H2S Donors and Their Use in Medicinal Chemistry
Abstract
:1. Introduction
1.1. Hydrogen Sulfide: Chemical Properties
1.2. Hydrogen Sulfide: Biosynthesis and Metabolic Pathways
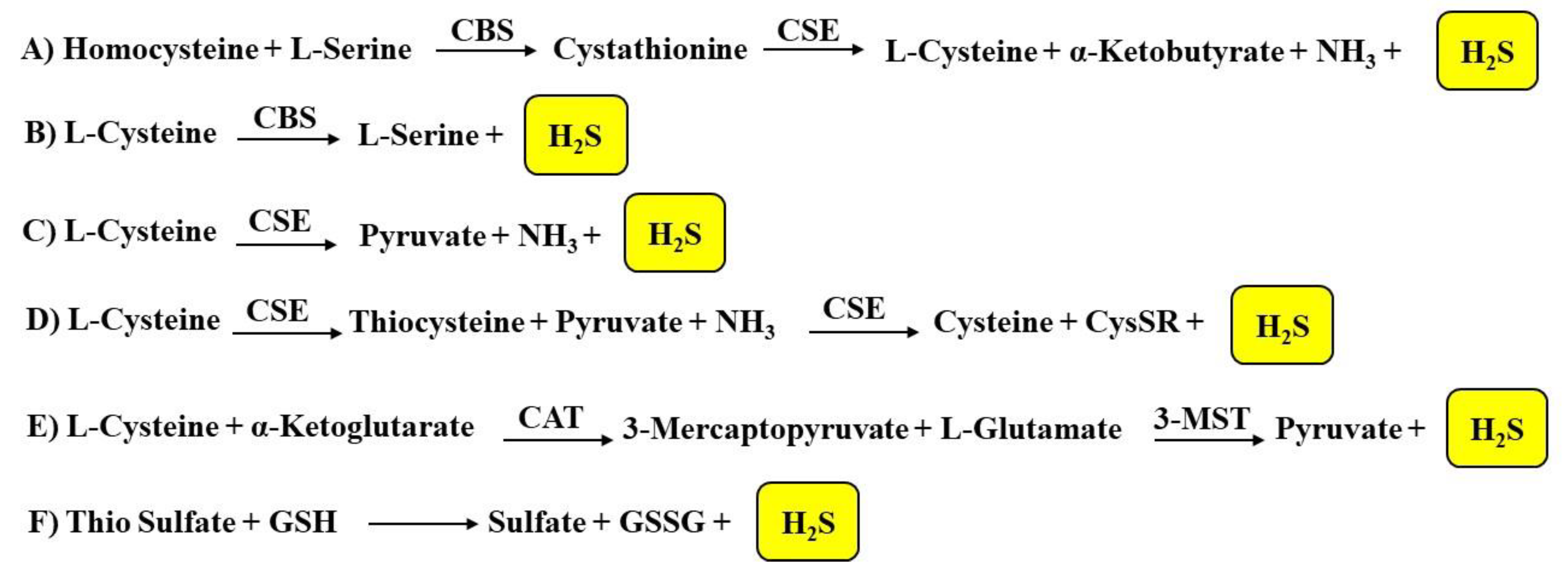
1.2.1. Biosynthesis of H2S
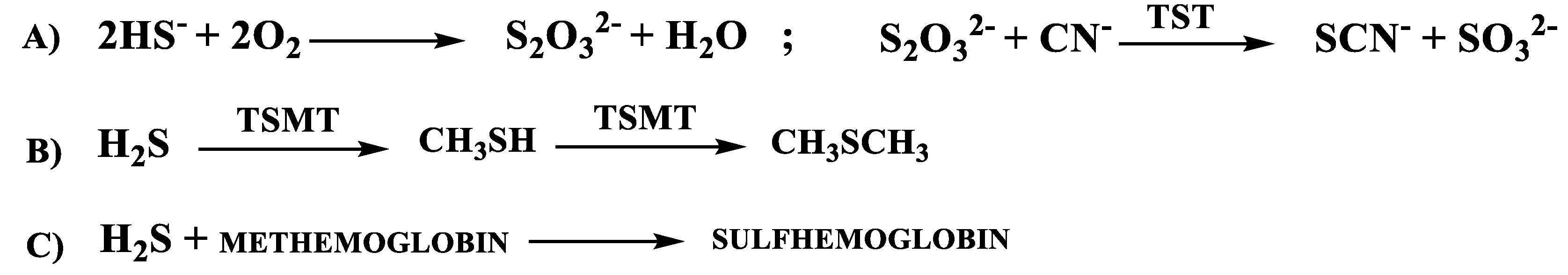
1.2.2. H2S Catabolic Pathways
1.3. Hydrogen Sulfide: Biological Functions
1.3.1. Cardiovascular System
1.3.2. Immune System and Inflammation
1.3.3. Respiratory System
1.3.4. Central Nervous System
1.3.5. Reproductive System
1.3.6. Gastrointestinal System
1.4. Hydrogen Sulfide and Diseases
2. H2S Donors
2.1. Gaseous H2S
2.2. Inorganic Salts
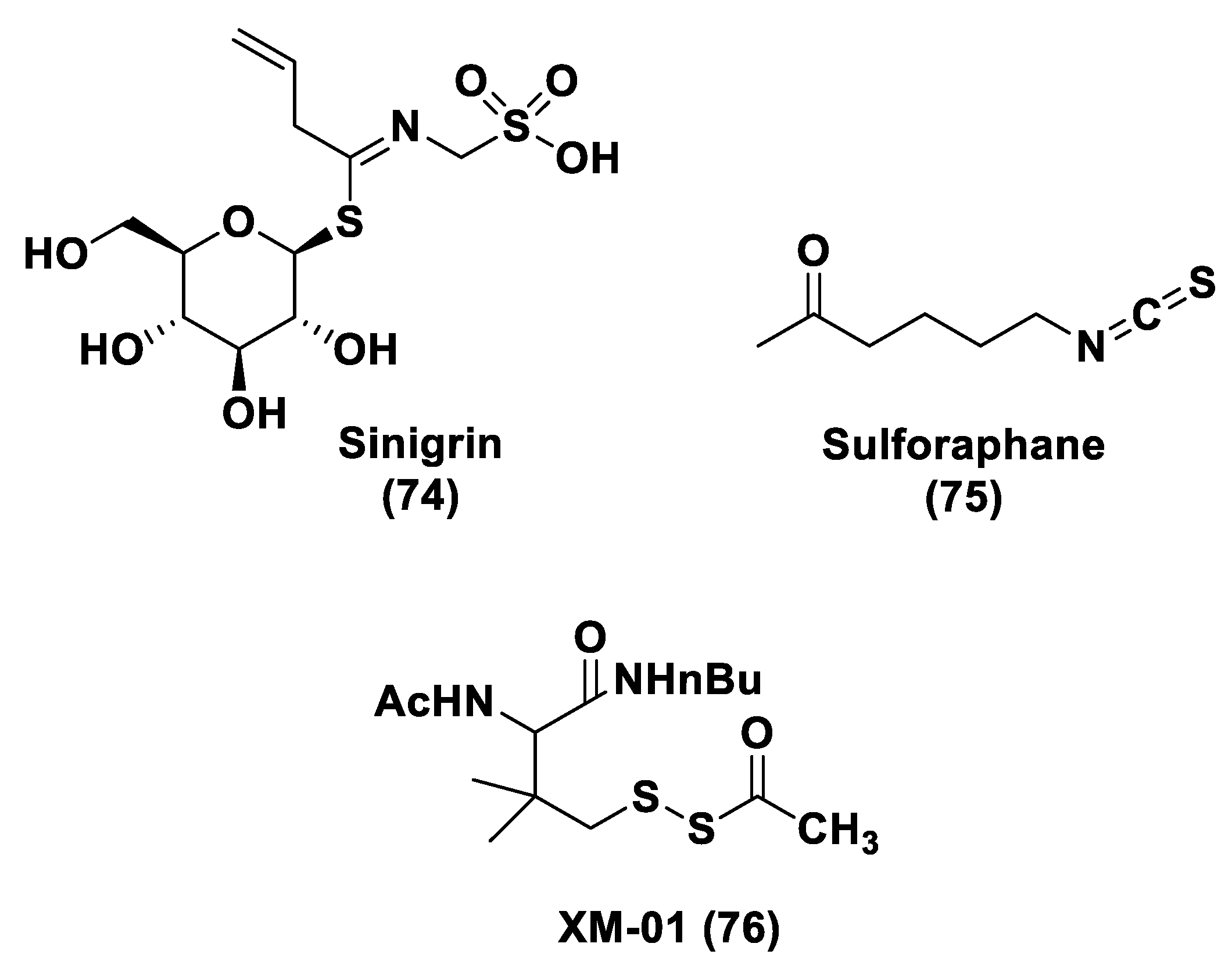
2.3. Natural H2S Donors
3. H2S Donors and Diseases
3.1. H2S Donors and Cardiovascular Diseases
3.1.1. Lawesson’s Reagent and Derivatives
3.1.2. DTTs
3.1.3. Thiol-Activated H2S Donors
N-Mercapto-Based Donors
S-Aroylthiooximes
Perthiol-Based Donors
Dithioperoxy-Anhydrides
Thioamide- and Aryl Isothiocyanate-Based Donors
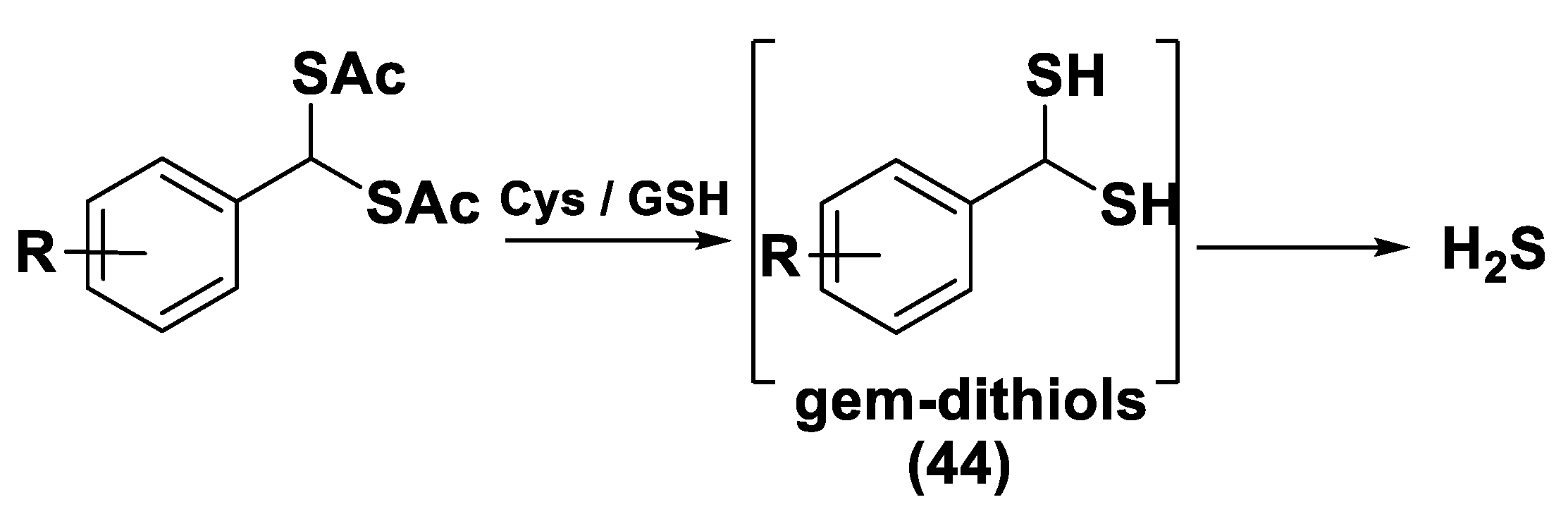
gem-Dithiol-Based H2S Donors
3.1.4. Enzyme-Triggered Donors
3.1.5. Other H2S Donors in Cardiovascular Diseases
3.2. H2S Donors and Cancer
3.3. H2S Donors and Nervous System Diseases
3.4. H2S Donors and Gastrointestinal Diseases
3.5. H2S Donors and Dermatological Diseases
3.6. H2S Donors and Viral Infections
3.7. H2S Donors and Other Diseases
3.8. Other H2S Donors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evans, C.L. The toxicity of hydrogen sulphide and other sulphides. J. Exp. Physiol. 1967, 52, 231–248. [Google Scholar] [CrossRef]
- Li, Q.; Lancaster, J.R., Jr. Chemical foundations of hydrogen sulfide biology. Nitric Oxide 2013, 35, 21–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindenmann, J.; Matzi, V.; Neuboeck, N.; Ratzenhofer-Komenda, B.; Maier, A.; Smolle-Juettner, F.M. Severe hydrogen sulphide poisoning treated with 4-dimethylaminophenol and hyperbaric oxygen. Diving Hyperb. Med. 2010, 40, 213–217. [Google Scholar] [PubMed]
- Vandiver, M.S.; Snyder, S.H. Hydrogen sulfide: A gasotransmitter of clinical relevance. J. Mol. Med. 2012, 90, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Wang, R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Moore, P.K. Hydrogen sulfide and cell signaling. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 169–187. [Google Scholar] [CrossRef] [Green Version]
- Szabo, C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007, 6, 917–935. [Google Scholar] [CrossRef]
- Kolluru, G.K.; Shen, X.; Bir, S.C.; Kevil, C.G. Hydrogen sulfide chemical biology: Pathophysiological roles and detection. Nitric Oxide 2013, 35, 5–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuto, J.M.; Carrington, S.J.; Tantillo, D.J.; Harrison, J.G.; Ignarro, L.J.; Freeman, B.A.; Chen, A.; Wink, D.A. Small molecule signaling agents: The integrated chemistry and biochemistry of nitrogen oxides, oxides of carbon, dioxygen, hydrogen sulfide, and their derived species. Chem. Res. Toxicol. 2012, 25, 769–793. [Google Scholar] [CrossRef]
- Olson, K.R.; Donald, J.A.; Dombkowski, R.A.; Perry, S.F. Evolutionary and comparative aspects of nitric oxide, carbon monoxide and hydrogen sulfide. Respir. Physiol. Neurobiol. 2012, 184, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R. A practical look at the chemistry and biology of hydrogen sulfide. Antioxid. Redox Signal. 2012, 17, 32–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caliendo, G.; Cirino, G.; Santagada, V.; Wallace, J.L. Synthesis and biological effects of hydrogen sulfide (H2S): Development of H2S-releasing drugs as pharmaceuticals. J. Med. Chem. 2010, 53, 6275–6286. [Google Scholar] [CrossRef]
- Vitvitsky, V.; Miljkovic, J.L.; Bostelaar, T.; Adhikari, B.; Yadav, P.K.; Steiger, A.K.; Torregrossa, R.; Pluth, M.D.; Whiteman, M.; Banerjee, R.; et al. Cytochrome c Reduction by H2S Potentiates Sulfide Signaling. ACS Chem. Biol. 2018, 13, 2300–2307. [Google Scholar] [CrossRef]
- Filipovic, M.R.; Miljkovic, J.L.; Nauser, T.; Royzen, M.; Klos, K.; Shubina, T.; Koppenol, W.H.; Lippard, S.J.; Ivanovic-Burmazovic, I. Chemical characterization of the smallest S-nitrosothiol, HSNO; cellular cross-talk of H2S and S-nitrosothiols. J. Am. Chem. Soc. 2012, 134, 12016–12027. [Google Scholar] [CrossRef]
- Carballal, S.; Trujillo, M.; Cuevasanta, E.; Bartesaghi, S.; Moller, M.N.; Folkes, L.K.; Garcia-Bereguian, M.A.; Gutierrez-Merino, C.; Wardmann, P.; Denicola, A.; et al. Reactivity of hydrogen sulfide with peroxynitrite and other oxidants of biological interest. Free Radic. Biol. Med. 2011, 50, 196–205. [Google Scholar] [CrossRef]
- Eberhardt, M.; Dux, M.; Namer, B.; Miljkovic, J.; Cordasic, N.; Will, C.; Kichko, T.I.; de la Roche, J.; Fischer, M.; Suárez, S.A.; et al. H2S and NO cooperatively regulate vascular tone by activating a neuroendocrine HNO–TRPA1–CGRP signalling pathway. Nat. Commun. 2014, 5, 4381. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical Biology of H2S Signaling through Persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, C.E.; Carroll, K.S. Cysteine-mediated redox signaling: Chemistry, biology, and tools for discovery. Chem. Rev. 2013, 113, 4633–4679. [Google Scholar] [CrossRef]
- Pan, J.; Carroll, K.S. Persulfide reactivity in the detection of protein S-sulfhydration. ACS Chem. Biol. 2013, 8, 1110–1116. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Macinkovic, I.; Devarie-Baez, N.O.; Pan, J.; Park, C.M.; Carroll, K.S.; Filipovic, M.R.; Xian, M. Detection of protein S-sulfhydration by a tag-switch technique. Angew. Chem. Int. Ed. 2014, 53, 575–581. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, A.K.; Gadalla, M.M.; Sen, N.; Kim, S.; Mu, W.; Gazi, S.K.; Barrow, R.K.; Yang, G.; Wang, R.; Snyder, S.H. H2S signals through protein S-sulfhydration. Sci. Signal. 2009, 2, ra72. [Google Scholar] [CrossRef] [Green Version]
- Gadalla, M.M.; Snyder, S.H. Hydrogen sulfide as a gasotransmitter. J. Neurochem. 2010, 113, 14–26. [Google Scholar] [CrossRef] [Green Version]
- Sen, N.; Paul, B.D.; Gadalla, M.M.; Mustafa, A.K.; Sen, T.; Xu, R.; Kim, S.; Snyder, S.H. Hydrogen sulfide-linked sulfhydration of NF-κb mediates its antiapoptotic actions. Mol. Cell 2012, 45, 13–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, N.; Fu, C.; Pappin, D.J.; Tonks, N.K. H2S-Induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Sci. Signal. 2011, 4, ra86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandiver, M.S.; Paul, B.D.; Xu, R.; Karuppagounder, S.; Rao, F.; Snowman, A.M.; Ko, H.S.; Lee, Y.I.; Dawson, V.L.; Dawson, T.M.; et al. Sulfhydration mediates neuroprotective actions of parkin. Nat. Commun. 2013, 4, 1626. [Google Scholar] [CrossRef] [Green Version]
- Paul, B.D.; Snyder, S.H. H2S signaling through protein sulfhydration and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhao, K.; Ju, Y.; Mani, S.; Cao, Q.; Puukila, S.; Khaper, N.; Wu, L.; Wang, R. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid. Redox Signal. 2013, 18, 1906–1919. [Google Scholar] [CrossRef]
- Sasakura, K.; Hanaoka, K.; Shibuya, N.; Mikami, Y.; Kimura, Y.; Komatsu, T.; Ueno, T.; Terai, T.; Kimura, H.; Nagano, T. Development of a highly selective fluorescence probe for hydrogen sulfide. J. Am. Chem. Soc. 2011, 133, 18003–18005. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Block, E.; Li, Z.; Connelly, T.; Zhang, J.; Huang, Z.; Su, X.; Pan, Y.; Wu, L.; Chi, Q.; et al. Crucial role of copper in detection of metal-coordinating odorants. Proc. Natl. Acad. Sci. USA 2012, 109, 3492–3497. [Google Scholar] [CrossRef] [Green Version]
- Blackstone, E.; Morrison, M.; Roth, M.B. H2S induces a suspended animation-like state in mice. Science 2005, 308, 518. [Google Scholar] [CrossRef] [Green Version]
- Collman, J.P.; Ghosh, S.; Dey, A.; Decre´au, R.A. Using a functional enzyme model to understand the chemistry behind hydrogen sulfide induced hibernation. Proc. Natl. Acad. Sci. USA 2009, 106, 22090–22095. [Google Scholar] [CrossRef] [Green Version]
- Kraus, J.P.; Williamson, C.L.; Firgaira, F.A.; Yang-Feng, T.L.; Münke, M.; Francke, U.; Bukovska, G.; Rosenberg, L.E. Cloning and sequencing nanogram amounts of immunopurified mRNAs: cDNA cloning and chromosomal mapping of cystathionine β-synthase and the β subunit of propionyl CoA carboxylase. Proc. Natl. Acad. Sci. USA 1986, 83, 2047–2051. [Google Scholar] [CrossRef] [Green Version]
- Alexander, F.W.; Sandmeier, E.; Mehta, P.K.; Christen, P. Evolutionary relationships among pyridoxal-5′-phosphate-dependent enzymes. Regio-specific alpha, beta and gamma families. Eur. J. Biochem. 1994, 219, 953–960. [Google Scholar] [CrossRef]
- Sun, Q.; Collins, R.; Huang, S.; Holmberg-Schiavone, L.; Anand, G.S.; Tan, C.H.; van-den-Berg, S.; Deng, L.W.; Moore, P.K.; Karlberg, T.; et al. Structural basis for the inhibition mechanism of human cystathionine-gammalyase: An enzyme responsible for the production of H2S. J. Biol. Chem. 2009, 284, 3076–3085. [Google Scholar] [CrossRef] [Green Version]
- Messerschmidt, A.; Worbs, M.; Steegborn, C.; Wahl, M.C.; Huber, R.; Laber, B.; Clausen, T. Determinants of enzymatic specificity in the Cys-Met metabolism PLPdependent enzyme family: Crystal structure of cystathionine γ-lyase from yeast and intrafamiliar structure comparison. Biol. Chem. 2003, 384, 373–386. [Google Scholar] [CrossRef]
- Huang, S.; Chua, J.H.; Yew, W.S.; Sivaraman, J.; Moore, P.K.; Tan, C.H.; Deng, L.W. Site-directed mutagenesis on human Cystathionine-γ-lyase reveals insights into the modulation of H2S production. J. Mol. Biol. 2010, 396, 708–718. [Google Scholar] [CrossRef]
- DeLano, W.L. The PyMOL Molecular Graphics System; DeLano Scientific LLC: Palo Alto, CA, USA, 2002. [Google Scholar]
- Stipanuk, M.H. Sulfur amino acid metabolism: Pathways for production and removal of homocysteine and cysteine. Annu. Rev. Nutr. 2004, 24, 539–577. [Google Scholar] [CrossRef]
- Singh, S.; Padovani, D.; Leslie, R.A.; Chiku, T.; Banerjee, R. Relative contributions of cystathionine beta-synthase and gamma-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J. Biol. Chem. 2009, 284, 22457–22466. [Google Scholar] [CrossRef] [Green Version]
- Kabil, O.; Banerjee, R. The redox biochemistry of hydrogen sulfide. J. Biol. Chem. 2010, 285, 21903–21907. [Google Scholar] [CrossRef] [Green Version]
- Stipanuk, M.H.; Beck, P.W. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem. J. 1982, 206, 267–277. [Google Scholar] [CrossRef] [Green Version]
- Kimura, H. Hydrogen sulfide: Its production, release and functions. Amino Acids 2011, 41, 113–121. [Google Scholar] [CrossRef]
- Olson, K.R.; Whitfield, N.L.; Bearden, S.E.; St Leger, J.; Nilson, E.; Gao, Y.; Madden, J.A. Hypoxic pulmonary vasodilation: A paradigm shift with a hydrogen sulfide mechanism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Kamoun, P. Endogenous production of hydrogen sulfide in mammals. Amino Acids 2004, 26, 243–254. [Google Scholar] [CrossRef]
- Mikami, Y.; Shibuya, N.; Kimura, Y.; Nagahara, N.; Ogasawara, Y.; Kimura, H. Thioredoxin and dihydrolipoic acid are required for 3-mercaptopyruvate sulfurtransferase to produce hydrogen sulfide. Biochem. J. 2011, 439, 479–485. [Google Scholar] [CrossRef] [Green Version]
- Whiteman, M.; Le Trionnaire, S.; Chopra, M.; Fox, B.; Whatmore, J. Emerging role of hydrogen sulfide in health and disease: Critical appraisal of biomarkers and pharmacological tools. Clin. Sci. 2011, 121, 459–488. [Google Scholar] [CrossRef]
- Morikawa, T.; Kajimura, M.; Nakamura, T.; Hishiki, T.; Nakanishi, T.; Yukutake, Y.; Nagahata, Y.; Ishikawa, M.; Hattori, K.; Takenouchi, T.; et al. Hypoxic regulation of the cerebral microcirculation is mediated by a carbon monoxide-sensitive hydrogen sulfide pathway. Proc. Natl. Acad. Sci. USA 2012, 109, 1293–1298. [Google Scholar] [CrossRef] [Green Version]
- Searcy, D.G.; Lee, S.H. Sulfur reduction by human erythrocytes. J. Exp. Zool. 1998, 282, 310–322. [Google Scholar] [CrossRef]
- Benavides, G.A.; Squadrito, G.L.; Mills, R.W.; Patel, H.D.; Isbell, T.S.; Patel, R.P.; DarleyUsmar, V.M.; Doeller, J.; Kraus, D.W. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. USA 2007, 104, 17977–17982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belardinelli, M.C.; Chabli, A.; Chadefaux-Vekemans, B.; Kamoun, P. Urinary sulfur compounds in Down syndrome. Clin. Chem. 2001, 47, 1500–1501. [Google Scholar] [CrossRef]
- Levitt, M.D.; Furne, J.; Springfield, J.; Suarez, F.; DeMaster, E. Detoxification of hydrogen sulfide and methanethiol in the cecal mucosa. J. Clin. Investig. 1999, 104, 1107–1114. [Google Scholar] [CrossRef] [Green Version]
- Picton, R.; Eggo, M.C.; Merrill, G.A.; Langman, M.J.; Singh, S. Mucosal protection against sulfide: Importance of the enzyme rhodanese. Gut 2002, 50, 201–205. [Google Scholar] [CrossRef] [Green Version]
- Kurzban, G.P.; Chu, L.; Ebersole, J.L.; Holt, S.C. Sulfhemoglobin formation in human erythrocytes by cystalysin, an l-cysteine desulfhydrase from Treponema denticola. Oral Microbiol. Immunol. 1999, 14, 153–164. [Google Scholar] [CrossRef]
- Zanardo, R.C.; Brancaleone, V.; Distrutti, E.; Fiorucci, S.; Cirino, G.; Wallace, J.L. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006, 20, 2118–2120. [Google Scholar] [CrossRef]
- Yonezawa, D.; Sekiguchi, F.; Miyamoto, M.; Taniguchi, E.; Honjo, M.; Masuko, T.; Nishikawa, H.; Kawabata, A. A protective role of hydrogen sulfide against oxidative stress in rat gastric mucosal epithelium. Toxicology 2007, 241, 11–18. [Google Scholar] [CrossRef]
- Abe, K.; Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996, 16, 1066–1071. [Google Scholar] [CrossRef] [Green Version]
- Laggner, H.; Hermann, M.; Esterbauer, H.; Muellner, M.K.; Exner, M.; Gmeiner, B.M.; Kapiotis, S. The novel gaseous vasorelaxant hydrogen sulphide inhibits angiotensin-converting enzyme activity of endothelial cells. J. Hypertens. 2007, 25, 2100–2104. [Google Scholar] [CrossRef] [PubMed]
- Sivarajah, A.; Collino, M.; Yasin, M.; Benetti, E.; Gallicchio, M.; Mazzon, E.; Cuzzocrea, S.; Fantozzi, R.; Thiemermann, C. Anti-apoptotic and anti-inflammatory effects of hydrogen sulfide in a rat model of regional myocardial I/R. Shock 2009, 31, 267–274. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Y.; Chen, S.; Tang, C.; Wang, G.; Du, J.; Jin, J. Hydrogen sulfide regulates insulin secretion and insulin resistance in diabetes mellitus, a new promising target for diabetes mellitus treatment? A review. J. Adv. Res. 2021, 27, 19–30. [Google Scholar] [CrossRef]
- Lefer, D.J. A new gaseous signaling molecule emerges: Cardioprotective role of hydrogen sulfide. Proc. Natl. Acad. Sci. USA 2007, 46, 17907–17908. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Ndisang, J.F.; Tang, G.; Cao, K.; Wang, R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H2316–H2323. [Google Scholar] [CrossRef]
- Yang, G.; Wu, L.; Jiang, B.; Yang, W.; Qi, J.; Cao, K.; Meng, Q.; Mustafa, A.K.; Mu, W.; Zhang, S.; et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionin gamma-lyase. Science 2008, 322, 587–590. [Google Scholar] [CrossRef] [Green Version]
- Zagli, G.; Patacchini, R.; Trevisani, M.; Abbate, R.; Cinotti, S.; Gensini, G.F.; Masotti, G.; Geppetti, P. Hydrogen sulfide inhibits human platelet aggregation. Eur. J. Pharmacol. 2007, 559, 65–68. [Google Scholar] [CrossRef]
- Fiorucci, S.; Antonelli, E.; Distrutti, E.; Rizzo, G.; Mencarelli, A.; Orlandi, S.; Zanardo, R.; Renga, B.; Di Sante, M.; Morelli, A.; et al. Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastroenterology 2005, 129, 1210–1224. [Google Scholar] [CrossRef] [Green Version]
- Perini, R.; Fiorucci, S.; Wallace, J.L. Mechanisms of nonsteroidal anti-inflammatory drug-induced gastrointestinal injury and repair: A window of opportunity for cyclooxygenase-inhibiting nitric oxide donors. Can. J. Gastroenterol. 2004, 18, 229–236. [Google Scholar] [CrossRef]
- Wallace, J.L.; Caliendo, G.; Santagada, V.; Cirino, G.; Fiorucci, S. Gastrointestinal safety and anti-inflammatory effects of a hydrogen sulfide-releasing diclofenac derivative in the rat. Gastroenterology 2007, 132, 261–271. [Google Scholar] [CrossRef]
- Fu, Z.; Liu, X.; Geng, B.; Fang, L.; Tang, C. Hydrogen sulfide protects rat lung from ischemia-reperfusion injury. Life Sci. 2008, 82, 1196–1202. [Google Scholar] [CrossRef]
- Zhang, C.; Du, J.; Bu, D.; Yan, H.; Tang, X.; Tang, C. The regulatory effect of hydrogen sulphide on hypoxic pulmonary hypertension in rats. Biochem. Biophys. Res. Commun. 2003, 302, 810–816. [Google Scholar]
- Wei, H.L.; Zhang, C.Y.; Jin, H.F.; Tang, C.S.; Du, J.B. Hydrogen sulphide regulates lung tissue oxidized glutathione and total antioxidant capacity in hypoxic pulmonary hypertensive rats. Acta Pharmacol. Sin. 2008, 29, 670–676. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.F.; Liang, C.; Liang, J.M.; Tang, C.S.; Du, J.B. Effects of hydrogen sulphide on vascular inflammation in pulmonary hypertension induced by high pulmonary blood flow: Experiment with rats. Zhonghua Yi Xue Za Zhi 2008, 88, 2235–2239. [Google Scholar]
- Chen, Y.H.; Wu, R.; Geng, B.; Qi, Y.F.; Wang, P.P.; Yao, W.Z.; Tang, C.S. Endogenous hydrogen sulfide reduces airway inflammation and remodeling in a rat model of asthma. Cytokine 2009, 45, 117–123. [Google Scholar] [CrossRef]
- Nicholson, C.K.; Calvert, J.W. Hydrogen Sulfide and Ischemia—Reperfusion Injury. Pharmacol. Res. 2010, 62, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.; Yang, W.; Zhang, H.; Song, Z.; Liu, T.; Lv, X. Hydrogen Sulfide Ameliorates Lung Ischemia-Reperfusion Injury through SIRT1 Signaling Pathway in Type 2 Diabetic Rats. Front. Physiol. 2020, 11, 596. [Google Scholar] [CrossRef]
- Kimura, H. Hydrogen sulfide induces cyclic AMP and modulates the NMDA receptor. Biochem. Biophys. Res. Commun. 2000, 267, 129–133. [Google Scholar] [CrossRef]
- Kimura, H.; Nagai, Y.; Umemura, K.; Kimura, Y. Physiological roles of hydrogen sulfide: Synaptic modulation, neuroprotection, and smooth muscle relaxation. Antioxid. Redox Signal. 2005, 7, 795–803. [Google Scholar] [CrossRef]
- Tan, B.H.; Wong, P.T.-H.; Bian, J.S. Hydrogen sulphide: A novel signalling molecule in the central nervous system. Neurochem. Int. 2010, 56, 3–10. [Google Scholar] [CrossRef]
- Hu, L.F.; Wong, P.T.; Moore, P.K.; Bian, J.S. Hydrogen sulphide attenuates lipopolysaccharide-induced inflammation by inhibition of p38 mitogen-activated protein kinase in microglia. J. Neurochem. 2007, 100, 1121–1128. [Google Scholar] [CrossRef]
- Roux, P.P.; Blenis, J. ERK and p38 MAPK-activated protein kinases: A family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 2004, 68, 320–344. [Google Scholar] [CrossRef] [Green Version]
- d’Emmanuele di Villa Bianca, R.; Sorrentino, R.; Maffia, P.; Mirone, V.; Imbimbo, C.; Fusco, F.; De Palma, R.; Ignarro, L.J.; Cirino, G. Hydrogen sulfide as a mediator of human corpus cavernosum smooth-muscle relaxation. Proc. Natl. Acad. Sci. USA 2009, 106, 4513–4518. [Google Scholar] [CrossRef] [Green Version]
- Fiorucci, S.; Santucci, L.; Distrutti, E. NSAIDs, coxibs, CINOD and H2S-releasing NSAIDs: What lies beyond the horizon. Dig. Liver Dis. 2007, 39, 1043–1051. [Google Scholar] [CrossRef]
- Wallace, J.L.; Vong, L. NSAID-induced gastrointestinal damage and the design of GI-sparing NSAIDs. Curr. Opin. Investig. Drugs 2008, 9, 1151–1156. [Google Scholar] [CrossRef]
- Wallace, J.L. Building a better aspirin: Gaseous solutions to a century-old problem. Br. J. Pharmacol. 2007, 152, 421–428. [Google Scholar] [CrossRef] [Green Version]
- Wallace, J.L.; Ianaro, A.; de Nucci, G. Gaseous Mediators in Gastrointestinal Mucosal Defense and Injury. Dig. Dis. Sci. 2017, 62, 2223–2230. [Google Scholar] [CrossRef] [Green Version]
- Glowacka, U.; Brzozowski, T.; Magierowski, M. Synergisms, discrepancies and interactions between hydrogen sulfide and carbon monoxide in the gastrointestinal and digestive system physiology, pathophysiology and pharmacology. Biomolecules 2020, 10, 445. [Google Scholar] [CrossRef] [Green Version]
- Sulaieva, O.; Wallace, J.L. Gaseous mediator-based anti-inflammatory drugs. Curr. Opin. Pharm. 2015, 25, 1–6. [Google Scholar] [CrossRef]
- Distrutti, E.; Sediari, L.; Mencarelli, A.; Renga, B.; Orlandi, S.; Russo, G.; Caliendo, G.; Santagada, V.; Cirino, G.; Wallace, J.L.; et al. 5-Amino-2-hydroxybenzoic acid 4-(5-thioxo-5H-[1,2]dithiol-3yl)-phenyl ester (ATB-429), a hydrogen sulfide-releasing derivative of mesalamine, exerts antinociceptive effects in a model of postinflammatory hypersensitivity. J. Pharm. Exp. 2006, 319, 447–458. [Google Scholar] [CrossRef] [Green Version]
- Ekundi-Valentim, E.; Santos, K.T.; Camargo, E.A.; Denadai-Souza, A.; Teixeira, S.A.; Zanoni, C.I.; Grant, A.D.; Wallace, J.; Muscara, M.N.; Costa, S.K. Differing effects of exogenous and endogenous hydrogen sulphide in carrageenan-induced knee joint synovitis in the rat. Br. J. Pharmacol. 2010, 159, 1463–1474. [Google Scholar] [CrossRef] [Green Version]
- Distrutti, E.; Sediari, L.; Mencarelli, A.; Renga, B.; Orlandi, S.; Antonelli, E.; Roviezzo, F.; Morelli, A.; Cirino, G.; Wallace, J.L.; et al. Evidence that hydrogen sulfide exerts antinociceptive effects in the gastrointestinal tract by activating KATP channels. J. Pharmacol. Exp. Ther. 2006, 316, 325–335. [Google Scholar] [CrossRef] [Green Version]
- Citi, V.; Martelli, A.; Gorica, E.; Brogi, S.; Testai, L.; Calderone, V. Role of hydrogen sulfide in endothelial dysfunction: Pathophysiology and therapeutic approaches. J. Adv. Res. 2021, 27, 99–113. [Google Scholar] [CrossRef]
- Testai, L.; Citi, V.; Martelli, A.; Brogi, S.; Calderone, V. Role of hydrogen sulfide in cardiovascular ageing. Pharmacol. Res. 2020, 160, 105–125. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, F.; Yin, J.; Wu, S.; Zhou, X. Protective mechanisms of hydrogen sulfide in myocardial ischemia. J. Cell. Physiol. 2020, 235, 9059–9070. [Google Scholar] [CrossRef]
- Kang, S.C.; Sohn, E.H.; Lee, S.R. Hydrogen Sulfide as a Potential Alternative for the Treatment of Myocardial Fibrosis. Oxid. Med. Cell. Longev. 2020, 23, 4105382. [Google Scholar] [CrossRef]
- Roorda, M.; Miljkovic, J.L.; van Goor, H.; Henning, R.H.; Bouma, H.R. Spatiotemporal regulation of hydrogen sulfide signaling in the kidney. Redox Biol. 2021, 43, 101961. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Tain, Y.L. Hydrogen Sulfide in Hypertension and Kidney Disease of Developmental Origins. Int. J. Mol. Sci. 2018, 19, 1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corvino, A.; Frecentese, F.; Magli, E.; Perissutti, E.; Santagada, V.; Scognamiglio, A.; Caliendo, G.; Fiorino, F.; Severino, B. Trends in H2S-Donors Chemistry and Their Effects in Cardiovascular Diseases. Antioxidants 2021, 10, 429. [Google Scholar] [CrossRef] [PubMed]
- Pieretti, J.C.; Cruz Junho, C.V.; Sorelli Carneiro-Ramos, M.; Barozzi Seabra, A. H2S- and NO-releasing gasotransmitter platform: A crosstalk signaling pathway in the treatment of acute kidney injury. Pharmacol. Res. 2020, 161, 105121. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, W.; Moore, P.K.; Bian, J. Protective Smell of Hydrogen Sulfide and Polysulfide in Cisplatin-Induced Nephrotoxicity. Int. J. Mol. Sci. 2019, 20, 313. [Google Scholar] [CrossRef] [Green Version]
- Zhong, H.; Yu, H.; Chen, J.; Sun, J.; Guo, L.; Huang, P.; Zhong, Y. Hydrogen Sulfide and Endoplasmic Reticulum Stress: A Potential Therapeutic Target for Central Nervous System Degeneration Diseases. Front. Pharmacol. 2020, 11, 702. [Google Scholar] [CrossRef]
- Gambari, L.; Grigolo, B.; Grassi, F. Hydrogen Sulfide in Bone Tissue Regeneration and Repair: State of the Art and New Perspectives. Int. J. Mol. Sci. 2019, 20, 5231. [Google Scholar] [CrossRef] [Green Version]
- Coavoy-Sánchez, S.A.; Costa, S.K.P.; Muscará, M.N. Hydrogen sulfide and dermatological diseases. Br. J. Pharmacol. 2020, 177, 857–865. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Ding, L.; Xie, Z.Z.; Yang, Y.; Whiteman, M.; Moore, P.K.; Bian, J.S. A Review of Hydrogen Sulfide Synthesis, Metabolism, and Measurement: Is Modulation of Hydrogen Sulfide a Novel Therapeutic for Cancer? Antioxid. Redox Signal. 2019, 31, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Dattilo, M. The role of host defences in Covid 19 and treatments thereof. Mol. Med. 2020, 26, 90. [Google Scholar] [CrossRef] [PubMed]
- Citi, V.; Martelli, A.; Brancaleone, V.; Brogi, S.; Gojon, G.; Montanaro, R.; Morales, G.; Testai, L.; Calderone, V. Anti-inflammatory and antiviral roles of hydrogen sulfide: Rationale for considering H2S donors in COVID-19 therapy. Br. J. Pharmacol. 2020, 177, 4931–4941. [Google Scholar] [CrossRef]
- Liu, H.; Anders, F.; Thanos, S.; Mann, C.; Liu, A.; Grus, F.H.; Pfeiffer, N.; Prokosch-Willing, V. Hydrogen Sulfide Protects Retinal Ganglion Cells Against Glaucomatous Injury In Vitro and In Vivo. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5129–5141. [Google Scholar] [CrossRef] [Green Version]
- Struve, M.F.; Brisbois, J.N.; James, R.A.; Marshall, M.W.; Dorman, D.C. Neurotoxicological effects associated with short-term exposure of Sprague-Dawley rats to hydrogen sulfide. Neurotoxicology 2001, 22, 375–385. [Google Scholar] [CrossRef]
- Volpato, G.P.; Searles, R.; Yu, B.; Scherrer-Crosbie, M.; Bloch, K.D.; Ichinose, F.; Zapol, W.M. Inhaled Hydrogen Sulfide: A Rapidly Reversible Inhibitor of Cardiac and Metabolic Function in the Mouse. Anesthesiology 2008, 108, 659–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faller, S.; Ryter, S.W.; Choi, A.M.K.; Loop, T.; Schmidt, R.; Hoetzel, A. Inhaled hydrogen sulfide protects against ventilator-induced lung injury. Anesthesiology 2010, 113, 104–115. [Google Scholar] [CrossRef] [Green Version]
- Wagner, F.; Wagner, K.; Weber, S.; Stahl, B.; Knöferl, M.W.; Huber-Lang, M.; Seitz, D.H.; Asfar, P.; Calzia, E.; Senftleben, U.; et al. Inflammatory Effects of Hypothermia and Inhaled H2S During Resuscitated, Hyperdynamic Murine Septic Shock. Shock 2011, 35, 396–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackstone, E.; Roth, M.B. Suspended animation-like state protects mice from lethal hypoxia. Shock 2007, 27, 370–372. [Google Scholar] [CrossRef]
- Xue, R.; Hao, D.; Sun, J.; Li, W.; Zhao, M.; Li, X.; Chen, Y.; Zhu, J.; Ding, Y.; Liu, J.; et al. Hydrogen Sulfide Treatment Promotes Glucose Uptake by Increasing Insulin Receptor Sensitivity and Ameliorates Kidney Lesions in Type 2 Diabetes. Antioxid. Redox Signal. 2013, 19, 5. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.Y.; Morris, J.C. Kinetics of oxidation of aqueous sulfide by oxygen. Environ. Sci. Technol. 1972, 6, 537. [Google Scholar] [CrossRef]
- Hughes, M.N.; Centelles, M.N.; Moore, K.P. Making and working with hydrogen sulfide. Free Radic. Biol. Med. 2009, 47, 1346–1353. [Google Scholar] [CrossRef]
- Calvert, J.W.; Elston, M.; Nicholson, C.K.; Gundewar, S.; Jha, S.; Elrod, J.W.; Ramachandran, A.; Lefer, D.J. Genetic and Pharmacologic Hydrogen Sulfide Therapy Attenuates Ischemia-Induced Heart Failure in Mice. Circulation 2010, 122, 11. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef] [Green Version]
- Mard, S.A.; Neisi, N.; Solgi, G.; Hassanpour, M.; Darbor, M.; MaMaleki, M. Gastroprotective effect of NaHS against mucosal lesions induced by ischemia-reperfusion injury in rat. Dig. Dis. Sci. 2012, 57, 1496–1503. [Google Scholar] [CrossRef]
- Gao, C.; Xu, D.Q.; Gao, C.J.; Ding, Q.; Yao, L.N.; Li, Z.C.; Chai, W. An exogenous hydrogen sulphide donor, NaHS, inhibits the nuclear factor κB inhibitor kinase/nuclear factor κB inhibitor/nuclear factor- κB signaling pathway and exerts cardioprotective effects in a rat hemorrhagic shock model. Biol. Pharm. Bull. 2012, 35, 1029–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Wang, M.J.; Jin, S.; Bai, Y.D.; Hou, C.L.; Ma, F.F.; Li, X.H.; Zhu, Y.C. The H2S donor NaHS changes the expression pattern of H2S-Producing enzymes after myocardial infarction. Oxid. Med. Cell. Longev. 2016, 2016, 6492469. [Google Scholar] [CrossRef] [Green Version]
- Esechie, A.; Kiss, L.; Olah, G.; Horvath, E.M.; Hawkins, H.; Szabo, C.; Traber, D.L. Protective effect of hydrogen sulfide in a murine model of combined burn and smoke inhalation-induced acute lung injury. Clin. Sci. 2008, 115, 91. [Google Scholar] [CrossRef] [Green Version]
- Kloesch, B.; Liszt, M.; Steiner, G.; Broll, J. Inhibitors of p38 and ERK1/2 MAPkinase and hydrogen sulphide block constitutive and IL-1β-induced IL-6 and IL-8 expression in the human chondrocyte cell line C-28/I2. Rheumatol. Int. 2012, 32, 729. [Google Scholar] [CrossRef] [PubMed]
- Andruski, B.; McCafferty, D.M.; Ignacy, T.; Millen, B.; McDougall, J.J. Leukocyte trafficking and pain behavioral responses to a hydrogen sulfide donor in acute monoarthritis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, 814–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Bhatia, M.; Zhu, Y.Z.; Zhu, Y.C.; Ramnath, R.D.; Wang, Z.J.; Anuar, F.B.M.; Whiteman, M.; Salto-Tellez, M.; Moore, P.K. Hydrogen sulfide is a novel mediator of lipopolysaccharideinduced inflammation in the mouse. FASEB J. 2005, 19, 1196–1198. [Google Scholar] [CrossRef]
- Bhatia, M.; Wong, F.L.; Fu, D.; Lau, H.Y.; Moochhala, S.M.; Moore, P.K. Role of hydrogen sulfide in acute pancreatitis and associated lung injury. FASEB J. 2005, 19, 623–625. [Google Scholar] [CrossRef]
- Mok, Y.Y.; Moore, P.K. Hydrogen sulfide is pro-inflammatory in haemorrhagic shock. Inflamm. Res. 2008, 57, 512–528. [Google Scholar] [CrossRef]
- Mok, Y.Y.; Atan, M.S.; Yoke Ping, C.; Zhong Jing, W.; Bhatia, M.; Moochhala, S.; Moore, P.K. Role of hydrogen sulfide in haemorrhagic shock in the rat: Protective effect of inhibitors of hydrogen sulfide biosynthesis. Br. J. Pharmacol. 2004, 143, 881–889. [Google Scholar] [CrossRef]
- Li, T.; Zhao, B.; Wang, C.; Wang, H.; Liu, Z.; Li, W.; Jin, H.; Tang, C.; Du, J. Regulatory Effects of Hydrogen Sulfide on IL-6, IL-8 and IL-10 Levels in the Plasma and Pulmonary Tissue of Rats with Acute Lung Injury. Exp. Biol. Med. 2008, 233, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.J.; West, A.B.; Dawson, V.L.; Dawson, T.M. Molecular pathophysiology of Parkinson’s disease. Annu. Rev. Neurosci. 2005, 28, 57–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, L.F.; Lu, M.; Tiong, C.X.; Dawe, G.S.; Hu, G.; Bian, J.S. Neuroprotective effects of hydrogen sulfide on Parkinson’s disease rat models. Aging Cell 2010, 9, 135–146. [Google Scholar] [CrossRef]
- Giuliani, D.; Ottani, A.; Zaffe, D.; Galantucci, M.; Strinati, F.; Lodi, R.; Guarini, S. Hydrogen sulfide slows down progression of experimental Alzheimer’s disease by targeting multiple pathophysiological mechanisms. Neurobiol. Learn. Mem. 2013, 104, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Bayan, L.; Koulivand, P.H.; Gorji, A. Garlic: A review of potential therapeutic effects. Avicenna J. Phytomed. 2014, 4, 1–14. [Google Scholar] [PubMed]
- Lawson, L.D.; Wood, S.G.; Hughes, B.G. HPLC analysis of allicin and other thiosulfinates in garlic clove homogenates. Planta Med. 1991, 57, 263–270. [Google Scholar] [CrossRef]
- Brodnitz, M.H.; Pascale, J.V.; Van Derslice, L. Flavor components of garlic extract. J. Agric. Food Chem. 1971, 19, 273–275. [Google Scholar] [CrossRef]
- Pluth, M.D.; Bailey, T.S.; Hammers, M.D.; Hartle, M.D.; Henthorn, H.A.; Steiger, A.K. Natural Products Containing Hydrogen Sulfide Releasing Moieties. Synlett 2015, 26, 2633–2643. [Google Scholar] [CrossRef]
- Liang, D.; Wu, H.; Wong, M.W.; Huang, D. Diallyl Trisulfide Is a Fast H2S Donor, but Diallyl Disulfide Is a Slow One: The Reaction Pathways and Intermediates of Glutathione with Polysulfides. Org. Lett. 2015, 17, 4196–4199. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Citi, V.; Martelli, A.; Testai, L.; Marino, A.; Breschi, M.C.; Calderone, V. Hydrogen Sulfide Releasing Capacity of Natural Isothiocyanates: Is It a Reliable Explanation for the Multiple Biological Effects of Brassicaceae? Planta Med. 2014, 80, 610–613. [Google Scholar] [CrossRef]
- Martelli, A.; Testai, L.; Citi, V.; Marino, A.; Bellagambi, F.G.; Ghimenti, S.; Breschi, M.C.; Calderone, V. Pharmacological characterization of the vascular effects of aryl isothiocyanates: Is hydrogen sulfide the real player? Vascul. Pharmacol. 2014, 60, 32–41. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, X.; Lu, Y.; Liang, D.; Huang, D. Isothiocyanates as H2S Donors Triggered by Cysteine: Reaction Mechanism and Structure and Activity Relationship. Org. Lett. 2019, 21, 5977–5980. [Google Scholar] [CrossRef]
- Ozturk, T.; Ertas, E.; Mert, O. Use of Lawesson’s Reagent in Organic Syntheses. Chem. Rev. 2007, 107, 5210–5278. [Google Scholar] [CrossRef] [PubMed]
- Lecher, H.Z.; Greenwood, R.A.; Whitehouse, K.C.; Chao, T.H. The Phosphonation of Aromatic Compounds with Phosphorus Pentasulfide. J. Am. Chem. Soc. 1956, 78, 5018. [Google Scholar] [CrossRef]
- Thomsen, I.; Clausen, K.; Scheibye, S.; Lawesson, S.O. Thiation with 2,4-bis(4-methoxyphenyl)-1,3,2,4-dithiadiphosphetane 2,4-disulfide: N-methylthiopyrrolidone. Org. Synth. 1984, 62, 158. [Google Scholar]
- Nicolau, L.A.D.; Silva, R.O.; Damasceno, S.R.B.; Carvalho, N.S.; Costa, N.R.D.; Aragão, K.S.; Barbosa, A.L.R.; Soares, P.M.G.; Souza, M.H.L.P.; Medeiros, J.V.R. The hydrogen sulfide donor, Lawesson’s reagent, prevents alendronate-induced gastric damage in rats. Braz. J. Med. Biol. Res. 2013, 46, 708–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiller, F.; Orrico, M.I.L.; Nascimento, D.C.; Czaikoski, P.G.; Souto, F.O.; Alves-Filho, J.C.; Freitas, A.; Carlos, D.; Montenegro, M.F.; Neto, A.F.; et al. Hydrogen Sulfide Improves Neutrophil Migration and Survival in Sepsis via K+ATP channel activation. Am. J. Respir. Crit. Care Med. 2010, 182, 360–368. [Google Scholar] [CrossRef]
- Li, L.; Whiteman, M.; Guan, Y.Y.; Neo, K.L.; Cheng, Y.; Lee, S.W.; Zhao, Y.; Baskar, R.; Tan, C.H.; Moore, P.K. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): New insights into the biology of hydrogen sulfide. Circulation 2008, 117, 2351–2360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, P.; Dymock, B.W.; Moore, P.K. GYY4137, a novel water-soluble, H2S-releasing molecule. Methods Enzymol. 2015, 554, 143–167. [Google Scholar]
- Zhao, Y.; Biggs, T.D.; Xian, M. Hydrogen sulfide (H2S) releasing agents: Chemistry and biological applications. Chem. Commun. 2014, 50, 11788–11805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martelli, A.; Testai, L.; Citi, V.; Marino, A.; Pugliesi, I.; Barresi, E.; Nesi, G.; Rapposelli, S.; Taliani, S.; Da Settimo, F.; et al. Arylthioamides as H2S donors: l-cysteine-activated releasing properties and vascular effects in vitro and in vivo. ACS Med. Chem. Lett. 2013, 4, 904–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karwi, Q.G.; Whiteman, M.; Wood, M.E.; Torregrossa, R.; Baxter, G.F. Pharmacological postconditioning against myocardial infarction with a slow-releasing hydrogen sulfide donor, GYY4137. Pharmacol. Res. 2016, 111, 442–451. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Wu, Y.; Meng, M.; Luo, M.; Zhao, H.; Sun, H.; Gao, S. GYY4137 protects against myocardial ischemia/reperfusion injury via activation of the PHLPP-1/Akt/Nrf2 signaling pathway in diabetic mice. J. Surg. Res. 2018, 225, 29–39. [Google Scholar] [CrossRef]
- Han, X.; Mao, Z.C.; Wang, S.; Xin, Y.; Li, P.; Maharjan, S.; Zhang, B. GYY4137 protects against MCAO via p38 MAPK mediated anti-apoptotic signaling pathways in rats. Brain Res. Bull. 2020, 158, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.E.; Coles, S.J.; Fox, B.C.; Khan, T.F.; Maliszewski, J.; Perry, A.; Pitak, M.B.; Whiteman, M.; Wood, M.E. Investigating the generation of hydrogen sulfide from the phosphonamidodithioate slow-release donor GYY4137. Med. Chem. Commun. 2015, 6, 1649. [Google Scholar] [CrossRef]
- Moore, P.K.; Whiteman, M. Chemistry, Biochemistry and Pharmacology of Hydrogen Sulfide; Springer: Berlin/Heidelberg, Germany, 2015; pp. 344–354. [Google Scholar]
- Park, C.; Zhao, Y.; Zhu, Z.; Pacheco, A.; Peng, B.; Devarie-Baez, N.O.; Bagdon, P.; Zhang, H.; Xian, M. Synthesis and evaluation of phosphorodithioate-based hydrogen sulfide donors. Mol. Biosyst. 2013, 9, 2430–2434. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Li, Z.; Organ, C.L.; Park, C.M.; Yang, C.T.; Pacheco, A.; Wang, D.; Lefer, D.J.; Xian, M. pH-controlled hydrogen sulfide release for myocardial ischemia-reperfusion injury. J. Am. Chem. Soc. 2016, 138, 6336–6339. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; Skeldon, P.; Thompson, G.E.; Wood, G.C. Synthesis and characterization of molybdenum disulphide formed from ammonium tetrathiomolybdate. J. Mater. Sci. 1997, 32, 497. [Google Scholar] [CrossRef]
- Xu, S.; Yang, C.T.; Meng, F.H.; Pacheco, A.; Chen, L.; Xian, M. Ammonium tetrathiomolybdate as a water-soluble and slow-release hydrogen sulfide donor. Bioorg. Med. Chem. Lett. 2016, 26, 1585–1588. [Google Scholar] [CrossRef] [Green Version]
- Dyson, A.; Dal-Pizzol, F.; Sabbatini, G.; Lach, A.B.; Galfo, F.; Dos Santos Cardoso, J.; Pescador Mendonça, B.; Hargreaves, I.; Bollen Pinto, B.; Bromage, D.I.; et al. Ammonium tetrathiomolybdate following ischemia/reperfusion injury: Chemistry, pharmacology, and impact of a new class of sulfide donor in preclinical injury models. PLoS Med. 2017, 14, 1002310. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhang, A.; Guo, C.; Shi, C.; Zhang, Y.; Liu, Q.; Sparatore, A.; Wang, C. S-diclofenac protects against doxorubicin-induced cardiomyopathy in mice via ameliorating cardiac gap junction remodeling. PLoS ONE 2011, 6, e26441. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Guo, W.; Lin, S.Z.; Wang, Z.J.; Kan, J.T.; Chen, S.Y.; Zhu, Y.Z. Gp130-mediated STAT3 activation by S-propargyl-cysteine, an endogenous hydrogen sulfide initiator, prevents doxorubicin-induced cardiotoxicity. Cell. Death Dis. 2016, 7, e2339. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.W.; Liang, C.; Jin, H.F.; Tang, X.Y.; Han, W.; Chai, L.J.; Zhang, C.Y.; Geng, B.; Tang, C.S.; Du, J.B. Hydrogen sulfide regulates cardiac function and structure in adriamycin-induced cardiomyopathy. Circ. J. 2009, 73, 741–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, N.; Willis, A.; Kornhauser, N.; Ward, M.M.; Lee, S.B.; Nackos, E.; Seo, B.R.; Chuang, E.; Cigler, T.; Moore, A.; et al. Influencing the tumor microenvironment: A phase II study of copper depletion using tetrathiomolybdate in patients with breast cancer at high risk for recurrence and in preclinical models of lung metastases. Clin. Cancer Res. 2017, 23, 666–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanatta, S.D.; Jarrott, B.; Williams, S.J. Synthesis and preliminary pharmacological evaluation of aryl dithiolethiones with cyclooxygenase-2-selective inhibitory activity and hydrogen sulfide-releasing properties. Aust. J. Chem. 2010, 63, 946–957. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Rossoni, G.; Sparatore, A.; Lee, L.C.; Del Soldato, P.; Moore, P.K. Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative. Free Radic. Biol. Med. 2007, 42, 706. [Google Scholar] [CrossRef]
- Rakitin, O.A. Synthesis and Reactivity of 3H-1,2-dithiole-3-thiones. Molecules 2021, 26, 3595. [Google Scholar] [CrossRef] [PubMed]
- Landis, P.S. The chemistry of 1,2-dithiole-3-thiones. Chem. Rev. 1965, 65, 237. [Google Scholar] [CrossRef]
- Curphey, T.J.; Libby, A.A. Dianions of 3-Oxo dithioic Acids: Preparation and Conversion to 3H-1,2-Dithiole-3-thiones. Tetrahedron Lett. 2000, 41, 6977. [Google Scholar] [CrossRef]
- Pedersen, B.S.; Lawesson, S.O. Syntheses of 3H-1,2-dithiole-3-thiones and 4H-1,3,2-oxazaphosphorine derivatives from the dimer of p-methoxyphenyl-thionophosphine sulfide and der. Tetrahedron 1979, 35, 2433–2437. [Google Scholar] [CrossRef]
- Hamada, T.; Nakane, T.; Kimura, T.; Arisawa, K.; Yoneda, K.; Yamamoto, T.; Osaki, T. Treatment of xerostomia with the bile secretion-stimulating drug anethole trithione: A clinical trial. Am. J. Med. Sci. 1999, 318, 146–151. [Google Scholar] [CrossRef]
- Perrino, E.; Cappelletti, G.; Tazzari, V.; Giavini, E.; Soldato, P.D.; Sparatore, A. New sulfurated derivatives of valproic acid with enhanced histone deacetylase inhibitory activity. Bioorg. Med. Chem. Lett. 2008, 18, 1893–1897. [Google Scholar] [CrossRef] [PubMed]
- Tazzari, V.; Cappelletti, G.; Casagrande, M.; Perrino, E.; Renzi, L.; Del Soldato, P.; Sparatore, A. New aryldithiolethione derivatives as potent histone deacetylase inhibitors. Bioorg. Med. Chem. 2010, 18, 4187–4194. [Google Scholar] [CrossRef]
- Sen, C.K.; Traber, K.E.; Packer, L. Inhibition of NF-jB activation in human T-cell lines by anetholdithiolthione. Biochem. Biophys. Res. Commun. 1996, 218, 148–153. [Google Scholar] [CrossRef]
- Qandil, A.M. Prodrugs of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs), More Than Meets the Eye: A Critical Review. Int. J. Mol. Sci. 2012, 13, 17244. [Google Scholar] [CrossRef] [Green Version]
- Sparatore, A.; Perrino, E.; Tazzari, V.; Giustarini, D.; Rossi, R.; Rossoni, G.; Erdman, K.; Schröder, H.; Del Soldato, P. Pharmacological profile of a novel H2S-releasing aspirin. Free Radic. Biol. Med. 2009, 46, 586–592. [Google Scholar] [CrossRef]
- Rossoni, G.; Manfredi, B.; Tazzari, V.; Sparatore, A.; Trivulzio, S.; Del Soldato, P.; Berti, F. Activity of a new hydrogen sulfide-releasing aspirin (ACS14) on pathological cardiovascular alterations induced by glutathione depletion in rats. Eur. J. Pharmacol. 2010, 648, 139–145. [Google Scholar] [CrossRef]
- Kodela, R.; Chattopadhyay, M.; Kashfi, K. NOSH-Aspirin: A novel nitric oxide–hydrogen sulfide-releasing hybrid: A new class of anti-inflammatory pharmaceuticals. ACS Med. Chem. Lett. 2012, 3, 257–262. [Google Scholar] [CrossRef] [Green Version]
- Rossoni, G.; Berti, M.; Colonna, V.D.; Bernareggi, M.; Del Soldato, P.; Berti, F. Myocardial protection by the nitroderivative of aspirin, NCX 4016: In vitro and in vivo experiments in the rabbit. Italian Heart J. 2000, 1, 146–155. [Google Scholar]
- Ahmed, A.; Rezai, H.; Broadway-Stringer, S. Evidence-Based Revised View of the Pathophysiology of Preeclampsia. Adv. Exp. Med. Biol. Adv. Intern. Med. 2017, 2, 355–374. [Google Scholar] [CrossRef]
- Sanchez-Aranguren, L.C.; Rezai, H.; Ahmad, S.; Alzahrani, F.A.; Sparatore, A.; Wang, K.; Ahmed, A. MZe786 Rescues Cardiac Mitochondrial Activity in High sFlt-1 and Low HO-1 Environment. Antioxidants 2020, 9, 598. [Google Scholar] [CrossRef] [PubMed]
- Rezai, H.; Ahmad, S.; Alzahrani, F.A.; Sanchez-Aranguren, L.; Dias, I.H.K.; Agrawal, S.; Sparatore, A.; Wang, K.; Ahmed, A. MZe786, a hydrogen sulfide-releasing aspirin prevents preeclampsia in heme oxygenase-1 haplodeficient pregnancy under high soluble flt-1 environment. Redox Biol. 2021, 38, 101768. [Google Scholar] [CrossRef] [PubMed]
- Fiorucci, S.; Orlandi, S.; Mencarelli, A.; Caliendo, G.; Santagada, V.; Distrutti, E.; Santucci, L.; Cirino, G.; Wallace, J.L. Enhanced activity of a hydrogen sulphide releasing derivative of mesalamine (ATB-429) in a mouse model of colitis. Br. J. Pharmacol. 2007, 150, 996–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahn, S. An open trial of high-dosage antioxidants in early Parkinson’s disease. Am. J. Clin. Nutr. 1991, 53, 380–382. [Google Scholar] [CrossRef]
- Rascol, O.; Brooks, D.J.; Korczyn, A.D.; De Deyn, P.P.; Clarke, C.E.; Lang, A.E. A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. N. Engl. J. Med. 2000, 342, 1484–1491. [Google Scholar] [CrossRef]
- Lee, M.; Tazzari, V.; Giustarini, D.; Rossi, R.; Sparatore, A.; Del Soldato, P.; McGeer, E.; McGeer, P.L. Effects of hydrogen sulfide-releasing L-DOPA derivatives on glial activation. J. Biol. Chem. 2010, 285, 17318–17328. [Google Scholar] [CrossRef] [Green Version]
- Kashfi, K. Anti-inflammatory agents as cancer therapeutics. Adv. Pharmacol. 2009, 57, 31–89. [Google Scholar] [PubMed]
- Chattopadhyay, M.; Kodela, R.; Olson, K.R.; Kashfi, K. NOSH-aspirin (NBS-1120), a novel nitric oxide- and hydrogen sulfide-releasing hybrid is a potent inhibitor of colon cancer cell growth in vitro and in a xenograft mouse model. Biochem. Biophys. Res. Commun. 2012, 419, 523–528. [Google Scholar] [CrossRef]
- Kashfi, K. Anti-cancer activity of new designer hydrogen sulfide-donating hybrids. Antioxid. Redox Signal. 2014, 20, 831–846. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, U.; van del Vlies, A.J. Design and synthesis of polymeric hydrogen sulfide donors. Bioconjug. Chem. 2014, 25, 1290–1300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Munday, R. Dithiolethiones for cancer chemoprevention: Where do we stand? Mol. Cancer Ther. 2008, 7, 3470–3479. [Google Scholar] [CrossRef] [Green Version]
- Wood, M.E.; Whiteman, M.; Perry, A. Hydrogen Sulfide Releasing Compounds and Their Use. U.S. Patent No. WO2013045951, 4 April 2013. [Google Scholar]
- Szczesny, B.; Módis, K.; Yanagi, K.; Coletta, C.; Le Trionnaire, S.; Perry, A.; Wood, M.E.; Whiteman, M.; Szabo, C. AP39, a novel mitochondria-targeted hydrogen sulfide donor, stimulates cellular bioenergetics, exerts cytoprotective effects and protects against the loss of mitochondrial DNA integrity in oxidatively stressed endothelial cells in vitro. Nitric Oxide 2014, 41, 120–130. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, K.; Marutani, E.; Hirai, S.; Wood, M.E.; Whiteman, M.; Ichinose, F. Mitochondria-targeted hydrogen sulfide donor AP39 improves neurological outcomes after cardiac arrest in mice. Nitric Oxide 2015, 49, 90–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, A.; Olah, G.; Szczesny, B.; Wood, M.E.; Whiteman, M.; Szabo, C. AP39, a mitochondrially targeted hydrogen sulfide donor, exerts protective effects in renal epithelial cells subjected to oxidative stress in vitro and in acute renal injury in vivo. Shock 2016, 45, 88–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatzianastasiou, A.; Bibli, S.I.; Andreadou, I.; Efentakis, P.; Kaludercic, N.; Wood, M.E.; Whiteman, M.; Di Lisa, F.; Daiber, A.; Manolopoulos, V.G.; et al. Cardioprotection by H2S donors: Nitric oxide-dependent and independent mechanisms. J. Pharmacol. Exp. Ther. 2016, 358, 431–440. [Google Scholar] [CrossRef] [Green Version]
- Karwi, Q.G.; Bornbaum, J.; Boengler, K.; Torregrossa, R.; Whiteman, M.; Wood, M.E.; Schulz, R.; Baxter, G.F. AP39, a mitochondria-targeting hydrogen sulfide (H2S) donor, protects against myocardial reperfusion injury independently of salvage kinase signalling. Br. J. Pharmacol. 2017, 174, 287–301. [Google Scholar] [CrossRef] [Green Version]
- Gerő, D.; Torregrossa, R.; Perry, A.; Waters, A.; Le-Trionnaire, S.; Whatmore, J.L.; Wood, M.; Whiteman, M. The novel mitochondria-targeted hydrogen sulfide (H2S) donors AP123 and AP39 protect against hyperglycemic injury in microvascular endothelial cells in vitro. Pharmacol. Res. 2016, 113, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Talalay, P.; De Long, M.J.; Prochaska, H.J. Identification of a common chemical signal regulating the induction of enzymes that protect against chemical carcinogenesis. Proc. Natl. Acad. Sci. USA 1988, 85, 8261–8265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, W.; Gates, K.S. Evidence for thiol-dependent production of oxygen radicals by 4-methyl-5-pyrazinyl-3H-1, 2-dithiole-3-thione: Possible relevance to the anticarcinogenic properties of 1, 2-dithiole-3-thiones. Chem. Res. Toxicol. 1997, 10, 296–301. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Xian, M. Cysteine-activated hydrogen sulfide (H2S) donors. J. Am. Chem. Soc. 2011, 133, 15–17. [Google Scholar] [CrossRef] [Green Version]
- Foster, J.C.; Powell, C.R.; Radzinski, S.C.; Matson, J.B. S-Aroylthiooximes: A facile route to hydrogen sulfide releasing compounds with structure-dependent release kinetics. Org. Lett. 2014, 16, 1558–1561. [Google Scholar] [CrossRef]
- Zhao, Y.; Bhushan, S.; Yang, C.; Otsuka, H.; Stein, J.D.; Pacheco, A.; Peng, B.; Devarie-Baez, N.O. Aguilar myocardial ischemia-reperfusion injury. ACS Chem. Biol. 2013, 8, 1283–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roger, T.; Raynaud, F.; Bouillaud, F.; Ransy, C.; Simonet, S.; Crespo, C.; Bourguignon, M.P.; Villeneuve, N.; Vilaine, J.P.; Artaud, I.; et al. New biologically active hydrogen sulfide donors. ChemBioChem 2013, 14, 2268–2271. [Google Scholar] [CrossRef] [PubMed]
- Cairns, T.L.; Evans, G.L.; Larchar, A.W.; Mckusick, B.C. gem-Dithiols. J. Am. Chem. Soc. 1952, 74, 3982–3989. [Google Scholar] [CrossRef]
- Berchtold, G.A.; Edwards, B.E.; Campaigne, E.; Carmack, M. The preparation of a crystalline gem-dithiol under mild conditions. J. Am. Chem. Soc. 1959, 81, 3148. [Google Scholar] [CrossRef]
- Zhao, Y.; Kang, J.; Park, C.M.; Bagdon, P.E.; Peng, B.; Xian, M. Thiol-activated gem-dithiols: A new class of controllable hydrogen sulfide (H2S) donors. Org. Lett. 2014, 16, 4536–4539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Yueqin, Z.; Ji, K.; Pan, Z.; Chittavong, V.; Wang, B. Esterase-Sensitive Prodrugs with Tunable Release Rates and Direct Generation of Hydrogen Sulfide. Angew. Chem. Int. Ed. 2016, 55, 4514–4518. [Google Scholar] [CrossRef]
- Borchardt, R.T.; Cohen, L.A. Stereopopulation Control. III. Facilitation of Intramolecular Conjugate Addition of the Carboxyl Group. J. Am. Chem. Soc. 1972, 94, 9175–9182. [Google Scholar] [CrossRef]
- Rao, Y.; Li, X.; Nagorny, P.; Hayashida, J.; Danishefsky, S.J. A Simple Method for the Conversion of Carboxylic Acids into Thioacids with Lawesson’s Reagent. Tetrahedron Lett. 2009, 50, 6684–6686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Organ, C.L.; Zheng, Y.; Wang, B.; Lefer, D.J. Abstract 17903: Novel Esterase Activated Hydrogen Sulfide Donors Attenuate Myocardial Ischemia/ Reperfusion Injury. Circulation 2016, 134, A17903. [Google Scholar]
- Chengelis, C.P.; Neal, R.A. Studies of Carbonyl Sulfide Toxicity: Metabolism by Carbonic Anhydrase. Toxicol. Appl. Pharmacol. 1980, 55, 198–202. [Google Scholar] [CrossRef]
- Chengelis, C.P.; Neal, R.A. Hepatic Carbonyl Sulfide Metabolism. Biochem. Biophys. Res. Commun. 1979, 90, 993–999. [Google Scholar] [CrossRef]
- Steiger, A.K.; Marcatti, M.; Szabo, C.; Szczesny, B.; Pluth, M.D. Inhibition of Mitochondrial Bioenergetics by Esterase-Triggered COS/H2S Donors. ACS Chem. Biol. 2017, 12, 2117–2123. [Google Scholar] [CrossRef] [Green Version]
- Steiger, A.K.; Pardue, S.; Kevil, C.G.; Pluth, M.D. Self-Immolative Thiocarbamates Provide Access to Triggered H2S Donors and Analyte Replacement Fluorescent Probes. J. Am. Chem. Soc. 2016, 138, 7256–7259. [Google Scholar] [CrossRef]
- Powell, C.R.; Foster, J.C.; Okyere, B.; Theus, M.H.; Matson, J.B. Therapeutic Delivery of H2S via COS: Small Molecule and Polymeric Donors with Benign Byproducts. J. Am. Chem. Soc. 2016, 138, 13477–13480. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Deng, Y.; Shen, Z.; Ling, J. Controlled Polymerization of N-Substituted Glycine N-Thiocarboxyanhydrides Initiated by Rare Earth Borohydrides toward Hydrophilic and Hydrophobic Polypeptoids. Macromolecules 2014, 47, 6173–6180. [Google Scholar] [CrossRef]
- Zhao, Y.; Pluth, M.D. Hydrogen Sulfide Donors Activated by Reactive Oxygen Species. Angew. Chem. Int. Ed. 2016, 55, 14638–14642. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, B.C.; Huynh, C.; Chang, C.J. A Palette of Fluorescent Probes with Varying Emission Colors for Imaging Hydrogen Peroxide Signaling in Living Cells. J. Am. Chem. Soc. 2010, 132, 5906–5915. [Google Scholar] [CrossRef] [Green Version]
- Miller, E.W.; Albers, A.E.; Pralle, A.; Isacoff, E.Y.; Chang, C.J. Boronate-Based Fluorescent Probes for Imaging Cellular Hydrogen Peroxide. J. Am. Chem. Soc. 2005, 127, 16652–16659. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Rekowski, M.V.W.; Coletta, C.; Szabo, C.; Bucci, M.; Cirino, G.; Topouzis, S.; Papapetropoulos, A.; Giannis, A. Thioglycine and L-Thiovaline: Biologically Active H2S-donors. Bioorganic Med. Chem. 2012, 20, 2675–2678. [Google Scholar] [CrossRef]
- Pan, J.; Devarie-Baez, N.O.; Xian, M. Facile Amide Formation viaS-Nitrosothioacids. Org. Lett. 2011, 13, 1092–1094. [Google Scholar] [CrossRef] [Green Version]
- Rao, Y.; Li, X.; Danishefsky, S.J. Thio FCMA Intermediates as Strong Acyl Donors: A General Solution to the Formation of Complex Amide Bonds. J. Am. Chem. Soc. 2009, 131, 12924–12926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Danishefshy, S.J. A Promising General Solution to the Problem of Ligating Peptides and Glycopeptides. J. Am. Chem. Soc. 2010, 132, 17045–17051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Orgel, L.E. Oxidative Acylation Using Thioacids. Nat. Cell Biol. 1997, 389, 52–54. [Google Scholar] [CrossRef]
- Dawson, E.P.; Muir, T.W.; Clark-Lewis, I.; Kent, S.B. Synthesis of Proteins by Native Chemical Ligation. Science 1994, 266, 776–779. [Google Scholar] [CrossRef]
- Crich, D.; Sharma, I. Epimerization-Free Block Synthesis of Peptides from Thioacids and Amines with the Sanger and Mukaiyama Reagents. Angew. Chem. Int. Ed. 2009, 48, 2355–2358. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Hu, Q.; Ma, F.; Zhu, Y.Z. Vasorelaxant Effect of a New Hydrogen Sulfide-Nitric Oxide Conjugated Donor in Isolated Rat Aortic Rings through cGMP Pathway. Oxidative Med. Cell. Longev. 2016, 2016, 7075682 . [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Guo, W.; Shi, X.; Kaium, M.; Gu, X.; Zhu, Y.Z. Leonurine-Cysteine Analog Conjugates as a New Class of Multifunctional Anti-myocardial Ischemia Agent. Eur. J. Med. Chem. 2011, 46, 3996–4009. [Google Scholar] [CrossRef] [PubMed]
- Ignarro, L.J.; Napoli, C.; Loscalzo, J. Nitric Oxide Donors and Cardiovascular Agents Modulating the Bioactivity of Nitric Oxide: An Overview. Circ. Res. 2002, 90, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Chang, L.-L.; Tran, B.; Dai, T.; Zhong, R.; Mao, Y.C.; Zhu, Y.Z. ZYZ-803, a Novel Hydrogen SulfiDe-nitric Oxide Conjugated Donor, Promotes Angiogenesis via Cross-Talk between STAT3 and CaMKII. Acta Pharmacol. Sin. 2020, 41, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Wu, D.; Ma, F.; Yang, S.; Tan, B.; Xin, H.; Gu, X.; Chen, X.; Chen, S.; Mao, Y.; et al. Novel Angiogenic Activity and Molecular Mechanisms of ZYZ-803, a Slow-Releasing Hydrogen Sulfide-Nitric Oxide Hybrid Molecule. Antioxid. Redox Signal. 2016, 25, 498–514. [Google Scholar] [CrossRef]
- Wu, D.; Hu, Q.; Xiong, Y.; Zhu, D.; Mao, Y.; Zhu, Y.Z. Novel H2S-NO Hybrid Molecule (ZYZ-803) Promoted Synergistic Effects Against Heart Failure. Redox Biol. 2018, 15, 243–252. [Google Scholar] [CrossRef]
- Bucci, M.; Vellecco, V.; Cantalupo, A.; Brancaleone, V.; Zhou, Z.; Evangelista, S.; Calderone, V.; Papapetropoulos, A.; Cirino, G. Hydrogen Sulfide Accounts for the Peripheral Vascular Effects of Zofenopril Independently of ACE Inhibition. Cardiovasc. Res. 2014, 102, 138–147. [Google Scholar] [CrossRef] [Green Version]
- Terzuoli, E.; Monti, M.; Vellecco, V.; Bucci, M.; Cirino, G.; Ziche, M.; Morbidelli, L. Characterization of Zofenoprilat as an Inducer of Functional Angiogenesis through Increased H2S Availability. Br. J. Pharmacol. 2015, 172, 2961–2973. [Google Scholar] [CrossRef] [Green Version]
- Monti, M.; Terzuoli, E.; Ziche, M.; Morbidelli, L. The Sulphydryl Containing ACE Inhibitor Zofenoprilat Protects Coronary Endothelium from Doxorubicin-Induced Apoptosis. Pharmacol. Res. 2013, 76, 171–181. [Google Scholar] [CrossRef]
- Monti, M.; Terzuoli, E.; Ziche, M.; Morbidelli, L. H2S Dependent and Independent Anti-inflammatory Activity of Zofenoprilat in Cells of the Vascular Wall. Pharmacol. Res. 2016, 113, 426–437. [Google Scholar] [CrossRef]
- Furuta, S.; Kiyosawa, K.; Higuchi, M.; Kasahara, H.; Saito, H.; Shioya, H.; Oguchi, H. Pharmacokinetics of Temocapril, an ACE Inhibitor with Preferential Biliary Excretion, in Patients with Impaired Liver Function. Eur. J. Clin. Pharmacol. 1993, 44, 383–385. [Google Scholar] [CrossRef]
- Donnarumma, E.; Ali, M.J.; Rushing, A.M.; Scarborough, A.L.; Bradley, J.M.; Organ, C.L.; Islam, K.N.; Polhemus, D.J.; Evangelista, S.; Cirino, G.; et al. Zofenopril Protects Against Myocardial Ischemia–Reperfusion Injury by Increasing Nitric Oxide and Hydrogen Sulfide Bioavailability. J. Am. Hear. Assoc. 2016, 5, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Ngowi, E.E.; Afzal, A.; Sarfra, M.; Khattak, S.; Zaman, S.U.; Khan, N.H.; Li, T.; Jiang, Q.Y.; Zhang, X.; Duan, S.F.; et al. Role of hydrogen sulfide donors in cancer development and progression. Int. J. Biol. Sci. 2021, 17, 73–88. [Google Scholar] [CrossRef]
- Li, H.; Xu, F.; Gao, G.; Gao, X.; Wu, B.; Zheng, C.; Wang, P.; Li, Z.; Hua, H.; Li, D. Hydrogen sulfide and its donors: Novel antitumor and antimetastatic therapies for triple-negative breast cancer. Redox Biol. 2020, 34, 101564. [Google Scholar] [CrossRef]
- Cardoso Amorim Reis, A.K.; Stern, A.; Pequeno Monteiro, H. S-nitrosothiols and H2S donors: Potential chemo-therapeutic agents in Cancer. Redox Biol. 2019, 27, 101190. [Google Scholar] [CrossRef]
- Fortunato, S.; Lenzi, C.; Granchi, C.; Citi, V.; Martelli, A.; Calderone, V.; Di Pietro, S.; Signore, G.; Di Bussolo, V.; Minutolo, F. First Examples of H2S-Releasing Glycoconjugates: Stereoselective Synthesis and Anticancer Activities. Bioconjugate Chem. 2019, 30, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Citi, V.; Piragine, E.; Pagnotta, E.; Ugolini, L.; Di Cesare Mannelli, L.; Testai, L.; Ghelardini, C.; Lazzeri, L.; Calderone, V.; Martelli, A. Anticancer properties of erucin, an H2S-releasing isothiocyanate, on human pancreatic adenocarcinoma cells (AsPC-1). Phytother. Res. 2019, 33, 845–855. [Google Scholar]
- Sestito, S.; Pruccoli, l.; Runfola, M.; Citi, V.; Martelli, A.; Saccomanni, G.; Calderone, V.; Tarozzi, T.; Rapposelli, S. Design and synthesis of H2S-donor hybrids: A new treatment for Alzheimer’s disease? Eur. J. Med. Chem. 2019, 184, 111745. [Google Scholar] [CrossRef] [PubMed]
- Sestito, S.; Daniele, S.; Pietrobono, D.; Citi, V.; Bellusci, L.; Chiellini, G.; Calderone, V.; Martini, C.; Rapposelli, S. Memantine prodrug as a new agent for Alzheimer’s Disease. Sci. Rep. 2019, 9, 4612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlovskiy, Y.; Yashchenko, A.; Zayachkivska, O. H2S Donors Reverse Age-Related Gastric Malfunction Impaired Due to Fructose-Induced Injury via CBS, CSE, and TST expression. Front. Pharmacol. 2020, 11, 1134. [Google Scholar] [CrossRef] [PubMed]
- Ercolano, G.; De Cicco, P.; Frecentese, F.; Saccone, I.; Corvino, A.; Giordano, F.; Magli, E.; Fiorino, F.; Severino, B.; Calderone, V.; et al. Anti-metastatic Properties of Naproxen-HBTA in a Murine Model of Cutaneous Melanoma. Front. Pharmacol. 2019, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Melino, S.; Leo, S.; Papajani, V.T. Natural Hydrogen Sulfide Donors from Allium sp. As a Nutraceutical Approach in Type 2 Diabetes Prevention and Therapy. Nutrients 2019, 11, 1581. [Google Scholar] [CrossRef] [Green Version]
- Cenac, N.; Castro, M.; Desormeaux, C.; Colin, P.; Sie, M.; Ranger, M.; Vergnolle, N. A novel orally administered trimebutine compound (GIC-1001) is anti-nociceptive and features peripheral opioid agonistic activity and Hydrogen Sulphide-releasing capacity in mice. Eur. J. Pain 2016, 20, 723–730. [Google Scholar] [CrossRef] [Green Version]
- Rapposelli, S.; Gambari, L.; Digiacomo, M.; Citi, V.; Lisignoli, G.; Manferdini, C.; Calderone, V.; Grassi, F. A Novel H2S-releasing Amino- Bisphosphonate which combines bone anti-catabolic and anabolic functions. Sci. Rep. 2017, 7, 11940. [Google Scholar] [CrossRef] [PubMed]
- Corvino, A.; Severino, B.; Fiorino, F.; Frecentese, F.; Magli, E.; Perissutti, E.; Santagada, V.; Bucci, M.; Cirino, G.; Kelly, G.; et al. Fragment-based de novo design of a cystathionine γ-lyase selective inhibitor blocking hydrogen sulfide production. Sci. Rep. 2016, 6, 34398. [Google Scholar] [CrossRef] [Green Version]
- Severino, B.; Corvino, A.; Fiorino, F.; Luciano, P.; Frecentese, F.; Magli, E.; Saccone, I.; Di Vaio, P.; Citi, V.; Calderone, V.; et al. 1,2,4-Thiadiazolidin-3,5-diones as novel hydrogen sulfide donors. Eur. J. Med. Chem. 2018, 143, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.; Martelli, A.; Citi, V.; Fu, M.; Wang, R.; Calderone, V.; Levi, R. The novel H2S donor 4-carboxy-pheny isothiocyanate inhibits mast cell degranulation and renin release by decreasing intracellular calcium. Br. J. Pharmacol. 2016, 173, 3222–3234. [Google Scholar] [CrossRef] [Green Version]
- Barresi, E.; Nesi, G.; Citi, V.; Piragine, E.; Piano, I.; Taliani, S.; Da Settimo, F.; Rapposelli, S.; Testai, L.; Breschi, M.C.; et al. Iminothioethers as Hydrogen Sulfide Donors: From the Gasotransmitter Release to the Vascular Effects. J. Med. Chem. 2017, 60, 7512–7523. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.; Wang, Y.; Li, J.; Bai, Z.; Zhao, Q.; He, D.; Wang, Z.; Zhang, J.; Chen, Y. Toxicity, bioactivity, release of H2S in vivo and pharmaco-kinetics of H2S-donors with thiophosphamide structure. Eur. J. Med. Chem. 2019, 176, 456–475. [Google Scholar] [CrossRef]
- Zhao, Y.; Steiger, A.K.; Pluth, M.D. Cyclic Sulfenyl Thiocarbamates Release Carbonyl Sulfide and Hydrogen Sulfide Independently in Thiol-Promoted Pathways. J. Am. Chem. Soc. 2019, 141, 13610–13618. [Google Scholar] [CrossRef] [PubMed]
- Levinn, C.M.; Cerda, M.M.; Pluth, M.D. Development and Application of Carbonyl Sulfide-Based Donors for H2S Delivery. Acc. Chem. Res. 2019, 52, 2723–2731. [Google Scholar] [CrossRef]
- Zhou, S.; Mou, Y.; Liu, M.; Du, Q.; Ali, B.; Ramprasad, J.; Qiao, C.; Hu, L.F.; Ji, X. Insights into the Mechanism of Thiol-Triggered COS/H2S Release from N-Dithiasuccinoyl Amine. J. Org. Chem. 2020, 85, 8352–8359. [Google Scholar] [CrossRef]
- Connal, L.A. The benefits of macromolecular hydrogen sulfide prodrugs. J. Mater. Chem. B 2018, 6, 7122. [Google Scholar] [CrossRef] [PubMed]
- Gojon, G.; Morales, G.A. SG1002 and Catenated Divalent Organic Sulfur Compounds as Promising Hydrogen Sulfide Prodrugs. Antioxid. Redox Signal. 2020, 33. [Google Scholar] [CrossRef] [PubMed]
- Giordano, F.; Corvino, A.; Scognamiglio, A.; Citi, V.; Gorica, E.; Fattorusso, C.; Persico, M.; Caliendo, G.; Fiorino, F.; Magli, E.; et al. Hybrids Between H2S-donors And Betamethasone 17-Valerate or Triamcinolone Acetonide Inhibit Mast Cell Degranulation and Promote Hyperpolarization of Bronchial Smooth Muscle Cells. Eur. J. Med. Chem. 2021, 221, 113517. [Google Scholar] [CrossRef]
- Corvino, A.; Citi, V.; Fiorino, F.; Frecentese, F.; Magli, E.; Perissutti, E.; Santagada, V.; Calderone, V.; Martelli, A.; Gorica, E.; et al. H2S donating corticosteroids: Design, synthesis and biological evaluation in a murine model of asthma. J. Adv. Res. 2021, in press. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, Y.; Ma, L.; Ji, R.; Qu, Y.; Xin, Y.; Lv, G. Chemopreventive activity of sulforaphane. Drug Des. Dev. Ther. 2018, 12, 2905–2913. [Google Scholar] [CrossRef] [PubMed] [Green Version]








































Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magli, E.; Perissutti, E.; Santagada, V.; Caliendo, G.; Corvino, A.; Esposito, G.; Esposito, G.; Fiorino, F.; Migliaccio, M.; Scognamiglio, A.; et al. H2S Donors and Their Use in Medicinal Chemistry. Biomolecules 2021, 11, 1899. https://doi.org/10.3390/biom11121899
Magli E, Perissutti E, Santagada V, Caliendo G, Corvino A, Esposito G, Esposito G, Fiorino F, Migliaccio M, Scognamiglio A, et al. H2S Donors and Their Use in Medicinal Chemistry. Biomolecules. 2021; 11(12):1899. https://doi.org/10.3390/biom11121899
Chicago/Turabian StyleMagli, Elisa, Elisa Perissutti, Vincenzo Santagada, Giuseppe Caliendo, Angela Corvino, Gianluca Esposito, Giovanna Esposito, Ferdinando Fiorino, Marco Migliaccio, Antonia Scognamiglio, and et al. 2021. "H2S Donors and Their Use in Medicinal Chemistry" Biomolecules 11, no. 12: 1899. https://doi.org/10.3390/biom11121899
APA StyleMagli, E., Perissutti, E., Santagada, V., Caliendo, G., Corvino, A., Esposito, G., Esposito, G., Fiorino, F., Migliaccio, M., Scognamiglio, A., Severino, B., Sparaco, R., & Frecentese, F. (2021). H2S Donors and Their Use in Medicinal Chemistry. Biomolecules, 11(12), 1899. https://doi.org/10.3390/biom11121899












