Abstract
Women experience a dramatical raise in cardiovascular events after menopause. The decline in estrogens is pointed to as the major responsible trigger for the increased risk of cardiovascular disease (CVD). Indeed, the menopausal transition associates with heart macro-remodeling, which results from a fine-tuned cell micro-remodeling. The remodeling of cardiomyocytes is a biomolecular response to several physiologic and pathologic stimuli, allowing healthy adaptation in normal conditions or maladaptation in an unfavorable environment, ending in organ architecture disarray. Estrogens largely impinge on cardiomyocyte remodeling, but they cannot fully explain the sex-dimorphism of CVD risk. Albeit cell remodeling and adaptation are under multifactorial regulation, vitamin D emerges to exert significant protective effects, controlling some intracellular paths, often shared with estrogen signaling. In post-menopause, the unfavorable association of hypoestrogenism-D hypovitaminosis may converge towards maladaptive remodeling and contribute to increased CVD risk. The aim of this review is to overview the role of estrogens and vitamin D in female cardiac health, speculating on their potential synergistic effect in cardiomyocyte remodeling, an issue that is not yet fully explored. Further learning the crosstalk between these two steroids in the biomolecular orchestration of cardiac cell fate during adaptation may help the translational approach to future cardioprotective strategies for women health.
1. Introduction
Sex-related differences consistently contribute to the clinical heterogeneity characterizing aging in respect to cardiovascular diseases (CVD), which currently represent the leading cause of illness and death in the Western world [1]. Indeed, women show a higher prevalence of age-related cardiac defects, including left ventricular hypertrophy, increased end-diastolic pressure, diastolic dysfunction, fibrosis, inflammation, oxidative stress and lower exercise capacity [2].
The midlife estrogen withdrawal is recognized as being the main traditional cause of CVD increase and heart failure (HF) in post-menopausal women [3]. Indeed, the menopausal transition associates with adverse organ macro-remodeling, which is in turn ascribable to cardiac and endothelial cell micro-remodeling (which occurs in a sex-specific mode [4,5]). Those effects largely depend on the presence of estrogen receptors (ER) within the myocardium and endothelium, and encompass many cellular biological functions by genomic and non-genomic mechanisms [6,7,8]. Nevertheless, estrogens/ER alone are unlikely to entirely explain heart disease presentations and outcomes in women, so that the identification of bio-factors contributing to the higher risk in females is still subject to ongoing debate.
Low vitamin D can critically promote molecular alterations toward an aberrant cell remodeling. Indeed, vitamin D deficiency increases the risk of CVD development, impacting cell morphology, metabolism and function [9,10]. Upon binding with its specific receptor VDR present in vascular and cardiac cells, vitamin D affects several biomolecular and cellular processes. As for estrogens, whereas vitamin D actions onto vascular cells are quite exhaustively covered in literature, its molecular effect onto cardiomyocytes is still incompletely understood, especially from sex-dependent standpoint. Yet, an aberrant remodeling of cardiomyocytes, as occurring in unfavorable environment, is pointed to as the main trigger of a compromised cardiac function [11,12]. Conversely, in normal conditions, cardiac cell remodeling allows adaptive responses and favors cardio-protection. Women after menopause tend to have pronounced hypovitaminosis D, which, along with hypoestrogenism, seems to permit more relevant negative effects on heart health.
This review aims to offer first an overview on the role of estrogens and vitamin D in cardiovascular female health, focusing on cardiomyocyte remodeling. Then, the potential synergistic effect of low estrogens-low vitamin D combination, both hormone-deficient conditions typically occurring in postmenopausal life, is speculated. Brief comments on the impact of possible protective interventions, such as a combined supplementation of these hormones or physical activity are mentioned in the conclusive part.
2. Cardiovascular Health: A Sex Hormone Matter
Although sex-dependent differences in cardiac aging and CVD development are multi-factorial, there is evidence pointing out the role of estrogens/ERs. Indeed, the drastic difference between aging women and men is unquestionably related to the rapid decline of female sex hormones, associated with menses ending. Female heart is known to better resist different insults, and, accordingly, women are better protected from heart diseases compared to men. This remarkable “female advantage” is lost with menopausal transition or metabolic diseases such as diabetes, which significantly increased the incidence of cardiovascular morbidity and mortality in post-fertile women life, as summarized elsewhere [13,14]. Indeed, some functional and structural sex-dependent intrinsic differences, including heart contractility and rhythm, which affect contraction and relaxation, less left ventricular mass, lower chamber volume, thinner wall thickness or electro-mechanical function as seen in females, might explain at least in part, the sex dimorphism in clinical manifestations and responses to treatments. In general, women present less severe clinical symptoms, but the prognosis after heart attack is worse, with about 40% mortality in women vs. 25% in men within a year [14]. Women are more frequently affected by hypertension (which likely contributes to the different left ventricular dysfunction and arterial stiffness) and are more prone to cardiometabolic syndrome (which exacerbates sex-related bias in cardiac disease occurrence) and disease sequelae, as addressed later in this manuscript. In particular, left ventricular diastolic dysfunction, the typical clinical presentation of HF with preserved ejection fraction (HFpEF) predominant in women, seems to be facilitated by estrogen deficiency, affecting intra-cellular calcium homeostasis, cyto-skeleton and extra-cellular matrix rearrangements [15].
From the pioneering studies, the role of estrogens in cardioprotection has been essentially related to the arterial vasodilation through direct genomic and non-genomic actions onto vascular cells [16,17,18,19].
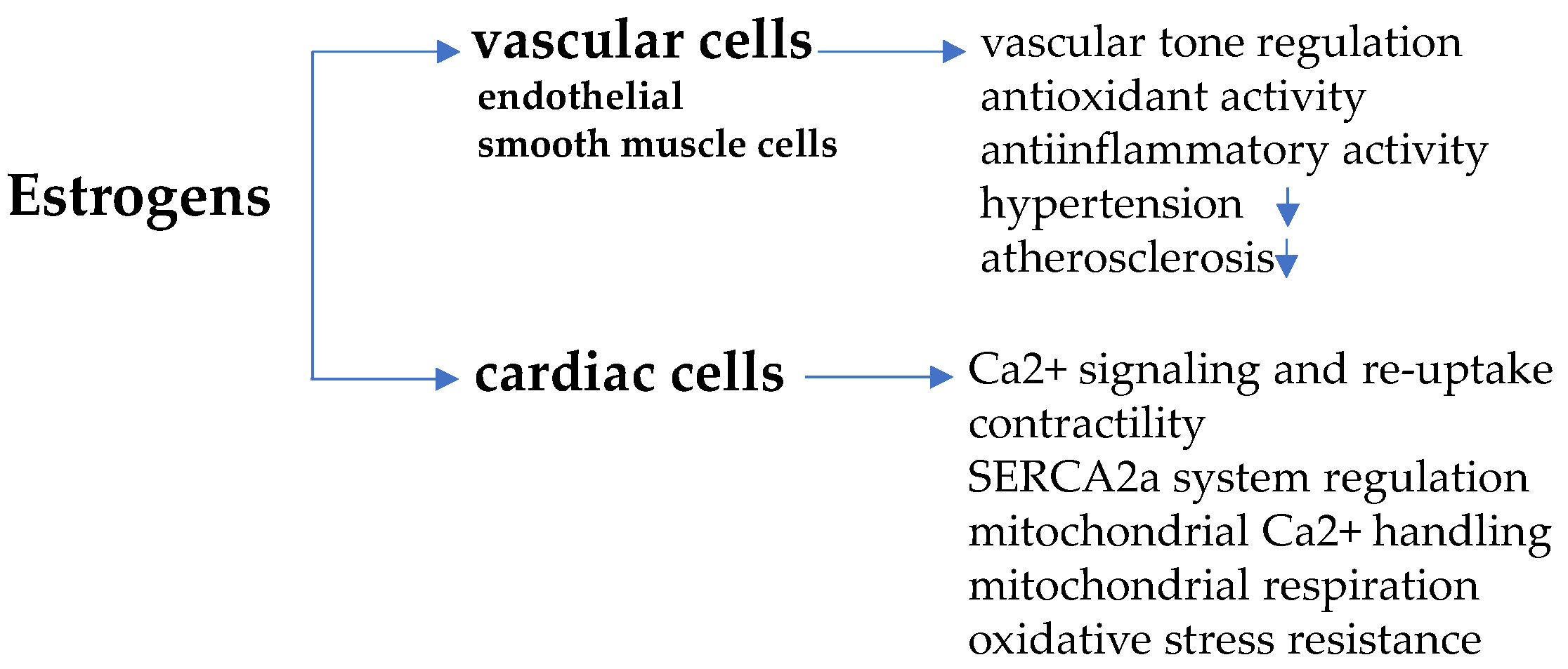
In endothelial and vascular smooth muscle cells, in fact, estrogens—especially 17β-estradiol/E2, the predominant biologically active form—upon binding with α and β subtype ER (ERα and ERβ) and with G-protein coupled estrogen receptor (GPER), activate a cascade of intra-cellular signaling paths, such as phosphoinositide 3-kinase-serin/threonine-specific kinase B (PI3K/Akt)/endothelial nitric oxide synthase (eNOS) and mitogen-activated protein kinases (MAPK)/eNOS, allowing nitric oxide (NO) release, vascular relaxation and vasodilation [20,21,22]. These effects converge towards the regulation of the vascular tone against hypertension and protect from high pressure-induced damage of arteries and atherosclerosis [23]. In addition, atherosclerosis prevention also depends on the anti-oxidant action of estrogens, which reduces the deposition of circulating cholesterol in arteries wall and limits inflammation [24]. With the menopausal transition, estrogen-induced protection is lost, as endogenous hormone concentration in women drops to the small amount produced in extra-gonadal sites (adrenal cortex cells, aortic smooth muscle cells, adipose tissue, brain, bone), similarly to ovariectomized women or men [25]. The scheme in Figure 1 summarizes the main effects of estrogens in vascular and cardiac cells.
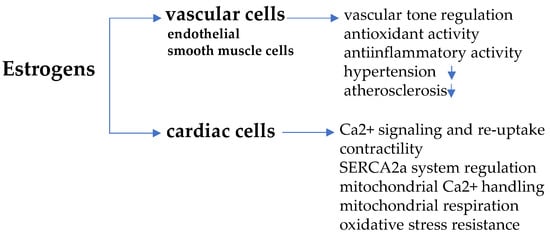
Figure 1.
Estrogen-induced regulation in vascular and cardiac cells. Estrogens work against hypertension and atherosclerosis through anti-oxidant/anti-inflammatory activity and regulate vascular tone, acting on endothelial and smooth muscle cells. In cardiomyocytes, estrogens regulate cell contractility affecting Ca2+ dependent signaling, mitochondria function and sarcoplasmic/endoplasmic reticulum Ca2+-ATPase 2a (SERCA2a) system.
Estrogens in Males, Androgens in Females
The conversion of testosterone to estrogens by aromatase seems to exert some protecting effects in males as well [26]. In fact, men with E2 deficiency or E2 resistance, due to specific mutations in the cytochrome P450 aromatase gene (Cyp19a1) or in the ERα gene (ESR1), respectively, show an increased risk of CDV in association with total cholesterol level rise, insulin resistance (IR) and type 2 diabetes (T2D) development, defects in glucose tolerance and vasodilation [27,28,29,30,31]. Treatment with estrogens can normalize the cardiac function in male mice with HF, induced by aromatase activity suppression [32]; in line with this experimental observation, a reduced risk of CVD events from endogenous estrogens is reported in elder men [33].
To date, whereas in men the exact role of E2 with regard to heart function remains questionable, estrogens seem undeniably protective in women, considering the CVD risk before and after menopause when disease incidence becomes equal or even greater than that one observed in men-making CVD the leading cause of death in both sexes [26,34,35].
Nevertheless, it should be recalled that long-term treatment with estrogens (especially synthetic drugs), as contraceptives or hormone replacement therapy (HRT), is associated with super-oxide radical accumulation, inflammation and hypertension [36,37], processes that can worsen cardiac myopathic changes. According to the “theory of timing and opportunity”—based on menopausal stage and time of hormone administration—the cardiovascular benefit seems limited to younger women, who initiated HRT in early peri-menopausal stage [21,38,39,40]. Particular attention should be given to HRT in women with a condition known as metabolic or cardiometabolic syndrome, a cluster of diseases, including obesity, dyslipidemia, hypertension and IR, which increases with menopause (present in 40% of post-menopausal women) and shows some sex dimorphism as well. In fact, although this condition represents a primary risk factor for diabetes and CVD in both sexes, it is speculated that it contributes to the different cardiovascular sequelae in men and women. Whereas HRT confers beneficial effects on metabolism, acting onto abdominal fat, blood lipid, adipokine profile, vascular resistance and oxidative stress, it does not provide sufficient cardio-protection in women with pre-existing diabetes, CVD or related risks [13,14]. This discrepancy might be partially due to estrogen bio-molecular interactions with the cardiovascular system, leading to differences in response to the treatment [41]. Further studies are mandatory to fully explain the underlying mechanism(s). Meanwhile, given the existing controversy on HRT pros and cons, a careful evaluation of potential real benefits is recommended when considering this therapeutic strategy.
The cardiovascular health in females seems under androgen control as well. The excess of androgens produced by ovary in menopause, not balanced by estrogen production, is hypothesized to negatively affect women cardiovascular function and to increase the cardiovascular risk in association with T2D and diabetic cardiomyopathy [13,42]. The link between hypoestrogenism, hyper-androgenism and cardiometabolic risk in women is still a challenging issue and surely deserves further studies [43,44]. Nevertheless, it is undeniable that estrogens broadly impact women’s cardiovascular health through rapid and genomic mechanisms exerted onto endothelium and vascular cells, as exhaustively covered in a recent report [19] and not discussed in this review. Herein, the attention is on some significant direct effects of estrogens onto cardiac cell remodeling, which represents the critical event driving toward health maintenance or disease development.
The physiological remodeling of the cardiomyocyte, both in males and females, is under control of different factors finely orchestrated to allow compensatory functional adaptation in response to various stimuli, i.e., aging, stress, physical exercise or pathological challenges. If, for any reason, an adaptive cell remodeling is not properly maintained, maladaptive processes take place, and, consequently, cardiac function is not safeguarded.
3. Estrogens and Cardiomyocyte Remodeling
The protective effect of estrogens on female heart against various stress challenges, such as hypertrophic, ischemic or cytotoxic stimuli, involves direct actions of these hormones in the cardiomyocyte. The sex difference underlying this process undoubtedly includes multi-factorial reasons, but the major evidence points to a causal role of the sex steroid hormone E2 and its receptors (ER) in the physiology and pathophysiology of the heart. Interestingly, key events like cardiac calcium (Ca2+) ion channel activity and mitochondrial function are regulated in a sex-specific manner, as discussed below.
3.1. Estrogen Receptors
The presence of α and β cardiac ERs in ventricular and atrial cells of adult and neonatal heart is known since quite ago, and successively confirmed in female and male mice [45,46,47,48]. ER subtypes are present in cardiac cell cytosol with different subcellular localization (being ERα located in or adjacent to plasma membrane) and exert translational and post-translational regulatory mechanisms [48,49,50]. Studies in animals carrying cardiomyocyte-specific deletion or overexpression of ERα (ERKO-mice or csERα-OE mice, respectively) documented that this receptor subtype leads female cardiomyocytes to more efficient recover after cardiac injury, albeit it is dedicated to heart mass regulation in both sexes [50,51,52,53].
ERβ dysfunction is linked to cardiomyocyte disarray and important alterations in tissue architecture, including nuclear structures and gap junctions [54]. Of note, females, not males, lacking ERβ show a significantly reduced post-ischemic cardiac recovery [55,56]. These findings support an ERβ-dependent protective role after cardiac injury in females. In presence of cardiomyocyte-specific ERβ over-expression (csERβ-OE mice), differences between sexes were present neither in basal cardiac morphology/function/weight, nor in recovery/survival after cardiac injury [54]. Female and male mice, indeed, showed the same improvements in several cardiac parameters, except for left ventricular volume and ejection fraction, being both more pronounced in males. This effect likely depends on a reduced cardiomyocyte remodeling towards fibrosis, as observed in males. The main protective action of ERβ seems to rely on a better protection of the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase 2a (SERCA2a) system and Ca2+ reuptake post-injury.
In addition to ER α and β, cardiomyocytes express GPER, a membrane receptor essentially mediating non-genomic rapid actions [57]. Only male GPER-knock out (KO)-mice seem to develop impairments in cardiac function. Defects in heart structure and function observed in cardiomyocyte-specific GPER-KO animals were exacerbated in aging males [58]. This difference observed between males and females likely mirrors the difference found in gene expression profile related to cardiomyocyte GPER-deficiency and sex, as mitochondrial genes were enriched only in female GPER-deleted cardiomyocytes vs. wild type [58,59].
Of note, mitochondria seem to play one of the major roles in orchestrating biomolecular events in cardiac cell remodeling and function.
3.2. Sex-Dimorphic Mitochondrial Function
A marked sexual dimorphism is reflected in different mitochondrial calcium handling, higher oxidative capacities, and greater resistance to oxidative stress. Mitochondria content from female cardiomyocytes seems lower but with higher efficiency and differentiation levels, as compared to male ones [60]. Mitochondria from cardiac cells express both ER subtypes, which contribute to ER-related regulation of these organelles by affecting contractility (involving ATP supply), Ca2+ homeostasis, reactive oxygen species (ROS) formation and cell apoptosis [61,62,63]. E2 are reported to enhance mitochondrial respiration and reduce ROS, both processes associated with a lower incidence of CVD in women before menopause; accordingly, during aging, estrogen decline is associated with mitochondrial damage, tissue/cell loss of function and increased risk of disease, as exhaustively described elsewhere [60]. Estrogens and ER play a pivotal role also in the regulation of Ca2+ ion channel signaling and contractility. Sex difference in contracting function, i.e., excitation–contraction (EC) coupling, involving cardiac L-type channels, are observed in humans and animals. Human ventricular cardiomyocytes of female failing heart retain greater contractility and enhanced L-type Ca2+ current, as compared to men [64,65,66]. So far, estrogen decline is largely engaged in Ca2+ signaling deregulation and mitochondria defective functioning, the two main mechanisms pointed to as the most important events involved in aging heart and CVD development/progression [63,67,68].
A study in ovariectomized mice documents the importance of AMP-activated protein kinase (AMPK) in estrogen-mediated cardio-protection via intra-cellular Ca2+ and cell contractility regulation [69]. This signaling molecule involved in energy metabolism and cardiac function regulation is documented to be permissive for estrogen-mediated maintenance of cardiac homeostasis, and, noticeably, can restore a correct cardiac glucose transport, impaired after ovariectomy. The effect of estrogens in cardiomyocytes are schematized in Figure 1.
Hence, the role of estrogens onto these cellular processes within cardiac myocytes is unquestionable; nevertheless, it should be underlined once more that sex hormones are not the unique steroids controlling cardiomyocyte function. In particular, the following paragraph will describe how cardiovascular health and cardiac cell remodeling depend on vitamin D, another steroid hormone exerting important biological actions, beyond its classical skeletal effects. The main functions mediated by ER different subtypes and VDR are summarized in Table 1.

Table 1.
Estrogen receptor (ER) and vitamin D receptor (VDR) in cardiomyocytes. The table summarizes the main intra-cellular effects mediated by ER sub-types and VDR, affecting cardiomyocyte remodeling.
4. Cardiovascular Health beyond Sex Hormones: Vitamin D Matters
The effect of vitamin D on cardiac aging, function and disease still represents a hot issue in research. Since quite a long time ago, vitamin D is known to encompass a wide spectrum of biological actions, which significantly affect cardiovascular homeostasis, as suggested by the clinical association between vitamin D deficiency and different cardiovascular events [77,78,79].
It should be kept in mind that vitamin D is a molecule with quite a special identity, presenting features typical of a nutrient, a hormone and a rapid regulating factor; it is able to affect human health through a fine-tuned regulation of cell functions, by actively participating in biomolecule networking, as recently reported [80,81,82].
Concerning basal vitamin D level, males seem to retain higher serum hormones than females (likely due to body composition [83]), albeit some controversial data exist, maybe depending on several variables. Data from cross-sectional studies in more than 2000 Norwegian morbidly obese subjects and in about 4000 Indian obese and diabetic patients document higher odds of vitamin D deficiency in men, likely related to abdominal adiposity, and higher cardiometabolic risk in association with lower vitamin D, respectively [84,85]. Conversely, studies in coronary artery disease report that lower vitamin D levels, as found in women associate with disease severity [86]. However, rather than absolute differences between male and female vitamin D basal levels, some sex-specific determinants of vitamin D status (i.e., body fat, presence/absence of pathologies, exposure to sun, diet and hormone supplementation and sedentary lifestyle) should be considered, especially in scenarios evaluating preventive strategies in diseases with a strong female bias, as shown, i.e., in auto-immune diseases [87,88,89].
Despite data controversy on sex-related difference in basal vitamin D and unsubstantiated remarks regarding D level and heart health in females and males, the need to improve vitamin D status related to cardiovascular health in the general population is unequivocally recognized [90].
From previous human and experimental studies, the last ones performed in restriction diet models, vitamin D deficiency emerges to associate with increased arterial blood pressure, vascular oxidative stress, modifications in cardiac gene expression, left ventricular hypertrophy, cardiac inflammation, coronary artery disease severity, fibrosis and apoptosis [78,91,92]. In humans, low serum vitamin D level associates with impairments of left ventricular structure and function [93,94]. The scheme in Figure 2 summarizes some of the main detrimental effects of vitamin D deficiency on CVD.

Figure 2.
Vitamin D deficiency-induced effects on cardiovascular system.
Hypovitaminosis D enables several cardiovascular alterations associated with CVD development and severity (CAD: coronary artery disease).
Indeed, vitamin D, upon binding VDR, can control cardiovascular homeostasis by affecting a variety of mechanisms, including cellular proliferation/hypertrophy, blood pressure and renin–angiotensin system. Clinical evidence indicates that vitamin D, like estrogens, directly impacts on vasculature and endothelial cells, as shown by the positive correlation between vitamin D level and arterial compliance, improvements in endothelial function, a reduction in vascular fibrosis in response to injury and a decrease in those inflammatory cytokines underlying HF development [79,95,96,97,98,99]. To date, concerning cardiovascular disease prevention based on endothelial function protection, the beneficial effects of vitamin D on the vasculature are still under debate, especially in light of the possible dose-related vasculature calcification during hormone supplementation. This specific issue is beyond the aim of this review and is plenty covered elsewhere [100,101,102].
Conversely, vitamin D actions directed onto the cardiomyocyte are less covered in literature and, understandably, the main reports are on animal models, which not always can be translated to humans. Another gap (even greater) in literature concerns possible sex-specific associations between vitamin D deficiency and CVD. Those topics are addressed in the following paragraphs.
5. Vitamin D and Cardiomyocyte Remodeling
Nowadays, it is widely recognized that vitamin D level affects cardiovascular health and cardiac cell adaptation, impinging on several intra-cellular processes, including Ca2+-dependent mechanisms such as Ca2+-binding protein synthesis, adenylate cyclase activation, voltage-dependent Ca2+ channel rapid activation and sarcoplasmic reticulum Ca2+ uptake and release [70]. Although the heart is not considered a traditional target tissue of vitamin D, functional vitamin D receptors (VDR) are present in human and animal cardiac myocytes and exert some biological protective actions [103,104] (please see Table 1).
Some investigations suggest the importance of vitamin D/VDR system in controlling cardiac hypertrophy, a dominant feature of several heart disease, and diastolic function.
I.e., cardiomyocyte-specific VDR deleted mice (VDRKO mice) and VDR-deleted rats exhibit ventricular hypertrophy, increased matrix turnover and kinetics alteration [71,105,106]. In those experimental models, liganded VDR works against hypertrophy by counteracting the pro-hypertrophic calcineurin/nuclear factor of activated T-cells (NFAT) and modulatory calcineurin inhibitory protein 1 (MCIP 1), and ameliorates contractility and relaxation kinetics, respectively [70,71,72]. Another example of heart cell protection upon VDR activation comes from a model of diabetic fatty rats (Zucker), in which the treatment with vitamin D can limit cardiomyocyte autophagic activity and damage through the inhibition of FoxO1 translocation and transcriptional activity [73]—the same mechanisms described in osteoblasts (the classical cell target of this hormone) [107].
In human cultured cardiomyocytes exposed to maximal pro-inflammatory challenge, a VDR agonist blunts the intra-cellular activation of signal transducer and activator of transcription 1 (Stat1), induced by interferon (IFN)γ, and notably, almost prevents phosphorylation/nuclear translocation of nuclear factor-kB (NF-kB), induced by tumor necrosis (TNF)α [74]. The latter effect seems particularly intriguing, considering the relevant role played by this prototypic inflammatory cytokine in adverse cardiac remodeling toward heart failure and disease outcome [108,109].
Deficits in VDR expression and low vitamin D are shown to allow detrimental alterations in metabolism, signaling and ionic currents of cardiomyocytes. As an example, in presence of vitamin D insufficiency, ROS generation is enhanced, and, in turn, promotes a cascade of pro-hypertrophic intracellular signaling, i.e., MAP kinase cascade, extra-cellular signal-regulated kinase 1/2 or ERK 1/2, ERK 5, and NFκ-B, c-Jun NH2-terminal kinase 1/2 (JNK), p38 mitogen-activated protein kinase [110], all converging towards fibrosis development. The length of vitamin D deficiency seems to determine the intensity of cardiomyocyte alterations, and, noticeably, the restoration of adequate vitamin D level can protect cardiomyocytes from aberrant signaling [111].
Membrane-bound VDR is shown to mediate rapid non-genomic processes and control the contraction of cardiomyocyte sarcomere, through caveolin 3 interaction, an integrated mechanism also reported in other cells [112,113,114,115].
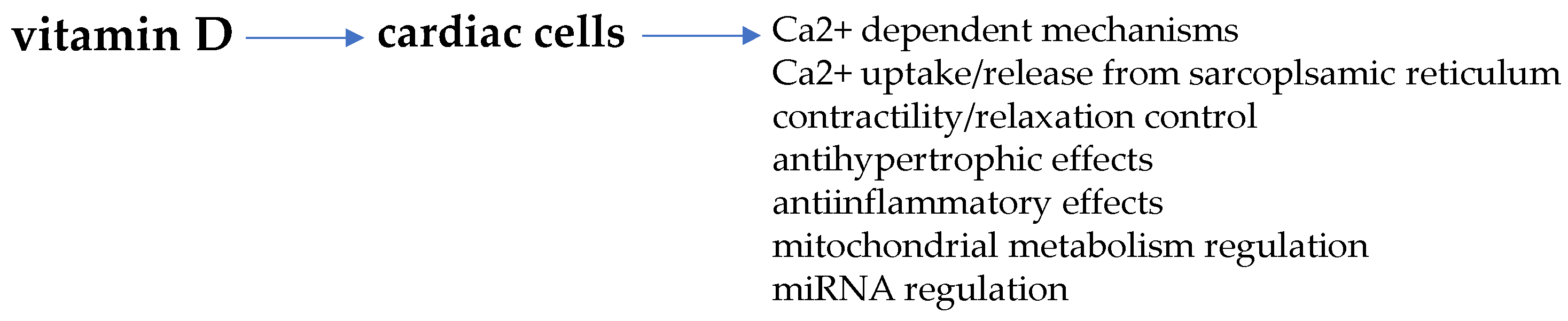
Non-genomic VDR activation is also described to regulate post-translational events through epigenetic effects by the generation of specific microRNA (miRNA) [116,117]. Of note, miRNA gene regulation by VDR may represent an important mechanism involved in the control of signal transduction through the recognition and degradation of target mRNAs level and translated proteins [118]. Indeed, the miRNA regulatory network may largely affect signaling molecules that exert pleiotropic effects all through the body, such as vitamin D. In fact, since miRNA targets may number up to hundred, it is conceivable that this mechanism can largely amplify the pleiotropic effects of vitamin D/VDR in cardiac cells, as occurs in other tissue and cells, i.e., adipose, cancer or bone cells, striated muscle cells, in which a mutual interaction is described [119,120,121,122,123,124,125]. Figure 3 schematically reports some of the main regulatory actions of vitamin D in cardiomyocytes.

Figure 3.
Vitamin D-mediated regulation of cardiac cells. Some important cardiomyocyte functions regulated by vitamin D are targeted by estrogens as well.
Vitamin D and Sex-Dimorphic Cardiac Metabolic Flexibility
Another cardioprotective mechanism of vitamin D is the regulation of mitochondrial metabolism and energy supply [75].
The energetic regulation of the cardiomyocyte is a quite complex process characterized by substrate promiscuity (fatty acids, carbohydrates, amino acids, lactates and ketons) to allow for multiple substrate utilization for energy production, in view of the high energy demand. Cardiac cells predominantly utilize fatty acids and rapidly shift to other substrates as they become abundantly available, to warranty an adequate ATP supply [126]. This process, known as metabolic substrate flexibility, permits the cell to adapt in response to physiologic conditions (i.e., during exercise) or pathologic challenge, such as hyperglycemia or inflammation [12]. Cardiac energetics and metabolic substrate flexibility display some degree of sex-dimorphism as well. As an example, according to human in vivo studies in healthy young adults, a women’s heart appears to utilize more oxygen and less glucose as compared with the heart of age-matched man [76]. This effect is dependent, in part, on estrogen-induced eNOS upregulation, which decreases glucose transporter (GLUT)-4 translocation to cell surface and, in turn, reduces glucose uptake/utilization by the cardiomyocyte [127,128]. Thus, female cardiac cell energetics depends more on fatty acid oxidation, which requires more oxygen consumption. The well-perfused oxygenation is one of the mechanisms underlying cardio-protection in healthy female heart. However, recent studies on exercise-induced remodeling in males and females show that female cardiac cells likely retain a lesser degree of metabolic flexibility to adaptation in stress or disease conditions [129]. Noticeably, in cardiac cells vitamin D is reported to impinge on the energy substrate balance, regulating fat uptake/fatty acid β-oxidation via sirtuin 3 [75].
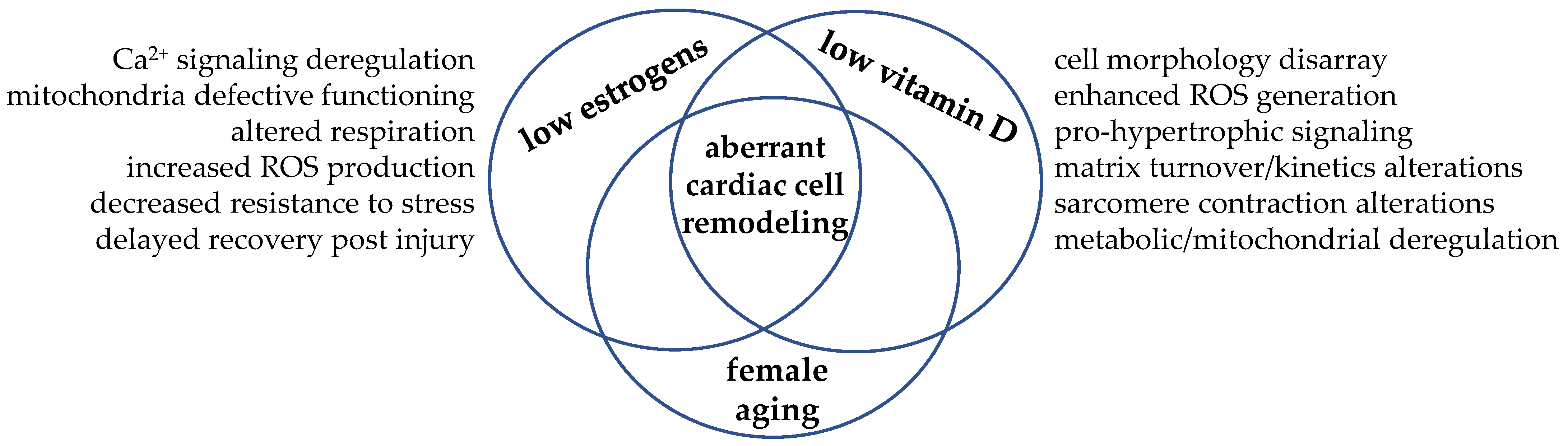
In this scenario, we could figure the cardiomyocyte as the cellular crossroad where the hormonal signaling from estrogens and vitamin D may meet and intersect to regulate cell remodeling and drive cardiac function towards adaptation or, in case of simultaneous hormone deficiency, maladaptation, as depicted in Figure 4.
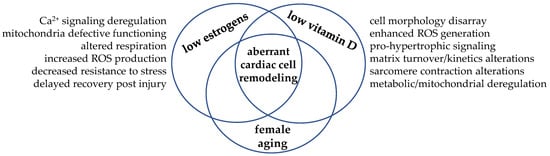
Figure 4.
Hypoestrogenism and hypovitaminosis D may intersect in the cardiomyocyte and drive aberrant remodeling. The simultaneous low level of these steroid molecules—a typical hormonal condition experienced post menopause—allows biomolecular aberrant signaling and cell maladaptative response. As these hormones often share the same biomolecular signaling, the hypothesis of interdependent synergic actions within the cardiomyocyte has taken place.
To date, the research on this specific topic is still in its infancy, but the cooperation between vitamin D and estrogen in the regulation of some other important biological functions may provide mechanisms and models as examples.
6. Vitamin D and Estrogen Cooperation: Learning from Examples
The classical example of interaction between vitamin D and estrogens comes from bone health in women: both hormones act in synergy to promote osteoblast proliferation and differentiation, in association with E2-induced VDR upregulation, via MAPK, ERK1/2 signaling, as well documented in past studies [130,131,132].
The cooperation between vitamin D and estrogens in mice striated muscle cells results in a potentiated induction of cAMP response element binding (CREB) phosphorylation and c-Fos protein expression, via MAPK and ERK-dependent intracellular cascade activation [133]; this observation discloses hypothesis on fast non-transcriptional responses evoked by hormone combination to promote muscle recovery and function.
Another example of vitamin D and estrogen signal cooperation is elegantly shown by human and experimental studies in autoimmune multiple sclerosis (MS), a disease with higher female bias and marked hypovitaminosis D. In mice with autoimmune encephalomyelitis (EAE, resembling human MS), only intact females can achieve protective effects via vitamin D [134,135]. After estrogen implants, EAE ovariectomized females fully retrieve vitamin D-induced beneficial effects. The protective effect relies on an enhanced E2 biosynthesis promoted by vitamin D and an increased VDR expression induced by E2, allowing the two hormones to act together toward disease remission [135]. This vitamin D-mediated protection is female specific and is observed neither in ovariectomized females nor in males. Of interest, similar results come from a human large prospective study in MS subjects: the higher level of vitamin D naturally occurring in summer inversely associates with MS incidence and MS disability only in women, suggesting that estrogens, somehow, are selectively permissive for the beneficial effects of vitamin D [136].
The importance of vitamin D-estrogens mutual crosstalk in health/disease discrimination is not so surprising when thinking of some pioneering investigations in women, which showed that vitamin D level is higher in high estrogen conditions (i.e., pregnancy, ovulation, HRT in post-menopause) [137,138]. Hypovitaminosis D and low free testosterone associate with particularly adverse clinical outcome in men referred for coronary angiography, suggesting some interplay of these hormones in males [139]. Low vitamin D and testosterone deficiency are considered typical features in men with advanced HF; however, vitamin D supplementation in this group of patients cannot prevent the decline in testosterone indices [140].
Concerning the specific topic on female heart function, a quite recent study postulates a synergistic role of vitamin D and E2 in postmenopausal women with metabolic syndrome, a cluster of simultaneous cardiovascular risk factors, leading to an increased risk of heart disease, stroke and T2D, as previously mentioned [141]. The study shows that low vitamin D likely increases the risk of disease in women with hypoestrogenism, documenting a stronger inverse correlation E2-metabolic syndrome in women with D hypovitaminosis vs. women with a normal vitamin D level. This observation is in line with a previous study performed in African American women, who are most vulnerable than other age-matched race or ethnic groups because of their higher risk for this disease, likely related to further reduction in estrogen and vitamin D levels [142].
Although these papers are far from confirming that a low estrogen level favors the disease via vitamin D deficiency, some mechanisms are conceivable, involving hormone-dependent merged actions onto endothelial cells and cardiomyocytes. Meanwhile, in vascular (endothelial and smooth muscle) cells, vitamin D and estrogens are known to interdependently regulate blood pressure by the release of potent vasodilators, such as NO, prostaglandins (PGs), nitric oxide and calcitonin gene related peptide (CGRP) [142,143], the potential cooperating mechanism(s) of these hormones in cardiomyocytes are scarcely described. However, as addressed before in this review, it is well recognized that vitamin D can directly affect signal transduction mediators and ion channels in cardiomyocytes, often sharing the same biomolecular signaling with E2, such as NO, eNOS, CGRP and peroxisome proliferator activated receptor (PPAR)α—the latter one is involved in lipid and glucose metabolism regulation [142]. Thus far, the recognition that cardiomyocytes express all the isoforms of nitric oxide synthase (NOS), the three isoforms of PPAR, CGRP and progesterone (PG) receptors [144,145] can hopefully open further hypothesis and perspectives on possible synergic mechanisms in these cells, whose structure and function likely respond to vitamin D-other hormone interplay.
7. Conclusions
Although sex undeniably matters in cardiac health, vitamin D tightly affects heart function in females and males. The detrimental convergence of hypovitaminosis D and estrogen deficiency, naturally occurring with menses ending, reveals the higher vulnerability of postmenopausal women experiencing this condition, associated, indeed, with a significant increase in CVD rate vs. age-matched men. Estrogens, the molecules classically considered as the main responsible for the sex-dimorphism in cardiac function, unlikely can fully cover this difference. Vitamin D, like estrogens, finely impinges on heart remodeling in response to different challenges (i.e., aging, volume or pressure overload, exercise, necrosis). Nowadays, cardiomyocyte remodeling, the cellular event driving whole organ macro-remodeling (via adaptive or maladaptive responses) is recognized to be sex-dimorphic and affected by vitamin D. In this scenario, supplementation with both hormones apparently would offer a promising approach in women CVD prevention, especially in post-menopause life. However, beside the limit of HRT (useful only when taken within a limited timeframe), data on the efficacy of vitamin D supplementation in CVD prevention are contradictory [146,147,148]. In the U-shaped relationship between vitamin D and cardiovascular risk, 20 ng/mL is considered the vitamin D serum level associated with an apparent minimum risk, and a dose > 4000 IU/day as supplement seems necessary to affect heart remodeling in vitamin D deficient subjects with HF [90,149,150]. Indeed, general important concerns still exist in several aspects, including the lack of a clear indication of the optimal dose requirement to respond to extra-skeletal needs, or the lack of a clear definition of vitamin D insufficiency/deficiency, based on well-defined serum ranges (still missing as well) [151,152,153]. Data from trials on D hormone supplementation, either alone or combined, are unsatisfactory likely due to the high variability in protocols and heterogeneity of the studied populations, since general health status, ethnicity, sedentary habit are often not defined, as recently summarized elsewhere [80,81]. Furthermore, recent evidence highlights the major role of exercise-induced sex-dependent heart remodeling during life of men and women at different ages, either sedentary or physically active [129]. This topic is not addressed in the present review, both for length limits and because, in our opinion, it would deserve a dedicated issue.
Thus far, albeit epidemiological studies and metanalyses report an unequivocal association between vitamin D status and heart remodeling, there are still inconclusive remarks from the sex-specific standpoint [90,150]. Research on this specific field is still in its infancy and we are aware of the difficulty to consider, present and discuss hypovitaminosis D and sex-related CVD as not separate but interconnected issues, within more complex scenario(s).
Herein, the interest is confined and focused on the importance of vitamin D-estrogen interplay in cardiomyocyte adaptation, to drive the attention on an issue still not fully explored and, maybe, to be hypothesis generating.
While waiting for mandatory well-designed trials to overcome the existing bias, further progress of basic research on estrogen–vitamin D crosstalk and their orchestration of the cardiomyocyte remodeling would represent an important step forward to narrow the large gap in the knowledge of this topic, in consideration of the translational approach to future strategies in women health prevention and therapy.
Funding
Paper supported by project PRIN (“Progetti di Ricerca di Interesse Nazionale”) 2017ATZ2YK.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Merz, A.A.; Cheng, S. Sex differences in cardiovascular ageing. Heart 2016, 102, 825–831. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Kararigas, G. Mechanistic pathways of sex differences in cardiovascular disease. Physiol. Rev. 2017, 97, 1–37. [Google Scholar] [CrossRef]
- Crandall, C.J.; Barrett-Connor, E. Endogenous sex steroid levels and cardiovascular disease in relation to the menopause: A systematic review. Endocrinol. Metab. Clin. 2013, 42, 227–253. [Google Scholar] [CrossRef]
- Kessler, E.L.; Rivaud, M.R.; Vos, M.A.; Van Veen, T.A. Sex-specific influence on cardiac structural remodeling and therapy in cardiovascular disease. Biol. Sex Differ. 2019, 10, 7. [Google Scholar] [CrossRef]
- Miller, R.J.H.; Mikami, Y.; Heydari, B.; Wilton, S.B.; James, M.T.; Howarth, A.G.; White, J.A.; Lydell, C.P. Sex-specific relationships between patterns of ventricular remodelling and clinical outcomes. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 983–990. [Google Scholar] [CrossRef]
- Oneglia, A.; Nelson, M.D.; Merz, C.N.B. Sex Differences in Cardiovascular Aging and Heart Failure. Curr. Heart Fail. Rep. 2020, 17, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Piro, M.; Bona, D.R.; Abbate, A.; Biasucci, L.M.; Crea, F. Sex-related differences in myocardial remodeling. J. Am. Coll. Cardiol. 2010, 55, 1057–1065. [Google Scholar] [CrossRef]
- Kararigas, G.; Bito, V.; Tinel, H.; Becher, E.; Baczko, I.; Knosalla, C.; Albrecht-Küpper, B.; Sipido, K.R.; Regitz-Zagrosek, V. Transcriptome Characterization of Estrogen-Treated Human Myocardium Identifies Myosin Regulatory Light Chain Interacting Protein as a Sex-Specific Element Influencing Contractile Function. J. Am. Coll. Cardiol. 2012, 59, 410–417. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Vitamin D and cardiovascular diseases: Causality. J. Steroid Biochem. Mol. Biol. 2018, 175, 29–43. [Google Scholar] [CrossRef]
- Kim, I.M.; Norris, K.C.; Artaza, J.N. Vitamin D and cardiac differentiation. Vitam. Horm. 2016, 100, 299–320. [Google Scholar] [CrossRef]
- Sithara, T.; Drosatos, K. Metabolic Complications in Cardiac Aging. Front. Physiol. 2021, 12, 579. [Google Scholar] [CrossRef]
- Filardi, T.; Ghinassi, B.; Di Baldassarre, A.; Tanzilli, G.; Morano, S.; Lenzi, A.; Basili, S.; Crescioli, C. Cardiomyopathy Associated with Diabetes: The Central Role of the Cardiomyocyte. Int. J. Mol. Sci. 2019, 20, 3299. [Google Scholar] [CrossRef]
- Ren, J.; Ceylan-Isik, A.F. Diabetic cardiomyopathy. Endocrine 2004, 25, 73–83. [Google Scholar] [CrossRef]
- Ren, J.; Kelley, R.O. Cardiac health in women with metabolic syndrome: Clinical aspects and pathophysiology. Obesity 2009, 17, 1114–1123. [Google Scholar] [CrossRef]
- Adekunle, A.O.; Adzika, G.K.; Mprah, R.; Ndzie Noah, M.L.; Adu-Amankwaah, J.; Rizvi, R.; Akhter, N.; Sun, H. Predominance of Heart Failure with Preserved Ejection Fraction in Postmenopausal Women: Intra-and Extra-Cardiomyocyte Maladaptive Alterations Scaffolded by Estrogen Deficiency. Front. Cell Dev. Biol. 2021, 9, 2729. [Google Scholar] [CrossRef]
- Mendelsohn, M.E.; Karas, R.H. The protective effects of estrogen on the cardiovascular system. N. Engl. J. Med. 1999, 340, 1801–1811. [Google Scholar] [CrossRef]
- Farhat, M.Y.; Lavigne, M.C.; Ramwell, P.W. The vascular protective effects of estrogen. FASEB J. 1996, 10, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Rafikova, O.; Sullivan, J.C. Estrogen: Good, bad, or both? Hypertension 2014, 63, 449–450. [Google Scholar] [CrossRef]
- Nită, A.R.; Knock, G.A.; Heads, R.J. Signalling Mechanisms in the Cardiovascular Protective Effects of Estrogen: With a focus on rapid/membrane signalling. Curr. Res. Physiol. 2021, 4, 103–118. [Google Scholar] [CrossRef]
- Nilsson, S.; Mäkelä, S.; Treuter, E.; Tujague, M.; Thomsen, J.; Andersson, G.; Enmark, E.; Pettersson, K.; Warner, M.; Gustafsson, J.Å. Mechanisms of Estrogen Action. Physiol. Rev. 2001, 81, 1535–1565. [Google Scholar] [CrossRef] [PubMed]
- Menazza, S.; Murphy, E. The expanding complexity of estrogen receptor signaling in the cardiovascular system. Circ. Res. 2016, 118, 994–1007. [Google Scholar] [CrossRef]
- Zimmerman, M.A.; Budish, R.A.; Kashyap, S.; Lindsey, S.H. GPER-novel membrane estrogen receptor. Clin. Sci. 2016, 130, 1005. [Google Scholar] [CrossRef]
- Nakanishi, R.; Baskaran, L.; Gransar, H.; Budoff, M.J.; Achenbach, S.; Al-Mallah, M.; Cademartiri, F.; Callister, T.Q.; Chang, H.; Chinnaiyan, K.; et al. Relationship of Hypertension to Coronary Atherosclerosis and Cardiac Events in Patients With Coronary Computed Tomographic Angiography. Hypertension 2017, 70, 293–299. [Google Scholar] [CrossRef]
- Austin, C.E. Chronic and acute effects of oestrogens on vascular contractility. J. Hypertens. 2000, 18, 1365–1378. [Google Scholar] [CrossRef]
- Barakat, R.; Oakley, O.; Kim, H.; Jin, J.; Ko, C.J. Extra-gonadal sites of estrogen biosynthesis and function. BMB Rep. 2019, 49, 488. [Google Scholar] [CrossRef]
- Vandenplas, G.; De Bacquer, D.; Calders, P.; Fiers, T.; Kaufman, J.M.; Ouwens, D.M.; Ruige, J.B. Endogenous oestradiol and cardiovascular disease in healthy men: A systematic review and meta-analysis of prospective studies. Heart 2012, 98, 1478–1482. [Google Scholar] [CrossRef]
- Carani, C.; Qin, K.; Simoni, M.; Faustini-Fustini, M.; Serpente, S.; Boyd, J.; Korach, K.; Simpson, E.R. Effect of Testosterone and Estradiol in a Man with Aromatase Deficiency. N. Engl. J. Med. 1997, 337, 91–95. [Google Scholar] [CrossRef]
- Smith, E.P.; Boyd, J.; Frank, G.R.; Takahashi, H.; Cohen, R.M.; Specker, B.; Williams, T.C.; Lubahn, D.B.; Korach, K. Estrogen Resistance Caused by a Mutation in the Estrogen-Receptor Gene in a Man. N. Engl. J. Med. 1994, 331, 1056–1061. [Google Scholar] [CrossRef]
- Morishima, A.K.I.R.A.; Grumbach, M.M.; Simpson, E.R.; Fisher, C.; Qin, K.E.N.A.N. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J. Clin. Endocrinol. Metab. 1995, 80, 3689–3698. [Google Scholar] [CrossRef]
- Vikan, T.; Schirmer, H.; Njølstad, I.; Svartberg, J. Low testosterone and sex hormone-binding globulin levels and high estradiol levels are independent predictors of type 2 diabetes in men. Eur. J. Endocrinol. 2010, 162, 747. [Google Scholar] [CrossRef][Green Version]
- Sudhir, K.; Chou, T.M.; Messina, L.M.; Hutchison, S.J.; Korach, K.S.; Chatterjee, K.; Rubanyi, G.M. Endothelial dysfunction in a man with disruptive mutation in oestrogen-receptor gene. Lancet 1977, 349, 1146–1147. [Google Scholar] [CrossRef]
- Iorga, A.; Cunningham, C.M.; Moazeni, S.; Ruffenach, G.; Umar, S.; Eghbali, M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol. Sex Differ. 2017, 8, 33. [Google Scholar] [CrossRef]
- Ärnlöv, J.; Pencina, M.J.; Amin, S.; Nam, B.-H.; Benjamin, E.J.; Murabito, J.M.; Wang, T.J.; Knapp, P.E.; D’Agostino, R.B.; Bhasin, S.; et al. Endogenous sex hormones and cardiovascular disease incidence in men. Ann. Intern. Med. 2006, 145, 176–184. [Google Scholar] [CrossRef]
- Vogel, B.; Acevedo, M.; Appelman, Y.; Merz, C.N.B.; Chieffo, A.; Figtree, G.A.; Guerrero, M.; Kunadian, V.; Lam, C.S.P.; Maas, A.H.E.M.; et al. The Lancet women and cardiovascular disease Commission: Reducing the global burden by 2030. Lancet 2021, 397, 2385–2438. [Google Scholar] [CrossRef]
- Kannel, W.B. The Framingham Study: Historical insight on the impact of cardiovascular risk factors in men versus women. J. Gend. Specif. Med. JGSM Off. J. Partnersh. Women’s Health Columbia 2002, 5, 27–37. [Google Scholar]
- Subramanian, M.; Balasubramanian, P.; Garver, H.; Northcott, C.; Zhao, H.; Haywood, J.R.; Fink, G.D.; Mohankumar, S.M.J.; Mohankumar, P.S. Chronic estradiol-17β exposure increases superoxide production in the rostral ventrolateral medulla and causes hypertension: Reversal by resveratrol. Am. J. Physiol. Integr. Comp. Physiol. 2011, 300, R1560–R1568. [Google Scholar] [CrossRef]
- Gurney, E.P.; Nachtigall, M.J.; Nachtigall, L.E.; Naftolin, F. The Women’s Health Initiative trial and related studies: 10 years later: A clinician’s view. J. Steroid Biochem. Mol. Biol. 2014, 142, 4–11. [Google Scholar] [CrossRef]
- Dehaini, H.; Fardoun, M.; Abou-Saleh, H.; El-Yazbi, A.; Eid, A.A.; Eid, A.H. Estrogen in vascular smooth muscle cells: A friend or a foe? Vasc. Pharmacol. 2018, 111, 15–21. [Google Scholar] [CrossRef]
- Manson, J.E. The ‘timing hypothesis’ for estrogen therapy in menopausal symptom management. Women’s Health 2015, 11, 437–440. [Google Scholar] [CrossRef]
- Hodis, H.N.; Mack, W.J.; Shoupe, D.; Azen, S.P.; Stanczyk, F.Z.; Hwang-Levine, J.; Budoff, M.J.; Henderson, V.W. Methods and baseline cardiovascular data from the Early versus Late Intervention Trial with Estradiol testing the menopausal hormone timing hypothesis. Menopause 2015, 22, 391–401. [Google Scholar] [CrossRef]
- Ueda, K.; Fukuma, N.; Adachi, Y.; Numata, G.; Tokiwa, H.; Toyoda, M.; Otani, A.; Hashimoto, M.; Liu, P.Y.; Takimoto, E. Sex Differences and Regulatory Actions of Estrogen in Cardiovascular System. Front. Physiol. 2021, 12, 738218. [Google Scholar] [CrossRef]
- Korytkowski, M.T.; Krug, E.I.; Daly, M.A.; DeRiso, L.; Wilson, J.W.; Winters, S.J. Does androgen excess contribute to the cardiovascular risk profile in postmenopausal women with type 2 diabetes? Metabolism 2005, 54, 1626–1631. [Google Scholar] [CrossRef]
- Macut, D.; Antić, I.B.; Bjekić-Macut, J. Cardiovascular risk factors and events in women with androgen excess. J. Endocrinol. Investig. 2015, 38, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; Kim, J.Y.; Wan, C.; Xiong, J.D.; Parry, S.A.; Azziz, R.; Lujan, M.E. Comprehensive evaluation of disparities in cardiometabolic and reproductive risk between Hispanic and White women with polycystic ovary syndrome in the United States: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Grohé, C.; Kahlert, S.; Löbbert, K.; Stimpel, M.; Karas, R.H.; Vetter, H.; Neyses, L. Cardiac myocytes and fibroblasts contain functional estrogen receptors. FEBS Lett. 1977, 416, 107–112. [Google Scholar] [CrossRef]
- Mahmoodzadeh, S.; Eder, S.; Nordmeyer, J.; Ehler, E.; Huber, O.; Martus, P.; Weiske, J.; Pregla, R.; Hetzer, R.; Regitz-Zagrosek, V. Estrogen receptor alpha up-regulation and redistribution in human heart failure. FASEB J. 2006, 20, 926–934. [Google Scholar] [CrossRef]
- Taylor, A.H.; Al-Azzawi, F. Immunolocalisation of oestrogen receptor beta in human tissues. J. Mol. Endocrinol. 2020, 24, 145–155. [Google Scholar] [CrossRef]
- Lizotte, E.; Grandy, S.A.; Tremblay, A.; Allen, B.G.; Fiset, C. Expression, distribution and regulation of sex steroid hormone receptors in mouse heart. Cell. Physiol. Biochem. 2009, 23, 075–086. [Google Scholar] [CrossRef]
- Knowlton, A.A.; Lee, A.R. Estrogen and the cardiovascular system. Pharmacol. Ther. 2012, 135, 54–70. [Google Scholar] [CrossRef]
- Ropero, A.B.; Eghbali, M.; Minosyan, T.Y.; Tang, G.; Toro, L.; Stefani, E. Heart estrogen receptor alpha: Distinct membrane and nuclear distribution patterns and regulation by estrogen. J. Mol. Cell. Cardiol. 2016, 41, 496–510. [Google Scholar] [CrossRef]
- Mahmoodzadeh, S.; Leber, J.; Zhang, X.; Jaisser, F.; Messaoudi, S.; Morano, I.; Furth, P.A.; Dworatzek, E.; Regitz-Zagrosek, V. Cardiomyocyte-specific estrogen receptor alpha increases angiogenesis, lymphangiogenesis and reduces fibrosis in the female mouse heart post-myocardial infarction. J. Cell Sci. Ther. 2014, 5, 153. [Google Scholar] [CrossRef] [PubMed]
- Devanathan, S.; Whitehead, T.; Schweitzer, G.G.; Fettig, N.; Kovacs, A.; Korach, K.S.; Finck, B.N.; Shoghi, K.I. An Animal Model with a Cardiomyocyte-Specific Deletion of Estrogen Receptor Alpha: Functional, Metabolic, and Differential Network Analysis. PLoS ONE 2014, 9, e101900. [Google Scholar] [CrossRef] [PubMed]
- Kararigas, G.; Nguyen, B.T.; Jarry, H. Estrogen modulates cardiac growth through an estrogen receptor α-dependent mechanism in healthy ovariectomized mice. Mol. Cell. Endocrinol. 2014, 382, 909–914. [Google Scholar] [CrossRef]
- Schuster, I.; Mahmoodzadeh, S.; Dworatzek, E.; Jaisser, F.; Messaoudi, S.; Morano, I.; Regitz-Zagrosek, V. Cardiomyocyte-specific overexpression of oestrogen receptor β improves survival and cardiac function after myocardial infarction in female and male mice. Clin. Sci. 2016, 130, 365–376. [Google Scholar] [CrossRef]
- Forster, C.; Kietz, S.; Hultenby, K.; Warner, M.; Gustafsson, J.-A. Characterization of the ERβ−/-mouse heart. Proc. Natl. Acad. Sci. USA 2004, 101, 14234–14239. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Y.; Weil, B.; Abarbanell, A.; Herrmann, J.; Tan, J.; Kelly, M.; Meldrum, D.R. Estrogen receptor β mediates increased activation of PI3K/Akt signaling and improved myocardial function in female hearts following acute ischemia. Am. J. Physiol. Integr. Comp. Physiol. 2009, 296, R972–R978. [Google Scholar] [CrossRef]
- Deschamps, A.; Murphy, E. Activation of a novel estrogen receptor, GPER, is cardioprotective in male and female rats. Am. J. Physiol. Circ. Physiol. 2009, 297, H1806–H1813. [Google Scholar] [CrossRef]
- Wang, H.; Sun, X.; Chou, J.; Lin, M.; Ferrario, C.M.; Zapata-Sudo, G.; Groban, L. Cardiomyocyte-specific deletion of the G protein-coupled estrogen receptor (GPER) leads to left ventricular dysfunction and adverse remodeling: A sex-specific gene profiling analysis. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2017, 1863, 1870–1882. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, X.; Chou, J.; Lin, M.; Ferrario, C.M.; Zapata-Sudo, G.; Groban, L. Inflammatory and mitochondrial gene expression data in GPER-deficient cardiomyocytes from male and female mice. Data Brief 2017, 10, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Clapier, R.; Moulin, M.; Piquereau, J.; Lemaire, C.; Mericskay, M.; Veksler, V.; Garnier, A. Mitochondria: A central target for sex differences in pathologies. Clin. Sci. 2017, 131, 803–822. [Google Scholar] [CrossRef] [PubMed]
- Jazbutyte, V.; Kehl, F.; Neyses, L.; Pelzer, T. Estrogen receptor alpha interacts with 17β-hydroxysteroid dehydrogenase type 10 in mitochondria. Biochem. Biophys. Res. Commun. 2009, 384, 450–454. [Google Scholar] [CrossRef]
- Yang, S.H.; Liu, R.; Perez, E.J.; Wen, Y.; Stevens, S.M.; Valencia, T.; Brun-Zinkernagel, A.-M.; Prokai, L.; Will, Y.; Dykens, J.; et al. Mitochondrial localization of estrogen receptor β. Proc. Natl. Acad. Sci. USA 2004, 101, 4130–4135. [Google Scholar] [CrossRef] [PubMed]
- Bertero, E.; Maack, C. Calcium signaling and reactive oxygen species in mitochondria. Circ. Res. 2018, 122, 1460–1478. [Google Scholar] [CrossRef] [PubMed]
- Verkerk, A.O.; Wilders, R.; Veldkamp, M.W.d.G.W.; Kirkels, J.H.; Tan, H.L. Gender disparities in cardiac cellular electrophysiology and arrhythmia susceptibility in human failing ventricular myocytes. Int. Heart J. 2005, 46, 1105–1118. [Google Scholar] [CrossRef] [PubMed]
- Papp, R.; Bett, G.C.; Lis, A.; Rasmusson, R.L.; Baczkó, I.; Varró, A.; Salama, G. Genomic upregulation of cardiac Cav1. 2α and NCX1 by estrogen in women. Biol. Sex Differ. 2017, 8, 1105–1118. [Google Scholar] [CrossRef] [PubMed]
- Curl, C.L.; Delbridge, L.M.; Wendt, I.R. Sex differences in cardiac muscle responsiveness to Ca2+ and L-type Ca2+ channel modulation. Eur. J. Pharmacol. 2008, 586, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Shkurat, T.P.; Melnichenko, A.A.; Grechko, A.V.; Orekhov, A.N. The role of mitochondrial dysfunction in cardiovascular disease: A brief review. Ann. Med. 2018, 50, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Bers, D.M. Altered cardiac myocyte Ca regulation in heart failure. Physiology 2006, 21, 380–387. [Google Scholar] [CrossRef]
- Turdi, S.; Huff, A.F.; Pang, J.; He, E.Y.; Chen, X.; Wang, S.; Chen, Y.; Zhang, Y.; Ren, J. 17-β estradiol attenuates ovariectomy-induced changes in cardiomyocyte contractile function via activation of AMP-activated protein kinase. Toxicol. Lett. 2015, 232, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Tishkoff, D.X.; Nibbelink, K.A.; Holmberg, K.H.; Dandu, L.; Simpson, R.U. Functional vitamin D receptor (VDR) in the t-tubules of cardiac myocytes: VDR knockout cardiomyocyte contractility. Endocrinology 2008, 149, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Law, C.S.; Grigsby, C.; Olsen, K.; Hong, T.-T.; Zhang, Y.; Yeghiazarians, Y.; Gardner, D.G. Cardiomyocyte-Specific Deletion of the Vitamin D Receptor Gene Results in Cardiac Hypertrophy. Circulation 2011, 124, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gardner, D.G. Liganded vitamin D receptor displays anti-hypertrophic activity in the murine heart. J. Steroid Biochem. Mol. Biol. 2013, 136, 150–155. [Google Scholar] [CrossRef]
- Guo, X.; Lin, H.; Liu, J.; Wang, D.; Li, D.; Jiang, C.; Tang, Y.; Wang, J.; Zhang, T.; Li, Y.; et al. 1,25-Dihydroxyvitamin D attenuates diabetic cardiac autophagy and damage by vitamin D receptor-mediated suppression of FoxO1 translocation. J. Nutr. Biochem. 2020, 80, 108380. [Google Scholar] [CrossRef] [PubMed]
- Sottili, M.; Cosmi, L.; Borgogni, E.; Sarchielli, E.; Maggi, L.; Francalanci, M.; Vannelli, G.; Ronconi, E.; Adorini, L.; Annunziato, F.; et al. Immunomodulatory effects of BXL-01-0029, a less hypercalcemic vitamin D analogue, in human cardiomyocytes and T cells. Exp. Cell Res. 2009, 315, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Y.; Pan, Y.; Sun, C.; Liu, Z.; Liu, N.; Fu, Y.; Li, X.; Li, Y.; Kong, J. The Protective Effect of 1,25(OH)2D3 on Myocardial Function is Mediated via Sirtuin 3-Regulated Fatty Acid Metabolism. Front. Cell Dev. Biol. 2021, 9, 966. [Google Scholar] [CrossRef]
- Peterson, L.R.; Soto, P.F.; Herrero, P.; Schechtman, K.B.; Dence, C.; Gropler, R.J. Sex differences in myocardial oxygen and glucose metabolism. J. Nucl. Cardiol. 2007, 14, 573–581. [Google Scholar] [CrossRef]
- Liu, E.; Meigs, J.B.; Pittas, A.G.; Economos, C.D.; McKeown, N.M.; Booth, S.L.; Jacques, P.F. Predicted 25-hydroxyvitamin D score and incident type 2 diabetes in the Framingham Offspring Study. Am. J. Clin. Nutr. 2010, 91, 1627–1633. [Google Scholar] [CrossRef]
- van Schouten, F.J.; Hirvonen, A.; Maas, L.M.; De Mol, B.A.; Kleinjans, J.C.S.; Bell, D.; Durrer, J.D. Putative susceptibility markers of coronary artery disease: Association between VDR genotype, smoking, and aromatic DNA adduct levels in human right atrial tissue. FASEB J. 1998, 12, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Schleithoff, S.S.; Zittermann, A.; Tenderich, G.; Berthold, H.; Stehle, P.; Koerfer, R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: A double-blind, randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2006, 83, 754–759. [Google Scholar] [CrossRef]
- Crescioli, C. Vitamin D Restores Skeletal Muscle Cell Remodeling and Myogenic Program: Potential Impact on Human Health. Int. J. Mol. Sci. 2021, 22, 1760. [Google Scholar] [CrossRef] [PubMed]
- Crescioli, C. Targeting Age-Dependent Functional and Metabolic Decline of Human Skeletal Muscle: The Geroprotective Role of Exercise, Myokine IL-6, and Vitamin D. Int. J. Mol. Sci. 2020, 21, 1010. [Google Scholar] [CrossRef] [PubMed]
- Crescioli, C. Vitamin D Merging into Immune System-Skeletal Muscle Network: Effects on Human Health. Appl. Sci. 2020, 10, 5592. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Di Somma, C.; Laudisio, D.; Salzano, C.; Pugliese, G.; De Alteriis, G.; Colao, A.; Savastano, S. Sex Differences of Vitamin D Status across BMI Classes: An Observational Prospective Cohort Study. Nutrients 2019, 11, 3034. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.K.; Hofsø, D.; Aasheim, E.T.; Tanbo, T.; Holven, K.B.; Andersen, L.F.; Røislien, J.; Hjelmesaeth, J. Impact of gender on vitamin D deficiency in morbidly obese patients: A cross-sectional study. Eur. J. Clin. Nutr. 2012, 66, 83–90. [Google Scholar] [CrossRef]
- Sanghera, D.K.; Sapkota, B.R.; Aston, C.E.; Blackett, P.R. Vitamin D status, gender differences, and cardiometabolic health disparities. Ann. Nutr. Metab. 2017, 70, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Verdoia, M.; Schaffer, A.; Barbieri, L.; Di Giovine, G.; Marino, P.; Suryapranata, H.; De Luca, G. Impact of gender difference on vitamin D status and its relationship with the extent of coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 464–470. [Google Scholar] [CrossRef]
- Jungert, A.; Neuhäuser-Berthold, M. Sex-specific determinants of serum 25-hydroxyvitamin D3 concentrations in an elderly German cohort: A cross-sectional study. Nutr. Metab. 2015, 12, 2. [Google Scholar] [CrossRef]
- Vasile, M.; Corinaldesi, C.; Antinozzi, C.; Crescioli, C. Vitamin D in autoimmune rheumatic diseases: A view inside gender differences. Pharmacol. Res. 2017, 117, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Crescioli, C.; Minisola, S. Vitamin D: Autoimmunity and gender. Curr. Med. Chem. 2017, 24, 2671–2686. [Google Scholar] [CrossRef] [PubMed]
- Kouvari, M.; Panagiotakos, D.B. Vitamin D status, gender and cardiovascular diseases: A systematic review of prospective epidemiological studies. Expert Rev. Cardiovasc. Ther. 2019, 17, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Argacha, J.-F.; Egrise, D.; Pochet, S.; Fontaine, D.; Lefort, A.; Libert, F.; Goldman, S.; van de Borne, P.; Berkenboom, G.; Moreno-Reyes, R. Vitamin D Deficiency-induced Hypertension Is Associated with Vascular Oxidative Stress and Altered Heart Gene Expression. J. Cardiovasc. Pharmacol. 2011, 58, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Roffe-Vazquez, D.N.; Huerta-Delgado, A.S.; Castillo, E.C.; Calderon, J.R.V.; Gonzalez-Gil, A.M.; Enriquez, C.; Garcia-Rivas, G.; Elizondo-Montemayor, L. Correlation of Vitamin D with Inflammatory Cytokines, Atherosclerotic Parameters, and Lifestyle Factors in the Setting of Heart Failure: A 12-Month Follow-Up Study. Int. J. Mol. Sci. 2019, 20, 5811. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Henry, R.M.A.; Snijder, M.B.; Van Dam, R.M.; Nijpels, G.; Stehouwer, C.D.A.; Kamp, O.; Tomaschitz, A.; Pieber, T.R.; Dekker, J.M. Vitamin D deficiency and myocardial structure and function in older men and women: The Hoorn Study. J. Endocrinol. Investig. 2010, 33, 612–617. [Google Scholar] [CrossRef]
- Akin, F.; Ayça, B.; Köse, N.; Celik, O.; Yilmaz, Y.; Akin, M.N.; Arinc, H.; Ozkok, A.; Covic, A.; Kanbay, M. Serum Vitamin D and C-Reactive Protein Levels Are Independently Associated with Diastolic Dysfunction. J. Investig. Med. 2014, 62, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Gouni-Berthold, I.; Berthold, H.K. Vitamin D and Vascular Disease. Curr. Vasc. Pharmacol. 2021, 19, 250–268. [Google Scholar] [CrossRef] [PubMed]
- Judd, S.E.; Tangpricha, V. Vitamin D therapy and cardiovascular health. Curr. Hypertens. Rep. 2011, 13, 187–191. [Google Scholar] [CrossRef] [PubMed]
- London, G.M.; Guérin, A.P.; Verbeke, F.H.; Pannier, B.; Boutouyrie, P.; Marchais, S.J.; Mëtivier, F. Mineral metabolism and arterial functions in end-stage renal disease: Potential role of 25-hydroxyvitamin D deficiency. J. Am. Soc. Nephrol. 2007, 18, 613–620. [Google Scholar] [CrossRef]
- Artaza, J.N.; Norris, K.C. Vitamin D reduces the expression of collagen and key profibrotic factors by inducing an antifibrotic phenotype in mesenchymal multipotent cells. J. Endocrinol. 2009, 200, 207. [Google Scholar] [CrossRef]
- Sugden, J.A.; Davies, J.I.; Witham, M.D.; Morris, A.D.; Struthers, A.D. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabet. Med. 2008, 25, 320–325. [Google Scholar] [CrossRef]
- Tintut, Y.; Demer, L.L. Potential Impact of the Steroid Hormone, Vitamin D, on the Vasculature Vitamin D-hormones and cardiovascular disease. Am. Heart J. 2021, 239, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Michos, E.D.; Cainzos-Achirica, M.; Heravi, A.S.; Appel, L.J. Vitamin D, calcium supplements, and implications for cardiovascular health: JACC focus seminar. J. Am. Coll. Cardiol. 2021, 77, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Verheyen, N.; Grübler, M.R.; Tomaschitz, A.; März, W. Vitamin D and cardiovascular disease prevention. Nat. Rev. Cardiol. 2016, 13, 404–417. [Google Scholar] [CrossRef]
- Wang, T.J. Vitamin D and cardiovascular disease. Annu. Rev. Med. 2016, 67, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Hershey, S.; Ahmed, S.; Nibbelink, K.; Simpson, R.U. Heart extracellular matrix gene expression profile in the vitamin D receptor knockout mice. J. Steroid Biochem. Mol. Biol. 2007, 103, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Tomaschitz, A.; Drechsler, C.; Dekker, J.M.; März, W. Vitamin D deficiency and myocardial diseases. Mol. Nutr. Food Res. 2010, 54, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Luo, W.; Wang, B.; Yi, Z.; Gong, P.; Xiong, Y. 1α,25-Dihydroxyvitamin D3 ameliorates diabetes-induced bone loss by attenuating FoxO1-mediated autophagy. J. Biol. Chem. 2021, 296, 100287. [Google Scholar] [CrossRef] [PubMed]
- Torre-Amione, G.; Kapadia, S.; Benedict, C.; Oral, H.; Young, J.B.; Mann, D. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: A report from the studies of left ventricular dysfunction (SOLVD). J. Am. Coll. Cardiol. 1996, 27, 1201–1206. [Google Scholar] [CrossRef]
- Schumacher, S.M.; Prasad, S.V.N. Tumor Necrosis Factor-α in Heart Failure: An Updated Review. Curr. Cardiol. Rep. 2018, 20, 117. [Google Scholar] [CrossRef]
- Rababa’H, A.M.; Guillory, A.N.; Mustafa, R.; Hijjawi, T. Oxidative Stress and Cardiac Remodeling: An Updated Edge. Curr. Cardiol. Rev. 2018, 14, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Assalin, H.B.; Rafacho, B.P.M.; dos Santos, P.P.; Ardisson, L.P.; Roscani, M.G.; Chiuso-Minicucci, F.; Barbisan, L.F.; Fernandes, A.A.H.; Azevedo, P.S.; Minicucci, M.F.; et al. Impact of the Length of Vitamin D Deficiency on Cardiac Remodeling. Circ. Heart Fail. 2013, 6, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Simpson, R.U. Interaction between vitamin D receptor with caveolin-3 and regulation by 1,25-dihydroxyvitamin D3 in adult rat cardiomyocytes. J. Steroid Biochem. Mol. Biol. 2010, 121, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorsi, L.; Marchiani, S.; Ferruzzi, P.; Muratori, M.; Crescioli, C.; Forti, G.; Maggi, M.; Baldi, E. Non-genomic effects of the androgen receptor and Vitamin D agonist are involved in suppressing invasive phenotype of prostate cancer cells. Steroids 2006, 71, 304–309. [Google Scholar] [CrossRef]
- Antinozzi, C.; Corinaldesi, C.; Giordano, C.; Pisano, A.; Cerbelli, B.; Migliaccio, S.; Di Luigi, L.; Stefanantoni, K.; Vannelli, G.B.; Minisola, S.; et al. Potential role for the VDR agonist elocalcitol in metabolic control: Evidences in human skeletal muscle cells. J. Steroid Biochem. Mol. Biol. 2017, 167, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Campbell, M.J. Vitamin D receptor signaling mechanisms: Integrated actions of a well-defined transcription factor. Steroids 2013, 78, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Karlic, H.; Varga, F. Impact of vitamin D metabolism on clinical epigenics. Clin Epigenet. 2011, 2, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Inui, M.; Martello, G.; Piccolo, S. MicroRNA control of signal transduction. Nat. Rev. Mol. Cell Biol. 2010, 11, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Assalin, H.B.; Gontijo, J.A.R.; Boer, P.A. miRNAs, target genes expression and morphological analysis on the heart in gestational protein-restricted offspring. PLoS ONE 2019, 14, e0210454. [Google Scholar] [CrossRef]
- Hobert, O. Gene regulation by transcription factors and microRNAs. Science 2008, 319, 1785–1786. [Google Scholar] [CrossRef] [PubMed]
- Jonas, M.I.; Kuryłowicz, A.; Bartoszewicz, Z.; Lisik, W.; Jonas, M.; Kozniewski, K.; Puzianowska-Kuznicka, M. Vitamin D receptor gene expression in adipose tissue of obese individuals is regulated by miRNA and correlates with the pro-inflammatory cytokine level. Int. J. Mol. Sci. 2019, 20, 5272. [Google Scholar] [CrossRef]
- Singh, P.K.; Long, M.D.; Battaglia, S.; Hu, Q.; Liu, S.; Sucheston-Campbell, L.E.; Campbell, M.J. VDR regulation of microRNA differs across prostate cell models suggesting extremely flexible control of transcription. Epigenetics 2015, 10, 40–49. [Google Scholar] [CrossRef]
- Singh, T.; Adams, B.D. The regulatory role of miRNAs on VDR in breast cancer. Transcription 2017, 8, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Ryan, Z.C.; Craig, T.A.; Folmes, C.; Wang, X.; Lanza, I.; Schaible, N.S.; Salisbury, J.; Nair, K.S.; Terzic, A.; Sieck, G.; et al. 1α,25-Dihydroxyvitamin D3 Regulates Mitochondrial Oxygen Consumption and Dynamics in Human Skeletal Muscle Cells. J. Biol. Chem. 2016, 291, 1514–1528. [Google Scholar] [CrossRef]
- Aguennouz, M.; Giudice, C.L.; Licata, N.; Rodolico, C.; Musumeci, O.; Fanin, M.; Migliorato, A.; Ragusa, M.; Macaione, V.; Di Giorgio, R.M.; et al. MicroRNA signatures predict dysregulated vitamin D receptor and calcium pathways status in limb girdle muscle dystrophies (LGMD) 2A/2B. Cell Biochem. Funct. 2016, 34, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Lisse, T.S.; Adams, J.S.; Hewison, M. Vitamin D and microRNAs in bone. Crit. Rev. Eukaryot. Gene Expr. 2013, 23, 195–214. [Google Scholar] [CrossRef] [PubMed]
- Kolwicz, S.C., Jr.; Purohit, S.; Tian, R. Cardiac Metabolism and its Interactions with Contraction, Growth, and Survival of Cardiomyocytes. Circ. Res. 2013, 113, 603–616. [Google Scholar] [CrossRef]
- dos Santos, R.L.; Da Silva, F.B.; Ribeiro, R.F.; Stefanon, I. Sex hormones in the cardiovascular system. Horm. Mol. Biol. Clin. Investig. 2014, 18, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Wittnich, C.; Tan, L.; Wallen, J.; Belanger, M. Sex differences in myocardial metabolism and cardiac function: An emerging concept. Pflügers Arch.-Eur. J. Physiol. 2013, 465, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.A.; Khalsa, S.S.S.; Vyas, A.K.; Rahimian, R. Sex-Specific Impacts of Exercise on Cardiovascular Remodeling. J. Clin. Med. 2021, 10, 3833. [Google Scholar] [CrossRef] [PubMed]
- Ishibe, M.; Nojima, T.; Ishibashi, T.; Koda, T.; Kaneda, K.; Rosier, R.N.; Puzas, J.E. 17β-Estradiol increases the receptor number and modulates the action of 1,25-dihydroxyvitamin D3 in human osteosarcoma-derived osteoblast-like cells. Calcif. Tissue Int. 1995, 57, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Padwa, B.L.; Zhou, S.; Mullokandova, J.; LeBoff, M.S.; Glowacki, J. Synergistic effect of 1α,25-dihydroxyvitamin D3 and 17β-estradiol on osteoblast differentiation of pediatric MSCs. J. Steroid Biochem. Mol. Biol. 2018, 177, 103–108. [Google Scholar] [CrossRef]
- Song, L.; Zhang, X.; Zhou, Y. A synergetic role of 1,25-dihydroxyvitamin D3 in 17β-estradial induced-proliferation and differentiation of osteoblastic MC3T3-E1 cells. Eur. J. Pharmacol. 2011, 659, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Ronda, A.C.; Buitrago, C.; Colicheo, A.; de Boland, A.R.; Roldán, E.; Boland, R. Activation of MAPKs by 1α,25(OH)2-Vitamin D3 and 17β-estradiol in skeletal muscle cells leads to phosphorylation of Elk-1 and CREB transcription factors. J. Steroid Biochem. Mol. Biol. 2007, 103, 462–466. [Google Scholar] [CrossRef]
- Spach, K.M.; Hayes, C.E. Vitamin D3 Confers Protection from Autoimmune Encephalomyelitis Only in Female Mice. J. Immunol. 2005, 175, 4119–4126. [Google Scholar] [CrossRef] [PubMed]
- Nashold, F.E.; Spach, K.M.; Spanier, J.A.; Hayes, C.E. Estrogen Controls Vitamin D3-Mediated Resistance to Experimental Autoimmune Encephalomyelitis by Controlling Vitamin D3 Metabolism and Receptor Expression. J. Immunol. 2009, 183, 3672–3681. [Google Scholar] [CrossRef] [PubMed]
- Kragt, J.J.; Van Amerongen, B.M.; Killestein, J.; Dijkstra, C.D.; Uitdehaag, B.M.J.; Polman, C.H.; Lips, P. Higher levels of 25-hydroxyvitamin D are associated with a lower incidence of multiple sclerosis only in women. Mult. Scler. J. 2009, 15, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Aarskog, D.; Aksnes, L.; Markestad, T.; Rødland, O. Effect of Estrogen on Vitamin D Metabolism in Tall Girls. J. Clin. Endocrinol. Metab. 1983, 57, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
- Gray, T.; McAdoo, T.; Hatley, L.; Lester, G.; Thierry, M. Fluctuation of serum concentration of 1,25-dihydroxyvitamin D3 during the menstrual cycle. Am. J. Obstet. Gynecol. 1982, 144, 880–884. [Google Scholar] [CrossRef]
- Lerchbaum, E.; Pilz, S.; Boehm, B.O.; Grammer, T.B.; Obermayer-Pietsch, B.; März, W. Combination of low free testosterone and low vitamin D predicts mortality in older men referred for coronary angiography. Clin. Endocrinol. 2012, 77, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A.; Ernst, J.B.; Prokop, S.; Fuchs, U.; Dreier, J.; Kuhn, J.; Knabbe, C.; Berthold, H.K.; Gouni-Berthold, I.; Gummert, J.F.; et al. Vitamin D supplementation does not prevent the testosterone decline in males with advanced heart failure: The EVITA trial. Eur. J. Nutr. 2018, 58, 673–680. [Google Scholar] [CrossRef]
- Huang, H.; Guo, J.; Chen, Q.; Chen, X.; Yang, Y.; Zhang, W.; Liu, Y.; Chen, X.; Yang, D. The synergistic effects of vitamin D and estradiol deficiency on metabolic syndrome in Chinese postmenopausal women. Menopause 2019, 26, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Gangula, P.R.; Dong, Y.L.; Al-Hendy, A.; Richard-Davis, G.; Montgomery-Rice, V.; Haddad, G.; Millis, R.; Nicholas, S.B.; Moseberry, D. Protective cardiovascular and renal actions of vitamin D and estrogen. Front. Biosci. 2013, 5, 134–148. [Google Scholar] [CrossRef]
- Somjen, D.; Katzburg, S.; Baz, M.; Stern, N.; Posner, G.H. Modulation of the response to estradiol-17β of rat vascular tissues by a non calcemic vitamin D analog. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-S.; Kim, J. Peroxisome Proliferator-Activated Receptors and the Heart: Lessons from the Past and Future Directions. PPAR Res. 2015, 2015, 271983. [Google Scholar] [CrossRef] [PubMed]
- Massion, P.B.; Feron, O.; Dessy, C.; Balligand, J.L. Nitric oxide and cardiac function: Ten years after, and continuing. Circ. Res. 2003, 93, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Kheiri, B.; Abdalla, A.; Osman, M.; Ahmed, S.; Hassan, M.; Bachuwa, G. Vitamin D deficiency and risk of cardiovascular diseases: A narrative review. Clin. Hypertens. 2018, 24, 9. [Google Scholar] [CrossRef] [PubMed]
- Norman, P.E.; Powell, J.T. Vitamin D and cardiovascular disease. Circ. Res. 2014, 114, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Barbarawi, M.; Kheiri, B.; Zayed, Y.; Barbarawi, O.; Dhillon, H.; Swaid, B.; Yelangi, A.; Sundus, S.; Bachuwa, G.; Alkotob, M.L.; et al. Vitamin D supplementation and cardiovascular disease risks in more than 83 000 individuals in 21 randomized clinical trials: A meta-analysis. JAMA Cardiol. 2019, 4, 765–776. [Google Scholar] [CrossRef]
- Wang, T.J.; Pencina, M.J.; Booth, S.L.; Jacques, P.F.; Ingelsson, E.; Lanier, K.; Benjamin, E.; D’Agostino, R.B.; Wolf, M.; Vasan, R.S. Vitamin D Deficiency and Risk of Cardiovascular Disease. Circulation 2008, 117, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.D.; Jia, J.J.; Dong, P.S.; Zhao, D.; Li, D.L.; Zhang, H.F. Effect of vitamin D on ventricular remodelling in heart failure: A meta-analysis of randomised controlled trials. BMJ Open 2018, 8, e020545. [Google Scholar] [CrossRef] [PubMed]
- Autier, P.; Mullie, P.; Macacu, A.; Dragomir, M.; Boniol, M.; Coppens, K.; Pizot, C.; Boniol, M. Effect of vitamin D supplementation on non-skeletal disorders: A systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017, 5, 986–1004. [Google Scholar] [CrossRef]
- Fuleihan, G.E.; Bouillon, R.; Clarke, B.; Chakhtoura, M.; Cooper, C.; McClung, M.R.; Singh, R. Serum 25-hydroxyvitamin D levels: Variability, knowledge gaps and the concept of a desirable range. J. Bone Miner. Res. 2015, 30, 1119–1133. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).