Potential Mechanisms of Plant-Derived Natural Products in the Treatment of Cervical Cancer
Abstract
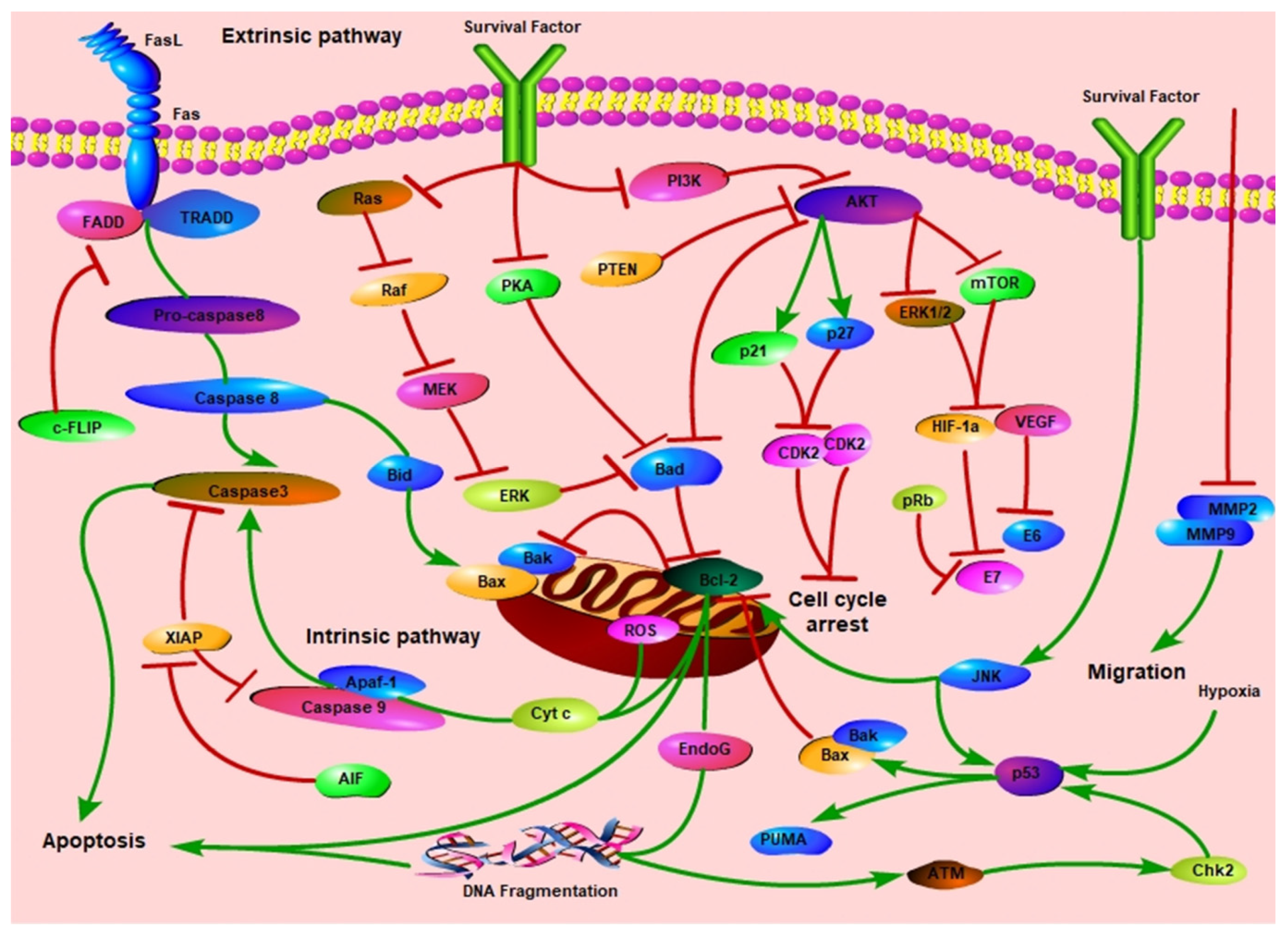
:1. Introduction
2. Methods
3. Flavonoids
3.1. Flavones
3.2. Flavanones
3.3. Flavonols
3.4. Flavanols
3.5. Isoflavones
3.6. Anthocyanins
4. Terpenoids
4.1. Monoterpenoids
4.2. Sesquiterpenoids
4.3. Diterpenoids and Terperpenoids
5. Alkaloids
5.1. Piperine
5.2. Matrine
5.3. Berberine
6. Phenols
6.1. Curcumin
6.2. Ellagic Acid
6.3. Resveratrol
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, L.; Hua, K. Cervical Cancer: Emerging Immune Landscape and Treatment. OncoTargets Ther. 2020, 13, 8037–8047. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Xu, L.; Simoens, C.; Martin-Hirsch, P.P. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst. Rev. 2018, 5, CD009069. [Google Scholar] [CrossRef] [PubMed]
- José, F.X.B.; Quint, W.G.; Alemany, L.; Geraets, D.T.; Klaustermeier, J.E.; Lloveras, B.; Tous, S.; Felix, A.; Bravo, L.E.; Shin, H.-R.; et al. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010, 11, 1048–1056. [Google Scholar] [CrossRef]
- Rogers, L.; Siu, S.S.N.; Luesley, D.; Bryant, A.; O Dickinson, H. Radiotherapy and chemoradiation after surgery for early cervical cancer. Cochrane Database Syst. Rev. 2012, 5, CD007583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tambaro, R.; Scambia, G.; Di Maio, M.; Pisano, C.; Barletta, E.; Iaffaioli, V.R.; Pignata, S. The role of chemotherapy in locally advanced, metastatic and recurrent cervical cancer. Crit. Rev. Oncol. 2004, 52, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Mann, J. Natural products in cancer chemotherapy: Past, present and future. Nat. Rev. Cancer 2002, 2, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Aung, T.N.; Qu, Z.; Kortschak, R.D.; Adelson, D.L. Understanding the Effectiveness of Natural Compound Mixtures in Cancer through Their Molecular Mode of Action. Int. J. Mol. Sci. 2017, 18, 656. [Google Scholar] [CrossRef]
- Harvey, A.L. Natural products in drug discovery. Drug Discov. Today 2008, 13, 894–901. [Google Scholar] [CrossRef]
- Ouyang, L.; Luo, Y.; Tian, M.; Zhang, S.-Y.; Lu, R.; Wang, J.-H.; Kasimu, R.; Li, X. Plant natural products: From traditional compounds to new emerging drugs in cancer therapy. Cell Prolif. 2014, 47, 506–515. [Google Scholar] [CrossRef]
- Kikuchi, H.; Yuan, B.; Hu, X.; Okazaki, M. Chemopreventive and anticancer activity of flavonoids and its possibility for clinical use by combining with conventional chemotherapeutic agents. Am. J. Cancer Res. 2019, 9, 1517–1535. [Google Scholar]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Alseekh, S.; de Souza, L.P.; Benina, M.; Fernie, A.R. The style and substance of plant flavonoid decoration; towards defining both structure and function. Phytochemistry 2020, 174, 112347. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, W.; Liu, J.; Liu, H.; Lv, Z.; Zhang, C.; Chen, D.; Jiao, Z. Identification of Six Flavonoids as Novel Cellular Antioxidants and Their Structure-Activity Relationship. Oxid. Med. Cell. Longev. 2020, 2020, 4150897. [Google Scholar] [CrossRef]
- Serafini, M.; Peluso, I.; Raguzzini, A. Flavonoids as anti-inflammatory agents. Proc. Nutr. Soc. 2010, 69, 273–278. [Google Scholar] [CrossRef] [Green Version]
- Khater, M.; Ravishankar, D.; Greco, F.; Osborn, H.M. Metal complexes of flavonoids: Their synthesis, characterization and enhanced antioxidant and anticancer activities. Future Med. Chem. 2019, 11, 2845–2867. [Google Scholar] [CrossRef]
- Ma, Y.; Zeng, M.; Sun, R.; Hu, M. Disposition of Flavonoids Impacts their Efficacy and Safety. Curr. Drug Metab. 2015, 15, 841–864. [Google Scholar] [CrossRef]
- Dinda, B.; Dinda, S.; Das Sharma, S.; Banik, R.; Chakraborty, A.; Dinda, M. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur. J. Med. Chem. 2017, 131, 68–80. [Google Scholar] [CrossRef]
- Liao, H.; Ye, J.; Gao, L.; Liu, Y. The main bioactive compounds of Scutellaria baicalensis Georgi. for alleviation of inflammatory cytokines: A comprehensive review. Biomed. Pharmacother. 2021, 133, 110917. [Google Scholar] [CrossRef]
- Cheng, C.-S.; Chen, J.; Tan, H.-Y.; Wang, N.; Chen, Z.; Feng, Y. Scutellaria baicalensis and Cancer Treatment: Recent Progress and Perspectives in Biomedical and Clinical Studies. Am. J. Chin. Med. 2018, 46, 25–54. [Google Scholar] [CrossRef]
- Xia, X.; Xia, J.; Yang, H.; Li, Y.; Liu, S.; Cao, Y.; Tang, L.; Yu, X. Baicalein blocked cervical carcinoma cell proliferation by targeting CCND1 via Wnt/β-catenin signaling pathway. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2729–2736. [Google Scholar] [CrossRef]
- Wu, X.; Yang, Z.; Dang, H.; Peng, H.; Dai, Z. Baicalein Inhibits the Proliferation of Cervical Cancer Cells Through the GSK3β-Dependent Pathway. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2018, 26, 645–653. [Google Scholar] [CrossRef]
- Li, J.; Ma, J.; Wang, K.S.; Mi, C.; Wang, Z.; Piao, L.X.; Xu, G.H.; Li, X.; Lee, J.J.; Jin, X. Baicalein inhibits TNF-α-induced NF-κB activation and expression of NF-κB-regulated target gene products. Oncol. Rep. 2016, 36, 2771–2776. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Guo, C.; Yang, Y.; Li, F.; Zhang, Y.; Jiang, B.; Li, Q. Baicalein induces apoptosis of human cervical cancer HeLa cells in vitro. Mol. Med. Rep. 2014, 11, 2129–2134. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Lu, K.; Xia, J.; Mao, X. Baicalein induces HeLa cell growth inhibition by down-regulation of matrix metalloproteinases and activating extracellular signal-regulated kinase. Chin. J. Cell. Mol. Immunol. 2014, 30, 798–801. [Google Scholar]
- Hu, J.; Wang, R.; Liu, Y.; Zhou, J.; Shen, K.; Dai, Y. Baicalein Represses Cervical Cancer Cell Growth, Cell Cycle Progression and Promotes Apoptosis via Blocking AKT/mTOR Pathway by the Regulation of circHIAT1/miR-19a-3p Axis. OncoTargets Ther. 2021, 14, 905–916. [Google Scholar] [CrossRef]
- Yu, X.; Yang, Y.; Li, Y.; Cao, Y.; Tang, L.; Chen, F.; Xia, J. Baicalein inhibits cervical cancer progression via downregulating long noncoding RNA BDLNR and its downstream PI3 K/Akt pathway. Int. J. Biochem. Cell Biol. 2018, 94, 107–118. [Google Scholar] [CrossRef]
- He, L.; Lu, N.; Dai, Q.; Zhao, Y.; Zhao, L.; Wang, H.; Li, Z.; You, Q.; Guo, Q. Wogonin induced G1 cell cycle arrest by regulating Wnt/β-catenin signaling pathway and inactivating CDK8 in human colorectal cancer carcinoma cells. Toxicology 2013, 312, 36–47. [Google Scholar] [CrossRef]
- Kim, M.S.; Bak, Y.; Park, Y.S.; Lee, D.H.; Kim, J.H.; Kang, J.W.; Song, H.-H.; Oh, S.-R.; Yoon, D.Y. Wogonin induces apoptosis by suppressing E6 and E7 expressions and activating intrinsic signaling pathways in HPV-16 cervical cancer cells. Cell Biol. Toxicol. 2013, 29, 259–272. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, H.-W.; Hu, R.; Yang, Y.; Qi, Q.; Lu, N.; Liu, W.; Chu, Y.-Y.; You, Q.-D.; Guo, Q.-L. Wogonin induces G1 phase arrest through inhibiting Cdk4 and cyclin D1 concomitant with an elevation in p21Cip1 in human cervical carcinoma HeLa cells. Biochem. Cell Biol. 2009, 87, 933–942. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Wang, Q.; Zheng, X.-L.; Yan, J.-Q.; Yang, L.; Sun, H.; Hu, L.-N.; Lin, Y.; Wang, X. Wogonin potentiates cisplatin-induced cancer cell apoptosis through accumulation of intracellular reactive oxygen species. Oncol. Rep. 2012, 28, 601–605. [Google Scholar] [CrossRef]
- Madunić, J.; Madunic, I.V.; Gajski, G.; Popić, J.; Garaj-Vrhovac, V. Apigenin: A dietary flavonoid with diverse anticancer properties. Cancer Lett. 2018, 413, 11–22. [Google Scholar] [CrossRef]
- Wang, W.; Yue, R.-F.; Jin, Z.; He, L.-M.; Shen, R.; Du, D.; Tang, Y.-Z. Efficiency comparison of apigenin-7-O-glucoside and trolox in antioxidative stress and anti-inflammatory properties. J. Pharm. Pharmacol. 2020, 72, 1645–1656. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-M.; Ma, R.-H.; Ni, Z.-J.; Thakur, K.; Cespedes-Acuña, C.L.; Jiang, L.; Wei, Z.-J. Apigenin 7-O-glucoside promotes cell apoptosis through the PTEN/PI3K/AKT pathway and inhibits cell migration in cervical cancer HeLa cells. Food Chem. Toxicol. 2020, 146, 111843. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.P.; Bonfim-Mendonça, P.D.S.; Gimenes, F.; Ratti, B.A.; Kaplum, V.; Bruschi, M.L.; Nakamura, C.V.; Silva, S.O.; Maria-Engler, S.; Consolaro, M.E.L. Oxidative Stress Triggered by Apigenin Induces Apoptosis in a Comprehensive Panel of Human Cervical Cancer-Derived Cell Lines. Oxidative Med. Cell. Longev. 2017, 2017, 1512745. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Zhang, Y.; Fan, Z.; Cheng, L.; Han, S.; Che, H. Apigenin Inhibits Histamine-Induced Cervical Cancer Tumor Growth by Regulating Estrogen Receptor Expression. Molecules 2020, 25, 1960. [Google Scholar] [CrossRef] [Green Version]
- Seelinger, G.; Merfort, I.; Schempp, C.M. Anti-Oxidant, Anti-Inflammatory and Anti-Allergic Activities of Luteolin. Planta Med. 2008, 74, 1667–1677. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a Flavonoid with Potential for Cancer Prevention and Therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef]
- Krifa, M.; Alhosin, M.; Muller, C.D.; Gies, J.-P.; Chekir-Ghedira, L.; Ghedira, K.; Mély, Y.; Bronner, C.; Mousli, M. Limoniastrum guyonianum aqueous gall extract induces apoptosis in human cervical cancer cells involving p16INK4A re-expression related to UHRF1 and DNMT1 down-regulation. J. Exp. Clin. Cancer Res. 2013, 32, 30. [Google Scholar] [CrossRef] [Green Version]
- Shi, R.-X.; Ong, C.-N.; Shen, H.-M. Luteolin sensitizes tumor necrosis factor-α-induced apoptosis in human tumor cells. Oncogene 2004, 23, 7712–7721. [Google Scholar] [CrossRef] [Green Version]
- Shao, J.; Wang, C.; Li, L.; Liang, H.; Dai, J.; Ling, X.; Tang, H. Luteoloside Inhibits Proliferation and Promotes Intrinsic and Extrinsic Pathway-Mediated Apoptosis Involving MAPK and mTOR Signaling Pathways in Human Cervical Cancer Cells. Int. J. Mol. Sci. 2018, 19, 1664. [Google Scholar] [CrossRef] [Green Version]
- Horinaka, M.; Yoshida, T.; Shiraishi, T.; Nakata, S.; Wakada, M.; Nakanishi, R.; Nishino, H.; Sakai, T. The combination of TRAIL and luteolin enhances apoptosis in human cervical cancer HeLa cells. Biochem. Biophys. Res. Commun. 2005, 333, 833–838. [Google Scholar] [CrossRef]
- Bharti, S.; Rani, N.; Krishnamurthy, B.; Arya, D.S. Preclinical Evidence for the Pharmacological Actions of Naringin: A Review. Planta Med. 2014, 80, 437–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanbari-Movahed, M.; Jackson, G.; Farzaei, M.H.; Bishayee, A. A Systematic Review of the Preventive and Therapeutic Effects of Naringin Against Human Malignancies. Front. Pharmacol. 2021, 12, 639840. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lin, R.; Hu, X.; Shi, Q.; Chen, H. Naringin induces endoplasmic reticulum stress-mediated apoptosis, inhibits β-catenin pathway and arrests cell cycle in cervical cancer cells. Acta Biochim. Pol. 2020, 67, 181–188. [Google Scholar] [CrossRef]
- Ramesh, E.; Alshatwi, A.A. Naringin induces death receptor and mitochondria-mediated apoptosis in human cervical cancer (SiHa) cells. Food Chem. Toxicol. 2013, 51, 97–105. [Google Scholar] [CrossRef]
- Zeng, L.; Zhen, Y.; Chen, Y.; Zou, L.; Zhang, Y.; Hu, F.; Feng, J.; Shen, J.; Wei, B. Naringin inhibits growth and induces apoptosis by a mechanism dependent on reduced activation of NF-κB/COX-2-caspase-1 pathway in HeLa cervical cancer cells. Int. J. Oncol. 2014, 45, 1929–1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, P.; Khan, F. A mechanistic review of the anticancer potential of hesperidin, a natural flavonoid from citrus fruits. Nutr. Res. 2021, 92, 21–31. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.M.P.F.; Santos, C.; Fernandes, E. Therapeutic potential of hesperidin and its aglycone hesperetin: Cell cycle regulation and apoptosis induction in cancer models. Phytomedicine 2020, 73, 152887. [Google Scholar] [CrossRef] [PubMed]
- Alshatwi, A.A.; Ramesh, E.; Periasamy, V.; Subash-Babu, P. The apoptotic effect of hesperetin on human cervical cancer cells is mediated through cell cycle arrest, death receptor, and mitochondrial pathways. Fundam. Clin. Pharmacol. 2013, 27, 581–592. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, H.; Zhang, J.; Gao, J.; Ge, X.; Lou, G. Hesperidin inhibits HeLa cell proliferation through apoptosis mediated by endoplasmic reticulum stress pathways and cell cycle arrest. BMC Cancer 2015, 15, 682. [Google Scholar] [CrossRef] [Green Version]
- Abenavoli, L.; Capasso, R.; Milic, N.; Capasso, F. Milk thistle in liver diseases: Past, present, future. Phytother. Res. 2010, 24, 1423–1432. [Google Scholar] [CrossRef]
- Chu, C.; Li, D.; Zhang, S.; Ikejima, T.; Jia, Y.; Wang, D.; Xu, F. Role of silibinin in the management of diabetes mellitus and its complications. Arch. Pharmacal. Res. 2018, 41, 785–796. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; He, Q.; Lu, H.; Zhou, X.; Chen, L.; Liu, H.; Lu, Z.; Liu, D.; Liu, Y.; Zuo, D.; et al. Silibinin Induces G2/M Cell Cycle Arrest by Activating Drp1-Dependent Mitochondrial Fission in Cervical Cancer. Front. Pharmacol. 2020, 11, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Maceira, P.; Mateo, J.; Garc, P. Silibinin inhibits hypoxia-inducible factor-1α and mTOR/p70S6K/4E-BP1 signalling pathway in human cervical and hepatoma cancer cells: Implications for anticancer therapy. Oncogene 2008, 28, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Ge, Y.; Chen, Y.; Li, Q.; Chen, J.; Dong, Y.; Shi, W. Cellular and molecular mechanisms of silibinin induces cell-cycle arrest and apoptosis on HeLa cells. Cell Biochem. Funct. 2011, 30, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Li, L.; Chen, S.; Yü, Y.; Qi, M.; Tashiro, S.-I.; Onodera, S.; Ikejima, T. Silibinin induced-autophagic and apoptotic death is associated with an increase in reactive oxygen and nitrogen species in HeLa cells. Free Radic. Res. 2011, 45, 1307–1324. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Qi, M.; Yü, Y.; Li, L.; Yao, G.; Tashiro, S.-I.; Onodera, S.; Ikejima, T. P53 activation plays a crucial role in silibinin induced ROS generation via PUMA and JNK. Free Radic. Res. 2012, 46, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Yu, Y.; Qi, M.; Sun, Z.; Li, L.; Yao, G.; Tashiro, S.-I.; Onodera, S.; Ikejima, T. P53-mediated GSH depletion enhanced the cytotoxicity of NO in silibinin-treated human cervical carcinoma HeLa cells. Free Radic. Res. 2012, 46, 1082–1092. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Rauf, A.; Shah, Z.A.; Saeed, F.; Imran, A.; Arshad, M.U.; Ahmad, B.; Bawazeer, S.; Atif, M.; Peters, D.G.; et al. Chemo-preventive and therapeutic effect of the dietary flavonoid kaempferol: A comprehensive review. Phytother. Res. 2019, 33, 263–275. [Google Scholar] [CrossRef]
- Tu, L.-Y.; Bai, H.-H.; Cai, J.-Y.; Deng, S.-P. The mechanism of kaempferol induced apoptosis and inhibited proliferation in human cervical cancer SiHa cell: From macro to nano. Scanning 2016, 38, 644–653. [Google Scholar] [CrossRef] [Green Version]
- Kashafi, E.; Moradzadeh, M.; Mohamadkhani, A.; Erfanian, S. Kaempferol increases apoptosis in human cervical cancer HeLa cells via PI3K/AKT and telomerase pathways. Biomed. Pharmacother. 2017, 89, 573–577. [Google Scholar] [CrossRef]
- Kumar, A.; Jaitak, V. Natural products as multidrug resistance modulators in cancer. Eur. J. Med. Chem. 2019, 176, 268–291. [Google Scholar] [CrossRef]
- Limtrakul, P.; Khantamat, O.; Pintha, K. Inhibition of P-Glycoprotein Function and Expression by Kaempferol and Quercetin. J. Chemother. 2005, 17, 86–95. [Google Scholar] [CrossRef]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schäfer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62, 1700447. [Google Scholar] [CrossRef]
- Mrkus, L.; Batinić, J.; Bjeliš, N.; Jakas, A. Synthesis and biological evaluation of quercetin and resveratrol peptidyl derivatives as potential anticancer and antioxidant agents. Amino Acids 2018, 51, 319–329. [Google Scholar] [CrossRef]
- Sundaram, M.K.; Raina, R.; Afroze, N.; Bajbouj, K.; Hamad, M.; Haque, S.; Hussain, A. Quercetin modulates signaling pathways and induces apoptosis in cervical cancer cells. Biosci. Rep. 2019, 39, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidya Priyadarsini, R.; Senthil Murugan, R.; Maitreyi, S.; Ramalingam, K.; Karunagaran, D.; Nagini, S. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-κB inhibition. Eur. J. Pharmacol. 2010, 649, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Soto, A.F.; Salas-Vidal, E.; Milan-Pacheco, C.; Sánchez-Carranza, J.N.; Peralta-Zaragoza, O.; González-Maya, L. Quercetin induces G2 phase arrest and apoptosis with the activation of p53 in an E6 expression-independent manner in HPV-positive human cervical cancer-derived cells. Mol. Med. Rep. 2019, 19, 2097–2106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishayee, K.; Ghosh, S.; Mukherjee, A.; Sadhukhan, R.; Mondal, J.; Khuda-Bukhsh, A.R. Quercetin induces cytochrome-c release and ROS accumulation to promote apoptosis and arrest the cell cycle in G2/M, in cervical carcinoma: Signal cascade and drug-DNA interaction. Cell Prolif. 2013, 46, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Xie, S.; Chen, X.; Pan, S.; Qian, H.; Zhu, X. Effects of Quercetin on the Efficacy of Various Chemotherapeutic Drugs in Cervical Cancer Cells. Drug Des. Dev. Ther. 2021, 15, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz-Gil, J.; Paduch, R.; Piersiak, T.; Głowniak, K.; Gawron, A.; Kandefer-Szerszeń, M. The effect of quercetin on pro-apoptotic activity of cisplatin in HeLa cells. Biochem. Pharmacol. 2005, 69, 1343–1350. [Google Scholar] [CrossRef]
- Adhami, V.M.; Syed, D.N.; Khan, N.; Mukhtar, H. Dietary flavonoid fisetin: A novel dual inhibitor of PI3K/Akt and mTOR for prostate cancer management. Biochem. Pharmacol. 2012, 84, 1277–1281. [Google Scholar] [CrossRef] [Green Version]
- Kashyap, D.; Sharma, A.; Sak, K.; Tuli, H.S.; Buttar, H.S.; Bishayee, A. Fisetin: A bioactive phytochemical with potential for cancer prevention and pharmacotherapy. Life Sci. 2018, 194, 75–87. [Google Scholar] [CrossRef]
- Chou, R.-H.; Hsieh, S.-C.; Yu, Y.-L.; Huang, M.-H.; Huang, Y.-C.; Hsieh, Y.-H. Fisetin inhibits migration and invasion of human cervical cancer cells by down-regulating urokinase plasminogen activator expression through suppressing the p38 MAPK-dependent NF-κB signaling pathway. PLoS ONE 2013, 8, e71983. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.-Q.; Guo, W.; Yu, W.-D.; You, Z.-S. Fisetin Simultaneously Targets Apaf-1, ERK, and COX-2 Signaling Leading to Growth Inhibition and Apoptosis in Human Cervical Carcinoma Cell In Vitro. J. Sun Yat-Sen Univ. Med. Sci. 2013, 34, 75. [Google Scholar]
- Ying, T.-H.; Yang, S.-F.; Tsai, S.-J.; Hsieh, S.-C.; Huang, Y.-C.; Bau, D.-T.; Hsieh, Y.-H. Fisetin induces apoptosis in human cervical cancer HeLa cells through ERK1/2-mediated activation of caspase-8-/caspase-3-dependent pathway. Arch. Toxicol. 2012, 86, 263–273. [Google Scholar] [CrossRef]
- Lin, M.-T.; Lin, C.-L.; Lin, T.-Y.; Cheng, C.-W.; Yang, S.-F.; Lin, C.-L.; Wu, C.-C.; Hsieh, Y.-H.; Tsai, J.-P. Synergistic effect of fisetin combined with sorafenib in human cervical cancer HeLa cells through activation of death receptor-5 mediated caspase-8/caspase-3 and the mitochondria-dependent apoptotic pathway. Tumor Biol. 2015, 37, 6987–6996. [Google Scholar] [CrossRef] [PubMed]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Hazzani, A.A.; Alshatwi, A.A. Catechin hydrate inhibits proliferation and mediates apoptosis of SiHa human cervical cancer cells. Food Chem. Toxicol. 2011, 49, 3281–3286. [Google Scholar] [CrossRef]
- Singh, M.; Singh, R.; Bhui, K.; Tyagi, S.; Mahmood, Z.; Shukla, Y. Tea polyphenols induce apoptosis through mitochondrial pathway and by inhibiting nuclear factor-kappaB and Akt activation in human cervical cancer cells. Oncol. Res. 2011, 19, 245–257. [Google Scholar] [CrossRef]
- Panji, M.; Behmard, V.; Zare, Z.; Malekpour, M.; Nejadbiglari, H.; Yavari, S.; Dizaj, T.N.; Safaeian, A.; Maleki, N.; Abbasi, M.; et al. Suppressing effects of green tea extract and Epigallocatechin-3-gallate (EGCG) on TGF-β- induced Epithelial-to-mesenchymal transition via ROS/Smad signaling in human cervical cancer cells. Gene 2021, 794, 145774. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, X.; Lu, Q.; Zhang, Z.; Rao, J.; Le, A.D. Green tea extract and (−)-epigallocatechin-3-gallate inhibit hypoxia- and serum-induced HIF-1α protein accumulation and VEGF expression in human cervical carcinoma and hepatoma cells. Mol. Cancer Ther. 2006, 5, 1227–1238. [Google Scholar] [CrossRef] [Green Version]
- Kilic, U.; Sahin, K.; Tuzcu, M.; Basak, N.; Orhan, C.; Elibol-Can, B.; Kilic, E.; Sahin, F.; Kucuk, O. Enhancement of Cisplatin sensitivity in human cervical cancer: Epigallocatechin-3-gallate. Front. Nutr. 2014, 1, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokoyama, M.; Noguchi, M.; Nakao, Y.; Pater, A.; Iwasaka, T. The tea polyphenol, (-)-epigallocatechin gallate effects on growth, apoptosis, and telomerase activity in cervical cell lines. Gynecol. Oncol. 2004, 92, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Ferreira, D. Genistein. Phytochemistry 2002, 60, 205–211. [Google Scholar] [CrossRef]
- Ghaemi, A.; Soleimanjahi, H.; Razeghi, S.; Gorji, A.; Tabarraei, A.; Moradi, A.; Alizadeh, A.; Vakili, M.A. Genistein induces a protective immunomodulatory effect in a mouse model of cervical cancer. Iran. J. Immunol. 2012, 9, 119–127. [Google Scholar] [PubMed]
- Sahin, K.; Tuzcu, M.; Basak, N.; Caglayan, B.; Kilic, U.; Sahin, F.; Kucuk, O. Sensitization of Cervical Cancer Cells to Cisplatin by Genistein: The Role of NFκB and Akt/mTOR Signaling Pathways. J. Oncol. 2012, 2012, 461562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molton, S.A.; Todd, D.E.; Cook, S.J. Selective activation of the c-Jun N-terminal kinase (JNK) pathway fails to elicit Bax activation or apoptosis unless the phosphoinositide 3′-kinase (PI3K) pathway is inhibited. Oncogene 2003, 22, 4690–4701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguirre-Ghiso, J.A.; Estrada, Y.; Liu, D.; Ossowski, L. ERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK). Cancer Res. 2003, 63, 1684–1695. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, Y.-B.; Jeon, Y.-T.; Lee, S.-C.; Song, Y.-S. Genistein Inhibits Cell Growth by Modulating Various Mitogen-Activated Protein Kinases and AKT in Cervical Cancer Cells. Ann. N. Y. Acad. Sci. 2009, 1171, 495–500. [Google Scholar] [CrossRef]
- Hsieh, C.J.; Hsu, Y.L.; Huang, Y.F.; Tsai, E.M. Molecular Mechanisms of Anticancer Effects of Phytoestrogens in Breast Cancer. Curr. Protein Pept. Sci. 2018, 19, 323–332. [Google Scholar] [CrossRef]
- Chen, H.-H.; Chen, S.-P.; Zheng, Q.-L.; Nie, S.-P.; Li, W.-J.; Hu, X.-J.; Xie, M.-Y. Genistein Promotes Proliferation of Human Cervical Cancer Cells Through Estrogen Receptor-Mediated PI3K/Akt-NF-κB Pathway. J. Cancer 2018, 9, 288–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Zhang, C.; Qing, Y.; Cheng, Y.; Jiang, X.; Li, M.; Yang, Z.; Wang, N. Genistein induces apoptosis by stabilizing intracellular p53 protein through an APE1-mediated pathway. Free Radic. Biol. Med. 2015, 86, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Bharti, R.; Chopra, B.S.; Raut, S.; Khatri, N. A Review on Traditional Uses, Pharmacology, and Phytochemistry. Front. Pharmacol. 2020, 11, 582506. [Google Scholar] [CrossRef] [PubMed]
- Gan, M.; Yin, X. Puerarin Induced in Mantle Cell Lymphoma Apoptosis and its Possible Mechanisms Involving Multi-signaling Pathway. Cell Biophys. 2015, 71, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-J.; Sun, L.; Wang, Z.-D. Effects of Pueraria Mirifica on immune and oxidative functions in U14 cervical cancer mice. Heilongjiang Med. Pharm. 2011, 34, 42–43. [Google Scholar]
- Hu, Y.-L.; Li, G.-L.; Lin, Z.-X. Effect and mechanism of Puerarin on the proliferation of human cervical cancer HeLa cells in vitro. Chin. J. Control Endem. Dis. 2017, 32, 1427–1428. [Google Scholar]
- Jia, L.; Hu, Y.; Yang, G.; Li, P. Puerarin suppresses cell growth and migration in HPV-positive cervical cancer cells by inhibiting the PI3K/mTOR signaling pathway. Exp. Ther. Med. 2019, 18, 543–549. [Google Scholar] [CrossRef] [Green Version]
- Jiang, D.; Rasul, A.; Batool, R.; Sarfraz, I.; Hussain, G.; Tahir, M.M.; Qin, T.; Selamoglu, Z.; Ali, M.; Li, J.; et al. Potential Anticancer Properties and Mechanisms of Action of Formononetin. BioMed Res. Int. 2019, 2019, 5854315. [Google Scholar] [CrossRef]
- Tay, K.-C.; Tan, L.T.-H.; Chan, C.K.; Hong, S.L.; Chan, K.G.; Yap, W.H.; Pusparajah, P.; Lee, L.-H.; Goh, B.-H. Formononetin: A Review of Its Anticancer Potentials and Mechanisms. Front. Pharmacol. 2019, 10, 820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, Y.-L.; Wang, W. Formononetin potentiates epirubicin-induced apoptosis via ROS production in HeLa cells in vitro. Chem. Interact. 2013, 205, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Liao, C.; Liu, X.; Yi, J. Preliminary study on conjugation of formononetin with multiwalled carbon nanotubes for inducing apoptosis via ROS production in HeLa cells. Drug Des. Dev. Ther. 2018, 12, 2815–2826. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, C.; Zhang, J. Effects and significance of formononetin on expression levels of HIF-1α and VEGF in mouse cervical cancer tissue. Oncol. Lett. 2019, 18, 2248–2253. [Google Scholar] [CrossRef]
- Jin, Y.-M.; Xu, T.-M.; Zhao, Y.-H.; Wang, Y.-C.; Cui, M.-H. In vitro and in vivo anti-cancer activity of formononetin on human cervical cancer cell line HeLa. Tumor Biol. 2014, 35, 2279–2284. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, B.; Gul, K.; Wani, A.A.; Singh, P. Health Benefits of Anthocyanins and Their Encapsulation for Potential Use in Food Systems: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2223–2230. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, J.; Liu, F.; Tong, L.; Chen, Z.; Chen, J.; He, H.; Xu, R.; Ma, Y.; Huang, C. Neuroprotective effects of anthocyanins and its major component cyanidin-3-O-glucoside (C3G) in the central nervous system: An outlined review. Eur. J. Pharmacol. 2019, 858, 172500. [Google Scholar] [CrossRef]
- Li, X.; Zhao, J.; Yan, T.; Mu, J.; Lin, Y.; Chen, J.; Deng, H.; Meng, X. Cyanidin-3-O-glucoside and cisplatin inhibit proliferation and downregulate the PI3K/AKT/mTOR pathway in cervical cancer cells. J. Food Sci. 2021, 86, 2700–2712. [Google Scholar] [CrossRef]
- Li, X.; Mu, J.; Lin, Y.; Zhao, J.; Meng, X. Combination of cyanidin-3-O-glucoside and cisplatin induces oxidative stress and apoptosis in HeLa cells by reducing activity of endogenous antioxidants, increasing bax/bcl-2 mRNA expression ratio, and downregulating Nrf2 expression. J. Food Biochem. 2021, 45, e13806. [Google Scholar] [CrossRef]
- Zhou, Y.-X.; Gong, X.-H.; Zhang, H.; Peng, C. A review on the pharmacokinetics of paeoniflorin and its anti-inflammatory and immunomodulatory effects. Biomed. Pharmacother. 2020, 130, 110505. [Google Scholar] [CrossRef]
- Wang, J.-S.; Huang, Y.; Zhang, S.; Yin, H.-J.; Zhang, L.; Zhang, Y.-H.; Song, Y.-W.; Li, D.-D. A Protective Role of Paeoniflorin in Fluctuant Hyperglycemia-Induced Vascular Endothelial Injuries through Antioxidative and Anti-Inflammatory Effects and Reduction of PKC1. Oxid. Med. Cell Longev. 2019, 2019, 5647219. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, F.; Wang, H.; Wang, Y.; Wu, Y.; Xu, H.; Su, C. Paeoniflorin inhibits proliferation of endometrial cancer cells via activating MAPK and NF-κB signaling pathways. Exp. Ther. Med. 2017, 14, 5445–5451. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.-B.; Xiao, G.-C.; Tong, S.-L.; Ding, Y.; Wang, Q.-S.; Li, S.-B.; Hao, Z.-N. Paeoniflorin inhibits human gastric carcinoma cell proliferation through up-regulation of microRNA-124 and suppression of PI3K/Akt and STAT3 signaling. World J. Gastroenterol. 2015, 21, 7197–7207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, S. Modulating Bcl-2 Family Proteins and Caspase-3 in Induction of Apoptosis by Paeoniflorin in Human Cervical Cancer Cells. Phytother. Res. 2011, 25, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Suntres, Z.E.; Coccimiglio, J.; Alipour, M. The Bioactivity and Toxicological Actions of Carvacrol. Crit. Rev. Food Sci. Nutr. 2014, 55, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.R.; De Carvalho, F.O.; Teixeira, L.G.B.; Santos, N.G.L.; Felipe, F.A.; Santana, H.S.R.; Shanmugam, S.; Júnior, L.J.Q.; Araujo, A.A.d.S.; Nunes, P.S. Pharmacological Effects of Carvacrol in In vitro Studies: A Review. Curr. Pharm. Des. 2018, 24, 3454–3465. [Google Scholar] [CrossRef]
- Potočnjak, I.; Gobin, I.; Domitrović, R. Carvacrol induces cytotoxicity in human cervical cancer cells but causes cisplatin resistance: Involvement of MEK-ERK activation. Phytother. Res. 2018, 32, 1090–1097. [Google Scholar] [CrossRef]
- Gökalp, F. The effective natural compounds for inhibiting Cervical cancer. Med. Oncol. 2021, 38, 1–4. [Google Scholar] [CrossRef]
- Cui, L.; Su, X.-Z. Discovery, mechanisms of action and combination therapy of artemisinin. Expert Rev. Anti-Infect. Ther. 2009, 7, 999–1013. [Google Scholar] [CrossRef]
- Ji, P.; Huang, H.; Yuan, S.; Wang, L.; Wang, S.; Chen, Y.; Feng, N.; Veroniaina, H.; Wu, Z.; Wu, Z.; et al. ROS-Mediated Apoptosis and Anticancer Effect Achieved by Artesunate and Auxiliary Fe(II) Released from Ferriferous Oxide-Containing Recombinant Apoferritin. Adv. Health Mater. 2019, 8, e1900911. [Google Scholar] [CrossRef]
- Wong, Y.K.; Xu, C.; Kalesh, K.A.; Chengchao, X.; Lin, Q.; Wong, W.S.F.; Shen, H.-M.; Wang, J. Artemisinin as an anticancer drug: Recent advances in target profiling and mechanisms of action. Med. Res. Rev. 2017, 37, 1492–1517. [Google Scholar] [CrossRef]
- Chen, H.-H.; Zhou, H.-J.; Fang, X. Inhibition of human cancer cell line growth and human umbilical vein endothelial cell angiogenesis by artemisinin derivatives in vitro. Pharmacol. Res. 2003, 48, 231–236. [Google Scholar] [CrossRef]
- Hu, C.-J.; Zhou, L.; Cai, Y. Dihydroartemisinin induces apoptosis of cervical cancer cells via upregulation of RKIP and downregulation of bcl-2. Cancer Biol. Ther. 2013, 15, 279–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, T.; Xia, Q.; Xi, M. Dihydroartemisinin and its anticancer activity against endometrial carcinoma and cervical cancer: Involvement of apoptosis, autophagy and transferrin receptor. Singap. Med. J. 2021, 62, 96–103. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Shi, X.; Li, S.; Tang, P.M.K.; Li, Z.; Li, H.; Wei, C. Antimalarial Dihydroartemisinin triggers autophagy within HeLa cells of human cervical cancer through Bcl-2 phosphorylation at Ser70. Phytomedicine 2019, 52, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Hu, Y.; Wang, T.; Cai, P. Dihydroartemisinin inhibits the viability of cervical cancer cells by upregulating caveolin 1 and mitochondrial carrier homolog 2: Involvement of p53 activation and NAD(P)H:quinone oxidoreductase 1 downregulation. Int. J. Mol. Med. 2017, 40, 21–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanaketpaisarn, O.; Waiwut, P.; Sakurai, H.; Saiki, I. Artesunate enhances TRAIL-induced apoptosis in human cervical carcinoma cells through inhibition of the NF-κB and PI3K/Akt signaling pathways. Int. J. Oncol. 2011, 39, 279–285. [Google Scholar]
- Luo, J.; Zhu, W.; Tang, Y.; Cao, H.; Zhou, Y.; Ji, R.; Zhou, X.; Lu, Z.; Yang, H.; Zhang, S.; et al. Artemisinin derivative artesunate induces radiosensitivity in cervical cancer cells in vitro and in vivo. Radiat. Oncol. 2014, 9, 84. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.-X.; Liu, Z.-N.; Ye, J.; Sha, M.; Qian, H.; Bu, X.-H.; Luan, Z.-Y.; Xu, X.-L.; Huang, A.-H.; Yuan, D.-L.; et al. Artesunate exerts an anti-immunosuppressive effect on cervical cancer by inhibiting PGE2production and Foxp3 expression. Cell Biol. Int. 2014, 38, 639–646. [Google Scholar] [CrossRef]
- Chen, H.-H.; Zhou, H.-J.; Wu, G.-D.; Lou, X.-E. Inhibitory Effects of Artesunate on Angiogenesis and on Expressions of Vascular Endothelial Growth Factor and VEGF Receptor KDR/flk-1. Pharmacology 2004, 71, 1–9. [Google Scholar] [CrossRef]
- Yen, J.-H.; Huang, S.-T.; Huang, H.-S.; Fong, Y.-C.; Wu, Y.-Y.; Chiang, J.-H.; Su, Y.-C. HGK-sestrin 2 signaling-mediated autophagy contributes to antitumor efficacy of Tanshinone IIA in human osteosarcoma cells. Cell Death Dis. 2018, 9, 1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.M.; Xu, S.W.; Liu, P.Q. Salvia miltiorrhizaBurge (Danshen): A golden herbal medicine in cardiovascular therapeutics. Acta Pharmacol. Sin. 2018, 39, 802–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Tanshinone IIA inhibits viral oncogene expression leading to apoptosis and inhibition of cervical cancer. Cancer Lett. 2015, 356, 536–546. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, W.; Kong, X.; Chen, X.; Sun, X.; Zhang, W.; Zhang, R. Tanshinone IIA inhibits glucose metabolism leading to apoptosis in cervical cancer. Oncol. Rep. 2019, 42, 1893–1903. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.-L.; Wang, P.-W.; Hung, Y.-C.; Huang, C.-H.; Rau, K.-M. Proteomic analysis reveals tanshinone IIA enhances apoptosis of advanced cervix carcinoma CaSki cells through mitochondria intrinsic and endoplasmic reticulum stress pathways. Proteomics 2013, 13, 3411–3423. [Google Scholar] [CrossRef]
- Qin, J.; Shi, H.; Xu, Y.; Zhao, F.; Wang, Q. Tanshinone IIA inhibits cervix carcinoma stem cells migration and invasion via inhibiting YAP transcriptional activity. Biomed. Pharmacother. 2018, 105, 758–765. [Google Scholar] [CrossRef]
- Cheng, W.; Huang, C.; Ma, W.; Tian, X.; Zhang, X. Recent Development of Oridonin Derivatives with Diverse Pharmacological Activities. Mini-Rev. Med. Chem. 2018, 19, 114–124. [Google Scholar] [CrossRef]
- Song, M.; Liu, X.; Liu, K.; Zhao, R.; Huang, H.; Shi, Y.; Zhang, M.; Zhou, S.; Xie, H.; Chen, H.; et al. Targeting AKT with Oridonin Inhibits Growth of Esophageal Squamous Cell Carcinoma In Vitro and Patient-Derived Xenografts In Vivo. Mol. Cancer Ther. 2018, 17, 1540–1553. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-N.; Zhang, Z.-Y.; Liu, H.-Y.; Wang, S.-H. Effects of oridonin on mitochondrial apoptotic pathway of human cervical carcinoma Hela cells. China J. Mod. Med. 2021, 31, 1–7. [Google Scholar]
- Hu, H.-Z.; Yang, Y.-B.; Xu, X.-D.; Shen, H.-W.; Shu, Y.-M.; Ren, Z.; Li, X.-M.; Shen, H.-M.; Zeng, H.-T. Oridonin induces apoptosis via PI3K/Akt pathway in cervical carcinoma HeLa cell line. Acta Pharmacol. Sin. 2007, 28, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-L.; Wu, L.-J.; Tashiro, S.-I.; Onodera, S.; Ikejima, T. Oridonin induced A375-S2 cell apoptosis VIA BAX-regulated caspase pathway activation, dependent on the cytochromeC/CASPASE-9 apoptosome. J. Asian Nat. Prod. Res. 2004, 6, 127–138. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Wu, Y.-L.; Tashiro, S.-I.; Onodera, S.; Ikejima, T. Reactive oxygen species contribute to oridonin-induced apoptosis and autophagy in human cervical carcinoma HeLa cells. Acta Pharmacol. Sin. 2011, 32, 1266–1275. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.; Hu, H.; Zeng, H.; Ke, P.; Yang, Y.; Li, X.; Ren, Z. Oridonin Induces Apoptosis Via PI3K/AKT Pathway in Cervical Car cinoma HeLa Cell Line. J. Sun Yat-sen Univ. Med. Sci. 2007, 28, 1819–1826. [Google Scholar]
- Li, X.; Chu, S.; Lin, M.; Gao, Y.; Liu, Y.; Yang, S.; Zhou, X.; Zhang, Y.; Hu, Y.; Wang, H.; et al. Anticancer property of ginsenoside Rh2 from ginseng. Eur. J. Med. Chem. 2020, 203, 112627. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Qiao, J.; Liu, S.; Wang, S.; Zhao, D.; Bai, X.; Liu, M. Ginsenoside Rh2 inhibits HeLa cell energy metabolism and induces apoptosis by upregulating voltage-dependent anion channel 1. Int. J. Mol. Med. 2020, 46, 1695–1706. [Google Scholar] [CrossRef]
- Shi, X.; Yang, J.; Wei, G. Ginsenoside 20(S)-Rh2 exerts anti-cancer activity through the Akt/GSK3β signaling pathway in human cervical cancer cells. Mol. Med. Rep. 2018, 17, 4811–4816. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, Y.; Wang, X.; Fang, X.; He, K.; Guo, X.; Zhan, Z.; Sun, C.; Jin, Y.-H. Co-treatment with ginsenoside Rh2 and betulinic acid synergistically induces apoptosis in human cancer cells in association with enhanced capsase-8 activation, bax translocation, and cytochrome c release. Mol. Carcinog. 2011, 50, 760–769. [Google Scholar] [CrossRef]
- Fulda, S. Betulinic acid: A natural product with anticancer activity. Mol. Nutr. Food Res. 2009, 53, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.L.; Máñez, S. New Pharmacological Opportunities for Betulinic Acid. Planta Med. 2018, 84, 8–19. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Pang, Q.; Wang, Y.; Yan, X. Betulinic acid induces apoptosis by regulating PI3K/Akt signaling and mitochondrial pathways in human cervical cancer cells. Int. J. Mol. Med. 2017, 40, 1669–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.-J.; Cho, H.-S.; Ban, H.S.; Nakamura, H. Suppression of HIF-1α accumulation by betulinic acid through proteasome activation in hypoxic cervical cancer. Biochem. Biophys. Res. Commun. 2020, 523, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Rzeski, W.; Stepulak, A.; Szymański, M.; Sifringer, M.; Kaczor, J.; Wejksza, K.; Zdzisińska, B.; Kandefer-Szerszeń, M. Betulinic acid decreases expression of bcl-2 and cyclin D1, inhibits proliferation, migration and induces apoptosis in cancer cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2006, 374, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Sarek, J.; Klinot, J.; Dzubák, P.; Klinotová, E.; Nosková, V.; Krecek, V.; Korínková, G.; Thomson, J.O.; Janost’áková, A.; Wang, S.; et al. New lupane derived compounds with pro-apoptotic activity in cancer cells: Synthesis and structure-activity relationships. J. Med. Chem. 2003, 46, 5402–5415. [Google Scholar] [CrossRef] [PubMed]
- Aumeeruddy, M.Z.; Mahomoodally, M.F. Combating breast cancer using combination therapy with 3 phytochemicals: Piperine, sulforaphane, and thymoquinone. Cancer 2019, 125, 1600–1611. [Google Scholar] [CrossRef] [PubMed]
- Haq, I.U.; Imran, M.; Nadeem, M.; Tufail, T.; Gondal, T.A.; Mubarak, M.S. Piperine: A review of its biological effects. Phytother. Res. 2020, 35, 680–700. [Google Scholar] [CrossRef] [PubMed]
- Jafri, A.; Siddiqui, S.; Rais, J.; Ahmad, S.; Kumar, S.; Jafar, T.; Afzal, M.; Arshad, M. Induction of apoptosis by piperine in human cervical adenocarcinoma via ROS mediated mitochondrial pathway and caspase-3 activation. EXCLI J. 2019, 18, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Wei, Y.; Xu, J.; Lei, J.; Yu, J. Alkaloids from Piper nigrum Synergistically Enhanced the Effect of Paclitaxel against Paclitaxel-Resistant Cervical Cancer Cells through the Downregulation of Mcl-1. J. Agric. Food Chem. 2019, 67, 5159–5168. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-Z.; Liu, H.-X.; Yang, L.-Q.; Cui, L.-d.; Xu, Y. Piperine (PP) enhanced mitomycin-C (MMC) therapy of human cervical cancer through suppressing Bcl-2 signaling pathway via inactivating STAT3/NF-κB. Biomed. Pharmacother. 2017, 96, 1403–1410. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, L.; Sun, X.; Yang, Q.; Wan, L.; Guo, C. Matrine: A Promising Natural Product with Various Pharmacological Activities. Front. Pharmacol. 2020, 11, 588. [Google Scholar] [CrossRef]
- You, L.; Yang, C.; Du, Y.; Liu, Y.; Chen, G.; Sai, N.; Dong, X.; Yin, X.; Ni, J. Matrine Exerts Hepatotoxic Effects via the ROS-Dependent Mitochondrial Apoptosis Pathway and Inhibition of Nrf2-Mediated Antioxidant Response. Oxid. Med. Cell. Longev. 2019, 2019, 1045345. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wang, T.; Wen, X.; Wei, Y.; Peng, X.; Li, H.; Wei, L. Effect of matrine on HeLa cell adhesion and migration. Eur. J. Pharmacol. 2007, 563, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhou, J.; Cai, D.; Xiaoling, W. Matrine inhibits the metastatic properties of human cervical cancer cells via downregulating the p38 signaling pathway. Oncol. Rep. 2017, 38, 1312–1320. [Google Scholar] [CrossRef]
- Imenshahidi, M.; Hosseinzadeh, H. Berberis Vulgaris and Berberine: An Update Review. Phytother. Res. 2016, 30, 1745–1764. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Feng, X.; Chai, L.; Cao, S.; Qiu, F. The metabolism of berberine and its contribution to the pharmacological effects. Drug Metab. Rev. 2017, 49, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Mahata, S.; Bharti, A.C.; Shukla, S.; Tyagi, A.; A Husain, S.; Das, B.C. Berberine modulates AP-1 activity to suppress HPV transcription and downstream signaling to induce growth arrest and apoptosis in cervical cancer cells. Mol. Cancer 2011, 10, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, S.-C.; Yu, C.-C.; Hsu, L.-S.; Chen, K.-S.; Su, M.-Y.; Chen, P.-N. Berberine Reverses Epithelial-to-Mesenchymal Transition and Inhibits Metastasis and Tumor-Induced Angiogenesis in Human Cervical Cancer Cells. Mol. Pharmacol. 2014, 86, 609–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, B.; Hu, M.; Liu, K.; Peng, J. Cytotoxicity of berberine on human cervical carcinoma HeLa cells through mitochondria, death receptor and MAPK pathways, and in-silico drug-target prediction. Toxicol. Vitr. 2010, 24, 1482–1490. [Google Scholar] [CrossRef]
- Mistry, B.M.; Shin, H.-S.; Keum, Y.-S.; Kim, D.H.; Moon, S.H.; Kadam, A.A.; Shinde, S.K.; Patel, R.V. Synthesis and Evaluation of Antioxidant and Cytotoxicity of the N-Mannich Base of Berberine Bearing Benzothiazole Moieties. Anti-Cancer Agents Med. Chem. 2018, 17, 1652–1660. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, T. Targeting cancer stem cells by curcumin and clinical applications. Cancer Lett. 2014, 346, 197–205. [Google Scholar] [CrossRef]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Singh, N. Molecular mechanism of curcumin induced cytotoxicity in human cervical carcinoma cells. Mol. Cell. Biochem. 2009, 325, 107–119. [Google Scholar] [CrossRef]
- Yoysungnoen-Chintana, P.; Bhattarakosol, P.; Patumraj, S. Antitumor and Antiangiogenic Activities of Curcumin in Cervical Cancer Xenografts in Nude Mice. BioMed Res. Int. 2014, 2014, 817972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.; Kim, H.S.; Jung, E.-J.; Lee, J.Y.; K Tsang, B.; Lim, J.M.; Song, Y.S. Curcumin induces ER stress-mediated apoptosis through selective generation of reactive oxygen species in cervical cancer cells. Mol. Carcinog. 2016, 55, 918–928. [Google Scholar] [CrossRef]
- Wang, T.; Wu, X.; Al Rudaisat, M.; Song, Y.; Cheng, H. Curcumin induces G2/M arrest and triggers autophagy, ROS generation and cell senescence in cervical cancer cells. J. Cancer 2020, 11, 6704–6715. [Google Scholar] [CrossRef] [PubMed]
- Anuchapreeda, S.; Leechanachai, P.; Smith, M.M.; Ambudkar, S.V.; Limtrakul, P.-N. Modulation of P-glycoprotein expression and function by curcumin in multidrug-resistant human KB cells. Biochem. Pharmacol. 2002, 64, 573–582. [Google Scholar] [CrossRef]
- Sajomsang, W.; Gonil, P.; Saesoo, S.; Ruktanonchai, U.R.; Srinuanchai, W.; Puttipipatkhachorn, S. Synthesis and anticervical cancer activity of novel pH responsive micelles for oral curcumin delivery. Int. J. Pharm. 2014, 477, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Ceci, C.; Lacal, P.M.; Tentori, L.; De Martino, M.G.; Miano, R.; Graziani, G. Experimental Evidence of the Antitumor, Antimetastatic and Antiangiogenic Activity of Ellagic Acid. Nutrients 2018, 10, 1756. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.-M.; Zhao, L.; Li, H.; Xu, H.; Chen, W.-W.; Tao, L. Research progress on the anticarcinogenic actions and mechanisms of ellagic acid. Cancer Biol. Med. 2014, 11, 92–100. [Google Scholar] [CrossRef]
- Chen, W.; Shen, X.; Ma, L.; Chen, R.; Yuan, Q.; Zheng, Y.; Li, C.; Peng, G. Phenolic Compounds from Polygonum chinense Induce Growth Inhibition and Apoptosis of Cervical Cancer SiHa Cells. BioMed Res. Int. 2020, 2020, 8868508. [Google Scholar] [CrossRef]
- Li, L.; Na, C.; Tian, S.; Chen, J.; Ma, R.; Gao, Y.; Lou, G. Ellagic acid induces HeLa cell apoptosis via regulating signal transducer and activator of transcription 3 signaling. Exp. Ther. Med. 2018, 16, 29–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, H.; Zhang, D.; Fu, Q. Inhibition of Cervical Cancer by Promoting IGFBP7 Expression Using Ellagic Acid from Pomegranate Peel. Med. Sci. Monit. 2016, 22, 4881–4886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, D.; Basu, S.; Parija, L.; Rout, D.; Manna, S.; Dandapat, J.; Debata, P.R. Curcumin and Ellagic acid synergistically induce ROS generation, DNA damage, p53 accumulation and apoptosis in HeLa cervical carcinoma cells. Biomed. Pharmacother. 2016, 81, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A. Cancer Prevention and Treatment with Resveratrol: From Rodent Studies to Clinical Trials. Cancer Prev. Res. 2009, 2, 409–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Fu, P.; Xie, L.; Chai, S.; Xu, Q.; Zeng, L.; Wang, X.; Jiang, N.; Sang, M. Resveratrol inhibits the progression of cervical cancer by suppressing the transcription and expression of HPV E6 and E7 genes. Int. J. Mol. Med. 2020, 47, 335–345. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, B.; Yao, Y.-Y.; Zhong, L.-X.; Chen, X.-Y.; Kong, Q.-Y.; Wu, M.-L.; Li, C.; Li, H.; Liu, J. PIAS3, SHP2 and SOCS3 Expression patterns in Cervical Cancers: Relevance with activation and resveratrol-caused inactivation of STAT3 signaling. Gynecol. Oncol. 2015, 139, 529–535. [Google Scholar] [CrossRef]
- Sun, X.; Xu, Q.; Zeng, L.; Xie, L.; Zhao, Q.; Xu, H.; Wang, X.; Jiang, N.; Fu, P.; Sang, M. Resveratrol suppresses the growth and metastatic potential of cervical cancer by inhibiting STAT3 Tyr705 phosphorylation. Cancer Med. 2020, 9, 8685–8700. [Google Scholar] [CrossRef] [PubMed]
- He, M.-J.; Yang, S.-Y.; Lu, J.; Han, S.-S.; Liu, R.; Lian, F.-Z. Effects of resveratrol on telomerase activity and gene expression in human cervical cancer HeLa cells. J. Hyg. Res. 2019, 48, 1001–1003. [Google Scholar]
- Tang, X.; Zhang, Q.; Nishitani, J.; Brown, J.; Shi, S.; Le, A.D. Overexpression of Human Papillomavirus Type 16 Oncoproteins Enhances Hypoxia-Inducible Factor 1α Protein Accumulation and Vascular Endothelial Growth Factor Expression in Human Cervical Carcinoma Cells. Clin. Cancer Res. 2007, 13, 2568–2576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Yuan, X.; Li, X.; Zhang, Y. Resveratrol significantly inhibits the occurrence and development of cervical cancer by regulating phospholipid scramblase 1. J. Cell. Biochem. 2019, 120, 1527–1531. [Google Scholar] [CrossRef]
- Epelbaum, R.; Schaffer, M. Curcumin as an Anti-Cancer Agent: Review of the Gap Between Basic and Clinical Applications. Curr. Med. Chem. 2010, 17, 190–197. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, Y.-H.; Lin, C.-W.; Wang, P.-H.; Hsin, M.-C.; Yang, S.-F. The Potential of Chinese Herbal Medicines in the Treatment of Cervical Cancer. Integr. Cancer Ther. 2019, 18, 1534735419861693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.-H.; Kim, M.; Lee, S.; Jung, W.; Kim, B. Therapeutic Potential of Natural Products in Treatment of Cervical Cancer: A Review. Nutrients 2021, 13, 154. [Google Scholar] [CrossRef] [PubMed]





| Chemical Family | Molecule Name | Concentration | Cell Line | Mechanism |
|---|---|---|---|---|
| Flavones | Baicalein | 100 μmol/L | HeLa | Increased: activation of caspase 3; PARP cleavage Decreased: cIAP-1, cIAP-2, FLIP, Bcl-2, MMP2, MMP9, caspase 8, Fas, FasL, VEGF, COX-2, cyclin D1, IL-8, MCP1; Inhibit NF-κB, ERK1/2; G1 phase cell block |
| 10 mg/kg for 28 d | Xenografts of cervical cancer HeLa cells in female BALB/c mice | Decreased: tumor growth | ||
| Wogonin | 0–100 μmol/L | SiHa and CaSki | Increased: Bax; activation of caspase 3 and 9; Cyt c release; PARP cleavage Decreased: MMP; Bcl-2 | |
| 40–160 μmol/L | SiHa and CaSki | Increased: Bax, p53, p21, p27, pRb Decreased: E6, E7 | ||
| Apigenin 7-glucoside | IC50 = 47.26 μmol/L | HeLa | Increased: ROS, Fas, FasL, TNF-α, TNF-r1, FADD, RADD; activation of caspase 3 and 9 Decreased: Bcl-2, Bcl-xl, Cyclin (A, D, E), CDK2/6, MMP; Inhibit PTEN/PI3K/AKT; | |
| Apigenin | IC50 = 10, 68, 76 and 40 μmol/L | HeLa, SiHa, CaSki and C33A | Increased: ROS, H2O2 Decreased: MMP | |
| 100 mg/kg for 30 d | Xenografts of cervical cancer HeLa cells in female BALB/c mice | Decreased: ERβ/ERα, tumor growth | ||
| Luteolin | IC50 = 21.8 μmol/L | HeLa | Increased: p16 INK4A, JNK Decreased: UHRF1, DNMT1, A20, c-IAP1; G2/M phase cell block | |
| Flavanones | Naringin | IC50 = 750 μmol/L | SiHa | Increased: p53, Bax, Fas, FADD; activation of caspase 3, 8 and 9 |
| Hesperidin | 0–100 μmol/L | HeLa | Increased: AIF, Cyt c release; activation of caspase 3 Decreased: MMP, cyclin D1, cyclin E1, CDK2; G0/G1 phase cell block | |
| Silibinin | IC50 = 250,195 μmol/L | HeLa and SiHa | Increased: activation of Drp1 Decreased: ATP, MMP, ROS, CDK1, Cdc25C, cyclinB1; G2/M phase cell block | |
| Flavonols | Kaempferol | IC50 = 10.48 μmol/L | HeLa | Increased: Bax, p53 Decreased: Bcl-2, hTERT; Inhibit PI3K/AKT |
| Quercetin | IC50 = 110.38 ± 0.66 μmol/L | HeLa | Increased: Bax, p53, ROS; activation of caspase 3; Cyt c release Decreased: Bcl-2, AKT, MMP; G2/M cell block | |
| 0–200 μmol/L | HeLa and SiHa | Increased: Bax, p53, p21 Decreased: E6/E6AP, G2 phase cell block | ||
| Fisetin | IC50 = 36.0 ± 0.5 μmol/L | HeLa | Increased: ERK1/2, activation of caspase 3 and 8 | |
| 2–4 mg/kg for 35 d | Xenografts of cervical cancer HeLa cells in male BALB/c mice | Decreased: tumor growth, with inhibition rates of 82.65% and 92.62% | ||
| Flavanols | Epigallocatechin gallate | IC50 = 20 μmol/L | HeLa | Increased: Bax, p53, ROS; Cyt c release Decreased: Bcl-2, COX-2; inhibition AKT and NF-κB |
| Isoflavones | Genistein | IC50 = 20 and 60 μmol/L | HeLa and CaSki | Increased: p38 MAPK, p38-JNK Decreased: ERK1/2, AKT |
| 20 mg/kg | C57BL/6 cervical cancer cell mice model | Decreased: tumor growth | ||
| Puerarin | 12.5–50 μmol/L | HeLa | Increased: Bax Decreased: Wnt/β-catenin, p21, p53, Bcl-2 | |
| 0–2000 μmol/L | HeLa | Increased: Bax Decreased: Inhibit PI3K/AKT/mTOR | ||
| 500 mg/kg for 15 d | cervical cancer cell line U14 mice models | Increased: IL-2, SOD Decreased: tumor growth | ||
| Formononetin | 0–100 μmol/L | HeLa | Increased: Bax, ROS; activation of caspase 3 and 9 Decreased: Bcl-2, MRP1 and MRP2, MMP | |
| 20 and 40 mg/kg for 35 d | Xenografts of cervical cancer HeLa cells in BALB/c nude mice | Decreased: tumor growth | ||
| Anthocyanins | Cyanidin 3-O-glucoside | 400 μmol/L | HeLa | Increased: Bax, p53, TIMP-1 Decreased: Bcl-2, cyclin D1; G2/M phase cell block; PI3K/AKT/mTOR |
| Chemical Family | Molecule Name | Concentration | Cell Line | Mechanism |
|---|---|---|---|---|
| Monoterpenoids | Paeoniflorin | IC50 = 2459 μg/mL | HeLa | Increased: Bax, Apaf-1; activation of caspase 3; Cyt c release Decreased: Bcl-2 |
| Carvacrol | IC50 = 556 ± 39 μmol/L | HeLa | Increased: LC3β-I/II; activation of caspase 9; PARP cleavage Decreased: ERK | |
| Sesquiterpenoids | Dihydroartemisinin | IC50 = 22.08 and 18.20 μmol/L | HeLa and Caski | Increased: RKIP Decreased: Bcl-2 |
| 20 μmol/L for 15 d | Xenografts of cervical cancer HeLa or Caski cells in BALB/c mice | Decreased: tumor growth, with inhibition rates of 70–80% | ||
| Artesunate | 60 μg/mL | HeLa | Increased: AKT; activation of caspase 3; PARP cleavage Decreased: survivin, XIAP, Bcl-xl; G2/M phase cell block; Inhibit NF-κB | |
| 100 mg/kg for 15 d | Xenografts of cervical cancer HeLa cells in BALB/c nude mice | Decreased: tumor growth and inhibition of angiogenesis | ||
| Diterpenoids | Tanshinone IIA | IC50 = 6.97, 14.47, 5.51, and 9.89 μmol/L | HeLa, SiHa, CaSki and C33A | Increased: Bax, PERK, IRE1, p38, JNK; activation of caspase 3 and 9; PARP cleavage; Cyt c and Ca2+ release Decreased: Bcl-2 |
| 0–10 μmol/L | HeLa, SiHa, CaSki | Increased: p53, p21, p130, pRb Decreased: E6, E7 | ||
| 40 mg/kg for 20 d | Cervical cancer cell line U14 mice models | Decreased: metastasis and tumor growth with inhibition rates of 72.7% | ||
| Oridonin | C50 = 4.13 μmol/L | HeLa | Increased: Bax; activation of caspase 3 and 9; Cyt c release Decreased: Bcl-2, MMP | |
| Triterpenoids | Ginsenoside Rh2 | C50 = 35 μmol/L | HeLa | Increased: Bax, ROS, VDAC1; Cyt c release Decreased: MMP |
| Betulinic acid | C50 = 30.42 ± 2.39 μmol/L | HeLa | Increased: Bad, ROS, p27Kip and p21Waf1/Cip1; activation of caspase 9 Decreased: G0/G1 phase cell block; Inhibit PI3K/AKT |
| Chemical Family | Molecule Name | Concentration | Cell Line | Mechanism |
|---|---|---|---|---|
| Alkaloids | Piperine | 0–200 μmol/L | HeLa | Increased: ROS; activation of caspase 3 Decreased: MMP; G2/M phase cell block |
| Matrine | 50 mg/kg for 21 d | Xenografts of cervical cancer HeLa cells in BALB/c nude mice | Decreased: p38, MMP2 and 9; tumor growth with inhibition rates of 58.33% | |
| Berberine | IC50 = 300 μmol/L | HeLa | Increased: Bax, Fas, FasL, TNF-α, TRAF-1, p53, MAPK; DNA fragmentation; activation of caspase 3 Decreased: Bcl-2; S phase cell block | |
| 0–250 μg/mL | HeLa and SiHa | Increased: p53, pRb Decreased: E6, E7, AP-1, c-Jun, c-Fos |
| Chemical Family | Molecule Name | Concentration | Cell Line | Mechanism |
|---|---|---|---|---|
| Phenols | Curcumin | 50 and 100 μmol/L | HeLa, SiHa and CaSki | Increased: AIF; activation of caspase 3 and 9; Cyt c release Decreased: COX-2, iNOS, cyclin D1; Inhibit Ras/Raf |
| 500, 1000 and 1500 mg/kg | Xenografts of cervical cancer CaSki cells in BALB/c mice | Decreased: tumor growth, VEGF and EGFR | ||
| Ellagic acid | C50 = 48.7 ± 2.5 μmol/L | SiHa | Increased: Bcl-2, p53; activation of caspase 3 and 9 Decreased: Bax; G2 phase cell block | |
| Resveratrol | C50 = 40.06, 59.07 μmol/L | HeLa | Increased: Bax, p53, p16 Decreased: G1/S phase cell block | |
| 5–250 μmol/L | HeLa and CaSki | Decreased: Inhibit PI3K/AKT, ERK1/2, VEGF, HIF-1α accumulation | ||
| 10 kg/mg for 28 d | Xenografts of cervical cancer HeLa cells in nude mice | Decreased: tumor growth |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, M.; Xia, L.; Li, J. Potential Mechanisms of Plant-Derived Natural Products in the Treatment of Cervical Cancer. Biomolecules 2021, 11, 1539. https://doi.org/10.3390/biom11101539
He M, Xia L, Li J. Potential Mechanisms of Plant-Derived Natural Products in the Treatment of Cervical Cancer. Biomolecules. 2021; 11(10):1539. https://doi.org/10.3390/biom11101539
Chicago/Turabian StyleHe, Meizhu, Lijie Xia, and Jinyao Li. 2021. "Potential Mechanisms of Plant-Derived Natural Products in the Treatment of Cervical Cancer" Biomolecules 11, no. 10: 1539. https://doi.org/10.3390/biom11101539
APA StyleHe, M., Xia, L., & Li, J. (2021). Potential Mechanisms of Plant-Derived Natural Products in the Treatment of Cervical Cancer. Biomolecules, 11(10), 1539. https://doi.org/10.3390/biom11101539






