Antibacterial and Antifungal Properties of Silver Nanoparticles—Effect of a Surface-Stabilizing Agent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Microorganisms
2.3. Synthesis of AgNPs
2.4. Physicochemical Characteristics of AgNPs
2.5. Exposure of Microorganisms to AgNPs
2.6. Statistical Analysis
3. Results and Discussion
| Symbol | Reducing Agent | Stabilizing Agent | T (°C) | pH | Agent for pH Adjustment | Ref. |
|---|---|---|---|---|---|---|
| CHSB1AgNPs | sodium borohydride (SB) | cysteamine hydrochloride (CH) | 20 | 5.2 | - | [53] |
| CHSB2AgNPs | sodium borohydride (SB) | cysteamine hydrochloride (CH) | 20 | 5.3 | - | [53] |
| CYSSBAgNPs | sodium borohydride (SB) | cysteine (CYS) | 20 | 3.4 | - | [27,54] |
| LYZSBAgNPs | sodium borohydride (SB) | lysine (LYZ) | 20 | 3.7 | - | - |
| ARGSBAgNPs | sodium borohydride (SB) | arginine (ARG) | 20 | 3.4 | - | - |
| TCSBAgNPs | sodium borohydride (SB) | trisodium citrate (TC) | 20 | 7.9 | - | [41] |
| TCAgNPs | trisodium citrate (TC) | 88 | 9.1 | - | [42] | |
| TCAAAgNPs | ascorbic acid (AA) | trisodium citrate (TC) | 25 | 9.5 | aq. ammonia | - |
| GAAgNPs | gallic acid (GA) | 25 | 8.8 | aq. ammonia | [55,56] | |
| EGCGAgNPs | (−)-epigallocatechin gallate (EGCG) | 25 | 8.9 | aq. ammonia | [50] | |
| TAAgNPs | tannic acid (TA) | 25 | 8.5 | aq. ammonia | [49] | |
| CFGAAgNPs | gallic acid (GA) | caffeine (CF) | 25 | 8.8 | aq. ammonia | [50] |
| GLAgNPs | D-glucose | 25 | 5.3 | aq. ammonia | [57] | |
| HHAgNPs | hydroxylamine hydrochloride (HH) | 25 | 10.5 | sodium hydroxide | [51] | |
| SHSHAgNPs | sodium hypophosphite (SH) | sodium hexameta-phosphate (SH) | 40 | 2.2 | sulfuric acid | [33,52,58] |
| Name of Compound | Examples of Biological Activity of the Compound and Its Derivatives | Ref. |
|---|---|---|
| cysteamine hydrochloride |
| [87,88] |
| L-cysteine |
| [89,90,91,92,93] |
| L-lysine |
| [94,95,96,97,98] |
| L-arginine |
| [86,99,100,101] |
| trisodium citrate |
| [102] |
| ascorbic acid |
| [103,104,105,106] |
| gallic acid |
| [106,107] |
| (−)-epicatechin-3-gallate |
| [108,109,110] |
| tannic acid |
| [111,112,113,114,115] |
| caffeine |
| [116,117,118,119] |
| hydroxylamine mine hydrochloride |
| [120] |
| sodium hexametaphosphate |
| [121,122,123,124] |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khtoon, U.T.; Rao, G.V.S.N.; Mantravadi, K.M.; Oztekin, Y. Strategies to synthesize various nanostructures of silver and their applications—A review. RSC Adv. 2018, 8, 19739–19753. [Google Scholar] [CrossRef] [Green Version]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver nanoparticles and their antibacterial applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.-H. Silver nanoparticles: Synthesis and application for nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahcelioglu, E.; Unalan, H.E.; Erguder, T.H. Silver-based nanomaterials: A critical review on factors affecting water disinfection performance and silver release. Crit. Rev. Environ. Sci. Technol. 2020, 51, 2389–2423. [Google Scholar] [CrossRef]
- Lashin, I.; Fouda, A.; Gobouri, A.A.; Azab, E.; Mohammedsaleh, Z.M.; Makharita, R.R. Antimicrobial and in vitro cytotoxic efficacy of biogenic silver nanoparticles (Ag-NPs) fabricated by callus extract of Solanum incanum L. Biomolecules 2021, 11, 341. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and cytotoxic properties of s ilver nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grelich, C.; Braun, D.; Peetsch, A.; Diendorf, J.; Siebers, B.; Epple, M.; Köller, M. The toxic effect of silver ions and silver nanoparticles towards bacteria and human cells occurs in the same concentration range. RSC Adv. 2012, 2, 6981–6987. [Google Scholar] [CrossRef]
- Ahamed, M.; Karns, M.; Goodson, M.; Rowe, J.; Hussain, S.M.; Schlager, J.J.; Hong, Y. DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. Toxicol. Appl. Pharmacol. 2008, 233, 404–410. [Google Scholar] [CrossRef]
- Luter, E.M.; Koehler, Y.; Diendorf, J.; Epple, M.; Dringen, R. Accumulation of silver nanoparticles by cultured primary brainastrocytes. Nanotechnology 2011, 22, 375101. [Google Scholar] [CrossRef]
- Kittler, S.; Greulich, C.; Diendorf, J.; Koller, M.; Epple, M. Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions. Chem. Mater. 2010, 22, 4548–4554. [Google Scholar] [CrossRef]
- Chambers, B.A.; Afrooz, N.; Bae, S.; Aich, N.; Katz, L.; Saleh, N.B.; Kirisits, M.J. Effects of chloride and ionic strength on physical morphology, dissolution, and bacterial toxicity of silver nanoparticles. Environ. Sci. Technol. 2013, 48, 761–769. [Google Scholar] [CrossRef]
- Gondikas, A.; Morris, A.; Reinsch, B.C.; Marinakos, S.M.; Lowry, G.V.; Hsu-Kim, H. Cysteine-induced modifications of zero-valent silver nanomaterials: Implications for particle surface chemistry, aggregation, dissolution, and silver speciation. Environ. Sci. Technol. 2012, 46, 7037–7045. [Google Scholar] [CrossRef] [PubMed]
- Peretyazhko, T.S.; Zhang, Q.; Colvin, V.L. Size-controlled dissolution of silver nanoparticles at neutral and acidic pH conditions: Kinetics and size changes. Environ. Sci. Technol. 2014, 48, 11954–11961. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhou, Y.-T.; Wmer, W.G.; Boudreau, M.D.; Yin, J.-J. Mechanisms of the pH dependent generation of hydroxylradicals and oxygen induced by Ag nanoparticles. Biomaterials 2012, 33, 7547–7555. [Google Scholar] [CrossRef] [PubMed]
- Mikoliunaite, L.; Rodriguez, R.D.; Sheremet, E.; Kolchuzhin, V.; Mehner, J.; Ramanavicius, A.; Zahn, D.R. The substrate matters in the Raman spectroscopy analysis of cells. Sci. Rep. 2015, 5, 13150. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.; Zheng, J. Antibacterial activity of silver nanoparticles: Structural effects. Adv. Heal. Mater. 2018, 7, e1701503. [Google Scholar] [CrossRef]
- Baker, C.; Pradhan, A.; Pakstis, L.; Pochan, D.; Shah, S.I. Synthesis and antibacterial properties of silver nanoparticles. J. Nanosci. Nanotechnol. 2005, 5, 244–249. [Google Scholar] [CrossRef]
- Kong, I.C.; Ko, K.-S.; Koh, D.-C. Evaluation of the effects of particle sizes of silver nanoparticles on various biological systems. Int. J. Mol. Sci. 2020, 21, 8465. [Google Scholar] [CrossRef]
- Kim, T.-H.; Kim, M.; Park, H.-S.; Shin, U.S.; Gong, M.-S.; Kim, H.-W. Size-dependent cellular toxicity of silver nanoparticles. J. Biomed. Mater. Res. Part A 2012, 100, 1033–1043. [Google Scholar] [CrossRef]
- Bae, E.; Park, H.-J.; Yoon, J.; Kim, Y.; Choi, K.; Yi, J. Bacterial uptake of silver nanoparticles in the presence of humic acid and AgNO3. Korean J. Chem. Eng. 2010, 28, 267–271. [Google Scholar] [CrossRef]
- Kettler, K.; Giannakou, C.; DeJong, W.H.; Hendriks, A.J.; Krystek, P. Uptake of silver nanoparticles by monocytic THP-1 cells depends on particle size and presence of serum proteins. J. Nanopart. Res. 2016, 18, 286. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.-M.; Wang, W.-X. Size-dependent uptake of silver nanoparticles in Daphnia magna. Environ. Sci. Technol. 2012, 46, 11345–11351. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Levard, C.; Marinakos, S.M.; Cheng, Y.; Liu, J.; Michel, F.M.; Brown, G.E.; Lowry, G.V. Size-controlled Dissolution of organic-coated silver nanoparticles. Environ. Sci. Technol. 2011, 46, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Helmlinger, J.; Sengstock, C.; Groß-Heitfeld, C.; Mayer, C.; Schildhauer, T.A.; Köller, M.; Epple, M. Silver nanoparticles withdifferent size and shape: Equal cytotoxicity, but different antibacterial effects. RSC Adv. 2016, 6, 18490–18501. [Google Scholar] [CrossRef] [Green Version]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.-L.; Cattoni, A.; DeLépinau, R.; Walker, A.W.; Höhn, O.; Lackner, D.; Siefer, G.; Faustini, M.; Vandamme, N.; Goffard, J.; et al. A 19.9%-efficient ultra thin solar cell based on a 205-nm-thick GaAs absorber and a silver nanostructured back mirror. Nat. Energy 2019, 4, 761–767. [Google Scholar] [CrossRef]
- Oćwieja, M.; Morga, M. Electrokinetic properties of cysteine-stabilized silver nanoparticles dispersed in suspensions and deposited on solid surfaces in the form of monolayers. Electrochim. Acta 2018, 297, 1000–1010. [Google Scholar] [CrossRef]
- Durán, N.; Silveira, C.; Durán, M.; Martinez, D.S.T. Silver nanoparticle protein corona and toxicity: Amini-review. J. Nanobiotechnol. 2015, 13, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, I.-L.; Hsieh, Y.-K.; Wang, C.-F.; Chen, I.-C.; Huang, Y.-J. Trojan-Horse mechanism in the cellular uptake of silver nanoparticles verified by direct intra-and extracellular silver speciation analysis. Environ. Sci. Technol. 2015, 49, 3813–3821. [Google Scholar] [CrossRef]
- Li, X.; Lenhart, J.J.; Walker, H.W. Aggregation kinetics and dissolution of coated silver nanoparticles. Langmuir 2011, 28, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Herting, G.; Wallinder, I.O.; Blomberg, E. Adsorption of bovine serum albumin on silver surfaces enhances the release of silver at pH neutral conditions. Phys. Chem. Chem. Phys. 2015, 17, 18524–18534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oćwieja, M.; Barbasz, A. Sodium hexametaphosphate–induced enhancement of silver nanoparticle toxicity towards leukemia cells. J. Nanopart. Res. 2020, 22, 167. [Google Scholar] [CrossRef]
- Ruden, S.; Hilpert, K.; Berditsch, M.; Wadhwani, P.; Ulrich, A.S. Synergistic interaction between silver nanoparticles and membrane-permeabilizing antimicrobial peptides. Antimicrob. Agents Chemother. 2009, 53, 3538–3540. [Google Scholar] [CrossRef] [Green Version]
- Hoppens, M.A.; Sylvester, C.; Qureshi, A.T.; Scherr, T.; Czapski, D.R.; Duran, R.S.; Savage, P.B.; Hayes, D. Ceragenin mediated selectivity of antimicrobial silver nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 13900–13908. [Google Scholar] [CrossRef]
- Duraipandy, N.; Lakra, R.; Vinjimur, S.K.; Samanta, D.; Purna, K.S.; Kiran, M.S. Caging of plumbagin on silver nanoparticles imparts selectivity and sensitivity to plumbagin for targeted cancer cell apoptosis. Metallomics 2014, 6, 2025–2033. [Google Scholar] [CrossRef] [Green Version]
- Gallón, S.N.; Alpaslan, E.; Wang, M.; Larese-Casanova, P.; Londoño, M.E.; Atehortúa, L.; Pavón, J.J.; Webster, T.J. Characterization and study of the antibacterial mechanisms of silver nanoparticles prepared with microalgal exopolysaccharides. Mater. Sci. Eng. C 2019, 99, 685–695. [Google Scholar] [CrossRef]
- Yousaf, H.; Mehmood, A.; Ahmad, K.S.; Raffi, M. Green synthesis of silver nanoparticles and their applications as analternative antibacterial and antioxidant agents. Mater. Sci. Eng. C 2020, 112, 110901. [Google Scholar] [CrossRef]
- Dhand, V.; Soumya, L.; Bharadwaj, S.; Chakra, S.; Bhatt, D.; Sreedhar, B. Green synthesis of silver nanoparticles using Coffea arabica seed extract and its antibacterial activity. Mater. Sci. Eng. C 2016, 58, 36–43. [Google Scholar] [CrossRef]
- Sankar, R.; Karthik, A.; Prabu, A.; Karthik, S.; Shivashangari, K.S.; Ravikumar, V. Origanum vulgare mediated biosynthesis of silver nanoparticles for its antibacterial and anticancer activity. Colloids Surf. B Biointerfaces 2013, 108, 80–84. [Google Scholar] [CrossRef]
- Lee, P.C.; Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidalgold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Oćwieja, M.; Maciejewska-Prończuk, J.; Adamczyk, Z.; Roman, M. Formation of positively charged gold nanoparticle monolayers on silica sensors. J. Colloid Interface Sci. 2017, 501, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Creighton, J.A.; Blatchford, C.G.; Albrecht, M.G. Plasma resonance enhancement of Raman scattering by pyridine adsorbed on silver or gold sol particles of size comparable to the excitation wavelength. J. Chem. Soc. Faraday Trans. 1979, 75, 790–798. [Google Scholar] [CrossRef]
- Kamyshny, A.; Magdassi, S. Aqueous dispersion of metallic nanoparticles. Preparation, stabilization and application. In Nanoscience Colloidal and Interfacial Aspects; Starov, V.M., Ed.; CRC Press: Boca Raton, FL, USA, 2010; Volume 147, pp. 747–779. [Google Scholar]
- Zhong, Z.; Patskovskyy, S.; Bouvrette, P.; Luong, A.J.H.T.; Gedanken, A. The surface chemistry of Au colloids and their interactions with functional amino acids. J. Phys. Chem. B 2004, 108, 4046–4052. [Google Scholar] [CrossRef]
- Martínez-Castañón, G.A.; Niño-Martínez, N.; Martínez-Gutierrez, F.; Martínez-Mendoza, J.R.; Ruiz, F. Synthesis and antibacterial activity of silver nanoparticles with different sizes. J. Nanopart. Res. 2008, 10, 1343–1348. [Google Scholar] [CrossRef]
- Yin, I.X.; Yu, O.Y.; Zhao, I.S.; Mei, M.L.; Li, Q.-L.; Tang, J.; Chu, C.-H. Developing biocompatible silver nanoparticles using epigallocatechingallate for dental use. Arch. Oral Biol. 2019, 102, 106–112. [Google Scholar] [CrossRef]
- Dadosh, T. Synthesis of uniform silver nanoparticles with a controllable size. Mater. Lett. 2009, 63, 2236–2238. [Google Scholar] [CrossRef]
- Barbasz, A.; Czyżowska, A.; Piergies, N.; Oćwieja, M. Design cytotoxicity: The effect of silver nanoparticles stabilized by selected antioxidants on melanoma cells. J. Appl. Toxicol. 2021. [Google Scholar] [CrossRef]
- Leopold, A.N.; Lendl, B.A. New method for fast preparation of highly surface-enhanced Raman scattering(SERS) active silver colloids at room temperature by reduction of silver nitrate with hydroxyl amine hydrochloride. J. Phys. Chem. B 2003, 107, 5723–5727. [Google Scholar] [CrossRef]
- Kujda, M.; Oćwieja, M.; Adamczyk, Z.; Bocheńska, O.; Braś, G.; Kozik, A.; Bielańska, E.; Barbasz, J. Charge stabilized silvernanoparticles applied as antibacterial agents. J. Nanosci. Nanotechnol. 2015, 15, 3574–3583. [Google Scholar] [CrossRef]
- Barbasz, A.; Oćwieja, M.; Roman, M. Toxicity of silver nanoparticles towards tumoral human cell lines U-937 and HL-60. Colloids Surf. B Biointerfaces 2017, 156, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Perni, S.; Hakala, V.; Prokopovich, P. Biogenic synthesis of antimicrobial silver nanoparticles cappedwith L-cysteine. Colloids Surf. A Physicochem. Eng. Asp. 2014, 460, 219–224. [Google Scholar] [CrossRef]
- Li, D.; Liu, Z.; Yuan, Y.; Liu, Y.; Niu, F. Green synthesis of gallic acid-coated silver nanoparticles with high antimicrobial activity and low cytotoxicity to normal cells. Process. Biochem. 2015, 50, 357–366. [Google Scholar] [CrossRef]
- Barbasz, A.; Oćwieja, M.; Piergies, N.; Duraczyńska, D.; Nowak, A. Antioxidant-modulated cytotoxicity of silver nanoparticles. J. Appl. Toxicol. 2021. [Google Scholar] [CrossRef]
- Panacek, A.; Kvitek, L.; Prucek, R.; Kolář, M.; Večeřová, R.; Pizfuirova, N.; Sharma, V.K.; Nevecna, T.; Zboril, R. Silver colloid nanoparticles: Synthesis, characterization, and their antibacterial activity. J. Phys. Chem. B 2006, 110, 16248–16253. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Kujda, M.; Oćwieja, M. Sposób Wytwarzania Stabilnych Suspensji Nanocząstek Srebra Oraz Zastosowanie Stabilnych Suspensji Nanocząstek Srebra Do Celów Biobójczych. Polish Patent PL 224713, 15 June 2016. [Google Scholar]
- Kreibig, U.; Vollmer, M. Optical Properties of Metal Clusters; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- Munro, C.H.; Smith, W.E.; Garner, M.; Clarkson, J.; White, P.C. Characterization of the surface of a citrate-reduced colloid optimized for use as a substrate for surface-enhanced resonance Raman scattering. Langmuir 1995, 11, 3712–3720. [Google Scholar] [CrossRef]
- Khatoon, U.T.; Rao, G.N.; Mohan, K.M.; Ramanaviciene, A.; Ramanavicius, A. Antibacterial and antifungal activity of silvernanospheres synthesized b trisodium citrate assisted chemical approach. Vacuum 2017, 146, 259–265. [Google Scholar] [CrossRef]
- Sivaraman, S.K.; Elango, I.; Kumar, S.; Santhanam, V. A green protocol for room temperature synthesis of silver nanoparticles inseconds. Curr. Sci. 2009, 97, 00113891. [Google Scholar]
- Alvarez-Ros, M.C.; Sanchez-Cortes, S.; Francioso, O.; García-Ramos, J.V. Adsorption and chemical modification of gallic acid on silver nanoparticles studied by Raman spectroscopy: Effect of anions and cationic pesticide paraquat. Can. J. Anal. Sci. Spectrosc. 2003, 48, 132–138. [Google Scholar]
- Hong, J.; Lu, H.; Meng, X.; Ryu, J.H.; Hara, Y.; Yang, C.S. Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (−)-epigallocatechin-3-gallatein HT-29 human colonadeno carcinoma cells. Cancer Res. 2002, 62, 7241–7246. [Google Scholar] [PubMed]
- Sharma, V.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Y.; Yu, Q. Significant parameters in the optimization of synthesis of silver nanoparticles by chemical reductionMethod. J. Mater. Eng. Perform. 2009, 19, 252–256. [Google Scholar] [CrossRef]
- Hwang, I.-S.; Lee, J.; Hwang, J.H.; Kim, K.-J.; Lee, D.G. Silver nanoparticles induce apoptotic cell death in Candida albican through the increase of hydroxyl radicals. FEBS J. 2012, 279, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Asghari, S.; Johari, S.A.; Lee, J.H.; Kim, Y.S.; Jeon, Y.B.; Choi, H.J.; Moon, M.C.; Yu, I.J. Toxicity of various silver nanoparticles compared to silver ions in Daphnia magna. J. Nanobiotechnol. 2012, 10, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, O.; Hu, Z. Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ. Sci. Technol. 2008, 42, 4583–4588. [Google Scholar] [CrossRef] [PubMed]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.; Morones-Ramirez, J.R.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [Green Version]
- Raza, M.A.; Kanwal, Z.; Rauf, A.; Sabri, A.N.; Riaz, S.; Naseem, S. Size- andshape-dependent antibacterial studies of silver nanoparticles synthesized by wet chemical routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef] [Green Version]
- LeOuay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef] [Green Version]
- Beer, C.; Foldbjerg, R.; Hayashi, Y.; Sutheraland, D.S.; Autrup, H. Toxicity of silver nanoparticles—Nanoparticles f silver ions? Toxicol. Lett. 2012, 208, 286–292. [Google Scholar] [CrossRef]
- DeLima, R.; Seabra, A.; Durán, N. Silver nanoparticles: A brie review of cytotoxicity and genotoxicity of chemically and biogenically synthesized nanoparticles. J. Appl. Toxicol. 2012, 32, 867–879. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Gustafsson, J.; Cronholm, P.; Möller, L. Size-dependent toxicity of metal oxide particles—A comparison between nano- and micrometer size. Toxicol. Lett. 2009, 188, 112–118. [Google Scholar] [CrossRef]
- Pasquina-Lemoche, L.; Burns, J.; Turner, R.D.; Kumar, S.; Tank, R.; Mullin, N.; Wilson, J.S.; Chakrabarti, B.; Bullough, P.A.; Foster, S.J.; et al. The architecture of the Gram-positive bacterial cell wall. Nature 2020, 582, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Chaffin, W.L.; López-Ribot, J.L.; Casanova, M.; Gozalbo, D.; Martínez, J.P. Cell wall and secreted proteins of Candida albicans: Identification, function, and expression. Microbiol. Mol. Biol. Rev. 1998, 62, 130–180. [Google Scholar] [CrossRef] [Green Version]
- ElBadawy, A.M.; Silva, R.G.; Morris, B.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Surfacecharge-dependent toxicity of silver nanoparticles. Environ. Sci. Technol. 2010, 45, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.; Pokhrel, L.R.; Dubey, B.; Tolaymat, T.M.; Maier, K.J.; Liu, X. Particle size, surface charge and concentration dependent ecotoxicity of three organo-coated silver nanoparticles: Comparison between general linear model predicted and observe toxicity. Sci. Total Environ. 2014, 468, 968–976. [Google Scholar] [CrossRef]
- Wigginton, N.; deTitta, A.; Piccapietra, F.; Dobias, J.; Nesatyy, V.J.; Suter, M.J.-F.; Bernier-Latmani, R. Binding of silver nanoparticles to bacterial proteins depends on surface modifications and inhibits enzymatic activity. Environ. Sci. Technol. 2010, 44, 2163–2168. [Google Scholar] [CrossRef] [Green Version]
- Suresh, A.K.; Pelletier, D.A.; Wang, W.; Morrell-Falvey, J.L.; Gu, B.; Doktycz, M.J. Cytotoxicity induced by engineered silver nanocrystallites is dependent on surface coatings and cell types. Langmuir 2012, 28, 2727–2735. [Google Scholar] [CrossRef] [PubMed]
- Storey, R.F.; Wiggins, J.S.; Mauritz, K.A.; Puckett, A.D. Bioabsorbable composites. II: Nontoxic, L-lysine-based poly (es-ter-urethane) matrix composites. Polym. Compos. 1993, 14, 17–25. [Google Scholar] [CrossRef]
- Fahey, J.L.; Perry, R.S.; McCoy, P.F. Blood ammonia elevation and toxicity from intravenous L-amino acid administration to dogs: The protective role of L-arginine. Am. J. Physiol. Content 1958, 192, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Mutschler, M.A.; Chu, C.-C. Synthesis and characterization of ionic charged water soluble arginine-based poly (esteramide). J. Mater. Sci. Mater. Electron. 2011, 22, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Shis, I.-L.; Van, Y.T.; Shen, M.H. Biomedical applications of chemically and microbiologically synthesized poly (glutamic acid) and poly (lysine). Mini Rev. Med. Chem. 2004, 4, 179–188. [Google Scholar] [CrossRef]
- Tanvir, F.; Yaqub, A.; Tanvir, S.; Anderson, W.A. Poly-L-arginine coated silver nanoprisms and their anti-bacterial properties. Nanomaterials 2017, 7, 296. [Google Scholar] [CrossRef] [Green Version]
- Jeitner, T.M. Mechanisms for the cytotoxicity of cysteamine. Toxicol. Sci. 2001, 63, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Pandey, S.H.; Patni, P.M.; Jain, P.; Sanwatsarkar, G.; Bardia, C. Cysteamine improves the bactericidal efficacy of intra-canal medicaments against Enterococcus faecalis. Med. Pharm. Rep. 2018, 91, 448–451. [Google Scholar] [CrossRef]
- El-Baky, R.M.A.; ElEla, D.M.M.A.; Gad, G.F.M. N-acetylcysteine inhibits and eradicates Candida albicans biofilms. Am. J. Infect. Dis. Microbiol. 2014, 2, 122–130. [Google Scholar] [CrossRef] [Green Version]
- Berglin, E.H.; Edlund, M.B.; Nyberg, G.K.; Carlsson, J. Potentiation by L-cysteine of the bactericidal effect of hydrogen peroxide in Escherichia coli. J. Bacteriol. 1982, 152, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, J.; Granberg, G.P.; Nyberg, G.K.; Edlund, M.B. Bactericidal effect of cysteine exposed to atmospheric oxygen. Appl. Environ. Microbiol. 1979, 37, 383–390. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yang, K.; Jia, Y.; Shi, J.; Tong, Z.; Wang, Z. Cysteine potentiates bactericidal antibiotics activity against Gram-Negative bacterial persisters. Infect. Drug Resist. 2020, 13, 2593–2599. [Google Scholar] [CrossRef]
- Octave, S.; Amborabé, B.-E.; Luini, E.; Ferreira, T.; Fleurat-Lessard, P.; Roblin, G. Antifungal effects of cysteine towards Eutypa lata, a pathogen of vineyards. Plant Physiol. Biochem. 2005, 43, 1006–1013. [Google Scholar] [CrossRef]
- Alkekhia, D.; Shukla, A. Influence of poly-l-lysine molecular weigh on antibacterial efficacy in polymer multilayer films. J. Biomed. Mater. Res. Part A 2019, 107, 1324–1339. [Google Scholar] [CrossRef]
- Gopal, R.; Seo, C.H.; Song, P.I.; Park, Y. Effect of repetitive lysine tryptophan motifs on the bactericidal activity of antimicrobial peptides. Amino Acids 2012, 44, 645–660. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, B.; Morais, T.P.; Zaini, P.A.; Campos, C.S.; Almeida-Souza, H.O.; Dandekar, A.M.; Nascimento, R.; Goulart, L.R. Antimicrobial activity of Epsilon-Poly-l-lysine against phytopathogenic bacteria. Sci. Rep. 2020, 10, 11324. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, P.; Wu, Y.; Tan, L.; Wei, W.; Liu, S.; Huang, Q.; Chen, J. Epsilon-poly-l-lysine decorated ordered mesoporous silica contributes to the synergistic antifungal effect and enhanced solubility of a lipophilic drug. Mater. Sci. Eng. C 2019, 99, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Yang, Q.; Zhang, A.; Guo, J.; Liu, X.; Wang, Y.; Ma, Q. Synergistic effect of the combinedbio-fungicidesε-poly-l-lysine and chitooligosaccharide in controlling grey mould (Botrytis cinerea) in tomatoes. Int. J. Food Microbiol. 2018, 276, 46–53. [Google Scholar] [CrossRef]
- Chan, D.I.; Prenner, E.J.; Vogel, H.J. Tryptophan-andarginine-rich antimicrobial peptides: Structures and mechanisms of action. Biochim. Biophys. Acta BBA Biomembr. 2006, 1758, 1184–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mutschler, A.; Tallet, L.; Rabineau, M.; Dollinger, C.; Metz-Boutigue, M.-H.; Schneider, F.; Senger, B.; Vrana, N.E.; Schaaf, P.; LaValle, P. Unexpected bactericidal activity of poly (arginine)/hyaluronan nanolayered coatings. Chem. Mater. 2016, 28, 8700–8709. [Google Scholar] [CrossRef]
- Taniguchi, M.; Ochiai, A.; Takahashi, K.; Nakamichi, S.-I.; Nomoto, T.; Saitoh, E.; Kato, T.; Tanaka, T. Effect of alanine, leucine, and arginine substitution on antimicrobial activity against Candida albicans and action mechanism of a cationic octadecapeptide derived from α-amylase of rice. Biopolymers 2016, 106, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Weijmer, M.C.; Debets-Ossenkopp, Y.J.; van de Vondervoort, F.J.; TerWee, P.M. Superior antimicrobial activity of trisodium citrate over heparin for catheter locking. Nephrol. Dial. Transplant. 2002, 17, 2189–2195. [Google Scholar] [CrossRef] [Green Version]
- Khalil, O.A.K.; Oliveira, O.M.M.D.F.; Vellosa, J.C.R.; deQuadros, A.U.; Dalposso, L.M.; Karam, T.K.; Mainardes, R.M.; Khalil, N.M. Curcumin antifungal and antioxidant activities are increased in the presence of ascorbic acid. Food Chem. 2012, 133, 1001–1005. [Google Scholar] [CrossRef]
- Myrvik, Q.N.; Volk, W.A. Comparative study of the antibacterial properties of ascorbic acid and reductogenic compounds. J. Bacteriol. 1954, 68, 622–626. [Google Scholar] [CrossRef] [Green Version]
- Ojha, R.; Manzoor, N.; Khan, L.A. Ascorbic acid modulates pathogenecity markers of Candida albicans. Indian J. Med. Res. 2009, 1, 19–24. [Google Scholar] [CrossRef]
- Yen, G.-C.; Duh, P.-D.; Tsai, H.-L. Antioxidant and prooxidant properties of ascorbic acid and gallic acid. Food Chem. 2002, 79, 307–313. [Google Scholar] [CrossRef]
- Li, Z.-J.; Liu, M.; Dawuti, G.; Dou, Q.; Ma, Y.; Liu, H.-G.; Aibai, S. Antifungal activity of gallic acid in vitro and in vivo. Phytother. Res. 2017, 31, 1039–1045. [Google Scholar] [CrossRef]
- Chakrawarti, L.; Agrawal, R.; Dang, S.; Gupta, S.; Gabrani, R. Therapeutic effects of EGCG: Apatent review. Expert Opin. Ther. Pat. 2016, 26, 907–916. [Google Scholar] [CrossRef]
- Das, S.; Tanwar, J.; Hameed, S.; Fatima, Z.; Manesar, G. Antimicrobial potential of epigallocatechin-3-gallate (EGCG): A green tea polyphenol. J. Biochem. Pharmacol. Res. 2014, 2, 167–174. [Google Scholar]
- Murtiastutik, D.; Sigit, P.; Cita, R.; Tantular, I.S.; Wibisono, Y.; Hidayati, A.N.; Sawitri, L.; Muhammad, Y. Epigallocathecingallate (EGCG) antifungal properties for Candida isolates from HIV/AIDS patients with oral Candidiasisin compare with fluconazole. Indian J. Forensic Med. Toxicol. 2021, 15, 1021–1026. [Google Scholar] [CrossRef]
- Chung, K.T.; Zhao, G.; Stevens, E., Jr.; Simco, B.A.; Wie, C.I. Growth inhibition of selected aquatic bacteria by tannic acid and related compounds. J. Aquat. Anim. Health 1995, 7, 46–49. [Google Scholar] [CrossRef]
- Gülçin, I.; Huyut, Z.; Elmastaş, M.; Aboul-Enein, H.Y. Radicalscavengingandantioxidantactivityoftannicacid. Arab. J. Chem. 2010, 3, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Ono, K.; Hasegawa, K.; Naiki, H.; Yamada, M. Anti-amyloidogenic activity of tannic acid and its activity to destabilize Alzheimer′s β-amyloid fibrils in vitro. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2004, 1690, 193–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimoi, K.; Nakamura, Y.; Tomita, I.; Kada, T. Bio-antimutagenic effects of tannic acid on UV and chemically induced mutagenesis in Escherichia coli B/r. Mutat. Res. Mol. Mech. Mutagen. 1985, 149, 17–23. [Google Scholar] [CrossRef]
- Zhu, C.; Lei, M.; Andargie, M.; Zeng, J.; Li, J. Antifungal activity and mechanism of action of tannic acid against Penicilliu digitatum. Physiol. Mol. Plant Pathol. 2019, 107, 46–50. [Google Scholar] [CrossRef]
- Azam, S.; Hadi, N.; Khan, N.U.; Hadi, S.M. Antioxidant and prooxidant properties of caffeine, theobromine and xanthine. Med. Sci. Monit. 2003, 9, BR325–BR330. [Google Scholar]
- Raut, J.S.; Chauhan, N.M.; Shinde, R.B.; Karuppayil, S.M. Inhibition of planktonic and biofilm growth of Candida albicans reveals novel antifungal activity of caffeine. J. Med. Plants Res. 2013, 7, 777–782. [Google Scholar] [CrossRef]
- Sledz, W.; Los, E.; Paczek, A.; Rischka, J.; Motyka-Pomagruk, A.; Zoledowska, S.; Piosik, J.; Lojkowska, E. Antibacterial activity of caffeine against plant pathogenic bacteria. Acta Biochim. Pol. 2015, 62, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Z.; Tagaya, I. Antiviral effects of atropine and caffeine. J. Gen. Virol. 1980, 50, 429–431. [Google Scholar] [CrossRef]
- Gross, P.; Smith, R.P. Biologic activity of hydroxylamine: A review. CRC Crit. Rev. Toxicol. 1985, 14, 87–99. [Google Scholar] [CrossRef]
- Fukao, T.; Sawada, H.; Ohta, Y. Combined effect of hop resins and sodium hexametaphosphate against certain strains of Escherichia coli. J. Food Prot. 2000, 63, 735–740. [Google Scholar] [CrossRef]
- Vaara, M. Agents that increase the permeability of the outer membrane. Microbiol. Mol. Biol. Rev. 1992, 56, 395–411. [Google Scholar] [CrossRef]
- Vaara, M.; Jaakkola, J. Sodium hexametaphosphate sensitizes Pseudomonas aeruginosa, several other species of Pseudomonas, and Escherichia coli to hydrophobic drugs. Antimicrob. Agents Chemother. 1989, 33, 1741–1747. [Google Scholar] [CrossRef] [Green Version]
- Mendes-Gouvêa, C.C.; Amaral, J.G.D.; Fernandes, R.A.; Fernandes, G.L.; Gorup, L.F.; Camargo, E.R.; Delbem, A.C.B.; Barbosa, D.B. Sodium trimetaphosphate and hexametaphosphate impregnated with silver nanoparticles: Characteristics and antimicrobial efficacy. Biofouling 2018, 34, 299–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
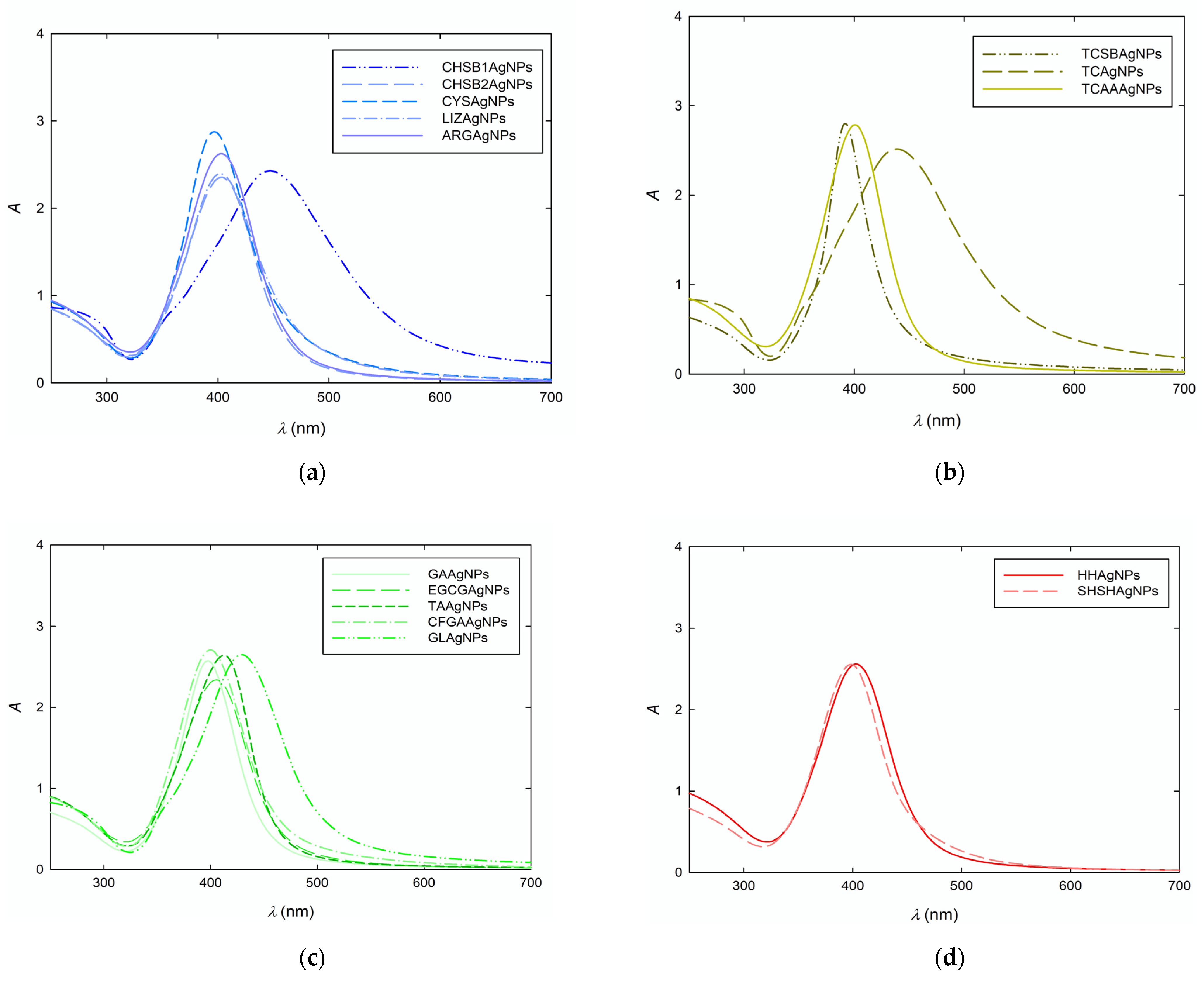
| Symbol | λmax | d (nm) | PdI | D (×10−7 cm2 s−1) | dH (nm) | μe (μm cm (Vs)) | ζ (mV) |
|---|---|---|---|---|---|---|---|
| CHSB1AgNPs | 447 | 55 ± 9 | 0.17 | 1.29 | 50 ± 5 | 4.55 ± 0.12 | 70 ± 2 |
| CHSB2AgNPs | 403 | 12 ± 4 | 0.33 | 5.37 | 12 ± 1 | 3.21 ± 0.17 | 51 ± 2 |
| CYSSBAgNPs | 396 | 12 ± 3 | 0.25 | 5.85 | 11 ± 2 | 2.54 ± 0.28 | 40 ± 4 |
| LYZSBAgNPs | 402 | 16 ± 5 | 0.31 | 4.61 | 14 ± 3 | 1.26 ± 0.09 | 25 ± 2 |
| ARGSBAgNPs | 403 | 13 ± 5 | 0.38 | 4.96 | 13 ± 3 | 1.61 ± 0.06 | 31 ± 2 |
| TCSBAgNPs | 392 | 13 ± 5 | 0.38 | 5.85 | 11 ± 3 | −2.78 ± 0.14 | −45 ± 3 |
| TCAgNPs | 438 | 57 ± 10 | 0.18 | 1.22 | 53 ± 4 | −3.03 ± 0.11 | −47 ± 2 |
| TCAAAgNPs | 400 | 12 ± 4 | 0.33 | 6.44 | 10 ± 2 | −2.53 ± 0.03 | −40 ± 1 |
| GAAgNPs | 397 | 12 ± 4 | 0.33 | 6.44 | 10 ± 3 | 3.29 ± 0.02 | −52 ± 2 |
| EGCGAgNPs | 405 | 15 ± 4 | 0.27 | 4.29 | 15 ± 2 | −3.87 ± 0.06 | −61 ± 1 |
| TAAgNPs | 412 | 13 ± 5 | 0.39 | 5.37 | 12 ± 1 | −3.30 ± 0.23 | −52 ± 3 |
| CFGAAgNPs | 400 | 17 ± 4 | 0.24 | 4.29 | 15 ± 1 | −3.16 ± 0.09 | −49 ± 2 |
| GLAgNPs | 429 | 23 ± 8 | 0.35 | 2.93 | 22 ± 2 | −3.17 ± 0.05 | −50 ± 1 |
| HHAgNPs | 403 | 13 ± 3 | 0.23 | 5.85 | 11 ± 1 | −3.65 ± 0.09 | −55 ± 2 |
| SHSHAgNPs | 398 | 11 ± 3 | 0.27 | 6.44 | 10 ± 2 | −3.76 ± 0.12 | −57 ± 4 |
| Symbol | d (nm) | Escherichia coli | Staphylococcus aureus | Candida albicans | |||
|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | ||
| CHSB1AgNPs | 55 ± 9 | 25 | 45 | 45 | 45 | 45 | 45 |
| CHSB2AgNPs | 12 ± 4 | 35 | 75 | 45 | 100 | 20 | 65 |
| CYSSBAgNPs | 12 ± 3 | 45 | 100 | 80 | 100 | 100 | 100 |
| LYZSBAgNPs | 16 ± 5 | 5 | 10 | 5 | 100 | 10 | 60 |
| ARGSBAgNPs | 13 ± 5 | 20 | 30 | 25 | 30 | 25 | 30 |
| TCSBAgNPs | 13 ± 5 | 40 | 45 | 50 | 80 | 100 | 100 |
| TCAgNPs | 57 ± 10 | 15 | 35 | 100 | 100 | 50 | 50 |
| TCAAAgNPs | 12 ± 4 | 25 | 90 | 75 | 100 | 100 | 100 |
| GAAgNPs | 12 ± 4 | 40 | 100 | 40 | 100 | 100 | 100 |
| EGCGAgNPs | 15 ± 4 | 15 | 40 | 30 | 70 | 10 | 10 |
| TAAgNPs | 13 ± 5 | 5 | 15 | 5 | 100 | 80 | 100 |
| CFGAAgNPs | 17 ± 4 | 10 | 60 | 10 | 85 | 30 | 50 |
| GLAgNPs | 23 ± 8 | 25 | 60 | 50 | 100 | 10 | 100 |
| HHAgNPs | 13 ± 3 | 25 | 25 | 100 | 100 | 100 | 100 |
| SHSHAgNPs | 11 ± 3 | 5 | 40 | 15 | 55 | 35 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gibała, A.; Żeliszewska, P.; Gosiewski, T.; Krawczyk, A.; Duraczyńska, D.; Szaleniec, J.; Szaleniec, M.; Oćwieja, M. Antibacterial and Antifungal Properties of Silver Nanoparticles—Effect of a Surface-Stabilizing Agent. Biomolecules 2021, 11, 1481. https://doi.org/10.3390/biom11101481
Gibała A, Żeliszewska P, Gosiewski T, Krawczyk A, Duraczyńska D, Szaleniec J, Szaleniec M, Oćwieja M. Antibacterial and Antifungal Properties of Silver Nanoparticles—Effect of a Surface-Stabilizing Agent. Biomolecules. 2021; 11(10):1481. https://doi.org/10.3390/biom11101481
Chicago/Turabian StyleGibała, Agnieszka, Paulina Żeliszewska, Tomasz Gosiewski, Agnieszka Krawczyk, Dorota Duraczyńska, Joanna Szaleniec, Maciej Szaleniec, and Magdalena Oćwieja. 2021. "Antibacterial and Antifungal Properties of Silver Nanoparticles—Effect of a Surface-Stabilizing Agent" Biomolecules 11, no. 10: 1481. https://doi.org/10.3390/biom11101481
APA StyleGibała, A., Żeliszewska, P., Gosiewski, T., Krawczyk, A., Duraczyńska, D., Szaleniec, J., Szaleniec, M., & Oćwieja, M. (2021). Antibacterial and Antifungal Properties of Silver Nanoparticles—Effect of a Surface-Stabilizing Agent. Biomolecules, 11(10), 1481. https://doi.org/10.3390/biom11101481








