Abstract
To reduce anthropological pressure on the environment, the implementation of novel technologies in present and future economies is needed for sustainable development. The food industry, with dairy and meat production in particular, has a significant environmental impact. Global poultry production is one of the fastest-growing meat producing sectors and is connected with the generation of burdensome streams of manure, offal and feather waste. In 2020, the EU alone produced around 3.2 million tonnes of poultry feather waste composed primarily of keratin, a protein biopolymer resistant to conventional proteolytic enzymes. If not managed properly, keratin waste can significantly affect ecosystems, contributing to environmental pollution, and pose a serious hazard to human and livestock health. In this article, the application of keratinolytic enzymes and microorganisms for promising novel keratin waste management methods with generation of new value-added products, such as bioactive peptides, vitamins, prion decontamination agents and biomaterials were reviewed.
1. Introduction
In order to reduce anthropological pressure on the environment and to mitigate threats connected with progressing climate change, many governmental, business, social and scientific organizations proposed actions and concepts that could facilitate positive changes and the implementation of novel models and technologies in present and future economies. Sustainable development (SD) is seen as one of the most ambitious and broad models formulated to date [1]. The United Nations seventeen SD goals (SDGs) with their targets and indicators (as a part of the 2030 Agenda) were designed to address and to connect environmental protection issues with economic growth and social advancement [2,3]. Since the framework’s announcement, many have identified proposed specific focus points, actions and transformations needed for successful implementation of SDGs on national and global levels [1,4,5]. Decarbonization of energy production systems, limitation of greenhouse-gas emissions, combating land, water and air pollutions as well as securing access to food and fresh water without compromising natural habitats and loss of biodiversity, are thought to be the main challenges and areas requiring heavy transformations in achieving environmental sustainability [1].
The food industry, with dairy and meat production in particular, is known to pose a significant environmental impact consisting of land and water use, carbon dioxide, nitrogen oxide and methane emissions, soil erosion, eutrophication of water reservoirs and considerable amounts of generated waste. Poultry meat production is not an exception [6,7,8].
In 2020, The European Union (EU-27) produced over 13.5 million tonnes of poultry meat, with Poland (2.6 million tonnes), Spain (1.7 million tonnes), France (1.7 million tonnes), Germany (1.6 million tonnes) and Italy (1.4 million tonnes) as the main producers. In the EU, the poultry industry is dominated by production of broiler chicken meat (82%), followed by turkey (14%), duck (3%) and others (1%) [9]. Global poultry production is one of the fastest-growing meat producing sectors and is connected with generation of burdensome streams of manure, offal and feather waste [10,11,12]. It is estimated that in 2020, the EU’s poultry meat production of 13.5 million tonnes created around 3.2 million tonnes of feather waste [13].
Poultry feather waste is mainly composed of keratin—a protein biopolymer [14]. Thanks to its complex structure, keratin is characterized by its high stability, durability and resistance to hydrolysis by common proteolytic enzymes such as pepsin, trypsin and chymotrypsin [15]. Traditionally keratin waste is disposed in the landfills, incinerated or converted into animal feed, using physical and chemical treatments [14]. If not managed properly, keratin waste, including feathers, can significantly affect ecosystems, contributing to environmental pollution, and pose a serious hazard to human and livestock health [16].
As the sustainability of the global economy, including closing of production cycles, decarbonization and bioeconomy development, has become of a great need, rational, unconventional and innovative keratin waste management methods are now subject of extensive international research, with India, China and Brazil, followed by South Korea, the United States, Egypt and Japan leading in research productivity of that field [12,17].
Keratinolytic microorganisms and keratinases can be applied in various industries, such as leather, textile, chemical, pharmaceutical, food and nutrition industries, as well as in biotechnology and environmental protection [18,19,20].
2. Keratin Characterization
Keratins are a heterogeneous family of proteins and the third most abundant biomass in nature, after chitin and cellulose [18,21]. Their structures, occurring in all vertebrates, have been thoroughly described in the literature [21,22,23,24]. Keratins, synthesized in epithelial cells, are an integral part of the epidermis and its appendages, including hair, wool, feathers, nails, horns, hooves and cuticles [22]. Keratins form solid structures, held by disulfide bonds formed between the thiol groups (–SH) of cysteine amino acid residues, hydrogen bonds and hydrophobic interactions [21,24]. The multi-level structure and a high number of cross-linkages between various types of keratins results in high resistance to mechanical, chemical and physical factors [23]. They are characterized based on their secondary structures (mainly α-helixes and β-sheets), sulphur content (soft and hard), amino acid composition (basic, acidic or neutral), molecular weight and a source of origin [21,22,23,24].
Based on their secondary structure, three main types of keratin have been recognized–α-keratin (with α-helices), β-keratin (with β-sheets) and γ-keratins (Table 1) [21,24,25]. The keratin-associated proteins (KAPs) that create a matrix between keratin fibrils are occasionally distinguished as a separate group from γ-keratins [24,26].

Table 1.
Keratin types characteristics [21,23,24].
However, the content of α- and β-keratin, as well as the exact amino acid composition, can vary depending on the origin of the biomass, and also based on the material’s role and its place of growth [21,23,24]. The feathers of the outer layer, called cover feathers, usually consist mostly of the harder β-keratin. The keratins of bird feathers contain about 2.5 times more cystine residues formed as a result of joining two cysteine residues with a disulfide bridge than the keratins of nails, horns or the epidermis [21,22,24].
Based on the sulfur content derived from cysteine residues, keratins can be also divided into soft and hard keratins [21,24]. Soft keratins, usually containing up to 1% of sulfur (or up to 10% of cysteine residues) in their composition, are found in the cytoskeleton materials of epithelial tissues, e.g., in the intermediate filaments. Due to the lower content of cysteine and thus disulfide bridges, soft keratins are more flexible than the hard keratins. They play an important role in cell division, transmission of signals in and out of cells and its spatial organization [22,27,28]. Hard keratins, an essential component of hair, wool, nails, feathers, beaks and claws, can contain from 1% to 5% of sulfur (10–14% cysteine residues). Their role is to protect against mechanical disruptions, predators and abiotic factors, such as low or high temperature or high doses of radiation [21,22,24]. Keratins are hygroscopic, but insoluble in water, weak acids, alkalis and most organic solvents [29,30].
3. Conventional Keratin Degradation
The main physicochemical methods of keratin treatment are solubilization in organic solvents or ionic liquids, hydrothermal treatment, acid or alkaline hydrolysis, oxidation or reduction in disulfide bridges by the addition of chemicals, and degradation of hydrogen bonds, e.g., by urea [23,24,31,32,33]. The choice of the treatment method has a significant impact on the composition of the extracted keratin and keratin hydrolysate and largely depends on the intended future use of the final product [30,31,34,35,36]. A comparison of various physicochemical treatment methods with their advantages and disadvantages are presented in Table 2.

Table 2.
Comparative analysis of various physicochemical treatment methods with their advantages and disadvantages [23,24,28,31,32,33,36].
Although physicochemical methods are relatively versatile and efficient, they usually lead to a significant modification of the amino acid composition of the extracted keratin or the hydrolysate. The final product contains free amino acids and peptides; however, depending on the method used, also specific amino acid derivatives or their stereoisomers [24,37]. Such products usually require further, often costly, isolation and purification. Therefore, the traditional waste management (e.g., incineration or landfilling) as well as physicochemical treatments are regarded as unsustainable, as they contribute to space shortages, increased usage of concentrated and hazardous chemicals, eutrophication, elevated emissions of greenhouse gases, high energy consumption and the necessity of potentially hazardous waste hydrolysate management. Therefore, degradation with the use of keratinolytic microorganisms and their enzymes is seen as a potentially beneficial alternative to conventional management methods [8,24,33].
4. Microbial and Enzymatic Biodegradation
Keratinases (E.C. 3.4.21/E.C. 3.4.24/E.C. 3.4.99.11) are a group of proteolytic enzymes that catalyze the breakdown of peptide bonds in keratins [38]. Even though keratins are one of the most abundant biomass produced, they do not accumulate significantly in ecologically stable environments [39]. This, along with an extensive amount of already classified keratin-degrading microbial strains shows that the presence of keratinases or keratin-degrading proteases is not limited to very specialized ecological niches, but rather common throughout the globe. Keratinases are synthesized by many bacteria and fungi [39]. Up to date, the most important producers of keratinolytic enzymes are bacteria of the genus Bacillus, including B. subtilis and B. licheniformis. Other bacterial producers belong to Micrococcus sp., actinomycetes (Streptomyces and Thermoactinomycetes genera), fungal Aspergillus, Alternaria, Penicillium and Chrysosporium genera and many more [18,38]. Keratinases are also produced by fungal pathogens, such as Trichophyton rubrum, T. mentagrophytes, Microsporum gypseum and Epidermophyton floccosum [40]. Reportedly, black carpet beetle (Attagenus unicolor) might be a possible producer of keratinolytic enzymes, as its transcriptome holds many gene sequences homologous to known microbial keratinases, and was altered when insects have been fed with poultry feathers [41].
Every year, new microorganisms and microbial strains with keratin-degrading abilities are isolated from various environments, including feather waste landfills, skin surface, soil and water bodies near slaughterhouses, industrial wastewaters and organisms such as insects and spiders [39,42].
4.1. Keratinases Properties
Keratinolytic enzymes have various optimal temperature and pH ranges [18,43]. Keratinase from Brevibacterium luteolum is characterized by optimal temperature (Topt) of 30 °C [44], whereas the enzyme from a thermophilic bacterium Fervidobacterium islandicum AW-1 [45] exhibits Topt of 100 °C. Even though most keratinases have an optimum activity at or above pH 7, Trichophyton mentagrophytes produces the keratinolytic enzyme with the highest activity in slightly acidic pH 5.5 [46]. On the other hand, the highly-alkaline bacterial keratinase from Brevibacillus sp. AS-S10-11 has been characterized by optimal pH (pHopt) of 12.5 [47]. Molecular weight of microbial keratinolytic enzymes can range from 20 [48] to even 240 kDa [18,49]. Recently isolated keratinolytic enzymes and their biochemical properties are shown in Table 3.

Table 3.
Biochemical properties and potential applications of recently isolated keratinolytic enzymes (examples from 2016 to 2021).
Most keratinases are typically synthesized in the late exponential and/or in stationary phase and it is most probably related to the time needed for metabolism adaptation to the lack of nutrients in a given environment. As keratinolytic enzymes are regarded as inductive biocatalysts, their synthesis is carried out mainly in response to a keratinous substrate presence [18,44,46,72,73,74,75,76]. Reportedly, other protein components of microbiological media, such as tryptone, casein or yeast extract are, however, less effective kertinases inducers [18,19,66,77]. Keratinolytic enzymes exhibit a broad substrate specificity, as they can catalyze the breakdown of hair, feathers, wool, horns, hooves, scales keratins and even other fiber-forming proteins, such as collagen and elastin [18,19,66]. The degradation of non-fibrous proteins, such as casein and albumin, is also well documented, and is often used in microbial isolation and screening procedures [19,39,78]. Many keratinolytic enzymes are active against synthetic peptides such as Z-Ala-Ala-Leu-pNA, Z-Gly-Gly-Leu-pNA, Z-Gly-Pro-Cit-pNA and Suc-Ala-Ala-Pro-Phe-pNA, corresponding to the subtilisin substrate specificity [22]. Enzymatic activity of keratinases is diverse and generally falls in the range of 10 to 18,000 U/mL [72]. However, it is worth noting that methods of keratinase activity assay are a subject of greater discussion, as there are many available [75] (see Table 4) and each is characterized by different advantages and disadvantages with little possibility for comparison of final results [19,74,79].

Table 4.
Substrates used for proteolytic activity assay of keratinases.
Framework for keratinolytic enzyme characterization is shown at Figure 1.
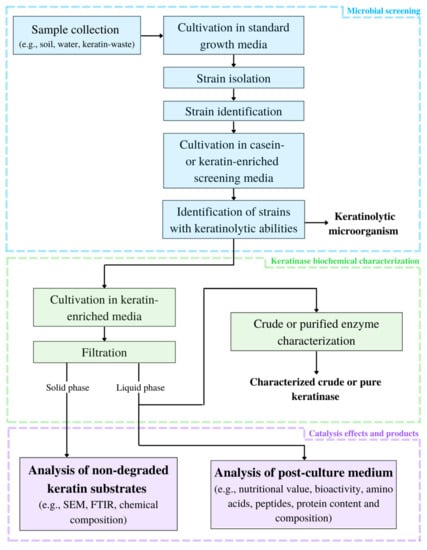
Figure 1.
Framework for keratinolytic enzyme characterization (SEM—scanning electron microscope; FTIR—Fourier transform infrared spectroscopy).
4.2. Catalytic Mechanism
Keratins, due to their compact structure (with compromised access to specific, recognized peptide bonds and residues) and insolubility, are a challenging substrate to hydrolyze for most common proteases. Even though many keratinolytic enzymes have been isolated over the years, the precise mechanism of keratin biodegradation is not fully understood [62,77]. A vast majority of keratinases are extracellular enzymes [56,59,60,62]. However, intracellular enzymes with keratinolytic activity from B. licheniformis RGI and Trichophyton gallinae were also reported [95,96]. Keratin biodegradation is thought to be composed of enzyme’s adsorption to the macromolecule’s surface (possibly by electrostatic and hydrophobic interactions) followed by the proper catalytic action. This action can be further divided into reduction in disulfide bonds, also called sulfitolysis, and disruption of the peptide chain (proteolysis). Based on proteolysis catalytic strategy, the majority of keratinases are representatives of serine- or metalloproteases. Sulfitolysis is thought to happen only in the presence of reducing agents, such as sodium sulfite, β-mercaptoethanol, dithiothreitol (DTT), thioglycolic acid, glutathione or cysteine. Enzymatic sulfitolysis is also possible [62,77]. It is also suggested that proteolytic enzymes might be involved in a catalytic synergy with disulfide reductases (oxidoreductases) acting as another group of disulfide-bond-reducing agents and a facilitators of keratin biodegradation [42,62,77]. Nevertheless, many keratinases from the Bacillus genus have been described as the sole catalysts of keratin degradation [19,97]. Therefore it is not ruled out that various microorganisms developed different mechanisms of keratin degradation, using self-produced reducing agents, membrane-associated, extracellular keratinolytic enzymes or their combinations, with intracellular proteases further degrading proteins and peptides after they are transported into cells [42,77]. However, it is not proven whether such cross-class enzymatic synergy is more common for keratin-degrading microorganisms or a highly specific mechanism developed by the thermophilic bacterium, Fervidobacterium islandicum AW-1 [77], as a response to extreme environmental conditions. Moreover, it was stated that a group of enzymes, called lytic polysaccharide monooxygenases (LPMOs), associated for some time with cellulose, chitin, starch and hemicellulose polymer degradation, may also contribute to the disruption of α- and β-keratins [98,99,100].
4.3. Keratinases Immobilization Methods
Though enzymes are a group of catalysts often praised by their effectiveness, selectiveness and sustainability, their application in many industrial scale processes has been limited due to their relatively high production costs, challenging recovery and difficulties with maintaining sufficient stability in catalysis conditions. To mitigate such drawbacks, many strategies, including enzyme immobilization methods, have been developed [101,102,103]. The main techniques used for biocatalysts immobilization on/with solid supporting materials are (1) adsorption, (2) entrapment, (3) encapsulation, (4) covalent binding and (5) cross-linking, and their combinations [101,102,103,104,105,106,107,108,109,110]. Specific immobilization conditions and choice of suitable matrix material have to be optimized and tailored to the given enzyme and its targeted application. Keratinolytic enzymes have also been subjected to immobilization methods employing various materials, e.g., charcoal, glass beads, silica gel, cellulose, chitosan, chitin and alginates [101,102,104].
Commercially available recombinant keratinase from B. licheniformis was successfully immobilized with chitosan-glutaraldehyde (chitosan-E) and chitosan-cyclodextrin-glutaraldehyde (chitosan-β-CD-E) beads with high immobilization yield (90 and 93%, respectively), resulting in increased storage stability and allowing for biocatalyst reuse in wool conditioning processes [103]. Calcium alginate gel beads has been used to entrap B. licheniformis LMUB05 keratinase. Immobilized enzyme, implemented in generation of keratin-derived feed, exhibited increased pH tolerance and thermal stability in comparison with the crude enzyme [105]. Another recombinant keratinase KMT-wt from thermophilic Meiothermus taiwanensis WR-220 was immobilized on modified bagasse cellulose and utilized in wastewater treatment [106]. Immobilizing B. subtilis RM-01 keratinase with poly(vinyl alcohol)-assisted silver-nanoparticles (PVA-AgNPs) resulted in increase in specific activity, thermostability and storage stability of the biocatalyst. Additionally, due to immobilization the enzyme exhibited enhanced antibacterial and dehairing activity [107].
Biocatalyst immobilization with polyethylene glycol-assisted (PEG) iron oxide (Fe3O4) magnetic nanoparticles (MNPs) was proven to facilitate recovery of immobilized keratinases of B. subtilis [108] and B. licheniformis AS-S24-I using magnets [109]. Interestingly genetic engineering can also be introduced in keratinolytic enzymes immobilization strategies. Recombinant keratinase from B. licheniformis PWD-1 was expressed as keratinase-streptavidin fusion protein resulting in affinity of fusion biocatalyst to a biotinylated materials. Moreover, such immobilization methods resulted in an increase in thermostability and improvement of the pH tolerance of the enzyme [110].
4.4. Genetic Engineering of Keratinolytic Strains and Enzymes
Pressure for agro-industries to produce more food for continuously growing human population is causing an increased generation of agricultural waste, including keratin-based waste [111]. Keratinases have many potential applications (described below) in various industries; however, their production from non-enhanced native hosts is generally regarded as insufficient to meet the large demand of the global market [112]. Moreover, many of efficiently keratin-degrading microorganisms and enzymes are thermophilic in nature [59,60,77,113,114], which might pose a threat to their economic and environmental sustainability. Several genetic engineering strategies have been developed and tested to (1) increase the amounts of secreted enzymes, (2) to facilitate the enzymes production, (3) to improve their biochemical properties and activity as well as (h4) to alter the substrate specificity [60,115,116,117,118,119,120,121,122,123,124,125].
Reportedly, a production of keratinolytic enzymes is significantly correlated with a composition of culturing medium, resulting in highest secretion at the relatively low keratin-substrate concentrations of about 1–5% (w/v). Further increase often decrease amounts of secreted enzymes. However, such determination is generally strain-specific and has to be examined with other culturing conditions [47,61,63,66,126,127,128]. To overcome this obstruction and to improve better cost-effectiveness of keratinase production, enzyme overexpression in the native host can be performed, using vector-integrated enzyme genes (possible to control thanks to plasmid construction), which can increase biocatalyst production even up to 5–6 times [115,116]. Similarly, random or specific mutagenesis of native strains, caused, e.g., by UV-radiation or ethyl methanesulfonate, can result in obtaining strains with better keratinolytic abilities [117,118]. Another strain engineering strategy is to produce metabolically impaired native strain, unable to produce certain intra- and extracellular proteases, signal peptides and RNases [125]. Such approach can result in obtaining hosts more suitable for secretion of heterologous, as well as homologous proteins [72,129]. Keratinolytic enzymes can be also cloned from newly isolated, often demanding, strains and expressed in a well-known and widely-used heterologous bacterial, e.g., Escherichia coli and B. subtilis, and fungal hosts, such as Pichia pastoris [18,72,130]. Heterologous expression of B. lichenifomis PWD-1 keratinase (KerA) have also been reported in insect cells of Spodoptera frugiperda (Sf9) [131].
Many molecular strategies, dedicated for each heterologous host, have been developed over the years, for further improvement of keratinases production, biochemical property enhancement and down-stream processes, including adjustment of available vectors to a given host, choice of suitable promoter, modification of enzyme propeptide sequences and chromosomal integration [72,112,129]. Examples of keratinolytic strains, enzyme engineering strategies and their outcomes are presented in Table 5.

Table 5.
Chosen examples of engineering strategies for keratinolytic strain and/or enzyme improvement.
5. Application
Keratinolytic enzymes and their microbial producers have been already been found to be applicable in several industrial sectors. Some microbial keratinases, especially from Bacillus spp., are available as purified enzymes, enzyme preparations or as bioactive ingredients in commercial products. Examples of some commercially available keratinolytic enzymes and their biotechnological applications are listed in Table 6 [17,19,39,132].

Table 6.
Commercial biotechnological applications of keratinases [17,19,39,132].
In many studies, when dealing with keratinous waste management, keratin substrate is transformed into protein-, peptide- and amino acid-rich hydrolysate as the main product of enzymatic or full-cell hydrolysis [34,37,97,133,134,135]. As keratin waste streams are constantly growing, keratinases and keratin-degrading microbes, can be utilized not only as waste decontamination agent, but also as novel tools for more sustainably obtained products with added value, ranging from animal feed, biomaterials and highly efficient dehairing agents to pharmaceuticals and bioactive peptides of therapeutic importance (see Figure 2).
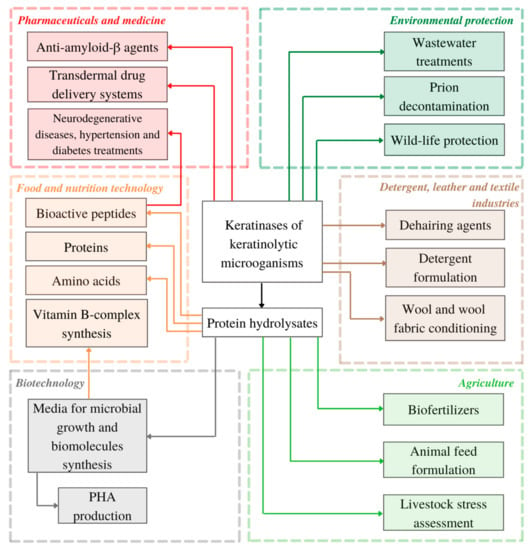
Figure 2.
Present and potential applications of keratinolytic microorganisms and keratinases reviewed in this article (PHA—polyhydroxyalkanoates).
5.1. Nutrition and Food Technology
Bioactive Peptides
Conventional raw substrates and food are nowadays regarded not only as a source of necessary nutrients crucial for maintaining organism’s homeostasis, but also as a source of industrially important bioactive molecules [136]. Bioactive peptides are fragments of the amino acid sequences of native proteins, which remain inactive in their precursors. They can also be synthesized by single-cell bacteria [137]. When released, they can act as regulators of numerous physiological processes and endocrine (oxytocin, adrenocorticotropic hormone, and calcitonin), immune, circulatory, nervous and digestive systems [136,137,138]. Type of biological activity, displayed by biopeptides, is main determinant of their division (see Table 7) [136]. Many bioactive peptides can exhibit antioxidant, antimicrobial (gramicidins, polymyxins, bacitracins), anti-amnesic or even opioid-like activities. Some can lower blood pressure (angiotensin-I converting enzyme (ACE) inhibitors; dipeptidyl peptidase-IV (DPP-IV) inhibitors; Ile-Tyr, Lys-Trp, Ile-Trp dipeptides), whereas others can bind metal ions or shape sensory properties of food (nisin, aspartame, lipopeptides). They are currently used in the generation of functional products and nutritional formulations [136,137]. The bioactive peptides are also attractive molecules for pharmaceutical and cosmetic applications [136,137,138]. It is estimated that the peptide-based product market value exceeds USD 40 billion [139].

Table 7.
Bioactive peptides—examples of groups, functions and sources [136,137,139].
Technologies for bioactive peptides production include (1) natural source extraction, (2) genetic engineering, (3) cell-free expression systems, (4) transgenic animals and plants and (5) chemical and enzymatic synthesis [137,138,140,141,142,143,144].
Event though, most of the bioactive peptides were identified in food and food by-products [136,139], waste biomass can also become a source of molecules with bioactive properties [34,37,133,142,145]. Extraction from natural sources is a commonly used strategy for releasing and obtaining peptides from food proteins, based on (a) in vitro enzymatic hydrolysis, (b) cultivation of protein-degrading microorganisms (fermentation) and (c) animal digestive enzymes hydrolysis [136,138]. For in vitro food-protein hydrolysis, proteases with broad substrate specificity, from plant (ficin, bromelain, papain), animal (pepsin, chymotrypsin, trypsin) and microbial (proteinase K, collagenase, subtilisin A) sources, are needed [136]. As the representatives of proteolytic enzymes, keratinases can be potentially used for bioactive peptides production via enzymatic hydrolysis of keratins and other proteins, or by full cell fermentation of microbial media supplemented with poultry feather and non-keratin plant proteins [68,142,145].
Several studies, exploring the possibility of obtaining bioactive hydrolysates and peptides from keratin waste have been conducted. Three previously evaluated Bacillus strains of keratinolytic activities, CL18, CL33A and CL14, were used for full-cell bioconversion of chicken feathers into protein hydrolysates [133] via submerged cultivation in feather broth (FB) medium (0.5 g/L NaCl, 0.3 g/L K2HPO4, and 0.4 g/L KH2PO4) with whole chicken feathers (10, 20, 30, 40 and 50 g/L). Fermentation was conducted at 30 °C, pH 7 and with 125 rpm for at least 13 days (different for each strain). Obtained hydrolysates were tested for DPPH and ABTS radical scavenging activities, Fe2+-chelating ability, as well as for inhibition of dipeptidyl peptidase IV (DPP IV) and angiotensin I-converting enzyme (ACE). Out of all three strains, Bacillus sp. CL18 degraded feathers most effectively, resulting in degradation of 98.9% of feathers after 7 days of cultivation in FB medium supplemented with 10% of feathers (FB10), followed by Bacillus sp. CL33A (56.2% after 9 days) and Bacillus sp. CL14 (71.4% after 13 days). All hydrolysates showed a wide range of bioactive activities in all tested concentrations of chicken feathers in the FB. Maximum of 95.7% of DPP IV and 89.7% of ACE inhibition was observed in feather hydrolysate after 5-day cultivation of Bacillus sp. CL18 in FB40, as well as significant antioxidant and reducing activities. Therefore protein hydrolysates after Bacillus sp. CL18 full-cell fermentation, might be potentially a rich source of bioactive peptides of nutritional and pharmaceutical importance [133].
In another study, keratinase from Bacillus sp. CL18 was partially purified and applied for conversion of various protein substrates into bioactive hydrolysates [142] at optimal conditions (55 °C; pH 8.0; 5 mM Ca2+). From all tested substrates, casein was the most preferable for hydrolysis (100% relative activity), followed by soy protein isolate (83%), albumin (26.8%), feather meal (19.4%) and non-pretreated keratinous substrates such as human nails (7.6%), chicken nails (5.2%), chicken feathers (4.5%) and human hair 2.8% [142]. Soy protein isolate by itself had antioxidant properties; however, after 4 h of keratinolytic hydrolysis, the ability to scavenge the ABTS radicals increased from around 17.5% to almost 69% and from 27% to around 40% of DDPH radicals. Moreover, increased inhibition of ACE (from 10% to maximum 89.1%) and DPP IV (form 4.7% to maximum 34.8%) were also observed as another result of soy proteins hydrolysis with partially purified keratinase from Bacillus sp. CL18 [142].
Feather hydrolysates were also obtained by full-cell fermentation of Bacillus cytotoxicus LT-1 and B. cytotoxicus Oll-15 [68]. Minimal mineral media (2.48 g/L K2HPO4, 0.49 g/L NaH2PO4, 0.01 g/L MgCl2, 0.02 g/L FeCl3, 0.1 mg/L CaCl2 and 0.013 g/L ZnCl2; pH 6.0) with 10 g/L of chicken feathers and given bacterium, were incubated 48 h at 50 °C with 150 rpm. Enzyme production of strain Oll-15 was the highest after 24 h of cultivation (14.4 U/mL), whereas strain LT-1 reached maximum production (16.6 U/mL) after 33 h. Hydrolysate from strain Oll-15 exhibited slightly better reducing activity than ascorbic acid (used as a positive control), whereas LT-1 hydrolysate did not show any significant reducing properties. Both Oll-15 and LT-1 hydrolysates showed noticeable scavenging activity of 85 and 64%, respectively, suggesting the presence of bioactive peptides in post-culture media [68].
Recombinant keratinase KerZ1 from B. licheniformis BBE11-1, expressed in B. subtilis WB600 (with maximum activity of 426.6 kU/mL in 15-L bioreactor), was used for feather degradation in an optimized fermentation medium (10 g/L yeast extract, 20 g/L tryptone, 20 g/L sucrose, 3 g/L KH2PO4, 6 g/L Na2HPO4, and 0.3 g/L MgSO4) supplemented with disulfide-bonds reducing agent, 1% sodium sulfate. Such combination allowed for efficient degradation of poultry feathers at 60 °C and pH 7.0 within 12 h, which resulted in a release of a wide variety of amino acids (with relatively high content of Glu, Ala, Tyr, Phe, Leu and Lys) with maximal conversion rate of 56.6%, when 100 g/L of feather were used [145]. Beside amino acids, hydrolysate contained a mixture of short peptides (~1.3 kDa) with at least 12 of them exhibiting catalytic and antioxidant activities. However, the exact sequences and properties of those bioactive peptides have yet to be determined [145].
Even though production processes of waste-derived bioactive hydrolysates and peptides require further research, especially in terms of product purity, stability and safety, with optimized production methods and dedicated delivery systems [139], they may become a promising and more sustainable alternative to food-derived [136], GMO/GMM-derived and enzymatically or chemically synthesized [137] peptides for human and animal consumption.
5.2. Agriculture
Agriculture is one of the most significant sectors of global economy. However, animal husbandry, especially industrial meat and dairy production, is greatly contributing to climate change by the native habitat uptake, biodiversity loss, generation of hazardous waste, eutrophication and significant green-house gases emissions. As current and future challenges, connected with satisfying the demands of growing human population and rise of environmental issues are more apparent, more sustainable methods for food, feed and nutrient generation are of great interest [7,8,12,146].
5.2.1. Biofertilizers and Plant Biostimulants
Despite being a valuable source of proteins, peptides and amino acids (both essential and non-essential), keratin hydrolysates, including feather-derived ones, are also regarded as a good source of nitrogen (N), phosphorus (P), and other minerals, e.g., potassium (K), calcium (Ca) and magnesium (Mg) [146]. As use of synthetic fertilizers can negatively impact the environment, feather-derived hydrolysates are studied for their biofertilizing proposes. Biologically obtained keratin hydrolysates generated during full-cell hydrolysis are of notable interest, mainly due to the possibility of additional plant biostimulants synthesis by fungi or bacteria during fermentation period (see Table 8). Plant biostimulants (PBs) are a wide group of molecules, often microbial metabolites, such as indole-3-acetic acid (IAA) with beneficial effects on plants growth and safety, including uptake of nutrients, improvement of stress tolerance and nutritional value of crops, as well as antimicrobial activity [24]. Moreover, utilization of feather waste biofertilizers can also improve quality of soil by increasing water retention and inorganic phosphorus solubilization [146].

Table 8.
Some plant cultivation promoting properties of microbial-derived keratin hydrolysates [34,146,147,148,149].
Studies showed that biofertilizer produced by full-cell hydrolysis of feather waste using Bacillus aerius NSMk2 and minimal salt medium with chicken feather as sole carbon source (3 days, at 35 °C, pH 7.5), stimulated germination of seeds and further growth of mung beans (Vigna radiata) when supplemented to the soil [147]. Similar observations were noted with activation of proton pump when tomato (Solanum lycopersicum) seedlings were cultivated in a soil enriched with keratin-based fertilizers, obtained by Trichoderma asperellum T50 and T. atroviride full-cell hydrolysis of wool or feathers conducted on minimal medium (0.5 g/L NaH2PO4, 0.5 g/L KH2PO4, 0.016 g/L FeCl3, 0.1 g/L CaCl2, 0.01 g/L MgCl2) with 1% w/w of keratin substrates for 21 days at around 26 °C and pH 7.0 [148].
Many amino acids can act as biostimulants, as they regulate normal and stress-induced metabolism of plants [149]. IAA is a phytohormone synthesized by both plants and keratinolytic microbes from tryptophan; therefore, the presence of this amino acid or IAA itself in biofertilizer can greatly contribute to faster and more efficient plant development. However, taking into consideration various chemical composition of keratin-rich animal waste can significantly affect final content and availability of certain proteins, peptides and amino acids in keratin hydrolysate, tailored approach for each application might be required [20,132].
5.2.2. Animal Feed
The Life Cycle Assessment (LCA) studies focused on environmental impact (EI) of meat and dairy industries in Europe and other regions have shown that animal feed production and supply are two major contributors of global warming, eutrophication and acidification potential [12,150,151]. Depending on the origin and supply chain of feed ingredients, land use change and pesticides utilization have become other factors of great concern. This is especially significant for protein sources, such as soy and its derivatives, mostly imported from South America [12]. Moreover, the heavy dependence of the poultry sector on soy creates a non-negligible food security risk for European countries. Research focused on transforming the waste streams such as chicken feather or animal hair into novel sources of nutrients seems to be especially valid in the EU, as up to 77% of required protein for feed and food supplies is imported from the outside of the EU member states [152]. As poultry feathers are among the most widely distributed keratin-rich industrial waste globally, attempts to converse it to novel and potentially beneficial protein products are not surprising. Examples of keratinolytic microorganisms or enzymes utilized for bioconversion of keratin waste into animal feed are presented in Table 9.

Table 9.
Examples of keratinolytic microorganism utilized for bioconversion of keratin waste into animal feed [135,153].
As study shows, steam hydrolyzed feather meal can be a more sustainable substitute for a fish meal in aquaculture [150]. However, little is yet known about the LCA and potential EI of feather-waste-derived feed hydrolysates obtained by microbial or enzymatic degradation. Reportedly, supplementing animal feed with keratinases improves its digestibility, as well as promoting microbiota growth and immune response in livestock [72]. Therefore, bioconversion of keratin waste into animal feed using whole cells and/or keratinolytic enzymes, might be a promising approach for better sustainability of the meat industry, as feed or protein digestibility positively impact the feed conversion ratio, which is strongly correlated with the animal feed environmental impact [150,151].
Microbial (bacterial, yeast, microalgal), insect and waste-derived proteins are amongst the top options regarded as more sustainable sources of nutrients than soy and its derivatives; therefore, their implementation needs further exploration and research [151]. However, some of microbial strains used for a small scale full-cell hydrolysis of keratin-rich substrates for enzyme, peptide and amino acid production, are known human and animal pathogens [84]. The presence of endotoxins and risk of the spread of infectious diseases are main concerns for the usage of some native keratinolytic microbes; therefore, their direct applicability on a larger scale is compromised [25].
5.2.3. Livestock Stress Assessment
Unsuitable and improper breeding conditions in large-scale industrial meat plants are among the factors that can affect the levels of steroid stress hormones, glucocorticoids (GCCs), in animal bloodstreams [154]. Increased blood concentrations of GCCs can lead to suppression of growth and reproduction, as well as overall immunodeficiency. Therefore, stress management should be a necessity for improving and maintaining animals’ well-being. As GCCs are secreted in response to many, potentially stressful, environmental factors, their levels can be used for an overall assessment of population stress, an assessment of long-term response to a newly introduced factor or for an effective control of stress-mitigating solutions. The main GCC checked in birds, including avian livestock, is called corticosterone (CORT) [155]. However, current methods of CORT indexing are mostly based on blood samples, which collecting is an event stressful itself and have to be repeated multiple times for long-term stress assessment. The CORT levels in feather tissue corresponds with its blood concentration during its period of growth [155]. Moreover, GCCs deposited in feathers are stable, thus samples do not need refrigeration or immediate extraction and hormone measurement [156]. Due to known resistance of keratins to proteolysis with conventional enzymes, keratinases have been used as promising agents for enhanced digestion of feathers and improved extraction of CORT from samples [154]. For that purpose, commercial keratinase from B. licheniformis (Cibenza IND900, Novus International, Inc., St. Charles, MO, USA) was applied as the enzyme-digesting keratin sample. Serial dilutions (with PBS buffer, pH 9.0) were performed to specify which enzyme concentration will be the most suitable for further extraction of radiolabeled GCCs. Feather samples have been incubated with enzymatic solutions of pH 9.0 at 45 °C for 5 days and observed [154]. From all tested concentrations, only undiluted keratinase solution (1:30) liquefied feathers to the point suitable for solid-phase extraction (SPE) techniques. Implementation of enzymatic digestion allowed for consistent and repetitive hormone extraction and development of a new accurate and reliable detection of CCS at very low concentrations (pg/mL). As study suggest, presented CORT assay protocol may be extended to different types of animal samples, as well as to other GCCs and biomolecules in various taxa [154].
5.3. Biotechnology
Microbial Media
The bacterium Pseudomonas sp. P5 has been utilized for the valorization of poultry feathers as a substrate for the production of protein hydrolysates with potential application as microbial media [37]. An isolated strain with the highest proteolytic and keratinolytic activity was cultured on mineral medium supplemented with raw wet feathers (form 30 g/L to 90 g/L). After 5 days of incubation, feather substrate hydrolysis rate varied from 70 to up to 93% and was correlated with initial concentration of feather supplement (highest rate observed for lowest feather concentration) [37]. However, with higher levels of keratin-substrate (70 and 90 g/L), the weight of bacterial biomass, the amount of extracellular enzyme and its activity increased, resulting in the maximum keratinolytic activity of 36.2 ± 8.4 U/mL (for 70 g/L). In this study, the final content of peptides and amino acids in the medium after full cell fermentation by Pseudomonas sp. P5 was compared with enzymatic (partially purified keratinase from the same bacterium; temp. 50 °C, pH 7.5, 24−48 h, with 1–2 U per 1 mg of dry feathers), weak alkaline hydrolysis (0.6% KOH, 70 °C, 24 h) and control conditions, with two same feather concentrations of 70 and 90 g/L. As the result, hydrolysate after full-cell fermentation contained significantly lower amounts of amino acids, 301.2 mg/L (90 g/L of wet feather) and 274.8 mg/L (70 g/L of wet feather), than the enzymatic hydrolysate, 1047.6 mg/L (90 g/L of wet feather) and 1090.7 mg/L (70 g/L of wet feather). However, peptides concentration was slightly higher in hydrolysate after full-cell fermentation (6.2 and 4.6 g/L compared with 3.2 and 3.3 g/L in enzymatic hydrolysate) [37]. Alkaline hydrolysis resulted in the highest number of peptides (17.2 g/L and 14.3 g/L) of all types of feather treatments, but lower concentrations of amino acids (326.9 mg/L and 377.8 mg/L) than in the enzymatic hydrolysate. Amino acid profiles were tested using ultra-high-performance liquid chromatography (UHPLC). Essential amino acids accounted for nearly 54% of all amino acids in post-culture liquid after enzymatic treatment, 44% after direct fermentation with Pseudomonas sp. P5 cells and only 12.5% after alkaline hydrolysis [37].
Obtained hydrolysates were further tested as culture medium for E. coli, a well-known and widely used model microorganism in biotechnology. Enzymatic and alkaline hydrolysates tested positive with no additional ingredients and operations (except pH adjustment) needed for satisfactory bacterial growth. The development of non-pretreated keratin substrate bioconversion (excluding the need for washing, milling or grounding) could positively affect cost of medium preparation; therefore, this increases the feasibility and applicability of the biotechnological process [37]. Though with time such hydrolysates can become a less expensive alternative for peptones in popular microbial media, more research is still required as commercially available protein ingredients are produced in well-optimized processes with product being filtrated for the removal of potential inhibitors, toxins and molecules of high molecular mass [37].
Keratin waste hydrolysates produced by enzymatic in vitro proteolysis or full cell fermentation of keratin substrates, can be also utilized for synthesis of organic micronutrients with crucial metabolic activities, such as vitamin B [25]. As the study shows, crude keratinase from newly isolated Bacillus thuringiens is MT1 was successfully used for preparation of amino-acid-rich hydrolysate from donkey hair [25]. The release of amino acid was obtained by incubation of previously washed and dried hair (0.5 g) with diluted crude enzyme (250 mL; glycine/NaOH buffer; final activity of 20 U/mL) at 50 °C, pH 9 and 100 rpm for 24 h. After incubation, the concentrations of amino acids were tested and their different levels were supplemented to YPD broth as precursors of vitamin B-complex (B1, B2 and B12) production by Sacharomyces cerevisiae (ATCC 64712). As a result, donkey hair hydrolysate was composed of 16 amino acids, half of them being essential amino acids (Met, Phe, Thr, Val, Leu, Ile, Lys and His) and other 8 the non-essential ones (Glu, Asp, Pro, Ala, Arg, Gly, Ser and Tyr) with concentrations ranging from 13.5 (Ile) to 32.3 (Lys) µg/mL and from 7.0 (Tyr) to 143.7 (Glu) µg/mL, respectively [25]. Analysis of enzyme-free post-incubation liquid revealed no free amino acids. Several concentrations of hair hydrolysate (from 0.0 to 2.5% of total amino acid) were used as supplement in S. cerevisiae culture for vitamin B-complex synthesis. The highest amounts of all three vitamins, B1, B2 and B12, were obtained with 1.5% of free amino acid solution at concentrations of 0.04, 0.06 and 0.06 mg/mL, respectively. In control culture (without hair hydrolysate supplementation), the presence of a vitamin B complex was not detected [25]. The bioconversion of donkey hair into a production medium for vitamins synthesis, though requiring a further optimization, seems to be a promising new method of keratin waste management. As the study suggests, other keratin-rich substrates can also be valorized for vitamin B complex production, as they often have a different chemical and protein structure, which may affect the final content and variety of free amino acids in potential production medium, as well as the amount of particular vitamins synthesized within the B complex [25].
5.4. Pharmaceuticals and Medicine
5.4.1. Transdermal Drug Delivery Systems
Many keratin-degrading microbes, especially fungal, are also human pathogens, often causing long-lasting and troublesome skin infections. Those microorganisms produce keratinolytic enzymes in order to infect and penetrate keratin-based tissues. However, such enzymatic virulence factors may also find novel application in transdermal drug delivery systems, where they could act as effectiveness-enhancing reagent for better and deeper delivery of antibiotics and other molecules for deep skin infections treatment [63]. Keratinase from Bacillus cereus was isolated from samples of farm soils in Egypt, biochemically characterized and tested in the transdermal drug delivery system (TDDS), composed of keratinolytic enzyme and fucidic acid cream. The effectiveness of the proposed TDDS was studied on mice, subcutaneously infected with 50 µL of Staphylococcus aureus ATCC 25,729 (2 × 109 CFU/mL) suspension—an equivalent to 1 × 108 CFU. Each group was observed, with complete healing periods being recorded. After that time, viable counts of S. aureus in skin sample homogenizates were examined using the mannitol salt agar plate cultivation method. As the results show, keratinase exhibited a relatively large thermal stability range by retaining up to 90% of primary activity after 16 h of incubation in temperatures between 4 °C and 50 °C. The enzyme maintained high activity after incubation for 16 h at pH 7.0, and about 90% after 2 h at pH 9.0. However, keratinase was quite sensitive towards more acidic pH. Implementation of keratinase into standard treatment of fucidic acid cream resulted in significant reduction in the time needed for complete healing from 7 days (for group treated only with fucidic acid) to only 5 days, with a notable decline in S. aureus viable count of about 2 log cycles on the fourth day of infection treatment [63].
Keratinolytic enzymes, acting as an additive for improved delivery of therapeutics and antibiotics, e.g., for deep skin bacterial infection treatments, opens yet another way for chicken feather waste management and rational keratinases application in novel industry.
5.4.2. Anti-Amyloid- β Agents
Amyloids are misfolded forms of regular proteins, that can interact with themselves and other agents, developing insoluble fibrous aggregates resistant to proteolysis. Deposition of such aggregates is main or co-responsible reason behind hereditary lysozyme amyloidosis, Alzheimer’s disease (AD), Huntington’s disease, Parkinson’s disease, prion diseases and many others [157]. When toxic peptide particles, from 39 to 43 amino acid residues long, known as amyloid-β peptides (Aβ), are aggregated in extracellular space between nerve cells in brain tissue, they block synaptic transmissions, what results in degeneration of neurons and AD symptoms and progress [157]. Because keratinases seem to be specialized in degrading β-sheet-abundant recalcitrant substrates, typically not available for common proteases, research on their potential application in degradation of Aβs could lead to promising results and development of novel therapeutic methods amyloidosis-associated diseases [157].
Two bacterial keratinolytic enzymes Ker1 and Ker2, derived from Amycolatopsis sp. MBRL 40, have been tested for degradation of Aβ fibrils, synthesized from hen egg white lysozyme (HEWL) used as a model system. Aβ fibrous precipitates were suspended in 50 mM phosphate buffer (pH 7.0) and incubated with purified keratinases at different concentrations (50, 100, 125, 250 and 500 mg/mL) at 40 °C and for various catalysis time (0, 3, 6, 12, and 24 h). Presence of amyloidal structures were then tested by immunoblotting with anti-Aβ antibody [157]. As the result, Ker1 (in conc. of 125 mg/mL) completely degraded Aβ fibrils after 24 h at 40 °C. Digestion was later confirmed by no signals in immunoblot (untreated fibrils showed a positive signal). Ker2 was less effective than Ker1 with partial solubilization observed after 24 h. For complete degradation, time of the process needed to be extended to 7 days. Neutral phosphatidylcholine and cationic DOPE/DOTAP liposomes, loaded with Ker1, were synthesized to determine, whether such formations can also degrade Aβ fibrils. Similar to soluble Ker1, both types of proteoliposomes exhibited excellent Aβ-digesting abilities, confirmed by immunoblotting [157].
Keratinolytic biomolecules could become an interesting alternative to other, currently tested drugs for amyloidoses-associated diseases, that can cause unwanted side-effects, such as hepatic, renal and retinal toxicity, subacute meningoencephalitis, as well as injection-site reactions [157].
5.5. Biomaterials
PHA Production
Microbial polyhydroxyalkanoates (PHA) are a group of biopolyesters heavily studied for their physiochemical, biochemical and mechanical properties [158]. Their mechanical properties and degradability can be changed and adjusted by monomeric composition of synthesized polymers, by manipulating production process parameters, substrates, down-stream processing modification and selection of microbial production strains. PHA have found numerous applications in medicine, e.g., drug delivery and tissue engineering [159,160]. Products composed of PHA polymers and copolymers have been already applied in agriculture [161] and in the food industry as biodegradable and renewable packaging materials [162]. In biotechnology, PHA scaffolds can be used for enzyme immobilization [163]. They are synthesized by various microorganisms, such as Cupriavidus necator [164] and Pseudomonas spp. [165], as carbon and energy source, when nitrogen availability in environment is limited. Protective and stress-resisting functions of PHA were also described [166]. To meet petroleum-based polymer’s market prices with sustainability, many efforts have been put into developing PHA production processes based on agricultural and food waste [167]. Keratinolytic microorganisms have been also tied up with synergetic production of medium-chain-length polyhydroxyalkanoates (mcl-PHA) using chicken feathers as sole carbon source for Pseudomonas putida KT2440 growth medium [168]. Bacterial biomass obtained after separation of degraded feathers was resuspended in PHA production medium, supplemented with waste frying oil. As a result of 72 h cultivation, 61.4% wt of mcl-PHA per cell dry weight and 1.42 g/l−1 mcl-PHA productivity yields were obtained. Biosynthesized polymer was composed of C-6 (27.2 %mol) and C-8 (72.8 %mol) monomers. As the study suggests, such approach may not only be used as a sustainable method for mcl-PHA production (after further optimization), but also as an enzyme (keratinase present in post-culture filtrate of growth medium) and animal feed (partially hydrolyzed chicken feather) coproduction/cogeneration [168].
5.6. Detergent, Leather and Textile Industries
Market of industrial enzymes is estimated to reach worth of USD 6.2 billion in this decade with market demand, partially driven by worldwide implementation of sustainable development policies, rising 7% annually [38]. Since the 1960s, many alkaline proteases were recognized as beneficial detergent additives and found application in formulations for stain removal [69].
In order to be applicable in detergent, leather and textile industries (see Table 10), keratinases should be stable in the presence of commercial detergent formulations, typically enzyme-denaturing (e.g., SDS or CTAB) or non-denaturing detergents (e.g., Triton X-100, Tween-20, Tween-80), alkaline pH (up to pH 12.5) and heavy metals [126]. In such conditions, most enzymes are unable to maintain their activity; however, many keratinolytic enzymes, when exposed to extreme abiotic factors, exhibit either good stability or even show higher enzymatic activity [19].

Table 10.
Keratinolytic microorganisms or enzymes with potential application in detergent, leather or textile industry.
In the leather industry, keratinolytic enzymes can be applied as dehairing agents thanks to (unlike in alkaline or sulfide treatments) exhibition of high substrate specificity towards hair-building keratins, thus allowing for proteolytic hair removal without damaging collagen-rich skin [91]. For example, the keratinase from Bacillus sp. RCM-SSR-102, known as KER102, had maximum observed activity at 50°C and pH 10.0 (retaining > 90% of activity in the range of pH 6.0 to 12.0). Moreover, KER102 could retain around 55% of its activity in highly saline environment of 3.4 M NaCl. Presence of Mg2+ and Ca2+ was stimulatory for the enzyme, whereas Fe2+, Pb2+, Ni2+, Cu2+, Zn2+, Mn2+ and Hg2+ ions had inhibitory effects. Both Tween-20 (1% v/v) and Tween-40 (1% v/v) have slightly increased enzyme activity, whereas 1% v/v of Triton X-100 have not changed it notably. KER102 showed K:C (keratinolytic to caseinolytic activity ratio) of 0.70 and 0.65 for feather and keratin azure, respectively. Such activity ratio determination is yet another method used for differentiating proteases from keratinases. Ratio exceeding 0.50 characterizes keratinolytic nature of a biocatalyst [97].
In the textile industry, keratinases may find applications mostly in pretreatments and conditioning of wool and woolen fabrics, as they are troublesome to process due to the felting shrinkage, compromised dyeing efficiency and degumming necessity [43]. Conventionally, excessive washing of the material leads to the felting and fibers entanglement, limiting fibers dyeing ability [82]. In addition, standard degumming process is regarded as hazardous to human health and environmentally problematic, thanks to utilization of concentrated chemicals, high process temperatures and generation of burdensome wastewater [43]. In result, keratinolytic enzymes might become a “green” alternative to conventional methods. Recombinant keratinase from B. licheniformis (500 U/mL) was incubated with wool fabric for 2 h, at 50 °C and pH 8.5 with addition 0.5 g/l non-ionic surfactant [103]. As the result, improvement of hydrophilicity and reduction in tensile strength was observed. Moreover, the enzyme was successfully immobilized with chitosan-E (chitosan + glutaraldehyde) and chitosan-β-CD-E (chitosan + cyclodextrin + glutaraldehyde) beads with high efficiency (immobilization yield of 90 and 93%, respectively), allowing for enzyme reusability and increased storage stability (assay with keratin azure), and thus potentially improving cost-effectiveness of enzymatic wool conditioning. However, enzymatic activity of immobilized keratinase towards wool and wool fabrics has yet to be determined [103].
Possibility of replacing environmentally unsustainable and hazardous chemicals with biodegradable enzymes in one of the highest polluting industries without loss of efficiency and generation of next hard-to-manage waste streams, seems to be especially promising for the implementation keratinases in leather and textile sectors of economy [18,19,75].
5.7. Environmental Protection
5.7.1. Wastewater Treatment
Keratinases were studied in relation to treatment of heavily polluted, industrial wastewaters, such as molasses wastewater (MWW) from sugar factories, composed of various hazardous contaminants, including melanoidins [106,171].
Due to high concentrations of contaminants and co-pollutants, oxidation resistance and often significant antimicrobial effect, conventional secondary aerobic and anaerobic biological treatments are not efficient in decolorization of MWW [171]. Melanoidins, phenols and caramel are major colorants in treated MWW (TMWW), with the first group being the most abundant [171]. Some physical and physicochemical treatments (flocculation precipitation, membrane methods, photocatalysis), though yielding an excellent decolorization performance, are not economically and environmentally sustainable (high energy consumption, generation of secondary pollutants) [106]. However, cultivation of Aspergillus citeromyces and Bacillus strains on TMWW was applied in decolorization processes with oxidoreductases, such as laccase, glucose oxidase and lignin peroxidase, acting as main agents. Due to the need of co-enzymes and the requirements of specific conditions, oxidoreductases are often seen as unviable for large scale processes [106]. Therefore, hydrolases such as cellulases, cutinases and proteases have been widely studied for wastewater treatment, but removal of TMWW pigments has not been reported.
Several immobilized commercial hydrolytic enzymes, including lipase, α-amylase, protease, α-dextranase, pectinase, cellulase and keratinase (Meiothermus sp.) as well as immobilized oxidoreductases—laccase, glucose oxidase and manganese peroxidase—, have been tested for decolorization of TMWW from cane sugar factories. Additionally, recombinant keratinase from wild-type of Meiothermus taiwanensis WR-220 thermophilic bacterium (KMT-wt), expressed in E. coli has been immobilized on modified bagasse cellulose and also valorized. Commercial immobilized enzymes (500 U each) were added to 1000 mL of TMWW and left at 30 °C, pH 7.5 at 100 rpm for 12 h. After that, decolorization yields were calculated. Interestingly, commercial immobilized keratinase, eliminated from 86.6% to 91.1% of all colorants in used TMWW with other commercial hydrolases and oxidoreductases obtaining lesser yields. Similarly, immobilized KMT-wt (150 U) removed from 84.7% to 90.2% of all color contaminants after 2 h, and up to 85.2% of melanoidins present in TMWW. KMT-wt exhibited better chemical oxygen demand (COD) and biochemical oxygen demand (BOD) removal efficiency after continuous 5-day treatment than the commercial keratinase. However, it had little to no effect on caramel and phenols removal [106].
Taking into consideration high efficiency and lower costs of purchase of studied keratinases, studied enzymes could pose a potentially viable alternatives to available oxidoreductases [106].
5.7.2. Prion Proteins Decontamination
Prions are infectious misfolded protein agents causing progressive neurological diseases known as transmissible spongiform encephalopathies (TSEs) [172]. Scrapie in sheep and goat, bovine spongiform encephalopathy (BSE) in cattle (also known as “mad cow disease”), chronic wasting disease (CWD) in deer (often called “zombie dear disease”) and Creutzfeldt-Jakob disease (CJD) and kuru disease in humans are all the examples of TSEs [157,172,173]. As TSEs are incurable and mostly fatal, management of prion transmission has become a crucial challenge for the public health. The danger of prion transmission through consumption of contaminated food and fodder impeded the animal waste usage in fodder formulations [18].
Due to high resistance to proteolysis performed by conventional proteases and traditional chemical and physicochemical sterilization methods, the protease-resistant prion protein (PrPSc) needs a special approach. Effective ways of prion decontamination include incineration, high-pressure hydrolysis (132°C for 18 min) and usage of concentrated chemicals, such as sodium hydroxide, hydrogen peroxide, phenolics, guanidine thiocyanate and peracetic acid [172,173,174]. However, those methods are not regarded as environmentally sustainable and often not suitable for decontamination of medical devices and materials or substrates that could be recycled and further processed into value added products. Over the years, several studies reported, that keratinolytic enzymes may be potentially applied in prion protein degradation [18,22,172,173,174], due to the fact that similar to β-keratin, accumulating PrPSc are also largely composed of fibers with β-pleated sheets.
The B. licheniformis N22 keratinase and Pseudomonas aeruginosa NCIMB 8626 biosurfactant formulation was found to partially degrade ME7 scrapie prions after 1 h at 50 °C and, when temperature was raised up to 65 °C, studied PrPSc were undetected (by Western blot analysis) after only 10 min of the process. Reportedly, sole B. licheniformis N22 keratinase (EF) could not fully digest ME7 prions at 65 °C even after 1 h. In such conditions, sample with both enzyme and biosurfactant led to a complete digestion of infectious proteins [173].
Purified keratinase MSK103 secreted by B. licheniformis effectively degraded PrPSc in homogenates of brain tissues infected with scrapie or BSE prions after 2 h at 50 °C, and degraded prions to the levels below the limits of used method, when time of decontamination was extended to 20 h. MSK103 keratinase could also perform PrPSc biodegradation in dry samples [172]. Recombinant E. coli keratinase from Pseudomonas aeruginosa KS-1 synergistically with γ-glutamyl transpeptidase (GGT) and glutathione (GSH) complex (producing cysteinyl-glycine, a strong disulfide bonds reducing agents) demonstrated effective degradation of prion-like protein Sup 35NM in 15 min at 37 °C and pH 7.0 [174] Lesser effect was observed when E. coli keratinase was replaced by proteinase K. No to little effect was noted when enzymes were not accompanied by γ-glutamyl transpeptidase [174].
These enzymes showed the ability to degrade the PrSc protein into harmless and immunochemically undetectable forms. Therefore, keratinases, when mixed with other enzymes or biomolecules, can be used as potentially safe, more sustainable, and environmentally friendly method of decontamination and disposal of hazardous waste from infected animals, recycling nutrients in animal agriculture or cleaning medical equipment.
5.7.3. Wild-Life Protection
As previously mentioned, keratinolytic enzymes can become a tool for stress assessment in industrially bred poultry, by determining levels of avian glucocorticoid stress hormone concentration deposited in feather tissue, as CORT hormone levels in epidermal keratin tissue corresponds with its concentrations in bloodstream during their period of growth [154,155].
Development of keratinase-involving methodology for improved steroid (glucocorticoids) extraction from chicken feathers [154] opened a way for implementing such approach towards samples of various epidermal tissue types of other species [175]. CORT tissue-deposited concentrations have been studied using B. licheniformis keratinase for sample digestion in whale (Balaenoptera musculus, Balaena mysticetus and Eubalaena australis) baleen, purple martin (Progne subis) feathers, arctic ground squirrel (Urocitellus parryii) hair, narrow-headed garter snake (Thamnophis rufipunctatus) and tegu lizard (Salvator merianae) shed skin and short-beaked echidna (Tachyloglossus aculeatus) spine, covering several vertebrate taxa [175]. A similar approach was also used to determine levels of reproductive hormones, progestagen and androgen in Temminick’s pangolin (Smutsia temminickii) scales [175,176]. As study shows, incorporation of keratin enzymatic digestion into standard protocol (after mechanical disruption and before organic solvent extraction) allows to obtain higher yields of CORT hormone from almost all samples. Relatively non-invasive, simple sample collection and possibility of analysis samples of limited mass and availability, seems to be especially beneficial in studying wild populations of endangered species [175,176]. However, differently than in industrially breed livestock, wild-life populations, notably animals leading mostly solitary lifestyle, may experience many stress-inducing periods unknown to researchers, which are impossible to detect or to catalogue during sample gathering and observational studies. Such obstacles can negatively affect comparability of hormone levels of certain individuals deposited in respective tissues [175,177]. The egg-laying process has been proven to rise CORT levels in blood and feather samples of red-legged partridge females (Alectoris rufa) with positive correlation between clutch sizes and hormone levels [155], posing theories that raised CORT concentration might be a natural cost of energy invested in reproduction or that the CORT hormone is needed for recovery after such a period [155].
Though promising, enzymatic digestion of keratin substrates possesses several concerns and limits [154,175,176]. As keratinases are proteolytic enzymes of diverse substrate specificity, not all enzymes are suitable for degrading a wide range of sampled tissues. Keratinase from B. licheniformis (FEED-0001; Creative Enzymes, Shirley, NY, USA) resulted in better yields of CORT hormones in hair, spine, baleen and feather samples varying from 30% to up to 377% higher hormone levels than in corresponding samples that were not subjected to enzymatic treatment. The highest change of CORT levels occurred in feather and hair samples, respectively. However, little to no change was recorded for snake and lizard shed skins samples. This may be due to many factors, e.g., sample age and composition, mainly α- and β-keratins ratios (very important in context of enzymatic digestion), distribution of hormones and their metabolites throughout sample, species differences, as well as sex and age of an tested individual [175,178]. Reportedly, CORT hormone and its metabolite concentrations can also differ based on body region that the sample was taken from [176]. As these differences seems to be species-specific, wildlife researchers should also take such premises into consideration when developing protocols for hormone assays deposited in keratin tissues [176,177].
6. Conclusions
Presented review explored various applications of keratinases and keratinolytic microorganisms in diverse industrial sectors, from environmental protection (decontamination of prion proteins, wastewater treatment, wildlife protection) to pharmaceuticals and medicine (anti-amyloid-β agents and transdermal drug delivery systems). Bioconversion of animal-derived keratin waste into biofertilizers, plant-growth stimulants and animal feedstuffs is in line with, at least partially, the enclosure of cross-sectoral production cycles, and caters to circular economy and bioeconomy models and policies. Application of keratinolytic microorganisms and enzymes, though still in a need of further investigation, gives an opportunity for the development of more sustainable methods of keratin waste management with generation of potentially high-end value-added products.
Author Contributions
Writing—original draft preparation, M.S.; writing—review and editing, A.M.B., I.J.; supervision, A.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sachs, J.D.; Schmidt-Traub, G.; Mazzucato, M.; Messner, D.; Nakicenovic, N.; Rockström, J. Six Transformations to achieve the Sustainable Development Goals. Nat. Sustain. 2019, 2, 805–814. [Google Scholar] [CrossRef]
- The United Nations. The Sustainable Development Goals. Available online: https://sdgs.un.org/goals (accessed on 5 October 2021).
- Zeng, Y.; Maxwell, S.; Runting, R.K.; Venter, O.; Watson, J.E.M.; Carrasco, L.R. Environmental destruction not avoided with the Sustainable Development Goals. Nat. Sustain. 2020, 3, 795–798. [Google Scholar] [CrossRef]
- Raszkowski, A.; Bartniczak, B. On the road to sustainability: Implementation of the 2030 Agenda sustainable development goals (SDG) in Poland. Sustainability 2019, 11, 366. [Google Scholar] [CrossRef]
- Khalid, A.M.; Sharma, S.; Dubey, A.K. Concerns of developing countries and the sustainable development goals: Case for India. Int. J. Sustain. Dev. World Ecol. 2021, 28, 303–315. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Aveyard, P.; Garnett, T.; Hall, J.W.; Key, T.J.; Lorimer, J.; Pierrehumbert, R.T.; Scarborough, P.; Springmann, M.; Jebb, S.A. Meat consumption, health, and the environment. Science 2018, 361. [Google Scholar] [CrossRef]
- Alsaffar, A.A. Sustainable diets: The interaction between food industry, nutrition, health and the environment. Food Sci. Technol. Int. 2016, 22, 102–111. [Google Scholar] [CrossRef]
- Cheng, D.; Liu, Y.; Hao, H.; Guo, W.; Woong, S.; Duc, D.; Zhang, S.; Luo, G.; Thanh, X. Sustainable enzymatic technologies in waste animal fat and protein management. J. Environ. Manag. 2021, 284, 112040. [Google Scholar] [CrossRef]
- European Commission. Poultry. Available online: https://ec.europa.eu/info/food-farming-fisheries/animals-and-animal-products/animal-products/poultry_en (accessed on 5 October 2021).
- Dróżdż, D.; Wystalska, K.; Malińska, K.; Grosser, A.; Grobelak, A.; Kacprzak, M. Management of poultry manure in Poland—Current state and future perspectives. J. Environ. Manag. 2020, 264, 110327. [Google Scholar] [CrossRef]
- Thyagarajan, D.; Barathi, M.; Sakthivadivu, R. Scope of Poultry Waste Utilization. J. Agric. Vet. Sci. 2013, 6, 29–35. [Google Scholar]
- Leinonen, I.; Kyriazakis, I. How can we improve the environmental sustainability of poultry production? Proc. Nutr. Soc. 2016, 265–273. [Google Scholar] [CrossRef]
- European Commission; CORDIS. Industrial Feather Waste Valorisation for Sustainable KeRatin Based MAterials. Available online: https://cordis.europa.eu/project/id/723268 (accessed on 5 October 2021).
- Aranberri, I.; Montes, S.; Azcune, I.; Rekondo, A.; Grande, H.J. Fully biodegradable biocomposites with high chicken feather content. Polymers 2017, 9, 593. [Google Scholar] [CrossRef]
- Alahyaribeik, S.; Sharifi, S.D.; Tabandeh, F.; Honarbakhsh, S.; Ghazanfari, S. Bioconversion of chicken feather wastes by keratinolytic bacteria. Process Saf. Environ. Prot. 2020, 135, 171–178. [Google Scholar] [CrossRef]
- Sharma, I.; Kango, N. Production and characterization of keratinase by Ochrobactrum intermedium for feather keratin utilization. Int. J. Biol. Macromol. 2021, 166, 1046–1056. [Google Scholar] [CrossRef]
- Nnolim, N.E.; Nwodo, U.U. Microbial keratinase and the bio-economy: A three-decade meta-analysis of research exploit. AMB Express 2021, 11. [Google Scholar] [CrossRef]
- Nnolim, N.E.; Udenigwe, C.C.; Okoh, A.I.; Nwodo, U.U. Microbial Keratinase: Next Generation Green Catalyst and Prospective Applications. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Martinez, J.P.D.O.; Cai, G.; Nachtschatt, M.; Navone, L.; Zhang, Z.; Robins, K.; Speight, R. Challenges and opportunities in identifying and characterising keratinases for value-added peptide production. Catalysts 2020, 10, 184. [Google Scholar] [CrossRef]
- Qiu, J.; Wilkens, C.; Barrett, K.; Meyer, A.S. Microbial enzymes catalyzing keratin degradation: Classification, structure, function. Biotechnol. Adv. 2020, 44. [Google Scholar] [CrossRef]
- Wang, B.; Yang, W.; McKittrick, J.; Meyers, M.A. Keratin: Structure, mechanical properties, occurrence in biological organisms, and efforts at bioinspiration. Prog. Mater. Sci. 2016, 76, 229–318. [Google Scholar] [CrossRef]
- Rodziewicz, A.; Łaba, W. Keratyny i ich biodegradacja. Biotechnologia 2006, 73, 130–147. [Google Scholar]
- Sinkiewicz, I.; Staroszczyk, H.; Śliwińska, A. Solubilization of keratins and functional properties of their isolates and hydrolysates. J. Food Biochem. 2018, 42, e12494. [Google Scholar] [CrossRef]
- Perta-Crisan, S.; Ursachi, C.S.; Gavrilas, S.; Oancea, F.; Munteanu, F.-D. Closing the Loop with Keratin-Rich Fibrous Materials. Polymers 2021, 13, 1896. [Google Scholar] [CrossRef]
- Hassan, M.A.; Taha, T.H.; Hamad, G.M.; Hashem, M.; Alamri, S.; Mostafa, Y.S. Biochemical characterisation and application of keratinase from Bacillus thuringiensis MT1 to enable valorisation of hair wastes through biosynthesis of vitamin B-complex. Int. J. Biol. Macromol. 2020, 153, 561–572. [Google Scholar] [CrossRef]
- Fraser, R.D.B.; Parry, D.A.D. Trichocyte Keratin-Associated Proteins (KAPs). In The Hair Fibre: Proteins, Structure and Development, Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2018; pp. 71–86. ISBN 9789811081958. [Google Scholar]
- Wang, J.; Hao, S.; Luo, T.; Cheng, Z.; Li, W.; Gao, F.; Guo, T.; Gong, Y.; Wang, B. Feather keratin hydrogel for wound repair: Preparation, healing effect and biocompatibility evaluation. Colloids Surf. B Biointerfaces 2017, 149, 341–350. [Google Scholar] [CrossRef]
- Hill, P.; Brantley, H.; Van Dyke, M. Some properties of keratin biomaterials: Kerateines. Biomaterials 2010, 31, 585–593. [Google Scholar] [CrossRef]
- Dhurai, B.; Saravanan, K. Exploration on Amino Acid Content and Morphological Structure in Chicken Feather Fiber. J. Text. Appar. Technol. Manag. 2012, 7, 1–6. [Google Scholar]
- Sinkiewicz, I.; Śliwińska, A.; Staroszczyk, H.; Kołodziejska, I. Alternative Methods of Preparation of Soluble Keratin from Chicken Feathers. Waste Biomass Valorization 2017, 8, 1043–1048. [Google Scholar] [CrossRef]
- Sakhno, T.V.; Barashkov, N.N.; Irgibaeva, I.S.; Mendigaliyeva, S.; Bostan, D.S. Ionic Liquids and Deep Eutectic Solvents and Their Use for Dissolving Animal Hair. Adv. Chem. Eng. Sci. 2020, 10, 40–51. [Google Scholar] [CrossRef]
- Shavandi, A.; Silva, T.H.; Bekhit, A.A.; Bekhit, A.E.D.A. Keratin: Dissolution, extraction and biomedical application. Biomater. Sci. 2017, 5, 1699–1735. [Google Scholar] [CrossRef] [PubMed]
- Rajabinejad, H.; Bucişcanu, I.I.; Maier, S.S. Practical ways of extracting keratin from keratinous wastes and by-products: A review. Environ. Eng. Manag. J. 2016, 15, 1131–1147. [Google Scholar] [CrossRef]
- Gaidau, C.; Stanca, M.; Niculescu, M.-D.; Alexe, C.-A.; Becheritu, M.; Horoias, R.; Cioineag, C.; Râpă, M.; Stanculescu, I.R. Wool Keratin Hydrolysates for Bioactive Additives Preparation. Materials 2021, 14, 4696. [Google Scholar] [CrossRef]
- Mu, B.; Hassan, F.; Yang, Y. Controlled assembly of secondary keratin structures for continuous and scalable production of tough fibers from chicken feathers. Green Chem. 2020, 22, 1726–1734. [Google Scholar] [CrossRef]
- Ding, S.; Sun, Y.; Chen, H.; Xu, C.; Hu, Y. An ultrasonic-ionic liquid process for the efficient acid catalyzed hydrolysis of feather keratin. Chin. J. Chem. Eng. 2019, 27, 660–667. [Google Scholar] [CrossRef]
- Stiborova, H.; Branska, B.; Vesela, T.; Lovecka, P.; Stranska, M.; Hajslova, J.; Jiru, M.; Patakova, P.; Demnerova, K. Transformation of raw feather waste into digestible peptides and amino acids. J. Chem. Technol. Biotechnol. 2016, 91, 1629–1637. [Google Scholar] [CrossRef]
- Błaszczyk, M.; Goryluk-Salmonowicz, A. Enzymy produkowane w skali przemysłowej przez mikroorganizmy. In Przemysłowe Wykorzystanie Mikroorganizmów; Kompanowska, M., Ed.; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2020; pp. 191–218. ISBN 978-83-01-21021-2. [Google Scholar]
- Vidmar, B.; Vodovnik, M. Microbial keratinases: Enzymes with promising biotechnological applications. Food Technol. Biotechnol. 2018, 56, 312–328. [Google Scholar] [CrossRef]
- Wawrzkiewicz, K.; Wolski, T.; Lobarzewski, J. Screening the keratinolytic activity of dermatophytes in vitro. Mycopathologia 1991, 1–8. [Google Scholar] [CrossRef]
- Keefe, M.; Lei, X.G. Unveiling the keratinolytic transcriptome of the black carpet beetle (Attagenus unicolor) for sustainable poultry feather recycling. Appl. Microbiol. Biotechnol. 2021, 105, 5577–5587. [Google Scholar] [CrossRef]
- Vilcinskas, A.; Schwabe, M.; Brinkrolf, K.; Plarre, R.; Wielsch, N. Larvae of the Clothing Moth Tineola bisselliella Maintain Gut Bacteria that Secrete Enzyme Cocktails to Facilitate the Digestion of Keratin. Microorganisms 2020, 8, 1415. [Google Scholar] [CrossRef]
- Srivastava, B.; Khatri, M.; Singh, G.; Kumar, S. Microbial keratinases : An overview of biochemical characterization and its eco-friendly approach for industrial applications. J. Clean. Prod. 2020, 252, 119847. [Google Scholar] [CrossRef]
- Thankaswamy, S.R.; Sundaramoorthy, S.; Palanivel, S.; Ramudu, K.N. Improved microbial degradation of animal hair waste from leather industry using Brevibacterium luteolum (MTCC 5982). J. Clean. Prod. 2018, 189, 701–708. [Google Scholar] [CrossRef]
- Lee, Y.; Dhanasingh, I.; Ahn, J.; Jin, H.; Choi, J.M.; Lee, S.; Lee, D. Biochemical and Structural Characterization of a Keratin-Degrading M32 Carboxypeptidase from Fervidobacterium islandicum AW-1. Biochem. Biophys. Res. Commun. 2015. [Google Scholar] [CrossRef]
- Muhsin, T.M.; Aubaid, A.H. Partial purification and some biochemical characteristics of exocellular keratinase from Trichophyton mentagrophytes var. erinacei. Mycopathologia 2001, 150, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.K.; Mukherjee, A.K. Optimization of production of an oxidant and detergent-stable alkaline β-keratinase from Brevibacillus sp. strain AS-S10-II: Application of enzyme in laundry detergent formulations and in leather industry. Biochem. Eng. J. 2011, 54, 47–56. [Google Scholar] [CrossRef]
- Mitsuiki, S.; Sakai, M.; Moriyama, Y.; Goto, M.; Furukawa, K. Purification and Some Properties of a Keratinolytic Enzyme from an Alkaliphilic Nocardiopsis sp. TOA-1. Biosci. Biotechnol. Biochem. 2002, 66, 164–167. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bernal, C.; Cair, J.; Coello, N. Purification and characterization of a novel exocellular keratinase from Kocuria rosea. Enzym. Microb. Technol. 2006, 38, 49–54. [Google Scholar] [CrossRef]
- Abdel-Fattah, A.M.; El-Gamal, M.S.; Ismail, S.A.; Emran, M.A.; Hashem, A.M. Biodegradation of feather waste by keratinase produced from newly isolated Bacillus licheniformis ALW1. J. Genet. Eng. Biotechnol. 2018, 16, 311–318. [Google Scholar] [CrossRef]
- Bhange, K.; Chaturvedi, V.; Bhatt, R. Simultaneous production of detergent stable keratinolytic protease, amylase and biosurfactant by Bacillus subtilis PF1 using agro industrial waste. Biotechnol. Rep. 2016, 10, 94–104. [Google Scholar] [CrossRef]
- Rieger, T.J.; de Oliveira, C.T.; Pereira, J.Q.; Brandelli, A.; Daroit, D.J. Proteolytic system of Bacillus sp. CL18 is capable of extensive feather degradation and hydrolysis of diverse protein substrates. Br. Poult. Sci. 2017, 58, 329–335. [Google Scholar] [CrossRef]
- Gegeckas, A.; Šimkutė, A.; Gudiukaitė, R.; Čitavičius, D.J. Characterization and application of keratinolytic paptidases from Bacillus spp. Int. J. Biol. Macromol. 2018, 113, 1206–1213. [Google Scholar] [CrossRef]
- Ferrareze, P.A.G.; Correa, A.P.F.; Brandelli, A. Purification and characterization of a keratinolytic protease produced by probiotic Bacillus subtilis. Biocatal. Agric. Biotechnol. 2016, 7, 102–109. [Google Scholar] [CrossRef]
- Emran, M.A.; Ismail, S.A.; Abdel-Fattah, A.M. Valorization of feather via the microbial production of multi-applicable keratinolytic enzyme. Biocatal. Agric. Biotechnol. 2020, 27, 101674. [Google Scholar] [CrossRef]
- Hamiche, S.; Mechri, S.; Khelouia, L.; Annane, R.; El Hattab, M.; Badis, A.; Jaouadi, B. Purification and biochemical characterization of two keratinases from Bacillus amyloliquefaciens S13 isolated from marine brown alga Zonaria tournefortii with potential keratin-biodegradation and hide-unhairing activities. Int. J. Biol. Macromol. 2019, 122, 758–769. [Google Scholar] [CrossRef]
- Tian, J.; Long, X.; Tian, Y.; Shi, B. Enhanced extracellular recombinant keratinase activity in Bacillus subtilis SCK6 through signal peptide optimization and site-directed mutagenesis. RSC Adv. 2019, 9, 33337–33344. [Google Scholar] [CrossRef]
- Zhang, R.X.; Gong, J.S.; Su, C.; Zhang, D.D.; Tian, H.; Dou, W.F.; Li, H.; Shi, J.S.; Xu, Z.H. Biochemical characterization of a novel surfactant-stable serine keratinase with no collagenase activity from Brevibacillus parabrevis CGMCC 10798. Int. J. Biol. Macromol. 2016, 93, 843–851. [Google Scholar] [CrossRef]
- Bouacem, K.; Bouanane-Darenfed, A.; Zaraî Jaouadi, N.; Joseph, M.; Hacene, H.; Ollivier, B.; Fardeau, M.-L.; Bejar, S.; Jaouadi, B. Novel serine keratinase from Caldicoprobacter algeriensis exhibiting outstanding hide dehairing abilities. Int. J. Biol. Macromol. 2016, 86, 321–328. [Google Scholar] [CrossRef]
- Wu, W.L.; Chen, M.Y.; Tu, I.F.; Lin, Y.C.; Eswarkumar, N.; Chen, M.Y.; Ho, M.C.; Wu, S.H. The discovery of novel heat-stable keratinases from Meiothermus taiwanensis WR-220 and other extremophiles. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Bokveld, A.; Nnolim, N.E.; Nwodo, U.U. Chryseobacterium aquifrigidense FANN1 Produced Detergent-Stable Metallokeratinase and Amino Acids Through the Abasement of Chicken Feathers. Front. Bioeng. Biotechnol. 2021, 9, 720176. [Google Scholar] [CrossRef]
- González, V.; Vargas-Straube, M.J.; Beys-da-Silva, W.O.; Santi, L.; Valencia, P.; Beltrametti, F.; Cámara, B. Enzyme Bioprospection of Marine-Derived Actinobacteria from the Chilean Coast and New Insight in the Mechanism of Keratin Degradation in Streptomyces sp. G11C. Mar. Drugs 2020, 18, 537. [Google Scholar] [CrossRef]
- Shalaby, M.M.; Samir, R.; Goma, F.A.Z.M.; Rammadan, M.A. Enhanced fusidic acid transdermal delivery achieved by newly isolated and optimized Bacillus cereus Keratinase. Biotechnol. Rep. 2021, 30, e00620. [Google Scholar] [CrossRef]
- Nnolim, N.E.; Nwodo, U.U. Bacillus sp. CSK2 produced thermostable alkaline keratinase using agro-wastes: Keratinolytic enzyme characterization. BMC Biotechnol. 2020, 20, 1–14. [Google Scholar] [CrossRef]
- Duffeck, C.E.; de Menezes, C.L.A.; Boscolo, M.; da Silva, R.; Gomes, E.; da Silva, R.R. Citrobacter diversus-derived keratinases and their potential application as detergent-compatible cloth-cleaning agents. Braz. J. Microbiol. 2020, 51, 969–977. [Google Scholar] [CrossRef]
- Barman, N.C.; Zohora, F.T.; Das, K.C.; Mowla, M.G.; Banu, N.A.; Salimullah, M.; Hashem, A. Production, partial optimization and characterization of keratinase enzyme by Arthrobacter sp. NFH5 isolated from soil samples. AMB Express 2017, 7. [Google Scholar] [CrossRef]
- Pawar, V.A.; Prajapati, A.S.; Akhani, R.C.; Patel, D.H.; Subramanian, R.B. Molecular and biochemical characterization of a thermostable keratinase from Bacillus altitudinis RBDV1. 3 Biotech 2018, 8, 2–8. [Google Scholar] [CrossRef]
- Cavello, I.; Bezus, B.; Cavalitto, S. The keratinolytic bacteria Bacillus cytotoxicus as a source of novel proteases and feather protein hydrolysates with antioxidant activities. J. Genet. Eng. Biotechnol. 2021, 19, 1–12. [Google Scholar] [CrossRef]
- Gurunathan, R.; Huang, B.; Ponnusamy, V.K.; Hwang, J.S.; Dahms, H.U. Novel recombinant keratin degrading subtilisin like serine alkaline protease from Bacillus cereus isolated from marine hydrothermal vent crabs. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Nnolim, N.E.; Mpaka, L.; Okoh, A.I.; Nwodo, U.U. Biochemical and molecular characterization of a thermostable alkaline metallo-keratinase from Bacillus sp. Nnolim-k1. Microorganisms 2020, 8, 1304. [Google Scholar] [CrossRef]
- Alwakeel, S.S.; Ameen, F.; Al Gwaiz, H.; Sonbol, H.; Alghamdi, S.; Moharram, A.M.; Al-Bedak, O.A. Keratinases produced by Aspergillus stelliformis, Aspergillus sydowii, and Fusarium brachygibbosum isolated from human hair: Yield and activity. J. Fungi 2021, 7, 471. [Google Scholar] [CrossRef]
- Yahaya, R.S.R.; Normi, Y.M.; Phang, L.Y.; Ahmad, S.A.; Abdullah, J.O.; Sabri, S. Molecular strategies to increase keratinase production in heterologous expression systems for industrial applications. Appl. Microbiol. Biotechnol. 2021, 105, 3955–3969. [Google Scholar] [CrossRef]
- Goda, D.A.; Bassiouny, A.R.; Abdel Monem, N.M.; Soliman, N.A.; Abdel Fattah, Y.R. Effective multi-functional biotechnological applications of protease/keratinase enzyme produced by new Egyptian isolate (Laceyella sacchari YNDH). J. Genet. Eng. Biotechnol. 2020, 18. [Google Scholar] [CrossRef]
- Letourneau, F.; Soussotte, V.; Bressollier, P.; Branland, P.; Verneuil, B. Keratinolytic activity of Streptomyces sp. S.K1-02: A new isolated strain. Lett. Appl. Microbiol. 1998, 26, 77–80. [Google Scholar] [CrossRef]
- Li, Q. Structure, Application, and Biochemistry of Microbial Keratinases. Front. Microbiol. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Dozie, I.N.S.; Okeke, C.N.; Unaeze, N.C. A thermostable, alkaline-active, keratinolytic proteinase from Chrysosporium keratinophilum. World J. Microbiol. Biotechnol. 1994, 10, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.; Jin, H.S.; La, J.W.; Sung, J.Y.; Park, S.Y.; Kim, W.C.; Lee, D.W. Identification of keratinases from Fervidobacterium islandicum AW-1 using dynamic gene expression profiling. Microb. Biotechnol. 2020, 13, 442–457. [Google Scholar] [CrossRef] [PubMed]
- Akhter, M.; Wal Marzan, L.; Akter, Y.; Shimizu, K. Microbial Bioremediation of Feather Waste for Keratinase Production: An Outstanding Solution for Leather Dehairing in Tanneries. Microbiol. Insights 2020, 13, 117863612091328. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.S.; Park, S.Y.; Kim, K.; Lee, Y.J.; Nam, G.W.; Kang, N.J.; Lee, D.W. Development of a keratinase activity assay using recombinant chicken feather keratin substrates. PLoS ONE 2017, 12, e0172712. [Google Scholar] [CrossRef]
- Herzog, B.; Overy, D.P.; Haltli, B.; Kerr, R.G. Discovery of keratinases using bacteria isolated from marine environments. Syst. Appl. Microbiol. 2015, 39, 49–57. [Google Scholar] [CrossRef]
- Tork, S.E.; Shahein, Y.E.; El-hakim, A.E.; Abdel-aty, A.M.; Aly, M.M. Production and characterization of thermostable metallo-keratinase from newly isolated Bacillus subtilis NRC 3. Int. J. Biol. Macromol. 2013, 55, 169–175. [Google Scholar] [CrossRef]
- Demirkan, E.; Kut, D.; Sevgi, T.; Dogan, M.; Baygin, E. Investigation of effects of protease enzyme produced by Bacillus subtilis 168 E6-5 and commercial enzyme on physical properties of woolen fabric. J. Text. Inst. 2020, 111, 26–35. [Google Scholar] [CrossRef]
- Iglesias, M.S.; Sequeiros, C.; García, S.; Olivera, N.L. Newly isolated Bacillus sp. G51 from Patagonian wool produces an enzyme combination suitable for felt-resist treatments of organic wool. Bioprocess Biosyst. Eng. 2017, 40, 833–842. [Google Scholar] [CrossRef]
- Bohacz, J.; Korniłowicz-Kowalska, T. Fungal diversity and keratinolytic activity of fungi from lignocellulosic composts with chicken feathers. Process Biochem. 2019, 80, 119–128. [Google Scholar] [CrossRef]
- Qu, F.; Chen, Q.; Ding, Y.; Liu, Z.; Zhao, Y.; Zhang, X.; Liu, Z.; Chen, J. Isolation of a feather-degrading strain of bacterium from spider gut and the purification and identification of its three key enzymes. Mol. Biol. Rep. 2018, 45, 1681–1989. [Google Scholar] [CrossRef]
- Gurung, S.K.; Adhikari, M.; Kim, S.W.; Bazie, S.; Kim, S.; Lee, H.G.; Kosol, S.; Lee, H.B.; Lee, Y.S. Discovery of Two Chrysosporium Species with Keratinolytic Activity from Field Soil in Korea. Mycobiology 2018, 46, 260–268. [Google Scholar] [CrossRef]
- Lowry, H.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Bockle, B.; Galunsky, B.; Muller, R. Characterization of a Keratinolytic Serine Proteinase from Streptomyces pactum DSM 40530. Appl. Environ. Microbiol. 1995, 61, 3705–3710. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, J.; Liu, B.; Du, G.; Chen, J. Enhancement of the catalytic efficiency and thermostability of Stenotrophomonas sp. keratinase KerSMD by domain exchange with KerSMF. Microb. Biotechnol. 2016, 9, 35–46. [Google Scholar] [CrossRef]
- Rozs, M.; Manczinger, L.; Vagvolgyi, C.; Kevei, F. Secretion of a trypsin-like thiol protease by a new keratinolytic strain of Bacillus licheniformis. FEMS Microbiol. Lett. 2001, 205, 221–224. [Google Scholar] [CrossRef]
- Macedo, A.J.; Beys da Silva, W.O.; Termignoni, C. Properties of a non collagen-degrading Bacillus subtilis keratinase. Can. J. Microbiol. 2008, 54, 180–188. [Google Scholar] [CrossRef]
- Bakhtiar, S.; Estiveira, R.J.; Hatti-Kaul, R. Substrate specificity of alkaline protease from alkaliphilic Nesterenkonia sp. AL20. Enzym. Microb. Technol. 2005, 37, 534–540. [Google Scholar] [CrossRef]
- Brandelli, A.; Daroit, D.J.; Riffel, A. Biochemical features of microbial keratinases and their production and applications. Appl. Microbiol. Biotechnol. 2010, 85, 1735–1750. [Google Scholar] [CrossRef]
- Makinen, P.; Makinen, K.K.; Syed, S.A. An Endo-Acting Proline-Specific Oligopeptidase from Treponema denticola ATCC 35405 : Evidence of Hydrolysis of Human Bioactive Peptides. Infect. Immun. 1994, 62, 4938–4947. [Google Scholar] [CrossRef]
- Wawrzkiewicz, K.; Łobarzewski, J.; Wolski, T. Intracellular keratinase of Trichophyton gallinae. J. Med. Vet. Mycol. 1987, 261–268. [Google Scholar] [CrossRef]
- Ramnani, P.; Gupta, R. Optimization of medium composition for keratinase production on feather by Bacillus licheniformis RG1 using statistical methods involving response surface methodology. Biotechnol. Appl. Biochem. 2004, 40, 191–196. [Google Scholar]
- Kshetri, P.; Roy, S.S.; Chanu, S.B.; Singh, T.S.; Tamreihao, K.; Sharma, S.K.; Ansari, M.A.; Prakash, N. Valorization of chicken feather waste into bioactive keratin hydrolysate by a newly purified keratinase from Bacillus sp. RCM-SSR-102. J. Environ. Manag. 2020, 273, 111195. [Google Scholar] [CrossRef]
- Busk, P.K.; Lange, L. Classification of fungal and bacterial lytic polysaccharide monooxygenases. BMC Genom. 2015, 16, 1–13. [Google Scholar] [CrossRef]
- Huang, Y.; Busk, P.K.; Herbst, F.A.; Lange, L. Genome and secretome analyses provide insights into keratin decomposition by novel proteases from the non-pathogenic fungus Onygena corvina. Appl. Microbiol. Biotechnol. 2015, 99, 9635–9649. [Google Scholar] [CrossRef]
- Lange, L.; Huang, Y.; Busk, P.K. Microbial decomposition of keratin in nature—A new hypothesis of industrial relevance. Appl. Microbiol. Biotechnol. 2016, 100, 2083–2096. [Google Scholar] [CrossRef]
- Farag, A.M.; Hassan, M.A. Purification, characterization and immobilization of a keratinase from Aspergillus oryzae. Enzym. Microb. Technol. 2004, 34, 85–93. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A General Overview of Support Materials for Enzyme Immobilization: Characteristics, Properties, Practical Utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- Srivastava, B.; Singh, H.; Khatri, M.; Singh, G.; Arya, S.K. Immobilization of keratinase on chitosan grafted-β-cyclodextrin for the improvement of the enzyme properties and application of free keratinase in the textile industry. Int. J. Biol. Macromol. 2020, 165, 1099–1110. [Google Scholar] [CrossRef]
- Husain, Q. Nanocarriers Immobilized Proteases and Their Industrial Applications: An Overview. J. Nanosci. Nanotechnol. 2018, 18, 486–499. [Google Scholar] [CrossRef]
- Adetunji, C.O.; Adejumo, I.O. Efficacy of crude and immobilised enzymes from Bacillus licheniformis for production of biodegraded feather meal and their assessment on chickens. Environ. Technol. Innov. 2018, 11, 116–124. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, D.; Zhang, X. Enzymatic decolorization of melanoidins from molasses wastewater by immobilized keratinase. Bioresour. Technol. 2019, 280, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.K.; Roy, J.K.; Mukherjee, A.K. Application of poly (vinyl alcohol)-assisted silver nanoparticles immobilized β-keratinase composite as topical antibacterial and dehairing agent. J. Proteins Proteom. 2020, 11, 119–134. [Google Scholar] [CrossRef]
- Konwarh, R.; Karak, N.; Rai, S.K. Polymer-assisted iron oxide magnetic nanoparticle immobilized keratinase. Nanotechnology 2009, 20, 225107. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.K.; Mukherjee, A.K. Optimization for production of liquid nitrogen fertilizer from the degradation of chicken feather by iron-oxide (Fe3O4) magnetic nanoparticles coupled β-keratinase. Biocatal. Agric. Biotechnol. 2015, 4, 632–644. [Google Scholar] [CrossRef]
- Wang, J.-J.; Swaisgood, H.E.; Shih, J.C.H. Bioimmobilization of Keratinase Using Bacillus subtilis and Escherichia coli Systems. Biotechnol. Bioeng. 2003, 81, 421–429. [Google Scholar] [CrossRef]
- Rubio, N.R.; Xiang, N.; Kaplan, D.L. Plant-based and cell-based approaches to meat production. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Gong, J.; Ye, J.; Tao, L.; Su, C.; Qin, J.; Zhang, Y.; Li, H. Enzyme and Microbial Technology E ffi cient keratinase expression via promoter engineering strategies for degradation of feather wastes. Enzym. Microb. Technol. 2020, 137, 109550. [Google Scholar] [CrossRef]
- Wang, L.; Qian, Y.; Cao, Y.; Huang, Y.; Chang, Z.; Huang, H. Production and characterization of keratinolytic proteases by a chicken feather-degrading Thermophilic strain, thermoactinomyces sp. YT06. J. Microbiol. Biotechnol. 2017, 27, 2190–2198. [Google Scholar] [CrossRef]
- Bhari, R.; Kaur, M.; Singh, R.S. Thermostable and halotolerant keratinase from Bacillus aerius NSMk2 with remarkable dehairing and laundary applications. J. Basic Microbiol. 2019, 59, 555–568. [Google Scholar] [CrossRef]
- Li, Z.-W.; Liang, S.; Ke, Y.; Deng, J.; Zhang, M.; Lu, D.; Li, J.; Luo, X. The feather degradation mechanisms of a new Streptomyces sp. isolate SCUT-3. Commun. Biol. 2020, 3, 191. [Google Scholar] [CrossRef]
- Yang, L.; Wang, H.; Lv, Y.; Bai, Y.; Luo, H.; Shi, P.; Huang, H.; Yao, B. Construction of a Rapid Feather-Degrading Bacterium by Overexpression of a Highly Efficient Alkaline Keratinase in Its Parent Strain Bacillus amyloliquefaciens K11. J. Agric. Food Chem. 2016, 64, 78–84. [Google Scholar] [CrossRef]
- Mukherjee, A.K.; Rai, S.K.; Bordoloi, N.K. Biodegradation of waste chicken-feathers by an alkaline b -keratinase (Mukartinase) purified from a mutant Brevibacillus sp. strain AS-S10-II. Int. Biodeterior. Biodegrad. 2011, 65, 1229–1237. [Google Scholar] [CrossRef]
- Duarte, T.R.; Oliveira, S.S.; Macrae, A.; Cedrola, S.M.L.; Mazotto, A.M.; Souza, E.P.; Melo, A.C.N. Increased expression of keratinase and other peptidases by Candida parapsilosis mutants. Braz. J. Med. Biol. Res. 2011, 43. [Google Scholar] [CrossRef]
- Hu, H.; He, J.; Yu, B.; Zheng, P.; Huang, Z.; Mao, X.; Yu, J.; Han, G.; Chen, D. Expression of a keratinase (kerA) gene from Bacillus licheniformis in Escherichia coli and characterization of the recombinant enzymes. Biotechnol. Lett. 2013, 35, 239–244. [Google Scholar] [CrossRef]
- Rajput, R.; Tiwary, E.; Sharma, R.; Gupta, R. Swapping of pro-sequences between keratinases of Bacillus licheniformis and Bacillus pumilus: Altered substrate specificity and thermostability. Enzym. Microb. Technol. 2012, 51, 131–138. [Google Scholar] [CrossRef]
- Yong, B.; Fei, X.; Shao, H.; Xu, P.; Hu, Y.; Ni, W.; Xiao, Q.; Tao, X.; He, X.; Feng, H. Recombinant expression and biochemical characterization of a novel keratinase BsKER71 from feather degrading bacterium Bacillus subtilis S1-4. AMB Express 2020, 10. [Google Scholar] [CrossRef]
- Hu, H.; Gao, J.; He, J.; Yu, B.; Zheng, P.; Huang, Z.; Mao, X.; Yu, J.; Han, G.; Chen, D. Codon Optimization Significantly Improves the Expression Level of a Keratinase Gene in Pichia pastoris. PLoS ONE 2013, 8, e005839. [Google Scholar] [CrossRef]
- Cao, H.; Villatoro-Hernandez, J.; Weme, R.D.O.; Frenzel, E.; Kuipers, O.P. Boosting heterologous protein production yield by adjusting global nitrogen and carbon metabolic regulatory networks in Bacillus subtilis. Metab. Eng. 2018, 49, 143–152. [Google Scholar] [CrossRef]
- Hertel, R.; Meyerjürgens, S.; Voigt, B.; Liesegang, H.; Volland, S. Small RNA mediated repression of subtilisin production in Bacillus licheniformis. Sci. Rep. 2017, 7, 5699. [Google Scholar] [CrossRef]
- Zhang, K.; Duan, X.; Wu, J. Multigene disruption in undomesticated Bacillus subtilis ATCC 6051a using the CRISPR/Cas9 system. Nat. Publ. Gr. 2016, 1–11. [Google Scholar] [CrossRef]
- Yusuf, I.; Ahmad, S.A.; Phang, L.Y.; Syed, M.A.; Shamaan, N.A.; Khalil, K.A.; Dahalan, F.A.; Shukor, M.Y. Keratinase production and biodegradation of polluted secondary chicken feather wastes by a newly isolated multi heavy metal tolerant. J. Environ. Manag. 2016, 183, 182–195. [Google Scholar] [CrossRef]
- Arokiyaraj, S.; Varghese, R.; Ali Ahmed, B.; Duraipandiyan, V.; Al-Dhabi, N.A. Optimizing the fermentation conditions and enhanced production of keratinase from Bacillus cereus isolated from halophilic environment. Saudi J. Biol. Sci. 2019, 26, 378–381. [Google Scholar] [CrossRef]
- Ramakrishna Reddy, M.; Sathi Reddy, K.; Ranjita Chouhan, Y.; Bee, H.; Reddy, G. Effective feather degradation and keratinase production by Bacillus pumilus GRK for its application as bio-detergent additive. Bioresour. Technol. 2017, 243, 254–263. [Google Scholar] [CrossRef]
- Burdette, L.A.; Leach, S.A.; Wong, H.T.; Ercek, D.T. Developing Gram—Negative bacteria for the secretion of heterologous proteins. Microb. Cell Fact. 2018, 1–16. [Google Scholar] [CrossRef]
- Baghban, R.; Farajnia, S.; Rajabibazl, M.; Ghasemi, Y.; Mafi, A.; Hoseinpoor, R.; Rahbarnia, L.; Aria, M. Yeast Expression Systems: Overview and Recent Advances. Mol. Biotechnol. 2019, 61, 365–384. [Google Scholar] [CrossRef]
- Huang, M.; Chen, R.; Ren, G. Secretory expression and purification of Bacillus licheniformis keratinase in insect cells. PLoS ONE 2017, 12, e0183764. [Google Scholar] [CrossRef]
- Sharma, R.; Devi, S. Versatility and commercial status of microbial keratinases : A review. Rev. Environ. Sci. Bio/Technol. 2017. [Google Scholar] [CrossRef]
- Callegaro, K.; Welter, N.; Daroit, D.J. Feathers as bioresource: Microbial conversion into bioactive protein hydrolysates. Process Biochem. 2018, 75, 1–9. [Google Scholar] [CrossRef]
- Bezus, B.; Ruscasso, F.; Garmendia, G.; Vero, S.; Cavello, I.; Cavalitto, S. Revalorization of chicken feather waste into a high antioxidant activity feather protein hydrolysate using a novel psychrotolerant bacterium. Biocatal. Agric. Biotechnol. 2021, 32. [Google Scholar] [CrossRef]
- Kshetri, P.; Roy, S.S.; Sharma, S.K.; Singh, T.S.; Ansari, M.A.; Prakash, N.; Ngachan, S.V. Transforming Chicken Feather Waste into Feather Protein Hydrolysate Using a Newly Isolated Multifaceted Keratinolytic Bacterium Chryseobacterium sediminis RCM-SSR-7. Waste Biomass Valorization 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Darewicz, M.; Borawska, J.; Minkiewicz, P.; Iwaniak, A.; Starowicz, P. Biologicznie aktywne peptydy uwalniane z białek żywności. Zywn. Nauk. Technol. Jakosc/Food. Sci. Technol. Qual. 2015, 22, 26–41. [Google Scholar] [CrossRef]
- Białkowska, A.M.; Morawski, K.; Florczak, T. Extremophilic proteases as novel and efficient tools in short peptide synthesis. J. Ind. Microbiol. Biotechnol. 2017, 44, 1325–1342. [Google Scholar] [CrossRef] [PubMed]
- Nwachukwu, I.D.; Aluko, R.E. Structural and functional properties of food protein-derived antioxidant peptides. J. Food Biochem. 2019, 43, e12761. [Google Scholar] [CrossRef] [PubMed]
- Bechaux, J.; Gatellier, P.; Page, J.L.; Drillet, Y.; Sante-lhoutellier, V. A comprehensive review of bioactive peptides obtained from animal byproducts and their applications. Food Funct. 2019, 6244–6266. [Google Scholar] [CrossRef]
- Qian, D.; Qiu, B.; Zhou, N.; Takaiwa, F.; Yong, W.; Qu, L.Q. Hypotensive Activity of Transgenic Rice Seed Accumulating Multiple Antihypertensive Peptides. J. Agric. Food Chem. 2020, 68, 7162–7168. [Google Scholar] [CrossRef]
- Origone, A.; Bersi, G.; Illanes, A.; Sturniolo, H.; Liggieri, C.; Guzmán, F.; Barberis, S. Enzymatic and chemical synthesis of new anticoagulant peptides. Biotechnol. Prog. 2018, 34, 1093–1101. [Google Scholar] [CrossRef]
- Lermen, A.M.; Clerici, N.J.; Daroit, D.J. Biochemical Properties of a Partially Purified Protease from Bacillus sp. CL18 and Its Use to Obtain Bioactive Soy Protein Hydrolysates. Appl. Biochem. Biotechnol. 2020, 192, 643–664. [Google Scholar] [CrossRef]
- Losurdo, L.; Quintieri, L.; Caputo, L.; Gallerani, R.; Mayo, B.; Leo, F. De Cloning and expression of synthetic genes encoding angiotensin-I converting enzyme (ACE)-inhibitory bioactive peptides in Bifidobacterium pseudocatenulatum. FEMS Microbiol. Lett. 2013, 340, 24–32. [Google Scholar] [CrossRef]
- El-Baky, N.A.; Ahmed, M.; Shawky, E.; Mohammed, M.; Mustafa, E.; Reda, H. De novo expression and antibacterial potential of four lactoferricin peptides in cell-free protein synthesis system. Biotechnol. Rep. 2021, 29, e00583. [Google Scholar] [CrossRef]
- Peng, Z.; Mao, X.; Zhang, J.; Du, G.; Chen, J. Biotransformation of keratin waste to amino acids and active peptides based on cell-free catalysis. Biotechnol. Biofuels 2020, 13, 1–12. [Google Scholar] [CrossRef]
- Tamreihao, K.; Mukaniherjee, S.; Khunjamayum, R.; Devi, L.J.; Asem, R.S.; Ningthoujam, D.S. Feather degradation by keratinolytic bacteria and biofertilizing potential for sustainable agricultural production. J. Basic Microbiol. 2019, 59, 4–13. [Google Scholar] [CrossRef]
- Kaur, M.; Bhari, R.; Singh, R.S. Chicken feather waste-derived protein hydrolysate as a potential biostimulant for cultivation of mung beans. Biologia 2021, 76, 1807–1815. [Google Scholar] [CrossRef]
- Calin, M.; Raut, I.; Arsene, M.L.; Capra, L.; Gurban, A.M.; Doni, M.; Jecu, L. Applications of Fungal Strains with Keratin-degrading and Plant Growth Promoting Characteristics. Agronomy 2019, 9, 543. [Google Scholar] [CrossRef]
- Bhari, R.; Kaur, M.; Sarup Singh, R. Chicken Feather Waste Hydrolysate as a Superior Biofertilizer in Agroindustry. Curr. Microbiol. 2021, 78, 2212–2230. [Google Scholar] [CrossRef]
- Campos, I.; Pinheiro Valente, L.M.; Matos, E.; Marques, P.; Freire, F. Life-cycle assessment of animal feed ingredients: Poultry fat, poultry by-product meal and hydrolyzed feather meal. J. Clean. Prod. 2020, 252. [Google Scholar] [CrossRef]
- Costantini, M.; Ferrante, V.; Guarino, M.; Bacenetti, J. Environmental sustainability assessment of poultry productions through life cycle approaches: A critical review. Trends Food Sci. Technol. 2021, 110, 201–212. [Google Scholar] [CrossRef]
- VALUEWASTE. Unlocking New Value from Urban Waste. Available online: https://valuewaste.eu/available-material/ (accessed on 5 October 2021).
- Callegaro, K.; Brandelli, A.; Daroit, D.J. Beyond plucking: Feathers bioprocessing into valuable protein hydrolysates. Waste Manag. 2019, 95, 399–415. [Google Scholar] [CrossRef]
- Alba, A.C.; Strauch, T.A.; Keisler, D.H.; Wells, K.D.; Kesler, D.C. Using a keratinase to degrade chicken feathers for improved extraction of glucocorticoids. Gen. Comp. Endocrinol. 2019, 270, 35–40. [Google Scholar] [CrossRef]
- Bortolotti, G.R.; Marchant, T.A.; Blas, J.; German, T. Corticosterone in feathers is a long-term, integrated measure of avian stress physiology. Funct. Ecol. 2008, 22, 494–500. [Google Scholar] [CrossRef]
- Bortolotti, G.R.; Marchant, T.; Blas, J.; Cabezas, S. Tracking stress: Localisation, deposition and stability of corticosterone in feathers. J. Exp. Biol. 2009, 212, 1477–1482. [Google Scholar] [CrossRef]
- Ningthoujam, D.S.; Mukherjee, S.; Devi, L.J.; Singh, E.S.; Tamreihao, K.; Khunjamayum, R.; Banerjee, S.; Mukhopadhyay, D. In vitro degradation of β-amyloid fibrils by microbial keratinase. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2019, 5, 154–163. [Google Scholar] [CrossRef]
- Tarrahi, R.; Fathi, Z.; Seydibeyoğlu, M.Ö.; Doustkhah, E.; Khataee, A. Polyhydroxyalkanoates (PHA): From production to nanoarchitecture. Int. J. Biol. Macromol. 2020, 146, 596–619. [Google Scholar] [CrossRef]
- Koller, M. Biodegradable and biocompatible polyhydroxy-alkanoates (PHA): Auspicious microbial macromolecules for pharmaceutical and therapeutic applications. Molecules 2018, 23, 362. [Google Scholar] [CrossRef]
- Elmowafy, E.; Abdal-Hay, A.; Skouras, A.; Tiboni, M.; Casettari, L.; Guarino, V. Polyhydroxyalkanoate (PHA): Applications in drug delivery and tissue engineering. Expert Rev. Med. Devices 2019, 16, 467–482. [Google Scholar] [CrossRef]
- Amelia, T.S.M.; Govindasamy, S.; Tamothran, A.M.; Vigneswari, S.; Bhubalan, K. Applications of PHA in Agriculture. In Biotechnological Applications of Polyhydroxyalkanoates; Springer: Singapore, 2019; pp. 347–361. ISBN 9789811337598. [Google Scholar]
- Nilsen-Nygaard, J.; Fernández, E.N.; Radusin, T.; Rotabakk, B.T.; Sarfraz, J.; Sharmin, N.; Sivertsvik, M.; Sone, I.; Pettersen, M.K. Current status of biobased and biodegradable food packaging materials: Impact on food quality and effect of innovative processing technologies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1333–1380. [Google Scholar] [CrossRef]
- Wong, J.X.; Ogura, K.; Chen, S.; Rehm, B.H.A. Bioengineered Polyhydroxyalkanoates as Immobilized Enzyme Scaffolds for Industrial Applications. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef]
- Volova, T.; Sapozhnikova, K.; Zhila, N. Cupriavidus necator B-10646 growth and polyhydroxyalkanoates production on different plant oils. Int. J. Biol. Macromol. 2020, 164, 121–130. [Google Scholar] [CrossRef]
- Rebocho, A.T.; Pereira, J.R.; Freitas, F.; Neves, L.A.; Alves, V.D.; Sevrin, C.; Grandfils, C.; Reis, M.A.M. Production of medium-chain length polyhydroxyalkanoates by Pseudomonas citronellolis grown in apple pulp waste. Appl. Food Biotechnol. 2019, 6, 71–82. [Google Scholar] [CrossRef]
- Obruca, S.; Sedlacek, P.; Slaninova, E.; Fritz, I.; Daffert, C.; Meixner, K.; Sedrlova, Z.; Koller, M. Novel unexpected functions of PHA granules. Appl. Microbiol. Biotechnol. 2020, 104, 4795–4810. [Google Scholar] [CrossRef]
- Koller, M.; Braunegg, G. Advanced approaches to produce polyhydroxyalkanoate (PHA) biopolyesters in a sustainable and economic fashion. EuroBiotech J. 2018, 2, 89–103. [Google Scholar] [CrossRef]
- Pernicova, I.; Enev, V.; Marova, I.; Obruca, S. Interconnection of waste chicken feather biodegradation and keratinase and mcl-PHA production employing Pseudomonas putida KT2440. Appl. Food Biotechnol. 2019, 6, 83–90. [Google Scholar] [CrossRef]
- Jana, A.; Halder, S.K.; Dasgupta, D.; Hazra, S.; Mondal, P.; Bhaskar, T.; Ghosh, D. Keratinase Biosynthesis from Waste Poultry Feathers for Proteinaceous Stain Removal. ACS Sustain. Chem. Eng. 2020, 8, 17651–17663. [Google Scholar] [CrossRef]
- Gunes, G.B.; Akkoyun, O.; Demir, T.; Bozaci, E.; Demir, A.; Esin Hames, E. Microbial Keratinase Production and Application to Improve the Properties of Wool Fabrics. Int. J. Text. Sci. 2018, 7, 43–47. [Google Scholar] [CrossRef]
- Chandra, R.; Kumar, V.; Tripathi, S. Evaluation of molasses-melanoidin decolourisation by potential bacterial consortium discharged in distillery effluent. 3 Biotech 2018, 8, 187. [Google Scholar] [CrossRef]
- Yoshioka, M.; Miwa, T.; Horii, H.; Takata, M.; Yokoyama, T.; Nishizawa, K.; Watanabe, M.; Shinagawa, M.; Murayama, Y. Characterization of a proteolytic enzyme derived from a Bacillus strain that effectively degrades prion protein. J. Appl. Microbiol. 2007, 102, 509–515. [Google Scholar] [CrossRef]
- Okoroma, E.A.; Purchase, D.; Garelick, H.; Morris, R.; Neale, M.H.; Windl, O.; Abiola, O.O. Enzymatic Formulation Capable of Degrading Scrapie Prion under Mild Digestion Conditions. PLoS ONE 2013, 8, e0068099. [Google Scholar] [CrossRef]
- Sharma, R.; Gupta, R. Coupled action of c -glutamyl transpeptidase-glutathione and keratinase effectively degrades feather keratin and surrogate prion protein, Sup 35NM. Bioresour. Technol. 2012, 120, 314–317. [Google Scholar] [CrossRef]
- Dillon, D.; Fernández Ajó, A.; Hunt, K.E.; Buck, C.L. Investigation of keratinase digestion to improve steroid hormone extraction from diverse keratinous tissues. Gen. Comp. Endocrinol. 2021, 309, 113795. [Google Scholar] [CrossRef]
- Blecher, A.S.; Scheun, J.; Ganswindt, A. Degradation of Temminck’s pangolin (Smutsia temminckii) scales with a keratinase for extraction of reproductive steroid hormones. MethodsX 2021, 8, 101229. [Google Scholar] [CrossRef]
- Lind, M.; Hõrak, P.; Sepp, T.; Meitern, R. Corticosterone levels correlate in wild-grown and lab-grown feathers in green finches (Carduelis chloris) and predict behaviour and survival in captivity. Horm. Behav. 2020, 118, 104642. [Google Scholar] [CrossRef]
- Stewart, N.D.; Reilly, A.; Gilman, C.; Mastromonaco, G.F.; Burness, G. Evidence of degradation of hair corticosterone in museum specimens. Gen. Comp. Endocrinol. 2018, 268, 128–133. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).