Molecules, Mechanisms, and Disorders of Self-Domestication: Keys for Understanding Emotional and Social Communication from an Evolutionary Perspective
Abstract
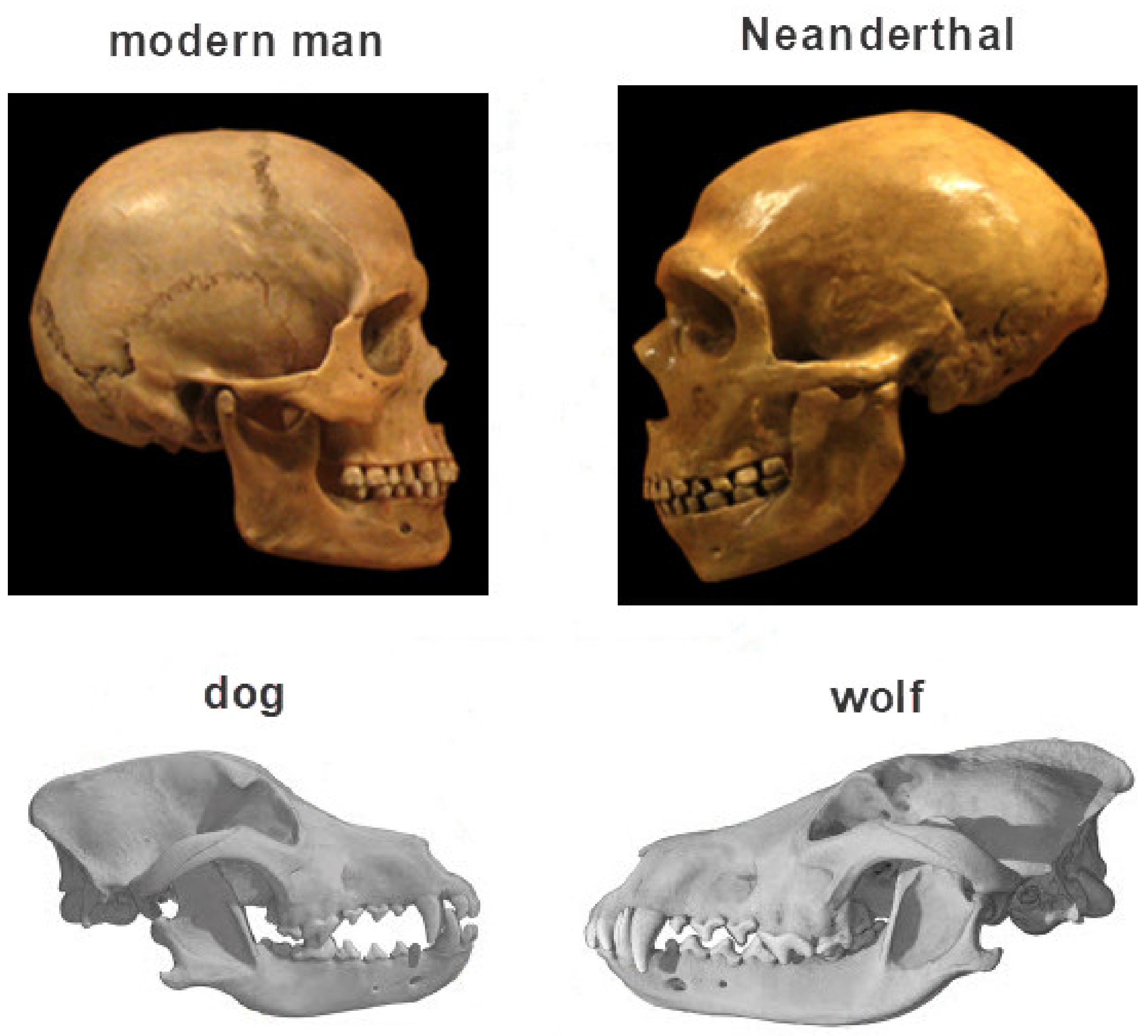
:1. The Concept of Self-Domestication and Its Possible Dependance on Neural Crest Cells (NCCs)
2. Basic Biology of NCCs
3. Human NCC-Dependent Disorders
4. Neoteny
5. Willams-Beuren Syndrome and Schizophrenia
6. Autism Spectrum Disorder (ASD)
“We think that the process of domestication, however, is fundamentally different (from evolution, authors’ comment): separation of a species from its natural ecological context, development of a form of this species via artificial selection (mostly eliminating natural selection, loss in genetic diversity) in an artificial environment that is dramatically different from the natural environment. In short: the nature of the domestication process appears as an attempt to minimize the driving forces of evolution as far as possible. Not surprisingly, the rules and laws of population ecology and population genetics are no longer applicable to domesticated forms (see temporal dimension, and numerical dimension).”(page 8, lines 4-9)
7. Evolutionary Importance of Emotions
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Darwin, C. The Variation of Plants and Animals under Domestication; John Murray: London, UK, 1868. [Google Scholar]
- Wilkins, A.S.; Wrangham, R.W.; Fitch, W.T. The “domestication syndrome“ in mammals: A unified explanation based on neural crest cell behavior and genetics. Genetics 2014, 197, 795–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Villagra, M.R.; Geiger, M.; Schneider, R.A. The taming of the neural crest: A developmental perspective on the origins of morphological covariation in domesticated mammals. R. Soc. Open Sci. 2016, 3, 160107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheat, C.H.; van der Bijl, W.; Wheat, C.W. Morphology does not covary with predicted behavioral correlations of the domestication syndrome in dogs. BioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Irving-Pease, E.K.; Ryan, H.; Jamieson, A.; Dimopoulos, E.A. Paleogenomics of animal domestication. In Paleogenomics. Population Genomics; Lindqvist, C., Rajora, O., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 225–272. [Google Scholar]
- Machugh, D.E.; Larson, G.; Ludovic, O. Taming the past: Ancient DNA and the study of animal domestication. Annu. Rev. Anim. Biosci. 2016, 5, 329–351. [Google Scholar] [CrossRef] [Green Version]
- Gregor, M. Versuche über Plflanzenhybriden. Verh. Naturf. Vereins Brünn 1866, 4, 3–47. [Google Scholar]
- Galton, D. Did Darwin read Mendel? Q. J. Med. 2009, 102, 587–589. [Google Scholar] [CrossRef]
- Kruska, D.C. On the evolutionary significance of encephalization in some eutherian mammals: Effects of adaptive radiation, domestication, and feralization. Brain Behav. Evol. 2005, 65, 73–108. [Google Scholar] [CrossRef]
- Belyaev, D.K. Domestication in animals. Sci. J. 1969, 5, 47–52. [Google Scholar]
- Dugatkin, L.A. The silver fox domestication experiment. Evo. Edu. Outreach. 2018, 11, 16. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Pipes, L.; Trut, L.N.; Herbeck, Y.; Vladimirova, A.V.; Gulevich, R.G.; Kharlamova, A.V.; Johnson, J.L.; Acland, G.M.; Kukekova, A.V.; et al. Genomic responses to selection for tame/aggressive behaviors in the silver fox (Vulpes vulpes). Proc. Natl. Acad. Sci. USA 2018, 115, 10398–10403. [Google Scholar] [CrossRef] [Green Version]
- Rosenfeld, C.S.; Hekman, J.P.; Johnson, J.L.; Lyu, Z.; Ortega, M.T.; Joshi, T.; Mao, J.; Vladimirova, A.V.; Gulevich, R.G.; Kharlamova, A.V.; et al. Hypothalamic transcriptome of tame and aggressive silver foxes (Vulpes vulpes) identifies gene expression differences shared across brain regions. Genes Brain Behav. 2020, 19, e12614. [Google Scholar] [CrossRef] [PubMed]
- Belyaev, D.K. Destabilizing selection as a factor of domestication. J. Hered. 1979, 70, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Feeney, A.; Nilsson, E.; Skinner, M.K. Epigenetics and transgenerational inheritance in domesticated farm animals. J. Anim. Sci. Biotechnol. 2014, 5, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoenebeck, J.J.; Hutchinson, S.A.; Byers, A.; Beale, H.C.; Carrington, B.; Faden, D.L.; Rimbault, M.; Decker, B.; Kidd, J.M.; Sood, R.; et al. Variation of BMP3 contributes to dog breed skull diversity. PLoS Genet. 2012, 8, e1002849. [Google Scholar] [CrossRef] [Green Version]
- Trut, L.N.; Plyusnina, I.; Oskina, I. An experiment on fox domestication and debatable issues of evolution of the dog. Russ. J. Genet. 2004, 40, 644–655. [Google Scholar] [CrossRef]
- Trut, L.N.; Oskina, I.; Kharlamova, I. Animal evolution during domestication: The domesticated fox as a model. Bioessays 2009, 31, 349–360. [Google Scholar] [CrossRef] [Green Version]
- Crockford, S.J. (Ed.) Dog evolution: A role for thyroid hormone physiology in domestication changes. In Dogs Through Time: An Archaeological Perspective; BAR International Series: Oxford, UK, 2000. [Google Scholar]
- Wright, D. The genetic architecture of domestication in animals. Bioinform. Biol. Insights 2015, 9 (Suppl. 4), 11–20. [Google Scholar]
- Gans, C.; Northcutt, R.G. Neural crest and the origin of vertebrates: A new head. Science 1983, 220, 268–273. [Google Scholar] [CrossRef]
- Crockford, S.J. Thyroid rhythm phenotypes and hominid evolution: A new paradigm implicates pulsatile hormone secretion in speciation and adaptation changes. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2003, 135, 105–129. [Google Scholar] [CrossRef]
- Forni, P.E.; Wray, S. Neural crest and olfactory system: New prospective. Mol. Neurobiol. 2012, 46, 349–360. [Google Scholar] [CrossRef] [Green Version]
- Northcutt, R. The new head hypothesis revisited. J. Exp. Zool. B. Mol. Dev. Evol. 2005, 304, 274–297. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.K. The neural crest as a fourth germ layer and vertebrates as quadroblastic not triploblastic. Evol. Dev. 2000, 2, 3–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shymala, K.; Yanduri, S.; Girish, H.C.; Murgod, S. Neural crest: The fourth germ layer. J. Oral. Maxillofac. Pathol. 2015, 19, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Marieb, E.N.; Hoehn, K. Human Anatomy and Physiology, 11th ed.; Pearson Publishers: New York, NY, USA, 2018. [Google Scholar]
- Kaltschmidt, B.; Kaltschmidt, C.; Widera, D. Adult craniofacial stem cells: Sources and relation to the neural crest. Stem Cell Rev. Rep. 2012, 8, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Strobl-Mazzulla, P.H.; Bronner, M.E. Epigenetic regulation of neural crest cells. In Neural Crest Cells. Evolution, Development and Disease; Trainor, P., Ed.; Academic Press: London, UK, 2014; pp. 89–100. [Google Scholar]
- His, W. Untersuchungen über die erste Anlage des Wirbelthierleibes. Die erste Entwicklung des Hühnchens im Ei; F. C. W. Vogel: Leipzig, Germany, 1868; p. 237. [Google Scholar]
- Szabó, A.; Mayor, R. Mechanisms of neural crest migration. Annu. Rev. Genet. 2018, 52, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Christ, B. Grundzüge der Entwicklung des Bewegungsapparates und der neuromuskulären Verknüpfungen. In Benninghoff Anatomie 1; Staubesand, J., Ed.; Urban Und Schwarzenberg: Munchen, Germany; Wien, NY, USA; Baltimore, MD, USA, 1985. [Google Scholar]
- McLennan, R.; Teddy, J.M.; Kasemeier-Kulesa, J.C.; Romine, M.H.; Kulesa, P.M. Vascular endothelial growth factor regulates cranial neural crest migration in vivo. Dev. Biol. 2010, 339, 114–125. [Google Scholar] [CrossRef] [Green Version]
- Schoenwolf, G.; Bleyl, S.; Brauer, P.; Francis-West, P. Larsen’s Human Embryology, 5th ed.; Churchill Livingstone: Philadelphia, PA, USA, 2014. [Google Scholar]
- Simões-Costa, M.; Bronner, M.E. Establishing neural crest identity: A gene regulatory recipe. Development 2015, 142, 242–257. [Google Scholar] [CrossRef] [Green Version]
- Crane, J.F.; Trainor, P.A. Neural crest stem and progenitor cells. Annu. Rev. Cell Dev. Biol. 2006, 22, 267–286. [Google Scholar] [CrossRef]
- Creuzet, S.; Schuler, B.; Couly, G.; Le Douarin, N.M. Reciprocal relationships between Fgf8 and neural crest cells in facial and forebrain development. Proc. Natl. Acad. Sci. USA 2004, 101, 4843–4847. [Google Scholar] [CrossRef] [Green Version]
- Kolesnikova, L.A. Circadian rhythm of biosynthetic activity of the epiphysis in relatively wild and domesticated silver foxes. Genetika 1997, 33, 1144–1148. [Google Scholar]
- Green, S.A.; Simões-Costa, M.; Bronner, M.E. Evolution of vertebrates as viewed from the crest. Nature 2015, 520, 474–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vega-Lopez, G.A.; Cerrizuela, S.; Tribulo, C.; Aybar, M.J. Neurocristopathies: New insights 150 years after the neural crest discovery. Dev. Biol. 2018, 444 (Suppl. 1), S110–S143. [Google Scholar] [CrossRef] [PubMed]
- Steel, K.P.; Barkway, C. Another role for melanocytes: Their importance for normal stria vascularis development in the mammalian inner ear. Development 1989, 107, 453–463. [Google Scholar] [PubMed]
- Locher, H.; de Groot, J.C.M.J.; van Iperen, L.; Huisman, M.A.; Frijns, J.H.M.; de Sousa Lopes, S.M.C. Development of the stria vascularis and potassium regulation in the human fetal coclea: Insights into hereditary sensorineural hearing loss. Dev. Neurobiol. 2015, 75, 1219–1240. [Google Scholar] [CrossRef] [Green Version]
- Sandell, L. Neural crest cells in ear development. In Neural Crest Cells. Evolution, Development and Disease; Trainor, P., Ed.; Academic Press: London, UK, 2014; pp. 167–187. [Google Scholar]
- Darwin, C. The Descent of Man, and Selection in Relation to Sex; John Murray: London, UK, 1871. [Google Scholar]
- Theofanopoulou, C.; Gastaldon, S.; O’Rourke, T.; Samuels, B.D.; Martins, P.T.; Delogu, F.; Alamri, S.; Boeckx, C. Self-domestication in Homo sapiens: Insights from comparative genomics. PLoS ONE 2017, 12, e0185306. [Google Scholar] [CrossRef] [Green Version]
- Gould, S.J. Ontogeny and Phylogeny; Belknap Press: Cambridge, MA, USA, 1977. [Google Scholar]
- Kollmann, J. Das Überwintern von europäischen Frosch- und Tritonlarven und die Umwandlung des mexikanischen Axolotl. Verh. Naturf. Ges. Basel 1885, 7, 387–398. [Google Scholar]
- Bogin, B. Evolutionary hypotheses for human childhood. Yrbk Phys. Anthropol. 1997, 40, 63–89. [Google Scholar] [CrossRef] [Green Version]
- Skulachev, V.P.; Holtze, S.; Vyssokikh, M.Y.; Bakeeva, L.E.; Skulachev, M.V.; Markov, A.V.; Hildebrandt, T.B.; Sadovnichii, V.A. Neoteny, prolongation of youth: From naked mole rats to „naked apes“ (humans). Physiol. Rev. 2017, 97, 699–720. [Google Scholar] [CrossRef] [Green Version]
- Safi, R.; Bertrand, S.; Marchand, O.; Duffraisse, M.; de Luze, A.; Vanacker, J.M.; Maraninchi, M.; Margotat, A.; Demeneix, B.; Laudet, V. The axolotl (Ambystoma mexicanum), a neotenic amphibian, expresses functional thyroid hormone receptors. Endocrinology 2004, 145, 760–772. [Google Scholar] [CrossRef] [Green Version]
- Rosenkilde, P.; Ussing, A.P. What mechanisms control neoteny and regulate induced metamorphosis in urodeles? Int. J. Dev. Biol. 1996, 40, 665–673. [Google Scholar]
- Leloup, J.; Buscaglia, M. La triiodothyronine hormone de la métamorphose des amphibiens. C. R. Acad. Sci. Ser. D. 1977, 284, 2261–2263. [Google Scholar]
- Bizjak-Mali Lj Sepčić, K.; Bulog, B. Long-term starvation in cave salamander effects on liver ultrastructure and energy reserve mobilization. J. Morphol. 2013, 274, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Voituron, Y.; de Fraipont, M.; Issartel, J.; Gaillaume, O.; Clobert, J. Extreme lifespan of the human fish (Proteus anguinus): A challenge for ageing mechanisms. Biol. Lett. 2011, 7, 105–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huynh, T.D.H.; Gallien, C.L.; Durand, J.P.; Chanoine, C. Cloning and expression of a thyroide hormone receptor α1 in the perennibranchiate amphibian Proteus anguinus. Int. J. Dev. Biol. 1996, 40, 537–543. [Google Scholar]
- McDaniel, T.V.; Martin, P.A.; Barrett, G.C.; Hughes, K.; Gendron, A.D.; Shirose, L.; Bishop, C.A. Relative abundance, age structure, and body size in mudpuppy populations in southwestern Ontario. J. Great Lakes Res. 2009, 35, 182–189. [Google Scholar] [CrossRef]
- Larson, J.; Drew, K.L.; Folkow, L.P.; Milton, S.L.; Park, T.J. No oxygen? No problem! Intrinsic brain tolerance to hypoxia in vertebrates. J. Exp. Biol. 2014, 217, 1024–1039. [Google Scholar] [CrossRef] [Green Version]
- Montagu, A. Growing Young, 2nd ed.; Bergin and Garvey: Granby, MA, USA, 1989; p. 226. [Google Scholar]
- Kollmann, J. Neue Gedanken über das Alter Problem von der Abstammung des Menschen. Bl. Dtsch. Ges. Anthropol. Ethnol. Urges. 1905, 36, 9–20. [Google Scholar]
- Naef, A. Über die Urformen der Anthropomorphen und die Stammesgeschichte des Menschenschädels. Naturwissenschaften 1926, 14, 445–452. [Google Scholar] [CrossRef]
- Starck, D.; Kummer, B. Zur Ontogenese des Schimpansenschädels. Anthropol. Anz. 1962, 25, 204–215. [Google Scholar]
- Verhulst, J. Louis Bolk revisited. II–Retardation, hypermorphosis and body proportions of humans. Med. Hypotheses 1993, 41, 100–114. [Google Scholar] [CrossRef]
- Schultz, A.H. Fetal growth of man and other primates. Quart. Rev. Biol. 1926, 1, 465–521. [Google Scholar] [CrossRef]
- Bolk, L. Das Problem der Menschwerdung; Gustav Fischer: Jena, Germany, 1926; pp. 1–44. [Google Scholar]
- Bolk, L. On the problem of anthropogenesis. Proc. Sect. Sci. Kon. Akad. Wetens Amst. 1926, 29, 465–475. [Google Scholar]
- Bolk, L. On the origin of human races. Proc. Sect. Sci. Kon. Akad. Wetens. Amst. 1927, 30, 320–328. [Google Scholar]
- Bolk, L. Origin of racial characteristics in man. Am. J. Phys. Anthropol. 1929, 13, 1–28. [Google Scholar] [CrossRef]
- Clark, G.; Henneberg, M. The life history of Ardipithecus ramidus: A heterochronic model of sexual and social maturation. Anthropol. Rev. 2015, 78, 109–132. [Google Scholar] [CrossRef] [Green Version]
- White, T.D.; Suwa, G.; Asfaw, B. Australopithecus ramidus, a new species of early hominid from Aramis, Ethiopia. Nature 1994, 371, 306–312. [Google Scholar] [CrossRef]
- Simpson, S.W.; Levin, N.E.; Quade, J.; Rogers, M.J.; Semaw, S. Ardipithecus ramidus postcrania from the Gona Project area, Afar Regional State, Ethiopia. J. Hum. Evol. 2019, 129, 1–45. [Google Scholar] [CrossRef]
- Lovejoy, C.O.; Suwa, G.; Spurlock, L.; Asfaw, B.; White, T.D. The pelvis and femur of Ardipithecus ramidus: The emergence of upright walking. Science 2009, 326, 71-71e6. [Google Scholar] [CrossRef] [Green Version]
- Kimbel, W.H.; Suwa, G.; Asfaw, B.; Rak, Y.; White, T.D. Ardipithecus ramidus and the evolution of the human cranial base. Proc. Natl. Acad. Sci. USA 2014, 111, 948–953. [Google Scholar] [CrossRef] [Green Version]
- Penin, X.; Berge, C.; Baylac, M. Ontogenetic study of the skull in modern humans and the common chimpanzees: Neotenic hypothesis reconsidered with a tridimensional Procrustes analysis. Am. J. Phys. Anthropol. 2002, 118, 50–62. [Google Scholar] [CrossRef]
- Bufill, E.; Agustí, J.; Blesa, R. Human neoteny revisited: The case of synaptic plasticity. Am. J. Hum. Biol. 2011, 23, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; Brace, C.L.; Jankowiak, W.; Laland, K.N.; Musselman, L.E.; Langlois, J.H.; Roggman, L.A.; Pérusse, D.; Schweder, B.; Symons, D. Sexual selection, physical attractiveness, and facial neoteny: Cross-cultural evidence and implications. Curr. Anthropol. 1995, 36, 723–748. [Google Scholar] [CrossRef] [Green Version]
- Simpson, J.A.; Kenrick, D.T. (Eds.) Evolutionary Social Psychology; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 1997. [Google Scholar]
- Greenspan, S.I.; Shanker, S. The First Idea: How Symbols, Language, and Intelligence Evolved from our Primate Ancestors to Modern Humans; Da Capo Press: Cambridge, MA, USA, 2004. [Google Scholar]
- Thomas, J. Self-Domestication and Language Evolution Disertation; University of Edinburgh: Edinburgh, UK, 2014. [Google Scholar]
- Petanjek, Z.; Judaš, M.; Šimić, G.; Rašin, M.R.; Uylings, H.B.; Rakic, P.; Kostović, I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc. Natl. Acad. Sci. USA 2011, 108, 13281–13286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somel, M.; Franz, H.; Yan, Z.; Lorenc, A.; Guo, S.; Giger, T.; Kelso, J.; Nickel, B.; Dannemann, M.; Bahn, S.; et al. Transcriptional neoteny in the human brain. Proc. Natl. Acad. Sci. USA 2009, 106, 5743–5748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cáceres, M.; Lachuer, J.; Zapala, M.A.; Redmond, J.C.; Kudo, L.; Geschwind, D.H.; Lockhart, D.J.; Preuss, T.M.; Barlow, C. Elevated gene expression levels distinguish human from non-human primate brains. Proc. Natl. Acad. Sci. USA 2003, 100, 13030–13035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blinkov, S.; Glezer, I. The Human Brain in Figures and Tables; BasicBooks: New York, NY, USA, 1968. [Google Scholar]
- Preuss, T.M. The human brain: Rewired and running hot. Ann. N. Y. Acad. Sci. 2011, 1225 (Suppl. 1), E182–E191. [Google Scholar] [CrossRef] [Green Version]
- Šimić, G.; Španić, E.; Langer Horvat, L.; Hof, P.R. Blood-brain barrier and innate immunity in the pathogenesis of Alzheimer’s disease. Prog. Mol. Biol. Transl. Sci. 2019, 168, 99–145. [Google Scholar]
- Bogin, B. Modern human life history: The evolution of human childhood and fertility. In The Evolution of Human Life History; Paine, R.R., Hawkes, K., Eds.; School of American Research Press: Santa Fe, NM, USA, 2006; pp. 197–230. [Google Scholar]
- Kleiber, M. Body size and metabolic rate. Physiol. Rev. 1947, 27, 511–541. [Google Scholar] [CrossRef]
- Bélanger, M.; Allaman, I.; Magistretti, P.J. Brain energy metabolism: Focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011, 14, 724–738. [Google Scholar] [CrossRef] [Green Version]
- Bak, L.K.; Schousboe, A.; Waagepetersen, H.S. The glutamate/GABA-glutamine cycle: Aspects of transport, neurotransmitter homeostasis and ammonia transfer. J. Neurochem. 2006, 98, 641–653. [Google Scholar] [CrossRef]
- Suárez, I.; Bodega, G.; Fernández, B. Glutamine synthetase in brain: Effect of ammonia. Neurochem. Int. 2002, 41, 123–142. [Google Scholar] [CrossRef]
- MacVicar, B.A.; Newman, E.A. Astrocyte regulation of blood flow in the brain. Cold Spring Harb. Perspect. Biol. 2015, 7, a020388. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, K. Über Tierisches und Menschliches Verhalten; Gesammelte Abhandlungen, Band 1. Werdegang des Verhaltenslehre; Piper: München, Germany, 1965. [Google Scholar]
- Glocker, M.L.; Langleben, D.D.; Ruparel, K.; Loughead, J.W.; Valdez, J.N.; Griffin, M.D.; Sachser, N.; Gur, R.C. Baby schema modulates the brain reward system in nulliparous women. Proc. Natl. Acad. Sci. USA 2009, 106, 9115–9119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gould, S.J. A biological homage to Mickey Mouse. Ecotone 2008, 1, 333–340. [Google Scholar] [CrossRef]
- Niego, A.; Benítez-Burraco, A. Williams syndrome, human self-domestication, and language evolution. Front. Psychol. 2019, 10, 521. [Google Scholar] [CrossRef] [Green Version]
- Merla, G.; Howald, C.; Henrichsen, C.N.; Lyle, R.; Wyss, C.; Zabot, M.T.; Antonarakis, S.E.; Reymond, A. Submicroscopic deletion in patients with Williams-Beuren syndrome influences expression levels of the nonhemizygous flanking genes. Am. J. Hum. Gen. 2006, 79, 332–341. [Google Scholar] [CrossRef] [Green Version]
- Von Holdt, B.M.; Shuldiner, E.; Koch, I.J.; Kartzinel, R.Y.; Hogan, A.; Brubaker, L.; Wanser, S.; Stahler, D.; Wynne, C.D.L.; Ostrander, E.A.; et al. Structural variants in genes associated with human Williams-Beuren syndrome underlie stereotypical hypersociability in domestic dogs. Sci. Adv. 2017, 3, e1700398. [Google Scholar] [CrossRef] [Green Version]
- Kukekova, A.V.; Johnson, J.L.; Xiang, X.; Feng, S.; Liu, S.; Rando, H.M.; Kharlamova, A.V.; Herbeck, Y.; Serdyukova, N.A.; Xiong, Z.; et al. Red fox genome assembly identifies genomic regions associated with tame and aggressive behaviors. Nat. Ecol. Evol. 2018, 2, 1479–1491. [Google Scholar] [CrossRef]
- Crespi, B.J.; Hurd, P.L. Cognitive-behavioral phenotypes of Williams syndrome are associated with genetic variation in the GTF2I gene, in a healthy population. BMC Neurosci. 2014, 15, 127. [Google Scholar] [CrossRef] [Green Version]
- Jabbi, M.; Chen, Q.; Turner, N.; Kohn, P.; White, M.; Kippenhan, J.S.; Dickinson, D.; Kolachana, B.; Mattay, V.; Weinberger, D.R.; et al. Variation in the Williams syndrome GTF2I gene and anxiety proneness interactively affect prefrontal cortical response to aversive stimuli. Transl. Psychiatry 2015, 5, e622. [Google Scholar] [CrossRef] [Green Version]
- Frangiskakis, J.M.; Ewart, A.K.; Morris, C.A.; Mervis, C.B.; Bertrand, J.; Robinskon, B.F.; Klein, B.P.; Ensing, G.J.; Everett, L.A.; Green, E.D.; et al. LIM-kinase1 hemizygosity implicated in impaired visuospatial constructive cognition. Cell 1996, 86, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Lowery, M.C.; Morris, C.A.; Ewart, A.; Brothman, L.J.; Zhu, X.L.; Leonard, C.O.; Carey, J.C.; Keating, M.; Brothman, A.R. Strong correlation of elastin deletions, detected by FISH, with Williams syndrome: Evaluation of 235 patients. Am. J. Hum. Genet. 1995, 57, 49–53. [Google Scholar] [PubMed]
- Tassabehji, M.; Hammond, P.; Karmiloff-Smith, A.; Thompson, P.; Thorgeirsson, S.S.; Durkin, M.E.; Popescu, N.C.; Hutton, T.; Metcalfe, K.; Rucka, A.; et al. GTF2IRD1 in craniofacial development of humans and mice. Science 2005, 310, 1184–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanella, M.; Vitriolo, A.; Andirko, A.; Martins, P.T.; Sturm, S.; O’Rourke, T.; Laugsch, M.; Malerba, N.; Skaros, A.; Trattaro, S.; et al. Dosage analysis of the 7q11.23 Williams region identifies BAZ1B as a major human gene patterning the modern human face and undrlying self-domestication. Sci. Adv. 2019, 5, eaaw7908. [Google Scholar] [CrossRef] [Green Version]
- Young, E.J.; Lipina, T.; Tam, E.; Mandel, A.; Clapcote, S.J.; Bechard, A.R.; Chambers, J.; Mount, H.T.; Fletcher, P.J.; Roder, J.C.; et al. Reduced fear and aggression and altered serotonin metabolism in Gtf2ird1-targeted mice. Genes Brain Behav. 2008, 7, 224–234. [Google Scholar] [CrossRef] [Green Version]
- De Vries, P.J. Targeted treatments for cognitive and neurodevelopmental disorders in tuberous sclerosis complex. Neurotherapeutics 2010, 7, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Hare, B.; Tomasello, M. Human-like social skills in dogs? Trends Cogn. Sci. 2005, 9, 439–444. [Google Scholar] [CrossRef]
- Benítez-Burraco, A.; Di Pietro, L.; Barba, M.; Lattanzi, W. Schizophrenia and human self-domestication: An evolutionary linguistic approach. Brain Behav. Evol. 2017, 89, 162–184. [Google Scholar] [CrossRef]
- Kagawa, H.; Suzuki, K.; Takahasi, M.; Okanoya, K. Domestication changes innate constraints for birdsong learning. Behav. Process. 2014, 106, 91–97. [Google Scholar] [CrossRef]
- Splawski, I.; Yoo, D.S.; Stotz, S.C.; Cherry, A.; Clapham, D.E.; Keating, M.T. CACNA1H mutations in autism spectrum disorders. J. Biol. Chem. 2006, 281, 22085–22091. [Google Scholar] [CrossRef] [Green Version]
- Nottebohm, F. Neural lateralization of vocal control in a passerine bird. J. Exp. Zool. 1971, 177, 229–261. [Google Scholar] [CrossRef] [PubMed]
- Nottebohm, F.; Nottebohm, M.E. Left hypoglossal dominance in the control of canary and white-crowned sparrow song. J. Comp. Physiol. 1976, 108, 171–192. [Google Scholar] [CrossRef]
- Crow, T.J. Schizophrenia as the price that Homo sapiens pays for language: A resolution of the central paradox in the origin of the species. Brain Res. Rev. 2000, 31, 118–129. [Google Scholar] [CrossRef]
- Yakovlev, P.I.; Rakic, P. Patterns of decussation of bulbar pyramids and distribution of pyramidal tracts on two sides of the spinal cord. Trans. Am. Neurol. Assoc. 1966, 91, 366–367. [Google Scholar]
- LeMay, M.; Kido, D.K. Asymmetries of the cerebral hemispheres on computed tomograms. J. Comput. Assist. Tomogr. 1978, 2, 471–476. [Google Scholar]
- Toga, A.W.; Thompson, P.M. Mapping brain asymmetry. Nat. Rev. Neurosci. 2003, 4, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Šimić, G.; Bexheti, S.; Kelović, Z.; Kos, M.; Grbić, K.; Hof, P.R.; Kostović, I. Hemispheric asymmetry, modular variability and age-related changes in the human entorhinal cortex. Neuroscience 2005, 130, 911–925. [Google Scholar] [CrossRef] [PubMed]
- Jamain, S.; Quach, H.; Betancur, C.; Råstam, M.; Colineaux, C.; Gillberg, I.C.; Soderstrom, H.; Giros, B.; Leboyer, M.; Gillberg, C.; et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat. Genet. 2003, 34, 27–29. [Google Scholar] [CrossRef] [Green Version]
- Angrilli, A.; Spironelli, C.; Elbert, T.; Crow, T.J.; Marano, G.; Stegagno, L. Schizophrenia as failure of left hemispheric dominance for the phonological component of language. PLoS ONE 2009, 4, e4507. [Google Scholar] [CrossRef] [Green Version]
- Durand, C.M.; Betancur, C.; Boeckers, T.M.; Bockmann, J.; Chaste, P.; Faucherau, F.; Nygren, G.; Rastam, M.; Gillberg, I.C.; Anckarsäter, H.; et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat. Genet. 2007, 39, 25–27. [Google Scholar] [CrossRef] [Green Version]
- Collinson, S.L.; Mackay, C.E.; Jiaqing, O.; James, A.C.D.; Crow, T.J. Dichotic listening impairments in early onset schizophrenia are associated with reduced left temporal lobe volume. Schizophr. Res. 2009, 112, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Petty, R.G.; Barta, P.E.; Pearlson, G.D.; McGilchrist, I.K.; Lewis, R.W.; Tien, A.Y.; Pulver, A.; Vaughn, D.D.; Casanova, M.F.; Powers, R.E. Reversal of asymmetry of the planum temporale in schizophrenia. Am. J. Psychiatry 1995, 152, 715–721. [Google Scholar] [PubMed]
- Douaud, G.; Mackay, C.; Andersson, J.; James, S.; Quested, D.; Ray, M.K.; Connell, J.; Roberts, N.; Crow, T.J.; Matthews, P.M.; et al. Schizophrenia delays and alters maturation of the brain in adolescence. Brain 2009, 132, 2437–2448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alarcón, M.; Abrahams, B.S.; Stone, J.L.; Duvall, J.A.; Perederiy, J.V.; Bomar, J.M.; Sebat, J.; Wigler, M.; Martin, C.L.; Ledbetter, D.H.; et al. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am. J. Hum. Genet. 2008, 82, 150–159. [Google Scholar] [CrossRef] [Green Version]
- Benayed, R.; Choi, J.; Matteson, P.G.; Gharani, N.; Kamdar, S.; Brzustowicz, L.M.; Millonig, J.H. Autism-associated haplotype affects the regulation of the homeobox gene, ENGRAILED2. Biol. Psychiatry 2009, 66, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Novosedlik, N.; Wang, A.; Hudson, M.L.; Cohen, I.L.; Chudley, A.E.; Forster-Gibson, C.J.; Lewis, S.M.; Holden, J.J. The DLX1 and DLX2 genes and susceptibility to autism spectrum disorders. Eur. J. Hum. Genet. 2009, 17, 228–235. [Google Scholar] [CrossRef] [Green Version]
- Butler, M.G.; Dasouki, M.J.; Zhou, X.P.; Talebizadeh, Z.; Brown, M.; Takahashi, T.N.; Miles, J.H.; Wang, C.H.; Stratton, R.; Pilarski, R.; et al. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumor suppressor gene mutations. J. Med. Genet. 2005, 42, 318–321. [Google Scholar] [CrossRef] [Green Version]
- Crow, T.J. Review: Brain weight is reduced in people with schizophrenia. Evid. Based Ment. Health. 2004, 7, 57. [Google Scholar] [CrossRef]
- Chance, S.A.; Casanova, M.F.; Switala, A.E.; Crow, T.J. Auditory cortex asymmetry, altered minicolumn spacing and absence of ageing effects in schizophrenia. Brain 2008, 131, 3178–3192. [Google Scholar] [CrossRef]
- Mladinov, M.; Sedmak, G.; Fuller, H.R.; Babić Leko, M.; Mayer, D.; Kirincich, J.; Štajduhar, A.; Borovečki, F.; Hof, P.R.; Šimić, G. Gene expression profiling of the dorsolateral and medial orbitofrontal cortex in schizophrenia. Transl. Neurosci. 2016, 7, 139–150. [Google Scholar] [CrossRef] [Green Version]
- Vignoli, A.; La Briola, F.; Peron, A.; Turner, K.; Vannicola, C.; Saccani, M.; Magnaghi, E.; Scornavacca, G.F.; Canevini, M.P. Autism spectrum disorder in tuberous sclerosis complex: Searching for risk markers. Orphanet J. Rare Dis. 2015, 10, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Liu, X.; Zhang, C.; Mundo, E.; Macciardi, F.; Grayson, D.R.; Guidotti, A.R.; Holden, J.J. Reelin gene alleles and susceptibility to autism spectrum disorders. Mol. Psychiatry 2002, 7, 1012–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McBride, K.L.; Varga, E.A.; Pastore, M.T.; Prior, T.W.; Manickam, K.; Atkin, J.F.; Herman, G.E. Confirmation study of PTEN mutations among individuals with autism or developmental delays/mental retardation and macrocephaly. Autism Res. 2010, 3, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Barešić, A.; Nash, A.J.; Dahoun, T.; Howes, O.; Lenhard, B. Understanding the genetics of neuropsychiatric disorders: The potential role of genomic regulatory blocks. Mol. Psychiatry 2020, 25, 6–18. [Google Scholar] [CrossRef]
- Zhou, J.; Blundell, J.; Ogawa, S.; Kwon, C.H.; Zhang, W.; Sinton, C.; Powell, C.M.; Parada, L.F. Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice. J. Neurosci. 2009, 29, 1773–1783. [Google Scholar] [CrossRef] [Green Version]
- Somerville, M.J.; Mervis, C.B.; Young, E.J.; Seo, E.J.; del Campo, M.; Bamforth, S.; Peregrine, E.; Loo, W.; Lilley, M.; Pérez-Jurado, L.A.; et al. Severe expressive-language delay related to duplication of the Williams-Beuren locus. N. Engl. J. Med. 2005, 353, 1694–1701. [Google Scholar] [CrossRef] [Green Version]
- Velleman, S.L.; Mervis, C.B. Children with 7q11.23 duplication syndrome: Speech, language, cognitive, and behavioral characteristics and their implications for intervention. Perspect. Lang. Learn. Educ. 2011, 18, 108–116. [Google Scholar] [CrossRef] [Green Version]
- Adamo, A.; Atashpaz, S.; Germain, P.L.; Zanella, M.; D’Agostino, G.; Albertin, V.; Chenoweth, J.; Micale, L.; Fusco, C.; Unger, C.; et al. 7q11.23 dosage-dependent dysregulation in human pluripotent stem cells affects transcriptional programs in disease-relevant lineages. Nat. Genet. 2015, 47, 132–141. [Google Scholar] [CrossRef]
- Kanner, L. Autistic disturbances of affective contact. Nervous Child. 1943, 2, 217–250. [Google Scholar]
- Garg, S.; Green, J.; Leadbitter, K.; Emsley, R.; Lehtonen, A.; Evans, D.G.; Huson, S.M. Neurofibromatosis type 1 and autism spectrum disorder. Pediatrics 2013, 132, e1642–e1648. [Google Scholar] [CrossRef] [Green Version]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Chakrabarti, S.; Fombonne, E. Pervasive developmental disorders in preschool children. JAMA 2001, 285, 3093–3099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kogan, M.D.; Vladutiu, C.J.; Schieve, L.A.; Ghandour, R.M.; Blumberg, S.J.; Zablotsky, B.; Perrin, J.M.; Shattuck, P.; Kuhlthau, K.A.; Harwood, R.L.; et al. The prevalence of parent-reportex autism spectrum disorder among US children. Pediatrics 2018, 142, e20174161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varghese, M.; Keshav, N.; Jacot-Descombes, S.; Warda, T.; Wicinski, B.; Dickstein, D.L.; Harony-Nicolas, H.; De Rubeis, S.; Drapeau, E.; Buxbaum, J.D.; et al. Autism spectrum disorder: Neuropathology and animal models. Acta Neuropathol. 2017, 134, 537–566. [Google Scholar] [CrossRef] [PubMed]
- Ramaswami, G.; Geschwind, D.H. Genetics of autism spectrum disorder. Handb. Clin. Neurol. 2018, 147, 321–329. [Google Scholar]
- Bernier, R.; Golzio, C.; Xiong, B.; Stessman, H.A.; Coe, B.P.; Penn, O.; Witherspoon, K.; Gerdts, J.; Baker, C.; Vulto-van Silfhout, A.T.; et al. Disruptive CHD8 mutations define a subtype of autism early in development. Cell 2014, 158, 263–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolevzon, A.; Smith, C.J.; Schmeidler, J.; Buxbaum, J.D.; Silverman, J.M. Familial symptom domains in monozygotic siblings with autism. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2004, 129B, 76–81. [Google Scholar] [CrossRef]
- Sztainberg, Y.; Zoghbi, H.Y. Lessons learned from studying syndromic autism spectrum disorders. Nat. Neurosci. 2016, 19, 1408–1417. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, L.Q.; He, Z.X.; He, X.X.; Wang, Y.J.; Jian, Y.L.; Wang, X.; Zhang, B.B.; Su, C.; Lu, J.; et al. Autism candidate gene DIP2A regulates spine morphogenesis via acetylation of cortactin. PLoS Biol. 2019, 17, e3000461. [Google Scholar] [CrossRef]
- Feliciano, P.; Zhou, X.; Astrovskaya, I.; Turner, T.N.; Wang, T.; Brueggeman, L.; Barnard, R.; Hsieh, A.; Snyder, L.G.; Muzny, D.M.; et al. Exome sequencing of 457 autism families recruited online provides evidence for autism risk genes. NPJ Genom. Med. 2019, 4, 19. [Google Scholar] [CrossRef] [Green Version]
- Cogné, B.; Ehresmann, S.; Beauregard-Lacroix, E.; Rousseau, J.; Besnard, T.; Garcia, T.; Petrovski, S.; Avni, S.; McWalter, K.; Blackburn, P.R.; et al. Missense variants in the histone acetyltransferase complex component gene TRRAP cause autism and syndromic intellectual disability. Am. J. Hum. Genet. 2019, 104, 530–541. [Google Scholar] [CrossRef] [Green Version]
- Bacchelli, E.; Cameli, C.; Viggiano, M.; Igliozzi, R.; Mancini, A.; Tancredi, R.; Battaglia, A.; Maestrini, E. An integrated analysis of rare CNV and exome vatiation in autism spectrum disorder using the Infinium PsychArray. Sci. Rep. 2020, 10, 3198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griesi-Oliveira, K.; Fogo, M.S.; Pinto, B.G.G.; Alves, A.Y.; Suzuki, A.M.; Morales, A.G.; Ezquina, S.; Sosa, O.J.; Sutton, G.J.; Sunaga-Franze, D.Y.; et al. Transcriptome of iPSC-derived neuronal cells reveals a module of co-expressed genes consistently associated with autism spectrum disorder. Mol. Psychiatry 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salcedo-Arellano, M.J.; Dufour, B.; McLennan, Y.; Martinez-Cerdeno, V.; Hagerman, R. Fragile X syndrome and associated disorders: Clinical aspects and pathology. Neurobiol. Dis. 2020, 136, 104740. [Google Scholar] [CrossRef] [PubMed]
- Šarac, H.; Henigsberg, N.; Markeljević, J.; Pavliša, G.; Hof, P.R.; Šimić, G. Fragile X-premutation tremor/ataxia syndrome (FXTAS) in a young woman: Clinical, genetic, MRI and 1H MR spectroscopy correlates. Coll. Antropol. 2011, 35 (Suppl. 1), 327–332. [Google Scholar]
- Richards, C.; Jones, C.; Groves, L.; Moss, J.; Oliver, C. Prevalence of autism spectrum disorder phenomenology in genetic disorders: A systematic review and meta-analysis. Lancet Psychiatry 2015, 2, 909–916. [Google Scholar] [CrossRef] [Green Version]
- Hagberg, B.; Aicardi, J.; Dias, K.; Ramos, O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett’s syndrome: Report of 35 cases. Ann. Neurol. 1983, 14, 471–479. [Google Scholar] [CrossRef]
- Ellison, K.A.; Fill, C.P.; Terwilliger, J.; DeGennaro, L.J.; Martin-Gallardo, A.; Anvret, M.; Percy, A.K.; Ott, J.; Zoghbi, H. Elimination of X chromosome markers in Rett syndrome: Exclusion mapping with a novel variation on multilocus linkage analysis. Am. J. Hum. Genet. 1992, 50, 278–287. [Google Scholar]
- Amir, R.E.; Van der Veyver, I.B.; Wan, M.; Tran, C.Q.; Francke, U.; Zoghbi, H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999, 23, 185–188. [Google Scholar] [CrossRef]
- Chahrour, M.; Jung, S.Y.; Shaw, C.; Zhou, X.; Wong, S.T.; Qin, J.; Zoghbi, H.Y. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 2008, 320, 1224–1229. [Google Scholar] [CrossRef] [Green Version]
- Prontera, P.; Ottaviani, V.; Toccaceli, D.; Rogaia, D.; Ardisia, C.; Romani, R.; Stangoni, G.; Pierini, A.; Donti, E. Recurent ~100 Kb microdeletion in the chromosomal region 14q11.2, involving CHD8 gene, is associated with autism and macrocephaly. Am. J. Med. Genet. A. 2014, 164A, 3137–3141. [Google Scholar] [CrossRef]
- Sugathan, A.; Biagioli, M.; Golzio, C.; Erdin, S.; Blumenthal, I.; Manavalan, P.; Ragavendran, A.; Brand, H.; Lucente, D.; Miles, J.; et al. CHD8 regulates neurodevelopmental pathways associated with autism spectrum disorder in neural progenitors. Proc. Natl. Acad. Sci. USA 2014, 111, E4468–E4477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, B.; Grepo, N.; Thompson, B.L.; Kim, J.; Wang, K.; Evgrafov, O.V.; Lu, W.; Knowles, J.A.; Campbell, D.B. The autism-associated gene chromodomain helicase DNA-binding protein 8 (CHD8) regulates noncoding RNAs and autism-related genes. Transl. Psychiatry 2015, 5, e568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.G.; Rosenfeld, J.A.; Scott, D.A.; Bénédicte, G.; Labonne, J.D.; Brown, J.; McGuire, M.; Mahida, S.; Naidu, S.; Gutierrez, J.; et al. Disruption of PHF21A causes syndromic intellectual disability with craniofacial anomalies, epilepsy, hypotonia, and neurobehavioral problems including autism. Mol. Autism. 2019, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- DiStefano, C.; Gulsrud, A.; Huberty, S.; Kasari, C.; Cook, E.; Reiter, L.T.; Thibert, R.; Jeste, S.S. Identification of a distinct developmental and behavioral profile in children with Dup15q syndrome. J. Neurodev. Disord. 2016, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Yuen, R.K.C.; Merico, D.; Bookman, M.; Howe, J.L.; Thiruvanhindrapuram, B.; Patel, R.V.; Whitney, J.; Deflaux, N.; Bingham, J.; Wang, Z.; et al. Whole genome sequencing resource identifies 18 new candidate genes for autism spectrum disorder. Nat. Neurosci. 2017, 20, 602–611. [Google Scholar] [CrossRef]
- Tillotson, R.; Bird, A. The molecular basis of MeCP2 function in the brain. J. Mol. Biol. 2019, S0022–S2836, 30595–30599. [Google Scholar] [CrossRef]
- Jin, X.R.; Chen, X.S.; Xiao, L. MeCP2 deficiency in neuroglia: New progress in the pathogenesis of Rett syndrome. Front. Mol. Neurosci. 2017, 10, 316. [Google Scholar] [CrossRef]
- Verkerk, A.J.; Pieretti, M.; Sutcliffe, J.S.; Fu, Y.-H.; Kuhl, D.P.; Pizzuti, A.; Reiner, O.; Richards, S.; Victoria, M.F.; Zhang, F.; et al. Identification of a gene (FMR1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 1991, 65, 905–914. [Google Scholar] [CrossRef]
- Schanen, N.C. Epigenetics of autism spectrum disorders. Hum. Mol. Genet 2006, 15, R138–R150. [Google Scholar] [CrossRef]
- Passarge, E. Color Atlas of Genetics, 4th ed.; Georg Thieme: New York, NY, USA; Stuttgart, Germany, 2007; pp. 410–411. [Google Scholar]
- Prader, A.; Labhart, A.; Willi, H. Ein Syndrom von Adipositas, Kleinwuchs, Kryptorchismus und Oligophrenie nach myatonieartigem Zustand im Neugeborenenalter. Schweiz. Med. Wochenschr 1956, 86, 1260–1261. [Google Scholar]
- Veltman, M.W.; Craig, E.E.; Bolton, P.F. Autism spectrum disorders in Prader-Willi and Angelman syndromes: A systematic review. Psychiatr Genet. 2005, 15, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Dykens, E.M.; Lee, E.; Roof, E. Prader-Willi syndrome and autism spectrum disorders: An evolving story. J. Neurodev. Disord. 2011, 3, 225–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelman, H. ‘Puppet’ children. A report on three cases. Dev. Med. Child Neurol. 1965, 7, 681–688. [Google Scholar] [CrossRef]
- Khatri, N.; Man, H.Y. The autism and Angelman syndrome protein Ube3A/E6AP: The gene, E3 ligase ubiquitination targets and neurobiological functions. Front. Mol. Neurosci. 2019, 12, 109. [Google Scholar] [CrossRef] [Green Version]
- Cook, E.H.; Lindren, V.; Leventhal, B.L.; Courchesne, R.; Lincoln, A.; Shulman, C.; Lord, C.; Courchesne, E. Autism or atypical autism in maternally but not paternally derived proximal 15q duplication. Am. J. Hum. Genet. 1997, 60, 928–934. [Google Scholar]
- Browne, C.E.; Dennis, N.R.; Maher, E.; Long, F.L.; Nicholson, J.C.; Sillibourne, J.; Barber, J.C. Inherited interstitial duplications of proximal 15q: Genotype-phenotype correlations. Am. J. Hum. Genet. 1997, 61, 1342–1352. [Google Scholar] [CrossRef] [Green Version]
- Abrahams, B.S.; Geschwind, D.H. Advances in autism genetics: On the threshold of a new neurobiology. Nat. Rev. Genet. 2008, 9, 341–355. [Google Scholar] [CrossRef] [Green Version]
- Kalsner, L.; Chamberlain, S.J. Prader-Willi, Angelman, and 15q11-q13 duplication syndromes. Pediatr. Clin. North Am. 2015, 62, 587–606. [Google Scholar] [CrossRef] [Green Version]
- Mercati, O.; Huguet, G.; Danckaert, A.; André-Leroux, G.; Maruani, A.; Bellinzoni, M.; Rolland, T.; Gouder, L.; Mathieu, A.; Buratti, J.; et al. CNTN6 mutations are risk factors for abnormal auditory sensory perception in autism spectrum disorders. Mol. Psychiatry 2017, 22, 625–633. [Google Scholar] [CrossRef] [Green Version]
- Hogart, A.; Wu, D.; LaSalle, J.M.; Schanen, N.C. The comorbidity of autism with the genomic disorders of chromosome 15q11.2-q13. Neurobiol. Dis. 2010, 38, 181–191. [Google Scholar] [CrossRef] [Green Version]
- Hamedani, S.Y.; Gharesouran, J.; Noroozi, R.; Sayad, A.; Omrani, M.D.; Mir, A.; Afjeh, S.S.A.; Toghi, M.; Manoochehrabadi, S.; Ghafouri-Fard, S.; et al. Ras-like without CAAX (RIT2): A susceptibility gene for autism spectrum disorder. Metab. Brain Dis. 2017, 32, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Bolton, P.F.; Dennis, N.R.; Browne, C.E.; Thomas, N.S.; Veltman, M.W.; Thompson, R.J.; Jacobs, P. The phenotypic manifestation of interstitial duplications of proximal 15q with special reference to the autistic spectrum disorders. Am. J. Med. Genet. 2001, 105, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Von Ehrenstein, O.S.; Aralis, H.; Cockburn, M.; Ritz, B. In utero exposure to toxic air pollutants and risk of childhood autism. Epidemiology 2014, 25, 851–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weisskopf, M.G.; Kioumourtzoglou, M.-A.; Roberts, A.L. Air pollution and autism spectrum disorders: Causal or confounded? Curr. Environ. Health Rep. 2015, 2, 430–439. [Google Scholar] [CrossRef]
- Costa, L.G.; Chang, L.-C.; Cole, T.B. Developmental neurotoxicity of traffic-related air pollution: Focus on autism. Curr. Environ. Health Rep. 2017, 4, 156–165. [Google Scholar] [CrossRef]
- Chai, X.; Förster, E.; Zhao, S.; Bock, H.H.; Frotscher, M. Reelin acts as a stop signal for radially migrating neurons by inducing phosphorylation on n-cofilin at the leading edge. Commun. Integr. Biol. 2009, 2, 375–377. [Google Scholar] [CrossRef] [Green Version]
- Grayson, D.R.; Jia, X.; Chen, Y.; Sharma, R.P.; Mithell, C.P.; Guidotti, A.; Costa, E. Reelin promoter hypermethylation in schizophrenia. Proc. Natl. Acad. Sci. USA 2005, 102, 9341–9346. [Google Scholar] [CrossRef] [Green Version]
- Cuchillo-Ibáñez, I.; Andreo-Lillo, P.; Pastor-Ferrándiz, L.; Carratalá-Marco, F.; Sáez-Valero, J. Elevated plasma reelin levels in children with autism. Front. Psychiatry 2020, 11, 242. [Google Scholar] [CrossRef]
- Testa, C.; Nuti, F.; Hayek, J.; De Felice, C.; Chelli, M.; Rovero, P.; Latini, G.; Papini, A.M. Di-(2-ethylhexyl) phthalate and autism spectrum disorders. ASN Neuro. 2012, 4, 223–229. [Google Scholar] [CrossRef]
- Fučić, A.; Šimić, G. Impact of Endocrine Disruptors on Androgen and Estrogen Receptors during Intrauterine Brain Development. In Zagreb: Nikola Škreb Symposium: New Platforms in Developmental Biology–Towards the Clinical; University of Zagreb School of Medicine: Zagreb, Croatia, 2018; p. 70. [Google Scholar]
- Mawson, A.R.; Croft, A.M. Rubella virus infection, the congenital rubella syndrome, and the link to autism. Int. J. Environ. Res. Public Health 2019, 16, E3543. [Google Scholar] [CrossRef] [Green Version]
- Bener, A.; Khattab, A.O.; Al-Dabbagh, M.M. Is high prevalence of vitamin D deficiency evidence for autism disorder? J. Pediatr. Neurosci. 2014, 9, 227–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, H.; Duyzend, M.H.; Coe, B.P.; Baker, C.; Hoekzema, K.; Gerdts, J.; Turner, T.N.; Zody, M.C.; Beighley, J.S.; Murali, S.C.; et al. Genome sequencing identifies multiple deleterious variants in autism patients with more severe phenotypes. Genet. Med. 2019, 21, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Rotem, R.S.; Chodick, G.; Shalev, V.; Davidovitch, M.; Koren, G.; Hauser, R.; Coull, B.A.; Seely, E.W.; Nguyen, V.T.; Weisskopf, M.G. Maternal thyroid disorders and risk of autism spectrum disorder in progeny. Epidemiology 2020, 31, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Courchesne, E.; Carper, R.; Akshoomoff, N. Evidence of brain overgrowth in the first year of life in autism. JAMA 2003, 290, 337–344. [Google Scholar] [CrossRef] [Green Version]
- Amaral, D.G.; Schumann, C.M.; Nordahl, C.W. Neuroanatomy of autism. Trends Neurosci. 2008, 31, 137–145. [Google Scholar] [CrossRef]
- Courchet, V.; Roberts, A.J.; Meyer-Dilhet, G.; Del Carmine, P.; Lewis TLJr Polleux, F.; Courchet, J. Haploinsufficiency of autism spectrum disorder candidate gene NUAK1 impairs cortical development and behavior in mice. Nat. Commun. 2018, 9, 4289. [Google Scholar] [CrossRef] [Green Version]
- Mosconi, M.W.; Cody-Hazlett, H.; Poe, M.D.; Gerig, G.; Gimpel-Smith, R.; Piven, J. Longitudinal study of amygdala volume and joint attention in 2- to 4-year-old children with autism. Arch. Gen. Psychiatry 2009, 66, 509–516. [Google Scholar] [CrossRef]
- Murphy, C.M.; Deeley, Q.; Daly, E.M.; Ecker, C.; O’Brien, F.M.; Hallahan, B.; Loth, E.; Toal, F.; Reed, S.; Hales, S.; et al. Anatomy and aging of the amygdala and hippocampus in autism spectrum disorder: An in vivo magnetic resonance imaging study of Asperger syndrome. Autism Res. 2012, 5, 3–12. [Google Scholar] [CrossRef]
- Munson, J.; Dawson, G.; Abbott, R.; Faja, S.; Webb, S.J.; Friedman, S.D.; Shaw, D.; Artru, A.; Dager, S.R. Amygdalar volume and behavioral development in autism. Arch. Gen. Psychiatry 2006, 63, 686–693. [Google Scholar] [CrossRef] [Green Version]
- Schumann, C.M.; Barnes, C.C.; Lord, C.; Courchesne, E. Amygdala enlargement in toddlers with autism related to severity of social and communication impairments. Biol. Psychiatry 2009, 66, 942–949. [Google Scholar] [CrossRef] [Green Version]
- Juranek, J.; Filipek, P.A.; Berenji, G.R.; Modahl, C.; Osann, K.; Spence, M.A. Association between amygdala volume and anxiety level: Magnetic resonance imaging (MRI) study in autistic children. J. Child Neurol. 2006, 21, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Sparks, B.F.; Friedman, S.D.; Shaw, D.W.; Aylward, E.H.; Echelard, D.; Artru, A.A.; Maravilla, K.R.; Giedd, J.N.; Munson, J.; Dawson, G.; et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology 2002, 59, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Mantilla, S.; Choe, M.S.; Flax, J.; Grant, P.E.; Benasich, A.A. Association between the size of the amygdala in infancy and language abilities during the preschool years in normally developing children. Neuroimage 2010, 49, 2791–2799. [Google Scholar] [CrossRef] [PubMed]
- Avino, T.A.; Barger, N.; Vargas, M.V.; Carlson, E.L.; Amaral, D.G.; Bauman, M.D.; Schumann, C.M. Neuron numbers increase in the human amygdala from birth to adulthood, but not in autism. Proc. Natl. Acad. Sci. USA 2018, 115, 3710–3715. [Google Scholar] [CrossRef] [Green Version]
- Santos, M.; Uppal, N.; Butti, C.; Wicinski, B.; Schmeidler, J.; Giannakopoulos, P.; Heinsen, H.; Schmitz, C.; Hof, P.R. Von Economo neurons in autism: A stereological study of the frontoinsular cortex in children. Brain Res. 2011, 1380, 206–217. [Google Scholar] [CrossRef]
- Casanova, M.F.; van Kooten, I.A.J.; Switala, A.E.; van Engeland, H.; Heinsen, H.; Steinbusch, H.W.M.; Hof, P.R.; Trippe, J.; Stone, J.; Schmitz, C. Minicolumnar abnormalities in autism. Acta Neuropathol. 2006, 112, 287–303. [Google Scholar] [CrossRef]
- Lovrečić, L.; Rajar, P.; Volk, M.; Bertok, S.; Gnidovec Stražišar, B.; Osredkar, D.; Jekovec Vrhovšek, M.; Peterlin, B. Diagnostic efficacy and new variants in isolated and complex autism spectrum disorder using molecular karyotyping. J. Appl. Genet. 2018, 59, 179–185. [Google Scholar] [CrossRef]
- Miyoshi, G. Elucidating the developmental trajectories of GABAergic cortical interneuron subtypes. Neurosci. Res. 2019, 138, 26–32. [Google Scholar] [CrossRef]
- Howell, B.W.; Smith, K.M. Synaptic structural protein dysfunction leads to altered excitation inhibition ratios in models of autism spectrum disorder. Pharmacol. Res. 2019, 139, 207–214. [Google Scholar] [CrossRef]
- Polšek, D.; Jagatic, T.; Cepanec, M.; Hof, P.R.; Šimić, G. Recent developments in neuropathology of autism spectrum disorders. Transl. Neurosci. 2011, 2, 256–264. [Google Scholar] [CrossRef]
- Mouridsen, S.E.; Brønnum-Hansen, H.; Rich, B.; Isager, T. Mortality and causes of death in autism spectrum disorders: An update. Autism 2008, 12, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Dinstein, I.; Pierce, K.; Eyler, L.; Solso, S.; Malach, R.; Behrmann, M.; Courchesne, E. Disrupted neural synchronization in toddlers with autism. Neuron 2011, 70, 1218–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redcay, E.; Courchesne, E. Deviant fMRI patterns of brain activity to speech in 2–3-year-old children with autism spectrum disorder. Biol. Psychiatry 2008, 64, 589–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knaus, T.A.; Silver, A.M.; Kennedy, M.; Lindgren, K.A.; Dominick, K.C.; Siegel, J.; Tager-Flusberg, H. Language laterality in autism spectrum disorder and typical controls: A functional, volumetric, and diffusion tension MRI study. Brain Lang. 2010, 112, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Postema, M.C.; van Rooij, D.; Anagnostou, E.; Arango, C.; Auzias, G.; Behrmann, M.; Filho, G.B.; Calderoni, S.; Calvo, R.; Daly, E.; et al. Altered structural brain asymmetry in autism spectrum disorder in a study of 54 datasets. Nat. Commun. 2019, 10, 4598. [Google Scholar] [CrossRef] [Green Version]
- Anderson, G.M.; Horne, W.C.; Chatterjee, D.; Cohen, D.J. The hyperserotonemia of autism. Ann. N. Y. Acad. Sci. 1990, 600, 331–340. [Google Scholar] [CrossRef]
- Leboyer, M.; Philippe, A.; Bouvard, M.; Guilloud-Bataille, M.; Bondoux, D.; Tabuteau, F.; Feingold, J.; Mouren-Simeoni, M.C.; Launay, J.M. Whole blood serotonin and plasma β-endorphin in autistic probands and their first-degree relatives. Biol. Psychiatry 1999, 45, 158–163. [Google Scholar] [CrossRef]
- Hranilović, D.; Bujas-Petković, Z.; Vragović, R.; Vuk, T.; Hock, K.; Jernej, B. Hyperserotonemia in adults with autistic disorder. J. Autism Dev. Disord. 2007, 37, 1934–1940. [Google Scholar] [CrossRef]
- Azmitia, E.C. Serotonin and brain: Evolution, neuroplasticity, and homeostasis. Int. Rev. Neurobiol. 2007, 77, 31–56. [Google Scholar]
- Fernandes, I.R.; Cruz, A.C.P.; Ferrasa, A.; Phan, D.; Herai, R.H.; Muotri, A.R. Genetic variations on SETD5 underlying autistic conditions. Dev. Neurobiol. 2018, 78, 500–518. [Google Scholar] [CrossRef]
- Šimić, G.; Babić Leko, M.; Wray, S.; Harrington, C.R.; Delalle, I.; Jovanov-Milošević, N.; Bažadona, D.; Buée, L.; de Silva, R.; Di Giovanni, G.; et al. Monoaminergic neuropathology in Alzheimer’s disease. Prog. Neurobiol. 2017, 151, 101–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabriele, S.; Canali, M.; Lintas, C.; Sacco, R.; Tirindelli, M.C.; Ricciardello, A.; Persico, A.M. Evidence that ITGB3 promoter variants increase serotonin blood levels by regulating platelet serotonin transporter trafficking. Hum. Mol. Genet. 2019, 28, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Walther, D.J.; Peter, J.U.; Bashammakh, S.; Hörtnagl, H.; Voits, M.; Fink, H.; Bader, M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 2003, 299, 76. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Burraco, A.; Lattanzi, W.; Murphy, E. Language impairments in ASD resulting from a failed domestication of the human brain. Front. Neurosci. 2016, 10, 373. [Google Scholar] [CrossRef] [Green Version]
- Khatri, N.; Gilbert, J.P.; Huo, Y.; Sharaflari, R.; Nee, M.; Qiao, H.; Man, H.Y. The autism protein Ube3A/E6AP remodels neuronal dendritic arborization via caspase-dependent microtubule destabilization. J. Neurosci. 2018, 38, 363–378. [Google Scholar] [CrossRef]
- Raaijmakers, M.A.; Smidts, D.P.; Seargeant, J.A.; Maassen, G.H.; Posthumus, J.A.; van Engeland, H.; Matthys, W. Executive function in preschool children with aggressive behavior: Impairment in inhibitory control. J. Abnorm. Child Psychol. 2008, 36, 1097–1107. [Google Scholar] [CrossRef] [Green Version]
- Darwin, C. The Expression of Emotions in Man and Animals; John Murray: London, UK, 1872. [Google Scholar]
- Berto, S.; Mendizabal, I.; Usui, N.; Toriumi, K.; Chatterjee, P.; Douglas, C.; Tamminga, C.A.; Preuss, T.M.; Yi, S.V.; Konopka, G. Accelerated evolution of oligodendrocytes in the human brain. Proc. Natl. Acad. Sci. USA 2019, 116, 24334–24342. [Google Scholar] [CrossRef]
- Shilton, D.; Breski, M.; Dor, D.; Jablonka, E. Human social evolution: Self-domestication or self-control? Front. Psychol. 2020, 11, 134. [Google Scholar] [CrossRef]
- Bohlken, H. Haustiere und zoologische Systematik. Z. Tierzuecht. Zuechtungsbiol 1961, 76, 107–113. [Google Scholar] [CrossRef]
- Herre, W.; Röhrs, M. Haustiere Zoologisch Gesehen, 2nd ed.; Gustav Fischer Verlag: Stuttgart, 1990. [Google Scholar]
- Zeller, U.; Göttert, T. The relations between evolution and domestication reconsidered–Implications for systematics, ecology, and nature conservation. Glob. Ecol. Conserv. 2019, 20, e00756. [Google Scholar] [CrossRef]
- Nesse, R.M.; Ellsworth, P.C. Evolution, emotions, and emotional disorders. Am. Psychol. 2009, 64, 129–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzaa, G.M.; Chong, J.X.; Piton, A.; Popp, B.; Foss, K.; Guo, H.; Harripaul, R.; Xia, K.; Scheck, J.; Aldinger, K.A.; et al. De novo and inherited variants in ZNF292 underlie a neurodevelopmental disorder with features of autism spectrum disorder. Genet. Med. 2020, 22, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Nesse, R.M. Emotional disorders in evolutionary perspective. Brit. J. Med. Psychol. 1999, 71, 397–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.-X.; Chen, X.; Shen, Y.-Q.; Li, L.; Chen, N.-X.; Zhu, Z.-C.; Castellanos, F.X.; Yan, C.-G. Rumination and the default mode network: Meta-analysis of brain imaging studies and implications for depression. Neuroimage 2020, 206, 116287. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Wu, H.; Xu, J.; Chen, F.; Wu, F.; Wang, C.; Wang, J. Abnormal large-scale resting-state functional networks in drug-free major depressive disorder. Brain Imaging Behav. 2020. [Google Scholar] [CrossRef] [PubMed]
- Fredrickson, B.L. The role of positive emotions in positive psychology. The broaden-and-build theory of positive emotions. Am. Psychol. 2001, 56, 218–226. [Google Scholar] [CrossRef]
- Dang, T.; Duan, W.Y.; Yu, B.; Tong, D.L.; Cheng, C.; Zhang, Y.F.; Wu, W.; Ye, K.; Zhang, W.X.; Wu, M.; et al. Autism-associated Dyrk1a truncation mutants impair neuronal dendritic and spine growth and interfere with postnatal cortical development. Mol. Psychiatry 2018, 23, 747–758. [Google Scholar] [CrossRef] [Green Version]
- Axelrod, R.; Hamilton, W.D. The evolution of cooperation. Science 1981, 211, 1390–1396. [Google Scholar] [CrossRef]
- Prisner, E. Game Theory Through Examples; Mathematical Association of America: Washington, DC, USA, 2014. [Google Scholar]
- Gross, J.J. (Ed.) Handbook of Emotional Regulation, 2nd ed.; Guilford Press: New York, NY, USA, 2014. [Google Scholar]
- Ochsner, K.N.; Silvers, J.A.; Buhle, J.T. Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Ann. N. Y. Acad. Sci. 2012, 1251, E1–E24. [Google Scholar] [CrossRef] [Green Version]
- Hajcak, G.; Nieuwenhuis, S. Reappraisal modulates the electrocortical response to unpleasant pictures. Cogn. Affect. Behav. Neurosci. 2006, 6, 291–297. [Google Scholar] [CrossRef]
- Foti, D.; Hajcak, G. Deconstructing reappraisal: Descriptions preceding arousing pictures modulate the subsequent neural response. J. Cogn. Neurosci. 2008, 20, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Krompinger, J.W.; Moses, J.S.; Simons, R.F. Modulations of the electrophysiological response to pleasant stimuli by cognitive reappraisal. Emotion 2008, 8, 132–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moser, J.S.; Krompinger, J.W.; Dietz, J.; Simons, R.F. Electrophysiological correlates of decreasing and increasing emotional responses to unpleasant pictures. Psychophysiology 2009, 46, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Kalisch, R.; Wiech, K.; Critchley, H.D.; Seymour, B.; O’Doherty, J.P.; Oakley, D.A.; Allen, P.; Dolan, R.J. Anxiety reduction through detachment: Subjective, physiological, and neural effects. J. Cogn. Neurosci. 2005, 17, 874–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiech, K.; Seymour, B.; Kalisch, R.; Stephan, K.E.; Koltzenburg, M.; Driver, J.; Dolan, R.J. Modulation of pain processing in hyperalgesia by cognitive demand. Neuroimage 2005, 27, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Buhle, J.T.; Silvers, J.A.; Wager, T.D.; Lopez, R.; Onyemekwu, C.; Kober, H.; Weber, J.; Ochsner, K.N. Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cereb. Cortex. 2014, 24, 2981–2990. [Google Scholar] [CrossRef]
- Beauregard, M.; Lévesque, J.; Bourgouin, P. Neural correlates of conscious self-regulation of emotion. J. Neurosci. 2001, 21, RC165. [Google Scholar] [CrossRef]
- Blair, K.S.; Smith, B.W.; Mitchell, D.G.; Morton, J.; Vythilingam, M.; Pessoa, L.; Fridberg, D.; Zametkin, A.; Sturman, D.; Nelson, E.E.; et al. Modulation of emotion by cognition and cognition by emotion. Neuroimage 2007, 35, 430–440. [Google Scholar] [CrossRef] [Green Version]
- Denny, B.T.; Inhoff, M.C.; Zerubavel, N.; Davachi, L.; Ochsner, K.N. Getting over it: Long-lasting effects of emotion regulation on amygdala response. Psychol. Sci. 2015, 26, 1377–1388. [Google Scholar] [CrossRef] [Green Version]
- Depue, B.E.; Curran, T.; Banich, M.T. Prefrontal regions orchestrate suppression of emotional memories via a two-phase process. Science 2007, 317, 215–219. [Google Scholar] [CrossRef] [Green Version]
- Feeser, M.; Prehn, K.; Kazzer, P.; Mungee, A.; Bajbouj, M. Transcranial direct current stimulation enhances cognitive control during emotion regulation. Brain Stimul. 2014, 7, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Inzlicht, M.; Gutsell, J.N. Running on empty: Neural signals for self-control failure. Psychol. Sci. 2007, 18, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Raio, C.M.; Orederu, T.A.; Palazzolo, L.; Shurick, A.A.; Phelps, E.A. Cognitive emotion regulation fails the stress test. Proc. Natl. Acad. Sci. USA 2013, 110, 15139–15144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnsten, A.F. Stress weakens prefrontal networks: Molecular insults to higher cognition. Nat. Neurosci. 2015, 18, 1376–1385. [Google Scholar] [CrossRef]
- Šimić, G. (Ed.) Mechanisms and disorders of self-domestication–the key to understanding emotional and social communication from an evolutionary perspective. In Introduction to Neuroscience of Emotions and Feelings; Naklada Ljevak: Zagreb, Croatia, 2020; pp. 211–280. (In Croatian) [Google Scholar]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šimić, G.; Vukić, V.; Kopić, J.; Krsnik, Ž.; Hof, P.R. Molecules, Mechanisms, and Disorders of Self-Domestication: Keys for Understanding Emotional and Social Communication from an Evolutionary Perspective. Biomolecules 2021, 11, 2. https://doi.org/10.3390/biom11010002
Šimić G, Vukić V, Kopić J, Krsnik Ž, Hof PR. Molecules, Mechanisms, and Disorders of Self-Domestication: Keys for Understanding Emotional and Social Communication from an Evolutionary Perspective. Biomolecules. 2021; 11(1):2. https://doi.org/10.3390/biom11010002
Chicago/Turabian StyleŠimić, Goran, Vana Vukić, Janja Kopić, Željka Krsnik, and Patrick R. Hof. 2021. "Molecules, Mechanisms, and Disorders of Self-Domestication: Keys for Understanding Emotional and Social Communication from an Evolutionary Perspective" Biomolecules 11, no. 1: 2. https://doi.org/10.3390/biom11010002
APA StyleŠimić, G., Vukić, V., Kopić, J., Krsnik, Ž., & Hof, P. R. (2021). Molecules, Mechanisms, and Disorders of Self-Domestication: Keys for Understanding Emotional and Social Communication from an Evolutionary Perspective. Biomolecules, 11(1), 2. https://doi.org/10.3390/biom11010002





