Mollusc N-glycosylation: Structures, Functions and Perspectives
Abstract
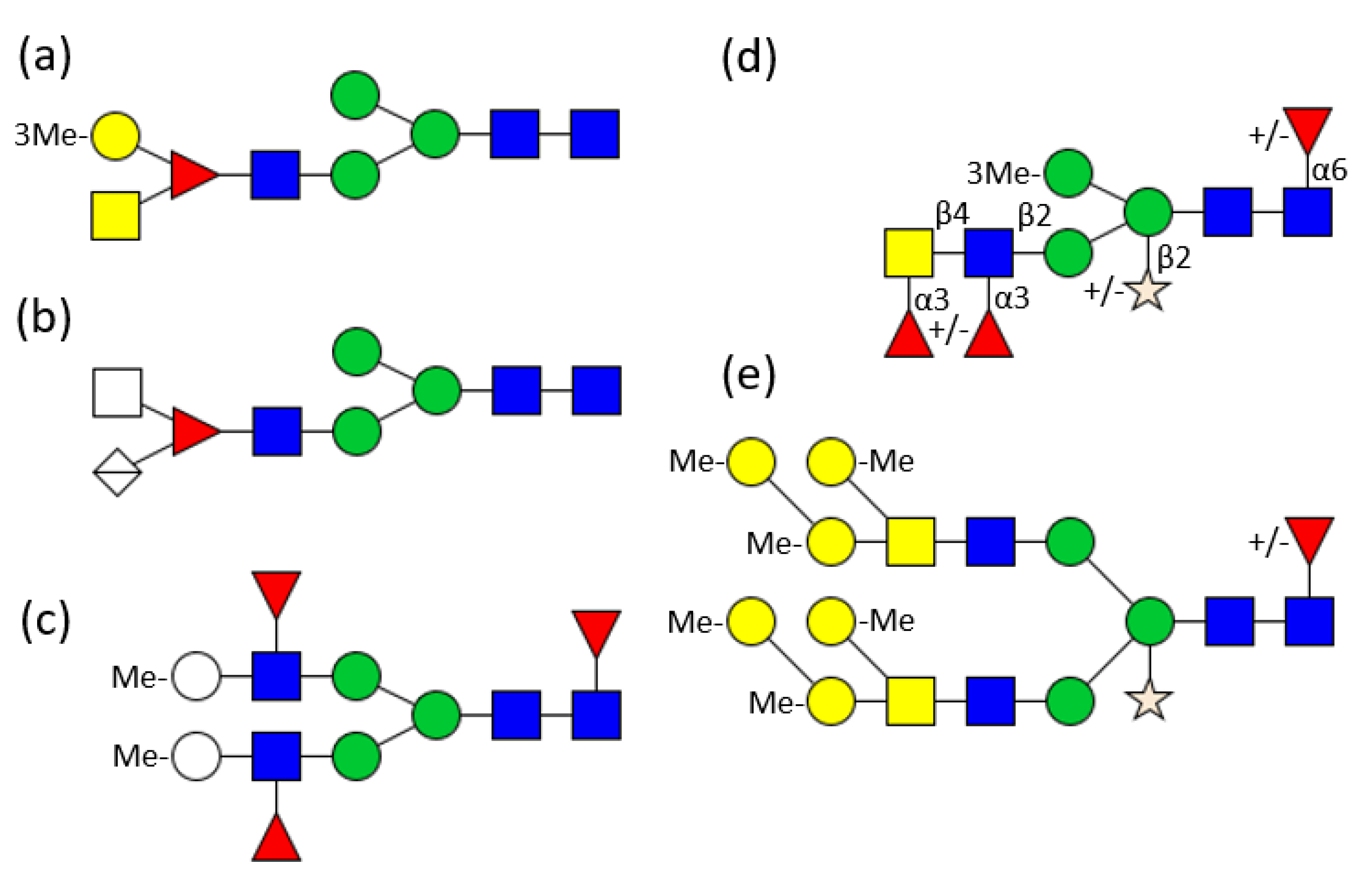
1. Introduction
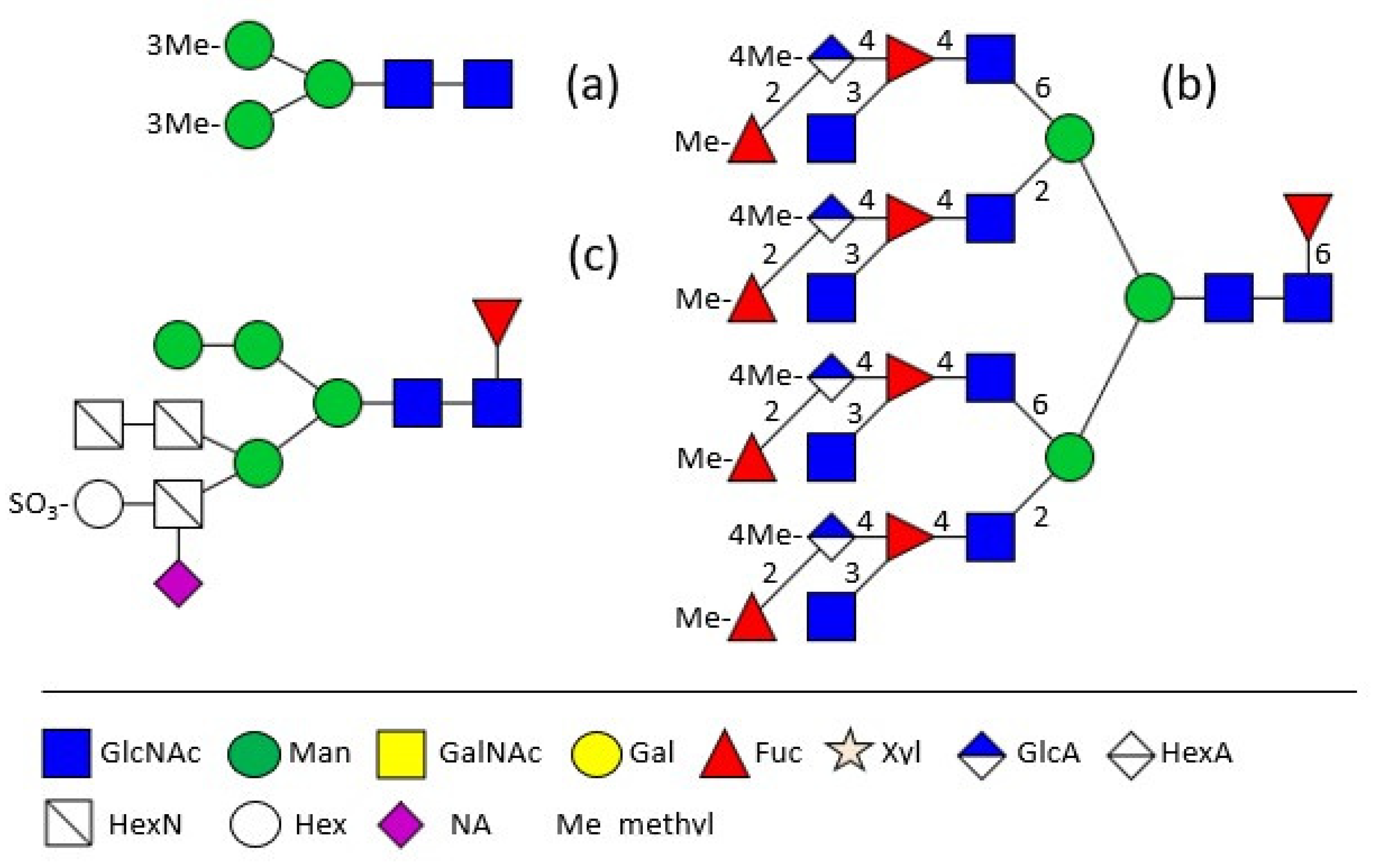
2. Shell Matrix Protein Glycosylation
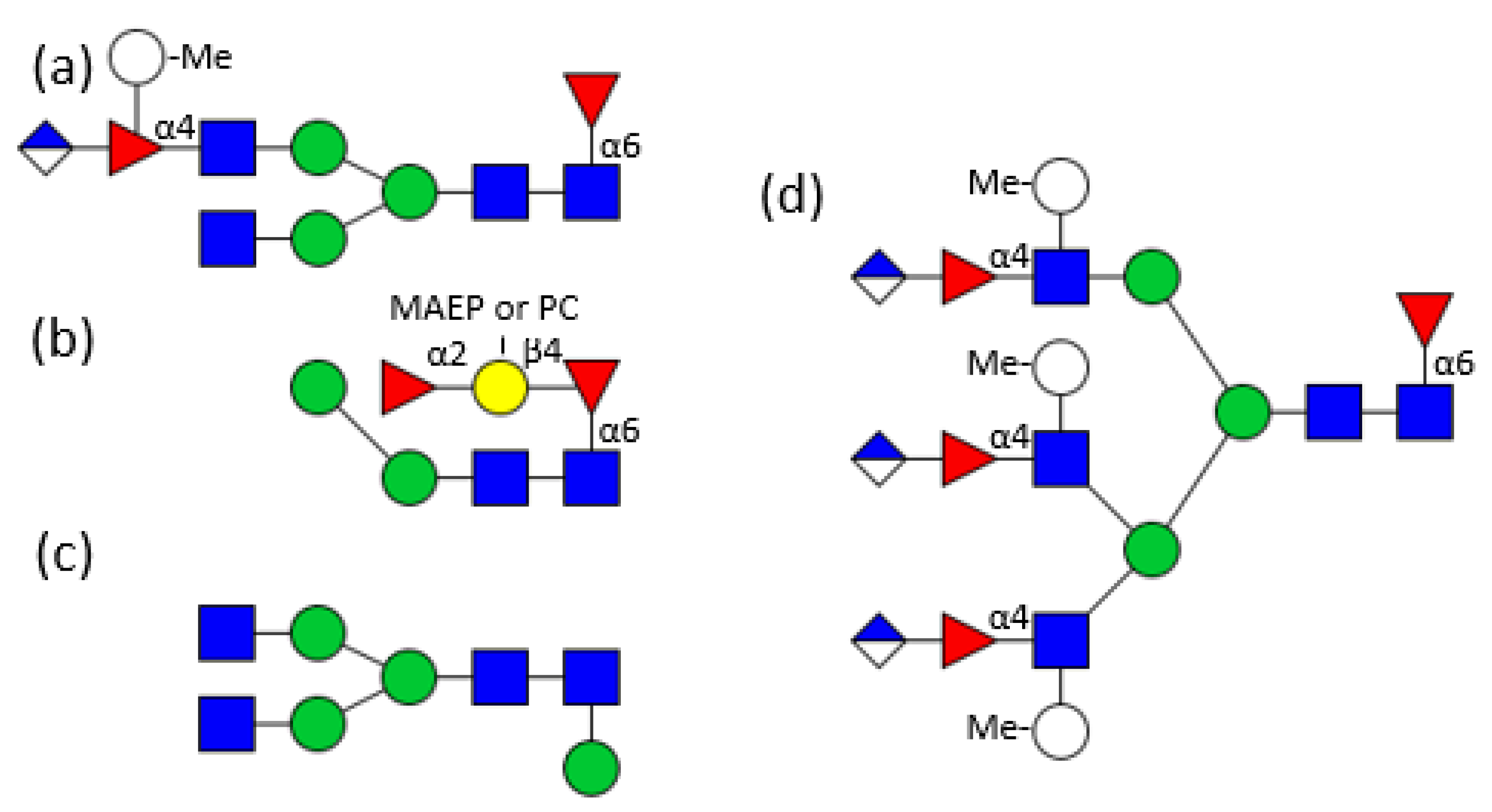
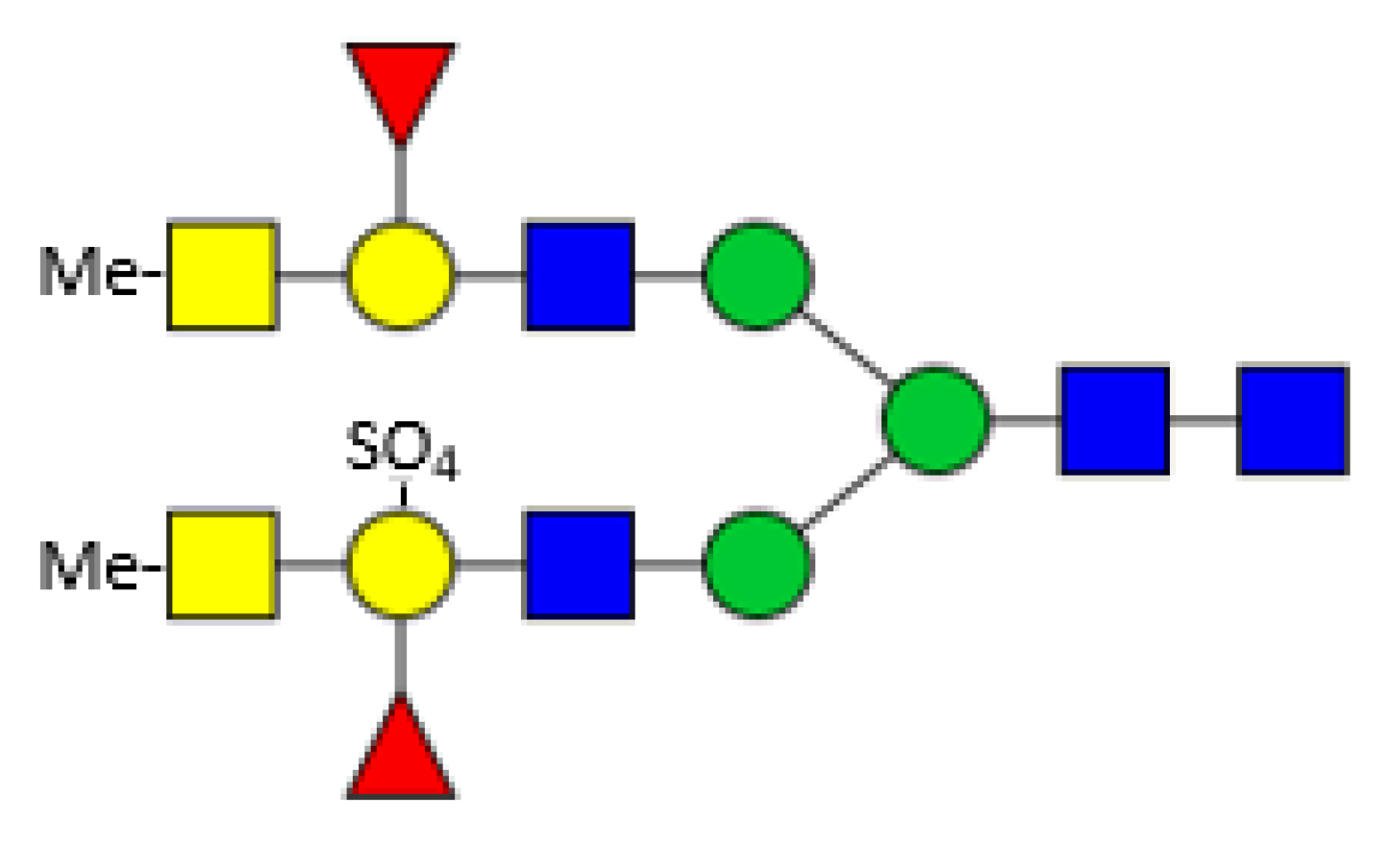
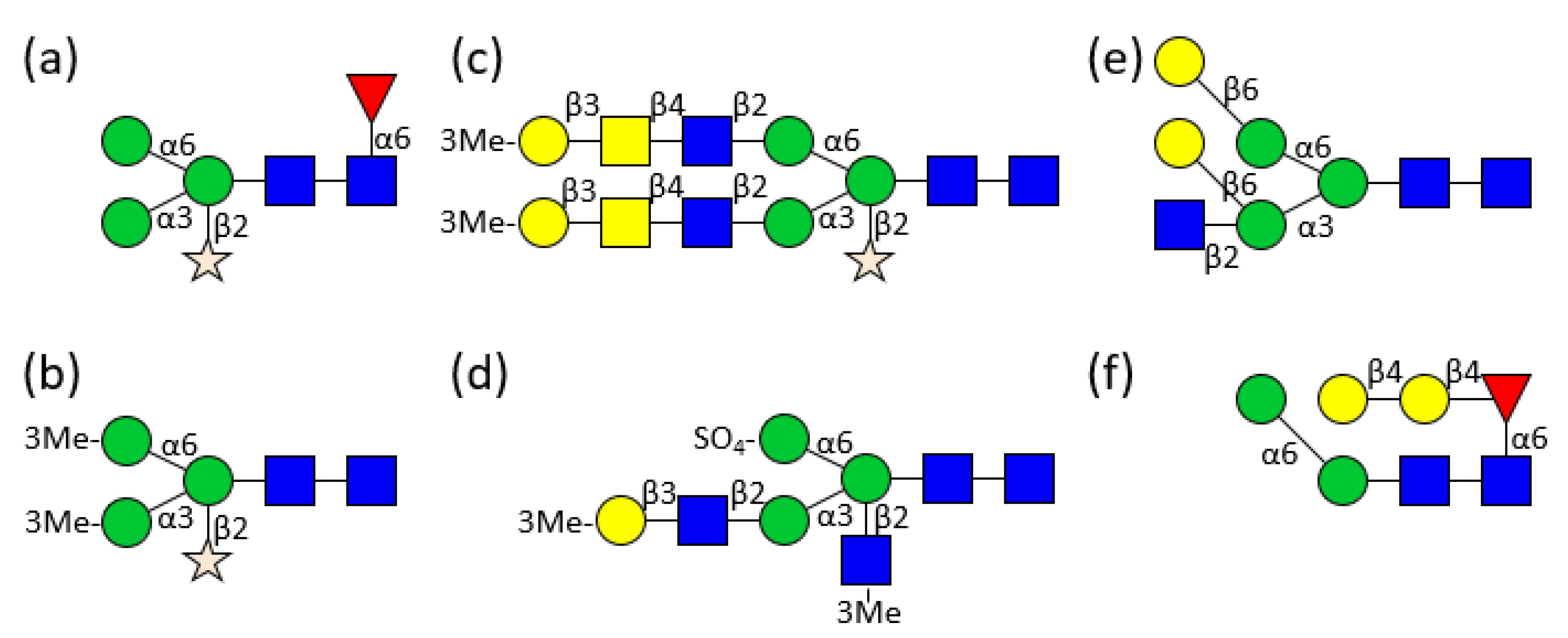
3. Hemolymph Proteins
4. Glycosylation of Other Mollusc Proteins
5. Molluscs as Intermediate Hosts for Parasites
6. Enzymes Involved in N-glycan Biosynthesis
7. Current and Prospective Applications of Mollusc Lectins
8. Glycopeptides from Conus Marine Molluscs
9. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wanninger, A.; Wollesen, T. The evolution of molluscs. Biol. Rev. Camb. Philos. Soc. 2018, 94, 102–115. [Google Scholar] [CrossRef]
- Gomes dos Santos, A.; Lopes-Lima, M.; Castro, L.F.C.; Froufe, E. Molluscan genomics: The road so far and the way forward. Hydrobiologia 2020, 847, 1705–1726. [Google Scholar] [CrossRef]
- Varki, A. Nothing in glycobiology makes sense, except in the light of evolution. Cell 2006, 126, 841–845. [Google Scholar] [CrossRef]
- Varki, A. Evolutionary forces shaping the Golgi glycosylation machinery: Why cell surface glycans are universal to living cells. Cold Spring Harb. Perspect. Biol. 2011, 3, a005462. [Google Scholar] [CrossRef]
- Aebi, M. N-linked protein glycoylation in the ER. Biochim. Biophys. Acta 2013, 1833, 2430–2437. [Google Scholar] [CrossRef]
- Stanley, P.; Taniguchi, N.; Aebi, M. N-glycans. Structure and Biosynthesis. In Essentials of Glycobiology, 3rd ed.; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2015. [Google Scholar]
- Staudacher, E. Methylation—An uncommon modification of glycans. Biol. Chem. 2012, 393, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Paschinger, K.; Wilson, I.B.H. Anionic and zwitterionic moieties as widespread glycan modifications in non-vertebrates. Glycoconj. J. 2020, 37, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Hykollari, A.; Paschinger, K.; Wilson, I.B.H. Negative-mode mass spectrometry in the analysis of invertebrate, fungal, and protist N-glycans. Mass Spec. Rev. 2021, 1–19. [Google Scholar] [CrossRef]
- Addadi, L.; Joester, D.; Nudelman, F.; Weiner, S. Mollusk shell formation: A source of new concepts for understanding biomineralization processes. Chemistry 2006, 12, 980–987. [Google Scholar] [CrossRef]
- Rivera-Pérez, C.; Hernández-Saavedra, N.Y. Post-translational modifications of marine shell matrix proteins. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2021, 256, 110641. [Google Scholar] [CrossRef]
- Marie, B.; Zanella-Cléon, I.; Corneillat, M.; Becchi, M.; Alcaraz, G.; Plasseraud, L.; Luquet, G.; Marin, F. Nautilin-63, a novel acidic glycoprotein from the shell nacre of Nautilus macromphalus. FEBS J. 2011, 278, 2117–2130. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.P.; Perovic, I.; Rao, A.; Cölfen, H.; Evans, J.S. Insect cell glycosylation and its impact on the functionality of a recombinant intracrystalline nacre protein, AP24. Biochemistry 2016, 55, 1024–1035. [Google Scholar] [CrossRef]
- Juan-Colas, J.; Jung, Y.S.; Johnson, S.; Evans, J.S. A complicated relationship: Glycosylation, Ca(II), and primary sequence affect the interactions and kinetics between two model mollusk shell intracrystalline nacre proteins. Biochemistry 2020, 59, 346–350. [Google Scholar] [CrossRef]
- Marxen, J.C.; Nimtz, M.; Becker, W.; Mann, K. The major soluble 19.6 kDa protein of the organic shell matrix of the freshwater snail Biomphalaria glabrata is an N-glycosylated dermatopontin. Biochim. Biophys. Acta. 2003, 1650, 92–98. [Google Scholar] [CrossRef]
- Zhou, H.; Hanneman, A.J.; Chasteen, N.D.; Reinhold, V.N. Anomalous N-glycan structures with an internal fucose branched to GlcA and GlcN residues isolated from a mollusk shell-forming fluid. J. Proteome. Res. 2013, 12, 4547–4555. [Google Scholar] [CrossRef]
- Takakura, D.; Norizuki, M.; Ishikawa, F.; Samata, T. Isolation and characterization of the N-linked oligosaccharides in nacrein from Pinctada fucata. Mar. Biotechnol. 2008, 10, 290–296. [Google Scholar] [CrossRef]
- Kato, S.; Matsui, T.; Gatsogiannis, C.; Tanaka, Y. Molluscan hemocyanin: Structure, evolution, and physiology. Biophys. Rev. 2018, 10, 191–202. [Google Scholar] [CrossRef]
- Kato, S.; Matsui, T.; Tanaka, Y. Molluscan Hemocyanins. Subcell. Biochem. 2020, 94, 195–218. [Google Scholar] [CrossRef]
- Hall, R.L.; Wood, E.J.; Kamerling, J.P.; Gerwig, G.J.; Vliegenthart, J.F.G. 3-O-methyl sugars as constituents of glycoproteins. Identification of 3-O-methylgalactose and 3-O-methylmannose in pulmonate gastropod haemocyanins. Biochem. J. 1977, 165, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Van Kuik, J.A.; van Halbeek, H.; Kamerling, J.P.; Vliegenthart, J.F. Primary structure of the low-molecular-weight carbohydrate chains of Helix pomatia α-hemocyanin. Xylose as a constituent of N-linked oligosaccharides in an animal glycoprotein. J. Biol. Chem. 1985, 260, 13984–13988. [Google Scholar] [CrossRef]
- Van Kuik, J.A.; Sijbesma, R.P.; Kamerling, J.P.; Vliegenthart, J.F.; Wood, E.J. Primary structure of a low-molecular-mass N-linked oligosaccharide from hemocyanin of Lymnaea stagnalis. 3-O-methyl-D-mannose as a constituent of the xylose-containing core structure in an animal glycoprotein. Eur. J. Biochem. 1986, 160, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Van Kuik, J.A.; Sijbesma, R.P.; Kamerling, J.P.; Vliegenthart, J.F.; Wood, E.J. Primary structure determination of seven novel N-linked carbohydrate chains derived from hemocyanin of Lymnaea stagnalis. 3-O-methyl-D-galactose and N-acetyl-D-galactosamine as constituents of xylose-containing N-linked oligosaccharides in an animal glycoprotein. Eur. J. Biochem. 1987, 169, 399–411. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stoeva, S.; Rachev, R.; Severov, S.; Voelter, W.; Genov, N. Carbohydrate content and monosaccharide composition of Rapana thomasiana grosse (Gastropoda) hemocyanin and its structural subunits. Comparison with gastropodan hemocyanins. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1995, 110, 761–765. [Google Scholar] [CrossRef]
- Lommerse, J.P.; Thomas-Oates, J.E.; Gielens, C.; Préaux, G.; Kamerling, J.P.; Vliegenthart, J.F. Primary structure of 21 novel monoantennary and diantennary N-linked carbohydrate chains from alpha D-hemocyanin of Helix pomatia. Eur. J. Biochem. 1997, 249, 195–222. [Google Scholar] [CrossRef]
- Puanglarp, N.; Oxley, D.; Currie, G.J.; Bacic, A.; Craik, D.J.; Yellowlees, D. Structure of the N-linked oligosaccharides from tridacnin, a lectin found in the haemolymph of the giant clam Hippopus hippopus. Eur. J. Biochem. 1995, 232, 873–880. [Google Scholar] [CrossRef]
- Dolashka-Angelova, P.; Beck, A.; Dolashki, A.; Beltramini, M.; Stevanovic, S.; Salvato, B.; Voelter, W. Characterization of the carbohydrate moieties of the functional unit RvH1-a of Rapana venosa haemocyanin using HPLC/electrospray ionization MS and glycosidase digestion. Biochem. J. 2003, 374, 185–192. [Google Scholar] [CrossRef]
- Kurokawa, T.; Wuhrer, M.; Lochnit, G.; Geyer, H.; Markl, J.; Geyer, R. Hemocyanin from the keyhole limpet Megathura crenulata (KLH) carries a novel type of N-glycan with Gal(β1,6)Man-motifs. Eur. J. Biochem. 2002, 269, 5459–5473. [Google Scholar] [CrossRef]
- Wuhrer, M.; Robijn, M.L.; Koeleman, C.A.; Balog, C.I.; Geyer, R.; Deelder, A.M.; Hokke, C.H. A novel Gal(β1,4)Gal(β1,4)Fuc(α1,6)-core modification attached to the proximal N-acetylglucosamine of keyhole limpet haemocyanin (KLH) N-glycans. Biochem. J. 2004, 378, 625–632. [Google Scholar] [CrossRef]
- Zhang, Y.; Iwasa, T.; Tsuda, M.; Kobata, A.; Takasaki, S. A novel monoantennary complex-type sugar chain found in octopus rhodopsin: Occurrence of the Gal beta 1-4Fuc group linked to the proximal N-acetylglucosamine residue of the trimannosyl core. Glycobiology 1997, 7, 1153–1158. [Google Scholar] [CrossRef][Green Version]
- Gielens, C.; Idakieva, K.; Van den Bergh, V.; Siddiqui, N.I.; Parvanova, K.; Compernolle, F. Mass spectral evidence for N-glycans with branching on fucose in a molluscan hemocyanin. Biochem. Biophys. Res. Commun. 2005, 331, 562–570. [Google Scholar] [CrossRef]
- Sandra, K.; Dolashka-Angelova, P.; Devreese, B.; Van Beeumen, J. New insights in Rapana venosa hemocyanin N-glycosylation resulting from on-line mass spectrometric analyses. Glycobiology 2007, 17, 141–156. [Google Scholar] [CrossRef]
- Dolashka, P.; Velkova, L.; Shishkov, S.; Kostova, K.; Dolashki, A.; Dimitrov, I.; Atanasov, B.; Devreese, B.; Voelter, W.; Van Beeumen, J. Glycan structures and antiviral effect of the structural subunit RvH2 of Rapana hemocyanin. Carbohydr. Res. 2010, 345, 2361–2367. [Google Scholar] [CrossRef] [PubMed]
- Velkova, L.; Dolashka, P.; Lieb, B.; Dolashki, A.; Voelter, W.; Van Beeumen, J.; Devreese, B. Glycan structures of the structural subunit (HtH1) of Haliotis tuberculata hemocyanin. Glycoconj. J. 2011, 28, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Lehr, T.; Geyer, H.; Maass, K.; Doenhoff, M.J.; Geyer, R. Structural characterization of N-glycans from the freshwater snail Biomphalaria glabrata cross-reacting with Schistosoma mansoni glycoconjugates. Glycobiology 2007, 17, 82–103. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.I.; Yigzaw, Y.; Préaux, G.; Gielens, C. Involvement of glycans in the immunological cross-reaction between alpha-macroglobulin and hemocyanin of the gastropod Helix pomatia. Biochimie 2009, 91, 508–516. [Google Scholar] [CrossRef]
- Velkova, L.; Dolashka, P.; Van Beeumen, J.; Devreese, B. N-glycan structures of beta-HlH subunit of Helix lucorum hemocyanin. Carbohydr. Res. 2017, 449, 1–10. [Google Scholar] [CrossRef]
- Dolashka, P.; Daskalova, A.; Dolashki, A.; Voelter, W. De Novo Structural Determination of the Oligosaccharide Structure of Hemocyanins from Molluscs. Biomolecules 2020, 10, 1470. [Google Scholar] [CrossRef]
- Dolashka, P.; Velkova, L.; Iliev, I.; Beck, A.; Dolashki, A.; Yossifova, L.; Toshkova, R.; Voelter, W.; Zacharieva, S. Antitumor activity of glycosylated molluscan hemocyanins via Guerin ascites tumor. Immunol. Investig. 2011, 40, 130–149. [Google Scholar] [CrossRef]
- Siddiqui, N.I.; Idakieva, K.; Demarsin, B.; Doumanova, L.; Compernolle, F.; Gielens, C. Involvement of glycan chains in the antigenicity of Rapana thomasiania hemocyanin. Biochem. Biophys. Res. Commun. 2007, 361, 705–711. [Google Scholar] [CrossRef]
- Salazar, M.L.; Jiménez, J.M.; Villar, J.; Rivera, M.; Báez, M.; Manubens, A.; Becker, M.I. N-Glycosylation of mollusk hemocyanins contributes to their structural stability and immunomodulatory properties in mammals. J. Biol. Chem. 2019, 294, 19546–19564. [Google Scholar] [CrossRef]
- Georgieva, A.; Todorova, K.; Iliev, I.; Dilcheva, V.; Vladov, I.; Petkova, S.; Toshkova, R.; Velkova, L.; Dolashki, A.; Dolashka, P. Hemocyanins from Helix and Rapana snails exhibit in vitro antitumor effects in human colorectal adenocarcinoma. Biomedicines 2020, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- Palacios, M.; Tampe, R.; Del Campo, M.; Zhong, T.Y.; López, M.N.; Salazar-Onfray, F.; Becker, M.I. Antitumor activity and carrier properties of novel hemocyanins coupled to a mimotope of GD2 ganglioside. Eur. J. Med. Chem. 2018, 150, 74–86. [Google Scholar] [CrossRef]
- Harris, J.R.; Markl, J. Keyhole limpet hemocyanin (KLH): A biomedical review. Micron 1999, 30, 597–623. [Google Scholar] [CrossRef]
- Pizarro-Bauerle, J.; Maldonado, I.; Sosoniuk-Roche, E.; Vallejos, G.; López, M.N.; Salazar-Onfray, F.; Aguilar-Guzmán, L.; Valck, C.; Ferreira, A.; Becker, M.I. Molluskan hemocyanins activate the classical pathway of the human complement system through natural antibodies. Front. Immunol. 2017, 8, 188. [Google Scholar] [CrossRef]
- Gutternigg, M.; Ahrer, K.; Grabher-Meier, H.; Bürgmayr, S.; Staudacher, E. Neutral N-glycans of the gastropod Arion lusitanicus. Eur. J. Biochem. 2004, 271, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Gutternigg, M.; Bürgmayr, S.; Pöltl, G.; Rudolf, J.; Staudacher, E. Neutral N-glycan patterns of the gastropods Limax maximus, Cepaea hortensis, Planorbarius corneus, Arianta arbustorum and Achatina fulica. Glycoconj. J. 2007, 24, 475–489. [Google Scholar] [CrossRef]
- Di Patrizi, L.; Capone, A.; Focarelli, R.; Rosati, F.; Gallego, R.G.; Gerwig, G.J.; Vliegenthart, J.F. Structural characterization of the N-glycans of gp273, the ligand for sperm-egg interaction in the mollusk bivalve Unio elongatulus. Glycoconj. J. 2001, 18, 511–518. [Google Scholar] [CrossRef]
- Park, Y.; Zhang, Z.; Laremore, T.N.; Li, B.; Sim, J.S.; Im, A.R.; Ahn, M.Y.; Kim, Y.S.; Linhardt, R.J. Variation of acharan sulfate and monosaccharide composition and analysis of neutral N-glycans in African giant snail (Achatina fulica). Glycoconj. J. 2008, 25, 863–877. [Google Scholar] [CrossRef][Green Version]
- Takahashi, N.; Masuda, K.; Hiraki, K.; Yoshihara, K.; Huang, H.-H.; Khoo, K.-H.; Kato, K. N-Glycan structures of rhodopsin. Existence of the α1,3 and α1,6 difucosylated innermost GlcNAc residue in a molluscan glycoprotein. Eur. J. Biochem. 2003, 270, 2617–2632. [Google Scholar] [CrossRef]
- Eckmair, B.; Jin, C.; Abed-Navandi, D.; Paschinger, K. Multistep fractionation and mass spectrometry reveal zwitterionic and anionic modifications of the N- and O-glycans of a marine snail. Mol. Cell Proteomics 2016, 15, 573–597. [Google Scholar] [CrossRef]
- Milanese, C.; Fiumara, F.; Bizzoca, A.; Giachello, C.; Leitinger, G.; Gennarini, G.; Montarolo, P.G.; Ghirardi, M. F3/contactin-related proteins in Helix pomatia nervous tissue (HCRPs): Distribution and function in neurite growth and neurotransmitter release. J. Neurosci. Res. 2008, 86, 821–831. [Google Scholar] [CrossRef]
- Silverman-Gavrila, L.B.; Senzel, A.G.; Charlton, M.P.; Feng, Z.P. Expression, phosphorylation, and glycosylation of CNS proteins in aversive operant conditioning associated memory in Lymnaea stagnalis. Neuroscience 2011, 186, 94–109. [Google Scholar] [CrossRef]
- Mitta, G.; Gourbal, B.; Grunau, C.; Knight, M.; Bridger, J.M.; Théron, A. The compatibility between Biomphalaria glabrata snails and Schistosoma mansoni: An increasingly complex puzzle. Adv. Parasitol. 2016, 97, 111–145. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-J.; Dinguirard, N.; Sabat, G.; Lui, H.; Gonzalez, L.; Gehring, M.; Bickham-Wright, U.; Yoshino, T.P. Proteomic analysis of Biomphalaria glabrata plasma proteins with binding affinity to those expressed by early developing larval Schistosoma mansoni. PLOS Pathoges 2017, 13, e1006081. [Google Scholar] [CrossRef] [PubMed]
- Famakinde, D.O. Molecular context of Schistosoma mansoni transmission in the molluscan environments: A mini-review. Acta Trop. 2017, 176, 98–104. [Google Scholar] [CrossRef]
- Odoemelan, E.; Raghavan, N.; Miller, A.; Bridger, J.M.; Knight, M. Revised karyotyping and gene mapping of the Biomphalaria glabrata embryonic (Bge) cell line. Int. J. Parasitol. 2009, 39, 675–681. [Google Scholar] [CrossRef]
- Martins -Souza, R.L.; Pereira, C.A.J.; Martins Filho, O.A.; Coelho, P.M.Z.; Corrêa Jr., A.; Negrão- Corrêa, D. Differential lectin labelling of circulating hemocytes from Biomphalaria glabrata and Biomphalaria tenagophila resistant or susceptible to Schistosoma mansoni infection. Mem. Inst. Oswaldeo Cruz 2006, 101, 185–192. [Google Scholar] [CrossRef]
- Yoshino, T.P.; Wu, X.J.; Liu, H.; Gonzalez, L.A.; Deelder, A.M.; Hokke, C.H. Glycotope sharing between snail hemolymph and larval schistosomes: Larval transformation products alter shared glycan patterns of plasma proteins. PLoS Negl. Trop. Dis. 2012, 6, e1569. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.P.; Wu, X.J.; Gonzalez, L.A.; Hokke, C.H. Circulating Biomphalaria glabrata hemocyte subpopulations possess shared schistosome glycans and receptors capable of binding larval glycoconjugates. Exp. Parasitol. 2013, 133, 28–36. [Google Scholar] [CrossRef]
- Mansour, M.H. Evidence for a family of schistosome glycan-binding lectins in Biomphalaria alexandrina. Dev. Comp. Immunol. 1995, 19, 365–376. [Google Scholar] [CrossRef]
- Mansour, M.H.; Abdul-Salam, F. Characterization of fucose-binding lectins in rock- and mud-dwelling snails inhabiting Kuwait Bay. Immunobiology 2009, 214, 77–85. [Google Scholar] [CrossRef]
- Yoshino, T.P.; Dinguirard, N.; Kunert, J.; Hokke, C.H. Molecular and functional characterization of a tandem-repeat galectin from the freshwater snail Biomphalaria glabrata, intermediate host of the human blood fluke Schistosoma mansoni. Gene 2008, 411, 46–58. [Google Scholar] [CrossRef]
- Lehr, T.; Beuerlein, K.; Doenhoff, M.J.; Grevelding, C.G.; Geyer, R. Localization of carbohydrate determinants common to Biomphalaria glabrata as well as to sporocysts and miracidia of Schistosoma mansoni. Parasitology 2008, 135, 931–942. [Google Scholar] [CrossRef]
- Castillo, M.G.; Wu, X.J.; Dinguirard, N.; Nyame, A.K.; Cummings, R.D.; Yoshino, T.P. Surface membrane proteins of Biomphalaria glabrata embryonic cells bind fucosyl determinants on the tegumental surface of Schistosoma mansoni primary sporocysts. J. Parasitol. 2007, 93, 832–840. [Google Scholar] [CrossRef]
- Lehr, T.; Frank, S.; Natsuka, S.; Geyer, H.; Beuerlein, K.; Doenhoff, M.J.; Hase, S.; Geyer, R. N-Glycosylation patterns of hemolymph glycoproteins from Biomphalaria glabrata strains expressing different susceptibility to Schistosoma mansoni infection. Exp. Parasitol. 2010, 126, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Faltýnková, A.; Nasincová, V.; Kablásková, L. Larval trematodes (Digenea) of the great pond snail, Lymnaea stagnalis (L.), (Gastropoda, Pulmonata) in Central Europe: A survey of species and key to their identification. Parasite 2007, 14, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Davison, A.; Blaxter, M.L. An expressed sequence tag survey of gene expression in the pond snail Lymnaea stagnalis, an intermediate vector of Fasciola hepatica. Parasitology 2005, 130, 539–552. [Google Scholar] [CrossRef]
- Plows, L.D.; Cook, R.T.; Davies, A.J.; Walker, A.J. Carbohydrates that mimic schistosome surface coat components affect ERK and PKC signaling in Lymnaea stagnalis haemocytes. Int. J. Parasitol. 2005, 35, 293–302. [Google Scholar] [CrossRef]
- Georgieva, K.; Georgieva, L.; Mizinska-Boevska, Y.; Stoitsova, S.R. Study of surface carbohydrates in Galba truncatula tissues before and after infection with Fasciola hepatica. Memórias Inst. Oswaldo Cruz 2016, 111, 475–483. [Google Scholar] [CrossRef]
- Feng, C.; Ghosh, A.; Amin, M.N.; Giomarelli, B.; Shridhar, S.; Banerjee, A.; Fernández-Robledo, J.A.; Bianchet, M.A.; Wang, L.-X.; Wilson, I.B.H.; et al. The galectin CvGal1 from Eastern oyster (Crassostrea virginica) binds to blood group A oligosaccharides on the hemocyte surface. J. Biol. Chem. 2013, 288, 24394–24409. [Google Scholar] [CrossRef] [PubMed]
- Tasumi, S.; Vasta, G.R. A galectin of unique domain organization from hemocytes of the Eastern oyster (Crassostrea virginica) is a receptor for the protistan parasite Perkinsus marinus. J. Immunol. 2007, 179, 3086–3098. [Google Scholar] [CrossRef]
- Feng, C.; Ghosh, A.; Amin, M.N.; Bachvaroff, T.R.; Tasumi, S.; Pasek, M.; Banerjee, A.; Shridhar, S.; Wang, L.-X.; Bianchet, M.A.; et al. Galectin CvGal2 from the Eastern oyster (Crassostrea virginica) displays unique specificity for ABH blood group oligosaccharides and differentially recognizes sympatric Perkinsus species. Biochemistry 2015, 54, 4711–4730. [Google Scholar] [CrossRef]
- Kurz, S.; Jin, C.; Hykollari, A.; Gregorich, D.; Giomarelli, B.; Vasta, G.R.; Wilson, I.B.; Paschinger, K. Hemocytes and plasma of the eastern oyster (Crassostrea virginica) display a diverse repertoire of sulfated and blood group A-modified N-glycans. J. Biol. Chem. 2013, 288, 24410–24428. [Google Scholar] [CrossRef]
- Thiengo, S.C.; Fernandez, M.A.; Torres, E.J.L.; Coelho, P.M.; Lanfredi, R.M. First record of a nematode Metastrongyloidea (Aelurostrongylus abstrusus larvae) in Achatina (Lissachatina) fulica (Mollusca, Achatinidae) in Brazil. J. Invertebr. Pathol. 2008, 98, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Geyer, H.; Wuhrer, M.; Resemann, A.; Geyer, R. Identification and characterization of keyhole limpet hemocyanin N-glycans mediating cross-reactivity with Schistosoma mansoni. J. Biol. Chem. 2005, 280, 40731–40748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Fang, X.; Guo, X.; Li, L.; Luo, R.; Xu, F.; Yang, P.; Zhang, L.; Wang, X.; Qi, H.; et al. The oyster genome reveals stress adaptation and complexity of shell formation. Nature 2012, 490, 49–54. [Google Scholar] [CrossRef]
- Adema, C.M.; Hillier, L.W.; Jones, C.S.; Loker, E.S.; Knight, M.; Minx, P.; Oliveira, G.; Raghavan, N.; Shedlock, A.; do Amaral, L.R.; et al. Whole genome analysis of a schistosomiasis-transmitting freshwater snail. Nat. Commun. 2017, 16, 15451. [Google Scholar] [CrossRef]
- Wheeler, N.J.; Dinguirard, N.; Marquez, J.; Gonzalez, A.; Zamanian, M.; Yoshino, T.P.; Castillo, M.G. Sequence and structural variation in the genome of the Biomphalaria glabrata embryonic (Bge) cell line. Parasites Vectors 2018, 11, 496. [Google Scholar] [CrossRef] [PubMed]
- Mulder, H.; Dideberg, F.; Schachter, H.; Spronk, B.A.; De Jong-Brink, M.; Kamerling, J.P.; Vliegenthart, J.F. In the biosynthesis of N-glycans in connective tissue of the snail Lymnaea stagnalis of incorporation GlcNAc by β2GlcNAc-transferase I is an essential prerequisite for the action of β2GlcNAc-transferase II and β2Xyl-transferase. Eur. J. Biochem. 1995, 232, 272–283. [Google Scholar] [CrossRef]
- Mulder, H.; Spronk, B.A.; Schachter, H.; Neeleman, A.P.; van den Eijnden, D.; De Jong-Brink, M.; Kamerling, J.P.; Vliegenthart, J.F.G. Identification of a novel UDP-GalNAc:GlcNAcβ-R β1,4 N-acetylgalactosaminyltransferase from the albumen gland and connective tissue of the snail Lymnaea stagnalis. Eur. J. Biochem. 1995, 227, 175–185. [Google Scholar] [CrossRef]
- Neeleman, A.P.; van den Eijnden, D.H. α-Lactalbumin affects the acceptor specificity of Lymnaea stagnalis albumen gland UDP-GalNAc:GlcNAcβ-R β1,4-N-acetylgalactosaminyltransferase: Synthesis of GalNAcβ1,4Glc. Proc. Natl. Acad. Sci. USA 1996, 93, 10111–10116. [Google Scholar] [CrossRef] [PubMed]
- Mulder, H.; Schachter, H.; De Jong-Brink, M.; Van der Ven, J.G.; Kamerling, J.P.; Vliegenthart, J.F. Identification of a novel UDP-Gal:GalNAc β1,4GlcNAc-R β1,3-galactosyltransferase in the connective tissue of the snail Lymnaea stagnalis. Eur. J. Biochem. 1991, 201, 459–465. [Google Scholar] [CrossRef]
- Mulder, H.; Schachter, H.; Thomas, J.R.; Halkes, K.M.; Kamerling, J.P.; Vliegenthart, J.F.G. Identification of a GDP-Fuc:Galβ1,3GalNAc-R(Fuc to Gal) α1,2 fucosyltransferase and a GDP-Fuc:Galβ1,4GlcNAc (Fuc to GlcNAc) α1,3 fucosyltransferase in connective tissue of the snail Lymnaea stagnalis. Glycoconj. J. 1996, 13, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Van Tetering, A.; Schiphorst, W.; van den Eijnden, D.H.; van Die, I. Characterization of a core alpha1-->3-fucosyltransferase from the snail Lymnaea stagnalis that is involved in the synthesis of complex-type N-glycans. FEBS Lett. 1999, 461, 311–314. [Google Scholar] [CrossRef][Green Version]
- Bakker, H.; Agterberg, M.; Van Tetering, A.; Koeleman, C.A.; Van den Eijnden, D.H.; Van Die, I. A Lymnaea stagnalis gene, with sequence similarity to that of mammalian beta 1-->4-galactosyltransferases, encodes a novel UDP-GlcNAc:GlcNAc beta-R beta 1-->4-N-acetylglucosaminyltransferase. J. Biol. Chem. 1994, 269, 30326–30333. [Google Scholar] [CrossRef]
- Van Die, I.; Cummings, R.D.; van Tetering, A.; Hokke, C.H.; Koeleman, C.A.; van den Eijnden, D.H. Identification of a novel UDP-Glc:GlcNAc beta1-->4-glucosyltransferase in Lymnaea stagnalis that may be involved in the synthesis of complex-type oligosaccharide chains. Glycobiology 2000, 10, 263–271. [Google Scholar] [CrossRef]
- Song, H.-B.; He, M.; Cai, Z.-P.; Huang, K.; Flitsch, S.L.; Liu, L.; Voglmeir, J. UDP-Glucose 4-epimerase and β-1,4-galactosyltransferase from the oyster Magallana gigas as valuable biocatalysts for the production of galactosylated products. Int. J. Mol. Sci. 2018, 19, 1600. [Google Scholar] [CrossRef] [PubMed]
- Robledo, Y.; Marigómez, I.; Angulo, E.; Cajaraville, M.P. Glycosylation and sorting pathways of lysosomal enzymes in mussel digestive cells. Cell Tissue Res. 2006, 324, 319–333. [Google Scholar] [CrossRef]
- Hu, Y.; Luan, H.; Liu, H.; Ge, G.; Zhou, K.; Liu, Y.; Yang, L. Acceptor specificity and transfer efficiency of a beta-glycosidase from the Chinese white jade snail. Biosci. Biotechnol. Biochem. 2009, 73, 671–676. [Google Scholar] [CrossRef]
- Biswas, C.; Sinha, D.; Mandal, C. Investigation on interaction of Achatinin, a 9-O-acetyl sialic acid-binding lectin, with lipopolysaccharide in the innate immunity of Achatina fulica snails. Mol. Immunol. 2000, 37, 745–754. [Google Scholar] [CrossRef]
- Brossmer, R.; Wagner, M.; Fischer, E. Specificity of the sialic acid-binding lectin from the snail Cepaea hortensis. J. Biol. Chem. 1992, 267, 8752–8756. [Google Scholar] [CrossRef]
- Gerlach, D.; Schlott, B.; Schmidt, K.H. Cloning and expression of a sialic acid-binding lectin from the snail Cepaea hortensis. FEMS Immunol. Med. Microbiol. 2004, 40, 215–221. [Google Scholar] [CrossRef][Green Version]
- Miller, R.L.; Collawn, J.F., Jr.; Fish, W.W. Purification and macromolecular properties of a sialic acid-specific lectin from the slug Limax flavus. J. Biol. Chem. 1982, 257, 7574–7580. [Google Scholar] [CrossRef]
- García-Maldonado, E.; Cano-Sánchez, P.; Hernández-Santoyo, A. Molecular and functional characterization of a glycosylated Galactose-Binding lectin from Mytilus californianus. Fish Shellfish Immunol. 2017, 66, 564–574. [Google Scholar] [CrossRef]
- He, X.; Zhang, Y.; Yu, F.; Yu, Z. A novel sialic acid binding lectin with anti-bacterial activity from the Hong Kong oyster (Crassostrea hongkongensis). Fish Shellfish Immunol. 2011, 31, 1247–1250. [Google Scholar] [CrossRef]
- Ito, S.; Shimizu, M.; Nagatsuka, M.; Kitajima, S.; Honda, M.; Tsuchiya, T.; Kanzawa, N. High molecular weight lectin isolated from the mucus of the Giant African Snail Achatina fulica. Biosci. Biotechnol. Biochem. 2011, 75, 20–25. [Google Scholar] [CrossRef]
- Brola, T.R.; Dreon, M.S.; Qiu, J.W.; Heras, H. A highly stable, non-digestible lectin from Pomacea diffusa unveils clade-related protection systems in apple snail eggs. J. Exp. Biol. 2020, 223, jeb231878. [Google Scholar] [CrossRef]
- Wang, W.; Song, X.; Wang, L.; Song, L. Pathogen-derived carbohydrate recognition in molluscs immune defense. Int. J. Mol. Sci. 2018, 19, 721. [Google Scholar] [CrossRef]
- Schumacher, U.; Adam, E.; Brooks, S.A.; Leathem, A.J. Lectin-binding properties of human breast cancer cell lines and human milk with particular reference to Helix pomatia agglutinin. J. Histochem. Cytochem. 1995, 43, 275–281. [Google Scholar] [CrossRef]
- Rambaruth, N.D.S.; Greenwell, P.; Dwek, M.V. The lectin Helix pomatia agglutinin recognizes O-GlcNAc containing glycoproteins in human breast cancer. Glycobiology 2012, 22, 839–848. [Google Scholar] [CrossRef]
- Pietrzyk, A.J.; Bujacz, A.; Mak, P.; Potempa, B.; Niedziela, T. Structural studies of Helix aspersa agglutinin complexed with GalNAc: A lectin that serves as a diagnostic tool. Int. J. Biol. Macromol. 2015, 81, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.M.; Suzuki, H.; Brooks, M.T.; Tomana, M.; Moldoveanu, Z.; Mestecky, J.; Julian, B.A.; Novak, J.; Herr, A.B. Recognition of galactose-deficient O-glycans in the hinge region of IgA1 by N-acetylgalactosamine-specific snail lectins: A comparative binding study. Biochemistry 2010, 49, 5671–5682. [Google Scholar] [CrossRef]
- Markiv, A.; Peiris, D.; Curley, G.P.; Odell, M.; Dwek, M.V. Identification, cloning, and characterization of two N-acetylgalactosamine-binding lectins from the albumen gland of Helix pomatia. J. Biol. Chem. 2011, 286, 20260–20266. [Google Scholar] [CrossRef]
- Sudhakar, G.R.L.; Vincent, S.G.P. Purification and characterization of a novel C-type lectin for clot lysis from the fresh water clam Villorita cyprinoides: A possible natural thrombolytic agent against myocardial infarction. Fish Shellfish Immunol. 2015, 36, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.G.; Zafaralla, G.; Cruz, L.J.; Santos, A.D.; Hillyard, D.R.; Dykert, J.; Rivier, J.E.; Gray, W.R.; Imperial, J.; DelaCruz, R.G.; et al. An O-glycosylated neuroexcitatory conus peptide. Biochemistry 1998, 37, 16019–16025. [Google Scholar] [CrossRef]
- Hocking, H.G.; Gerwig, G.J.; Dutertre, S.; Violette, A.; Favreau, P.; Stöcklin, R.; Kamerling, J.P.; Boelens, R. Structure of the O-glycosylated conopeptide CcTx from Conus consors venom. Chemistry 2013, 19, 870–879. [Google Scholar] [CrossRef]
- Gerwig, G.J.; Hocking, H.G.; Stöcklin, R.; Kamerling, J.P.; Boelens, R. Glycosylation of conotoxins. Mar. Drugs 2013, 11, 623–642. [Google Scholar] [CrossRef] [PubMed]
- Tsetlin, V.I.; Hucho, F. Snake and snail toxins acting on nicotinic acetylcholine receptors: Fundamental aspects and medical applications. FEBS Lett. 2004, 557, 9–13. [Google Scholar] [CrossRef]
- Lee, H.K.; Zhang, L.; Smith, M.D.; Walewska, A.; Vellore, N.A.; Baron, R.; McIntosh, J.M.; White, H.S.; Olivera, B.M.; Bulaj, G. A marine analgesic peptide, Contulakin-G, and neurotensin are distinct agonists for neurotensin receptors: Uncovering structural determinants of desensitization properties. Front. Pharmacol. 2015, 6, 11. [Google Scholar] [CrossRef]
- Jimenez, E.C. Post-translationally modified conopeptides: Biological activities and pharmacological applications. Peptides 2021, 139, 170525. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staudacher, E. Mollusc N-glycosylation: Structures, Functions and Perspectives. Biomolecules 2021, 11, 1820. https://doi.org/10.3390/biom11121820
Staudacher E. Mollusc N-glycosylation: Structures, Functions and Perspectives. Biomolecules. 2021; 11(12):1820. https://doi.org/10.3390/biom11121820
Chicago/Turabian StyleStaudacher, Erika. 2021. "Mollusc N-glycosylation: Structures, Functions and Perspectives" Biomolecules 11, no. 12: 1820. https://doi.org/10.3390/biom11121820
APA StyleStaudacher, E. (2021). Mollusc N-glycosylation: Structures, Functions and Perspectives. Biomolecules, 11(12), 1820. https://doi.org/10.3390/biom11121820