Transgenerational Effects of Hexavalent Chromium on Marine Medaka (Oryzias melastigma) Reveal Complex Transgenerational Adaptation in Offspring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experience Fishes
2.2. Experimental Design
2.3. Parameter Calculation
2.3.1. Parental Fecundity
2.3.2. Death Rate and Malformation Rate
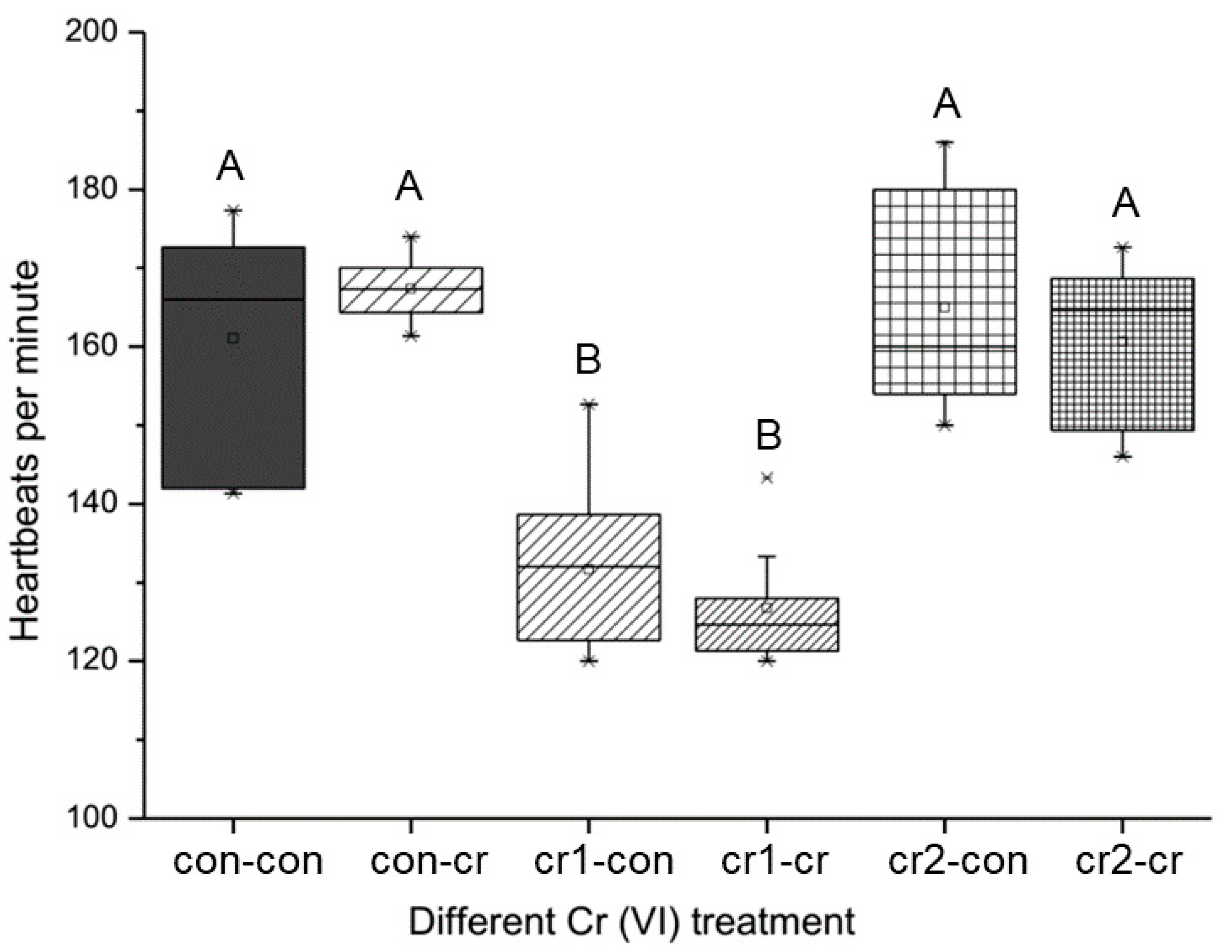
2.3.3. Heart Rate
2.3.4. Hatch Situation
2.4. Statistical Analysis
3. Results
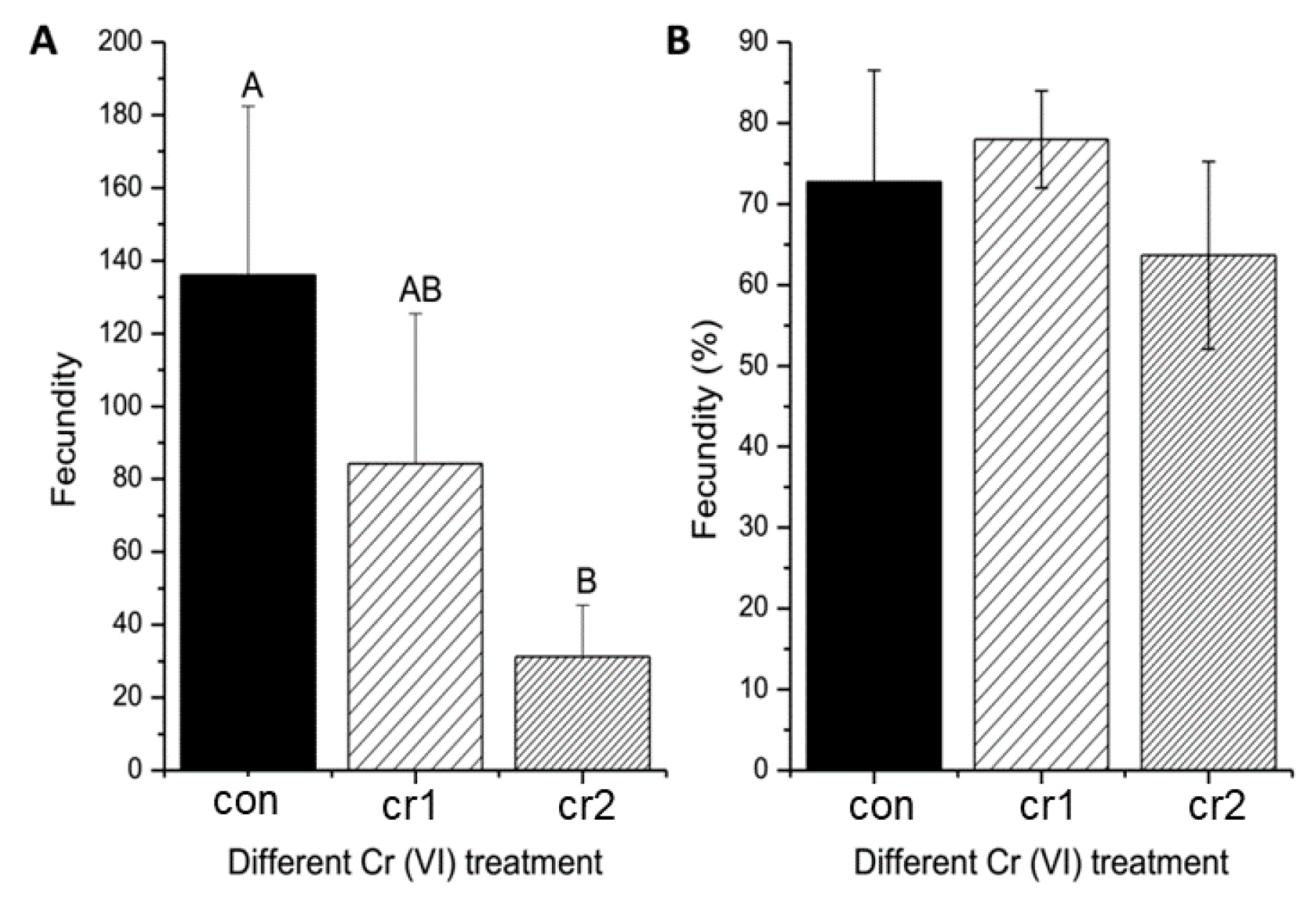
3.1. Cr(VI) Reduced Maternal Fecundity
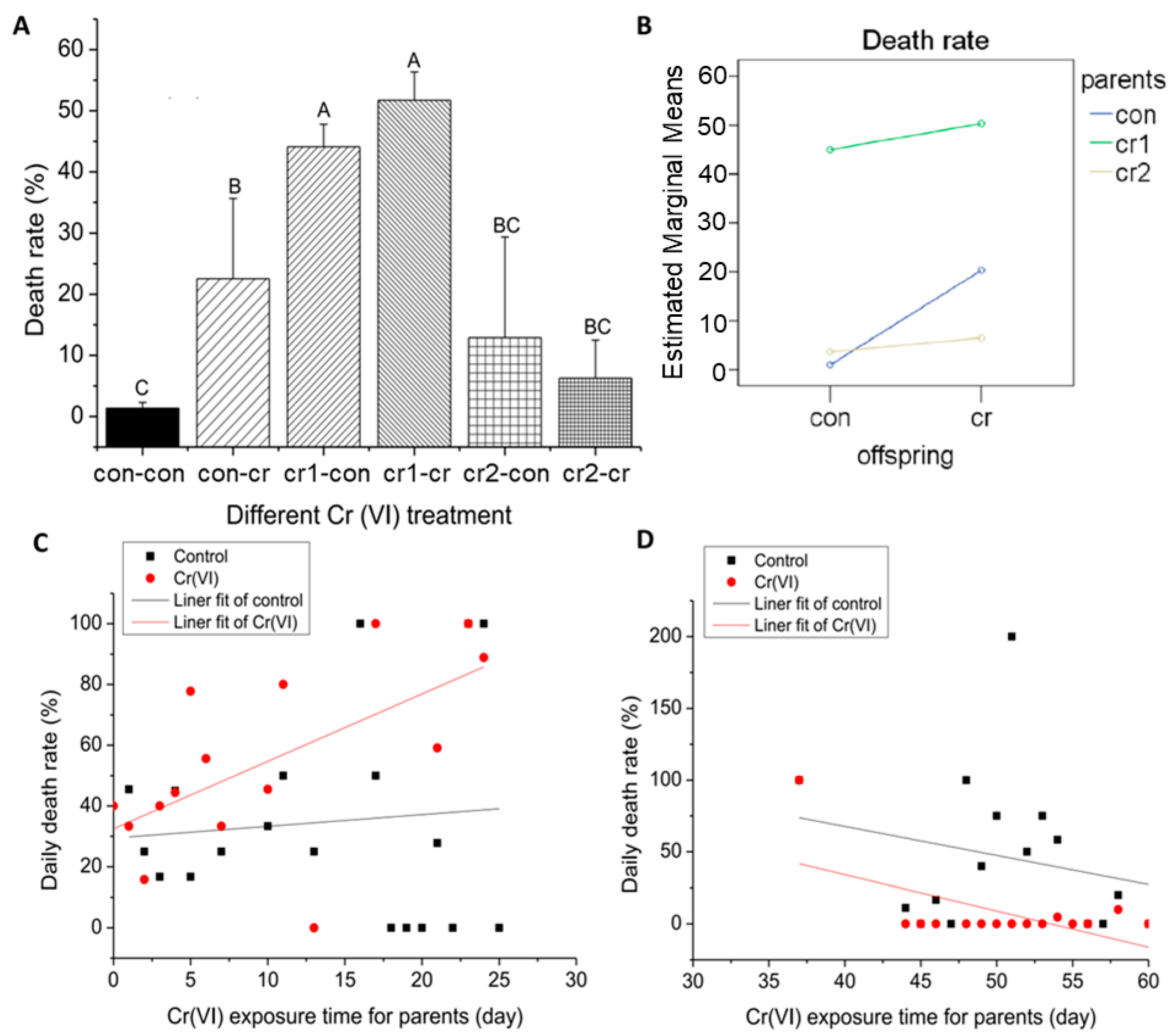
3.2. Cr(VI) Led to Increase of Death Rate
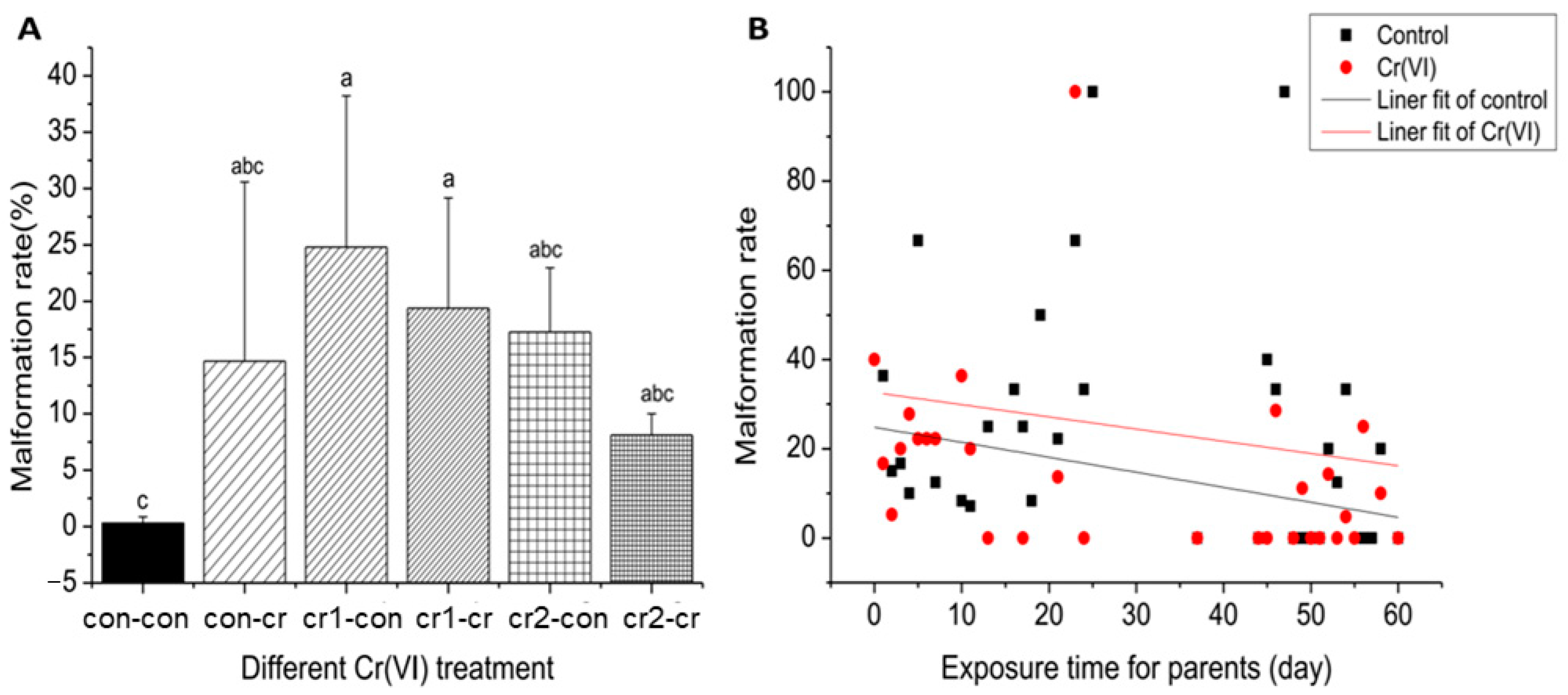
3.3. Cr(VI) Was Highly Teratogenic
3.4. Parental Cr(VI) Treatment Delayed Heat Development
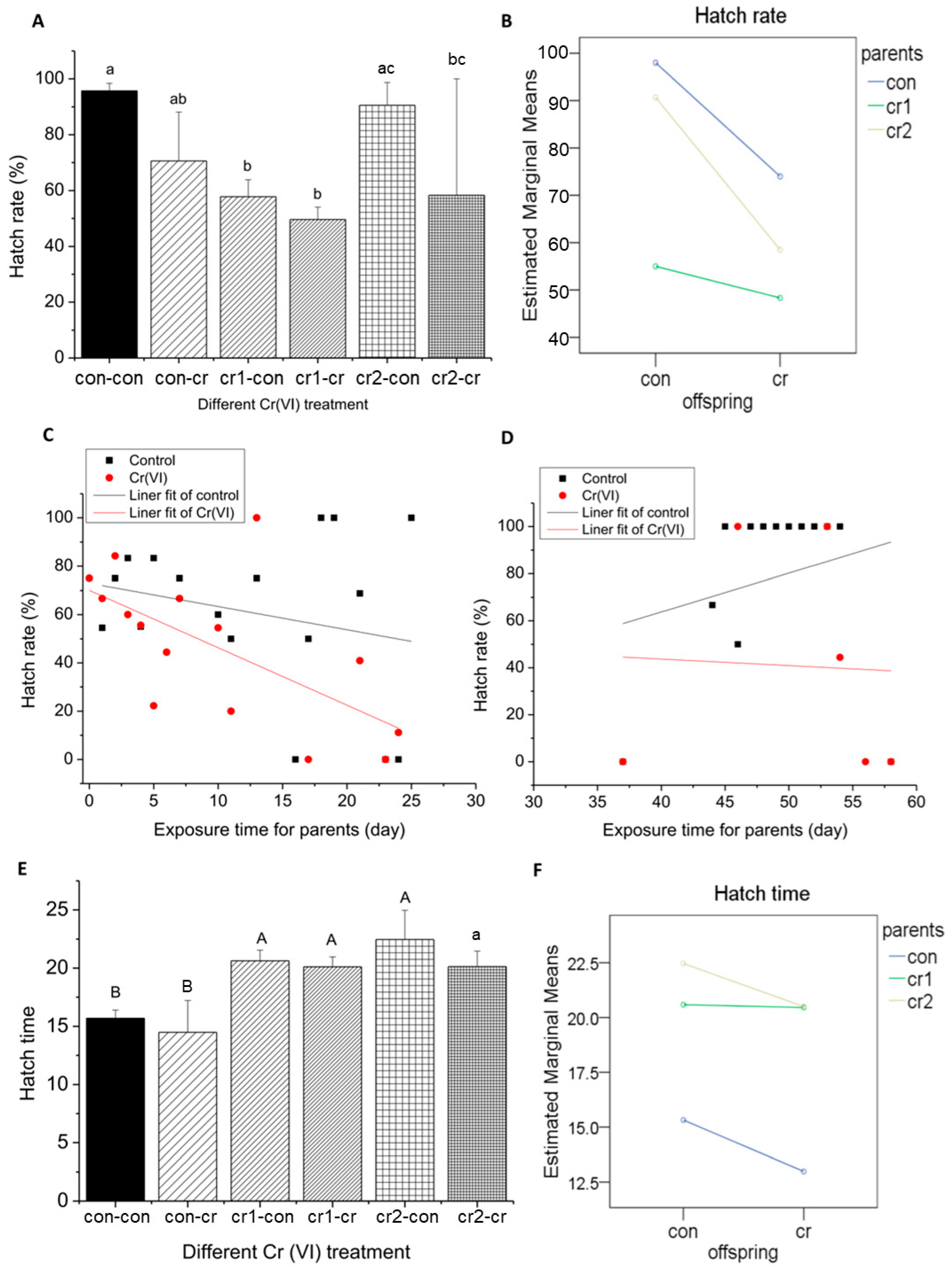
3.5. Parental Cr(VI) Treatment Delayed Hatch Process
3.5.1. Parental Cr(VI) Treatment Reduced Hatch Rate
3.5.2. Parental Cr(VI) Treatment Delayed Hatch Time
3.5.3. Parental Cr(VI) Treatment Increase Hatch Delay Rate
4. Discussion
4.1. Cr(VI) Induced Reproductive Compensation Mechanism
4.2. Parental Effects Were Stronger than Developmental Exposures
4.3. Longterm Exposure of Cr(VI) Induced Adaptation
4.3.1. Adaptation in the Same Generation
4.3.2. Improved Adaptation in the Next Generation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, H.; Zhou, T.; Li, Q.; Lu, L.; Lin, C. Anthropogenic chromium emissions in china from 1990 to 2009. PLoS ONE 2014, 9, e87753. [Google Scholar] [CrossRef]
- Owlad, M.; Aroua, M.K.; Ashri, W.D.W.; Baroutian, S. Removal of hexavalent chromium-contaminated water and wastewater: A review. Water Air Soil Pollut. 2009, 200, 59–77. [Google Scholar] [CrossRef]
- Cheng, S. Heavy metal pollution in China: Origin, pattern and control. Environ. Sci. Pollut. Res. Int. 2003, 10, 192–198. [Google Scholar] [CrossRef]
- Fu, J.; Wang, H.; Billah, S.M.; Yu, H.; Zhang, X. Heavy metals in seawater, sediments, and biota from the coastal area of Yancheng City, China. Environ. Toxicol. Chem. 2014, 33, 1697. [Google Scholar] [CrossRef] [PubMed]
- Costa, M. Potential hazards of hexavalent chromate in our drinking water. Toxicol. Appl. Pharmacol. 2003, 188, 1–5. [Google Scholar] [CrossRef]
- Yan, L.; Wang, B.; Li, Z.; Liu, Y.; Huo, W.; Wang, J.; Li, Z.; Ren, A. Association of essential trace metals in maternal hair with the risk of neural tube defects in offspring. Birth Defects Res. 2017, 109, 234–243. [Google Scholar] [CrossRef]
- Vignet, C.; Joassard, L.; Lyphout, L.; Guionnet, T.; Goubeau, M.; Le Menach, K.; Brion, F.; Kah, O.; Chung, B.-C.; Budzinski, H.; et al. Exposures of zebrafish through diet to three environmentally relevant mixtures of PAHs produce behavioral disruptions in unexposed F1 and F2 descendant. Environ. Sci. Pollut. Res. Int. 2015, 22, 16371–16383. [Google Scholar] [CrossRef] [Green Version]
- Tye, M.T.; Montgomery, J.E.; Hobbs, M.R.; Vanpelt, K.T.; Masino, M.A. An Adult Zebrafish Diet Contaminated with Chromium Reduces the Viability of Progeny. Zebrafish 2018, 15, 179–187. [Google Scholar] [CrossRef]
- Ni, X.; Wan, L.; Liang, P.; Zheng, R.; Lin, Z.; Chen, R.; Pei, M. The acute toxic effects of hexavalent chromium on the liver of marine medaka (Oryzias melastigma). Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP. 2020, 231, 108734. [Google Scholar] [CrossRef]
- Volkova, K.; Caspillo, N.R.; Porseryd, T.; Hallgren, S.; Dinnetz, P.; Olsén, H.; Hällström, I.P. Transgenerational effects of 17α-ethinyl estradiol on anxiety behavior in the guppy, Poecilia reticulata. Gen. Comp. Endocrinol. 2015, 223, 66. [Google Scholar] [CrossRef] [Green Version]
- Thomas, B. Towards an alternative for the acute fish LC(50) test in chemical assessment: The fish embryo toxicity test goes multi-species—an update. ALTEX 2005, 22, 87–102. [Google Scholar]
- Braunbeck, T.; Lammer, E. Detailed review paper “fish embryo toxicity assays”. UBA Rep. Contract 2006, 20385422, 298. [Google Scholar]
- Fort, D.J.; Mathis, M.; Fort, C.E.; Fort, H.M.; Bacon, J.P. Application of endocrine disruptor screening program fish short-term reproduction assay: Reproduction and endocrine function in fathead minnow (Pimephales promelas) and killifish (Fundulus heteroclitus) exposed to Bermuda pond sediment. Environ. Toxicol. Chem. 2015, 34, 1283–1295. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Fang, C.; Wu, X.; Fan, J.; Dong, S. Perfluorooctane sulfonate impairs the cardiac development of a marine medaka (Oryzias melastigma). Aquat. Toxicol. 2011, 105, 71. [Google Scholar] [CrossRef] [PubMed]
- Colman, J.R.; Dechraoui, M.Y.; Dickey, R.W.; Ramsdell, J.S. Characterization of the developmental toxicity of Caribbean ciguatoxins in finfish embryos. Toxicon Off. J. Int. Soc. Toxicol. 2004, 44, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Yusof, S. Effect of mercury and cadmium on early life stages of Java medaka (Oryzias javanicus): A potential tropical test fish. Mar. Pollut. Bull. 2011, 63, 347–349. [Google Scholar] [CrossRef]
- Chen, H.; Cao, J.; Li, L.; Wu, X.; Bi, R.; Klerks, P.L.; Xie, L. Maternal transfer and reproductive effects of Cr(VI) in Japanese medaka (Oryzias latipes) under acute and chronic exposures. Aquat. Toxicol. 2016, 171, 59–68. [Google Scholar] [CrossRef]
- Munkittrick, K.R.; Dixon, D.G. Effects of natural exposure to copper and zinc on egg size and larval copper tolerance in white sucker (Catostomus commersoni). Ecotoxicol. Environ. Saf. 1989, 18, 15–26. [Google Scholar] [CrossRef]
- Pickering, Q.H. Chronic toxicity of hexavalent chromium to the fathead minnow (Pimephales promelas). Arch. Environ. Contam. Toxicol. 1980, 9, 405–413. [Google Scholar] [CrossRef]
- Truong, L.; Harper, S.L.; Tanguay, R.L. Evaluation of embryotoxicity using the zebrafish model. Methods Mol. Biol. 2011, 691, 271–279. [Google Scholar]
- Wu, Y.; Zhou, Q. Dose-and time-related changes in aerobic metabolism, chorionic disruption, and oxidative stress in embryonic medaka (Oryzias latipes): Underlying mechanisms for silver nanoparticle developmental toxicity. Aquat. Toxicol. 2012, 124–125, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Liu, Z.; Liu, F.; Ye, Y.; Peng, T.; Fu, Z. Embryonic exposure to cadmium (II) and chromium (VI) induce behavioral alterations, oxidative stress and immunotoxicity in zebrafish (Danio rerio). Neurotoxicol. Teratol. 2015, 48, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.V.; Cahansky, A.V.; López Greco, L.S.; Rodríguez, E.M. Toxicity of mercury during the embryonic development of Chasmagnathus granulatus (Brachyura, Varunidae). Environ. Res. 2005, 99, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Kapur, K.; Yadav, N.A. The Effects of Certain Heavy Metal Salts on the Development of Eggs in Common Carp, Cyprinus Carpio var. communis. CLEAN-Soil Air Water 2010, 10, 517–522. [Google Scholar]
- Lin, S.; Zhao, Y.; Nel, A.E.; Lin, S. Zebrafish: An in vivo model for nano EHS studies. Small 2013, 9, 1608–1618. [Google Scholar] [CrossRef] [Green Version]
- Gowaty, P.A. Reproductive compensation. J. Evol. Biol. 2008, 21, 1189–1200. [Google Scholar] [CrossRef]
- Harder, L.D.; Richards, S.A.; Routley, M.B. Effects of reproductive compensation, gamete discounting and reproductive assurance on mating-system diversity in hermaphrodites. Evol. Int. J. Org. Evol. 2008, 62, 157–172. [Google Scholar] [CrossRef]
- Goncalves, I.B.; Mobley, K.B.; Ahnesjö, I.; Sagebakken, G.; Jones, A.G.; Kvarnemo, C. Reproductive compensation in broad-nosed pipefish females. Proc. Biol. Sci. 2010, 277, 1581–1587. [Google Scholar] [CrossRef] [Green Version]
- Laidlaw, C.T.; Condon, J.M.; Belk, M.C. Viability Costs of Reproduction and Behavioral Compensation in Western Mosquitofish (Gambusia affinis). PLoS ONE 2014, 9, e110524. [Google Scholar] [CrossRef] [Green Version]
- Fanson, B.G.; Fanson, K.V.; Taylor, P.W. Cost of reproduction in the Queensland fruit fly: Y-model versus lethal protein hypothesis. Proc. Biol. Sci. 2012, 279, 4893–4900. [Google Scholar] [CrossRef] [Green Version]
- Haag, W.R. The role of fecundity and reproductive effort in defining life-history strategies of North American freshwater mussels. Biol. Rev. Camb. Philos. Soc. 2013, 88, 745–766. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ye, R.; Xu, Y.; Gao, Z.; Au, D.W.; Qian, P.Y. Comparative safety of the antifouling compound butenolide and 4,5-dichloro-2-n-octyl-4-isothiazolin-3-one (DCOIT) to the marine medaka (Oryzias melastigma). Aquat. Toxicol. 2014, 149, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Domingues, I.; Oliveira, R.; Lourenco, J.; Grisolia, C.K.; Mendo, S.; Soares, A.M. Biomarkers as a tool to assess effects of chromium (VI): Comparison of responses in zebrafish early life stages and adults. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP. 2010, 152, 338–345. [Google Scholar] [CrossRef]
- Mishra, A.K.; Mohanty, B. Effect of sublethal hexavalent chromium exposure on the pituitary-ovarian axis of a teleost, Channa punctatus (Bloch). Environ. Toxicol. 2012, 27, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Vodicnik, M.J.; Vomachka, M.S.; Lech, J.J. The effect of estradiol benzoate on the association of 2,4,5,2′,4′,5′-hexachlorobiphenyl with rainbow trout plasma lipoproteins. Fundam. Appl. Toxicol. 1983, 3, 502–506. [Google Scholar] [CrossRef]
- Gallenberg, L.A.; Vodicnik, M.J. Potential mechanisms for redistribution of polychlorinated biphenyls during pregnancy and lactation. Xenobiotica 1987, 17, 299–310. [Google Scholar] [CrossRef]
- Hamm, J.T.; Wilson, B.W.; Hinton, D.E. Increasing uptake and bioactivation with development positively modulate diazinon toxicity in early life stage medaka (Oryzias latipes). Toxicol. Sci. Off. J. Soc. Toxicol. 2001, 61, 304–313. [Google Scholar] [CrossRef] [Green Version]
- Harvey, B.; Kelley, R.N.; Ashwood-Smith, M.J. Permeability of intact and dechorionated zebra fish embryos to glycerol and dimethyl sulfoxide. Cryobiology 1983, 20, 432–439. [Google Scholar] [CrossRef]
- Billard, R.; Roubaud, P. The Effect of Metals and Cyanide on Fertilization in Rainbow-Trout (Salmo-Gairdneri). Water Res. 1985, 19, 209–214. [Google Scholar] [CrossRef] [Green Version]
- Farag, A.M.; Harper, D.D.; Cleveland, L.; Brumbaugh, W.G.; Little, E.E. The potential for chromium to affect the fertilization process of Chinook salmon (Oncorhynchus tshawytscha) in the Hanford reach of the Columbia River, Washington, USA. Arch. Environ. Contam. Toxicol. 2006, 50, 575–579. [Google Scholar] [CrossRef]
- Angilletta, M.J., Jr. Thermal Adaptation: A Theoretical and Empirical Synthesis; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Somero, G.N. Linking biogeography to physiology: Evolutionary and acclimatory adjustments of thermal limits. Front. Zool. 2005, 2, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, I.; Maria, V.L.; Oliveira, M.; Pacheco, M.; Santos, M.A. Oxidative stress and genotoxic effects in gill and kidney of Anguilla anguilla L. exposed to chromium with or without pre-exposure to beta-naphthoflavone. Mutat. Res. 2006, 608, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Salinas, S. Non-genetic inheritance and changing environments. Non-Genet. Inherit. 2013, 1, 38–50. [Google Scholar] [CrossRef]
- Bonduriansky, R.; Day, T. Nongenetic Inheritance and Its Evolutionary Implications. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 103–125. [Google Scholar] [CrossRef] [Green Version]
- Bird, A. DNA methylation patterns and epigenetic memory. Genes. Dev. 2002, 16, 6–21. [Google Scholar] [CrossRef] [Green Version]
- Crews, D.; Gillette, R.; Scarpino, S.V.; Manikkam, M.; Savenkova, M.I.; Skinner, M.K. Epigenetic transgenerational inheritance of altered stress responses. Proc. Natl. Acad. Sci. USA 2012, 109, 9143–9148. [Google Scholar] [CrossRef] [Green Version]
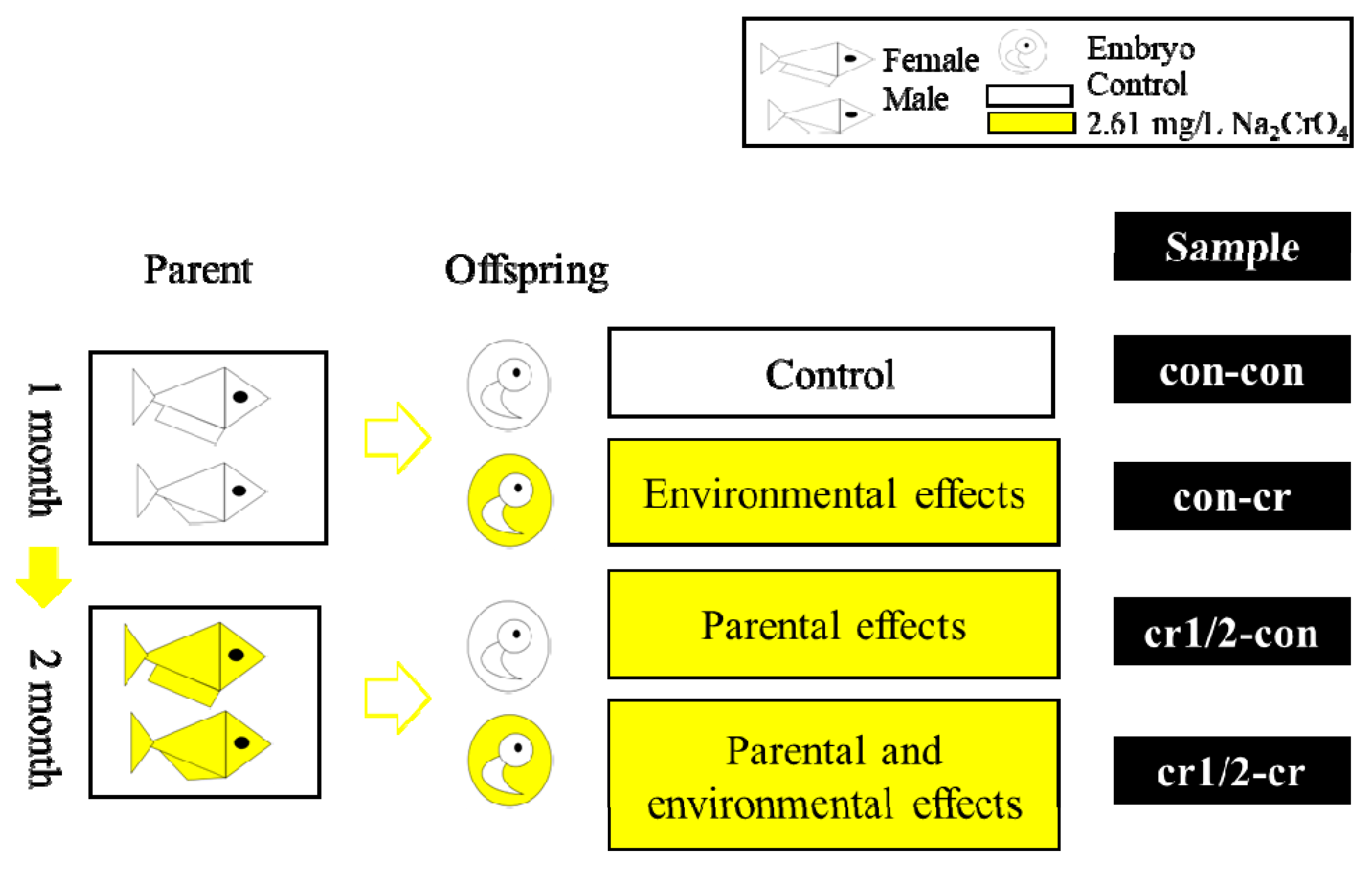



| Sample | Cr(VI) Treatment | |||
|---|---|---|---|---|
| Parents | Offspring | |||
| Concentration | Time | Concentration | Time | |
| con-con | 0 | 0 | 0 | 0 |
| con-cr | 0 | 0 | 2.61 mg/L | From fertilization to hatch |
| cr-con1 | 2.61 mg/L | 0–1 month | 0 | 0 |
| cr-cr1 | 2.61 mg/L | 0–1 month | 2.61 mg/L | From fertilization to hatch |
| cr-con2 | 2.61 mg/L | 1–2 month | 0 | 0 |
| cr-cr2 | 2.61 mg/L | 1–2 month | 2.61 mg/L | From fertilization to hatch |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, X.; Shen, Y. Transgenerational Effects of Hexavalent Chromium on Marine Medaka (Oryzias melastigma) Reveal Complex Transgenerational Adaptation in Offspring. Biomolecules 2021, 11, 138. https://doi.org/10.3390/biom11020138
Ni X, Shen Y. Transgenerational Effects of Hexavalent Chromium on Marine Medaka (Oryzias melastigma) Reveal Complex Transgenerational Adaptation in Offspring. Biomolecules. 2021; 11(2):138. https://doi.org/10.3390/biom11020138
Chicago/Turabian StyleNi, Xiaomin, and Yingjia Shen. 2021. "Transgenerational Effects of Hexavalent Chromium on Marine Medaka (Oryzias melastigma) Reveal Complex Transgenerational Adaptation in Offspring" Biomolecules 11, no. 2: 138. https://doi.org/10.3390/biom11020138






