5-HT Receptors and Temperature Homeostasis
Abstract
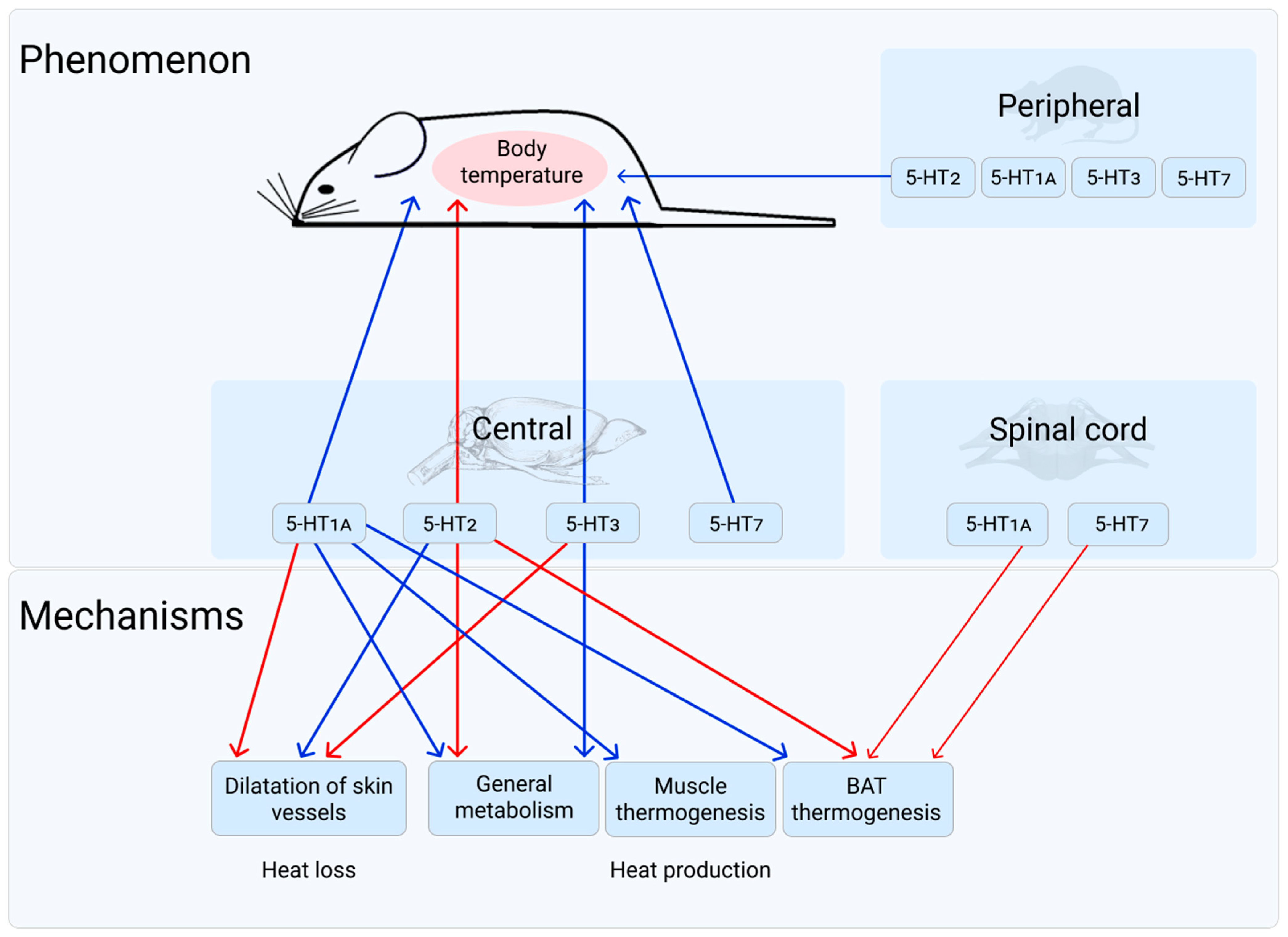
1. 5-HT1A Receptor
2. 5-HT2 Receptors
3. 5-HT3 Receptor
4. 5-HT7 Receptor
5. General Discussion and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ivanov, K.P. The principles of energetics of organism: Theoretical and practical aspects. In Modern Problems, Enigmas and Paradoxes of Regulation of Energetical Balance; Nauka: St-Petersburg, Russia, 2001; Volume 3, p. 278. [Google Scholar]
- Romanovsky, A.A. The thermoregulation system and how it works. In Handbook of Clinical Neurology, Thermoregulation: From Basic Neuroscience to Clinical Neurology, Part I; Romanovsky, A.A., Ed.; 3rd Series; Elsevier: Amsterdam, The Netherlands, 2018; Volume 156, pp. 3–43. [Google Scholar] [CrossRef]
- Tan, C.L.; Knight, Z.A. Regulation of Body Temperature by the Nervous System. Neuron 2018, 98, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Parent, A. Comparative anatomy of the serotoninergic systems. J. Physiol. 1981, 77, 147–156. [Google Scholar]
- Gaspar, P.; Lillesaar, C. Probing the diversity of serotonin neurons. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 2382–2394. [Google Scholar] [CrossRef] [PubMed]
- Twarog, B.M.; Page, I.H. Serotonin content of some mammalian tissues and urine and a method for its determination. Am. J. Physiol. 1953, 175, 157–161. [Google Scholar] [CrossRef]
- Amin, A.H.; Crawford, T.B.B.; Gaddum, J.H. The distribution of substance p and 5-hydroxytryptamine in the central nervous system of the dog. J. Physiol. 1954, I26, 596–618. [Google Scholar] [CrossRef]
- Bachtold, H.; Pletscher, A. Einfluss von isonikotinsaurehydraziden auf den Verlauf der Korpertemperatur nach Reserpin, Monoaminen und Clorpromazin. Experientia 1957, 13, 163–165. [Google Scholar] [CrossRef]
- Hillegaart, V. Effect of local application of 5-HT and 8-OH-DPAT into the dorsal and median raphe nuclei on core temperature in the rat. Psychopharmacology 1991, 103, 291–596. [Google Scholar] [CrossRef] [PubMed]
- Feldberg, W.; Myers, R.D. A new concept of temperature regulation by amines in the hypothalamus. Nature 1963, 200, 1325–1326. [Google Scholar] [CrossRef]
- Myers, R.D. Serotonin and thermoregulation: Old and new views. J. Physiol. 1981, 77, 505–513. [Google Scholar]
- Bligh, J.; Cottle, W.H.; Maskrey, M. Influence of ambient temperature on the thermoregulatory responses to 5-hydroxytryptamine, noradrenaline and acetylcholine injected into the lateral cerebral ventricles of sheep, goats and rabbits. J. Physiol. 1971, 212, 377–392. [Google Scholar] [CrossRef]
- Gurin, V.N. Central Mechanisms of Thermoregulation; USSR: Minsk, Belarus, 1980; p. 127. [Google Scholar]
- Koriakina, L.A.; Kulikov, A.V.; Figurova, M.I.; Popova, N.K. Effect of cold on the brain serotonin system and the corticosteroid level of the blood in different strains of mice. Fiziol. Zhurnal SSSR Im. IM Sechenova 1985, 71, 422–427. [Google Scholar]
- Popova, N.K.; Konusova, A.V. Brain and peripheral effects of serotonin on thermoregulation. Biog. Amines 1985, 3, 125–134. [Google Scholar]
- Sitnikov, V.D.; Kudriavtseva, N.N.; Iakimenko, M.A.; Popova, N.K. Effect of serotonin on thermoregulation in normothermic hibernators and during arousal from deep hypothermia. Biull. Eksp. Biol. Med. 1986, 101, 5–7. [Google Scholar] [CrossRef]
- Zeisberger, E. The roles of monoaminergic neurotransmitters in thermoregulation. Can. J. Physiol. Pharmacol. 1987, 65, 1395–1401. [Google Scholar] [CrossRef]
- Saudou, F.; Hen, R. 5-Hydroxytryptamine receptor subtypes: Molecular and functional diversity. Adv. Pharmacol. 1994, 30, 327–380. [Google Scholar]
- Barnes, N.M.; Sharp, T. A review of central 5-HT receptors and their function. Neuropharmacology 1999, 38, 1083–1152. [Google Scholar] [CrossRef]
- Peroutka, S.J.; Howell, T.A. The molecular evolution of G protein-coupled receptors: Focus on 5-hydroxytryptamine receptors. Neuropharmacology 1994, 33, 319–324. [Google Scholar] [CrossRef]
- Hoyer, D.; Hannon, J.P.; Martin, G.R. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol. Biochem. Behav. 2002, 71, 533–554. [Google Scholar] [CrossRef]
- Hannon, J.; Hoyer, D. Molecular biology of 5-HT receptors. Behav. Brain Res. 2008, 195, 198–213. [Google Scholar] [CrossRef]
- Nichols, D.E.; Nichols, C.D. Serotonin Receptors. Chem. Rev. 2008, 108, 1614–1641. [Google Scholar] [CrossRef]
- Filip, M.; Bader, M. Overview on 5-HT receptors and their role in physiology and pathology of the central nervous system. Pharmacol. Rep. 2009, 61, 761–777. [Google Scholar] [CrossRef]
- Andrade, R.; Barnes, N.M.; Baxter, G.; Bockaert, J.; Branchek, T.; Butler, A.; Cohen, M.L.; Dumuis, A.; Eglen, R.M.; Gothert, M.; et al. 5-Hydroxytryptamine receptors (version 2019.4) in the IUPHAR/BPS guide to pharmacology database. IUPHAR/BPS Guide Pharmacol. 2019, 2019. [Google Scholar] [CrossRef]
- Sharp, T.; Barnes, N.M. Central 5-HT receptors and their function; present and future. Neuropharmacology 2020, 177, 108155. [Google Scholar] [CrossRef]
- Barnes, N.M.; Ahern, G.P.; Becamel, C.; Bockaert, J.; Camilleri, M.; Chaumont-Dubel, S.; Claeysen, S.; Cunningham, K.A.; Fone, K.C.; Gershon, M.; et al. International Union of Basic and Clinical Pharmacology。 CX. Classification of Receptors for 5-hydroxytryptamine; Pharmacology and Function. Pharmacol. Rev. 2021, 73, 310–520. [Google Scholar] [CrossRef]
- Popova, N.K.; Kulikov, A.V. Multiple serotonin receptors as a molecular background underlying the multifunctionality of serotonin. In Successes of Functional Neurochemistry; Dambilova, S.A., Arutjunjan, A.V., Eds.; St.-Petersburg University: St.-Petersburg, Russia, 2003; pp. 56–73. [Google Scholar]
- Popova, N.K.; Naumenko, V.S. Polymorphism in 5-HT receptors as the background of serotonin functional diversity. Ross. Fiziol. Zhurnal Im. IM Sechenova 2010, 96, 778–786. [Google Scholar]
- Naumenko, V.S.; Kondaurova, E.M.; Popova, N.K. On the role of brain 5-HT7 receptor in the mechanism of hypothermia: Comparison with hypothermia mediated via 5-HT1A and 5-HT3 receptor. Neuropharmacology 2011, 61, 1360–1365. [Google Scholar] [CrossRef]
- Ishiwata, T. Role of serotonergic system in thermoregulation in rats. J. Phys. Fit. Sports Med. 2014, 3, 445–450. [Google Scholar] [CrossRef][Green Version]
- Ensemble Genome Browser. Available online: https://www.ensembl.org/index.html (accessed on 5 November 2021).
- Ou, X.M.; Jafar-Nejad, H.; Storring, J.M.; Meng, J.H.; Lemonde, S.; Albert, P.R. Novel dual repressor elements for neuronal cell-specific transcription of the rat 5-HT1A receptor gene. J. Biol. Chem. 2000, 275, 8161–8168. [Google Scholar] [CrossRef]
- Zhong, P.; Ciaranello, R.D. Transcriptional regulation of hippocampal 5-HT1a receptors by corticosteroid hormones. Mol. Brain Res. 1995, 29, 23–34. [Google Scholar] [CrossRef]
- Meijer, O.C.; Cole, T.J.; Schmid, W.; Schütz, G.; Joëls, M.; De Kloet, E.R. Regulation of hippocampal 5-HT1A receptor mRNA and binding in transgenic mice with a targeted disruption of the glucocorticoid receptor. Mol. Brain Res. 1997, 46, 290–296. [Google Scholar] [CrossRef]
- Ou, X.M.; Storring, J.M.; Kushwaha, N.; Albert, P.R. Heterodimerization of mineralocorticoid and glucocorticoid receptors at a novel negative response element of the 5-HT1A receptor gene. J. Biol. Chem. 2001, 276, 14299–14307. [Google Scholar] [CrossRef] [PubMed]
- Masson, J.; Emerit, M.B.; Hamon, M.; Darmon, M. Serotonergic signaling: Multiple effectors and pleiotropic effects. WIREs Membr. Transp. Signal 2012, 1, 685–713. [Google Scholar] [CrossRef]
- Naumenko, V.S.; Popova, N.K.; Lacivita, E.; Leopoldo, M.; Ponimaskin, E.G. Interplay between serotonin 5-HT1A and 5-HT7 receptors in depressive disorders. CNS Neurosci. Ther. 2014, 20, 582–590. [Google Scholar] [CrossRef]
- Weissman-Nanopoulos, D.; Mach, E.; Marge, J.; Demassey, Y.; Pujol, J.-F. Evidence for localization of 5-HT1A binding sites on serotonin containing neurons in the raphe dorsalis and raphe centralis nuclei on the rat brain. Neurochem. Int. 1985, 7, 1061–1072. [Google Scholar] [CrossRef]
- Verge, D.; Daval, G.; Patey, A.; Gozlan, H.; El Mestikawy, S.; Hammon, M. Presinaptic 5-HT autoreceptors on serotonergic cell bodies and/or dendrites but not terminals are of the 5-HT1A subtype. Eur. J. Pharmacol. 1985, 113, 463–464. [Google Scholar] [CrossRef]
- Hjorth, S.; Magnusson, T. The 5-HT1A receptor agonist, 8-OH-DPAT, preferentially activates cell body 5-HT autoreceptors in rat brain In Vivo. Naunyn Schmiedebergs Arch. Pharmacol. 1988, 338, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Pompeiano, M.; Palacios, J.M.; Mengod, G. Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: Correlation with receptor binding. J. Neurosci. 1992, 12, 440–453. [Google Scholar] [CrossRef]
- Burnet, P.W.; Eastwood, S.L.; Harrison, P.J. Detection and quantitation of 5-HT1A and 5-HT2A receptor mRNAs in human hippocampus using a reverse transcriptase-polymerase chain reaction (RT-PCR) technique and their correlation with binding site densities and age. Neurosci. Lett. 1994, 178, 85–89. [Google Scholar] [CrossRef]
- Kia, H.K.; Miquel, M.C.; Brisorgueil, M.J.; Daval, G.; Riad, M.; El Mestikawy, S.; Hamon, M.; Vergé, D. Immunocytochemical localization of serotonin1A receptors in the rat central nervous system. J. Comp. Neurol. 1996, 365, 289–305. [Google Scholar] [CrossRef]
- Knapp, D.J.; Overstreet, D.H.; Crews, F.T. Brain 5-HT1A receptor autoradiography and hypothermic responses in rats bred for differences in 8-OH-DPAT sensitivity. Brain Res. 1998, 782, 1–10. [Google Scholar] [CrossRef]
- Popova, N.K.; Naumenko, V.S. 5-HT1A receptor as a key player in the brain 5-HT system. Rev. Neurosci. 2013, 24, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Popova, N.K.; Naumenko, V.S.; Plyusnina, I.Z.; Kulikov, A.V. Reduction in 5-HT1A receptor density, 5-HT1A mRNA expression, and functional correlates for 5-HT1A receptors in genetically defined aggressive rats. J. Neurosci. Res. 2005, 80, 286–292. [Google Scholar] [CrossRef]
- Kondaurova, E.M.; Bazovkina, D.V.; Kulikov, A.V.; Popova, N.K. Selective breeding for catalepsy changes the distribution of microsatellite D13Mit76 alleles linked to the 5-HT serotonin receptor gene in mice. Genes Brain Behav. 2006, 5, 596–601. [Google Scholar] [CrossRef]
- Naumenko, V.S.; Bazovkina, D.V.; Kondaurova, E.M.; Zubkov, E.A.; Kulikov, A.V. The role of 5-HT2A receptor and 5-HT2A/5-HT1A receptor interaction in the suppression of catalepsy. Genes Brain Behav. 2010, 9, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Kelliher, P.; Kelly, J.P.; Leonard, B.E. Comparative effects of serotonergic agonists with varying efficacy at the 5-HT(1A) receptor on core body temperature: Modification by the selective 5-HT(1A) receptor antagonist WAY 100635. J. Psychopharmacol. 1999, 13, 278–283. [Google Scholar] [CrossRef]
- Richardson-Jones, J.W.; Craige, C.P.; Guiard, B.P.; Stephen, A.; Metzger, K.L.; Kung, H.F.; Gardier, A.M.; Dranovsky, A.; David, D.J.; Beck, S.G.; et al. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron 2010, 65, 40–52. [Google Scholar] [CrossRef]
- Nishitani, N.; Ohmura, Y.; Nagayasu, K.; Shibui, N.; Kaneko, S.; Ohashi, A.; Yoshida, T.; Yamanaka, A.; Yoshioka, M. CRISPR/Cas9-mediated In Vivo gene editing reveals that neuronal 5-HT1A receptors in the dorsal raphe nucleus contribute to body temperature regulation in mice. Brain Res. 2019, 1719, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Turcotte-Cardin, V.; Vahid-Ansari, F.; Luckhart, C.; Daigle, M.; Geddes, S.D.; Tanaka, K.F.; Hen, R.; James, J.; Merali, Z.; Beïque, J.-C. Loss of adult 5-HT1A autoreceptors results in a paradoxical anxiogenic response to antidepressant Treatment. J. Neurosci. 2019, 39, 1334–1346. [Google Scholar] [CrossRef]
- Goodwin, G.M.; De Souza, R.J.; Green, A.R. The pharmacology of the hypothermic response in mice to 8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT): A model of presynaptic 5-HT1 function. Neuropharmacology 1985, 24, 1187–1194. [Google Scholar] [CrossRef]
- Hjorth, S. Hypothermia in the rat induced by the potent serotonergic agent 8-OH-DPAT. J. Neural Transm. 1985, 61, 131–135. [Google Scholar] [CrossRef]
- Gudelsky, G.A.; Koenig, J.I.; Meltzer, H.Y. Thermoregulatory responses to serotonin (5-HT) receptor stimulation in the rat. Evidence for opposing roles of 5-HT2 and 5-HT1A receptors. Neuropharmacology 1986, 25, 1307–1313. [Google Scholar] [CrossRef]
- De Souza, R.J.; Goodwin, G.M.; Green, A.R.; Heal, D.J. Effect of chronic treatment with 5-HT1 agonist (8-OH-DPAT and RU 24969) and antagonist (isapirone) drugs on the behavioural responses of mice to 5-HT1 and 5-HT2 agonists. Br. J. Pharmacol. 1986, 89, 377–384. [Google Scholar] [CrossRef]
- Goodwin, G.M.; De Souza, R.J.; Green, A.R.; Heal, D.J. The pharmacology of the behavioural and hypothermic responses of rats to 8-hydroxy-2-(di-n-propylamino)-tetralin (8-OH-DPAT). Psychopharmacology 1987, 91, 506–511. [Google Scholar] [CrossRef]
- Hutson, P.H.; Donohoe, T.P.; Curzon, G. Hypothermia induced by the putative 5-HT1A agonists LY 165163 and 8-OHDPAT is not prevented by 5-HT depletion. Eur. J. Pharmacol. 1987, 143, 221–228. [Google Scholar] [CrossRef]
- Higgins, G.A.; Bradbury, A.J.B.; Jones, J.; Oakley, N.R. Behavioural and biochemical consequences following activation of 5HT1-like and GABA receptors in the dorsal raphe’ nucleus of the rat. Neurophormncology 1988, 27, 993–1001. [Google Scholar] [CrossRef]
- Wozniak, K.M.; Aulakh, C.S.; Hill, J.L.; Murphy, D.L. The effect of 8-OH-DPAT on temperature in the rat and its modification by chronic antidepressant treatments. Pharmacol. Biochem. Behav. 1988, 30, 451–456. [Google Scholar] [CrossRef]
- Nash, J.F.; Meltzer, H.Y.; Gudelsky, G.A. Antagonism of serotonin receptor mediated neuroendocrine and temperature responses by atypical neuroleptics in the rat. Eur. J. Pharmacol. 1988, 151, 463–469. [Google Scholar] [CrossRef]
- Berendsen, H.H.; Broekkamp, C.L. Behavioural evidence for functional interactions between 5-HT-receptor subtypes in rats and mice. Br. J. Pharmacol. 1990, 101, 667–673. [Google Scholar] [CrossRef]
- Bill, A.; Knight, M.; Forster, E.A.; Fletcher, A. Direct evidence for an important species difference in the mechanism of 8-OH-DPAT-induced hypothermia. Br. J. Pharmacol. 1991, 103, 1857–1864. [Google Scholar] [CrossRef]
- Meller, E.; Chalfin, M.; Bohmaker, K. Serotonin 5-HT1A receptor-mediated hypothermia in mice: Absence of spare receptors and rapid induction of tolerance. Pharmacol. Biochem. Behav. 1992, 43, 405–411. [Google Scholar] [CrossRef]
- Martin, K.F.; Phillips, I.; Hearson, M.; Prow, M.R.; Heal, D.J. Characterization of 8-OH-DPAT-induced hypothermia in mice as a 5-HT1A autoreceptor response and its evaluation as a model to selectively identify antidepressants. Br. J. Pharmacol. 1992, 107, 15–21. [Google Scholar] [CrossRef]
- Zamfir, O.; Broqua, P.; Baudrie, V.; Chaouloff, F. Effects of cold stress on some 5-HT1A, 5-HT1C and 5-HT2 receptor-mediated responses. Eur. J. Pharmacol. 1992, 219, 261–269. [Google Scholar] [CrossRef]
- Fletcher, A.; Forster, E.A.; Bill, D.J.; Brown, G.; Cliffe, I.A.; Hartley, J.E.; Jones, D.E.; McLenachan, A.; Stanhope, K.J.; Critchley, D.J.; et al. Electrophysiological, biochemical, neurohormonal and behavioural studies with WAY-100635, a potent, selective and silent 5-HT1A receptor antagonist. Behav. Brain Res. 1996, 73, 337–353. [Google Scholar] [CrossRef]
- Takao, K.; Nagatani, T.; Kitamura, Y.; Yamawaki, S. Effects of corticosterone on 5-HT1A and 5-HT2 receptor binding and on the receptor-mediated behavioral responses of rats. Eur. J. Pharmacol. 1997, 333, 123–128. [Google Scholar] [CrossRef]
- Heisler, L.K.; Chu, H.-M.; Brennan, T.J.; Danao, J.A.; Bajwa, P.; Parsons, L.H.; Tecott, L.H. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc. Natl. Acad. Sci. USA 1998, 95, 15049–15054. [Google Scholar] [CrossRef]
- Lin, M.T.; Tsay, H.J.; Su, W.H.; Chueh, F.Y. Changes in extracellular serotonin in rat hypothalamus affect thermoregulatory function. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 1998, 274, R1260–R1267. [Google Scholar] [CrossRef]
- Hensler, J.G.; Truett, K.A. Effect of chronic serotonin-2 receptor agonist or antagonist administration on serotonin-1A receptor sensitivity. Neuropsychopharmacology 1998, 19, 354–364. [Google Scholar] [CrossRef]
- Gardier, A.M.; Gruwez, B.; Trillat, A.-C.; Jacquot, C.; Hen, R.; Bourin, M. Interaction between 5-HT1A and 5-HT1B receptors: Effects of 8-OH-DPAT-induced hypothermia in 5-HT1B receptor knockout mice. Eur. J. Pharmacol. 2001, 421, 171–175. [Google Scholar] [CrossRef]
- Haddjeri, N.; Faure, C.; Lucas, G.; Mnie-Filali, O.; Chouvet, G.; Astier, B.; Renaud, B.; Blier, P.; Debonnel, G. In-vivo modulation of central 5-hydroxytryptamine (5-HT1A) receptor-mediated responses by the cholinergic system. Int. J. Neuropsychopharmacol. 2004, 7, 391–399. [Google Scholar] [CrossRef][Green Version]
- Hedlund, P.B.; Kelly, L.; Mazur, C.; Lovenberg, T.; Sutcliffe, J.G.; Bonaventure, P. 8-OH-DPAT acts on both 5-HT1A and 5-HT7 receptors to induce hypothermia in rodents. Eur. J. Pharmacol. 2004, 487, 125–132. [Google Scholar] [CrossRef]
- Popova, N.K.; Naumenko, V.S.; Plyusnina, I.Z. The involvement of brain 5-HT(1A)-receptors in genetically determined aggressive behavior. Zhurnal Vyss. Nervn. Deiatelnosti Im. IP Pavlov. 2006, 56, 537–542. [Google Scholar]
- Rusyniak, D.E.; Zaretskaia, M.V.; Zaretsky, D.V.; DiMicco, J.A. 3,4-Methylenedioxymethamphetamine- and 8-hydroxy-2-di-n-propylamino-tetralin-induced hypothermia: Role and location of 5-hydroxytryptamine 1A receptors. J. Pharmacol. Exp. Ther. 2007, 323, 477–487. [Google Scholar] [CrossRef]
- Popova, N.K.; Tibeikina, M.A.; Amstislavskaya, T.G. Hypothermic effect of 5-HT1A receptor agonist: Comparison of intranasal, intraperitoneal, and subcutaneous routes of administration. Bull. Exp. Biol. Med. 2008, 146, 433–435. [Google Scholar] [CrossRef]
- Naumenko, V.S.; Kondaurova, E.M.; Popova, N.K. Central 5-HT3 receptor-induced hypothermia in mice: Interstrain differences and comparison with hypothermia mediated via 5-HT1A receptor. Neurosci. Lett. 2009, 465, 50–54. [Google Scholar] [CrossRef]
- Voronova, I.P.; Naumenko, V.S.; Khramova, G.M.; Kozyreva, T.V.; Popova, N.K. Central 5-HT3 receptor-induced hypothermia is associated with reduced metabolic rate and increased heat loss. Neurosci. Lett. 2011, 504, 209–214. [Google Scholar] [CrossRef]
- Bagdy, G.; To, C.T. Comparison of relative potencies of i.v. and i.c.v. administered 8-OH-DPAT gives evidence of different sites of action for hypothermia, lower lip retraction and tail flicks. Eur. J. Pharmacol. 1997, 323, 53–58. [Google Scholar] [CrossRef]
- Overstreet, D.H.; Rezvani, A.H.; Knapp, D.J.; Crews, F.T.; Janowsky, D.S. Further selection of rat lines differing in 5-HT-1A receptor sensitivity: Behavioral and functional correlates. Psychiat. Genet. 1996, 6, 107–117. [Google Scholar] [CrossRef]
- Wesolowska, A.; Paluchowska, M.H.; Golembiowska, K.; Chojnacka-Wojcik, E. Pharmacological characterization of MP349, a novel 5HT1A-receptor antagonist with anxiolytic-like activity, in mice and rats. J. Pharm. Pharmacol. 2003, 55, 533–543. [Google Scholar] [CrossRef]
- Hjorth, S.; Carlsson, A.; Lindberg, P.; Sanchez, D.; Wikstrom, H.; Arvidsson, L.-E.; Hacksell, U.; Nilsson, J.L.G. 8-Hydroxy-2-(di-n-Propylamino)Tetralin, 8-OH-DPAT, a potent and selective simplified ergot congener with central 5-HT-receptor stimulating activity. J. Neural Transm. 1982, 55, 169–188. [Google Scholar] [CrossRef]
- Matsuda, T.; Kanda, T.; Seong, Y.H.; Baba, A.; Itawa, H. p-Chlorophenylalanine attenuates the pituitary-adrenocortical responses to 5-HT1A receptor agonist in mice. Eur. J. Pharmacol. 1990, 181, 295–297. [Google Scholar] [CrossRef]
- Blier, P.; Seletti, B.; Gilbert, F.; Young, S.N.; Benkelfat, C. Serotonin(1A) receptor activation and hypothermia in humans: Lack of evidence for a presynaptic mediation. Neuropsychopharmacology 2002, 27, 301–308. [Google Scholar] [CrossRef]
- Richardson-Jones, J.W.; Craig, C.P.; Nguyen, T.H.; Kung, H.F.; Gardier, A.M.; Dranovsky, A.; David, D.J.; Guiard, B.P.; Beck, S.G.; Hen, R.; et al. Serotonin-1A autoreceptors are necessary and sufficient for the normal formation of circuits underlying innate anxiety. J. Neurosci. 2011, 31, 6008–6018. [Google Scholar] [CrossRef]
- O’Connell, M.T.; Sarna, G.S.; Curzon, G. Evidence for postsynaptic mediation of the hypothermic effect of 5-HT1A receptor activation. Br. J. Pharmacol. 1992, 106, 603–609. [Google Scholar] [CrossRef] [PubMed]
- IUPHAR/BPS Guide to Pharmacology. Available online: https://www.guidetopharmacology.org (accessed on 14 November 2021).
- Koe, B.K.; Weissman, A. p-Chlorophenylalanine: A specific depletor of brain serotonin. J. Pharmacol. Exp. Ther. 1966, 154, 499–516. [Google Scholar]
- Slonim, A.D. Ecological Animal Physiology; Vysshaja shkola Edition: Moscow, Russia, 1971; p. 448. [Google Scholar]
- Prosser, L. Temperature. In Comperative Animal Physiology, 3rd ed.; Prosser, L., Ed.; W.B. Saunders Company: Philadelphia, PA, USA; London, UK; Toronto, ON, Canada, 1973; Volume 2, pp. 84–209. [Google Scholar]
- Schmidt-Nielsen, K. Animal Physiology: Adaptation and Environment, 5th ed.; Cambridge University Press: Cambridge, UK, 1997; p. 607. [Google Scholar]
- Gordon, C.J. Thermal biology of the laboratory rat. Physiol. Behav. 1990, 47, 963–991. [Google Scholar] [CrossRef]
- Gordon, C.J. Thermal physiology of laboratory mice: Defining thermoneutrality. J. Therm. Biol. 2012, 37, 654–685. [Google Scholar] [CrossRef]
- El Bitar, N.; Pollin, B.; Karroum, E.; Pincedé, I.; Mouraux, A.; Le Bars, D. Thermoregulatory vasomotor tone of the rat tail and paws in thermoneutral conditions and its impact on a behavioral model of acute pain. J. Neurophysiol. 2014, 112, 2185–2198. [Google Scholar] [CrossRef]
- Blessing, W.; McAllen, R.; McKinley, M. Control of the cutaneous circulation by the central nervous system. Compr. Physiol. 2016, 6, 1161–1197. [Google Scholar] [CrossRef] [PubMed]
- Blessing, W.W. 5-hydroxytryptamine 1A receptor activation reduces cutaneous vasoconstriction and fever associated with the acute inflammatory response in rabbits. Neuroscience 2004, 123, 1–4. [Google Scholar] [CrossRef]
- Ootsuka, Y.; Blessing, W.W. 5-Hydroxytryptamine 1A receptors inhibit cold-induced sympathetically mediated cutaneous vasoconstriction in rabbits. J. Physiol. 2003, 552, 303–314. [Google Scholar] [CrossRef]
- Ootsuka, Y.; Blessing, W.W. Activation of 5-HT1A receptors in rostral medullary raphe inhibits cutaneous vasoconstriction elicited by cold exposure in rabbits. Brain Res. 2006, 1073–1074, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Ootsuka, Y.; Blessing, W.W. Thermogenesis in brown adipose tissue: Increase by 5-HT2A receptor activation and decrease by 5-HT1A receptor activation in conscious rats. Neurosci. Lett. 2006, 395, 170–174. [Google Scholar] [CrossRef]
- Korovin, K.F. The functional state of the sympathoadrenal system during short-term and long-term air cooling of non-anesthetized rats. In Neurohumoral Mechanisms of the Body’s Response to Cooling; Nauka: Leningrad, Russia, 1973; Volume 121, p. 79. [Google Scholar]
- Jansky, L. Nonshivering thermogenesis and its thermoregulatory significance. Biol. Rev. 1973, 48, 85–132. [Google Scholar] [CrossRef]
- Jansky, L. Humoral thermogenesis and its role in maintaining energy balance. Physiol. Rev. 1995, 75, 237–259. [Google Scholar] [CrossRef] [PubMed]
- Hammel, H.T. Regulation of internal body temperature. Ann. Rev. Physiol. 1968, 30, 641–710. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, K.P. Basic principles of temperature homeostasis regulation. In Physiology of Thermoregulation. Physiology Manual; Ivanov, K.P., Ed.; Nauka: Leningrad, Russia, 1984; pp. 113–138. [Google Scholar]
- Ivanov, K.P. The Principles of Energetics of Organism: Theoretical and Practical Aspects. In The General Energetics, Heatexchange, Thermoregulation; Nauka: St-Petersburg, Russia, 1990; Volume 1, p. 307. [Google Scholar]
- Morrison, S.F. Efferent neural pathways for the control of brown adipose tissue thermogenesis and shivering. In Thermoregulation: From Basic Neuroscience to Clinical Neurology, Part I; Romanovsky, A.A., Ed.; 3rd Series; Elsevier: Amsterdam, The Netherlands, 2018; Volume 156, pp. 281–303. [Google Scholar]
- Nakamura, K. Afferent pathways for autonomic and shivering thermoeffectors. In Thermoregulation: From Basic Neuroscience to Clinical Neurology, Part I; Romanovsky, A.A., Ed.; 3rd Series; Elsevier: Amsterdam, The Netherlands, 2018; Volume 156, pp. 263–279. [Google Scholar]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- Oelkrug, R.; Polymeropoulos, E.T.; Jastroch, M.J. Brown adipose tissue: Physiological function and evolutionary significance. J. Comp. Physiol. B 2015, 185, 587–606. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, J.; Cannon, B. Brown adipose tissue as a heat-producing thermoeffector. In Thermoregulation: From Basic Neuroscience to Clinical Neurology, Part I; Romanovsky, A.A., Ed.; 3rd Series; Elsevier: Amsterdam, The Netherlands, 2018; Volume 156, pp. 137–152. [Google Scholar] [CrossRef]
- Morrison, S.F. Activation of 5-HT1A receptors in raphe pallidus inhibits leptin-evoked increases in brown adipose tissue thermogenesis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R832–R837. [Google Scholar] [CrossRef]
- Mota, C.M.D.; Branco, L.G.S.; Morrison, S.F.; Madden, C.J. Systemic serotonin inhibits brown adipose tissue sympathetic nerve activity via a GABA input to the dorsomedial hypothalamus, not via 5HT1A receptor activation in raphe pallidus. Acta Physiol. 2020, 228, e13401. [Google Scholar] [CrossRef]
- Nakamura, K.; Morrison, S.F. Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R127–R136. [Google Scholar] [CrossRef]
- Ivanov, K.P. Bioenergetics and Temperature Homeostasis; Nauka: Leningrad, Russia, 1972; p. 172. [Google Scholar]
- Yakimenko, M.A. Muscular thermogenesis during adaptation to cold. In Ecological Physiology of Animals. Physiology Manual; Slonim, A.D., Ed.; Nauka: Leningrad, Russia, 1982; pp. 80–83. [Google Scholar]
- Nakamura, K.; Morrison, S.F. Central efferent pathways for cold-defensive and febrile shivering. J. Physiol. 2011, 589, 3641–3658. [Google Scholar] [CrossRef]
- Blondin, D.P.; Haman, F. Shivering and nonshivering thermogenesis in skeletal muscles. In Thermoregulation: From Basic Neuroscience to Clinical Neurology, Part I; Romanovsky, A.A., Ed.; 3rd Series; Elsevier: Amsterdam, The Netherlands, 2018; Volume 156, pp. 153–173. [Google Scholar]
- Berner, N.J.; Grahn, D.A.; Heller, H.C. 8-OH-DPAT-sensitive neurons in the nucleus raphe magnus modulate thermoregulatory output in rats. Brain Res. 1999, 831, 155–164. [Google Scholar] [CrossRef]
- Hoffman, J.M.; Brown, J.W.; Sirlin, E.A.; Benoit, A.M.; Gill, W.H.; Harris, M.B.; Darnall, R.A. Activation of 5-HT1A receptors in the paragigantocellularis lateralis decreases shivering during cooling in the conscious piglet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R518–R527. [Google Scholar] [CrossRef][Green Version]
- Brown, J.W.; Sirlin, E.A.; Benoit, A.M.; Hoffman, J.M.; Darnall, R.A. Activation of 5-HT1a receptors in medullary raphe disrupts sleep and decreases shivering during cooling in the conscious piglet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R884–R894. [Google Scholar] [CrossRef][Green Version]
- Oerther, S. Temperature set-point changes induced by DA D2/3 and 5-HT1A receptor agonists in the rat. Neuroreport 2000, 11, 3949–3951. [Google Scholar] [CrossRef] [PubMed]
- Madden, C.J.; Morrison, S.F. Brown adipose tissue sympathetic nerve activity is potentiated by activation of 5-hydroxytryptamine (5-HT)1A/5-HT7 receptors in the rat spinal cord. Neuropharmacology 2008, 54, 487–496. [Google Scholar] [CrossRef]
- Madden, C.J.; Morrison, S.F. Endogenous activation of spinal 5-hydroxytryptamine (5-HT) receptors contributes to the thermoregulatory activation of brown adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R776–R783. [Google Scholar] [CrossRef] [PubMed]
- Voronova, I.P.; Kulikov, A.V.; Popova, N.K.; Kozyreva, T.V. Expression of the 1a and 2a serotonin receptor genes in the brain of rats adapted to warm and cold. J. Thermal. Biol. 2007, 32, 188–192. [Google Scholar] [CrossRef]
- Hart, J.S. Insulative and metabolic adaptations to cold in vertebrates. Symp. Soc. Exp. Biol. 1964, 18, 31–48. [Google Scholar] [PubMed]
- Werner, J.; Schingnitz, G.; Hensel, H. Influence of cold adaptation on the activity of thermoresponsive neurons in thalamus and midbrain of the rat. Pflugers Arch. 1981, 391, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Kozyreva, T.V.; Pierau, F.-K. Effect of cold adaptation and noradrenaline on thermosensitivity of rat hypothalamic neuron studied In Vitro. Neurophysiology 1994, 26, 142–146. [Google Scholar] [CrossRef]
- Kozyreva, T.V. Neurophysiological aspects of the long-term adaptation to cold in mammals: The role of central and peripheral thermoreceptors. J. Therm. Biol. 2006, 31, 105–114. [Google Scholar] [CrossRef]
- Hori, T.; Nakayama, T. Effects of biogenic amines on central thermoresponsive neurones in the rabbit. J. Physiol. 1973, 232, 71–85. [Google Scholar] [CrossRef][Green Version]
- Watanabe, T.; Morimoto, A.; Murakami, N. Effect of amine on temperature-responsive neuron in slice preparation of rat brain stem. Am. J. Physiol. 1986, 250, R553–R559. [Google Scholar] [CrossRef]
- Ulhoa, M.A.; da Silva, N.F.; Pires, J.G.; Futuro Neto Hde, A. Raphe obscurus neurons participate in thermoregulation in rats. Arq. Neuropsiquiatr. 2013, 71, 249–253. [Google Scholar] [CrossRef][Green Version]
- Nason, M.W., Jr.; Mason, P.J. Medullary raphe neurons facilitate brown adipose tissue activation. J. Neurosci. 2006, 26, 1190–1198. [Google Scholar] [CrossRef]
- Naumenko, V.S.; Tkachev, S.E.; Kulikov, A.V.; Semenova, T.P.; Amerhanov, Z.G.; Smirnova, N.P.; Popova, N.K. The Brain 5-HT1A Receptor Gene Expression in Hibernation. Genes Brain Behav. 2008, 7, 300–305. [Google Scholar] [CrossRef]
- Kalabukhov, N.I. Spyachka mlekopitayushchikh (Hibernation in Mammals); Nauka: Moscow, Russia, 1985; p. 248. [Google Scholar]
- Kusserow, H.; Davies, B.; Hfrtnagl, H.; Voigt, I.; Stroh, T.; Bert, B.; Deng, D.R.; Fink, H.; Veh, R.W.; Theuring, F. Reduced anxiety-related behaviour in transgenic mice overexpressing serotonin 1A receptors. Mol. Brain Res. 2004, 129, 104–116. [Google Scholar] [CrossRef]
- Olivier, B.; Pattij, T.; Wood, S.J.; Oosting, R.; Sarnyai, Z.; Toth, M. The 5-HT(1A) receptor knockout mouse and anxiety. Behav. Pharmacol. 2001, 12, 439–450. [Google Scholar] [CrossRef]
- Toth, M. 5-HT1A receptor knockout mouse as a genetic model of anxiety. Eur. J. Pharmacol. 2003, 463, 177–184. [Google Scholar] [CrossRef]
- Moutkine, I.; Collins, E.L.; Béchade, C.; Maroteaux, L. Evolutionary considerations on 5-HT2 receptors. Pharmacol. Res. 2019, 140, 14–20. [Google Scholar] [CrossRef]
- Pompeiano, M.; Palacios, J.M.; Mengod, G. Distribution of the serotonin 5-HT2 receptor family mRNAs: Comparison between 5-HT2A and 5-HT2C receptors. Mol. Brain Res. 1994, 23, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.E.; Seroogy, K.B.; Lundgren, K.H.; Davis, B.M.; Jennes, L. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J. Comp. Neurol. 1995, 351, 357–373. [Google Scholar] [CrossRef]
- Hoffman, B.J.; Mezey, E. Distribution of serotonin 5-HT1C receptor mRNA in adult rat brain. FEBS Lett. 1989, 247, 453–462. [Google Scholar] [CrossRef]
- Molineaux, S.M.; Jessell, T.M.; Axel, R.; Julius, D. 5-HT1c receptor is a prominent serotonin receptor subtype in the central nervous system. Proc. Natl. Acad. Sci. USA 1989, 86, 6793–6797. [Google Scholar] [CrossRef]
- Mengod, G.; Nguyen, H.; Le, H.; Waeber, C.; Lübbert, H.; Palacios, J.M. The distribution and cellular localization of the serotonin 1C receptor mRNA in the rodent brain examined by in situ hybridization histochemistry. Comparison with receptor binding distribution. Neuroscience 1990, 35, 577–591. [Google Scholar]
- Yadav, V.K.; Oury, F.; Suda, N.; Liu, Z.-W.; Gao, X.-B.; Confavreux, C.; Klemenhagen, K.C.; Tanaka, K.F.; Gingrich, J.A.; Guo, X.E.; et al. Leptin regulation of bone mass, appetite and energy expenditure relies on its ability to inhibit serotonin synthesis in the brainstem. Cell 2009, 138, 976–989. [Google Scholar] [CrossRef]
- Bonaventure, P.; Guo, H.; Tian, B.; Liu, X.; Bittner, A.; Roland, B.; Salunga, R.; Ma, X.J.; Kamme, F.; Meurers, B.; et al. Nuclei and subnuclei gene expression profiling in mammalian brain. Brain Res. 2002, 943, 38–47. [Google Scholar] [CrossRef]
- Falkenberg, V.R.; Gurbaxani, B.M.; Unger, E.R.; Rajeevan, M.S. Functional genomics of serotonin receptor 2A (HTR2A): Interaction of polymorphism, methylation, expression and disease association. Neuromol. Med. 2011, 13, 66–76. [Google Scholar] [CrossRef]
- De Lucchini, S.; Marracci, S.; Nardi, I. The serotonin 5-HT2B receptor from the puffer fish Tetraodon fluviatilis: cDNA cloning, genomic organization and alternatively spliced variants. Mol. Brain Res. 2001, 97, 89–93. [Google Scholar] [CrossRef]
- Palacios, J.M.; Pazos, A.; Hoyer, D. A short history of the 5-HT2C receptor: From the choroid plexus to depression, obesity and addiction treatment. Psychopharmacology 2017, 234, 1395–1418. [Google Scholar] [CrossRef]
- Burns, C.M.; Chu, H.; Rueter, S.M.; Hutchinson, L.K.; Canton, H.; Sanders-Bush, E.; Emerson, R.B. Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature 1997, 387, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Niswender, C.M.; Sanders-Bush, E.; Emeson, R.B. Identification and characterization of RNA editing events within the 5-HT2C receptor. Ann. N. Y. Acad. Sci. 1998, 861, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Niswender, C.M.; Copeland, S.C.; Herrick-Davis, K.; Emeson, R.B.; Sanders-Bush, E. RNA editing of the human serotonin 5-hydroxytryptamine 2C receptor silences constitutive activity. J. Biol. Chem. 1999, 274, 9472–9478. [Google Scholar] [CrossRef]
- Fitzgerald, L.W.; Iyer, G.; Conklin, D.S.; Krause, C.M.; Marshall, A.; Patterson, J.P.; Tran, D.P.; Jonak, G.J.; Hartig, P.R. Messenger RNA editing of the human serotonin 5-HT2C receptor. Neuropsychopharmacology 1999, 21, 82S–90S. [Google Scholar] [CrossRef][Green Version]
- Werry, T.D.; Loiacono, R.; Sexton, P.M.; Christopoulos, A. RNA editing of the serotonin 5HT2C receptor and its effects on cell signalling, pharmacology and brain function. Pharmacol. Ther. 2008, 119, 7–23. [Google Scholar] [CrossRef]
- Wang, Q.; O’Brien, P.J.; Chen, C.-X.; Cho, D.-S.; Murray, J.M.; Nishikura, K. Altered G protein-coupling functions of RNA editing isoform and splicing variant serotonin 2C receptors. J. Neurochem. 2000, 74, 1290–1300. [Google Scholar] [CrossRef]
- Nonogaki, K.; Memon, R.A.; Grunfeld, C.; Feingold, K.R.; Tecotta, L.H. Altered gene expressions involved in energy expenditure in 5-HT2C receptor mutant mice. Biochem. Biophys. Res. Commun. 2002, 295, 249–254. [Google Scholar] [CrossRef]
- Wozniak, K.M.; Aulakh, C.S.; Hill, J.L.; Murphy, D.L. Hyperthermia induced by m-CPP in the rat and its modification by antidepressant treatments. Psychopharmacology 1989, 97, 269–274. [Google Scholar] [CrossRef]
- Murphy, D.L.; Mueller, E.A.; Hill, J.L.; Tolliver, T.J.; Jacobsen, F.M. Comparative anxiogenic, neuroendocrine, and other physiologic effects of m-chlorophenylpiperazine given intravenously or orally to healthy volunteers. Psychopharmacology 1989, 98, 275–282. [Google Scholar] [CrossRef]
- Nash, J.F., Jr.; Meltzer, H.Y.; Gudelsky, G.A. Selective cross-tolerance to 5-HT1A and 5-HT2 receptor-mediated temperature and corticosterone responses. Pharmacol. Biochem. Behav. 1989, 33, 781–785. [Google Scholar] [CrossRef]
- Pranzatelli, M.R. Evidence for involvement of 5-HT2 and 5-HT1C receptors in the behavioral effects of the 5-HT agonist 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI). Neurosci. Lett. 1990, 115, 74–80. [Google Scholar] [CrossRef]
- Loscher, W.; Witte, U.; Fredow, G.; Ganter, M.; Bickhardt, K. Pharmacodynamic effects of serotonin (5-HT) receptor ligands in pigs: Stimulation of 5-HT2 receptors induces malignant hyperthermia. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1990, 341, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Klodzinska, A.; Chojnacka-Wojcik, E. Hyperthermia induced by m-trifluoromethylphenylpiperazine (TFMPP) or m-chlorophenylpiperazine (m-CPP) in heat-adapted rats. Psychopharmacology 1992, 109, 466–472. [Google Scholar] [CrossRef]
- Mazzola-Pomietto, P.; Aulakh, C.S.; Wozniak, K.M.; Hill, J.L.; Murphy, D.L. Evidence that 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI)-induced hyperthermia in rats is mediated by stimulation of 5-HT2A receptors. Psychopharmacology 1995, 117, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Aulakh, C.S.; Mazzola-Pomietto, P.; Murphy, D.L. Long-term antidepressant treatments alter 5-HT2A and 5-HT2C receptor-mediated hyperthermia in Fawn-Hooded rats. Eur. J. Pharmacol. 1995, 282, 65–70. [Google Scholar] [CrossRef]
- Mazzola-Pomietto, P.; Aulakh, C.S.; Wozniak, K.M.; Murphy, D.L. Evidence that m-chlorophenylpiperazine-induced hyperthermia in rats is mediated by stimulation of 5-HT2C receptors. Psychopharmacology 1996, 123, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Mazzola-Pomietto, P.; Aulakh, C.S.; Tolliver, T.; Murphy, D.L. Functional subsensitivity of 5-HT2A and 5-HT2C receptors mediating hyperthermia following acute and chronic treatment with 5-HT2A/2C receptor antagonists. Psychopharmacology 1997, 130, 144–151. [Google Scholar] [CrossRef][Green Version]
- Salmi, P.; Ahlenius, S. Evidence for functional interactions between 5-HT1A and 5-HT2A receptors in rat thermoregulatory mechanisms. Pharmacol. Toxicol. 1998, 82, 122–127. [Google Scholar] [CrossRef]
- Blessing, W.W.; Seaman, B. 5-hydroxytryptamine 2a receptors regulate sympathetic nerves constricting the cutaneous vascular bed in rabbits and rats. Neuroscience 2003, 117, 939–948. [Google Scholar] [CrossRef]
- Hayashi, A.; Sonoda, R.; Kimura, Y.; Takasu, T.; Suzuki, M.; Sasamata, M.; Miyata, K. Antiobesity effect of YM348, a novel 5-HT2C receptor agonist, in Zucker rats. Brain Res. 2004, 1011, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Tao, R. Enhanced responsivity of 5-HT2A receptors at warm ambient temperatures is responsible for the augmentation of the 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI)-induced hyperthermia. Neurosci. Lett. 2011, 490, 68–71. [Google Scholar] [CrossRef]
- Nakamura, M.; Shintani-Ishida, K.; Ikegaya, H. 5-HT2A receptor agonist-induced hyperthermia is induced via vasoconstriction by peripheral 5-ht2a receptors and brown adipose tissue thermogenesis by peripheral serotonin loss at a high ambient temperature. J. Pharmacol. Exp. Ther. 2018, 367, 356–362. [Google Scholar] [CrossRef]
- Won, S.J.; Lin, M.T. 5-Hydroxytryptamine receptors in the hypothalamus mediate thermoregulatory responses in rabbits. Naunyn. Schmiedeberg’s Arch. Pharmacol. 1988, 338, 256–261. [Google Scholar] [CrossRef]
- Yamada, J.; Sugimoto, Y.; Horisaka, K. Serotonin2 (5-HT2) receptor agonist 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) inhibits chlorpromazine- and haloperidol-induced hypothermia in mice. Biol. Pharm. Bull. 1995, 18, 1580–1583. [Google Scholar] [CrossRef]
- Morishima, Y.; Shibano, T. Evidence that 5-HT2A receptors are not involved in 5-HT-mediated thermoregulation in mice. Pharmacol. Biochem. Behav. 1995, 52, 755–758. [Google Scholar] [CrossRef]
- Fox, M.A.; French, H.T.; LaPorte, J.L.; Blackler, A.R.; Murphy, D.L. The serotonin 5-HT(2A) receptor agonist TCB-2: A behavioral and neurophysiological analysis. Psychopharmacology 2010, 212, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Voronova, I.P.; Khramova, G.M.; Kulikova, E.A.; Petrovskii, D.V.; Bazovkina, D.V.; Kulikov, A.V. 5-HT2A receptors control body temperature in mice during LPS-induced inflammation via regulation of NO production. Pharmacol. Res. 2016, 103, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Buchborn, T.; Lyons, T.; Song, C.; Feilding, A.; Knöpfel, T.J. The serotonin 2A receptor agonist 25CN-NBOH increases murine heart rate and neck-arterial blood flow in a temperature-dependent manner. J. Psychopharmacol. 2020, 34, 786–794. [Google Scholar] [CrossRef]
- Naumenko, V.S.; Bazovkina, D.V.; Kondaurova, E.M. On the Functional Cross-Talk between Brain 5-HT1A and 5-HT2A Receptors. Zhurnal Vyss. Nervn. Deiatelnosti Im. IP Pavlov. 2015, 65, 240–247. [Google Scholar]
- Voronova, I.; Khramova, G.; Bazovkina, D.; Kulikov, A. Involvement of serotonin2A (5-HT2A) receptor and terminal fragment of chromosome 13 in the development of hypothermia in lipopolysaccharide (LPS)-treated mice. Eur. Neuropsychopharmacol. 2019, 29, s277. [Google Scholar] [CrossRef]
- Dalpiaz, A.; Gessi, S.; Borea, P.A.; Gilli, G. Binding thermodynamics of serotonin to rat-brain 5-HT1A, 5-HT2A and 5-HT3 receptors. Life Sci. 1995, 57, PL141–PL146. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Yamada, J.; Horisaka, K. Activation of peripheral serotonin 2 receptors induces hypothermia in mice. Life Sci. 1991, 48, 419–423. [Google Scholar] [CrossRef]
- Ootsuka, Y.; Nalivaiko, E.; Blessing, W.W. Spinal 5-HT2A receptors regulate cutaneous sympathetic vasomotor outflow in rabbits and rats; relevance for cutaneous vasoconstriction elicited by MDMA (3,4-methylenedioxymethamphetamine, “Ecstasy”) and its reversal by clozapine. Brain Res. 2004, 1014, 34–44. [Google Scholar] [CrossRef]
- Nitanda, A.; Yasunami, N.; Tokumo, K.; Fujii, H.; Hirai, T.; Nishio, H. Contribution of the peripheral 5-HT2A receptor to mechanical hyperalgesia in a rat model of neuropathic pain. Neurochem. Int. 2005, 47, 394–400. [Google Scholar] [CrossRef]
- Nonogaki, K.; Nozue, K.; Oka, Y. Increased hypothalamic 5-HT2A receptor gene expression and effects of pharmacologic 5-HT2A receptor inactivation in obese Ay mice. Biochem. Biophys. Res. Commun. 2006, 351, 1078–1082. [Google Scholar] [CrossRef]
- Saitoh, Y. Suppression of Central post–stroke pain with sarpogrelate hydrochloride and paroxetine. Pain Res. 2012, 27, 1–6. [Google Scholar] [CrossRef][Green Version]
- Crane, J.D.; Palanivel, R.; Mottillo, E.; Bujak, A.L.; Wang, H.; Ford, R.J.; Collins, A.; Blümer, R.M.; Fullerton, M.D.; Yabut, J.M.; et al. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nat. Med. 2015, 21, 166–172. [Google Scholar] [CrossRef]
- Ootsuka, Y.; Blessing, W.W. Activation of slowly conducting medullary raphe-spinal neurons, including serotonergic neurons, increases cutaneous sympathetic vasomotor discharge in rabbit. Am. J. Physiol. 2005, 288, R909–R918. [Google Scholar] [CrossRef]
- Ootsuka, Y.; Blessing, W.W.; Nalivaiko, E. Selective blockade of 5-HT2A receptors attenuates the increased temperature response in brown adipose tissue to restraint stress in rats. Stress 2008, 11, 125–133. [Google Scholar] [CrossRef]
- Beig, M.I.; Baumert, M.; Walker, F.R.; Day, T.A.; Nalivaiko, E. Blockade of 5-HT2A receptors suppresses hyperthermic but not cardiovascular responses to psychosocial stress in rats. Neuroscience 2009, 159, 1185–1191. [Google Scholar] [CrossRef]
- Sinh, V.; Ootsuka, Y. Blockade of 5-HT2A receptors inhibits emotional hyperthermia in mice. J. Physiol. Sci. 2019, 69, 1097–1102. [Google Scholar] [CrossRef]
- Buckholtz, N.S.; Zhou, D.F.; Freedman, D.X. Serotonin2 agonist administration down-regulates rat brain serotonin2 receptors. Life Sci. 1988, 42, 2439–2445. [Google Scholar] [CrossRef]
- Lam, D.D.; Przydzial, M.J.; Ridley, S.H.; Yeo, G.S.H.; Rochford, J.J.; O’Rahilly, S.; Heisler, L.K. Serotonin 5-HT2C receptor agonist promotes hypophagia via downstream activation of melanocortin4 Receptors. Endocrinology 2008, 149, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Gray, J.A.; Roth, B.L. The Expanded Biology of Serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Sohle, J.; Machuy, N.; Smailbegovic, E.; Holtzmann, U.; Gronniger, E.; Wenck, H.; Stab, F.; Winnefeld, M. Identification of new genes involved in human adipogenesis and fat storage. PLoS ONE 2012, 7, e31193. [Google Scholar] [CrossRef]
- Oh, C.-M.; Namkung, J.; Go, Y.; Shong, K.E.; Kim, K.; Kim, H.; Park, B.-Y.; Lee, H.W.; Jeon, Y.H.; Song, J.; et al. Regulation of systemic energy homeostasis by serotonin in adipose tissues. Nat. Commun. 2015, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Stunes, A.K.; Reseland, J.E.; Hauso, Ø.; Kidd, M.; Tømmera, K.; Waldum, H.L.; Syversen, U.; Gustafsson, B.I. Adipocytes express a functional system for serotonin synthesis, reuptake and receptor activation. Diabetes Obes. Metab. 2011, 13, 551–558. [Google Scholar] [CrossRef] [PubMed]
- McGlashon, J.M.; Gorecki, M.C.; Kozlowski, A.E.; Thirnbeck, C.K.; Markan, K.R.; Leslie, K.L.; Kotas, M.E.; Potthoff, M.J.; Richerson, G.B.; Gillum, M.P. Central serotonergic neurons activate and recruit thermogenic brown and beige fat and regulate glucose and lipid homeostasis. Cell Metab. 2015, 21, 692–705. [Google Scholar] [CrossRef]
- Nebigil, C.G.; Etienne, N.; Messaddeq, N.; Maroteaux, L. Serotonin is a novel survival factor of cardiomyocytes: Mitochondria as a target of 5-HT2B receptor signaling. FASEB J. 2003, 17, 1373–1375. [Google Scholar] [CrossRef] [PubMed]
- Harmon, J.L.; Wills, L.P.; McOmish, C.E.; Demireva, E.Y.; Gingrich, J.A.; Beeson, C.C.; Schnellmann, R.G. 5-HT2 receptor regulation of mitochondrial genes: Unexpected pharmacological effects of agonists and antagonists. J. Pharmacol. Exp. Ther. 2016, 357, 1–9. [Google Scholar] [CrossRef]
- Bouillaud, F.; Alves-Guerra, M.C.; Ricquier, D. UCPs, at the interface between bioenergetics and metabolism. Biochim. Biophys. Acta. 2016, 1863, 2443–2456. [Google Scholar] [CrossRef] [PubMed]
- Derkach, V.; Surprenant, A.; North, R.A. 5-HT3 receptors are membrane ion channels. Nature 1989, 339, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Lummis, S.C.R. 5-HT3 Receptors. J. Biol. Chem. 2012, 287, 40239–40245. [Google Scholar] [CrossRef] [PubMed]
- Maricq, A.V.; Peterson, A.S.; Brake, A.J.; Myers, R.M.; Julius, D. Primary structure and functiomd expression of the 5-HT3 receptor, a serotonin-gated ion channel. Science 1991, 254, 432–437. [Google Scholar] [CrossRef]
- Thompson, A.J.; Lummis, S.C. 5-HT3 receptors. Curr. Pharm. Des. 2006, 12, 3615–3630. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Lummis, S.C. The 5-HT3 receptor as a therapeutic target. Expert Opin. Ther. Targets 2007, 11, 527–540. [Google Scholar] [CrossRef]
- Barnes, N.M.; Hales, T.G.; Lummis, S.C.; Peters, J.A. The 5-HT3 receptor--the relationship between structure and function. Neuropharmacology 2009, 56, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Werner, P.; Kawashima, E.; Reid, J.; Hussy, N.; Lundström, K.; Buell, G.; Humbert, Y.; Jones, K.A. Organization of the mouse 5-HT3 receptor gene and functional expression of two splice variants. Mol. Brain Res. 1994, 26, 233–241. [Google Scholar] [CrossRef]
- Butler, A.S.; Lindesay, S.A.; Dover, T.J.; Kennedy, M.D.; Patchell, V.B.; Levine, B.A.; Hope, A.G.; Barnes, N.M. Importance of the C-terminus of the human 5-HT3A receptor subunit. Neuropharmacology 2009, 56, 292–302. [Google Scholar] [CrossRef]
- Dubin, A.E.; Huvar, R.; D’Andrea, M.R.; Pyati, J.; Zhu, J.Y.; Joy, K.C.; Wilson, S.J.; Galindo, J.E.; Glass, C.A.; Luo, L.; et al. The pharmacological and functional characteristics of the serotonin 5-HT(3A) receptor are specifically modified by a 5-HT(3B) receptor subunit. J. Biol. Chem. 1999, 274, 30799–30810. [Google Scholar] [CrossRef]
- Yaakob, N.; Malone, D.T.; Exintaris, B.; Irving, H.R. Heterogeneity amongst 5-HT3 receptor subunits: Is this significant? Curr. Mol. Med. 2011, 11, 57–68. [Google Scholar] [CrossRef]
- Thompson, A.J.; Lummis, S.C. Discriminating between 5-HT3A and 5-HT3AB receptors. Br. J. Pharmacol. 2013, 169, 736–747. [Google Scholar] [CrossRef]
- Boyd, G.W.; Doward, A.I.; Kirkness, E.F.; Millar, N.S.; Connolly, C.N. Cell surface expression of 5-hydroxytryptamine type 3 receptors is controlled by an endoplasmic reticulum retention signal. J. Biol. Chem. 2003, 278, 27681–27687. [Google Scholar] [CrossRef]
- Massoura, A.N.; Dover, T.J.; Newman, A.S.; Barnes, N.M. The identification of N-glycosylated residues of the human 5-HT3B receptor subunit: Importance for cell membrane expression. J. Neurochem. 2011, 116, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Bloom, F.E. The 5-HT3 receptor is present in different subpopulations of GABAergic neurons in the rat telencephalon. J. Neurosci. 1997, 17, 3157–3167. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Kondo, M.; Shimada, S. Building a 5-HT3A Receptor Expression Map in the Mouse Brain. Sci. Rep. 2017, 7, 42884. [Google Scholar] [CrossRef]
- Chameau, P.; van Hooft, J.A. Serotonin 5-HT3 receptors in the central nervous system. Cell Tissue Res. 2006, 326, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Mazzola-Pomietto, P.; Aulakh, C.S.; Murphy, D.L. Temperature, food intake, and locomotor activity effects of a 5-HT3 receptor agonist and two 5-HT3 receptor antagonists in rats. Psychopharmacology 1995, 121, 488–493. [Google Scholar] [CrossRef]
- Kandasamy, S.B. Effect of ondansetron and ICS 205-930 on radiation-induced hypothermia in rats. Radiat. Res. 1997, 147, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.M.; Wilson, B.C.; Chen, X.; Takahashi, Y.; Poulin, P.; Pittman, Q.J. Vagal CCK and 5-HT(3) receptors are unlikely to mediate LPS or IL-1beta-induced fever. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R960–R965. [Google Scholar] [CrossRef] [PubMed]
- Ngampramuan, S.; Cerri, M.; Del Vecchio, F.; Corrigan, J.J.; Kamphee, A.; Dragic, A.S.; Rudd, J.A.; Romanovsky, A.A.; Nalivaiko, E. Thermoregulatory correlates of nausea in rats and musk shrews. Oncotarget 2014, 5, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Refinetti, R. Peripheral nervous control of cold-induced reduction in the respiratory quotient of the rat. Int. J. Biometeorol. 1990, 34, 24–27. [Google Scholar] [CrossRef]
- Tkachenko, E.Y.; Kozaruk, V.P.; Khramova, G.M.; Voronova, I.P.; Meyta, E.S.; Kozyreva, T.V. Different formation of thermoregulatory response to cooling in dependence on the type of the skin thermoreceptor activity. Bull. Sib. Branch Russ. Acad. Med. Sci. 2010, 30, 95–100. [Google Scholar]
- Wang, W.; Song, X.; Wang, T.; Zhang, C.; Sun, L. 5-HT3 receptor antagonists for the prevention of perioperative shivering: A meta-analysis. J. Clin. Pharmacol. 2017, 57, 428–439. [Google Scholar] [CrossRef]
- Shen, Q.-H.; Li, H.-F.; Zhou, X.; Lu, Y.; Yuan, X.-Z. 5-HT3 receptor antagonists for the prevention of perioperative shivering undergoing spinal anaesthesia: A systematic review and meta-analysis of randomised controlled trials. BMJ Open 2020, 10, e038293. [Google Scholar] [CrossRef] [PubMed]
- Ruat, M.; Traiffort, E.; Leurs, R.; Tardivel-Lacombe, J.; Diaz, J.; Arrang, J.M.; Schwartz, J.C. Molecular cloning, characterization, and localization of a high- affinity serotonin receptor (5-HT7) activating cAMP formation. Proc. Natl. Acad. Sci. USA 1993, 90, 8547–8551. [Google Scholar] [CrossRef]
- Lovenberg, T.W.; Baron, B.M.; de Lecea, L.; Miller, J.D.; Prosser, R.A.; Rea, M.A.; Foye, P.E.; Racke, M.; Slone, A.L.; Siegel, B.W.; et al. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron 1993, 11, 449–458. [Google Scholar] [CrossRef]
- Bard, J.A.; Zgombick, J.; Adham, N.; Vaysse, P.; Branchek, T.A.; Weinshank, R.L. Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. J. Biol. Chem. 1993, 268, 23422–23426. [Google Scholar] [CrossRef]
- Hedlund, P.B.; Sutcliffe, J.G. Functional, molecular and pharmacological advances in 5-HT7 receptor research. Trends Pharmacol. Sci. 2004, 25, 481–486. [Google Scholar] [CrossRef]
- Gellynck, E.; Heyninck, K.; Andressen, K.W.; Haegeman, G.; Levy, F.O.; Vanhoenacker, P.; Van Craenenbroeck, K. The serotonin 5--HT7 receptors: Two decades of research. Exp. Brain Res. 2013, 230, 555–568. [Google Scholar] [CrossRef]
- Nikiforuk, A. Targeting the serotonin 5-HT7 receptor in the search for treatments for CNS disorders: Rationale and progress to date. CNS Drugs 2015, 29, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Heidmann, D.E.; Metcalf, M.A.; Kohen, R.; Hamblin, M.W. Four 5-hydroxytryptamine7 (5-HT7) receptor isoforms in human and rat produced by alternative splicing: Species differences due to altered intron-exon organization. J. Neurochem. 1997, 68, 1372–1381. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M.A.; Schechter, L.E. Cloning, mRNA localization and evolutionary conservation of a human 5-HT7 receptor pseudogene. Gene 1999, 227, 63–69. [Google Scholar] [CrossRef]
- Leopoldo, M.; Lacivita, E.; Berardi, F.; Perrone, R.; Hedlund, P.B. Serotonin 5-HT7 receptor agents: Structure-activity relationships and potential therapeutic applications in central nervous system disorders. Pharmacol. Ther. 2011, 129, 120–148. [Google Scholar] [CrossRef]
- Matthys, A.; Haegeman, G.; Van Craenenbroeck, K.; Vanhoenacke, P. Role of the 5-HT7 receptor in the central nervous system: From current status to future perspectives. Mol. Neurobiol. 2011, 43, 228–253. [Google Scholar] [CrossRef]
- Guseva, D.; Wirth, A.; Ponimaskin, E. Cellular mechanisms of the 5-HT7 receptor-mediated signaling. Front. Behav. Neurosci. 2014, 8, 306. [Google Scholar] [CrossRef]
- Blattner, K.M.; Canney, D.J.; Pippin, D.A.; Blass, B.E. Pharmacology and therapeutic potential of the 5--HT7 receptor. ACS Chem. Neurosci. 2019, 10, 89–119. [Google Scholar] [CrossRef]
- Renner, U.; Zeug, A.; Woehler, A.; Niebert, M.; Dityatev, A.; Dityateva, G.; Gorinski, N.; Guseva, D.; Abdel-Galil, D.; Fröhlich, M. Heterodimerization of serotonin receptors 5-HT1A and 5-HT7 differentially regulates receptor signalling and trafficking. J. Cell Sci. 2012, 125, 2486–2499. [Google Scholar] [CrossRef]
- Hagan, J.J.; Price, G.W.; Jeffrey, P.; Deeks, N.J.; Stean, T.; Piper, D.; Smith, M.I.; Upton, N.; Medhurst, A.D.; Middlemiss, D.N.; et al. Characterization of SB-269970-A, a selective 5-HT7 receptor antagonist. Br. J. Pharmacol. 2000, 130, 539–548. [Google Scholar] [CrossRef]
- Thomas, D.R.; Melotto, S.; Massagrande, M.; Gribble, A.D.; Jeffrey, P.; Stevens, A.J.; Deeks, N.J.; Eddershaw, P.J.; Fenwick, S.H.; Riley, G.; et al. SB-656104-A, a novel selective 5-HT7 receptor antagonist, modulates REM sleep in rats. Br. J. Pharmacol. 2003, 139, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Guscott, M.R.; Egan, E.; Cook, G.P.; Stanton, J.A.; Beer, M.S.; Rosahl, T.W.; Hartmann, S.; Kulagowski, J.; McAllister, G.; Fone, K.C.F.; et al. The hypothermic effect of 5-CT in mice is mediated through the 5-HT7 receptor. Neuropharmacology 2003, 44, 1031–1037. [Google Scholar] [CrossRef]
- Hedlund, P.B.; Danielson, P.E.; Thomas, E.A.; Slanina, K.; Carson, M.J.; Sutcliffe, J.G. No hypothermic response to serotonin in 5-HT7 receptor knockout mice. Proc. Natl. Acad. Sci. USA 2003, 100, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, P.B.; Leopoldo, M.; Caccia, S.; Sarkisyan, G.; Fracasso, C.; Martelli, G.; Lacivita, E.; Berardi, F.; Perrone, R. LP-211 is a brain penetrant selective agonist for the serotonin 5-HT7 receptor. Neurosci. Lett. 2010, 481, 12–16. [Google Scholar] [CrossRef][Green Version]
- Brenchat, A.; Rocasalbas, M.; Zamanillo, D.; Hamon, M.; Vela, J.M.; Romero, L. Assessment of 5-HT7 receptor agonists selectivity using nociceptive and thermoregulation tests in knockout versus wild-type mice. Adv. Pharmacol. Sci. 2012, 2012, 312041. [Google Scholar] [CrossRef]
- Faure, C.; Mnie-Filali, O.; Scarna, H.; Debonnel, G.; Haddjeri, N. Effects of the 5-HT7 receptor antagonist SB-269970 on rat hormonal and temperature responses to the 5-HT1A/7 receptor agonist 8-OH-DPAT. Neurosci. Lett. 2006, 404, 122–126. [Google Scholar] [CrossRef]
- Di Pilato, P.; Niso, M.; Adriani, W.; Romano, E.; Travaglini, D.; Berardi, F.; Colabufo, N.A.; Perrone, R.; Laviola, G.; Lacivita, E.; et al. Selective agonists for serotonin 7 (5-HT7) receptor and their applications in preclinical models: An overview. Rev. Neurosci. 2014, 25, 401–415. [Google Scholar] [CrossRef]
- Yamada, J.; Sugimoto, Y.; Wakita, H.; Horisaka, K. The involvement of serotonergic and dopaminergic systems in hypothermia induced in mice by intracerebroventricular injection of serotonin. Jpn. J. Pharmacol. 1988, 48, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Kose, D.; Cadirci, E.; Halici, Z.; Sirin, B.; Dincer, B. The investigation of possible roles of central 5--HT7 receptors in antipyretic effect mechanism of paracetamol in LPS--induced hyperthermia model of mice. Inflammopharmacology 2019. [Google Scholar] [CrossRef]
- Gargaglioni, L.H.; Steiner, A.A.; Branco, L.G.S. Involvement of serotoninergic receptors in the anteroventral preoptic region on hypoxia-induced hypothermia. Brain Res. 2005, 1044, 16–24. [Google Scholar] [CrossRef]
- Hrvatin, S.; Sun, S.; Wilcox, O.F.; Yao, H.; Lavin-Peter, A.J.; Cicconet, M.; Assad, E.G.; Palmer, M.E.; Aronson, S.; Banks, A.S.; et al. Neurons that regulate mouse torpor. Nature 2020, 583, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Bielefeldt, K.; Gebhart, G.F. TRPV1 function in mouse colon sensory neurons is enhanced by metabotropic 5-hydroxytryptamine receptor activation. J. Neurosci. 2004, 24, 9521–9530. [Google Scholar] [CrossRef] [PubMed]
- Kayser, V.; Elfassi, I.E.; Aubel, B.; Melfort, M.; Julius, J.D.; Gingrich, A.; Hamon, M.; Bourgoin, S. Mechanical, thermal and formalin-induced nociception is differentially altered in 5-HT1A-/-, 5-HT1B-/-, 5-HT2A-/-,5-HT3A-/- and 5-HTT-/- knock-out male mice. Pain 2007, 130, 235–248. [Google Scholar] [CrossRef]
- Kupers, R.; Frokjaer, V.G.; Naert, A.; Christensen, R.; Budtz-Joergensen, E.; Kehlet, H.; Knudsen, G.M. A PET [18F]altanserin study of 5-HT2A receptor binding in the human brain and responses to painful heat stimulation. Neuroimage 2009, 44, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Brenchat, A.; Romero, L.; García, M.; Pujol, M.; Burgueño, J.; Torrens, A.; Hamon, M.; Baeyens, J.M.; Buschmann, H.; Zamanillo, D.; et al. 5-HT7 receptor activation inhibits mechanical hypersensitivity secondary to capsaicin sensitization in mice. Pain 2009, 141, 239–247. [Google Scholar] [CrossRef]
- Cortes-Altamirano, J.L.; Olmos-Hernández, A.; Jaime, H.B.; Carrillo-Mora, P.; Bandala, C.; Reyes-Long, S.; Alfaro-Rodríguez, A. 5-HT1, 5-HT2, 5-HT3 and 5-HT7 receptors and their role in the modulation of pain response in the central nervous system. Curr. Neuropharmacol. 2018, 16, 210–221. [Google Scholar] [CrossRef]
- Nagai, M. The role of serotonergic system in body temperature regulation. Physiol. Res. 1992, 41, 65–69. [Google Scholar]
- Uphouse, L. Multiple serotonin receptors: Too many, not enough, or just the right number? Neurosci. Biobehav. Rev. 1997, 21, 679–698. [Google Scholar] [CrossRef]
- Zhang, Y.; D’Souza, D.; Raap, D.K.; Garcia, F.; Battaglia, G.; Muma, N.A.; Van de Kar, L.D. Characterization of the functional heterologous desensitization of hypothalamic 5-HT1A receptors after 5-HT2A receptor activation. J. Neurosci. 2001, 21, 7919–7927. [Google Scholar] [CrossRef]
- Zhang, Y.; Gray, T.S.; D’Souza, D.N.; Carrasco, G.A.; Damjanoska, K.J.; Dudas, B.; Garcia, F.; Zainelli, G.M.; Hanley, N.R.S.; Battaglia, G.; et al. Desensitization of 5-HT1A receptors by 5-HT2A receptors in neuroendocrine neurons In Vivo. J. Pharmacol. Exp. Ther. 2004, 310, 59–66. [Google Scholar] [CrossRef]
- Ishiwata, T.; Hasegawac, H.; Greenwood, B.N. Involvement of serotonin in the ventral tegmental area in thermoregulation of freely moving rats. Neurosci. Lett. 2017, 653, 71–77. [Google Scholar] [CrossRef]
- Burton, A.C.; Edholm, O.G. Man in A Cold Environment. Physiological and Pathological Effects of Exposure to Low Temperatures; Edward Arnold Ltd.: London, UK, 1955; p. 273. [Google Scholar]
- Gordon, C.J. The therapeutic potential of regulated hypothermia. Emerg. Med. J. 2001, 18, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Johansen, F.F.; Hasseldam, H.; Smith, M.N.; Rasmussen, R.S. Drug-induced hypothermia by 5HT1A agonists provide neuroprotection in experimental stroke: New perspectives for acute patient treatment. J. Stroke Cerebrovasc. Dis. 2014, 23, 2879–2887. [Google Scholar] [CrossRef] [PubMed]
- Tarahovsky, Y.S.; Fadeeva, I.S.; Komelina, N.P.; Khrenov, M.O.; Zakharova, N.M. Antipsychotic inductors of brain hypothermia and torpor-like states: Perspectives of application. Psychopharmacology 2016, 234, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Puspitasari, A.; Cerri, M.; Takahashi, A.; Yoshida, Y.; Hanamura, K.; Tinganelli, W. Hibernation as a tool for radiation protection in space exploration. Life 2021, 11, 54. [Google Scholar] [CrossRef]
| Maximal Hypothermic Effect (°C) | Time of Its Achievements (min) | Object | Dose of 8-OH-DPAT | Route of Administration | References |
|---|---|---|---|---|---|
| 3.5 | 10 | C57/B16 mice | 3.0 µg | i.c.v. | [54] |
| 2.2 | 30 | 0.5 mg/kg | s.c. | ||
| 2.9 | 30 | 2.5 mg/kg | |||
| 3 | 18 | 5.0 mg/kg | |||
| 3 | 18 | 10.0 mg/kg | |||
| 2.4 | 30 | Sprague-Dawley rats | 0.03–0.1 mg/kg | s.c. | [55] |
| 3 | 30 | Sprague-Dawley rats | 0.3 mg/kg | s.c. | [56] |
| 3 | 15 | C57Bl6 mice | 5 mg/kg | s.c. | [57] |
| 3.5 | 10 | 2 µg | i.c.v. | ||
| 2 | 30 | Sprague-Dawley rats | 0.3 mg/kg | s.c. | [58] |
| 2.5 | 30 | Sprague-Dawley rats | 1 mg/kg | s.c. | [59] |
| 2.5 | 10 | Hooded Lister rats | 2500 ng | into DRN | [60] |
| 0.5 | 60 | Wistar rats | 0.125 mg/kg | s.c. | [61] |
| 0.9 | 30 | 0.25 mg/kg | |||
| 1.5 | 60 | 0.5 mg/kg | |||
| 1.3 | 30 | Sprague-Dawley rats | 0.1 mg/kg | s.c. | [62] |
| 1.6 | 10 | ICR mice | 0.25mg/kg | s.c. | [63] |
| 3.0 | 15–30 | Female albino Tuck (T/O strain) mice | 3.0 mg/kg | s.c. | [64] |
| 2.3 | 30 | Sprague-Dawley rats | |||
| 1.3 | 20 | Wistar rats | 5 µg | into DRN | [9] |
| no effect | into MRN | ||||
| 2.5 | 15 | Swiss-Webster mice | 2 mg/kg | s.c. | [65] |
| 2 | 20 | C57/Bl/6Ola mice | 5.0 mg/kg | s.c. | [66] |
| 1.3 | 0.5 mg/kg | ||||
| 2.5 | 30 | Wistar rats | 0.5 mg/kg | s.c. | [67] |
| 2 | 15–30 | Sprague-Dawley rats | 0.25 mg/kg | s.c. | [68] |
| 2.8 | 15–30 | T/O mice female | 0.5 mg/kg | ||
| 1.5 | 30 | Wistar rats | 0.3 mg/kg | s.c. | [69] |
| 4 | 20 | C57/Bl6Htr1a (+/+) | 1 mg/kg | s.c. | [70] |
| 3.3 | C57/Bl6 Htr1a (−/+) | ||||
| no effect | C57/Bl6 Htr1a (−/−) | ||||
| 1.2 | C57/Bl6 Htr1a (+/+) | 0.2 mg/kg | |||
| 1.3 | C57/Bl6 Htr1a (−/+) | ||||
| no effect | C57/Bl6 Htr1a (−/−) | ||||
| 5 | 45 | rats HDS * | 0.5 mg/kg | s.c. | [45] |
| 1.2 | rats RDS * | ||||
| 0.7 | rats LDS * | ||||
| 2.5 | 30 | rats HDS * | 20 µg | i.c.v. | |
| no effect | rats LDS * | ||||
| 1.27 | 90 | Sprague-Dawley rats | 500 µg/kg | s.c. | [71] |
| 1.15 | 0.5 µg | into the hypothalamus | |||
| 1.6 | 30 | Sprague-Dawley rats | 0.05 mg/kg | s.c. | [72] |
| 3.5 | 0.1 mg/kg | ||||
| 2 | 30 | Sprague-Dawley rats | 0.1 mg/kg | i.p. | [50] |
| 3 | 0.3 mg/kg | ||||
| 3.5 | 1 mg/kg | ||||
| 2.1 | 30 | ♀♂mice 129rSv WT | 0.2 mg/kg | s.c. | [73] |
| 3.1 | 30 | ♀♂ Htr1b KO mice | 0.6 mg/kg | ||
| 2.5 | 30 | Sprague-Dawley rats | 0.1 mg/kg | s.c. | [74] |
| 2 | 30 | Sprague-Dawley rats | 0.3 mg/kg | i.p. | [75] |
| 0.5 | 30 | C57BL/6J mice | 0.3 mg/kg | i.p. | |
| 1.2 | 0.6 mg/kg | ||||
| 1.5 | 0.1 mg/kg | ||||
| 1.58 | 30 | non aggressive rats | 0.5 mg/kg | i.p. | [47] |
| no effect | aggressive rats | ||||
| no effect | 30 | aggressive rats | 0.5 mg/kg | i.p. | [76] |
| 2.2 | non-aggressive rats | ||||
| 0.75 | 20 | Tg8 mice | 2 mg/kg | i.p. | |
| 2 | C3H mice | ||||
| 1.75 | 20 | CBA/Lac mice | 0.5 mg/kg | i.p. | [48] |
| 2.25 | 1 mg/kg | ||||
| no effect | 20 | AKR mice | 0.5 mg/kg1 mg/kg | ||
| 1.4 | 30 | Sprague-Dawley rats | 0.2 mg/kg | i.p. | [77] |
| 1.57 | 10–20 | CBA/Lac mice | 1 mg/kg | i.n. | [78] |
| 1.1 | i.p. | ||||
| 1.9 | s.c. | ||||
| 0.71 | DBA/2 mice | i.n. | |||
| 1.37 | i.p. | ||||
| 1.44 | s.c. | ||||
| 1.8 | PT mice | i.n. | |||
| 1.91 | i.p. | ||||
| 0.98 | C57Bl mice | i.n. | |||
| 0.67 | i.p. | ||||
| 2.62 | s.c. | ||||
| 1.2 | 20 | CBA/Lac mice | 1 mg/kg | i.p. | [79] |
| 1 | C57/Bl6 mice | ||||
| 1 | DBA/2 mice | ||||
| 2.2 | BALB/C mice | ||||
| 0.5 | AKR mice | ||||
| 0.5 | ICR mice | ||||
| 3 | 20 | 1A-High mice | 0.5 mg/kg | i.p. | [51] |
| 1 | 10 | 1A-Low mice | |||
| 2.25 | 20 | CBA mice | 1 mg/kg | i.p. | [49] |
| 2.5 | ASC mice | ||||
| 0.4ns | AKR mice | ||||
| 1.25 | AKR.CBA-D13Mit76C mice | ||||
| 2.5 | 30 | CBA/Lac mice | 40 nM | i.c.v. | [80] |
| 1.2 | 20 | CBA/Lac mice | 1 mg/kg | i.p. | [30] |
| 0.9 | C57/Bl6 mice | ||||
| 0.9 | DBA/2 mice | ||||
| 2.2 | BALB/C mice | ||||
| 0.5ns | AKR mice | ||||
| 0.5ns | ICR mice | ||||
| 1.2 | C3H mice | ||||
| 2.6 | Asn mice | ||||
| 3 | 20 | WT mice | 0.5 mg/kg | i.p. | [52] |
| 1.3 | KO Htr1a in DRN | ||||
| 1.5 | 20 | ♂ WT mice | 0.75 mg/kg | i.p. | [53] |
| 1.75 | ♀ WT mice | ||||
| no effect | 1AcKO mice ♂♀ |
| Conclusion | Argumentation | Object | References |
|---|---|---|---|
| Pre- | Hypothermic effect of 8-OH-DPAT was attenuated when 5-HT was depleted by repeated i.p. administration of pCPA or by injection of 5,7-DHT into the third ventricle | C57/B16/0la mice | [54] |
| Post- | After pretreatment by pCPA the minimal dose of 8-OH-DPAT (0.03 mg/kg s.c.), ineffective earlier, deveined effective. It may be the result of receptor sensitization that appeared after pCPA reducing of the whole brain 5-HT level. | Sprague-Dawley rats | [55] |
| Pre- | Hypothermic effect of 8-OH-DPAT was attenuated when 5-HT was depleted by repeated i.p. administration of pCPA or by injection of 5,7-DHT into the third ventricle | Sprague-Dawley rat | [58] |
| Post- | pCPA tended to increase the hypothermic effect of 8-OH-DPAT (−3 °C in comparing with −2,5 °C) | Sprague-Dawley rats | [59] |
| Post- | pCPA tended to increase the hypothermic effect of 8-OH-DPAT | ddY mice | [85] |
| Pre- | Lesioning of central 5-HT neurones by 5,7-DHT abolished the hypothermic response to 8-OH-DPAT. Depletion of brain 5-HT levels with p-CPA markedly attenuated the hypothermic response. | Female albino Tuck (T/O strain) mice | [64] |
| Post- | Lesioning of central 5-HT neurones by 5,7-DHT had no effect on the hypothermic responses to lower doses of 8-OH-DPAT. pCPA significantly increased hypothermic response. | Sprague-Dawley rats | [64] |
| Pre- Post-(?) | Injection of 8-OH-DPAT directly into the DRN evoked a clear hypothermic effect. Injection of 8-OH-DPAT directly into the MRN produced no hypothermic effect. | Wistar rat | [9] |
| ? | pCPA pretreatment reduced the 5-HT level in cortex and hippocampus, but did not affect the hypothermia | Swiss-Webster mice | [65] |
| Pre- | Destruction of 5-HT neurones with the neurotoxin 5,7-DHT abolished the hypothermic response to 8-OH-DPAT | C57/Bl/601a mice | [66] |
| Post- | Dose–response curve shows that i.c.v. administration of 8-OH-DPAT was more potent than direct injection to the DRN to causing hypothermia. | Sprague-Dawley rats | [81] |
| ? | Correlation between the severity of hypothermic response to 8-OH-DPAT and radio ligand 5-HT1A binding sites in the brain regions associated with thermoregulation was not found | rats HDS * rats LDS * | [45] |
| Post- | Buspirone produced the same significant increase in prolactin and growth hormone (effects mediated by post- 5HT1A receptors) in the tryptophan-depleted state as in the control condition. The degree of hypothermia produced by buspirone was not significantly different in the two experimental conditions. | healthy volunteers humen | [86] |
| Pre- | Htr1a auto-KO mice displayed no detectable body temperature decrease in response to 8-OH-DPAT | Genetically modified mice | [53,87] |
| Pre- | Htr1a hetero-KO mice displayed a full hypothermic response to 8-OH-DPAT | Genetically modified mice | [87] |
| Change in Thermoregulatory Response | Change in Registered Parameter | Ambient Temperature Conditions | Object | Dose and Route of Administration | References |
|---|---|---|---|---|---|
| ↓General metabolism | ↓O2 consumption | Room temperature | rat | 500 µg/kg s.c. | [71] |
| rat | 0.5 µg into the hypothalamus | [71] | |||
| mice | 40 nM left lateral ventricle | [80] | |||
| ↓excretion of CO2 increased via leptin injection | rat | 10 mM 60 nL raphe’ pallidus | [113] | ||
| ↓O2 consumption | Cooling | rat | 4 mg/mL 1 µL Nucleus raphe’ magnus | [120] | |
| ↓excretion of CO2 | rat | 10 mM 60 nL Rostral raphe’ pallidus | [115] | ||
| rat | 100 µg/500 µL i.v. | [114] | |||
| rat | 10 mM 60 nL Rostral raphe’ pallidus | [114] | |||
| ↑Heat loss | ↑skin temperature | Room temperature | rat | 500 µg/kg s.c. | [71] |
| rat | 0.5 µg into the hypothalamus | [71] | |||
| rat | 0.6 µmol/kg s.c. | [123] | |||
| ↑peripheral blood flow | rabbit | 0.1 mg/kg i.v. | [98] | ||
| ↑peripheral blood flow | Cooling | rabbit | 0.1 mg/kg i.v. | [99] | |
| rabbit | 3–5 nmol rostral medullary raphe’ | [100] | |||
| rat | 0.5 mg/kg, s.c. | [101] | |||
| ↑skin temperature | piglet | dialysis 30 mM 30 min medullary raphe’ | [122] | ||
| rat | 0.6 µmol/kg s.c. | [123] | |||
| ↓Heat production | ↓BAT temperature increased via leptin injection | Room temperature | rat | 10 mM 60 nL raphe’ pallidus | [113] |
| ↓shivering, i.e., muscle electrical activity | Cooling | rat | 4 mg/mL 1 µL Nucleus raphe’ magnus | [120] | |
| piglet | dialysis 30 mM 30 min medullary raphe’ | [122] | |||
| piglet | dialysis 30 mM 30 min paragigantocellularis lateralis | [121] | |||
| ↓BAT temperature | rat | 10 mM 60 nL Rostral raphe’ pallidus | [115] | ||
| rat | 0.5 mg/kg, s.c. | [101] | |||
| rat | 100 µg/500 µL i.v. | [114] | |||
| rat | 10 mM 60 nL Rostral raphe’ pallidus | [114] |
| Compound | Dose and Route of Administration | Temperature Effect (Time of Its Achievement) | Object | Ambient Temperature Conditions | References |
|---|---|---|---|---|---|
| Effect of 5-HT2A/C agonists | |||||
| MK-212 | 0.03; 0.1; mg/kg i.p. | no effect (30′) | Sprague- Dawley rats | 28–29.5 °C | [56] |
| 1.0 mg/kg i.p. | 0.8 °C (15′) | ||||
| 0.83 °C (30′) | |||||
| 3.0 mg/kg i.p. | 1.1 °C (30′) | ||||
| MK-212 | 2.5 mg/kg i.p | 1.4 °C (30′) | Sprague- Dawley rats | 29 °C | [62] |
| mCPP | 1.25 mg/kg i.p. | 0.5 °C (30′) | Wistar rats | 25 °C | [158] |
| 2.5 mg/kg i.p. | 0.75 °C (15′); | ||||
| 1.1 °C (30′); | |||||
| 1.1 °C (45′) | |||||
| 5 mg/kg i.p. | 0.75 °C (15′); | ||||
| 1.1 °C (30′); | |||||
| 1.2 °C (45′); | |||||
| 1.3 °C (60′); | |||||
| 1.3 °C (90′) | |||||
| mCPP | 0.5 mg/kg oral | 0.48 °C (≈190′) | Health volunteers | [159] | |
| 0.1 mg/kg i.v. | 0.32 °C (≈57′) | ||||
| DOI | 0.1 mg/kg i.p. | 0.4 °C (30′) | Sprague- Dawley rats | 29 °C | [160] |
| 0.3 mg/kg i.p | 1.3 °C (30′) | ||||
| 1.0 mg/kg i.p. | 1.8 °C (30′) | ||||
| DOI | 3 mg/kg s.c. | 1.5 °C (30′) | Sprague- Dawley rats | 20 °C | [161] |
| DOI | 0.8 mg/kg i.v. | 0.9 °C (5′); 1.5 °C (10′); 2 °C (15′); 2.5 °C (20′); 3 °C (30′) Malignant hyperthermia | pigs | [162] | |
| TFMPP | 1 mg/kg i.p. | 0.7 °C (30′); 0.6 °C (60′); | Wistar rats | 28 °C | [163] |
| 5 mg/kg i.p. | 0.9 °C (30′); 1.0 °C (60′); 0.9 °C (90′); 0.6 °C (120′); | ||||
| 10 mg/kg i.p. | 1.0 °C (30′); 1.3 °C (60′); 1.4 °C (90′); 1.1 °C (120′); | ||||
| 20 mg/kg i.p. | 0.8 °C (30′); 1.1 °C (60′); 1.3 °C (90′); 1.3 °C (120′); | ||||
| mCPP | 1 mg/kg i.p. | 0.6 °C (60′); −0.5 °C (90′) | |||
| 5 mg/kg i.p. | 0.6 °C (60′); 0.9 °C (90′) | ||||
| 10 mg/kg i.p. | 1.1 °C (30′); 1.2 °C (60′); | ||||
| 1.1 °C (90′); 0.9 °C (120′); | |||||
| 20 mg/kg i.p. | 1.3 °C (30′); 1.3 °C (60′); 1.2 °C (90′); 1.2 °C (120′); | ||||
| DOI | 1 mg/kg i.p. | 1.5 °C (60′) | Wistar rats | 21 °C | [164] |
| DOI | 2.5 mg/kg i.p. | 1.5 °C (60′) | Wistar rats | 22 °C | [165] |
| 0.6 °C (60′) | Fawn- Hooded rats | ||||
| mCPP | 2.5 mg/kg i.p. | 1 °C (30′) | Wistar rats | ||
| 0.3 °C (30′) | Fawn- Hooded rats | ||||
| mCPP | 2.5 mg/kg i.p. | 1.2 °C (30′) | Wistar rats | 21 °C | [166] |
| 0.4 °C (60′) | Fawn- Hooded rats | ||||
| 1.25 mg/kg i.p. | 0.5 °C (30′) | Wistar rats | |||
| no effect | Fawn- Hooded rats | ||||
| DOI | 2.5 mg/kg i.p. | 2 °C (60′) | Wistar rats | [167] | |
| mCPP | 1.2 °C (30′) | ||||
| DOI | 1 mg/kg s.c. | 1.2 °C (30′) | Wistar rats | 23 °C | [69] |
| DOI | Intrahypothalamic administration 0.2 µg | 1.38 °C (45′) | Sprague- Dawley rats | room temperature | [71] |
| DOI | 0.025; 0.1 mg/kg s.c. | no effect (20′) | Sprague- Dawley rats | 21 °C | [168] |
| 0.4; 1.6 mg/kg s.c. | 1.0 °C (20′) | ||||
| DOI | 5 µg/kg i.v. | 0.25 °C (20′–60′) | rabbits | 23–25 °C | [169] |
| 50 µg/kg i.v. | 1.0 °C (40′–60′) | ||||
| 100 µg/kg i.v. | >2 °C (90′–120′) | ||||
| 100 µg/kg s.c. | 1.0 °C (90′) | Sprague- Dawley rats | 27 °C | ||
| YM348 | 0.3 mg/kg per os | 0.9 °C (60′) | Zucker rats | [170] | |
| 1 mg/kg per os | 1.1 °C (60′) | ||||
| 3 mg/kg per os | 1.3 °C (60′) | ||||
| DOI | 0.1 mg/kg s.c. | 1.4 °C (30′) | Sprague- Dawley rats | 25–28 °C | [101] |
| DOI | 0.05 mg/kg s.c. | 0.29 °C ns | Sprague- Dawley rats | 22 °C | [171] |
| 0.1 mg/kg s.c;. | 0.64 °C (30′; 45′; 60′) | ||||
| 0.5 mg/kg s.c. | 0.79 °C (15′; 30′; 45′; 60′; 90′) | ||||
| 0.05 mg/kg s.c. | 0.5 °C ns | 32 °C | |||
| 0.1 mg/kg s.c. | 0.5 °C (15′); 1.7 °C (75′) | ||||
| 0.5 mg/kg s.c. | 1.2 °C (15′); 2.7 °C (75′) | ||||
| 0.1 mg/kg s.c. | 0.1°C ns | 12 °C | |||
| 0.1 mg/kg s.c. | 0.99 °C (30′; 45′; 60′) | 27 °C | |||
| 25B-NBOMe | 0.25 mg/kg i.p. | no effect | Sprague- Dawley rats | 23 °C | [172] |
| 0.25 mg/kg i.p. | 0.8 °C (30′); 1 °C (60′); 1.2 °C (90′); 0.9 °C (120′) | 29 °C | |||
| Effect of 5-HT2A/C antagonists | |||||
| pirenpirone | 0.01; 0.03; 0.1; 0.3 mg/kg i.p. | −0.15 °C ?s; −0.5 °C; −0.85 °C; −0.9 °C (<60′) | Sprague- Dawley rats | 28–29.5 °C | [56] |
| ketanserin | 0.1; 0.3; 1; 3 mg/kg i.p. | −0.2 °C ?s; −0.5 °C; −0.7 °C; −1.2 °C (<60′) | |||
| ritanserin | 0.63 mg/kg i.p. | no effect | Wistar rats | 25 °C | [158] |
| ritanserin | 0.5; 1; 2 mg/kg i.p | no effect alone, but dose-dependently reduces hyperthermic effect of mCPP and TFMPP | Wistar rats | 28 °C | [163] |
| ketanserin | 0.6; 1.25; 2.5 mg/kg i.p | ||||
| LY53857 | 1 mg/kg i.p. | −0.55 °C (90′) | Wistar rats | 21 °C | [164] |
| ketanserin | 1 mg/kg i.p. | no effect | |||
| ritanserin | 1 mg/kg s.c. | no effect (30′) | Sprague- Dawley rats | 21 °C | [168] |
| amperozide | 2 mg/kg s.c. | no effect (30′) | |||
| ketanserin | 5 mg/kg i.p | no effect | Sprague- Dawley rats | 22 °C | [171] |
| no effect alone, but blocks DOI | 32 °C | ||||
| ketanserin | 0.2 µg into preoptic anterior hypothalamus | 0.58 °C (180′) | rabbit | 2 °C | [173] |
| 0.54 °C (180′) | 22 °C | ||||
| 0.57 °C (180′) | 32°C | ||||
| 0.4 µg into preoptic anterior hypothalamus | 1.13 °C (180′) | 2 °C | |||
| 1.0 °C (180′) * | 22 °C | ||||
| 1.09 °C (180′) | 32 °C | ||||
| 2 µg into preoptic anterior hypothalamus | 1.42 °C (180′) | 2 °C | |||
| 1.37 °C (180′) | 22 °C | ||||
| 1.43 °C (180′) | 32°C | ||||
| Compound | Dose and route of Administration | Temperature Effect (Time of Its Achievement) | Object | Ambient Temperature Conditions | References |
|---|---|---|---|---|---|
| Effect of 5-HT2A/C agonists | |||||
| TFMPP | 1 mg/kg s.c. | no effect alone(10′), but reduces the effect of 8-OH-DPAT | ICR | 21 °C | [63] |
| mCPP | |||||
| MK-212 | |||||
| DOI | 0.1 mg/kg s.c. | ||||
| MK-212 | |||||
| DOI | 0.5, 1.0, 2.5 mg/kg i.p. | no effect (15′, 30′, 45′, 60′, 90′, 120′) | ICR | 24 °C | [174] |
| 5 mg/kg i.p. | −1 °C (30′, 45′, 60′, 90′) | ||||
| DOI | 1 mg/kg i.p; 5 mg/kg i.p. | no effect | Ddy | 24 °C | [175] |
| DV-7028 | 10 mg/kg per os | ||||
| TCB-2 | 0.1; 0.5; 1.0; 2.5; mg/kg i.p. | no effect (15′, 30′, 45′) | C57Bl6J | plexiglass containers 20 °C | [176] |
| 5.0 mg/kg i.p. | −4 °C ( ‘) | ||||
| DOI | 0.1; 0.5; 1.0; 2.5; 5.0 mg/kg i.p. | no effect (15′, 30′, 45′) | |||
| DOI | 1 mg/kg i.p. | no effect (each 5′ over 24 h) | C57BL/6J | 22.5–23.5 °C | [177] |
| 25CN-NBOH | 1.5 mg/kg, s.c. | no effect | Mice Mix genetic background | Temperature of heat-pad = 37 °C | [178] |
| 1 °C (15′) | Temperature of heat-pad = 41 °C | ||||
| Effect of 5-HT2A/C antagonists | |||||
| ritanserin | 1 mg/kg per os 10 mg/kg per os | no effect | Ddy | 24 °C | [175] |
| ketanserin | 1 mg/kg per os | no effect | |||
| 10 mg/kg per os | −2.2 °C (60′) | ||||
| MDL 11,939 | (1.0 mg/kg), i.p. | no effect (30′) | C57Bl6J | [176] | |
| ketanserin | 1.0 mg/kg i.p. (180 µM). | −2 °C | CBA/Lac | [179] | |
| 2.0 mg/kg i.p. (360 µM) | −2.3 °C | ||||
| 20 nM i.c.v. | −2.3 °C | ||||
| 40 nM i.c.v. | −3.5 °C | ||||
| ketanserin | 1 mg/kg i.p. | no effect (each 5′ over 24 h) | C57BL/6J | 22.5–23.5 °C | [177] |
| 40 nmol i.c.v. | no effect (60′) | ||||
| ketanserin | 1 mg/kg i.p. | no effect (60′) | AKR/J | [180] | |
| CBA/Lac | |||||
| AKR.CBA- D13Mit76 | |||||
| Change in Thermoregulatory Response | Change in Registered Parameter | Ambient Temperature Conditions | Object | Ligand (Dose and Route of Administration) | Reference |
|---|---|---|---|---|---|
| ↑General metabolism | ↑O2 consumption | Room temperature | Sprague- Dawley rat | DOI (0.2 µg into the hypothalamus) | [71] |
| ↑O2 consumption; CO2 excretion | Zucker fa/fa rats | YM348 (0.3; 1; 3 mg/kg per os) | [170] | ||
| ↑ Heat production | ↑BAT temperature | 25–28 °C | Sprague- Dawley rat | DOI (0.1 mg/kg s.c) | [101] |
| ↑BAT temperature | 29 °C | Sprague- Dawley rat | 25B-NBOMe (0.25 mg/kg i.p.) | [172] | |
| ↓Heat loss | ↓skin temperature | Room temperature | Sprague- Dawley rat | DOI (0.2 µg into the hypothalamus) | [71] |
| ↓peripheral blood flow | 23–25 °C | Rabbits | DOI (5; 50; 100 µg/kg, i.v.) | [169] | |
| 27 °C | Sprague- Dawley rat | DOI (100 µg/kg s.c.) | |||
| ↓peripheral blood flow | rabbits | DOI (50 µg/kg, i.v.) | [183] | ||
| ↓peripheral blood flow | 25–28 °C | Sprague- Dawley rat | DOI (0.1 mg/kg s.c) | [101] | |
| ↓skin temperature | 29 °C | Sprague- Dawley rat | 25B-NBOMe (0.25 mg/kg i.p.) | [172] |
| Compound | Dose (Route of Administration) | Maximal Effect (°C) | Time of Its Achievements | Object | References |
|---|---|---|---|---|---|
| mCPBG | 0.1 mg/kg (i.p.) | no effect | Wistar rats | [218] | |
| 1 mg/kg (i.p.) | 0.5 | ≈30′ | |||
| 10 mg/kg (i.p.) | 0.6 | ≈30′ | |||
| MDL72222 | 0.1; 1; 10 mg/kg (i.p.) | no effect | |||
| ondansetron | 0.1; 1 mg/kg(i.p.) | no effect | |||
| 2-Me-5-HT | 5 µg (i.c.v.) | ≈−0.62 | 45′ | Sprague- Dawley rats | [219] |
| 10 µg (i.c.v.) | −1.1 | 45′ | |||
| 20 µg (i.c.v.) | −1.5 | 45′ | |||
| 1–5 mg/kg (i.p.) | no effect | During 2 h | |||
| ondansetron | 5, 10, 20 µg (i.c.v.) | no effect | During 150′ | ||
| 50, 100, 300 mg/kg (i.p.) | no effect | ||||
| tropisetron | 50, 100, 300 mg/kg (i.p.) | no effect | |||
| 5, 10, 20 µg (i.c.v.) | no effect | ||||
| 2-Me-5-HT | 4 mg/kg (i.p.) | no effect | During 6 h | Sprague- Dawley rats | [220] |
| mCPBG | 160 nmol (i.c.v.) | −4 | 30′ | AKR/J mice | [79] |
| 80 nmol (i.c.v.) | −2 | ||||
| 40 nmol (i.c.v.) | −2 | ||||
| 20 nmol (i.c.v.) | no effect | ||||
| 0.5; 1; 5; 10 mg/kg (i.p.) | no effect | ||||
| mCPBG | 40 nmol (i.c.v.) | −4 | 30′ | DBA/2J mice | |
| −5.5 | CBA/Lac mice | ||||
| −2.6 | AKR/J mice | ||||
| −5.1 | C57Bl/6 mice | ||||
| −3.1 | BALB/c mice | ||||
| −3.2 | ICR mice | ||||
| mCPBG | 40 nmol (i.c.v.) | −2.3 | 30′ | CBA/Lac mice | [80] |
| mCPBG | 40 nmol (i.c.v.) | −5 | 30′ | C57Bl/6 mice | [30] |
| −5 | C3H mice | ||||
| −4 | DBA mice | ||||
| −5.9 | Asn mice | ||||
| −3 | BALB mice | ||||
| −5.1 | CBA mice | ||||
| −3.2 | ICR mice | ||||
| −2.5 | AKR mice |
| Hypothermia-Inducing Compound | Dosa (mg/kg) | ΔT Body (Time of Effect) | Object | Reference |
|---|---|---|---|---|
| 5-HT | 5 | −3.1 °C (30′) | WT | [242] |
| −2 °C (60′) | ||||
| no change | Htr7 KO | |||
| 5-CT | 0.5 | −2 °C (30′) | WT | |
| 3 | −4.5 °C (120′) | |||
| 0.5 | no change | Htr7 KO | ||
| 3 | −1.2 °C (120′) | |||
| 5-CT | 0.1 | −1.2 °C ns | WT | [241] |
| 0.3 | −2.8 °C | |||
| 1 | −3.9 °C | |||
| 0.1; 0.3; 1 | no change | Htr7 KO * | ||
| 8-OH-DPAT | 0.3 | −0.7 °C (30′) | WT | [75] |
| 0.6 | −1.2 °C (30′) | |||
| 1 | −1.5 °C (30′) | |||
| 0.3 | no change | Htr7 KO | ||
| 0.6 | no change | |||
| 1 | −1.1 °C (30′) |
| Pretreatment | Main Hypothermic Effect | Joint Effect of Two Compaunds ΔT Body | Object | References | ||||
|---|---|---|---|---|---|---|---|---|
| Pretreatment Compound | Dose (Route of Administration) | Time Interval | Compound | Dose (Route of Administration) | ΔT body (Time of Effect) | |||
| 5-CT | 0.3 mg/kg (i.p.) | −1.8 °C (75′) | Guinea pig | [239] | ||||
| SB-269970-A | 3 mg/kg (i.p.) | 60′ | 5-CT | −0.4 °C | ||||
| 5-CT | 0.3 mg/kg (i.p.) | −1.9 °C | Guinea pig | [240] | ||||
| SB-656104-A | 3 mg/kg (i.p.) | 60′ | 5-CT | −0.7 °C | ||||
| 5-CT | 0.3 mg/kg (i.p.) | ≈−1.8 °C | Swiss Webster mice | [241] | ||||
| WAY100635 | 0.1; 0.3; 1 mg/kg (i.p.) | 30′ | 5-CT | 0.3 mg/kg (i.p.) | ≈−1.4 °C | |||
| SB 258719 | 5; 10 mg/kg (i.p.) | 30′ | 5-CT | 0.3 mg/kg (i.p.) | ≈−1.7 °C | |||
| 20 mg/kg (i.p.) | ≈−0.2 °C | |||||||
| SB-269970 | 1 mg/kg (i.p.) | 30′ | 5-CT | 0.3 mg/kg (i.p.) | −1.2 °C | |||
| 3 mg/kg (i.p.) | −0.6 °C | |||||||
| 10; 30 mg/kg (i.p.) | −0.4 °C | |||||||
| 8-OH-DPAT | 0.3 mg/kg (i.p.) | -2°C (30′) | Sprague–Dawley rats | [75] | ||||
| WAY-100135 | 1 mg/kg (i.p.) | 20′ | 8-OH-DPAT | 0.3 mg/kg (i.p.) | −1.6 °C | |||
| 10 mg/kg (i.p.) | −0.5 °C | |||||||
| SB-269970 | 0.3 mg/kg (i.p.) | 20′ | −1.2 °C | |||||
| 3 mg/kg (i.p.) | −1.1 °C | |||||||
| DR-4004 | 3 mg/kg (i.p.) | 20′ | −1.2 °C | |||||
| 10 mg/kg (i.p.) | −1.1 °C | |||||||
| 8-OH-DPAT | 0.3 mg/kg (i.p.) | −0.7 °C (30′) | C57Bl6+/+ | |||||
| 0.6 mg/kg (i.p.) | −1.2 °C (30′) | |||||||
| 1 mg/kg (i.p.) | −1.5 °C (30′) | |||||||
| 0.3 mg/kg (i.p.) | no change | C57Bl6−/− | ||||||
| 0.6 mg/kg (i.p.) | no change | |||||||
| 1 mg/kg (i.p.) | −1.1 °C (30′) | |||||||
| WAY-100135 | 10 mg/kg (i.p.) | 30′ | 8-OH-DPAT | 0.3 mg/kg (i.p.); 0.6mg/kg (i.p.); 1 mg/kg (i.p.) | no change | C57Bl6+/+ | ||
| no change | C57Bl6−/− | |||||||
| SB-269970 | 10 mg/kg (i.p.) | 30′ | 8-OH-DPAT | 0.3 mg/kg (i.p.) | no change | C57Bl6+/+ | ||
| 0.6 mg/kg (i.p.) | −0.7 °C (30′) | |||||||
| 1 mg/kg (i.p.) | −1.9 °C (30′) | |||||||
| 0.3 mg/kg (i.p.) | no change | C57Bl6−/− | ||||||
| 0.6 mg/kg (i.p.) | −0.6 °C (30′) | |||||||
| 1 mg/kg (i.p.) | −1.2 °C (30′) | |||||||
| 8-OH-DPAT | 0.1 mg/kg (s.c.) | −3.4 °C (30′) | Sprague–Dawley rats | [245] | ||||
| WAY-100635 | 0.005 mg/kg, s.c.) | 8-OH-DPAT | −2 °C | |||||
| SB-269970 | 0.1 mg/kg (i.p.) | 8-OH-DPAT | −3.4 °C | |||||
| 0.5 mg/kg (i.p.) | −2.7 °C | |||||||
| 1 mg/kg (i.p.) | −2.3 °C | |||||||
| WAY-100635 (0.005 mg/kg, s.c.) + SB-269970(1 mg/kg, i.p.) | 8-OH-DPAT | −1 °C | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voronova, I.P. 5-HT Receptors and Temperature Homeostasis. Biomolecules 2021, 11, 1914. https://doi.org/10.3390/biom11121914
Voronova IP. 5-HT Receptors and Temperature Homeostasis. Biomolecules. 2021; 11(12):1914. https://doi.org/10.3390/biom11121914
Chicago/Turabian StyleVoronova, Irina P. 2021. "5-HT Receptors and Temperature Homeostasis" Biomolecules 11, no. 12: 1914. https://doi.org/10.3390/biom11121914
APA StyleVoronova, I. P. (2021). 5-HT Receptors and Temperature Homeostasis. Biomolecules, 11(12), 1914. https://doi.org/10.3390/biom11121914





