Innocuous, Highly Conductive, and Affordable Thermal Interface Material with Copper-Based Multi-Dimensional Filler Design
Abstract
:1. Introduction
2. Materials and Methods
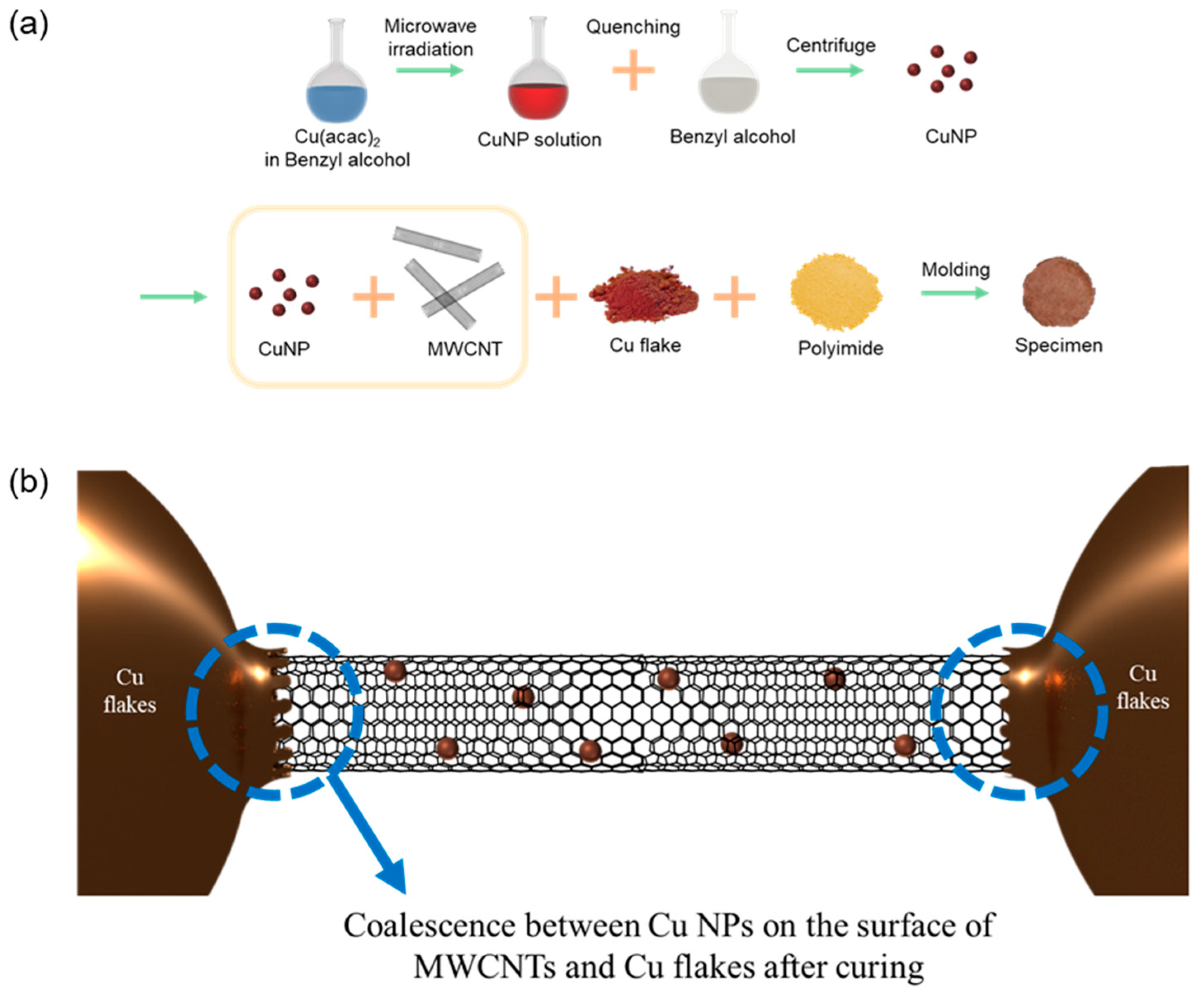
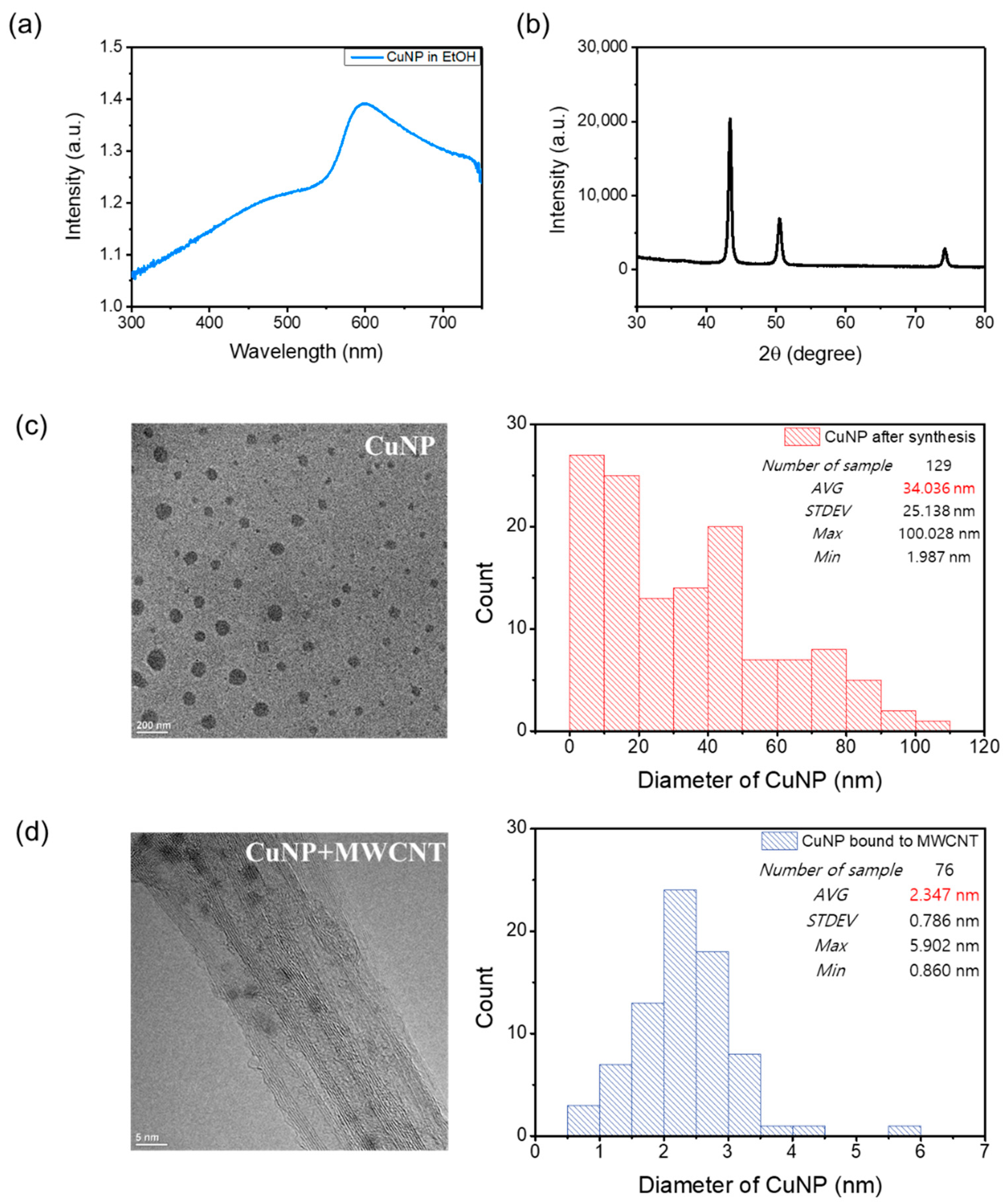
2.1. Synthesis of Cu NPs
2.2. Binding of Cu NPs with MWCNTs
2.3. Mixing of the Fillers with the Matrix and Preparation of Specimens
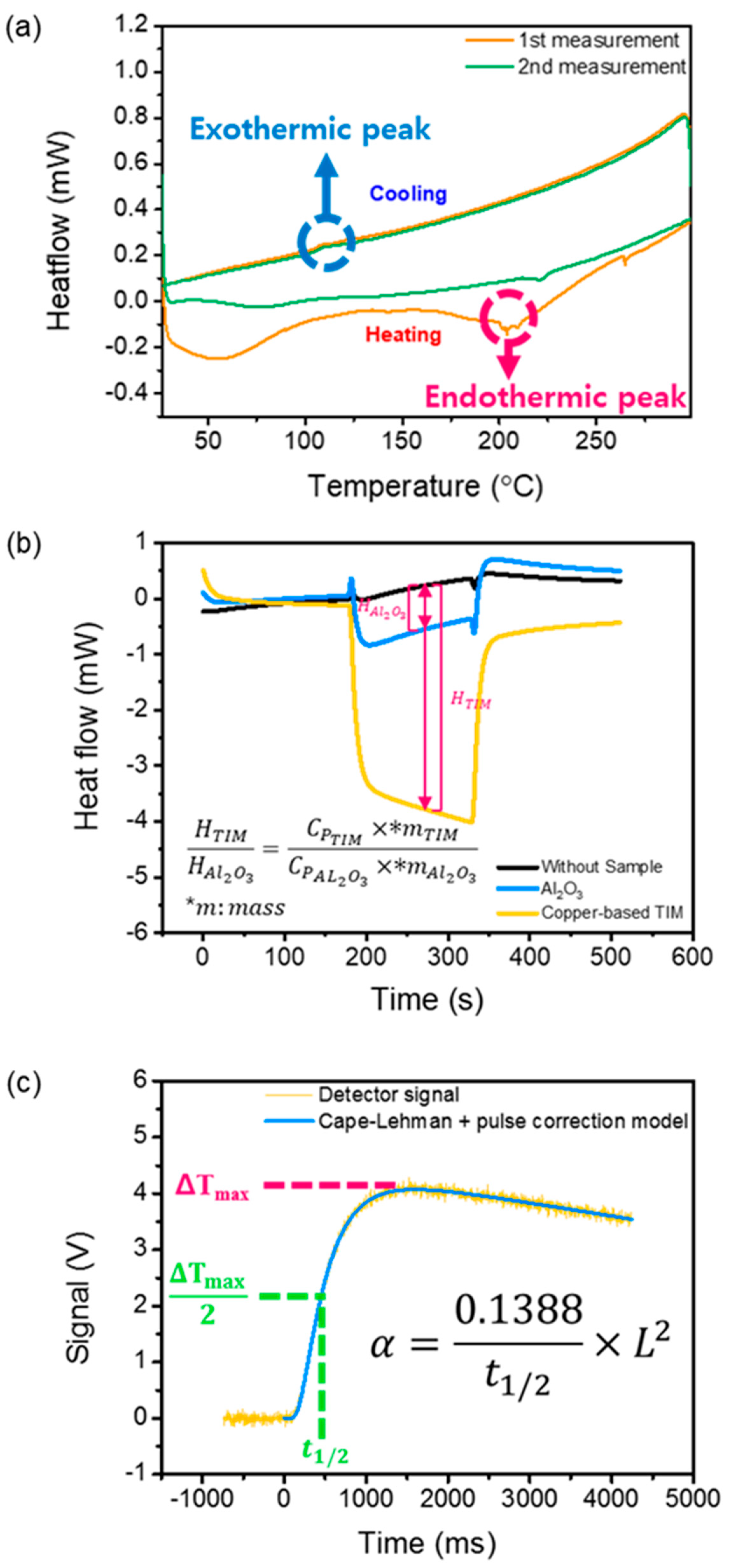
2.4. Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suh, D.; Moon, C.M.; Kim, D.; Baik, S. Ultrahigh thermal conductivity of interface materials by silver—Functionalized carbon nanotube phonon conduits. Adv. Mater. 2016, 28, 7220–7227. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, C.; Park, J.; Kim, T.Y. Highly enhanced thermoelectric energy harvesting from a high-temperature heat source by boosting thermal interface conduction. Energy Convers. Manag. 2019, 183, 360–368. [Google Scholar] [CrossRef]
- Cimbaluk, G.V.; Ramsdorf, W.A.; Perussolo, M.C.; Santos, H.K.F.; De Assis, H.C.D.S.; Schnitzler, M.C.; Schnitzler, D.C.; Carneiro, P.G.; Cestari, M.M. Evaluation of multiwalled carbon nanotubes toxicity in two fish species. Ecotoxicol. Environ. Saf. 2018, 150, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, M.; Nakajima, H.; Yudasaka, M.; Iijima, S.; Okazaki, T. Diameter-dependent degradation of 11 types of carbon nanotubes: Safety implications. ACS Appl. Nano Mater. 2019, 2, 4293–4301. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, M. Biodegradation of carbon nanotubes by macrophages. Front. Mater. 2019, 6, 225. [Google Scholar] [CrossRef]
- Silva, R.M.; Anderson, D.S.; Franzi, L.M.; Peake, J.L.; Edwards, P.C.; Van Winkle, L.S.; Pinkerton, K.E. Pulmonary effects of silver nanoparticle size, coating, and dose over time upon intratracheal instillation. Toxicol. Sci. 2015, 144, 151–162. [Google Scholar] [CrossRef] [Green Version]
- Seiffert, J.; Hussain, F.; Wiegman, C.; Li, F.; Bey, L.; Baker, W.; Porter, A.; Ryan, M.P.; Chang, Y.; Gow, A. Pulmonary toxicity of instilled silver nanoparticles: Influence of size, coating and rat strain. PLoS ONE 2015, 10, e0119726. [Google Scholar] [CrossRef] [Green Version]
- Wiemann, M.; Vennemann, A.; Blaske, F.; Sperling, M.; Karst, U. Silver nanoparticles in the lung: Toxic effects and focal accumulation of silver in remote organs. Nanomaterials 2017, 7, 441. [Google Scholar] [CrossRef] [Green Version]
- Botelho, D.; Leo, B.F.; Massa, C.; Sarkar, S.; Tetley, T.; Chung, K.F.; Chen, S.; Ryan, M.P.; Porter, A.; Atochina-Vasserman, E.N. Exposure to silver nanospheres leads to altered respiratory mechanics and delayed immune response in an in Vivo murine model. Front. Pharmacol. 2018, 9, 213. [Google Scholar] [CrossRef] [Green Version]
- Johari, S.; Kalbassi, M.; Soltani, M.; Yu, I. Toxicity comparison of colloidal silver nanoparticles in various life stages of rainbow trout (Oncorhynchus mykiss). Iran. J. Fish. Sci. 2013, 12, 76–95. [Google Scholar]
- Asghari, S.; Johari, S.A.; Lee, J.H.; Kim, Y.S.; Jeon, Y.B.; Choi, H.J.; Moon, M.C.; Yu, I.J. Toxicity of various silver nanoparticles compared to silver ions in Daphnia magna. J. Nanobiotechnol. 2012, 10, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, F.; Gallego-Urrea, J.A.; Jurkschat, K.; Crossley, A.; Hassellöv, M.; Taylor, C.; Soares, A.M.; Loureiro, S. Silver nanoparticles and silver nitrate induce high toxicity to Pseudokirchneriella subcapitata, Daphnia magna and Danio rerio. Sci. Total Environ. 2014, 466, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Araya, M.; Pizarro, F.; Olivares, M.; Arredondo, M.; Gonzalez, M.; Méndez, M. Understanding copper homeostasis in humans and copper effects on health. Biol. Res. 2006, 39, 183–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danks, D. Copper deficiency in humans. Annu. Rev. Nutr. 1988, 8, 235–257. [Google Scholar] [CrossRef] [PubMed]
- Olivares, M.; Uauy, R. Copper as an essential nutrient. Am. J. Clin. Nutr. 1996, 63, 791S–796S. [Google Scholar] [CrossRef] [PubMed]
- Dar, M.I.; Sampath, S.; Shivashankar, S. Microwave-assisted, surfactant-free synthesis of air-stable copper nanostructures and their SERS study. J. Mater. Chem. 2012, 22, 22418–22423. [Google Scholar] [CrossRef]
- Ramyadevi, J.; Jeyasubramanian, K.; Marikani, A.; Rajakumar, G.; Rahuman, A.A.; Santhoshkumar, T.; Kirthi, A.V.; Jayaseelan, C.; Marimuthu, S. Copper nanoparticles synthesized by polyol process used to control hematophagous parasites. Parasitol. Res. 2011, 109, 1403–1415. [Google Scholar] [CrossRef]
- Seku, K.; Reddy Ganapuram, B.; Pejjai, B.; Mangatayaru Kotu, G.; Golla, N. Hydrothermal synthesis of Copper nanoparticles, characterization and their biological applications. Int. J. Nano Dimens. 2018, 9, 7–14. [Google Scholar]
- Raja, M.; Subha, J.; Ali, F.B.; Ryu, S.H. Synthesis of copper nanoparticles by electroreduction process. Mater. Manuf. Process. 2008, 23, 782–785. [Google Scholar] [CrossRef]
- Yang, J.; Yang, J.; Dang, Z.; Chen, J. A controllable transformation in copper valence states and its applications. Dalton Trans. 2012, 41, 2393–2398. [Google Scholar] [CrossRef]
- Yeshchenko, O.A.; Dmitruk, I.M.; Alexeenko, A.A.; Dmytruk, A.M. Size-dependent melting of spherical copper nanoparticles embedded in a silica matrix. Phys. Rev. B 2007, 75, 085434. [Google Scholar] [CrossRef] [Green Version]
- Parker, W.; Jenkins, R.; Butler, C.; Abbott, G. Flash method of determining thermal diffusivity, heat capacity, and thermal conductivity. J. Appl. Phys. 1961, 32, 1679–1684. [Google Scholar] [CrossRef]
- Cape, J.; Lehman, G. Temperature and finite pulse—Time effects in the flash method for measuring thermal diffusivity. J. Appl. Phys. 1963, 34, 1909–1913. [Google Scholar] [CrossRef]
- Yu, A.; Ramesh, P.; Sun, X.; Bekyarova, E.; Itkis, M.E.; Haddon, R.C. Enhanced thermal conductivity in a hybrid graphite nanoplatelet–carbon nanotube filler for epoxy composites. Adv. Mater. 2008, 20, 4740–4744. [Google Scholar] [CrossRef]
- Barani, Z.; Mohammadzadeh, A.; Geremew, A.; Huang, C.Y.; Coleman, D.; Mangolini, L.; Kargar, F.; Balandin, A.A. Thermal properties of the binary—Filler hybrid composites with graphene and copper nanoparticles. Adv. Funct. Mater. 2020, 30, 1904008. [Google Scholar] [CrossRef]
- Yaman, K.; Taga, Ö. Thermal and electrical conductivity of unsaturated polyester resin filled with copper filler composites. Int. J. Polym. Sci. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Gwinn, J.P.; Webb, R.L. Performance and testing of thermal interface materials. Microelectron. J. 2003, 34, 215–222. [Google Scholar] [CrossRef]
- Narumanchi, S.; Mihalic, M.; Kelly, K.; Eesley, G. Thermal interface materials for power electronics applications. In Proceedings of the 2008 11th Intersociety Conference on Thermal and Thermomechanical Phenomena in Electronic Systems, Orlando, FL, USA, 28–31 May 2008; pp. 395–404. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, W.; Kim, C.; Lee, W.; Park, J.; Kim, D. Innocuous, Highly Conductive, and Affordable Thermal Interface Material with Copper-Based Multi-Dimensional Filler Design. Biomolecules 2021, 11, 132. https://doi.org/10.3390/biom11020132
Kim W, Kim C, Lee W, Park J, Kim D. Innocuous, Highly Conductive, and Affordable Thermal Interface Material with Copper-Based Multi-Dimensional Filler Design. Biomolecules. 2021; 11(2):132. https://doi.org/10.3390/biom11020132
Chicago/Turabian StyleKim, Woochang, Chihyun Kim, Wonseok Lee, Jinsung Park, and Duckjong Kim. 2021. "Innocuous, Highly Conductive, and Affordable Thermal Interface Material with Copper-Based Multi-Dimensional Filler Design" Biomolecules 11, no. 2: 132. https://doi.org/10.3390/biom11020132






