
- Kratochwill, K.; Boehm, M.; Herzog, R.; Gruber, K.; Lichtenauer, A.M.; Kuster, L.; Csaicsich, D.; Gleiss, A.; Alper, S.L.; Aufricht, C.; et al. Addition of Alanyl-Glutamine to Dialysis Fluid Restores Peritoneal Cellular Stress Responses—A First-In-Man Trial. PLoS ONE 2016, 11, e0165045. [Google Scholar] [CrossRef]
- Vychytil, A.; Herzog, R.; Probst, P.; Ribitsch, W.; Lhotta, K.; Machold-Fabrizii, V.; Wiesholzer, M.; Kaufmann, M.; Salmhofer, H.; Windpessl, M.; et al. A randomized controlled trial of alanyl-glutamine supplementation in peritoneal dialysis fluid to assess impact on biomarkers of peritoneal health. Kidney Int. 2018, 94, 1227–1237. [Google Scholar] [CrossRef]
- Krediet, R.T.; Lindholm, B.; Rippe, B. Pathophysiology of peritoneal membrane failure. Perit Dial. Int. 2000, 20, S22–S42. [Google Scholar]
- Horiuchi, T.; Matsunaga, K.; Banno, M.; Nakano, Y.; Nishimura, K.; Hanzawa, C.; Miyamoto, K.; Nomura, S.; Ohta, Y. HPMCs induce greater intercellular delocalization of tight junction-associated proteins due to a higher susceptibility to H2O2 compared with HUVECs. Perit. Dial. Int. 2009, 29, 217–226. [Google Scholar] [CrossRef]
- Rippe, B. A three-pore model of peritoneal transport. Perit. Dial. Int. 1993, 13, S35–S38. [Google Scholar] [CrossRef]
- Stachowska-Pietka, J.; Waniewski, J.; Flessner, M.F.; Lindholm, B. Computer simulations of osmotic ultrafiltration and small-solute transport in peritoneal dialysis: A spatially distributed approach. Am. J. Physiol Ren. Physiol. 2012, 302, F1331–F1341. [Google Scholar] [CrossRef] [PubMed]
- Devuyst, O.; Rippe, B. Water transport across the peritoneal membrane. Kidney Int. 2014, 85, 750–758. [Google Scholar] [CrossRef]
- Rippe, B.; Davies, S. Permeability of peritoneal and glomerular capillaries: What are the differences according to pore theory? Perit. Dial. Int. 2011, 31, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Rippe, B.; Rosengren, B.I.; Venturoli, D. The peritoneal microcirculation in peritoneal dialysis. Microcirculation 2001, 8, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Verbavatz, J.M.; Rippe, A.; Boisde, I.; Moulin, P.; Rippe, B.; Verkman, A.S.; Devuyst, O. Aquaporin-1 plays an essential role in water permeability and ultrafiltration during peritoneal dialysis. Kidney Int. 2006, 69, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Stefanidis, I.; Zarogiannis, S.; Hatzoglou, C.; Liakopoulos, V.; Kourti, P.; Poultsidi, A.; Mertens, P.R.; Gourgoulianis, K.; Molyvdas, P.A. Enhancement of the transmesothelial resistance of the parietal sheep peritoneum by epinephrine in vitro: Ussing-type chamber experiments. Artif. Organs 2005, 29, 919–922. [Google Scholar] [CrossRef]
- Kourti, P.; Zarogiannis, S.G.; Liakopoulos, V.; Karioti, A.; Eleftheriadis, T.; Hatzoglou, C.; Gourgoulianis, K.; Molyvdas, P.A.; Stefanidis, I. Endothelin-1 acutely reduces the permeability of visceral sheep peritoneum in vitro through both endothelin-A and endothelin-B receptors. Artif. Organs 2013, 37, 308–312. [Google Scholar] [CrossRef]
- Stefanidis, I.; Liakopoulos, V.; Kourti, P.; Zarogiannis, S.; Poultsidi, A.; Mertems, P.R.; Salmas, M.; Hatzoglou, C.; Gourgoulianis, K.; Molyvdas, P.A. Amiloride-sensitive sodium channels on the parietal human peritoneum: Evidence by ussing-type chamber experiments. ASAIO J. 2007, 53, 335–338. [Google Scholar] [CrossRef]
- Amasheh, S.; Schmidt, T.; Mahn, M.; Florian, P.; Mankertz, J.; Tavalali, S.; Gitter, A.H.; Schulzke, J.D.; Fromm, M. Contribution of claudin-5 to barrier properties in tight junctions of epithelial cells. Cell Tissue Res. 2005, 321, 89–96. [Google Scholar] [CrossRef]
- Van Itallie, C.M.; Tietgens, A.J.; Anderson, J.M. Visualizing the dynamic coupling of claudin strands to the actin cytoskeleton through ZO-1. Mol. Biol. Cell 2017, 28, 524–534. [Google Scholar] [CrossRef]
- Rodgers, L.S.; Beam, M.T.; Anderson, J.M.; Fanning, A.S. Epithelial barrier assembly requires coordinated activity of multiple domains of the tight junction protein ZO-1. J. Cell Sci. 2013, 126, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Mateo, G.T.; Loureiro, J.; Jimenez-Hefferman, J.A.; Bajo, M.A.; Selgas, R.; Lopez-Cabrera, M.; Aroeira, L.S. Chronic exposure of mouse peritoneum to peritoneal dialysis fluid: Structural and functional alterations of the peritoneal membrane. Perit. Dial. Int. 2009, 29, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Harvie, M.C.G.; McManus, D.P.; You, H.; Rivera, V.A.; Nawaratna, S.K.; MacDonald, K.P.A.; Ramm, G.A.; Gobert, G.N. Live imaging of collagen deposition during experimental hepatic schistosomiasis and recovery: A view on a dynamic process. Lab. Investig. 2019, 99, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Abramoff, M.D.; Magalhaes, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Eryilmaz, M.; Schmitt, E.; Krufczik, M.; Theda, F.; Lee, J.H.; Cremer, C.; Bestvater, F.; Schaufler, W.; Hausmann, M.; Hildenbrand, G. Localization Microscopy Analyses of MRE11 Clusters in 3D-Conserved Cell Nuclei of Different Cell Lines. Cancers 2018, 10, 25. [Google Scholar] [CrossRef]
- Hausmann, M.; Wagner, E.; Lee, J.H.; Schrock, G.; Schaufler, W.; Krufczik, M.; Papenfuß, F.; Port, M.; Bestvater, F.; Scherthan, H. Super-resolution localization microscopy of radiation-induced histone H2AX-phosphorylation in relation to H3K9-trimethylation in HeLa cells. Nanoscale 2018, 10, 4320–4331. [Google Scholar] [CrossRef]
- Hausmann, M.; Ilic, N.; Pilarczyk, G.; Lee, J.H.; Logeswaran, A.; Borroni, A.P.; Krufczik, M.; Theda, F.; Waltrich, N.; Bestvater, F.; et al. Challenges for Super-Resolution Localization Microscopy and Biomolecular Fluorescent Nano-Probing in Cancer Research. Int. J. Mol. Sci. 2017, 18, 2066. [Google Scholar] [CrossRef]
- Lemmer, P.; Gunkel, M.; Baddeley, D.; Kaufmann, R.; Urich, A.; Weiland, Y.; Reymann, J.; Müller, P.; Hausmann, M.; Cremer, C. SPDM: Light microscopy with single-molecule resolution at the nanoscale. Appl. Phys. B 2008, 93, 1. [Google Scholar] [CrossRef]
- Bender, T.O.; Bohm, M.; Kratochwill, K.; Vargha, R.; Riesenhuber, A.; Witowski, J.; Jorres, A.; Wieslander, A.; Aufricht, C. Peritoneal dialysis fluids can alter HSP expression in human peritoneal mesothelial cells. Nephrol. Dial. Transpl. 2011, 26, 1046–1052. [Google Scholar] [CrossRef]
- Heyland, D.; Muscedere, J.; Wischmeyer, P.E.; Cook, D.; Jones, G.; Albert, M.; Elke, G.; Berger, M.M.; Day, A.G. A randomized trial of glutamine and antioxidants in critically ill patients. N. Engl. J. Med. 2013, 368, 1489–1497. [Google Scholar] [CrossRef]
- Boyd, P.S.; Struve, N.; Bach, M.; Eberle, J.P.; Gote, M.; Schock, F.; Cremer, C.; Kriegs, M.; Hausmann, M. Clustered localization of EGFRvIII in glioblastoma cells as detected by high precision localization microscopy. Nanoscale 2016, 8, 20037–20047. [Google Scholar] [CrossRef] [PubMed]
- Gote, M.; Neitzel, C.; Bobkova, E.; Falková, E.; Falk, M.; Hausmann, M. Advanced DNA repair focus analyses by complex multiscale characterization of nano-distance frequency histograms. (unpublished; manuscript in preparation).
- Schaefer, B.; Bartosova, M.; Macher-Goeppinger, S.; Ujszaszi, A.; Wallwiener, M.; Nyarangi-Dix, J.; Sallay, P.; Burkhardt, D.; Querfeld, U.; Pfeifle, V.; et al. Quantitative Histomorphometry of the Healthy Peritoneum. Sci. Rep. 2016, 6, 21344. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.; Chang, M.; Liu, S.; Niu, M.; Zhang, Y.; Liu, X.; Yu, X. Peritoneal microvascular endothelial function and the microinflammatory state are associated with baseline peritoneal transport characteristics in uremic patients. Int. Urol. Nephrol. 2015, 47, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Morita, K.; Sasaki, H.; Furuse, M.; Tsukita, S. Endothelial claudin: Claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J. Cell Biol. 1999, 147, 185–194. [Google Scholar] [CrossRef]
- Lynn, K.S.; Peterson, R.J.; Koval, M. Ruffles and spikes: Control of tight junction morphology and permeability by claudins. Biochim. Et Biophys. Acta. Biomembr. 2020, 1862, 183339. [Google Scholar] [CrossRef]
- Retana, C.; Sanchez, E.; Perez-Lopez, A.; Cruz, A.; Lagunas, J.; Cruz, C.; Vital, S.; Reyes, J.L. Alterations of intercellular junctions in peritoneal mesothelial cells from patients undergoing dialysis: Effect of retinoic Acid. Perit. Dial. Int. 2015, 35, 275–287. [Google Scholar] [CrossRef]
- Li, N.; Lewis, P.; Samuelson, D.; Liboni, K.; Neu, J. Glutamine regulates Caco-2 cell tight junction proteins. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G726–G733. [Google Scholar] [CrossRef]
- Seth, A.; Basuroy, S.; Sheth, P.; Rao, R.K. L-Glutamine ameliorates acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G510–G517. [Google Scholar] [CrossRef]
- Kratochwill, K.; Lechner, M.; Siehs, C.; Lederhuber, H.C.; Rehulka, P.; Endemann, M.; Kasper, D.C.; Herkner, K.R.; Mayer, B.; Rizzi, A.; et al. Stress responses and conditioning effects in mesothelial cells exposed to peritoneal dialysis fluid. J. Proteome Res. 2009, 8, 1731–1747. [Google Scholar] [CrossRef]
- Boehm, M.; Herzog, R.; Klinglmüller, F.; Lichtenauer, A.M.; Wagner, A.; Unterwurzacher, M.; Beelen, R.H.J.; Alper, S.L.; Aufricht, C.; Kratochwill, K. The Peritoneal Surface Proteome in a Model of Chronic Peritoneal Dialysis Reveals Mechanisms of Membrane Damage and Preservation. Front. Physiol. 2019, 10, 472. [Google Scholar] [CrossRef]
- Krug, S.M.; Fromm, M. Special Issue on “The Tight Junction and Its Proteins: More than Just a Barrier”. Int. J. Mol. Sci. 2020, 21, 4612. [Google Scholar] [CrossRef] [PubMed]
- Lal-Nag, M.; Morin, P.J. The claudins. Genome Biol. 2009, 10, 235. [Google Scholar] [CrossRef] [PubMed]
- Rom, S.; Heldt, N.A.; Gajghate, S.; Seliga, A.; Reichenbach, N.L.; Persidsky, Y. Hyperglycemia and advanced glycation end products disrupt BBB and promote occludin and claudin-5 protein secretion on extracellular microvesicles. Sci. Rep. 2020, 10, 7274. [Google Scholar] [CrossRef] [PubMed]
- Huber, P. Targeting of the apical junctional complex by bacterial pathogens. Biochim. Et Biophys. Acta. Biomembr. 2020, 1862, 183237. [Google Scholar] [CrossRef]
- Debray-Garcia, Y.; Sanchez, E.I.; Rodriguez-Munoz, R.; Venegas, M.A.; Velazquez, J.; Reyes, J.L. Diabetes and exposure to peritoneal dialysis solutions alter tight junction proteins and glucose transporters of rat peritoneal mesothelial cells. Life Sci. 2016, 161, 78–89. [Google Scholar] [CrossRef]
- Lan, P.G.; Johnson, D.W.; McDonald, S.P.; Boudville, N.; Borlace, M.; Badve, S.V.; Sud, K.; Clayton, P.A. The association between peritoneal dialysis modality and peritonitis. Clin. J. Am. Soc. Nephrol. 2014, 9, 1091–1097. [Google Scholar] [CrossRef]

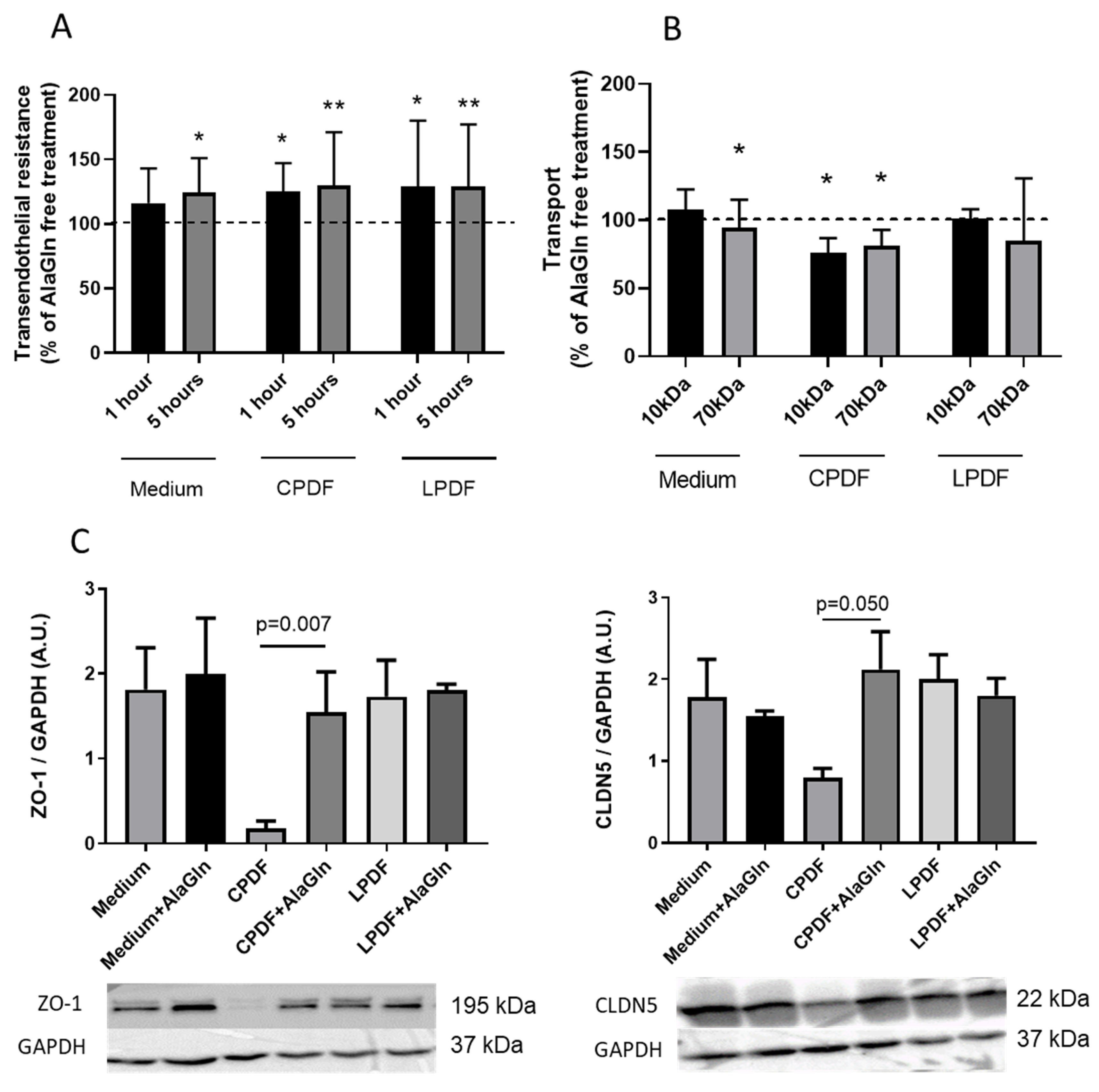

| Medium [%] | CPDF [%] | LPDF [%] | ||
|---|---|---|---|---|
| Resistance | 1 h | 116 ± 27 | 125 ± 22 * | 129 ± 51 * |
| 5 h | 123 ± 27 * | 130 ± 41 ** | 125 ± 30 ** | |
| Transport | 10 kDa | 107 ± 15 | 76 ± 11 * | 101 ± 7 |
| 70 kDa | 86 ± 20 * | 81 ± 12 * | 84 ± 46 |
| Saline (n = 10) | Saline + AlaGln (n = 9) | CPDF (n = 11) | CPDF + AlaGln (n = 11) | ANOVA | |
|---|---|---|---|---|---|
| Peritoneal thickness [µm] (IQR) | 30.1 (12, 55) | 12.2 (11, 27) | 32 (29, 54) | 44.2 (29, 66) | 0.72 |
| Collagen submesothelial area [%] | 7.1 (2.2, 19.1) | 19.6 (13.1, 29.9) | 18.5 (4.3, 39.2) | 3.0 (1.9, 10.9) * | 0.73 |
| Microvessel density [/mm2] | 47.8 (23, 83) | 16.6 (4, 119) | 33.2 (13, 89) | 60.4 (35, 73) | 0.39 |
| Microvessel number [/mm section length] | 1.2 (0.6, 2.7) | 0.5 (0.1, 1.3) | 0.7 (0.5, 4.8) | 2.2 (1.1, 4.0) | 0.90 |
| Cell density [/mm2] | 12544 (8032, 18970) | 9037 * (4833, 11115) | 6008 # (2861, 8885) | 8801 (6863, 11642) | 0.64 |
| Cell number [/mm section length] | 614 (127, 755) | 132 (63, 451) | 245 (92, 345) | 423 (304, 542) | 0.42 |
| Drained effluent [ml] | 1.3 (1.0, 1.4) | 1.4 (1.3, 1.5) | 2.2 (1.9, 2.5) ## | 1.8 (1.5, 2.2) | 0.45 |
| Effluent cells [× 106 cells/mL effluent] | 6.9 (4.9, 11.9) | 4.9 (3.0, 8.4) | 9.9 (3.8, 20.1) | 9.5 (4.5, 16.6) | 0.11 |
| Arteriolar CLDN5 [% pos. area] | 38 ± 9 | 45 ± 17 | 24 ± 8 ## | 32 ± 9 ** | 0.04 |
| Endothelial CLDN5 [% pos. area] | 69 ± 11 | 67 ± 14 | 43 ± 20 ## | 63 ± 14 ** | 0.07 |
| Mesothelial CLDN5 [% pos. area] | 58 ± 28 | 51 ± 28 | 34 ± 12 # | 29 ± 14 | 0.56 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartosova, M.; Herzog, R.; Ridinger, D.; Levai, E.; Jenei, H.; Zhang, C.; González Mateo, G.T.; Marinovic, I.; Hackert, T.; Bestvater, F.; et al. Alanyl-Glutamine Restores Tight Junction Organization after Disruption by a Conventional Peritoneal Dialysis Fluid. Biomolecules 2020, 10, 1178. https://doi.org/10.3390/biom10081178
Bartosova M, Herzog R, Ridinger D, Levai E, Jenei H, Zhang C, González Mateo GT, Marinovic I, Hackert T, Bestvater F, et al. Alanyl-Glutamine Restores Tight Junction Organization after Disruption by a Conventional Peritoneal Dialysis Fluid. Biomolecules. 2020; 10(8):1178. https://doi.org/10.3390/biom10081178
Chicago/Turabian StyleBartosova, Maria, Rebecca Herzog, David Ridinger, Eszter Levai, Hanna Jenei, Conghui Zhang, Guadalupe T. González Mateo, Iva Marinovic, Thilo Hackert, Felix Bestvater, and et al. 2020. "Alanyl-Glutamine Restores Tight Junction Organization after Disruption by a Conventional Peritoneal Dialysis Fluid" Biomolecules 10, no. 8: 1178. https://doi.org/10.3390/biom10081178
APA StyleBartosova, M., Herzog, R., Ridinger, D., Levai, E., Jenei, H., Zhang, C., González Mateo, G. T., Marinovic, I., Hackert, T., Bestvater, F., Hausmann, M., López Cabrera, M., Kratochwill, K., Zarogiannis, S. G., & Schmitt, C. P. (2020). Alanyl-Glutamine Restores Tight Junction Organization after Disruption by a Conventional Peritoneal Dialysis Fluid. Biomolecules, 10(8), 1178. https://doi.org/10.3390/biom10081178







