Aggrecan, the Primary Weight-Bearing Cartilage Proteoglycan, Has Context-Dependent, Cell-Directive Properties in Embryonic Development and Neurogenesis: Aggrecan Glycan Side Chain Modifications Convey Interactive Biodiversity
Abstract
:1. Introduction
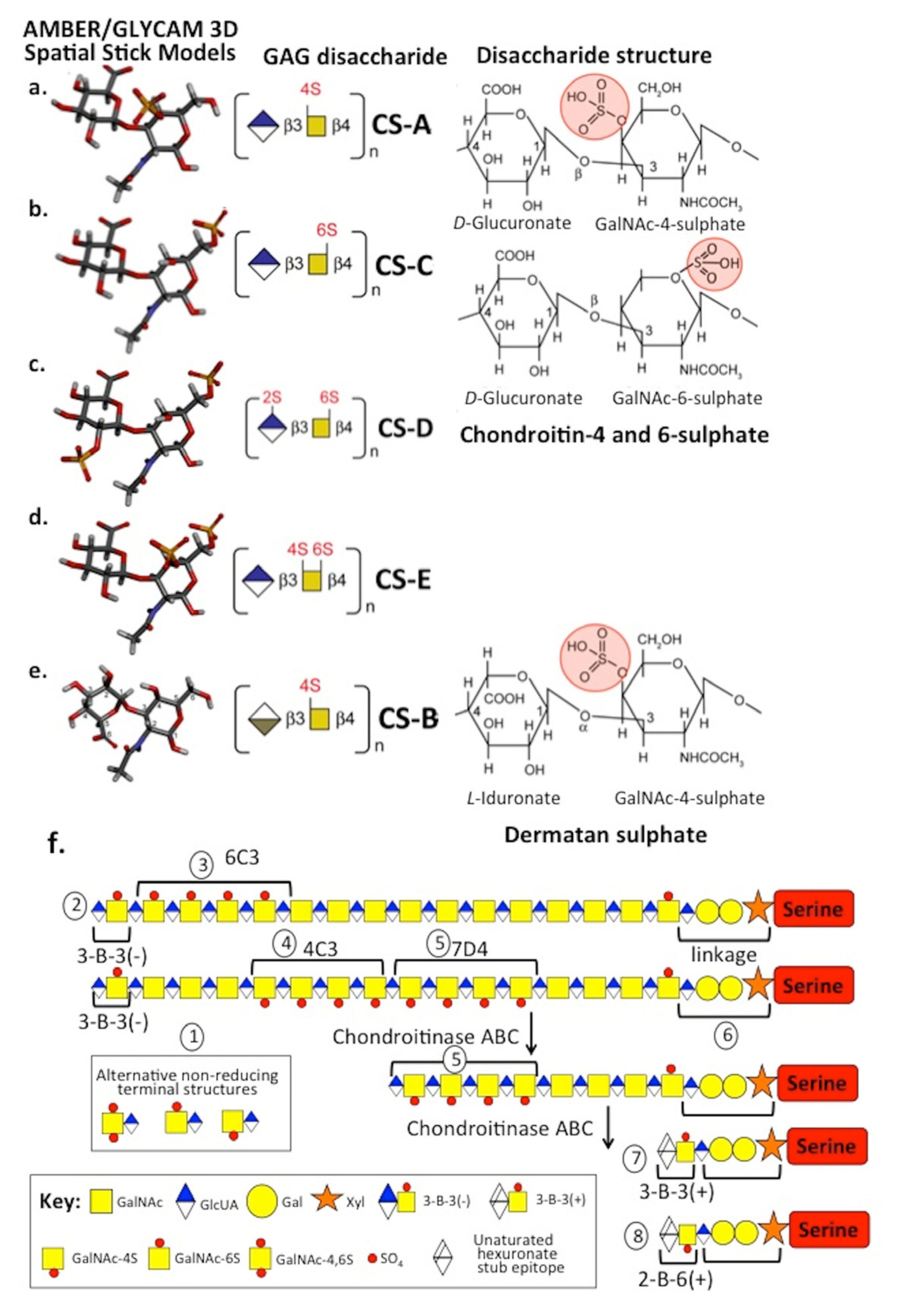
2. CS Sulphation on Aggrecan Is an Important Functional Determinant
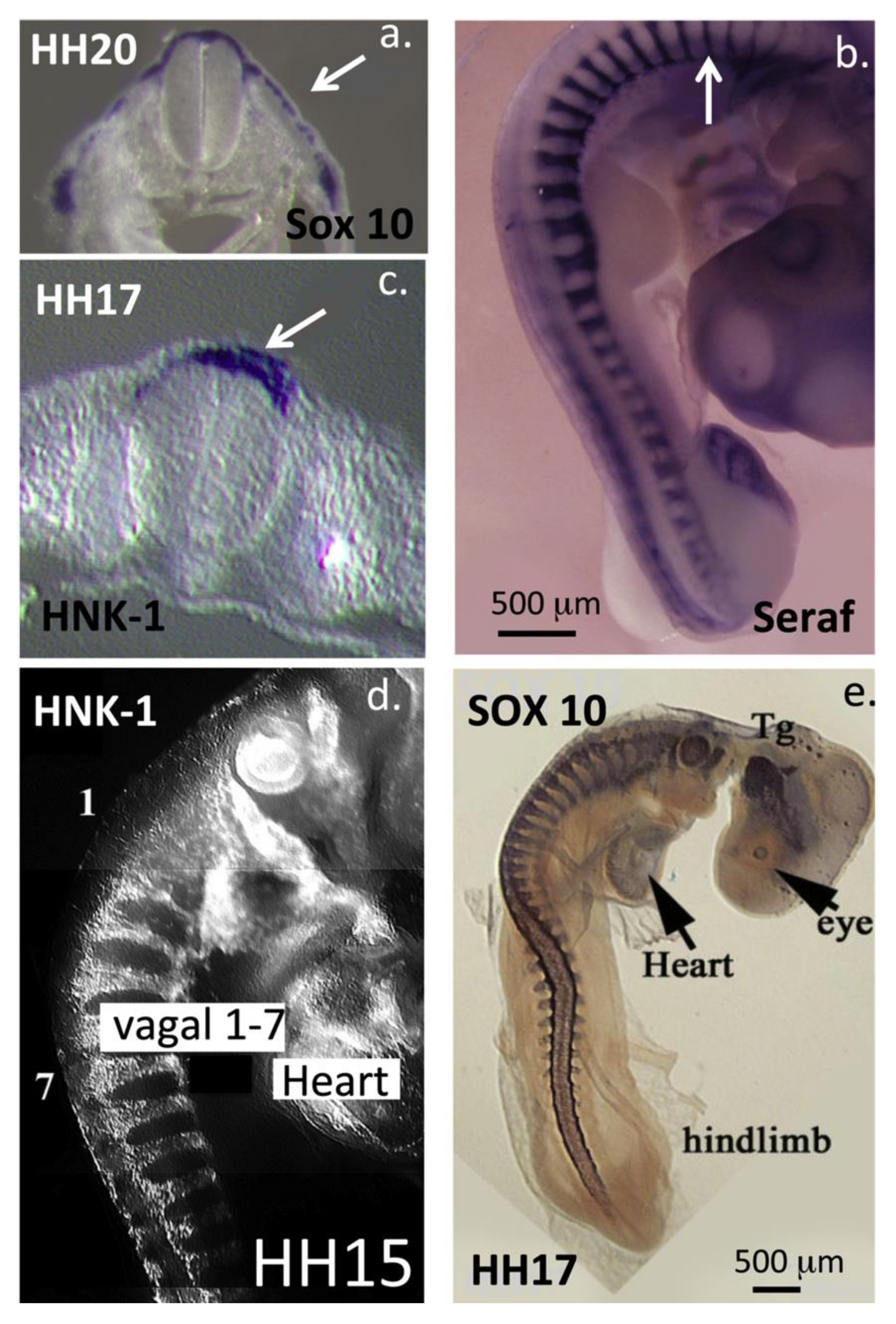
3. HNK-1 Aggrecan Regulates Neural Crest Cell Migration during Embryonic Development
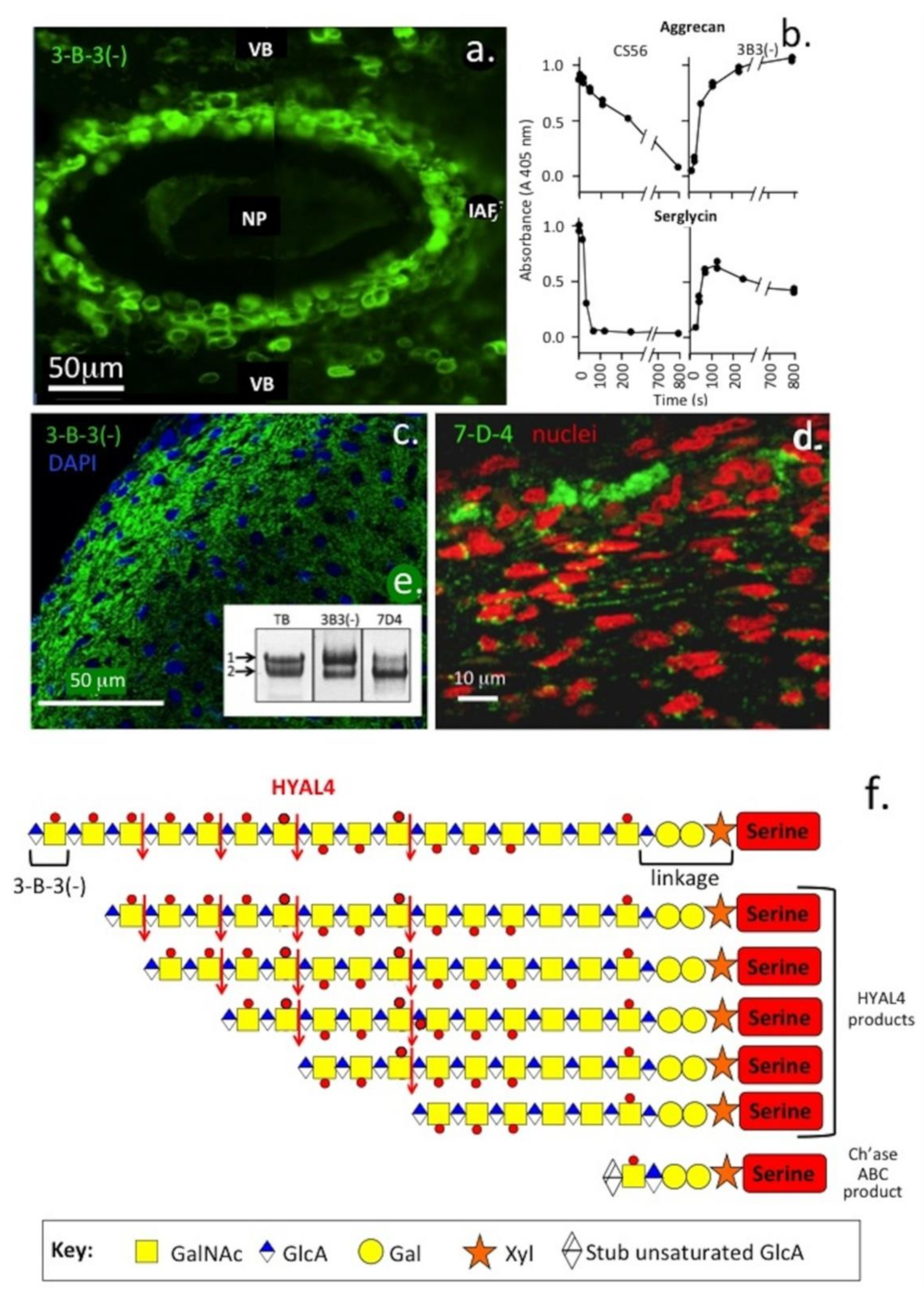
4. Variation in the CS Chain Fine Structure with Development and Pathology in Health and Disease
5. Effects of Modulation of CS Sulphation on Gene Expression and Cartilage Development
6. Aggrecans Roles in Articular Cartilage, Fibrocartilages, Heart and Neural Tissues
7. Co-Ordination of Weight-Bearing and Tension-Bearing Properties in Tissues
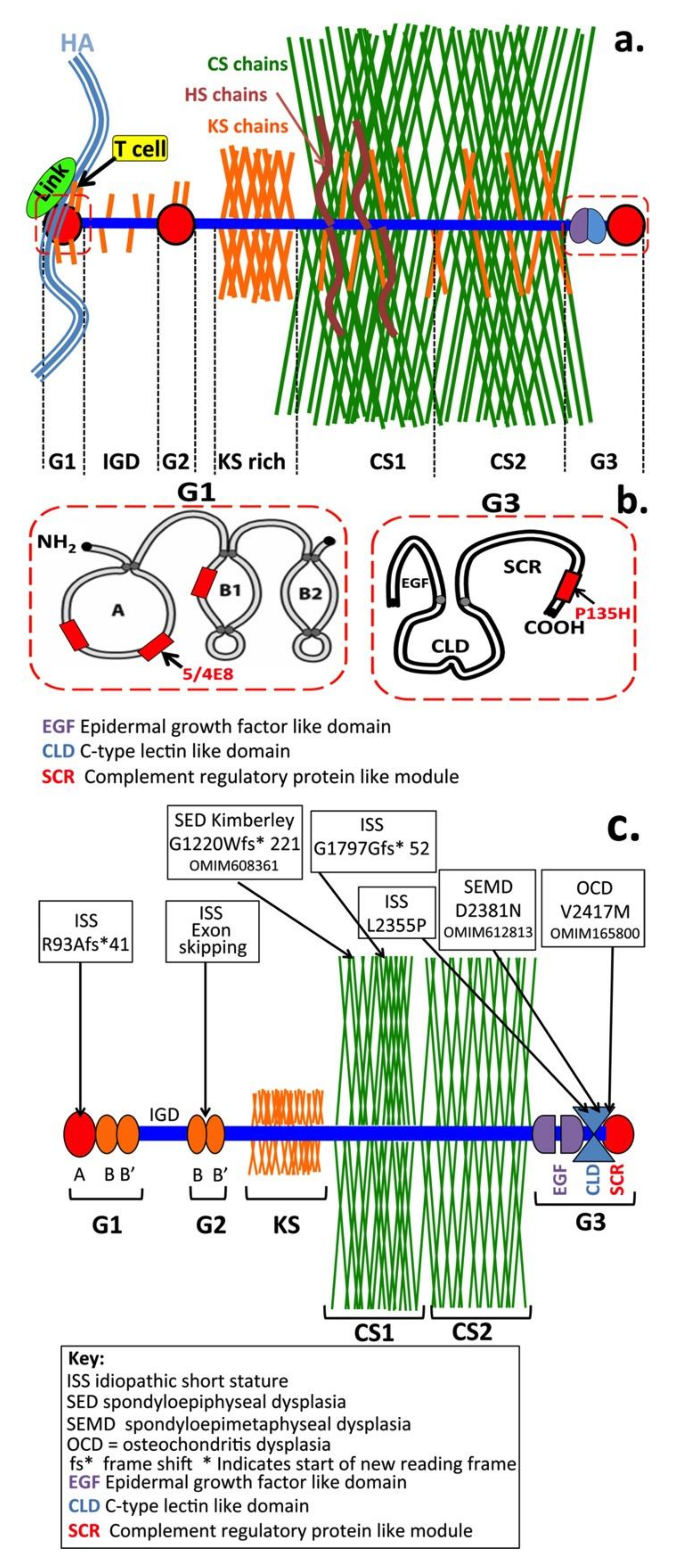
8. Modifications to Aggrecan Side Chain Structure Modifies Its Functional Properties in Tissues
9. Aggrecan–GAG Interactions Are of Importance in Heart Development
10. Aggrecan and Cellular Regulation
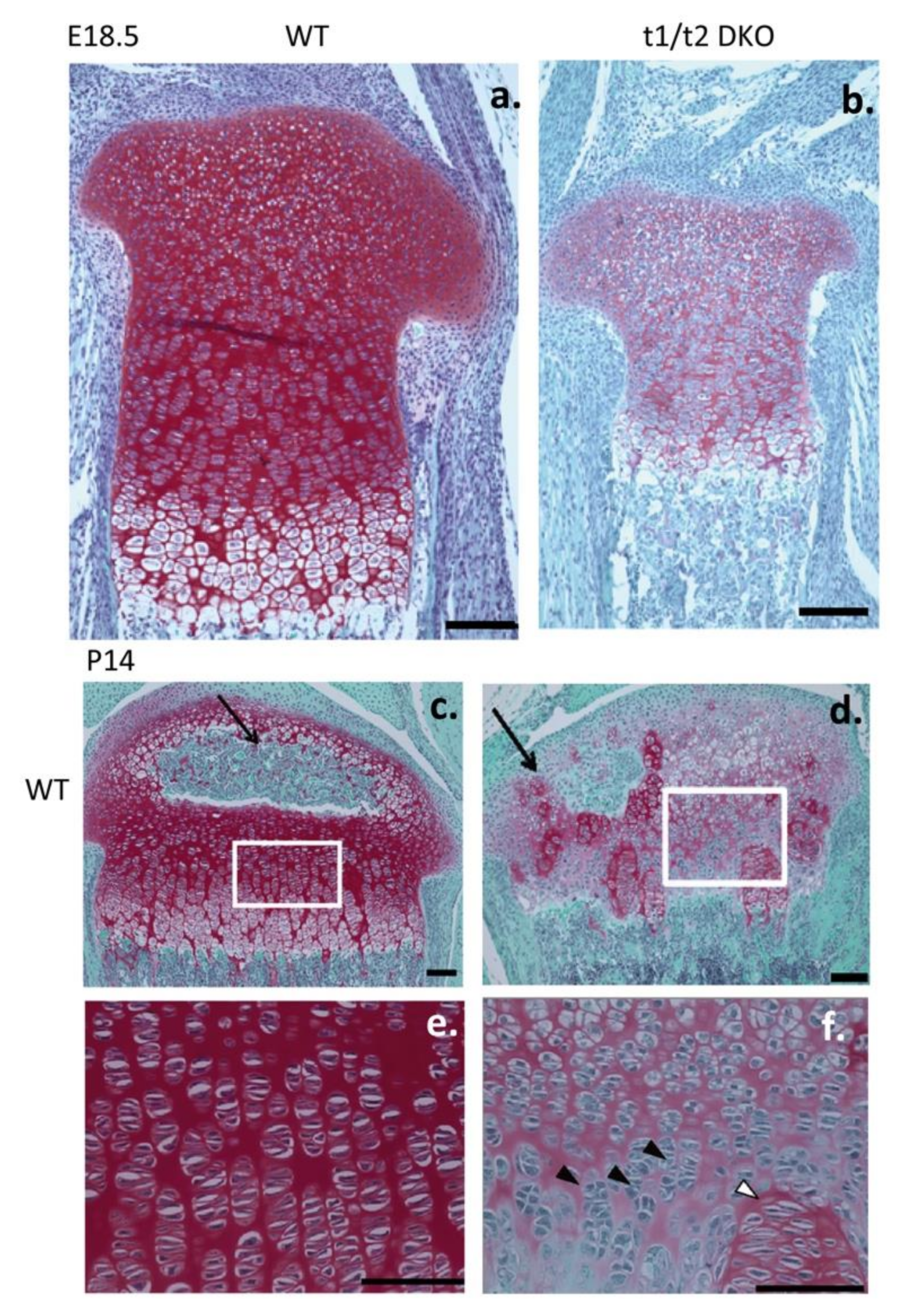
11. Role of IHH in Chondrogenesis
12. HNK-1 Carbohydrate Epitope as a Recognition Motif
13. The Therapeutic Potential of Aggrecan and Its GAG Side Chain Components
13.1. Analysis of Cartilage Aggrecan and Its GAG Side Chains
13.2. Aggrecan Isolation Procedures
13.3. Analysis of Aggrecan’s GAG Side Chains
14. Evaluation of the Aggrecan Content and Distribution in Pathological Cartilage Using Imaging Techniques
15. Tissue Therapeutic Interventions Involving CS
16. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Feng, H.; Danfelter, M.; Strömqvist, B.; Heinegård, D. Extracellular Matrix in Disc Degeneration. J. Bone Jt. Surg. Am. Vol. 2006, 88, 25. [Google Scholar] [CrossRef]
- Hardingham, T.E.; Fosang, A.J.; Dudhia, J. The structure, function and turnover of aggrecan, the large aggregating proteoglycan from cartilage. Eur. J. Clin. Chem. Clin. Biochem. 1994, 32, 249–257. [Google Scholar] [PubMed]
- Heinegard, D. Fell-Muir Lecture: Proteoglycans and More—From Molecules to Biology. Int. J. Exp. Pathol. 2009, 90, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Kiani, C.; Chen, L.; Wu, Y.J.; Yee, A.J.; Yang, B.B. Structure and function of aggrecan. Cell Res. 2002, 12, 19–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roughley, P.J.; Lee, E.R. Cartilage proteoglycans: Structure and potential functions. Microsc. Res. Tech. 1994, 28, 385–397. [Google Scholar] [CrossRef]
- Roughley, P.; Mort, J.S. The role of aggrecan in normal and osteoarthritic cartilage. J. Exp. Orthop. 2014, 1, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Sivan, S.S.; Wachtel, E.; Roughley, P. Structure, function, aging and turnover of aggrecan in the intervertebral disc. BBA Gen. Subj. 2014, 1840, 3181–3189. [Google Scholar] [CrossRef]
- Watanabe, H.; Yamada, Y.; Kimata, K. Roles of aggrecan, a large chondroitin sulfate proteoglycan, in cartilage structure and function. J. Biochem. 1998, 124, 687–693. [Google Scholar] [CrossRef] [Green Version]
- Halper, J. Proteoglycans and Diseases of Soft Tissues. Pharm. Biotechnol. 2013, 802, 49–58. [Google Scholar] [CrossRef]
- Sarbacher, C.A.; Halper, J. Connective Tissue and Age-Related Diseases. Sub-Cell. Biochem. 2019, 91, 281–310. [Google Scholar] [CrossRef]
- Lincoln, J.; Lange, A.W.; Yutzey, K.E. Hearts and bones: Shared regulatory mechanisms in heart valve, cartilage, tendon, and bone development. Dev. Biol. 2006, 294, 292–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson, C.S.; Solano, A.G.; Tilve, S.M.; Mencio, C.P.; Martin, K.R.; Geller, H.M. Spatiotemporal distribution of chondroitin sulfate proteoglycans after optic nerve injury in rodents. Exp. Eye Res. 2020, 190, 107859. [Google Scholar] [CrossRef] [PubMed]
- Rambeau, P.; Faure, E.; Faucherre, A.; Theron, A.; Avierinos, J.-F.; Jopling, C.; Zaffran, S. Reduced aggrecan expression affects cardiac outflow tract development in zebrafish and is associated with bicuspid aortic valve disease in humans. Int. J. Cardiol. 2017, 249, 340–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz, A.; Brendler, J.; Blaschuk, O.; Landgraf, K.; Krueger, M.; Ricken, A.M. Non-pathological Chondrogenic Features of Valve Interstitial Cells in Normal Adult Zebrafish. J. Histochem. Cytochem. 2019, 67, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Yasmin; Al Maskari, R.; McEniery, C.M.; Cleary, S.E.; Li, Y.; Siew, K.; Figg, N.L.; Khir, A.W.; Cockcroft, J.R.; Wilkinson, I.B.; et al. The matrix proteins aggrecan and fibulin-1 play a key role in determining aortic stiffness. Sci. Rep. 2018, 8, 8550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanin, M.K.; Bundy, J.; Ernst, H.; Wessels, A.; Conway, S.J.; Hoffman, S. Distinct spatial and temporal distributions of aggrecan and versican in the embryonic chick heart. Anat. Rec. 1999, 256, 366–380. [Google Scholar] [CrossRef]
- Fomovsky, G.M.; Thomopoulos, S.; Holmes, J.W. Contribution of extracellular matrix to the mechanical properties of the heart. J. Mol. Cell. Cardiol. 2010, 48, 490–496. [Google Scholar] [CrossRef] [Green Version]
- Miyata, S.; Nadanaka, S.; Igarashi, M.; Kitagawa, H. Structural Variation of Chondroitin Sulfate Chains Contributes to the Molecular Heterogeneity of Perineuronal Nets. Front. Integr. Neurosci. 2018, 12, 3. [Google Scholar] [CrossRef]
- Morawski, M.; Dityatev, A.; Hartlage-Rübsamen, M.; Blosa, M.; Holzer, M.; Flach, K.; Pavlica, S.; Dityateva, G.; Grosche, J.; Brückner, G.; et al. Tenascin-R promotes assembly of the extracellular matrix of perineuronal nets via clustering of aggrecan. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20140046. [Google Scholar] [CrossRef]
- Reichelt, A.C.; Hare, D.J.; Bussey, T.J.; Saksida, L.M. Perineuronal Nets: Plasticity, Protection, and Therapeutic Potential. Trends Neurosci. 2019, 42, 458–470. [Google Scholar] [CrossRef]
- Rowlands, D.; Lensjø, K.K.; Dinh, T.; Yang, S.; Andrews, M.R.; Hafting, T.; Fyhn, M.; Fawcett, J.W.; Dick, G. Aggrecan Directs Extracellular Matrix-Mediated Neuronal Plasticity. J. Neurosci. 2018, 38, 10102–10113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hering, T.M.; Beller, J.A.; Calulot, C.M.; Snow, D.M. Contributions of Chondroitin Sulfate, Keratan Sulfate and N-linked Oligosaccharides to Inhibition of Neurite Outgrowth by Aggrecan. Biology 2020, 9, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardingham, T.E.; Fosang, A.J.; Hey, N.J.; Hazell, P.K.; Kee, W.J.; Ewins, R.J.; Hardingham, T. The sulphation pattern in chondroitin sulphate chains investigated by chondroitinase ABC and ACII digestion and reactivity with monoclonal antibodies. Carbohydr. Res. 1994, 255, 241–254. [Google Scholar] [CrossRef]
- Midura, R.J.; Calabrò, A.; Yanagishita, M.; Hascall, V.C. Nonreducing End Structures of Chondroitin Sulfate Chains on Aggrecan Isolated from Swarm Rat Chondrosarcoma Cultures. J. Biol. Chem. 1995, 270, 8009–8015. [Google Scholar] [CrossRef] [Green Version]
- A West, L.; Roughley, P.; Nelson, F.R.; Plaas, A.H. Sulphation heterogeneity in the trisaccharide (GalNAcSbeta1, 4GlcAbeta1,3GalNAcS) isolated from the non-reducing terminal of human aggrecan chondroitin sulphate. Biochem. J. 1999, 342, 223–229. [Google Scholar] [CrossRef]
- Benito-Arenas, R.; Doncel-Perez, E.; Fernández-Gutiérrez, M.; Garrido, L.; García-Junceda, E.; Revuelta, J.; Bastida, A.; Fernández-Mayoralas, A. A holistic approach to unravelling chondroitin sulfation: Correlations between surface charge, structure and binding to growth factors. Carbohydr. Polym. 2018, 202, 211–218. [Google Scholar] [CrossRef]
- Cortes, M.; Baria, A.T.; Schwartz, N.B. Sulfation of chondroitin sulfate proteoglycans is necessary for proper Indian hedgehog signaling in the developing growth plate. Development 2009, 136, 1697–1706. [Google Scholar] [CrossRef] [Green Version]
- Miyachi, K.; Wakao, M.; Suda, Y. Syntheses of chondroitin sulfate tetrasaccharide structures containing 4,6-disulfate patterns and analysis of their interaction with glycosaminoglycan-binding protein. Bioorganic Med. Chem. Lett. 2015, 25, 1552–1555. [Google Scholar] [CrossRef] [Green Version]
- Mizumoto, S.; Yamada, S.; Sugahara, K. Molecular interactions between chondroitin–dermatan sulfate and growth factors/receptors/matrix proteins. Curr. Opin. Struct. Biol. 2015, 34, 35–42. [Google Scholar] [CrossRef]
- Nandini, C.D.; Sugahara, K. Role of the Sulfation Pattern of Chondroitin Sulfate in its Biological Activities and in the Binding of Growth Factors. Adv. Pharmacol. 2006, 53, 253–279. [Google Scholar] [CrossRef]
- Wakao, M.; Obata, R.; Miyachi, K.; Kaitsubata, Y.; Kondo, T.; Sakami, C.; Suda, Y. Synthesis of a chondroitin sulfate disaccharide library and a GAG-binding protein interaction analysis. Bioorganic Med. Chem. Lett. 2015, 25, 1407–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whalen, D.M.; Malinauskas, T.; Gilbert, R.J.C.; Siebold, C. Structural insights into proteoglycan-shaped Hedgehog signaling. Proc. Natl. Acad. Sci. USA 2013, 110, 16420–16425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L. Glycosaminoglycan (GAG) Biosynthesis and GAG-Binding Proteins. Prog. Mol. Biol. Transl. 2010, 93, 1–17. [Google Scholar] [CrossRef]
- Nadanaka, S.; Ishida, M.; Ikegami, M.; Kitagawa, H. Chondroitin 4-O-Sulfotransferase-1 Modulates Wnt-3a Signaling through Control of E Disaccharide Expression of Chondroitin Sulfate. J. Biol. Chem. 2008, 283, 27333–27343. [Google Scholar] [CrossRef] [Green Version]
- Reichsman, F.; Smith, L.; Cumberledge, S. Glycosaminoglycans can modulate extracellular localization of the wingless protein and promote signal transduction. J. Cell Biol. 1996, 135, 819–827. [Google Scholar] [CrossRef]
- Melrose, J.; Isaacs, M.D.; Smith, S.M.; Hughes, C.E.; Little, C.B.; Caterson, B.; Hayes, A.J. Chondroitin sulphate and heparan sulphate sulphation motifs and their proteoglycans are involved in articular cartilage formation during human foetal knee joint development. Histochem. Cell Biol. 2012, 138, 461–475. [Google Scholar] [CrossRef]
- Cao, J.; Li, S.; Shi, Z.; Yue, Y.; Sun, J.; Chen, J.; Fu, Q.; Hughes, C.E.; Caterson, B. Articular cartilage metabolism in patients with Kashin–Beck Disease: An endemic osteoarthropathy in China. Osteoarthr. Cartil. 2008, 16, 680–688. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.Q.; Guo, X.; Duan, C.; Ma, W.; Xu, P.; Wang, W.; Chen, J.C. Altered aggrecan synthesis and collagen expression profiles in chondrocytes from patients with Kashin-Beck disease and osteoarthritis. J. Int. Med. Res. 2012, 40, 1325–1334. [Google Scholar] [CrossRef]
- Luo, M.; Chen, J.; Li, S.-Y.; Sun, H.; Zhang, Z.; Fu, Q.; Li, J.; Wang, J.; Hughes, C.E.; Caterson, B.; et al. Changes in the metabolism of chondroitin sulfate glycosaminoglycans in articular cartilage from patients with Kashin–Beck disease. Osteoarthr. Cartil. 2014, 22, 986–995. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Xue, S.; Fang, Q.; Zhang, M.; He, Y.; Zhang, Y.; Lammi, M.J.; Cao, J.; Chen, J. Expression and localization of the small proteoglycans decorin and biglycan in articular cartilage of Kashin-Beck disease and rats induced by T-2 toxin and selenium deficiency. Glycoconj. J. 2019, 36, 1–9. [Google Scholar] [CrossRef]
- Wu, C.; Lei, R.; Tiainen, M.; Wu, S.-X.; Zhang, Q.; Pei, F.-X.; Guo, X. Disordered glycometabolism involved in pathogenesis of Kashin–Beck disease, an endemic osteoarthritis in China. Exp. Cell Res. 2014, 326, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Asada, M.; Shinomiya, M.; Suzuki, M.; Honda, E.; Sugimoto, R.; Ikekita, M.; Imamura, T. Glycosaminoglycan affinity of the complete fibroblast growth factor family. BBA Gen. Subj. 2009, 1790, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Prinz, R.D.; Willis, C.M.; Van Kuppevelt, T.H.; Kluppel, M. Biphasic Role of Chondroitin Sulfate in Cardiac Differentiation of Embryonic Stem Cells through Inhibition of Wnt/β-Catenin Signaling. PLoS ONE 2014, 9, e92381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirko, S.; Von Holst, A.; Weber, A.; Wizenmann, A.; Theocharidis, U.; Götz, M.; Faissner, A. Chondroitin Sulfates Are Required for Fibroblast Growth Factor-2-Dependent Proliferation and Maintenance in Neural Stem Cells and for Epidermal Growth Factor-Dependent Migration of Their Progeny. Stem Cells 2010, 28, 775–787. [Google Scholar] [CrossRef]
- Sterner, E.; Meli, L.; Kwon, S.J.; Dordick, J.S.; Linhardt, R.J. FGF–FGFR Signaling Mediated through Glycosaminoglycans in Microtiter Plate and Cell-Based Microarray Platforms. Biochemistry 2013, 52, 9009–9019. [Google Scholar] [CrossRef] [Green Version]
- Plaas, A.; West, L.A.; Wong-Palms, S.; Nelson, F.R. Glycosaminoglycan sulfation in human osteoarthritis. Disease-related alterations at the non-reducing termini of chondroitin and dermatan sulfate. J. Biol. Chem. 1998, 273, 12642–12649. [Google Scholar] [CrossRef] [Green Version]
- De Paz, J.L.; Nieto, P.M. Improvement on binding of chondroitin sulfate derivatives to midkine by increasing hydrophobicity. Org. Biomol. Chem. 2016, 14, 3506–3509. [Google Scholar] [CrossRef]
- Deepa, S.S.; Umehara, Y.; Higashiyama, S.; Itoh, N.; Sugahara, K. Specific Molecular Interactions of Oversulfated Chondroitin Sulfate E with Various Heparin-binding Growth Factors. J. Biol. Chem. 2002, 277, 43707–43716. [Google Scholar] [CrossRef] [Green Version]
- Solera, C.; Macchione, G.; Maza, S.; Kayser, M.M.; Corzana, F.; De Paz, J.L.; Nieto, P.M. Chondroitin Sulfate Tetrasaccharides: Synthesis, Three-Dimensional Structure and Interaction with Midkine. Chem. Eur. J. 2016, 22, 2356–2369. [Google Scholar] [CrossRef]
- Shu, C.C.; Hughes, C.E.; Smith, S.M.; Smith, M.M.; Hayes, A.J.; Caterson, B.; Little, C.B.; Melrose, J. The ovine newborn and human foetal intervertebral disc contain perlecan and aggrecan variably substituted with native 7D4 CS sulphation motif: Spatiotemporal immunolocalisation and co-distribution with Notch-1 in the human foetal disc. Glycoconj. J. 2013, 30, 717–725. [Google Scholar] [CrossRef]
- Caterson, B. Fell-Muir Lecture: Chondroitin sulphate glycosaminoglycans: Fun for some and confusion for others. Int. J. Exp. Pathol. 2012, 93, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, A.J.; Hughes, C.E.; Ralphs, J.; Caterson, B. Chondroitin sulphate sulphation motif expression in the ontogeny of the intervertebral disc. Eur. Cell Mater. 2011, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Belcher, C.; Yaqub, R.; Fawthrop, F.; Bayliss, M.; Doherty, M. Synovial fluid chondroitin and keratan sulphate epitopes, glycosaminoglycans, and hyaluronan in arthritic and normal knees. Ann. Rheum. Dis. 1997, 56, 299–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazell, P.K.; Dent, C.; Fairclough, J.A.; Bayliss, M.T.; Hardingham, T.E. Changes in glycosaminoglycan epitope levels in knee joint fluid following injury. Arthritis Rheum. 1995, 38, 953–959. [Google Scholar] [CrossRef]
- Ratcliffe, A.; Shurety, W.; Caterson, B. The quantitation of a native chondroitin sulfate epitope in synovial fluid lavages and articular cartilage from canine experimental osteoarthritis and disuse atrophy. Arthritis Rheum. 1993, 36, 543–551. [Google Scholar] [CrossRef]
- Ratcliffe, A.; Grelsamer, R.P.; Kiernan, H.; Saed-Nejad, F.; Visco, D. High levels of aggrecan aggregate components are present in synovial fluids from human knee joints with chronic injury or osteoarthrosis. Acta Orthop. Scand. 1995, 66, 111–115. [Google Scholar] [CrossRef]
- Farrugia, B.L.; Mizumoto, S.; Lord, M.S.; O’Grady, R.L.; Kuchel, R.P.; Yamada, S.; Whitelock, J.M. Hyaluronidase-4 is produced by mast cells and can cleave serglycin chondroitin sulfate chains into lower molecular weight forms. J. Biol. Chem. 2019, 294, 11458–11472. [Google Scholar] [CrossRef]
- Caterson, B.; Mahmoodian, F.; Sorrell, J.M.; Hardingham, T.E.; Bayliss, M.T.; Carney, S.L.; Ratcliffe, A.; Muir, H. Modulation of native chondroitin sulphate structure in tissue development and in disease. J. Cell Sci. 1990, 97, 411–417. [Google Scholar]
- Hayes, A.J.; Smith, S.M.; Caterson, B.; Melrose, J. Concise Review: Stem/Progenitor Cell Proteoglycans Decorated with 7-D-4, 4-C-3, and 3-B-3(-) Chondroitin Sulfate Motifs Are Morphogenetic Markers of Tissue Development. Stem Cells 2018, 36, 1475–1486. [Google Scholar] [CrossRef] [Green Version]
- Hayes, A.; Sugahara, K.; Farrugia, B.L.; Whitelock, J.M.; Caterson, B.; Melrose, J. Biodiversity of CS–proteoglycan sulphation motifs: Chemical messenger recognition modules with roles in information transfer, control of cellular behaviour and tissue morphogenesis. Biochem. J. 2018, 475, 587–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klüppel, M. Maintenance of chondroitin sulfation balance by chondroitin-4-sulfotransferase 1 is required for chondrocyte development and growth factor signaling during cartilage morphogenesis. Development 2005, 132, 3989–4003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mark, M.P.; Baker, J.R.; Kimata, K.; Ruch, J.V. Regulated changes in chondroitin sulfation during embryogenesis: An immunohistochemical approach. Int. J. Dev. Biol. 1990, 34, 191–204. [Google Scholar] [PubMed]
- Melrose, J.; Smith, S.M. The 7D4, 4C3 and 3B3 (-) Chondroitin Sulphation Motifs are expressed at Sites of Cartilage and Bone Morphogenesis during Foetal Human Knee Joint Development. J. Glycobiol. 2016, 5, 2–9. [Google Scholar] [CrossRef]
- Slater, R.R.; Bayliss, M.T.; Lachiewicz, P.F.; Visco, D.M.; Caterson, B. Monoclonal antibodies that detect biochemical markers of arthritis in humans. Arthritis Rheum. 1995, 38, 655–659. [Google Scholar] [CrossRef]
- Sorrell, J.M.; Lintala, A.M.; Mahmoodian, F.; Caterson, B. Epitope-specific changes in chondroitin sulfate/dermatan sulfate proteoglycans as markers in the lymphopoietic and granulopoietic compartments of developing bursae of Fabricius. J. Immunol. 1988, 140, 4263–4270. [Google Scholar]
- Curchoe, C.L.; Maurer, J.; McKeown, S.J.; Cattarossi, G.; Cimadamore, F.; Nilbratt, M.; Snyder, E.Y.; Bronner-Fraser, M.; Terskikh, A.V. Early Acquisition of Neural Crest Competence During hESCs Neuralization. PLoS ONE 2010, 5, e13890. [Google Scholar] [CrossRef] [Green Version]
- Tucker, G.; Delarue, M.; Zada, S.; Boucaut, J.C.; Thiery, J.P. Expression of the HNK-1/NC-1 epitope in early vertebrate neurogenesis. Cell Tissue Res. 1988, 251, 457–465. [Google Scholar] [CrossRef]
- Griffith, L.; Schmitz, B.; Schachner, M. L2/HNK-1 carbohydrate and protein-protein interactions mediate the homophilic binding of the neural adhesion molecule P0. J. Neurosci. Res. 1992, 33, 639–648. [Google Scholar] [CrossRef]
- Melrose, J. Keratan sulfate (KS)-proteoglycans and neuronal regulation in health and disease: The importance of KS-glycodynamics and interactive capability with neuroregulatory ligands. J. Neurochem. 2019, 149, 170–194. [Google Scholar] [CrossRef] [Green Version]
- Furley, A.J.; Morton, S.B.; Manalo, D.; Karagogeos, D.; Dodd, J.; Jessell, T.M. The axonal glycoprotein TAG-1 is an immunoglobulin superfamily member with neurite outgrowth-promoting activity. Cell 1990, 61, 157–170. [Google Scholar] [CrossRef]
- Hall, H.; Liu, L.; Schachner, M.; Schmitz, B. The L2/HNK-1 carbohydrate mediates adhesion of neural cells to laminin. Eur. J. Neurosci. 1993, 5, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Hall, H.; Carbonetto, S.; Schachner, M. L1/HNK-1 carbohydrate- and beta 1 integrin-dependent neural cell adhesion to laminin-1. J. Neurochem. 1997, 68, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Visco, D.M.; Johnstone, B.; Hill, M.A.; Jolly, G.A.; Caterson, B. Immunohistochemical analysis of 3-b-3(–) and 7-d-4 epitope expression in canine osteoarthritis. Arthritis Rheum. 1993, 36, 1718–1725. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, K.; Grogan, S.; Olee, T.; Lotz, M. Mesenchymal progenitor cells in adult human articular cartilage. Biorheology 2006, 43, 447–454. [Google Scholar] [PubMed]
- Dowthwaite, G.P.; Bishop, J.C.; Redman, S.N.; Khan, I.M.; Rooney, P.; Evans, D.J.R.; Haughton, L.; Bayram-Weston, Z.; Boyer, S.; Thomson, B.; et al. The surface of articular cartilage contains a progenitor cell population. J. Cell Sci. 2004, 117, 889–897. [Google Scholar] [CrossRef] [Green Version]
- Grogan, S.P.; Miyaki, S.; Asahara, H.; D’Lima, D.D.; Lotz, M.K. Mesenchymal progenitor cell markers in human articular cartilage: Normal distribution and changes in osteoarthritis. Arthritis Res. Ther. 2009, 11, R85. [Google Scholar] [CrossRef] [Green Version]
- Hollander, A.P.; Dickinson, S.C.; Kafienah, W. Stem Cells and Cartilage Development: Complexities of a Simple Tissue. Stem Cells 2010, 28, 1992–1996. [Google Scholar] [CrossRef] [Green Version]
- Akatsu, C.; Mizumoto, S.; Kaneiwa, T.; Maccarana, M.; Malmström, A.; Yamada, S.; Sugahara, K. Dermatan sulfate epimerase 2 is the predominant isozyme in the formation of the chondroitin sulfate/dermatan sulfate hybrid structure in postnatal developing mouse brain. Glycobiology 2010, 21, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Akita, K.; Von Holst, A.; Furukawa, Y.; Mikami, T.; Sugahara, K.; Faissner, A. Expression of Multiple Chondroitin/Dermatan Sulfotransferases in the Neurogenic Regions of the Embryonic and Adult Central Nervous System Implies That Complex Chondroitin Sulfates Have a Role in Neural Stem Cell Maintenance. Stem Cells 2008, 26, 798–809. [Google Scholar] [CrossRef]
- Mitsunaga, C.; Mikami, T.; Mizumoto, S.; Fukuda, J.; Sugahara, K. Chondroitin sulfate/dermatan sulfate hybrid chains in the development of cerebellum. Spatiotemporal regulation of the expression of critical disulfated disaccharides by specific sulfotransferases. J. Biol. Chem. 2006, 281, 18942–18952. [Google Scholar] [CrossRef] [Green Version]
- Hayes, A.J.; Tudor, D.; Nowell, M.A.; Caterson, B.; Hughes, C.E. Chondroitin Sulfate Sulfation Motifs as Putative Biomarkers for Isolation of Articular Cartilage Progenitor Cells. J. Histochem. Cytochem. 2007, 56, 125–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Hayes, A.J.; Caterson, B.; Hughes, C.E. The effect of beta-xylosides on the chondrogenic differentiation of mesenchymal stem cells. Histochem. Cell Biol. 2012, 139, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.J.; Hughes, C.E.; Smith, S.M.; Caterson, B.; Little, C.; Melrose, J. The CS Sulfation Motifs 4C3, 7D4, 3B3[−]; and Perlecan Identify Stem Cell Populations and Their Niches, Activated Progenitor Cells and Transitional Areas of Tissue Development in the Fetal Human Elbow. Stem Cells Dev. 2016, 25, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Skandalis, S.S.; Theocharis, A.D.; Vynios, D.H.; A Theocharis, D.; Papageorgakopoulou, N. Proteoglycans in human laryngeal cartilage. Identification of proteoglycan types in successive cartilage extracts with particular reference to aggregating proteoglycans. Biochimie 2004, 86, 221–229. [Google Scholar] [CrossRef]
- Novoselov, V.P.; Savchenko, S.V.; Pyatkova, E.V.; Nadeev, A.P.; Ageeva, T.A.; Chikinev, Y.V.; Polyakevich, A.S. Morphological Characteristics of the Cartilaginous Tissue of Human Auricle in Different Age Periods. Bull. Exp. Biol. Med. 2016, 160, 840–843. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Karamanos, N.K.; Papageorgakopoulou, N.; Tsiganos, C.P.; A Theocharis, D. Isolation and characterization of matrix proteoglycans from human nasal cartilage. Compositional and structural comparison between normal and scoliotic tissues. BBA Gen. Subj. 2002, 1569, 117–126. [Google Scholar] [CrossRef]
- Wilson, C.G.; Nishimuta, J.F.; Levenston, M.E. Chondrocytes and Meniscal Fibrochondrocytes Differentially Process Aggrecan During De Novo Extracellular Matrix Assembly. Tissue Eng. Part A 2009, 15, 1513–1522. [Google Scholar] [CrossRef] [Green Version]
- Lockhart, M.; Wirrig, E.; Phelps, A.; Wessels, A. Extracellular matrix and heart development. Birth Defects Res. Part A Clin. Mol. Teratol. 2011, 91, 535–550. [Google Scholar] [CrossRef] [Green Version]
- Miyata, S.; Kitagawa, H. Formation and remodeling of the brain extracellular matrix in neural plasticity: Roles of chondroitin sulfate and hyaluronan. BBA Gen. Subj. 2017, 1861, 2420–2434. [Google Scholar] [CrossRef]
- Domowicz, M.; Li, H.; Hennig, A.; Henry, J.; Vertel, B.M.; Schwartz, N.B. The Biochemically and Immunologically Distinct CSPG of Notochord Is a Product of the Aggrecan Gene. Dev. Biol. 1995, 171, 655–664. [Google Scholar] [CrossRef]
- Domowicz, M.; Mangoura, D.; Schwartz, N.B. Cell Specific-Chondroitin Sulfate Proteoglycan Expression During CNS Morphogenesis in the Chick Embryo. Int. J. Dev. Neurosci. 2000, 18, 629–641. [Google Scholar] [CrossRef]
- Domowicz, M.; Mueller, M.M.; Novak, T.E.; Schwartz, L.E.; Schwartz, N.B. Developmental expression of the HNK-1 carbohydrate epitope on aggrecan during chondrogenesis. Dev. Dyn. 2002, 226, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Morawski, M.; Bruckner, G.; Arendt, T.; Matthews, R. Aggrecan: Beyond cartilage and into the brain. Int. J. Biochem. Cell Biol. 2012, 44, 690–693. [Google Scholar] [CrossRef]
- Schwartz, N.B.; Domowicz, M.; Krueger, R.C.; Li, H.; Mangoura, D. Brain aggrecan. Perspect. Dev. Neurobiol. 1996, 3, 291–306. [Google Scholar] [PubMed]
- Trapani, V.; Bonaldo, P.; Corallo, D. Role of the ECM in notochord formation, function and disease. J. Cell Sci. 2017, 130, 3203–3211. [Google Scholar] [CrossRef] [Green Version]
- Corallo, D.; Trapani, V.; Bonaldo, P. The notochord: Structure and functions. Cell. Mol. Life Sci. 2015, 72, 2989–3008. [Google Scholar] [CrossRef]
- De Bree, K.; De Bakker, B.S.; Oostra, R.-J. The development of the human notochord. PLoS ONE 2018, 13, e0205752. [Google Scholar] [CrossRef] [Green Version]
- Tada, M. Notochord morphogenesis: A prickly subject for ascidians. Curr. Biol. 2005, 15, R14–R16. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Urban, J.P.G. The elastic network of articular cartilage: An immunohistochemical study of elastin fibres and microfibrils. J. Anat. 2010, 216, 533–541. [Google Scholar] [CrossRef]
- He, B.; Wu, J.; Chen, H.H.; Kirk, T.B.; Xu, J. Elastin fibers display a versatile microfibril network in articular cartilage depending on the mechanical microenvironments. J. Orthop. Res. 2013, 31, 1345–1353. [Google Scholar] [CrossRef] [Green Version]
- He, B.; Wu, J.; Chim, S.; Xu, J.; Kirk, T.B. Microstructural analysis of collagen and elastin fibres in the kangaroo articular cartilage reveals a structural divergence depending on its local mechanical environment. Osteoarthr. Cartil. 2013, 21, 237–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, B.; Wu, J.; Xu, J.; Day, R.E.; Kirk, T.B. Microstructural and Compositional Features of the Fibrous and Hyaline Cartilage on the Medial Tibial Plateau Imply a Unique Role for the Hopping Locomotion of Kangaroo. PLoS ONE 2013, 8, e74303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, M.K.; Kurtz, I. Physiologic Interrelationships between Gibbs-Donnan Equilibrium, Osmolality of Body Fluid Compartments, and Plasma Water Sodium Concentration. J. Appl. Physiol. 2006. [Google Scholar] [CrossRef] [PubMed]
- Nimer, E.; Schneiderman, R.; Maroudas, A. Diffusion and partition of solutes in cartilage under static load. Biophys. Chem. 2003, 106, 125–146. [Google Scholar] [CrossRef]
- Klüppel, M. The Roles of Chondroitin-4-Sulfotransferase-1 in Development and Disease. Prog. Mol. Biol. Transl. Sci. 2010, 93, 113–132. [Google Scholar] [CrossRef]
- Mikami, T.; Kitagawa, H. Biosynthesis and function of chondroitin sulfate. BBA Gen. Subj. 2013, 1830, 4719–4733. [Google Scholar] [CrossRef]
- Hardingham, T.E.; Fosang, A.J. The structure of aggrecan and its turnover in cartilage. J. Rheumatol. Suppl. 1995, 43, 86–90. [Google Scholar]
- Walcz, E.; Deák, F.; Erhardt, P.; Coulter, S.N.; Fülöp, C.; Horvath, P.; Doege, K.J.; Glant, T.T. Complete Coding Sequence, Deduced Primary Structure, Chromosomal Localization, and Structural Analysis of Murine Aggrecan. Genomics 1994, 22, 364–371. [Google Scholar] [CrossRef]
- Watanabe, H.; Gao, L.; Sugiyama, S.; Doege, K.; Kimata, K.; Yamada, Y. Mouse aggrecan, a large cartilage proteoglycan: Protein sequence, gene structure and promoter sequence. Biochem. J. 1995, 308, 433–440. [Google Scholar] [CrossRef]
- Antonsson, P.; Heinegård, D.; Oldberg, A. The keratan sulfate-enriched region of bovine cartilage proteoglycan consists of a consecutively repeated hexapeptide motif. J. Biol. Chem. 1989, 264, 16170–16173. [Google Scholar]
- Doege, K.J.; Sasaki, M.; Kimura, T.; Yamada, Y. Complete coding sequence and deduced primary structure of the human cartilage large aggregating proteoglycan, aggrecan. Human-specific repeats, and additional alternatively spliced forms. J. Biol. Chem. 1991, 266, 894–902. [Google Scholar] [PubMed]
- Fosang, A.; Rogerson, F.; East, C.; Stanton, H. ADAMTS-5: The story so far. Eur. Cells Mater. 2008, 15, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Conrad, A.H.; Zhang, Y.; Tasheva, E.S.; Conrad, G.W. Proteomic analysis of potential keratan sulfate, chondroitin sulfate A, and hyaluronic acid molecular interactions. Investig. Opthalmol. Vis. Sci. 2010, 51, 4500–4515. [Google Scholar] [CrossRef] [Green Version]
- Russo, V.C.; Bach, L.A.; Fosang, A.J.; Baker, N.L.; Werther, G.A. Insulin-like growth factor binding protein-2 binds to cell surface proteoglycans in the rat brain olfactory bulb. Endocrinology 1997, 138, 4858–4867. [Google Scholar] [CrossRef]
- Melrose, J. Functional Consequences of Keratan Sulphate Sulfation in Electrosensory Tissues and in Neuronal Regulation. Adv. Biosyst. 2019, 3, 1800327. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.S.; Mancera, R.L. The Structure of Glycosaminoglycans and their Interactions with Proteins. Chem. Biol. Drug Des. 2008, 72, 455–482. [Google Scholar] [CrossRef]
- Weyers, A.; Yang, B.; Solakyildirim, K.; Yee, V.; Li, L.; Zhang, F.; Linhardt, R.J. Isolation of bovine corneal keratan sulfate and its growth factor and morphogen binding. FEBS J. 2013, 280, 2285–2293. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Zheng, L.; Cheng, S.; Peng, Y.; Fu, L.; Zhang, X.; Linhardt, R.J. Comparison of the Interactions of Different Growth Factors and Glycosaminoglycans. Molecules 2019, 24, 3360. [Google Scholar] [CrossRef] [Green Version]
- Caterson, B.; Melrose, J. Keratan sulfate, a complex glycosaminoglycan with unique functional capability. Glycobiology 2018, 28, 182–206. [Google Scholar] [CrossRef]
- Caterson, B.; E Christner, J.; Baker, J.R. Identification of a monoclonal antibody that specifically recognizes corneal and skeletal keratan sulfate. Monoclonal antibodies to cartilage proteoglycan. J. Biol. Chem. 1983, 258, 8848–8854. [Google Scholar]
- Melrose, J. Mucin-like glycopolymer gels in electrosensory tissues generate cues which direct electrolocation in amphibians and neuronal activation in mammals. Neural Regen. Res. 2019, 14, 1191. [Google Scholar] [CrossRef]
- Day, J.M.; Murdoch, A.D.; Hardingham, T.E. The Folded Protein Modules of the C-terminal G3 Domain of Aggrecan Can Each Facilitate the Translocation and Secretion of the Extended Chondroitin Sulfate Attachment Sequence. J. Biol. Chem. 1999, 274, 38107–38111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundell, A.; Olin, A.I.; Mörgelin, M.; Al-Karadaghi, S.; Aspberg, A.; Logan, D.T. Structural Basis for Interactions between Tenascins and Lectican C-Type Lectin Domains. Structure 2004, 12, 1495–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fürst, C.M.; Mörgelin, M.; Vadstrup, K.; Heinegård, D.; Aspberg, A.; Blom, A.M. The C-Type Lectin of the Aggrecan G3 Domain Activates Complement. PLoS ONE 2013, 8, e61407. [Google Scholar] [CrossRef] [Green Version]
- Sjöberg, A.; Trouw, L.A.; Blom, A.M. Complement activation and inhibition: A delicate balance. Trends Immunol. 2009, 30, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 2010, 11, 785–797. [Google Scholar] [CrossRef] [Green Version]
- Wiberg, C.; Klatt, A.R.; Wagener, R.; Paulsson, M.; Bateman, J.; Heinegård, D.; Mörgelin, M. Complexes of Matrilin-1 and Biglycan or Decorin Connect Collagen VI Microfibrils to Both Collagen II and Aggrecan. J. Biol. Chem. 2003, 278, 37698–37704. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.H.; Herndon, M.E.; Patel, N.; Hecht, J.T.; Tuan, R.S.; Lawler, J. Interaction of Cartilage Oligomeric Matrix Protein/Thrombospondin 5 with Aggrecan. J. Biol. Chem. 2007, 282, 24591–24598. [Google Scholar] [CrossRef] [Green Version]
- Krueger, R.C.; Kurima, K.; Schwartz, N.B. Completion of the mouse aggrecan gene structure and identification of the defect in the cmd-Bc mouse as a near complete deletion of the murine aggrecan gene. Mamm. Genome 1999, 10, 1119–1125. [Google Scholar] [CrossRef]
- Watanabe, H.; Kimata, K.; Line, S.; Strong, D.; Gao, L.-Y.; Kozak, C.A.; Yamada, Y. Mouse cartilage matrix deficiency (cmd) caused by a 7 bp deletion in the aggrecan gene. Nat. Genet. 1994, 7, 154–157. [Google Scholar] [CrossRef]
- Yoo, T.J.; Cho, H.; Yamada, Y. Hearing Impairment in Mice with the cmd/cmd (Cartilage Matrix Deficiency) Mutant Gene. Ann. N. Y. Acad. Sci. 1991, 630, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Nakata, K.; Kimata, K.; Nakanishi, I.; Yamada, K.M. Dwarfism and age-associated spinal degeneration of heterozygote cmd mice defective in aggrecan. Proc. Natl. Acad. Sci. USA 1997, 94, 6943–6947. [Google Scholar] [CrossRef] [Green Version]
- Primorac, D.; Stover, M.; Clark, S.; Rowe, D. Molecular basis of nanomelia, a heritable chondrodystrophy of chicken. Matrix Biol. 1994, 14, 297–305. [Google Scholar] [CrossRef]
- Domowicz, M.; Cortes, M.; Henry, J.G.; Schwartz, N.B. Aggrecan modulation of growth plate morphogenesis. Dev. Biol. 2009, 329, 242–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stirpe, N.S.; Argraves, W.; Goetinck, P.F. Chondrocytes from the cartilage proteoglycan-deficient mutant, nanomelia, synthesize greatly reduced levels of the proteoglycan core protein transcript. Dev. Biol. 1987, 124, 77–81. [Google Scholar] [CrossRef]
- Vertel, B.M.; Grier, B.L.; Li, H.; Schwartz, N.B. The chondrodystrophy, nanomelia: Biosynthesis and processing of the defective aggrecan precursor. Biochem. J. 1994, 301, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Vertel, B.M.; Walters, L.M.; Grier, B.; Maine, N.; Goetinck, P.F. Nanomelic chondrocytes synthesize, but fail to translocate, a truncated aggrecan precursor. J. Cell Sci. 1993, 104, 104. [Google Scholar]
- Glant, T.T.; I Buzás, E.; Finnegan, A.; Negroiu, G.; Cs-Szabó, G.; Mikecz, K. Critical roles of glycosaminoglycan side chains of cartilage proteoglycan (aggrecan) in antigen recognition and presentation. J. Immunol. 1998, 160, 3812–3819. [Google Scholar]
- Glant, T.T.; Fülöp, C.; Cs-Szabo, G.; Buzás, E.I.; Ragasa, D.; Mikecz, K. Mapping of Arthritogenic/Autoimmune Epitopes of Cartilage Aggrecans in Proteoglycan-Induced Arthritis. Scand. J. Rheumatol. 1995, 24, 43–49. [Google Scholar] [CrossRef]
- Glant, T.T.; Radács, M.; Nagyeri, G.; Olasz, K.; László, A.; Boldizsár, F.; Hegyi, A.; Finnegan, A.; Mikecz, K. Proteoglycan-induced arthritis and recombinant human proteoglycan aggrecan G1 domain-induced arthritis in BALB/c mice resembling two subtypes of rheumatoid arthritis. Arthritis Rheum. 2011, 63, 1312–1321. [Google Scholar] [CrossRef] [Green Version]
- De Jong, H.; E Berlo, S.; Hombrink, P.; Otten, H.G.; Van Eden, W.; Lafeber, F.P.; Heurkens, A.H.M.; Bijlsma, J.W.J.; Glant, T.T.; Prakken, B. Cartilage proteoglycan aggrecan epitopes induce proinflammatory autoreactive T-cell responses in rheumatoid arthritis and osteoarthritis. Ann. Rheum. Dis. 2009, 69, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Murad, Y.; Szabó, Z.; Ludanyi, K.; Glant, T.T. Molecular manipulation with the arthritogenic epitopes of the G1 domain of human cartilage proteoglycan aggrecan. Clin. Exp. Immunol. 2005, 142, 303–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poon, C.J.; Plaas, A.H.; Keene, D.R.; McQuillan, D.J.; Last, K.; Fosang, A.J. N-Linked Keratan Sulfate in the Aggrecan Interglobular Domain Potentiates Aggrecanase Activity. J. Biol. Chem. 2005, 280, 23615–23621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- A Nieduszynski, I.; Huckerby, T.N.; Dickenson, J.M.; Brown, G.M.; Tai, G.H.; Morris, H.G.; Eady, S. There are two major types of skeletal keratan sulphates. Biochem. J. 1990, 271, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.C.; Haubeck, H.D.; Eich, K.; Kolbe-Busch, S.; Stocker, G.; Stuhlsatz, H.W.; Greiling, H. A novel keratan sulphate domain preferentially expressed on the large aggregating proteoglycan from human articular cartilage is recognized by the monoclonal antibody 3D12/H7. Biochem. J. 1996, 318 Pt 3, 1051–1056. [Google Scholar] [CrossRef] [Green Version]
- Gibson, B.G.; Briggs, M.D. The aggrecanopathies; An evolving phenotypic spectrum of human genetic skeletal diseases. Orphanet J. Rare Dis. 2016, 11, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, O.; Guo, M.H.; Dunbar, N.; Popovic, J.; Flynn, D.; Jacobsen, C.; Lui, J.C.; Hirschhorn, J.N.; Baron, J.; Dauber, A. Short stature, accelerated bone maturation, and early growth cessation due to heterozygous aggrecan mutations. J. Clin. Endocrinol. Metab. 2014, 99, E1510–E1518. [Google Scholar] [CrossRef]
- Schwartz, N.B.; Domowicz, M. Chondrodysplasias due to proteoglycan defects. Glycobiology 2002, 12, 57R–68R. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Schwartz, N.B.; Vertel, B.M. cDNA cloning of chick cartilage chondroitin sulfate (aggrecan) core protein and identification of a stop codon in the aggrecan gene associated with the chondrodystrophy, nanomelia. J. Biol. Chem. 1993, 268, 23504–23511. [Google Scholar]
- Kurima, K.; Warman, M.L.; Krishnan, S.; Domowicz, M.; Krueger, R.C.; Deyrup, A.; Schwartz, N.B. A member of a family of sulfate-activating enzymes causes murine brachymorphism. Proc. Natl. Acad. Sci. USA 1998, 95, 8681–8685. [Google Scholar] [CrossRef] [Green Version]
- Hästbacka, J.; De La Chapelle, A.; Mahtani, M.M.; Clines, G.; Reeve-Daly, M.P.; Daly, M.; Hamilton, B.A.; Kusumi, K.; Trivedi, B.; Weaver, A.; et al. The diastrophic dysplasia gene encodes a novel sulfate transporter: Positional cloning by fine-structure linkage disequilibrium mapping. Cell 1994, 78, 1073–1087. [Google Scholar] [CrossRef]
- Hästbacka, J.; Superti-Furga, A.; Wilcox, W.R.; Rimoin, D.L.; Cohn, D.H.; Lander, E.S. Atelosteogenesis type II is caused by mutations in the diastrophic dysplasia sulfate-transporter gene (DTDST): Evidence for a phenotypic series involving three chondrodysplasias. Am. J. Hum. Genet. 1996, 58, 255–262. [Google Scholar] [PubMed]
- Superti-Furga, A.; Rossi, A.; Steinmann, B.; Gitzelmann, R. A chondrodysplasia family produced by mutations in the diastrophic dysplasia sulfate transporter gene: Genotype/phenotype correlations. Am. J. Med. Genet. 1996, 63, 144–147. [Google Scholar] [CrossRef]
- Gkourogianni, A.; Andrew, M.; Tyzinski, L.; Crocker, M.; Douglas, J.; Dunbar, N.; Fairchild, J.; Funari, M.F.A.; Heath, K.E.; Jorge, A.A.L.; et al. Clinical characterization of patients with autosomal dominant short stature due to aggrecan mutations. J. Clin. Endocrinol. Metab. 2016, 102, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Sentchordi, L.; Aza-Carmona, M.; Benito-Sanz, S.; Bonis, A.C.B.-; Sánchez-Garre, C.; Prieto-Matos, P.; Ruiz-Ocaña, P.; Lechuga-Sancho, A.M.; Carcavilla-Urquí, A.; Mulero-Collantes, I.; et al. Heterozygous aggrecan variants are associated with short stature and brachydactyly: Description of 16 probands and a review of the literature. Clin. Endocrinol. 2018, 88, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Sun, C.; Zhou, Z.; Wu, B.-B.; Yang, L.; Chang, Z.; Zhang, M.; Xi, L.; Cheng, R.; Ni, J.; et al. Novel aggrecan variant, p. Gln2364Pro, causes severe familial nonsyndromic adult short stature and poor growth hormone response in Chinese children. BMC Med. Genet. 2018, 19, 79. [Google Scholar] [CrossRef] [PubMed]
- Bates, J.T.; Jacobs, J.C.; Shea, K.G.; Oxford, J. Emerging Genetic Basis of Osteochondritis Dissecans. Clin. Sports Med. 2014, 33, 199–220. [Google Scholar] [CrossRef] [Green Version]
- Stattin, E.-L.; Wiklund, F.; Lindblom, K.; Önnerfjord, P.; Jonsson, B.-A.; Tegner, Y.; Sasaki, T.; Struglics, A.; Lohmander, S.; Dahl, N.; et al. A Missense Mutation in the Aggrecan C-type Lectin Domain Disrupts Extracellular Matrix Interactions and Causes Dominant Familial Osteochondritis Dissecans. Am. J. Hum. Genet. 2010, 86, 126–137. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Stattin, E.-L.; Shaw, G.; Heinegård, D.; Sullivan, G.J.; Wilmut, I.; Colman, A.; Önnerfjord, P.; Khabut, A.; Aspberg, A.; et al. Chondrocytes Derived From Mesenchymal Stromal Cells and Induced Pluripotent Cells of Patients With Familial Osteochondritis Dissecans Exhibit an Endoplasmic Reticulum Stress Response and Defective Matrix Assembly. Stem Cells Transl. Med. 2016, 5, 1171–1181. [Google Scholar] [CrossRef]
- Dai, J.; Kim, O.-H.; Cho, T.-J.; Schmidt-Rimpler, M.; Tonoki, H.; Takikawa, K.; Haga, N.; Miyoshi, K.; Kitoh, H.; Yoo, W.-J.; et al. Novel and recurrent TRPV4 mutations and their association with distinct phenotypes within the TRPV4 dysplasia family. J. Med. Genet. 2010, 47, 704–709. [Google Scholar] [CrossRef] [Green Version]
- Hurd, L.; Kirwin, S.M.; Boggs, M.; MacKenzie, W.G.; Bober, M.B.; Funanage, V.L.; Duncan, R.L. A mutation in TRPV4 results in altered chondrocyte calcium signaling in severe metatropic dysplasia. Am. J. Med. Genet. Part A 2015, 167, 2286–2293. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S.; Shin, S.H.; Auh, C.-K.; Chun, J. Human skeletal dysplasia caused by a constitutive activated transient receptor potential vanilloid 4 (TRPV4) cation channel mutation. Exp. Mol. Med. 2012, 44, 707–722. [Google Scholar] [CrossRef] [PubMed]
- Phan, M.N.; Leddy, H.A.; Votta, B.J.; Kumar, S.; Levy, D.S.; Lipshutz, D.B.; Lee, S.H.; Liedtke, W.; Guilak, F. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum. 2009, 60, 3028–3037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saitta, B.; Passarini, J.; Sareen, D.; Ornelas, L.; Sahabian, A.; Argade, S.; Krakow, D.; Cohn, D.H.; Svendsen, C.N.; Rimoin, D.L. Patient-derived skeletal dysplasia induced pluripotent stem cells display abnormal chondrogenic marker expression and regulation by BMP2 and TGFbeta1. Stem Cells Dev. 2014, 23, 1464–1478. [Google Scholar] [CrossRef] [Green Version]
- Lauing, K.; Cortes, M.; Domowicz, M.S.; Henry, J.G.; Baria, A.T.; Schwartz, N.B. Aggrecan is required for growth plate architecture and differentiation. Dev. Biol. 2014, 396, 224–236. [Google Scholar] [CrossRef] [Green Version]
- Shimbo, M.; Suzuki, R.; Fuseya, S.; Sato, T.; Kiyohara, K.; Hagiwara, K.; Okada, R.; Wakui, H.; Tsunakawa, Y.; Watanabe, H.; et al. Postnatal Lethality and Chondrodysplasia in Mice Lacking Both Chondroitin Sulfate N-acetylgalactosaminyltransferase-1 and -2. PLoS ONE 2017, 12, e0190333. [Google Scholar] [CrossRef]
- Bekku, Y.; Saito, M.; Moser, M.; Fuchigami, M.; Maehara, A.; Nakayama, M.; Kusachi, S.; Ninomiya, Y.; Oohashi, T. Bral2 is indispensable for the proper localization of brevican and the structural integrity of the perineuronal net in the brainstem and cerebellum. J. Comp. Neurol. 2012, 520, 1721–1736. [Google Scholar] [CrossRef]
- Ueno, H.; Fujii, K.; Suemitsu, S.; Murakami, S.; Kitamura, N.; Wani, K.; Aoki, S.; Okamoto, M.; Ishihara, T.; Takao, K. Expression of aggrecan components in perineuronal nets in the mouse cerebral cortex. IBRO Rep. 2018, 4, 22–37. [Google Scholar] [CrossRef]
- Yamada, J.; Jinno, S. Molecular heterogeneity of aggrecan-based perineuronal nets around five subclasses of parvalbumin-expressing neurons in the mouse hippocampus. J. Comp. Neurol. 2016, 525, 1234–1249. [Google Scholar] [CrossRef]
- Bozzelli, P.L.; Alaiyed, S.; Kim, E.; Villapol, S.; Conant, K. Proteolytic Remodeling of Perineuronal Nets: Effects on Synaptic Plasticity and Neuronal Population Dynamics. Neural Plast. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Creazzo, T.L.; Godt, R.E.; Leatherbury, L.; Conway, S.J.; Kirby, M.L. ROLE OF CARDIAC NEURAL CREST CELLS IN CARDIOVASCULAR DEVELOPMENT. Annu. Rev. Physiol. 1998, 60, 267–286. [Google Scholar] [CrossRef] [PubMed]
- Arciniegas, E.; Neves, C.Y.; Candelle, D.; Parada, D. Differential versican isoforms and aggrecan expression in the chicken embryo aorta. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2004, 279, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Foglia, M.; Poss, K.D. Building and re-building the heart by cardiomyocyte proliferation. Development 2016, 143, 729–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, M.; Olson, E.N.; Bassel-Duby, R. Mending broken hearts: Cardiac development as a basis for adult heart regeneration and repair. Nat. Rev. Mol. Cell. Biol. 2013, 14, 529–541. [Google Scholar] [CrossRef] [Green Version]
- Wakamatsu, Y.; Osumi, N.; A Weston, J. Expression of a novel secreted factor, Seraf indicates an early segregation of Schwann cell precursors from neural crest during avian development. Dev. Biol. 2004, 268, 162–173. [Google Scholar] [CrossRef] [Green Version]
- Giovannone, D.; Ortega, B.; Reyes, M.; El-Ghali, N.; Rabadi, M.; Sao, S.; De Bellard, M.E. Chicken trunk neural crest migration visualized with HNK1. Acta Histochem. 2015, 117, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Krueger, R.C.; A Fields, T.; Mensch, J.R.; Schwartz, N.B. Chick cartilage chondroitin sulfate proteoglycan core protein. II. Nucleotide sequence of cDNA clone and localization of the S103L epitope. J. Biol. Chem. 1990, 265, 12088–12097. [Google Scholar]
- Krueger, R.C.; Hennig, A.K.; Schwartz, N.B. Two immunologically and developmentally distinct chondroitin sulfate proteolglycans in embryonic chick brain. J. Biol. Chem. 1992, 267, 12149–12161. [Google Scholar]
- Pettway, Z.; Domowicz, M.; Schwartz, N.B.; Bronner-Fraser, M. Age-Dependent Inhibition of Neural Crest Migration by the Notochord Correlates with Alterations in the S103L Chondroitin Sulfate Proteoglycan. Exp. Cell Res. 1996, 225, 195–206. [Google Scholar] [CrossRef]
- Hirotani, H. The Calcineurin/Nuclear Factor of Activated T Cells Signaling Pathway Regulates Osteoclastogenesis in RAW264.7 Cells. J. Biol. Chem. 2004, 279, 13984–13992. [Google Scholar] [CrossRef] [Green Version]
- De La Pompa, J.L.; Timmerman, L.A.; Takimoto, H.; Yoshida, H.; Elia, A.J.; Samper, E.; Potter, J.; Wakeham, A.; Marengere, L.; Langille, B.L.; et al. Role of the NF-ATc transcription factor in morphogenesis of cardiac valves and septum. Nature 1998, 392, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Ranger, A.M.; Grusby, M.J.; Hodge, M.R.; Gravallese, E.M.; De La Brousse, F.C.; Hoey, T.; Mickanin, C.; Baldwin, H.S.; Glimcher, L.H. The transcription factor NF-ATc is essential for cardiac valve formation. Nature 1998, 392, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.A.; Kim, Y.; Czubryt, M.P.; Phan, D.; McAnally, J.; Qi, X.; Shelton, J.M.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. MEF2C Transcription Factor Controls Chondrocyte Hypertrophy and Bone Development. Dev. Cell 2007, 12, 377–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, X.-H.; Wang, F.; Zhang, X.-L.; Huang, R.-T.; Xue, S.; Wang, J.; Qiu, X.-B.; Liu, X.-Y.; Yang, Y.-Q. MEF2C loss-of-function mutation contributes to congenital heart defects. Int. J. Med. Sci. 2017, 14, 1143–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wirrig, E.E.; Hinton, R.B.; Yutzey, K.E. Differential expression of cartilage and bone-related proteins in pediatric and adult diseased aortic valves. J. Mol. Cell. Cardiol. 2011, 50, 561–569. [Google Scholar] [CrossRef] [Green Version]
- Domowicz, M.; Sanders, T.A.; Ragsdale, C.W.; Schwartz, N.B. Aggrecan is expressed by embryonic brain glia and regulates astrocyte development. Dev. Biol. 2008, 315, 114–124. [Google Scholar] [CrossRef] [Green Version]
- Umehara, Y.; Yamada, S.; Nishimura, S.; Shioi, J.; Robakis, N.K.; Sugahara, K. Chondroitin sulfate of appican, the proteoglycan form of amyloid precursor protein, produced by C6 glioma cells interacts with heparin-binding neuroregulatory factors. FEBS Lett. 2003, 557, 233–238. [Google Scholar] [CrossRef]
- Pangalos, M.N.; Shioi, J.; Efthimiopoulos, S.; Wu, A.; Robakis, N.K. Characterization of Appican, the Chondroitin Sulfate Proteoglycan Form of the Alzheimer Amyloid Precursor Protein. Neurodegeneration 1996, 5, 445–451. [Google Scholar] [CrossRef]
- Shioi, J.; Pangalos, M.N.; Ripellino, J.A.; Vassilacopoulou, D.; Mytilineou, C.; Margolis, R.U.; Robakis, N.K. The Alzheimer Amyloid Precursor Proteoglycan (Appican) Is Present in Brain and Is Produced by Astrocytes but Not by Neurons in Primary Neural Cultures. J. Biol. Chem. 1995, 270, 11839–11844. [Google Scholar] [CrossRef] [Green Version]
- Tsuchida, K.; Shioi, J.; Yamada, S.; Boghosian, G.; Wu, A.; Cai, H.; Sugahara, K.; Robakis, N.K. Appican, the Proteoglycan Form of the Amyloid Precursor Protein, Contains Chondroitin Sulfate E in the Repeating Disaccharide Region and 4-O-Sulfated Galactose in the Linkage Region. J. Biol. Chem. 2001, 276, 37155–37160. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.; Pangalos, M.N.; Efthimiopoulos, S.; Shioi, J.; Robakis, N.K. Appican Expression Induces Morphological Changes in C6 Glioma Cells and Promotes Adhesion of Neural Cells to the Extracellular Matrix. J. Neurosci. 1997, 17, 4987–4993. [Google Scholar] [CrossRef] [PubMed]
- Thielen, N.G.; Van Der Kraan, P.M.; Van Caam, A. TGFβ/BMP Signaling Pathway in Cartilage Homeostasis. Cells 2019, 8, 969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salazar, V.S.; Gamer, L.W.; Rosen, V. BMP signalling in skeletal development, disease and repair. Nat. Rev. Endocrinol. 2016, 12, 203–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.N.; Green, J.; Wang, Z.; Deng, Y.; Qiao, M.; Peabody, M.; Zhang, Q.; Ye, J.; Yan, Z.; Denduluri, S.; et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014, 1, 87–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Zhao, M.; Mundy, G.R. Bone morphogenetic proteins. Growth Factors 2004, 22, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Gross, G. BMP signaling pathways in cartilage and bone formation. Crit. Rev. Eukaryot. Gene Expr. 2001, 11, 24. [Google Scholar] [CrossRef]
- Mak, K.K.; Kronenberg, H.M.; Chuang, P.-T.; Mackem, S.; Yang, Y. Indian hedgehog signals independently of PTHrP to promote chondrocyte hypertrophy. Development 2008, 135, 1947–1956. [Google Scholar] [CrossRef] [Green Version]
- Pathi, S.; Pagan-Westphal, S.; Baker, D.P.; A Garber, E.; Rayhorn, P.; Bumcrot, D.; Tabin, C.J.; Pepinsky, R.B.; Williams, K.P. Comparative biological responses to human Sonic, Indian, and Desert hedgehog. Mech. Dev. 2001, 106, 107–117. [Google Scholar] [CrossRef]
- Vortkamp, A.; Lee, K.; Lanske, B.; Segre, G.V.; Kronenberg, H.M.; Tabin, C.J. Regulation of Rate of Cartilage Differentiation by Indian Hedgehog and PTH-Related Protein. Science 1996, 273, 613–622. [Google Scholar] [CrossRef]
- Kronenberg, H.M. PTHrP and Skeletal Development. Ann. N. Y. Acad. Sci. 2006, 1068, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Grimsrud, C.D.; Romano, P.R.; D’Souza, M.; Puzas, J.E.; Schwarz, E.M.; Reynolds, P.R.; Roiser, R.N.; O’Keefe, R.J. BMP signaling stimulates chondrocyte maturation and the expression of Indian hedgehog. J. Orthop. Res. 2001, 19, 18–25. [Google Scholar] [CrossRef]
- Lai, L.P.; Mitchell, J. Indian hedgehog: Its roles and regulation in endochondral bone development. J. Cell. Biochem. 2005, 96, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Macica, C.M.; Nasiri, A.; Broadus, A.E. Regulation of articular chondrocyte proliferation and differentiation by indian hedgehog and parathyroid hormone-related protein in mice. Arthritis Rheum. 2008, 58, 3788–3797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, F.; Zhou, J.; Wei, X.; Zhang, J.; Fleming, B.C.; Terek, R.; Pei, M.; Chen, Q.; Liu, T.; Wei, L. Activation of Indian Hedgehog Promotes Chondrocyte Hypertrophy and Upregulation of MMP-13 in Human Osteoarthritic Cartilage. Osteoarthr. Cartil. 2012, 20, 755–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohba, S. Hedgehog Signaling in Endochondral Ossification. J. Dev. Biol. 2016, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Ruoslahti, E.; Yamaguchi, Y. Proteoglycans as modulators of growth factor activities. Cell 1991, 64, 867–869. [Google Scholar] [CrossRef]
- Yoshihara, Y.; Oka, S.; Watanabe, Y.; Mori, K. Developmentally and spatially regulated expression of HNK-1 carbohydrate antigen on a novel phosphatidylinositol-anchored glycoprotein in rat brain. J. Cell Biol. 1991, 115, 731–744. [Google Scholar] [CrossRef] [Green Version]
- Allory, Y.; Commo, F.; Boccon-Gibod, L.; Sibony, M.; Callard, P.; Ronco, P.; Debiec, H. Sulfated HNK-1 Epitope in Developing and Mature Kidney: A New Marker for Thin Ascending Loop of Henle and Tubular Injury in Acute Tubular Necrosis. J. Histochem. Cytochem. 2006, 54, 575–584. [Google Scholar] [CrossRef]
- A Blom, N.; Groot, A.C.G.-D.; DeRuiter, M.C.; E Poelmann, R.; Mentink, M.M.; Ottenkamp, J. Development of the cardiac conduction tissue in human embryos using HNK-1 antigen expression: Possible relevance for understanding of abnormal atrial automaticity. Circulation 1999, 99, 800–806. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, T.; Ikeda, T.; Shimokawa, I.; Inoue, Y.; Suematsu, T.; Sakai, H.; Iwasaki, K.; Matsuo, T. Distribution of acetylcholinesterase activity in the rat embryonic heart with reference to HNK-1 immunoreactivity in the conduction tissue. Anat. Embryol. Berl 1994, 190, 367–373. [Google Scholar] [CrossRef]
- Kivelä, T. Expression of the HNK-1 carbohydrate epitope in human retina and retinoblastoma. An immunohistochemical study with the anti-Leu-7 monoclonal antibody. Virchows Arch. A Pathol. Anat. Histopathol. 1986, 410, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, M.; Blosa, M.; Schmidt, S.; Rübsamen, R.; Morawski, M. Perineuronal nets in the auditory system. Hear. Res. 2015, 329, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Miura, R.; Aspberg, A.; Ethell, I.M.; Hagihara, K.; Schnaar, R.L.; Ruoslahti, E.; Yamaguchi, Y. The Proteoglycan Lectin Domain Binds Sulfated Cell Surface Glycolipids and Promotes Cell Adhesion. J. Biol. Chem. 1999, 274, 11431–11438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miura, R.; Ethell, I.M.; Yamaguchi, Y. Carbohydrate-protein interactions between HNK-1-reactive sulfoglucuronyl glycolipids and the proteoglycan lectin domain mediate neuronal cell adhesion and neurite outgrowth. J. Neurochem. 2001, 76, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.D.; Ginsburg, V. Sulfated glycolipids and cell adhesion. Arch. Biochem. Biophys. 1988, 267, 405–415. [Google Scholar] [CrossRef]
- Roberts, D.D.; Rao, C.N.; Magnani, J.L.; Spitalnik, S.L.; Liotta, L.A.; Ginsburg, V. Laminin binds specifically to sulfated glycolipids. Proc. Natl. Acad. Sci. USA 1985, 82, 1306–1310. [Google Scholar] [CrossRef] [Green Version]
- Balint, R.; Cassidy, N.J.; Cartmell, S. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef]
- Ong, E.; Suzuki, M.; Bélot, F.; Yeh, J.-C.; Franceschini, I.; Angata, K.; Hindsgaul, O.; Fukuda, M. Biosynthesis of HNK-1 Glycans onO-Linked Oligosaccharides Attached to the Neural Cell Adhesion Molecule (NCAM). J. Biol. Chem. 2002, 277, 18182–18190. [Google Scholar] [CrossRef] [Green Version]
- Ong, E. Structure and Function of HNK-1 Sulfotransferase. IDENTIFICATION OF DONOR AND ACCEPTOR BINDING SITES BY SITE-DIRECTED MUTAGENESIS. J. Biol. Chem. 1999, 274, 25608–25612. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, N.; Izumikawa, T.; Kitagawa, H.; Oka, S. Sulfation of glucuronic acid in the linkage tetrasaccharide by HNK-1 sulfotransferase is an inhibitory signal for the expression of a chondroitin sulfate chain on thrombomodulin. Biochem. Biophys. Res. Commun. 2011, 415, 109–113. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, N.; Manya, H.; Toda, T.; Endo, T.; Oka, S. Human Natural Killer-1 Sulfotransferase (HNK-1ST)-induced Sulfate Transfer Regulates Laminin-binding Glycans on α-Dystroglycan. J. Biol. Chem. 2012, 287, 30823–30832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashiguchi, T.; Mizumoto, S.; Nishimura, Y.; Tamura, J.-I.; Yamada, S.; Sugahara, K. Involvement of Human Natural Killer-1 (HNK-1) Sulfotransferase in the Biosynthesis of the GlcUA(3-O-sulfate)-Gal-Gal-Xyl Tetrasaccharide Found in α-Thrombomodulin from Human Urine. J. Biol. Chem. 2011, 286, 33003–33011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aspberg, A.; Miura, R.; Bourdoulous, S.; Shimonaka, M.; Heinegård, D.; Schachner, M.; Ruoslahti, E.; Yamaguchi, Y. The C-type lectin domains of lecticans, a family of aggregating chondroitin sulfate proteoglycans, bind tenascin-R by protein- protein interactions independent of carbohydrate moiety. Proc. Natl. Acad. Sci. USA 1997, 94, 10116–10121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yagi, H.; Yanagisawa, M.; Suzuki, Y.; Nakatani, Y.; Ariga, T.; Kato, K.; Yu, R.K. HNK-1 Epitope-carrying Tenascin-C Spliced Variant Regulates the Proliferation of Mouse Embryonic Neural Stem Cells. J. Biol. Chem. 2010, 285, 37293–37301. [Google Scholar] [CrossRef] [Green Version]
- Saghatelyan, A.; Gorissen, S.; Albert, M.; Hertlein, B.; Schachner, M.; Dityatev, A. The extracellular matrix molecule tenascin-R and its HNK-1 carbohydrate modulate perisomatic inhibition and long-term potentiation in the CA1 region of the hippocampus. Eur. J. Neurosci. 2000, 12, 3331–3342. [Google Scholar] [CrossRef]
- Suttkus, A.; Rohn, S.; Weigel, S.; Glöckner, P.; Arendt, T.; Morawski, M. Aggrecan, link protein and tenascin-R are essential components of the perineuronal net to protect neurons against iron-induced oxidative stress. Cell Death Dis. 2014, 5, e1119. [Google Scholar] [CrossRef] [Green Version]
- Melrose, J.; Ghosh, P. The quantitative discrimination of corneal type I, but not skeletal type II, keratan sulfate in glycosaminoglycan mixtures by using a combination of dimethylmethylene blue and endo-beta-D-galactosidase digestion. Anal. Biochem. 1988, 170, 293–300. [Google Scholar] [CrossRef]
- Hitchcock, A.; Yates, K.E.; Shortkroff, S.; Costello, C.E.; Zaia, J. Optimized extraction of glycosaminoglycans from normal and osteoarthritic cartilage for glycomics profiling. Glycobiology 2007, 17, 25–35. [Google Scholar] [CrossRef]
- Samiric, T.; Ilic, M.Z.; Handley, C.J. Characterisation of proteoglycans and their catabolic products in tendon and explant cultures of tendon. Matrix Biol. 2004, 23, 127–140. [Google Scholar] [CrossRef]
- Bayliss, M.T. The Organization of Aggrecan in Human Articular Cartilage. EVIDENCE FOR AGE-RELATED CHANGES IN THE RATE OF AGGREGATION OF NEWLY SYNTHESIZED MOLECULES. J. Biol. Chem. 2000, 275, 6321–6327. [Google Scholar] [CrossRef] [Green Version]
- Lyon, M.; Greenwood, J.; Sheehan, J.K.; A Nieduszynski, I. Isolation and characterization of high-buoyant-density proteoglycans from bovine femoral-head cartilage. Biochem. J. 1983, 213, 355–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilím, V.; Fosang, A.J. Proteoglycans isolated from dissociative extracts of differently aged human articular cartilage: Characterization of naturally occurring hyaluronan-binding fragments of aggrecan. Biochem. J. 1994, 304, 887–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hileman, R.E.; Fromm, J.R.; Weiler, J.M.; Linhardt, R.J. Glycosaminoglycan-protein interactions: Definition of consensus sites in glycosaminoglycan binding proteins. BioEssays 1998, 20, 156–167. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, F.; Linhardt, R.J. Analysis of the Glycosaminoglycan Chains of Proteoglycans. J. Histochem. Cytochem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Vallet, S.; Clerc, O.; Ricard-Blum, S. Glycosaminoglycan-Protein Interactions: The First Draft of the Glycosaminoglycan Interactome. J. Histochem. Cytochem. 2020. [Google Scholar] [CrossRef]
- Karamanos, N.; Hjerpe, A. Strategies for analysis and structure characterization of glycans/proteoglycans by capillary electrophoresis. Their diagnostic and biopharmaceutical importance. Biomed. Chromatogr. 1999, 13, 507–512. [Google Scholar] [CrossRef]
- Lamari, F.N.; Militsopoulou, M.; Mitropoulou, T.N.; Hjerpe, A.; Karamanos, N.K. Analysis of glycosaminoglycan-derived disaccharides in biologic samples by capillary electrophoresis and protocol for sequencing glycosaminoglycans. Biomed. Chromatogr. 2002, 16, 95–102. [Google Scholar] [CrossRef]
- Lu, G.; Crihfield, C.L.; Gattu, S.; Veltri, L.M.; Holland, L.A. Capillary Electrophoresis Separations of Glycans. Chem. Rev. 2018, 118, 7867–7885. [Google Scholar] [CrossRef] [Green Version]
- Gill, V.L.; Aich, U.; Rao, S.; Pohl, C.A.; Zaia, J. Disaccharide Analysis of Glycosaminoglycans Using Hydrophilic Interaction Chromatography and Mass Spectrometry. Anal. Chem. 2013, 85, 1138–1145. [Google Scholar] [CrossRef] [Green Version]
- Staples, G.O.; Zaia, J. Analysis of Glycosaminoglycans Using Mass Spectrometry. Curr. Proteom. 2011, 8, 325–336. [Google Scholar] [CrossRef] [Green Version]
- Zaia, J. Glycosaminoglycan glycomics using mass spectrometry. Mol. Cell. Proteom. 2013, 12, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Volpi, N. Disaccharide Analysis and Molecular Mass Determination to Microgram Level of Single Sulfated Glycosaminoglycan Species in Mixtures Following Agarose-Gel Electrophoresis. Anal. Biochem. 1999, 273, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Ernst, S.; Langer, R.; Cooney, C.L.; Sasisekharan, R. Enzymatic degradation of glycosaminoglycans. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 387–444. [Google Scholar] [CrossRef] [PubMed]
- Hernáiz, M.J.; Linhardt, R.J.; Iozzo, R.V. Degradation of Chondroitin Sulfate and Dermatan Sulfate with Chondroitin Lyases. Methods Mol. Biol. 2001, 171, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Kaneiwa, T.; Mizumoto, S.; Sugahara, K.; Yamada, S. Identification of human hyaluronidase-4 as a novel chondroitin sulfate hydrolase that preferentially cleaves the galactosaminidic linkage in the trisulfated tetrasaccharide sequence. Glycobiology 2009, 20, 300–309. [Google Scholar] [CrossRef]
- Linhardt, R.J.; Avci, F.Y.; Toida, T.; Kim, Y.S.; Cygler, M. CS Lyases: Structure, Activity, and Applications in Analysis and the Treatment of Diseases. Adv. Pharmacol. 2006, 53, 187–215. [Google Scholar] [CrossRef] [Green Version]
- Petit, E.; Delattre, C.; Papy–Garcia, D.; Michaud, P. Chondroitin Sulfate Lyases: Applications in Analysis and Glycobiology. Adv. Pharmacol 2006, 53, 167–186. [Google Scholar] [CrossRef]
- Plaas, A.H.; West, L.; Midura, R.J.; Hascall, V.C.; Iozzo, R.V. Disaccharide Composition of Hyaluronan and Chondroitin/Dermatan Sulfate: Analysis with Fluorophore-Assisted Carbohydrate Electrophoresis. Methods Mol. Biol. 2003, 171, 117–128. [Google Scholar] [CrossRef]
- Komarraju, A.; Goldberg-Stein, S.; Pederson, R.; McCrum, C.; Chhabra, A. Spectrum of common and uncommon causes of knee joint hyaline cartilage degeneration and their key imaging features. Eur. J. Radiol. 2020, 129, 109097. [Google Scholar] [CrossRef]
- Kim, J.; Mamoto, K.; Lartey, R.; Nakamura, K.; Shin, W.; Winalski, C.S.; Obuchowski, N.; Tanaka, M.; Bahroos, E.; Link, T.M.; et al. Multi-vendor multi-site T1ρ and T2 quantification of knee cartilage. Osteoarthr. Cartil. 2020. [Google Scholar] [CrossRef]
- Abrar, D.B.; Schleich, C.; Nebelung, S.; Frenken, M.; Ullrich, T.; Radke, K.L.; Antoch, G.; Vordenbäumen, S.; Brinks, R.; Schneider, M.; et al. Proteoglycan loss in the articular cartilage is associated with severity of joint inflammation in psoriatic arthritis—A compositional magnetic resonance imaging study. Arthritis Res. 2020, 22, 124. [Google Scholar] [CrossRef] [PubMed]
- Abrar, D.B.; Schleich, C.; Radke, K.L.; Frenken, M.; Stabinska, J.; Ljimani, A.; Wittsack, H.-J.; Antoch, G.; Bittersohl, B.; Hesper, T.; et al. Detection of early cartilage degeneration in the tibiotalar joint using 3 T gagCEST imaging: A feasibility study. Magn. Reson. Mater. Physics, Biol. Med. 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dou, W.; Lin, C.-Y.E.; Ding, H.; Shen, Y.; Dou, C.; Qian, L.; Wen, B.; Wu, B. Chemical exchange saturation transfer magnetic resonance imaging and its main and potential applications in pre-clinical and clinical studies. Quant. Imaging Med. Surg. 2019, 9, 1747–1766. [Google Scholar] [CrossRef] [PubMed]
- Müller-Lutz, A.; Kamp, B.; Nagel, A.M.; Ljimani, A.; Abrar, D.; Schleich, C.; Wollschläger, L.; Nebelung, S.; Wittsack, H.-J. Sodium MRI of human articular cartilage of the wrist: A feasibility study on a clinical 3T MRI scanner. Magn. Reson. Mater. Physics Biol. Med. 2020. [Google Scholar] [CrossRef]
- Zbyn, S.; Schreiner, M.; Juras, V.; Mlynarik, V.; Szomolanyi, P.; Laurent, D.; Scotti, C.; Haber, H.; Deligianni, X.; Bieri, O.; et al. Assessment of Low-Grade Focal Cartilage Lesions in the Knee with Sodium MRI at 7 T. Investig. Radiol. 2020, 55, 430–437. [Google Scholar] [CrossRef]
- Tibrewala, R.; Pedoia, V.; Bucknor, M.; Majumdar, S. Principal Component Analysis of Simultaneous PET-MRI Reveals Patterns of Bone-Cartilage Interactions in Osteoarthritis. J. Magn. Reson. Imaging 2020. [Google Scholar] [CrossRef]
- Noguerol, T.M.; Raya, J.G.; E Wessell, D.; Vilanova, J.C.; Rossi, I.; Luna, A. Functional MRI for evaluation of hyaline cartilage extracelullar matrix, a physiopathological-based approach. Br. J. Radiol. 2019, 92, 20190443. [Google Scholar] [CrossRef]
- Muir, E.M.; De Winter, F.; Verhaagen, J.; Fawcett, J. Recent advances in the therapeutic uses of chondroitinase ABC. Exp. Neurol. 2019, 321, 113032. [Google Scholar] [CrossRef]
- Rani, A.; Patel, S.; Goyal, A. Chondroitin Sulfate (CS) Lyases: Structure, Function and Application in Therapeutics. Curr. Protein Pept. Sci. 2017, 19, 22–33. [Google Scholar] [CrossRef]
- Morgenstern, D.; Asher, R.A.; Fawcett, J.W. Chondroitin sulphate proteoglycans in the CNS injury response. Prog. Brain Res. 2002, 137, 313–332. [Google Scholar] [CrossRef]
- Tachi, Y.; Okuda, T.; Kawahara, N.; Kato, N.; Ishigaki, Y.; Matsumoto, T. Expression of Hyaluronidase-4 in a Rat Spinal Cord Hemisection Model. Asian Spine J. 2015, 9, 7–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradbury, E.J.; Carter, L.M. Manipulating the glial scar: Chondroitinase ABC as a therapy for spinal cord injury. Brain Res. Bull. 2011, 84, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Barritt, A.W.; Davies, M.; Marchand, F.; Hartley, R.; Grist, J.; Yip, P.; McMahon, S.B.; Bradbury, E.J. Chondroitinase ABC promotes sprouting of intact and injured spinal systems after spinal cord injury. J. Neurosci. 2006, 26, 10856–10867. [Google Scholar] [CrossRef] [PubMed]
- Cafferty, W.B.J.; Bradbury, E.J.; Lidierth, M.; Jones, M.; Duffy, P.J.; Pezet, S.; McMahon, S.B. Chondroitinase ABC-mediated plasticity of spinal sensory function. J. Neurosci. 2008, 28, 11998–12009. [Google Scholar] [CrossRef]
- Bradbury, E.J.; Moon, L.; Popat, R.J.; King, V.R.; Bennett, G.S.; Patel, P.N.; Fawcett, J.W.; McMahon, S.B. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 2002, 416, 636–640. [Google Scholar] [CrossRef]
- James, N.D.; Shea, J.; Muir, E.M.; Verhaagen, J.; Schneider, B.; Bradbury, E.J. Chondroitinase gene therapy improves upper limb function following cervical contusion injury. Exp. Neurol. 2015, 271, 131–135. [Google Scholar] [CrossRef] [Green Version]
- Koh, C.H.; Pronin, S.; Hughes, M. Chondroitinase ABC for neurological recovery after acute brain injury: Systematic review and meta-analyses of preclinical studies. Brain Inj. 2018, 32, 715–729. [Google Scholar] [CrossRef]
- Day, P.; Alves, N.; Daniell, E.; Dasgupta, D.; Ogborne, R.; Steeper, A.; Raza, M.; Ellis, C.; Fawcett, J.; Keynes, R.; et al. Targeting chondroitinase ABC to axons enhances the ability of chondroitinase to promote neurite outgrowth and sprouting. PLoS ONE 2020, 15, e0221851. [Google Scholar] [CrossRef] [Green Version]
- Fawcett, J.W. The extracellular matrix in plasticity and regeneration after CNS injury and neurodegenerative disease. Prog. Brain Res. 2015, 218, 213–226. [Google Scholar] [CrossRef]
- Monfort, J.; Pelletier, J.-P.; Garcia-Giralt, N.; Martel-Pelletier, J. Biochemical basis of the effect of chondroitin sulphate on osteoarthritis articular tissues. Ann. Rheum. Dis. 2008, 67, 735–740. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Tat, S.K.; Pelletier, J.-P. Effects of chondroitin sulfate in the pathophysiology of the osteoarthritic joint: A narrative review. Osteoarthr. Cartil. 2010, 18, S7–S11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochberg, M.; Chevalier, X.; Henrotin, Y.; Hunter, D.; Uebelhart, D. Symptom and structure modification in osteoarthritis with pharmaceutical-grade chondroitin sulfate: What’s the evidence? Curr. Med. Res. Opin. 2013, 29, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Honvo, G.; Bruyère, O.; Reginster, J.-Y. Update on the role of pharmaceutical-grade chondroitin sulfate in the symptomatic management of knee osteoarthritis. Aging Clin. Exp. Res. 2019, 31, 1163–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pomin, V.H.; Vignovich, W.P.; Gonzales, A.V.; Vasconcelos, A.A.; Mulloy, B. Galactosaminoglycans: Medical Applications and Drawbacks. Molecules 2019, 24, 2803. [Google Scholar] [CrossRef] [Green Version]
- Reginster, J.-Y.; Veronese, N. Highly purified chondroitin sulfate: A literature review on clinical efficacy and pharmacoeconomic aspects in osteoarthritis treatment. Aging Clin. Exp. Res. 2020, 1–11. [Google Scholar] [CrossRef]
- Hayes, A.J.; Melrose, J. Glycosaminoglycan and Proteoglycan Biotherapeutics in Articular Cartilage Protection and Repair Strategies: Novel Approaches to Visco–supplementation in Orthobiologics. Adv. Ther. 2019, 2, 1900034. [Google Scholar] [CrossRef]
- Farrugia, B.; Hayes, A.J.; Melrose, J. Use of chondroitin sulphate to aid in-vitro stem cell differentiation In Proteoglycans in stem cells: From development to cancer. Biol. Extracell. Matrix 2020. submitted. [Google Scholar]
- Melrose, J. Glycosaminoglycans in Wound Healing. Bone Tissue Regen. Insights 2016, 7, 29–50. [Google Scholar] [CrossRef] [Green Version]
- Farrugia, B.L.; Lord, M.S.; Whitelock, J.M.; Melrose, J. Harnessing chondroitin sulphate in composite scaffolds to direct progenitor and stem cell function for tissue repair. Biomater. Sci. 2018, 6, 947–957. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayes, A.J.; Melrose, J. Aggrecan, the Primary Weight-Bearing Cartilage Proteoglycan, Has Context-Dependent, Cell-Directive Properties in Embryonic Development and Neurogenesis: Aggrecan Glycan Side Chain Modifications Convey Interactive Biodiversity. Biomolecules 2020, 10, 1244. https://doi.org/10.3390/biom10091244
Hayes AJ, Melrose J. Aggrecan, the Primary Weight-Bearing Cartilage Proteoglycan, Has Context-Dependent, Cell-Directive Properties in Embryonic Development and Neurogenesis: Aggrecan Glycan Side Chain Modifications Convey Interactive Biodiversity. Biomolecules. 2020; 10(9):1244. https://doi.org/10.3390/biom10091244
Chicago/Turabian StyleHayes, Anthony J, and James Melrose. 2020. "Aggrecan, the Primary Weight-Bearing Cartilage Proteoglycan, Has Context-Dependent, Cell-Directive Properties in Embryonic Development and Neurogenesis: Aggrecan Glycan Side Chain Modifications Convey Interactive Biodiversity" Biomolecules 10, no. 9: 1244. https://doi.org/10.3390/biom10091244
APA StyleHayes, A. J., & Melrose, J. (2020). Aggrecan, the Primary Weight-Bearing Cartilage Proteoglycan, Has Context-Dependent, Cell-Directive Properties in Embryonic Development and Neurogenesis: Aggrecan Glycan Side Chain Modifications Convey Interactive Biodiversity. Biomolecules, 10(9), 1244. https://doi.org/10.3390/biom10091244




