The Protective Role of Decorin in Hepatic Metastasis of Colorectal Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Microarray (TMA)
2.2. Plasmid Preparation
2.3. Animal Experiments
2.4. Immunostaining
2.5. Tissue Culture and Reagents
2.6. Western Blot, Dot Blot and Array Analysis
2.7. Real-Time qPCR
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Statistical Analysis
3. Results
3.1. Decorin Expression Decreased in Liver Metastases of Human CRC
3.2. Decorin Expression of Myofibroblasts Is Inhibited by Tumor Cells In Vitro
3.3. Overexpressed Decorin Reduces Tumor Formation in an Experimental Mouse Model
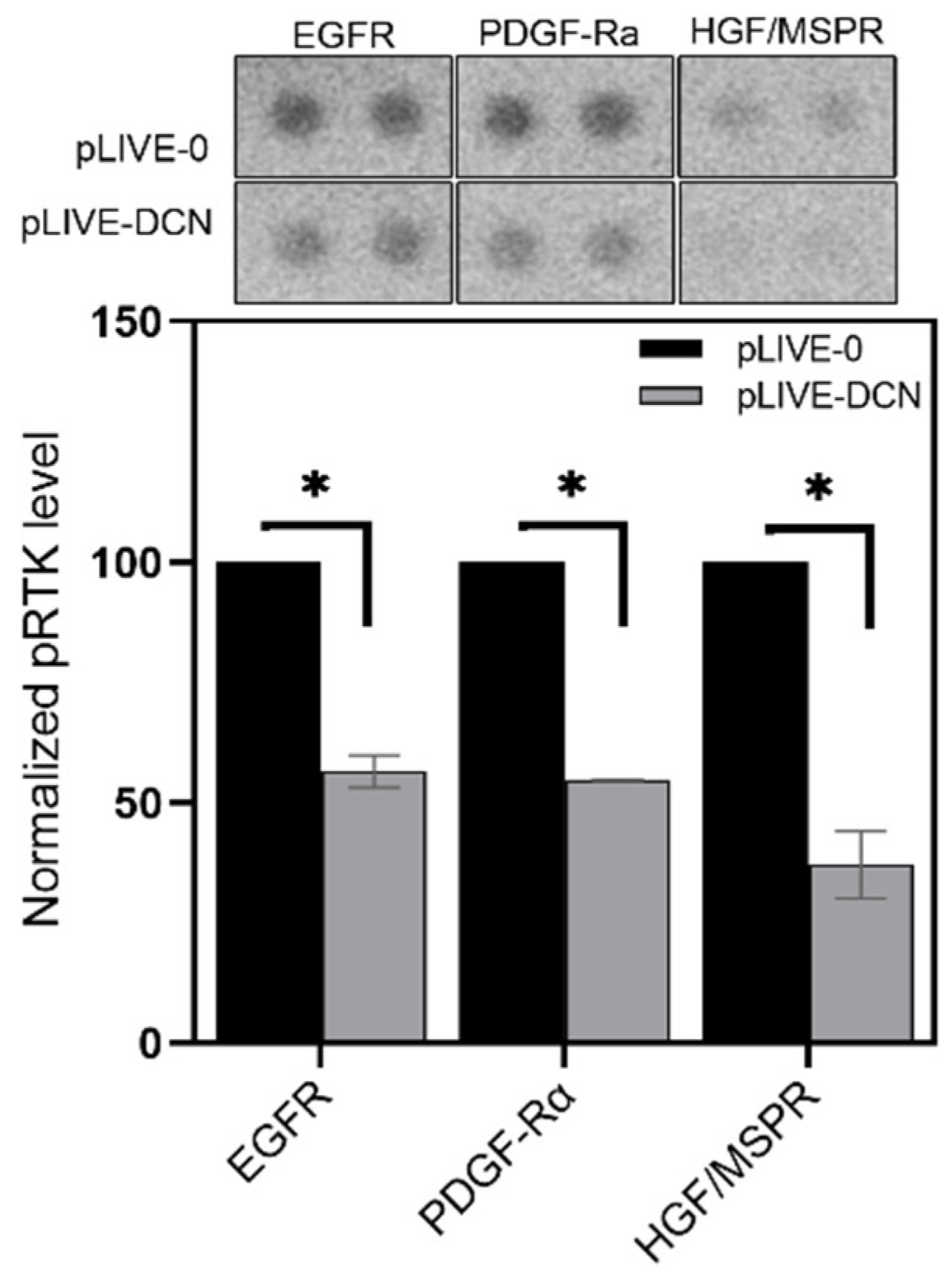
3.4. Signaling Pathways Affected by Decorin in Mouse Liver Metastases
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Farhood, B.; Raei, B.; Malekzadeh, R.; Shirvani, M.; Najafi, M.; Mortezazadeh, T.; Ameri, H.; Alizadeh, A. A review of incidence and mortality of colorectal, lung, liver, thyroid, and bladder cancers in Iran and compared to other countries. Współcz. Onkol. 2019, 23, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Golubnitschaja, O.; Sridhar, K.C. Liver metastatic disease: New concepts and biomarker panels to improve individual outcomes. Clin. Exp. Metast. 2016, 33, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Chow, F.C.-L.; Chok, K.S.H. Colorectal liver metastases: An update on multidisciplinary approach. World J. Hepatol. 2019, 11, 150–172. [Google Scholar] [CrossRef]
- Martinelli, E.; Ciardiello, F.; Martini, G.; Troiani, T.; Cardone, C.; Vitiello, P.; Normanno, N.; Rachiglio, A.; Maiello, E.; Latiano, T.; et al. Implementing anti-epidermal growth factor receptor (EGFR) therapy in metastatic colorectal cancer: Challenges and future perspectives. Ann. Oncol. 2020, 31, 30–40. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Baiocchini, A.; Montaldo, C.; Conigliaro, A.; Grimaldi, A.; Correani, V.; Mura, F.; Ciccosanti, F.; Rotiroti, N.; Brenna, A.; Montalbano, M.; et al. Extracellular Matrix Molecular Remodeling in Human Liver Fibrosis Evolution. PLoS ONE 2016, 11, e0151736. [Google Scholar] [CrossRef]
- Arriazu, E.; De Galarreta, M.R.; Cubero, F.J.; Varela-Rey, M.; De Obanos, M.P.P.; Leung, T.M.; Lopategi, A.; Benedicto, A.; Abraham-Enachescu, I.; Nieto, N. Extracellular Matrix and Liver Disease. Antioxid. Redox Signal. 2014, 21, 1078–1097. [Google Scholar] [CrossRef]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef]
- Wells, R.G. Cellular Sources of Extracellular Matrix in Hepatic Fibrosis. Clin. Liver Dis. 2008, 12, 759–768. [Google Scholar] [CrossRef]
- Neill, T.; Schaefer, L.; Iozzo, R.V. Decorin. Am. J. Pathol. 2012, 181, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Krusius, T.; Ruoslahti, E. Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proc. Natl. Acad. Sci. USA 1986, 83, 7683–7687. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, Z.; Lazaro, L.D.; Iozzo, R.V.; Höök, M.; Grande-Allen, K.J. Influence of cyclic strain and decorin deficiency on 3D cellularized collagen matrices. Biomaterials 2008, 29, 2740–2748. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rühland, C.; Schönherr, E.; Robenek, H.; Hansen, U.; Iozzo, R.V.; Bruckner, P.; Seidler, D.G. The glycosaminoglycan chain of decorin plays an important role in collagen fibril formation at the early stages of fibrillogenesis. FEBS J. 2007, 274, 4246–4255. [Google Scholar] [CrossRef]
- Weis, S.M.; Zimmerman, S.D.; Shah, M.; Covell, J.W.; Omens, J.H.; Rossjr, J.; Dalton, N.; Jones, Y.; Reed, C.C.; Iozzo, R.V.; et al. A role for decorin in the remodeling of myocardial infarction. Matrix Boil. 2005, 24, 313–324. [Google Scholar] [CrossRef]
- Brandan, E.; Cabello-Verrugio, C.; Vial, C. Novel regulatory mechanisms for the proteoglycans decorin and biglycan during muscle formation and muscular dystrophy. Matrix Boil. 2008, 27, 700–708. [Google Scholar] [CrossRef]
- Järveläinen, H.; Puolakkainen, P.; Pakkanen, S.H.; Brown, E.L.; Höök, M.; Iozzo, R.V.; Sage, E.; Wight, T.N. A role for decorin in cutaneous wound healing and angiogenesis. Wound Repair Regen. 2006, 14, 443–452. [Google Scholar] [CrossRef]
- Schaefer, L.; Macakova, K.; Raslik, I.; Micegova, M.; Gröne, H.-J.; Schönherr, E.; Robenek, H.; Echtermeyer, F.G.; Grässel, S.; Bruckner, P.; et al. Absence of Decorin Adversely Influences Tubulointerstitial Fibrosis of the Obstructed Kidney by Enhanced Apoptosis and Increased Inflammatory Reaction. Am. J. Pathol. 2002, 160, 1181–1191. [Google Scholar] [CrossRef]
- Breitkopf, K.; Van Roeyen, C.; Sawitza, I.; Wickert, L.; Floege, J.; Gressner, A.M. Expression patterns of PDGF-A, -B, -C and -D and the PDGF-receptors α and β in activated rat hepatic stellate cells (HSC). Cytokine 2005, 31, 349–357. [Google Scholar] [CrossRef]
- Schaefer, L.; Tredup, C.; Gubbiotti, M.A.; Iozzo, R.V. Proteoglycan neofunctions: Regulation of inflammation and autophagy in cancer biology. FEBS J. 2016, 284, 10–26. [Google Scholar] [CrossRef]
- Iozzo, R.V. The Family of the Small Leucine-Rich Proteoglycans: Key Regulators of Matrix Assembly and Cellular Growth. Crit. Rev. Biochem. Mol. Boil. 1997, 32, 141–174. [Google Scholar] [CrossRef]
- Lala, P.K.; Nandi, P. Mechanisms of trophoblast migration, endometrial angiogenesis in preeclampsia: The role of decorin. Cell Adhes. Migr. 2016, 10, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Baghy, K.; Horváth, Z.; Regős, E.; Kiss, K.; Schaff, Z.; Iozzo, R.V.; Kovalszky, I. Decorin interferes with platelet-derived growth factor receptor signaling in experimental hepatocarcinogenesis. FEBS J. 2013, 280, 2150–2164. [Google Scholar] [CrossRef] [PubMed]
- Baghy, K.; Dezsö, K.; Laszlo, V.; Fullár, A.; Péterfia, B.; Paku, S.; Nagy, P.; Schaff, Z.; Iozzo, R.V.; Kovalszky, I. Ablation of the decorin gene enhances experimental hepatic fibrosis and impairs hepatic healing in mice. Lab. Investig. 2010, 91, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Baghy, K.; Tátrai, P.; Regős, E.; Kovalszky, I. Proteoglycans in liver cancer. World J. Gastroenterol. 2016, 22, 379–393. [Google Scholar] [CrossRef]
- Santra, M.; Mann, D.M.; Mercer, E.W.; Skórski, T.; Calabretta, B.; Iozzo, R.V. Ectopic expression of decorin protein core causes a generalized growth suppression in neoplastic cells of various histogenetic origin and requires endogenous p21, an inhibitor of cyclin-dependent kinases. J. Clin. Investig. 1997, 100, 149–157. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Moscatello, D.K.; McQuillan, D.J.; Eichstetter, I. Decorin Is a Biological Ligand for the Epidermal Growth Factor Receptor. J. Boil. Chem. 1999, 274, 4489–4492. [Google Scholar] [CrossRef]
- Csordás, G.; Santra, M.; Reed, C.C.; Eichstetter, I.; McQuillan, D.J.; Gross, D.; Nugent, M.A.; Hajnóczky, G.; Iozzo, R.V. Sustained Down-regulation of the Epidermal Growth Factor Receptor by Decorin. J. Boil. Chem. 2000, 275, 32879–32887. [Google Scholar] [CrossRef]
- Goldoni, S.; Humphries, A.; Nyström, A.; Sattar, S.; Owens, R.T.; McQuillan, D.J.; Ireton, K.; Iozzo, R.V. Decorin is a novel antagonistic ligand of the Met receptor. J. Cell Boil. 2009, 185, 743–754. [Google Scholar] [CrossRef]
- Khan, G.A.; Girish, G.V.; Lala, N.; Di Guglielmo, G.M.; Lala, P.K. Decorin Is a Novel VEGFR-2-Binding Antagonist for the Human Extravillous Trophoblast. Mol. Endocrinol. 2011, 25, 1431–1443. [Google Scholar] [CrossRef]
- Schönherr, E.; Sunderkötter, C.; Iozzo, R.V.; Schaefer, L. Decorin, a Novel Player in the Insulin-like Growth Factor System. J. Boil. Chem. 2005, 280, 15767–15772. [Google Scholar] [CrossRef] [PubMed]
- Horváth, Z.; Kovalszky, I.; Fullár, A.; Kiss, K.; Schaff, Z.; Iozzo, R.V.; Baghy, K. Decorin deficiency promotes hepatic carcinogenesis. Matrix Boil. 2014, 35, 194–205. [Google Scholar] [CrossRef]
- Reszegi, A.; Horváth, Z.; Fehér, H.; Wichmann, B.; Tátrai, P.; Kovalszky, I.; Baghy, K. Protective Role of Decorin in Primary Hepatocellular Carcinoma. Front. Oncol. 2020, 10, 645. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Shinto, E.; Shimazaki, H.; Kajiwara, Y.; Sueyama, T.; Yamamoto, J.; Hase, K. Histologic Categorization of Desmoplastic Reaction: Its Relevance to the Colorectal Cancer Microenvironment and Prognosis. Ann. Surg. Oncol. 2014, 22, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Höppener, D.J.; Nierop, P.M.H.; Herpel, E.; Rahbari, N.N.; Doukas, M.; Vermeulen, P.B.; Grünhagen, D.J.; Verhoef, C. Histopathological growth patterns of colorectal liver metastasis exhibit little heterogeneity and can be determined with a high diagnostic accuracy. Clin. Exp. Metast. 2019, 36, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Regős, E.; Abdelfattah, H.H.; Reszegi, A.; Szilák, L.; Werling, K.; Szabo, G.; Kiss, A.; Schaff, Z.; Kovalszky, I.; Baghy, K. Syndecan-1 inhibits early stages of liver fibrogenesis by interfering with TGFβ1 action and upregulating MMP14. Matrix Boil. 2018, 474–489. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.; Sainio, A.; Kakko, T.; Savontaus, M.; Söderström, M.; Järveläinen, H. Localization of decorin gene expression in normal human breast tissue and in benign and malignant tumors of the human breast. Histochem. Cell Boil. 2012, 139, 161–171. [Google Scholar] [CrossRef]
- Nyman, M.C.; Sainio, A.; Pennanen, M.M.; Lund, R.; Vuorikoski, S.; Sundström, J.T.T.; Järveläinen, H.T. Decorin in Human Colon Cancer. J. Histochem. Cytochem. 2015, 63, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Sainio, A.; Nyman, M.; Lund, R.; Vuorikoski, S.; Boström, P.; Laato, M.; Boström, P.J.; Järveläinen, H. Lack of Decorin Expression by Human Bladder Cancer Cells Offers New Tools in the Therapy of Urothelial Malignancies. PLoS ONE 2013, 8, e76190. [Google Scholar] [CrossRef]
- Bi, X.; Pohl, N.M.; Qian, Z.; Yang, G.R.; Gou, Y.; Guzman, G.; Kajdacsy-Balla, A.; Iozzo, R.V.; Yang, W. Decorin-Mediated inhibition of colorectal cancer growth and migration is associated with E-cadherin in vitro and in mice. Carcinogenesis 2011, 33, 326–330. [Google Scholar] [CrossRef]
- Neill, T.; Schaefer, L.; Iozzo, R.V. Decorin as a multivalent therapeutic agent against cancer. Adv. Drug Deliv. Rev. 2016, 97, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.; Sainio, A.; Eigėlienė, N.; Jokilammi, A.; Elenius, K.; Koskivuo, I.; Järveläinen, H. Human Metaplastic Breast Carcinoma and Decorin. Cancer Microenviron. 2017, 10, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Tong, C.; Dockendorff, A.; Bancroft, L.; Gallagher, L.; Guzman, G.; Iozzo, R.V.; Augenlicht, L.H.; Yang, W. Genetic deficiency of decorin causes intestinal tumor formation through disruption of intestinal cell maturation. Carcinogenesis 2008, 29, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, Y.; Zhang, X.; Wang, H.; Xu, W.; Wang, H.; Xiao, F.; Bai, Z.; Yao, H.; Ma, X.; et al. An Oncolytic Adenovirus Encoding Decorin and Granulocyte Macrophage Colony Stimulating Factor Inhibits Tumor Growth in a Colorectal Tumor Model by Targeting Pro-Tumorigenic Signals and via Immune Activation. Hum. Gene Ther. 2017, 28, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Cawthorn, T.R.; Moreno, J.C.; Dharsee, M.; Tran-Thanh, D.; Ackloo, S.; Zhu, P.H.; Sardana, G.; Chen, J.; Kupchak, P.; Jacks, L.M.; et al. Proteomic Analyses Reveal High Expression of Decorin and Endoplasmin (HSP90B1) Are Associated with Breast Cancer Metastasis and Decreased Survival. PLoS ONE 2012, 7, e30992. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Goel, M.; Bhatia, V.; Chandra, S.; Makker, A.; Kumar, S.; Agrawal, S.; Mehrotra, D.; Rath, S.; S, N.; et al. Molecular and phenotypic expression of decorin as modulator of angiogenesis in human potentially malignant oral lesions and oral squamous cell carcinomas. Indian J. Pathol. Microbiol. 2013, 56, 204–210. [Google Scholar] [CrossRef]
- Satonaka, H.; Wakabayashi, H.; Iino, T.; Uchida, A.; Araki, K.; Wakabayashi, T.; Matsubara, T.; Matsumine, A.; Kusuzaki, K.; Morikawa, J.; et al. Decorin suppresses lung metastases of murine osteosarcoma. Oncol. Rep. 2008, 19, 1533–1539. [Google Scholar] [CrossRef]
- Goldoni, S.; Seidler, D.G.; Heath, J.; Fassan, M.; Baffa, R.; Thakur, M.L.; Owens, R.T.; McQuillan, D.J.; Iozzo, R.V. An Antimetastatic Role for Decorin in Breast Cancer. Am. J. Pathol. 2008, 173, 844–855. [Google Scholar] [CrossRef]
- Tralhão, J.G.; Schaefer, L.; Micegova, M.; Evaristo, C.; Schönherr, E.; Kayal, S.; Veiga-Fernandes, H.; Danel, C.; Iozzo, R.V.; Kresse, H.; et al. In Vivo selective and distant killing of cancer cells, using adenovirus-mediated decorin gene transfer. FASEB J. 2003, 17, 1–21. [Google Scholar] [CrossRef]
- Araki, K.; Wakabayashi, H.; Shintani, K.; Morikawa, J.; Matsumine, A.; Kusuzaki, K.; Sudo, A.; Uchida, A. Decorin Suppresses Bone Metastasis in a Breast Cancer Cell Line. Oncology 2009, 77, 92–99. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, W.; Neill, T.; Hu, Z.; Wang, C.-H.; Xiao, X.; Stock, S.R.; Guise, T.; Yun, C.-O.; Brendler, C.B.; et al. Systemic Delivery of an Oncolytic Adenovirus Expressing Decorin for the Treatment of Breast Cancer Bone Metastases. Hum. Gene Ther. 2015, 26, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Reed, C.C.; Gauldie, J.; Iozzo, R.V. Suppression of tumorigenicity by adenovirus-mediated gene transfer of decorin. Oncogene 2002, 21, 3688–3695. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Neill, T.; Yang, Y.; Hu, Z.; Cleveland, E.; Wu, Y.; Hutten, R.; Xiao, X.; Stock, S.R.; Shevrin, D.; et al. The systemic delivery of an oncolytic adenovirus expressing decorin inhibits bone metastasis in a mouse model of human prostate cancer. Gene Ther. 2014, 22, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.; Choi, J.-W.; Kasala, D.; Hong, J.; Oh, E.; Li, Y.; Jung, S.-J.; Kim, S.W.; Yun, C.-O. Potent antitumor effect of neurotensin receptor-targeted oncolytic adenovirus co-expressing decorin and Wnt antagonist in an orthotopic pancreatic tumor model. J. Control. Release 2015, 220, 766–782. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nature 2011, 13, 1016–1023. [Google Scholar] [CrossRef]
- Alers, S.; Löffler, A.S.; Wesselborg, S.; Stork, B. Role of AMPK-mTOR-Ulk1/2 in the Regulation of Autophagy: Cross Talk, Shortcuts, and Feedbacks. Mol. Cell. Boil. 2011, 32, 2–11. [Google Scholar] [CrossRef]
- Goyal, A.; Neill, T.; Owens, R.T.; Schaefer, L.; Iozzo, R.V. Decorin activates AMPK, an energy sensor kinase, to induce autophagy in endothelial cells. Matrix Boil. 2014, 34, 46–54. [Google Scholar] [CrossRef]
- Kleinert, M.; Parker, B.L.; Chaudhuri, R.; Fazakerley, D.J.; Serup, A.; Thomas, K.C.; Krycer, J.R.; Sylow, L.; Fritzen, A.M.; Hoffman, N.; et al. mTORC2 and AMPK differentially regulate muscle triglyceride content via Perilipin 3. Mol. Metab. 2016, 5, 646–655. [Google Scholar] [CrossRef]
- Kankanamalage, S.G.; Lee, A.-Y.; Wichaidit, C.; Lorente-Rodriguez, A.; Shah, A.M.; Stippec, S.; Whitehurst, A.W.; Cobb, M.H. WNK1 is an unexpected autophagy inhibitor. Autophagy 2017, 13, 969–970. [Google Scholar] [CrossRef]
- Kankanamalage, S.G.; Karra, A.; Cobb, M.H. WNK pathways in cancer signaling networks. Cell Commun. Signal. 2018, 16, 72. [Google Scholar] [CrossRef]
- Papa, S.; Choy, P.M.; Bubici, C. The ERK and JNK pathways in the regulation of metabolic reprogramming. Oncogene 2018, 38, 2223–2240. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Schaffer, B.E.; Brunet, A. AMPK: An Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends Cell Boil. 2016, 26, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Thornton, T.M. Non-Classical P38 Map Kinase Functions: Cell Cycle Checkpoints and Survival. Int. J. Boil. Sci. 2009, 5, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.S. The functional Interactions Between the MAPK and p53 Signaling Pathways. Cancer Boil. Ther. 2004, 3, 156–161. [Google Scholar] [CrossRef]
- Giebler, H.A.; Lemasson, I.; Nyborg, J.K. p53 Recruitment of CREB Binding Protein Mediated through Phosphorylated CREB: A Novel Pathway of Tumor Suppressor Regulation. Mol. Cell. Boil. 2000, 20, 4849–4858. [Google Scholar] [CrossRef]
- Gomes, A.S.; Ramos, H.; Soares, J.; Saraiva, L. p53 and glucose metabolism: An orchestra to be directed in cancer therapy. Pharmacol. Res. 2018, 131, 75–86. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, N.; Liu, P.; Xie, X. AMPK and Cancer. Exp. Suppl. 2016, 107, 203–226. [Google Scholar] [CrossRef]
- Bousoik, E.; Aliabadi, H.M. “Do We Know Jack” About JAK? A Closer Look at JAK/STAT Signaling Pathway. Front. Oncol. 2018, 8, 8. [Google Scholar] [CrossRef]
- Guo, Y.; Pan, W.; Liu, S.; Shen, Z.; Xu, Y.; Hu, L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef]
- Adiseshaiah, P.; Li, J.; Vaz, M.; Kalvakolanu, D.V.; Reddy, S.P. ERK signaling regulates tumor promoter induced c-Jun recruitment at the Fra-1 promoter. Biochem. Biophys. Res. Commun. 2008, 371, 304–308. [Google Scholar] [CrossRef]
- Fuchs, S.Y.; Adler, V.; Pincus, M.R.; Ronai, Z.A. MEKK1/JNK signaling stabilizes and activates p53. Proc. Natl. Acad. Sci. USA 1998, 95, 10541–10546. [Google Scholar] [CrossRef] [PubMed]
- Florescu-Ţenea, R.M.; Kamal, A.M.; Mitruţ, P.; Mitruţ, R.; Ilie, D.S.; Nicolaescu, A.C.; Mogoantă, L. Colorectal Cancer: An Update on Treatment Options and Future Perspectives. Curr. Health Sci. J. 2019, 45, 134–141. [Google Scholar] [PubMed]
- García-Foncillas, J.; Sunakawa, Y.; Aderka, D.; Wainberg, Z.; Ronga, P.; Witzler, P.; Stintzing, S. Distinguishing Features of Cetuximab and Panitumumab in Colorectal Cancer and Other Solid Tumors. Front. Oncol. 2019, 9, 849. [Google Scholar] [CrossRef] [PubMed]






| Protein | Phosphorylation Site | Relative Level in DCN Group (%) | p Value |
|---|---|---|---|
| Akt 1/2/3 (S473) | S473 | 139.277 | 0.085 |
| Akt 1/2/3 (T308) | T308 | 96.147 | 0.083 |
| AMPKα1 (T183) | T183 | 107.259 | 0.027 |
| AMPKα2 (T172) | T172 | 109.825 | 0.124 |
| Chk-2 | T68 | 105.490 | 0.517 |
| c-Jun | S63 | 80.634 | 0.016 |
| CREB | S133 | 114.239 | 0.103 |
| EGF R | Y1086 | 97.511 | 0.274 |
| eNOS | S1177 | 96.573 | 0.528 |
| ERK1/2 | T202/Y204, T185/Y187 | 79.370 | 0.045 |
| FAK | Y397 | 87.540 | 0.343 |
| Fgr | Y412 | 73.382 | 0.047 |
| Fyn | Y420 | 99.185 | 0.220 |
| GSK-3α/β | S21/S9 | 98.895 | 0.126 |
| Hck | Y411 | 103.026 | 0.210 |
| HSP27 | S78/S82 | 85.398 | 0.014 |
| HSP60 | - | 107.067 | 0.042 |
| JNK 1/2/3 | T183/Y185, T221/Y223 | 114.402 | 0.438 |
| Lck | Y394 | 100.697 | 0.567 |
| Lyn | Y397 | 109.082 | 0.371 |
| MSK1/2 | S376/S360 | 117.438 | 0.038 |
| p27 | T198 | 106.962 | 0.336 |
| p38α | T180/Y182 | 108.060 | 0.435 |
| p53 (S46) | S46 | 104.783 | 0.276 |
| p53 (S15) | S15 | 112.702 | 0.185 |
| p53 (S392) | S392 | 117.981 | 0.064 |
| p70 S6 Kinase | T421/S424 | 79.221 | 0.050 |
| p70 S6 Kinase (T389) | T389 | 78.876 | 0.059 |
| PDGF Rβ | Y751 | 70.647 | 0.012 |
| PLC-γ1 | Y783 | 75.498 | 0.026 |
| PRAS40 | T246 | 116.635 | 0.067 |
| PYK2 | Y402 | 79.806 | 0.083 |
| RSK1/2/3 | S380/S386/S377 | 90.656 | 0.072 |
| Src | Y419 | 110.985 | 0.089 |
| STAT2 | Y689 | 87.833 | 0.139 |
| STAT3 | Y705 | 84.852 | 0.117 |
| STAT3 | S727 | 96.977 | 0.462 |
| STAT5a | Y694 | 85.820 | 0.157 |
| STAT5a/b | Y694/Y699 | 82.277 | 0.287 |
| STAT5b | Y699 | 75.547 | 0.016 |
| STAT6 | Y641 | 92.892 | 0.083 |
| TOR | S2448 | 97.492 | 0.346 |
| WNK1 | T60 | 82.123 | 0.053 |
| Yes | Y426 | 97.223 | 0.313 |
| β-Catenin | - | 84.948 | 0.025 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reszegi, A.; Horváth, Z.; Karászi, K.; Regős, E.; Postniková, V.; Tátrai, P.; Kiss, A.; Schaff, Z.; Kovalszky, I.; Baghy, K. The Protective Role of Decorin in Hepatic Metastasis of Colorectal Carcinoma. Biomolecules 2020, 10, 1199. https://doi.org/10.3390/biom10081199
Reszegi A, Horváth Z, Karászi K, Regős E, Postniková V, Tátrai P, Kiss A, Schaff Z, Kovalszky I, Baghy K. The Protective Role of Decorin in Hepatic Metastasis of Colorectal Carcinoma. Biomolecules. 2020; 10(8):1199. https://doi.org/10.3390/biom10081199
Chicago/Turabian StyleReszegi, Andrea, Zsolt Horváth, Katalin Karászi, Eszter Regős, Victoria Postniková, Péter Tátrai, András Kiss, Zsuzsa Schaff, Ilona Kovalszky, and Kornélia Baghy. 2020. "The Protective Role of Decorin in Hepatic Metastasis of Colorectal Carcinoma" Biomolecules 10, no. 8: 1199. https://doi.org/10.3390/biom10081199
APA StyleReszegi, A., Horváth, Z., Karászi, K., Regős, E., Postniková, V., Tátrai, P., Kiss, A., Schaff, Z., Kovalszky, I., & Baghy, K. (2020). The Protective Role of Decorin in Hepatic Metastasis of Colorectal Carcinoma. Biomolecules, 10(8), 1199. https://doi.org/10.3390/biom10081199






