A Combination of Structural, Genetic, Phenotypic and Enzymatic Analyses Reveals the Importance of a Predicted Fucosyltransferase to Protein O-Glycosylation in the Bacteroidetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Construction of a Cladogram of Bacteroidetes Fucosyltransferases
2.2. Bacterial Strains and Cultivation Conditions
2.3. General Methods
2.4. Preparation of Bacteroides fragilis Glycopeptides for Glycan Structure Elucidation
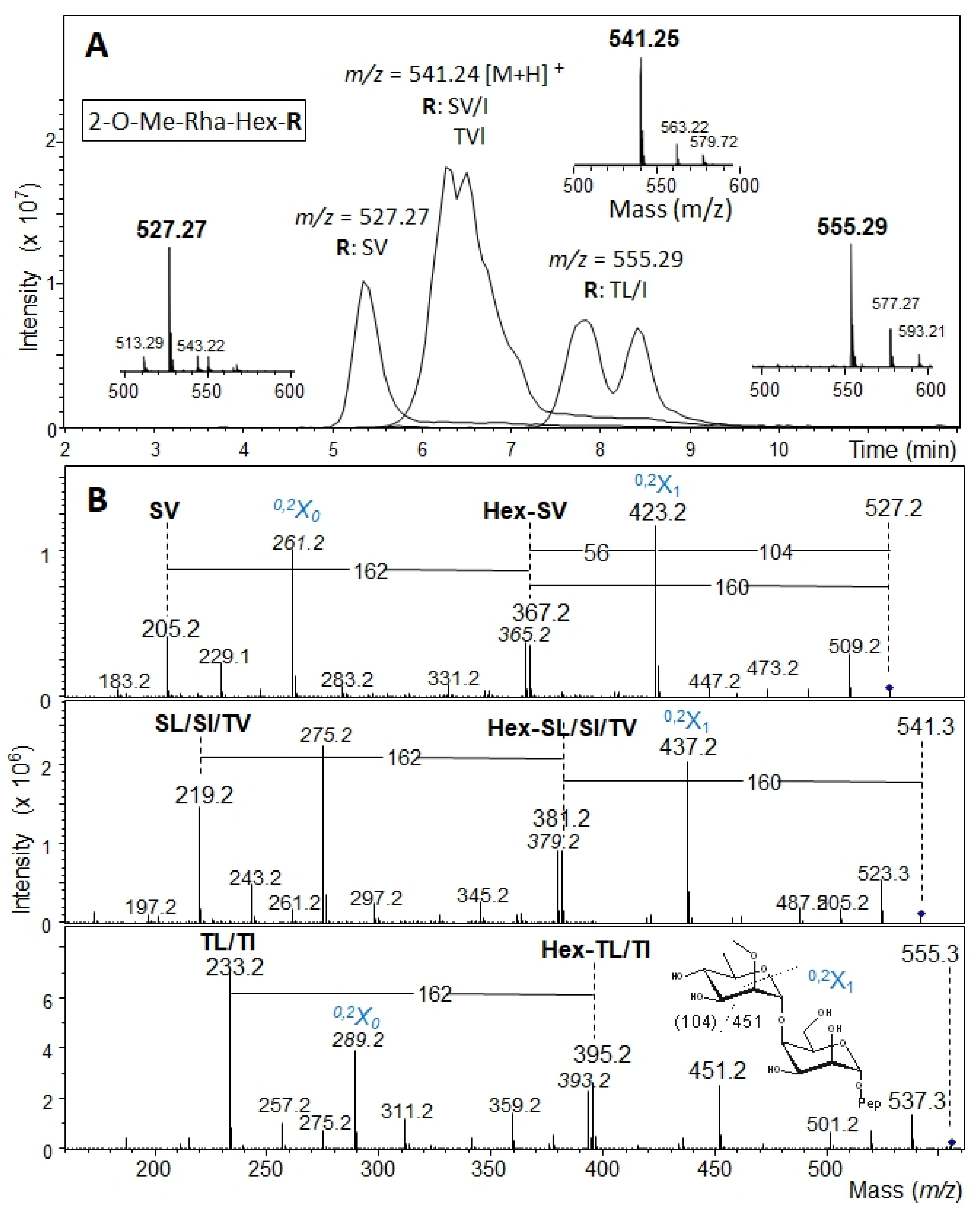
2.5. β-Elimination of Glycans and Mass Spectrometry Analysis
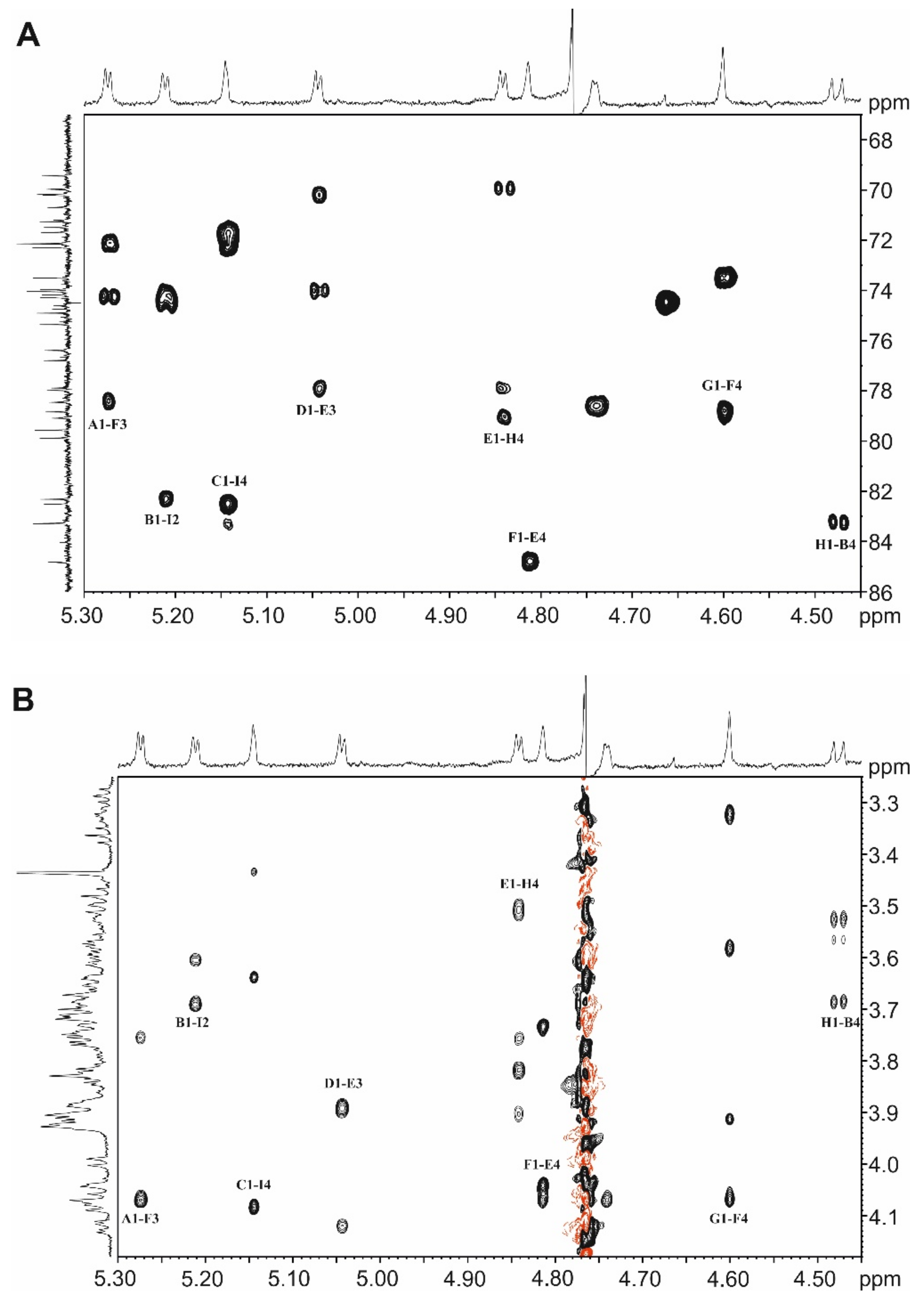
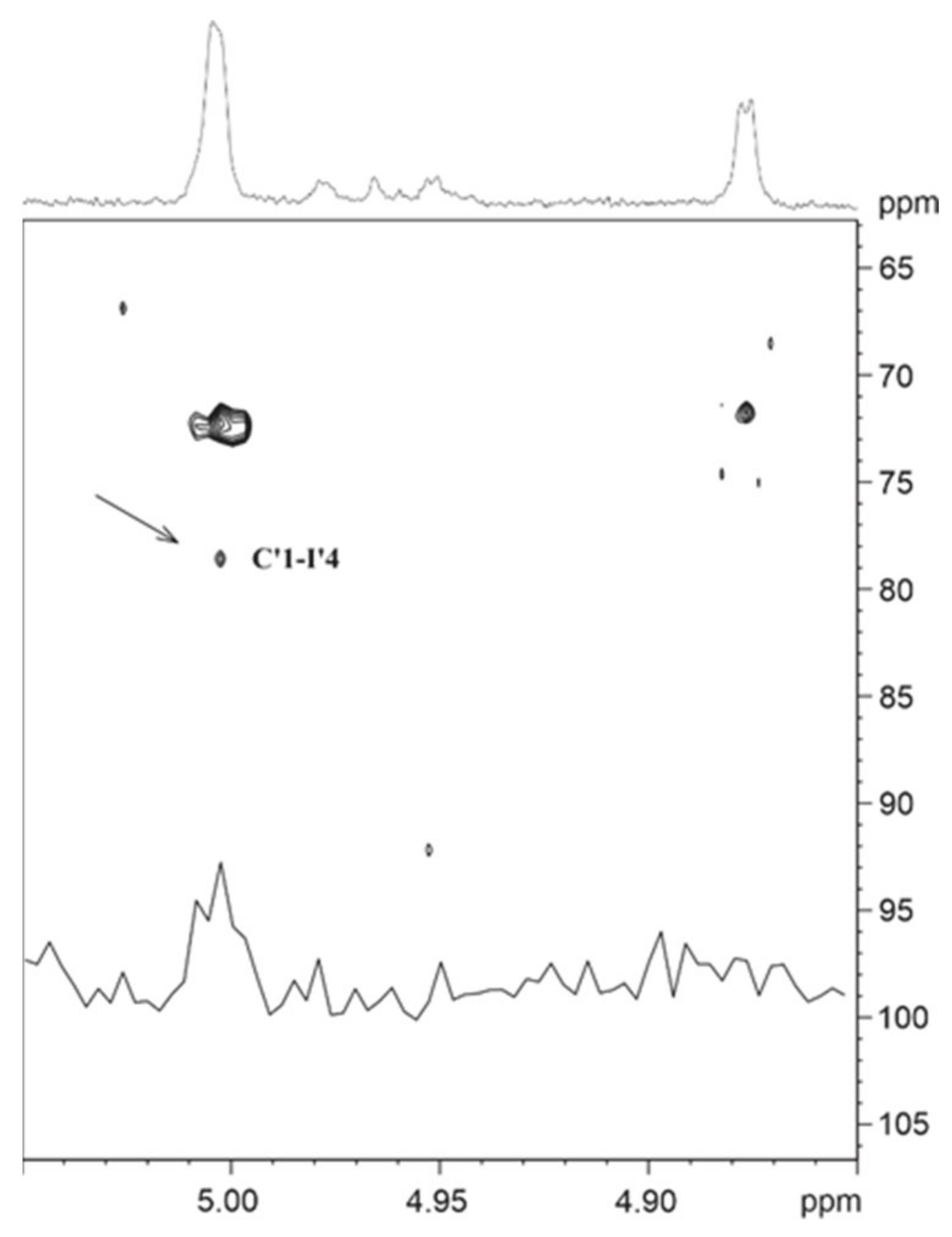
2.6. NMR Spectroscopy for Structure Analysis of the Bacteroides fragilis O-Glycan
2.7. Cross-Complementation in Tannerella forsythia
2.8. Cross-Complementation in Bacteroides fragilis
2.9. Construction of a T. forsythia GDP-l-Fucose Synthase Deletion Mutant
2.10. Cloning of Fucosyltransferase Genes
2.11. Expression and Purification of Recombinant Fucosyltransferases
2.12. In Vitro Fucosyltransferase Activity Assays Using pNP-Sugar Substrates
2.13. Preparation of pNP-α-d-Gal-Fuc as a Fucosidase Substrate
3. Results
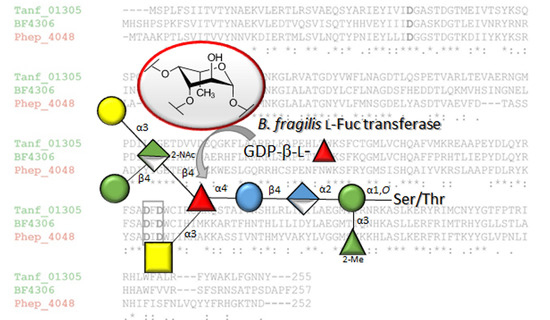
3.1. Presence of FucT Orthologs in Diverse Bacteroidetes Genomes
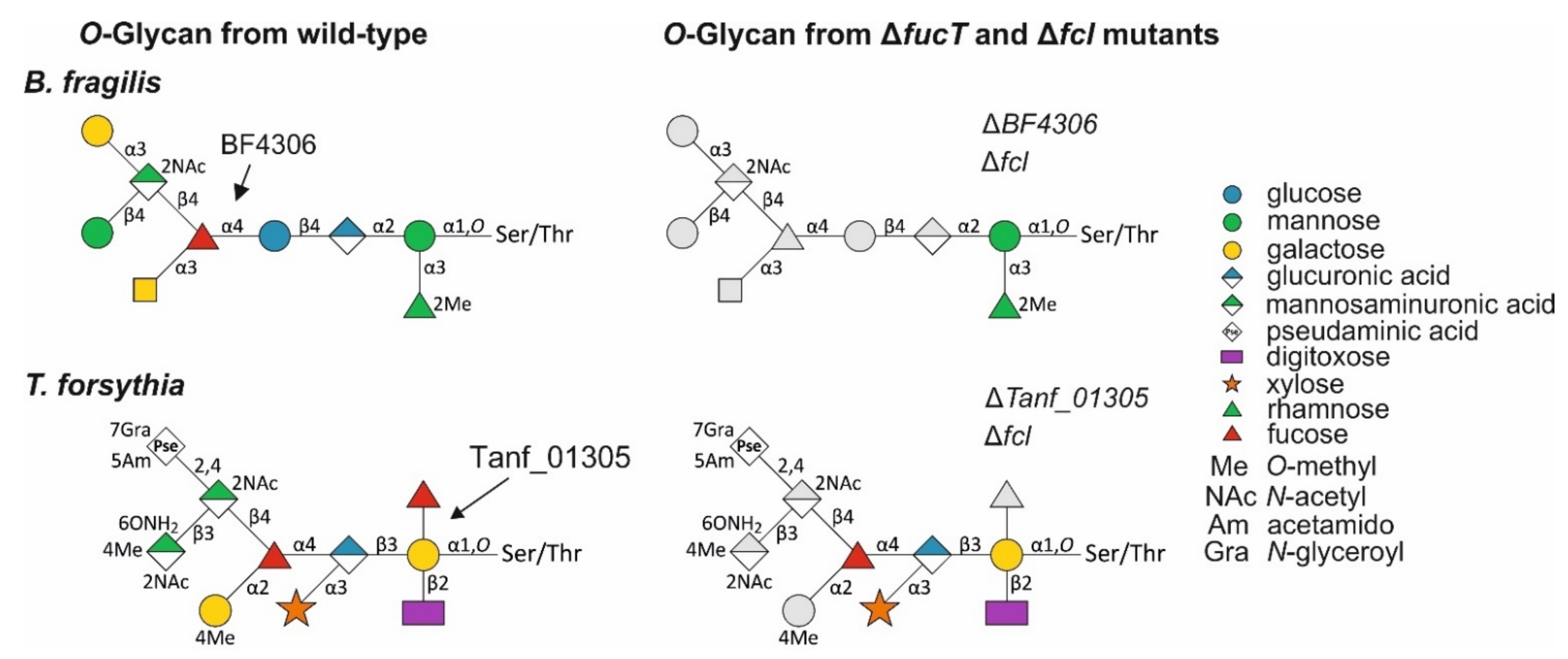
3.2. The Bacteroides fragilis O-Glycan Is a Complex Nonasaccharide Containing Fucose Linked α1,4 to Glucose
3.3. Determination of the Reducing-End Sugar of the Bacteroides fragilis O-Glycan
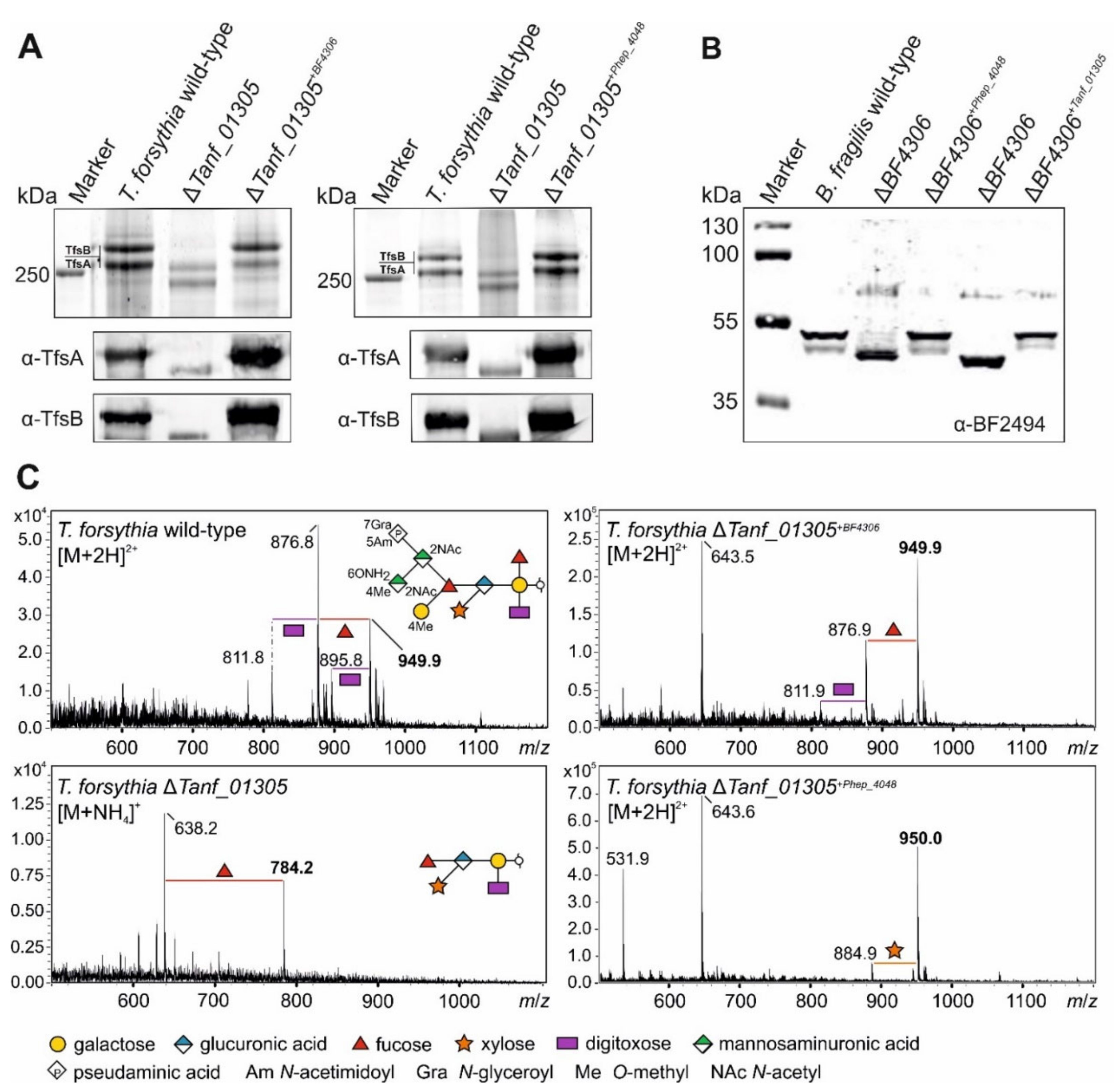
3.4. Bacteroidetes FucTs Can Cross-Complement
3.5. A T. forsythia GDP-l-Fucose Synthase fcl Knock-Out Strain Produces the Same Truncated O-Glycan Phenotype as the FucT-Deficient Strain
3.6. A T. forsythia GDP-l-Fucose Synthase fcl Knock-Out Strain Produces the Same O-Glycan Phenotype as the FucT-Deficient Strain
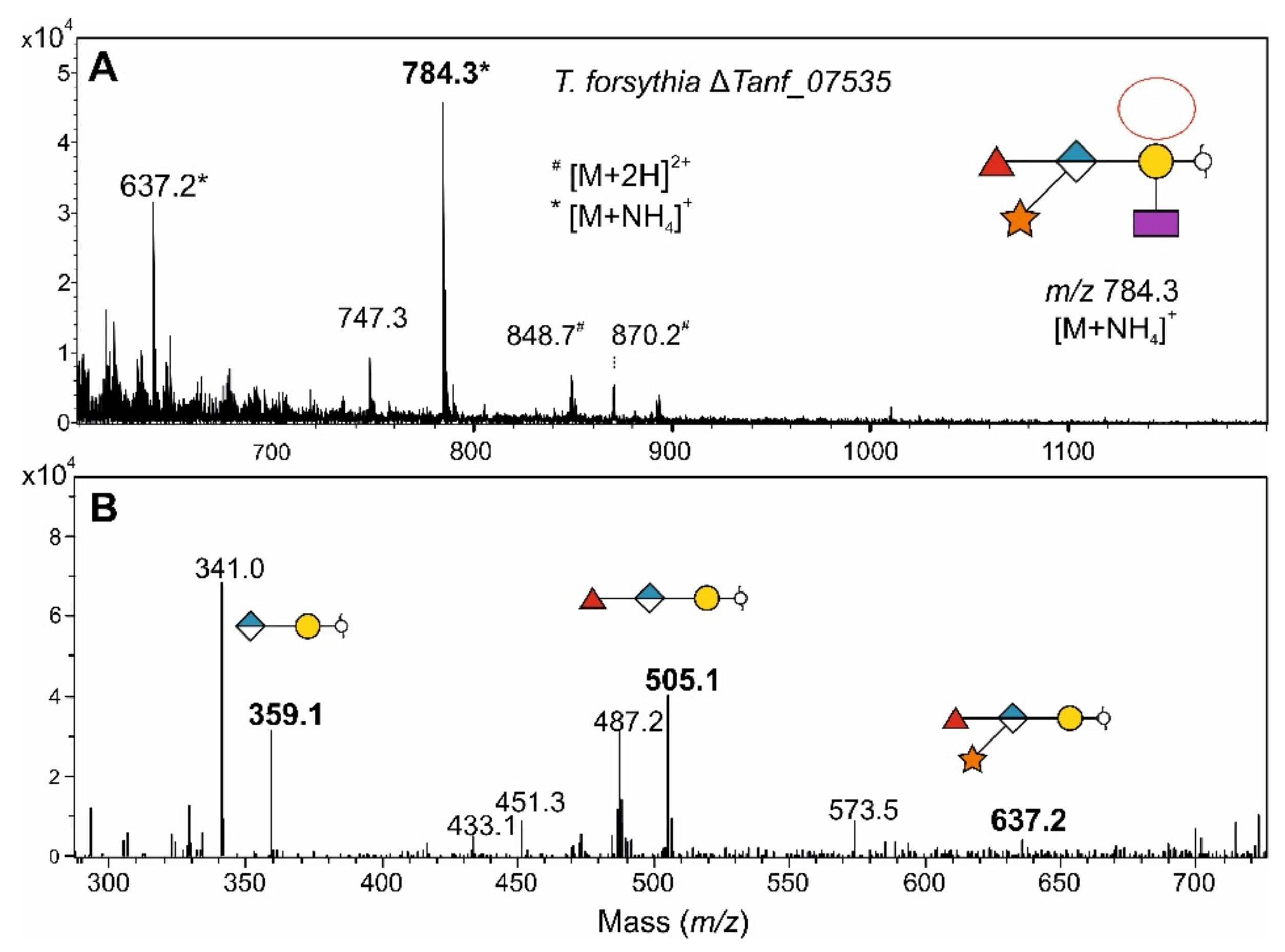
3.7. Fucosidase Treatment of pNP-α-d-Gal-Fuc Suggests an α1,6-Linkage of the l-Fuc in the T. forsythia O-Glycan
4. Discussion
5. Conclusions
- The structure of the protein-linked O-glycan of B. fragilis NCTC 9343 was elucidated by 1D and 2D NMR spectroscopy and shown to be a complex, branched nonasaccharide with an l-Fuc residue in the backbone structure.
- The described l-FucT is relatively conserved among different members of the bacterial phylum Bacteroidetes. Functional orthologs of the FucT BF4306 were demonstrated in the periodontal pathogen Tannerella forsythia (Tanf_01305) and the soil bacterium Pedobacter heparinus (Phep_4048), using in vivo cross-complementation and an in vitro enzyme assay.
- Enzymatic analyses revealed that these l-FucTs exhibit relaxed acceptor substrate specificity transferring l-Fuc from GDP-l-Fuc to galactose, glucose, and mannose residues, with α1,4/6 linkage specificity.
- Given the biological importance of fucosylated carbohydrates, the Bacteroidetes l-FucTs are promising candidates for glycobiology applications.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wexler, H.M. Bacteroides: The good, the bad, and the nitty-gritty. Clin. Microbiol. Rev. 2007, 4, 593–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wexler, A.G.; Goodman, A.L. An insider’s perspective: Bacteroides as a window into the microbiome. Nat. Microbiol. 2017, 5, 17026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 12, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.; Hehemann, J.H.; Rebuffet, E.; Czjzek, M.; Michel, G. Environmental and gut Bacteroidetes: The food connection. Front. Microbiol. 2011, 2, 93. [Google Scholar] [CrossRef] [Green Version]
- Krinos, C.M.; Coyne, M.J.; Weinacht, K.G.; Tzianabos, A.O.; Kasper, D.L.; Comstock, L.E. Extensive surface diversity of a commensal microorganism by multiple DNA inversions. Nature 2001, 6863, 555–558. [Google Scholar] [CrossRef]
- Baumann, H.; Tzianabos, A.O.; Brisson, J.R.; Kasper, D.L.; Jennings, H.J. Structural elucidation of two capsular polysaccharides from one strain of Bacteroides fragilis using high-resolution NMR spectroscopy. Biochemistry 1992, 16, 4081408–4081409. [Google Scholar] [CrossRef]
- Coyne, M.J.; Fletcher, C.M.; Chatzidaki-Livanis, M.; Posch, G.; Schäffer, C.; Comstock, L.E. Phylum-wide general protein O-glycosylation system of the Bacteroidetes. Mol. Microbiol. 2013, 4, 772–783. [Google Scholar] [CrossRef] [Green Version]
- Veith, P.D.; Scott, N.E.; Reynolds, E.C. Characterization of the O-glycoproteome of Tannerella forsythia. mSphere 2021, 6, e00649-21. [Google Scholar] [CrossRef]
- Posch, G.; Pabst, M.; Brecker, L.; Altmann, F.; Messner, P.; Schäffer, C. Characterization and scope of S-layer protein O-glycosylation in Tannerella forsythia. J. Biol. Chem. 2011, 44, 38714–38724. [Google Scholar] [CrossRef] [Green Version]
- Tomek, M.B.; Maresch, D.; Windwarder, M.; Friedrich, V.; Janesch, B.; Fuchs, K.; Neumann, L.; Nimeth, I.; Zwickl, N.F.; Dohm, J.C.; et al. A general protein O-glycosylation gene cluster encodes the species-specific glycan of the oral pathogen Tannerella forsythia: O-glycan biosynthesis and immunological implications. Front. Microbiol. 2018, 9, 2008. [Google Scholar] [CrossRef]
- Bloch, S.; Thurnheer, T.; Murakami, Y.; Belibasakis, G.N.; Schäffer, C. Behavior of two Tannerella forsythia strains and their cell surface mutants in multispecies oral biofilms. Mol. Oral Microbiol. 2017, 5, 404–418. [Google Scholar] [CrossRef] [Green Version]
- Sekot, G.; Posch, G.; Messner, P.; Matejka, M.; Rausch-Fan, X.; Andrukhov, O.; Schäffer, C. Potential of the Tannerella forsythia S-layer to delay the immune response. J. Dent. Res. 2011, 1, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Staudacher, E.; Altmann, F.; Wilson, I.B.; März, L. Fucose in N-glycans: From plant to man. Biochim. Biophys. Acta 1999, 1, 216–236. [Google Scholar] [CrossRef]
- Ma, B.; Simala-Grant, J.L.; Taylor, D.E. Fucosylation in prokaryotes and eukaryotes. Glycobiology 2006, 12, 158r–184r. [Google Scholar] [CrossRef] [Green Version]
- Garber, J.M.; Hennet, T.; Szymanski, C.M. Significance of fucose in intestinal health and disease. Mol. Microbiol. 2021, 6, 1086–1093. [Google Scholar] [CrossRef]
- Coyne, M.J.; Reinap, B.; Lee, M.M.; Comstock, L.E. Human symbionts use a host-like pathway for surface fucosylation. Science 2005, 5716, 1778–1781. [Google Scholar] [CrossRef]
- Comstock, L.E.; Kasper, D.L. Bacterial glycans: Key mediators of diverse host immune responses. Cell 2006, 5, 847–850. [Google Scholar] [CrossRef] [Green Version]
- Oriol, R.; Mollicone, R.; Cailleau, A.; Balanzino, L.; Breton, C. Divergent evolution of fucosyltransferase genes from vertebrates, invertebrates, and bacteria. Glycobiology 1999, 4, 323–334. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Ge, Z.; Rasko, D.A.; Taylor, D.E. Lewis antigens in Helicobacter pylori: Biosynthesis and phase variation. Mol. Microbiol. 2000, 6, 1187–1196. [Google Scholar] [CrossRef]
- Sun, H.Y.; Lin, S.W.; Ko, T.P.; Pan, J.F.; Liu, C.L.; Lin, C.N.; Wang, A.H.; Lin, C.H. Structure and mechanism of Helicobacter pylori fucosyltransferase. A basis for lipopolysaccharide variation and inhibitor design. J. Biol. Chem. 2007, 13, 9973–9982. [Google Scholar] [CrossRef] [Green Version]
- Brzezinski, K.; Dauter, Z.; Jaskolski, M. Structures of NodZ alpha1,6-fucosyltransferase in complex with GDP and GDP-fucose. Acta Crystallogr. D Biol. Crystallogr. 2012, 68, 160–168. [Google Scholar] [CrossRef] [Green Version]
- Posch, G.; Pabst, M.; Neumann, L.; Coyne, M.J.; Altmann, F.; Messner, P.; Comstock, L.E.; Schäffer, C. “Cross-glycosylation” of proteins in Bacteroidales species. Glycobiology 2013, 5, 568–577. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 6, 1547–1549. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 4, 783–791. [Google Scholar] [CrossRef]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 1992, 3, 275–282. [Google Scholar] [CrossRef]
- Friedrich, V.; Pabinger, S.; Chen, T.; Messner, P.; Dewhirst, F.E.; Schäffer, C. Draft genome sequence of Tannerella forsythia type strain ATCC 43037. Genome Announc. 2015, 3, e00660-15. [Google Scholar] [CrossRef] [Green Version]
- Cerdeno-Tarraga, A.M.; Patrick, S.; Crossman, L.C.; Blakely, G.; Abratt, V.; Lennard, N.; Poxton, I.; Duerden, B.; Harris, B.; Quail, M.A.; et al. Extensive DNA inversions in the B. fragilis genome control variable gene expression. Science 2005, 5714, 1463–1465. [Google Scholar] [CrossRef] [Green Version]
- Han, C.; Spring, S.; Lapidus, A.; Del Rio, T.G.; Tice, H.; Copeland, A.; Cheng, J.F.; Lucas, S.; Chen, F.; Nolan, M.; et al. Complete genome sequence of Pedobacter heparinus type strain (HIM 762-3(T)). Stand. Gen. Sci. 2009, 1, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Guiney, D.G.; Hasegawa, P.; Davis, C.E. Plasmid transfer from Escherichia coli to Bacteroides fragilis—Differential expression of antibiotic-resistance phenotypes. Proc. Natl. Acad. Sci. USA 1984, 22, 7203–7206. [Google Scholar] [CrossRef] [Green Version]
- Tomek, M.B.; Neumann, L.; Nimeth, I.; Koerdt, A.; Andesner, P.; Messner, P.; Mach, L.; Potempa, J.S.; Schäffer, C. The S-layer proteins of Tannerella forsythia are secreted via a type IX secretion system that is decoupled from protein O-glycosylation. Mol. Oral Microbiol. 2014, 6, 307–320. [Google Scholar] [CrossRef] [Green Version]
- Tomek, M.B.; Janesch, B.; Maresch, D.; Windwarder, M.; Altmann, F.; Messner, P.; Schäffer, C. A pseudaminic acid or a legionaminic acid derivative transferase is strain-specifically implicated in the general protein O-glycosylation system of the periodontal pathogen Tannerella forsythia. Glycobiology 2017, 6, 555–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, H.R.; Jiang, N. Extremely rapid extraction of DNA from bacteria and yeasts. Biotechnol. Lett. 2006, 1, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 5259, 680–685. [Google Scholar] [CrossRef]
- Hart, C.; Schulenberg, B.; Steinberg, T.H.; Leung, W.Y.; Patton, W.F. Detection of glycoproteins in polyacrylamide gels and on electroblots using Pro-Q Emerald 488 dye, a fluorescent periodate Schiff-base stain. Electrophoresis 2003, 4, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Sekot, G.; Posch, G.; Oh, Y.J.; Zayni, S.; Mayer, H.F.; Pum, D.; Messner, P.; Hinterdorfer, P.; Schäffer, C. Analysis of the cell surface layer ultrastructure of the oral pathogen Tannerella forsythia. Arch. Microbiol. 2012, 6, 525–539. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, C.M.; Coyne, M.J.; Villa, O.F.; Chatzidaki-Livanis, M.; Comstock, L.E. A general O-glycosylation system important to the physiology of a major human intestinal symbiont Bacteroides fragilis. Cell 2009, 2, 321–331. [Google Scholar] [CrossRef] [Green Version]
- Schonenberger, M.; Kellner, H.; Sudhof, H.; Haupt, H. Method of hexose determination in serum proteins with orcinol. Hoppe Seylers Z. Physiol. Chem. 1957, 309, 145–157. [Google Scholar] [PubMed]
- Zarschler, K.; Janesch, B.; Pabst, M.; Altmann, F.; Messner, P.; Schäffer, C. Protein tyrosine O-glycosylation -a rather unexplored prokaryotic glycosylation system. Glycobiology 2010, 6, 787–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pabst, M.; Kolarich, D.; Poltl, G.; Dalik, T.; Lubec, G.; Hofinger, A.; Altmann, F. Comparison of fluorescent labels for oligosaccharides and introduction of a new postlabeling purification method. Anal. Biochem. 2009, 2, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Ceroni, A.; Maass, K.; Geyer, H.; Geyer, R.; Dell, A.; Haslam, S.M. GlycoWorkbench: A tool for the computer-assisted annotation of mass spectra of glycans. J. Proteome Res. 2008, 4, 1650–1659. [Google Scholar] [CrossRef] [Green Version]
- Harris, R.K.; Becker, E.D.; De Menezes, S.M.C.; Granger, P.; Hoffman, R.E.; Zilm, K.W. Further conventions for NMR shielding and chemical shifts (IUPAC recommendations 2008). Pure Appl. Chem. 2008, 1, 59–84. [Google Scholar] [CrossRef]
- Megson, Z.A.; Koerdt, A.; Schuster, H.; Ludwig, R.; Janesch, B.; Frey, A.; Naylor, K.; Wilson, I.; Stafford, G.P.; Messner, P.; et al. Characterization of an α-L-fucosidase from the periodontal pathogen Tannerella forsythia. Virulence 2015, 3, 282–292. [Google Scholar] [CrossRef] [Green Version]
- Breton, C.; Šnajdrová, L.; Jeanneau, C.; Koča, J.; Imberty, A. Structures and mechanisms of glycosyltransferases. Glycobiology 2006, 2, 29R–37R. [Google Scholar] [CrossRef]
- Harvey, D.J.; Mattu, T.S.; Wormald, M.R.; Royle, L.; Dwek, R.A.; Rudd, P.M. "Internal residue loss": Rearrangements occurring during the fragmentation of carbohydrates derivatized at the reducing terminus. Anal. Chem. 2002, 4, 734–740. [Google Scholar] [CrossRef]
- Lettow, M.; Mucha, E.; Manz, C.; Thomas, D.A.; Marianski, M.; Meijer, G.; von Helden, G.; Pagel, K. The role of the mobile proton in fucose migration. Anal. Bioanal. Chem. 2019, 19, 4637–4645. [Google Scholar] [CrossRef] [Green Version]
- Wuhrer, M.; Koeleman, C.A.; Hokke, C.H.; Deelder, A.M. Mass spectrometry of proton adducts of fucosylated N-glycans: Fucose transfer between antennae gives rise to misleading fragments. Rapid Commun. Mass Spectrom. 2006, 11, 1747–1754. [Google Scholar] [CrossRef]
- Domon, B.; Costello, C.E. A systematic nomenclature for carbohydrate fragmentations in FAB-MS MS spectra of glycoconjugates. Glycoconj. J. 1988, 4, 397–409. [Google Scholar] [CrossRef]
- Neelamegham, S.; Aoki-Kinoshita, K.; Bolton, E.; Frank, M.; Lisacek, F.; Lütteke, T.; O’Boyle, N.; Packer, N.H.; Stanley, P.; Toukach, P.; et al. Updates to the Symbol Nomenclature for Glycans guidelines. Glycobiology 2019, 9, 620–624. [Google Scholar] [CrossRef]
- Fletcher, C.M.; Coyne, M.J.; Comstock, L.E. Theoretical and experimental characterization of the scope of protein O-glycosylation in Bacteroides fragilis. J. Biol. Chem. 2011, 5, 3219–3226. [Google Scholar] [CrossRef] [Green Version]
- Reinhold, B.B.; Hauer, C.R.; Plummer, T.H.; Reinhold, V.N. Detailed structural analysis of a novel, specific O-linked glycan from the prokaryote Flavobacterium meningosepticum. J. Biol. Chem. 1995, 22, 13197–13203. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Van Halbeek, H.; Eggimann, B.; Zimmcrmann, J. Structural characterization of the novel O-linked carbohydrate structure of Flavobacterium heparinum heparinase I. Glycobiology 1995, 5, 712. [Google Scholar] [CrossRef]
- Turska-Szewczuk, A.; Russa, R. Structural studies of the O-specific polysaccharide from the lipopolysaccharide of Mesorhizobium huakuii strain S-52, the symbiotic partner of Astragalus sinicus. Carbohydr. Res. 2011, 8, 1065–1069. [Google Scholar] [CrossRef]
- Yoshida, Y.; Nakano, Y.; Nezu, T.; Yamashita, Y.; Koga, T. A novel NDP-6-deoxyhexosyl-4-ulose reductase in the pathway for the synthesis of thymidine diphosphate-D-fucose. J. Biol. Chem. 1999, 24, 16933–16939. [Google Scholar] [CrossRef] [Green Version]
- Zayni, S.; Steiner, K.; Pföstl, A.; Hofinger, A.; Kosma, P.; Schäffer, C.; Messner, P. The dTDP-4-dehydro-6-deoxyglucose reductase encoding fcd gene is part of the surface layer glycoprotein glycosylation gene cluster of Geobacillus tepidamans GS5-97T. Glycobiology 2007, 4, 433–443. [Google Scholar] [CrossRef] [Green Version]
- Petschacher, B.; Nidetzky, B. Biotechnological production of fucosylated human milk oligosaccharides: Prokaryotic fucosyltransferases and their use in biocatalytic cascades or whole cell conversion systems. J. Biotechnol. 2016, 235, 61–83. [Google Scholar] [CrossRef]
- Heimburg-Molinaro, J.; Lum, M.; Vijay, G.; Jain, M.T.; Almogren, A.; Rittenhouse-Olson, K. Cancer vaccines and carbohydrate epitopes. Vaccine 2011, 48, 8802–8826. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Turnbull, W.B. Carbohydrate inhibitors of cholera toxin. Beilstein J. Org. Chem. 2018, 14, 484–498. [Google Scholar] [CrossRef]





| Strain or Plasmid | Genotype and Use or Description | Source or Reference |
|---|---|---|
| Escherichia coli Strains | ||
| DH5α | F− Φ80lacZ∆M15 ∆(lacZYA-argF) U169 recA1 endA1 hsdR17 (rK–, mK+) phoA supE44 λ- thi-1 gyrA96 relA1; cloning strain | Thermo Fisher Scientific |
| BL21(DE3) | F− ompT hsdSB (rB−mB−) gal dcm (DE3); expression strain | Thermo Fisher Scientific |
| Tannerella forsythia Strains | ||
| ATCC 43037 | Type strain, wild-type | ATCC; [26] |
| ATCC 43037 ΔTanf_01305 | ΔTanf_01305:(Perm)-ermF; knock-out strain of Tanf_01305 | [10] |
| ATCC 43037 ΔTanf_01305+BF4306 | ΔTanf_01305::BF4306 cat; cross-complemented knock-out strain | This study |
| ATCC 43037 ΔTanf_01305+Phep_4048 | ΔTanf_01305::Phep_4048 cat; cross-complemented knock-out strain | This study |
| ATCC 43037 ΔTanf_07535 | ΔTanf_07535::ermF; knock-out strain of Tanf_07535 | This study |
| Bacteroides fragilis Strains | ||
| NCTC 9343 | Type strain, wild-type | NCTC; [27] |
| NCTC 9343 ΔBF4306 | ΔBF4306; knock-out strain of BF4306 | [7] |
| Pedobacter heparinus strain | ||
| DSM 2366 | Type strain, wild-type | DSMZ; [28] |
| Plasmids | ||
| pJET1.2/blunt | Cloning vector; ampR | Thermo Fisher Scientific |
| pMAL_c2E | Expression vector, ampR | New England Biolabs |
| RK231 | Broad-host-range mobilizing IncP plasmid, RK2 derivative; kanR | [29] |
| pCMF118 | E. coli-Bacteroides shuttle vector, pFD340 derivative; ampR ermR | [22] |
| pJET/TF0955ko | Template for amplifying the erm gene | [30] |
| pJET1.2/ΔTanf_01245+ | Template for amplifying the cat gene; ampR catR | [31] |
| pJET1.2/ΔTanf_01305+BF4306 | Cassette for reconstitution of ΔTanf_01305 with BF4306; ampR catR | This study |
| pJET1.2/ΔTanf_01305+Phep_4048 | Cassette for reconstitution of ΔTanf_01305 with Phep_4048; ampR catR | This study |
| pMT2 | Cassette for reconstitution of ΔBF4306 with Tanf_01305; ampR catR | This study |
| pMT21 | Cassette for reconstitution of ΔBF4306 with Phep_4048; ampR catR | This study |
| pJET1.2/ΔTanf_07535 | ΔTanf_07535::ermF; Tanf_07535 knock-out cassette; ampR ermR | This study |
| pMAL_c2E/Tanf_01305 | MBP-Tanf_01305 fusion; 72.5 kDa; ampR | This study |
| pMAL_c2E/BF4306 | MBP-BF4306 fusion; 72.7 kDa; ampR | This study |
| pMAL_c2E/Phep_4048 | MBP-Phep_4048 fusion; 72.0 kDa; ampR | This study |
| Chemical Shift δ 1H (JHH) and 13C (JCH) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Unit | 1 | 2 | 3 | 4 | 5 | 6 | CH3 | CO |
| A | 5.274 (4.0) | 3.760 (4.0, 10.4) | 3.707 (10.4, 3.1) | 3.925 (3.1, 1.2) | 4.261 (1.2, 5.5, 7.0) | 3.719 (5.5, 11.5) | ||
| α-Galp | 3.712 (7.0, 11.5) | |||||||
| 102.87 (173.1) | 71.26 | 72.14 | 72.14 | 74.30 | 64.24 | |||
| B | 5.212 (4.1) | 3.606 (4.1, 10.0) | 3.828 (10.0, 8.8) | 3.687 (8.8, 9.7) | 4.205 (9.7) | |||
| α-GlcpA | 102.44 (171.6) | 73.97 | 74.18 | 83.28 | 74.62 | 178.51 | ||
| C | 5.145 (1.6) | 3.639 (1.6, 3.5) | 3.826 (3.5, 9.7) | 3.364 (9.7, 9.7) | 3.767 (9.7, 6.3) | 1.248 (6.3) | 3.436 | |
| α-(2-O-Me)-Rhap | 101.56 (168.4) | 83.30 | 72.31 | 74.91 | 71.69 | 19.29 | 61.09 | |
| D | 5.044 (4.0) | 4.122 (4.0, 11.2) | 3.896 (11.2, 3.3) | 3.930 (3.3, 0.8) | 4.225 (0.8, 8.7, 3.5) | 3.747 (8.7, 12.0) | 2.010 | |
| α-GalpNAc | 3.664 (3.5, 12.0) | |||||||
| 102.32 (172.1) | 52.51 | 70.21 | 71.50 | 74.04 | 64.58 | 24.80 | 177.24 | |
| E | 4.842 (4.1) | 3.819 (4.1, 10.3) | 3.893 (10.3, 3.2) | 4.042 (3.2, 0.8) | 4.384 (0.8, 6.2) | 1.147 (6.2) | ||
| α-Fucp | 102.25 (172.2) | 70.69 | 77.89 | 84.83 | 69.98 | 18.11 | ||
| F | 4.814 (1.2) | 4.741 (1.2, 3.3) | 4.077 (3.3, 9.8) | 4.059 (9.8, 9.3) | 3.736 (9.3) | 2.097 | ||
| β-ManpANAc | 103.23 (161.5) | 55.70 | 78.46 | 78.84 | 79.89 | 177.72 | 24.78 | 177.61 |
| G | 4.601 (1.0) | 3.914 (1.0, 3.7) | 3.583 (3.7, 9.8) | 3.482 (9.8, 9.7) | 3.323 (9.7, 2.2, 7.0) | 3.913 (2.2, 12.1) | ||
| β-Manp | 3.687 (7.0, 12.1) | |||||||
| 102.27 (160.4) | 73.51 | 75.36 | 69.44 | 79.57 | 63.70 | |||
| H | 4.476 (8.0) | 3.287 (8.0, 9.3) | 3.568 (9.3, 8.8) | 3.501 (8.8, 9.8) | 3.529 (9.8, 3.5, 4.0) | 3.911 (3.5, 11.4) | ||
| β-Glcp | 3.760 (4.0, 11.4) | |||||||
| 104.77 (161.2) | 76.39 | 76.80 | 79.09 | 77.99 | 62.42 | |||
| I | 3.914 | 3.691 | 3.998 | 4.085 | 3.884 | 3.837 | ||
| Alditol | 3.869 | 3.669 | ||||||
| 63.79 | 82.32 | 70.18 | 82.52 | 74.05 | 65.11 | |||
| C’ | 5.004 (1.5) | 3.608 (1.5, 3.5) | 3.770 (3.5, 9.4) | 3.365 (9.4, 9.4) | 3.956 (9.4, 6.3) | 1.240 (6.3) | 3.454 | |
| α-(2-O-Me)-Rhap | 100.49 (169.7) | 83.13 | 72.68 | 74.89 | 71.61 | 19.15 | 61.30 | |
| I’ | 4.877 (1.9) | 3.941 (1.9, 3.7) | 3.888 (3.7, 9.4) | 3.768 (9.4, 9.2) | 3.708 (9.2, 1.8, 5.2) | 3.855 (1.8, 12.3) | ||
| α-Manp | 3.773 (5.2, 12.3) | |||||||
| 103.09 (170.3) | 72.55 | 71.65 | 78.58 | 74.76 | 63.27 | |||
| A | β | γ | CO | |||||
| Ser | 4.335 (3.9, 5.7) | 4.181 (3.9, 11.5) | ||||||
| 3.916 (5.7, 11.5) | ||||||||
| 55.69 | 68.56 | n.d. | ||||||
| Val | 4.081 (6.1) | 2.099 (6.1, 6.9) | 0.934 (6.9) | 0.903 (6.9) | ||||
| 55.75 | 33.10 | 21.56 | 20.07 | n.d. | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomek, M.B.; Janesch, B.; Braun, M.L.; Taschner, M.; Figl, R.; Grünwald-Gruber, C.; Coyne, M.J.; Blaukopf, M.; Altmann, F.; Kosma, P.; et al. A Combination of Structural, Genetic, Phenotypic and Enzymatic Analyses Reveals the Importance of a Predicted Fucosyltransferase to Protein O-Glycosylation in the Bacteroidetes. Biomolecules 2021, 11, 1795. https://doi.org/10.3390/biom11121795
Tomek MB, Janesch B, Braun ML, Taschner M, Figl R, Grünwald-Gruber C, Coyne MJ, Blaukopf M, Altmann F, Kosma P, et al. A Combination of Structural, Genetic, Phenotypic and Enzymatic Analyses Reveals the Importance of a Predicted Fucosyltransferase to Protein O-Glycosylation in the Bacteroidetes. Biomolecules. 2021; 11(12):1795. https://doi.org/10.3390/biom11121795
Chicago/Turabian StyleTomek, Markus B., Bettina Janesch, Matthias L. Braun, Manfred Taschner, Rudolf Figl, Clemens Grünwald-Gruber, Michael J. Coyne, Markus Blaukopf, Friedrich Altmann, Paul Kosma, and et al. 2021. "A Combination of Structural, Genetic, Phenotypic and Enzymatic Analyses Reveals the Importance of a Predicted Fucosyltransferase to Protein O-Glycosylation in the Bacteroidetes" Biomolecules 11, no. 12: 1795. https://doi.org/10.3390/biom11121795
APA StyleTomek, M. B., Janesch, B., Braun, M. L., Taschner, M., Figl, R., Grünwald-Gruber, C., Coyne, M. J., Blaukopf, M., Altmann, F., Kosma, P., Kählig, H., Comstock, L. E., & Schäffer, C. (2021). A Combination of Structural, Genetic, Phenotypic and Enzymatic Analyses Reveals the Importance of a Predicted Fucosyltransferase to Protein O-Glycosylation in the Bacteroidetes. Biomolecules, 11(12), 1795. https://doi.org/10.3390/biom11121795