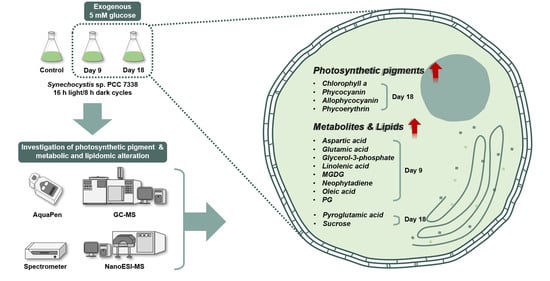
Enhanced Production of Photosynthetic Pigments and Various Metabolites and Lipids in the Cyanobacteria Synechocystis sp. PCC 7338 Culture in the Presence of Exogenous Glucose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture Conditions and Sample Collection
2.2. Measurements of Cell Growth
2.3. Measurement of Chlorophyll a Content
2.4. Measurement of Phycobiliprotein Content
2.5. Measurement of the Maximum Quantum Yield (QYmax) of Photosystem II
2.6. Metabolite Analysis Using GC-MS
2.7. Lipid Analysis Using nanoESI-MS
2.8. Data Processing and Multivariate Analysis
3. Results
3.1. Effect of Exogenous Glucose on the Growth of Synechocystis 7338
3.2. Effect of Exogenous Glucose on Chlorophyll a and Phycobiliprotein Content of Synechocystis 7338
3.3. Effect of Exogenous Glucose on Photosynthetic Activity of Synechocystis 7338
3.4. Effect of Exogenous Glucose on Comprehensive Metabolic and Lipidomic Profiles of Synechocystis 7338
3.5. Multivariate Statistical Analysis Based on GC-MS and nanoESI-MS Analyses of Synechocystis 7338 Treated with Exogenous Glucose
3.6. Relative Yields (Relative Intensity/L) of Various Metabolites and Intact Lipid Species in Synechocystis 7338 Treated with Exogenous Glucose
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parmar, A.; Singh, N.K.; Pandey, A.; Gnansounou, E.; Madamwar, D. Cyanobacteria and microalgae: A positive prospect for biofuels. Bioresour. Technol. 2011, 102, 10163–10172. [Google Scholar] [CrossRef] [PubMed]
- Wan, N.; Abernathy, M.; Tang, J.K.H.; Tang, Y.J.; You, L. Cyanobacterial photo-driven mixotrophic metabolism and its advantages for biosynthesis. Front. Chem. Sci. Eng. 2015, 9, 308–316. [Google Scholar] [CrossRef]
- Matson, M.M.; Atsumi, S. Photomixotrophic chemical production in cyanobacteria. Curr. Opin. Biotechnol. 2018, 50, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Francisco, É.C.; Franco, T.T.; Wagner, R.; Jacob-Lopes, E. Assessment of different carbohydrates as exogenous carbon source in cultivation of cyanobacteria. Bioprocess Biosyst. Eng. 2014, 37, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.L.; Choi, H.J.; Pawar, R.R.; Jung, S.P.; Lee, S.M. Enhanced biomass production through optimization of carbon source and utilization of wastewater as a nutrient source. J. Environ. Manag. 2016, 184, 585–595. [Google Scholar] [CrossRef]
- Pancha, I.; Chokshi, K.; Mishra, S. Enhanced biofuel production potential with nutritional stress amelioration through optimization of carbon source and light intensity in Scenedesmus sp. CCNM 1077. Bioresour. Technol. 2015, 179, 565–572. [Google Scholar] [CrossRef]
- Prasanna, R.; Pabby, A.; Singh, P.K. Effect of glucose and light-dark environment on pigmentation profiles in the cyanobacterium Calothrix elenkenii. Folia Microbiol. 2004, 49, 26–30. [Google Scholar] [CrossRef]
- Sharma, N.K.; Tiwari, S.P.; Tripathi, K.; Rai, A.K. Sustainability and cyanobacteria (blue-green algae): Facts and challenges. J. Appl. Phycol. 2011, 23, 1059–1081. [Google Scholar] [CrossRef]
- Kaneko, T.; Nakamura, Y.; Sasamoto, S.; Watanabe, A.; Kohara, M.; Matsumoto, M.; Shimpo, S.; Yamada, M.; Tabata, S. Structural analysis of four large plasmids harboring in a unicellular cyanobacterium, Synechocystis sp. PCC 6803. DNA Res. 2003, 10, 221–228. [Google Scholar] [CrossRef]
- Reyes, J.C.; Muro-Pastor, M.I.; Florencio, F.J. Transcription of glutamine synthetase genes (glnA and glnN) from the cyanobacterium Synechocystis sp. strain PCC 6803 is differently regulated in response to nitrogen availability. J. Bacteriol. 1997, 179, 2678–2689. [Google Scholar] [CrossRef] [Green Version]
- Su, X.; Fraenkel, P.G.; Bogorad, L. Excitation energy transfer from phycocyanin to chlorophyll in an apcA- defective mutant of Synechocystis sp. PCC 6803. J. Biol. Chem. 1992, 267, 22944–22950. [Google Scholar] [PubMed]
- Yu, Y.; You, L.; Liu, D.; Hollinshead, W.; Tang, Y.J.; Zhang, F. Development of Synechocystis sp. PCC 6803 as a phototrophic cell factory. Mar. Drugs 2013, 11, 2894–2916. [Google Scholar] [CrossRef] [PubMed]
- Varman, A.M.; Xiao, Y.; Pakrasi, H.B.; Tang, Y.J. Metabolic engineering of Synechocystis sp. Strain PCC 6803 for isobutanol production. Appl. Environ. Microbiol. 2013, 79, 908–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noh, Y.J.; Lee, H.; Hong, S.J.; Lee, H.; Cho, B.K.; Lee, C.G.; Choi, H.K. Comparative primary metabolic and lipidomic profiling of freshwater and marine Synechocystis strains using by GC-MS and nanoESI-MS analyses. Biotechnol. Bioprocess Eng. 2020, 25, 308–319. [Google Scholar] [CrossRef]
- Pagels, F.; Guedes, A.C.; Amaro, H.M.; Kijjoa, A.; Vasconcelos, V. Phycobiliproteins from cyanobacteria: Chemistry and biotechnological applications. Biotechnol. Adv. 2019, 37, 422–443. [Google Scholar] [CrossRef]
- Chakdar, H.; Pabbi, S. Cyanobacterial phycobilins: Production, purification, and regulation. In Frontier Discoveries and Innovations in Interdisciplinary Microbiology; Springer: New Delhi, India, 2016; pp. 45–69. [Google Scholar]
- Li, W.; Su, H.N.; Pu, Y.; Chen, J.; Liu, L.N.; Liu, Q.; Qin, S. Phycobiliproteins: Molecular structure, production, applications, and prospects. Biotechnol. Adv. 2019, 37, 340–353. [Google Scholar] [CrossRef]
- Mioso, R.; Marante, F.J.T.; González, J.E.G.; Rodríguez, J.J.S.; de Laguna, I.H.B. Metabolite profiling of Schizochytrium sp. by GC-MS, an oleaginous microbial source of biodiesel. Braz. J. Microbiol. 2014, 45, 403–409. [Google Scholar] [CrossRef] [Green Version]
- Wase, N.; Tu, B.; Allen, J.W.; Black, P.N.; DiRusso, C.C. Identification and metabolite profiling of chemical activators of lipid accumulation in green algae. Plant Physiol. 2017, 174, 2146–2165. [Google Scholar] [CrossRef] [Green Version]
- Vu, H.S.; Tamura, P.; Galeva, N.A.; Chaturvedi, R.; Roth, M.R.; Williams, T.D.; Wang, X.; Shah, J.; Welti, R. Direct infusion mass spectrometry of oxylipin-containing arabidopsis membrane lipids reveals varied patterns in different stress responses. Plant Physiol. 2012, 158, 324–339. [Google Scholar] [CrossRef]
- Yang, D.; Song, D.; Kind, T.; Ma, Y.; Hoefkens, J.; Fiehn, O. Lipidomic analysis of Chlamydomonas reinhardtii under nitrogen and sulfur deprivation. PLoS ONE 2015, 10, e137948. [Google Scholar] [CrossRef] [Green Version]
- Pittera, J.; Jouhet, J.; Breton, S.; Garczarek, L.; Partensky, F.; Maréchal, É.; Nguyen, N.A.; Doré, H.; Ratin, M.; Pitt, F.D.; et al. Thermoacclimation and genome adaptation of the membrane lipidome in marine Synechococcus. Environ. Microbiol. 2018, 20, 612–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhadauriya, P.; Gupta, R.; Singh, S.; Bisen, P.S. NaCl induced metabolic changes in the diazotrophic cyanobacterium Anabaena cylindrica. World J. Microbiol. Biotechnol. 2009, 25, 341–345. [Google Scholar] [CrossRef]
- Hasunuma, T.; Kikuyama, F.; Matsuda, M.; Aikawa, S.; Izumi, Y.; Kondo, A. Dynamic metabolic profiling of cyanobacterial glycogen biosynthesis under conditions of nitrate depletion. J. Exp. Bot. 2013, 64, 2943–2954. [Google Scholar] [CrossRef] [Green Version]
- Plohnke, N.; Seidel, T.; Kahmann, U.; Rögner, M.; Schneider, D.; Rexroth, S. The proteome and lipidome of Synechocystis sp. PCC 6803 cells grown under light-activated heterotrophic conditions. Mol. Cell. Proteomics 2015, 14, 572–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshikawa, K.; Hirasawa, T.; Ogawa, K.; Hidaka, Y.; Nakajima, T.; Furusawa, C.; Shimizu, H. Integrated transcriptomic and metabolomic analysis of the central metabolism of Synechocystis sp. PCC 6803 under different trophic conditions. Biotechnol. J. 2013, 8, 571–580. [Google Scholar] [CrossRef]
- Anderson, S.L.; McIntosh, L. Light-activated heterotrophic growth of the cyanobacterium Synechocystis sp. strain PCC 6803: A blue-light-requiring process. J. Bacteriol. 1991, 173, 2761–2767. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, T.; Kajihata, S.; Yoshikawa, K.; Matsuda, F.; Furusawa, C.; Hirasawa, T.; Shimizu, H. Integrated metabolic flux and omics analysis of Synechocystis sp. PCC 6803 under mixotrophic and photoheterotrophic conditions. Plant Cell Physiol. 2014, 55, 1606–1612. [Google Scholar] [CrossRef]
- Knothe, G.; Van Gerpen, J.; Krahl, J. The Biodiesel Handbook; AOCS Press: Champaign, IL, USA, 2005. [Google Scholar]
- De Marsac, N.T.; Houmard, J. Complementary chromatic adaptation: Physiological conditions and action spectra. Methods Enzymol. 1988, 167, 318–328. [Google Scholar] [CrossRef]
- Hong, S.J.; Lee, C.G. Statistical optimization of culture media for production of phycobiliprotein by Synechocystis sp. PCC 6701. Biotechnol. Bioprocess Eng. 2008, 13, 491–498. [Google Scholar] [CrossRef]
- Osanai, T.; Kuwahara, A.; Iijima, H.; Toyooka, K.; Sato, M.; Tanaka, K.; Ikeuchi, M.; Saito, K.; Hirai, M.Y. Pleiotropic effect of sigE over-expression on cell morphology, photosynthesis and hydrogen production in Synechocystis sp. PCC 6803. Plant J. 2013, 76, 456–465. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, H.Y.; Jeon, J.Y.; Kim, D.M.; Zhou, Y.; Lee, J.S.; Lee, H.; Choi, H.K. Effects of coronatine elicitation on growth and metabolic profiles of Lemna paucicostata culture. PLoS ONE 2017, 12, e0187622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Lim, S.R.; Hong, S.J.; Cho, B.K.; Lee, H.; Lee, C.G.; Choi, H.K. Effect of ethephon as an ethylene-releasing compound on the metabolic profile of Chlorella vulgaris. J. Agric. Food Chem. 2016, 64, 4807–4816. [Google Scholar] [CrossRef] [PubMed]
- Lüttge, U.; Büdel, B. Resurrection kinetics of photosynthesis in desiccation-tolerant terrestrial green algae (Chlorophyta) on tree bark. Plant Biol. 2010, 12, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.; Johansson, E.; Kettaneh-Wold, N.; Trygg, J.; Wikström, C.; Wold, S. PLS. In Multi-and Megavariate Data Analysis; Eriksson, L., Ed.; Umetrics AB: Umeå, Sweden, 2006; pp. 63–101. [Google Scholar]
- Kim, T.J.; Park, J.G.; Kim, H.Y.; Ha, S.-H.; Lee, B.; Park, S.U.; Seo, W.D.; Kim, J.K. Metabolite profiling and chemometric study for the discrimination analyses of geographic origin of perilla (Perilla frutescens) and sesame (Sesamum indicum) seeds. Foods 2020, 9, 989. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, S.Y.; Chun, Y.S.; Chun, Y.J.; Shin, S.Y.; Choi, C.H.; Choi, H.K. Characteristics of fecal metabolic profiles in patients with irritable bowel syndrome with predominant diarrhea investigated using 1H-NMR coupled with multivariate statistical analysis. Neurogastroenterol. Motil. 2020, 32, 1–13. [Google Scholar] [CrossRef]
- Nakamura, C.E.; Whited, G.M. Metabolic engineering for the microbial production of 1,3-propanediol. Curr. Opin. Biotechnol. 2003, 14, 454–459. [Google Scholar] [CrossRef]
- Da Silva, G.P.; Mack, M.; Contiero, J. Glycerol: A promising and abundant carbon source for industrial microbiology. Biotechnol. Adv. 2009, 27, 30–39. [Google Scholar] [CrossRef]
- Rattanapoltee, P.; Kaewkannetra, P. Utilization of agricultural residues of pineapple peels and sugarcane bagasse as cost-saving raw materials in Scenedesmus acutus for lipid accumulation and biodiesel production. Appl. Biochem. Biotechnol. 2014, 173, 1495–1510. [Google Scholar] [CrossRef]
- Perez-Garcia, O.; Bashan, Y.; Esther Puente, M. Organic carbon supplementation of sterilized municipal wastewater is essential for heterotrophic growth and removing ammonium by the microalga Chlorella Vulgaris. J. Phycol. 2011, 47, 190–199. [Google Scholar] [CrossRef]
- Wang, J.; Yang, H.; Wang, F. Mixotrophic cultivation of microalgae for biodiesel production: Status and prospects. Appl. Biochem. Biotechnol. 2014, 172, 3307–3329. [Google Scholar] [CrossRef]
- Yu, G.; Shi, D.; Cai, Z.; Cong, W.; Ouyang, F. Growth and physiological features of cyanobacterium Anabaena sp. strain PCC 7120 in a glucose-mixotrophic culture. Chin. J. Chem. Eng. 2011, 19, 108–115. [Google Scholar] [CrossRef]
- Marquez, F.J.; Nishio, N.; Nagai, S.; Sasaki, K. Enhancement of biomass and pigment production during growth of Spirulina platensis in mixotrophic culture. J. Chem. Technol. Biotechnol. 1995, 62, 159–164. [Google Scholar] [CrossRef]
- Znachor, P.; Nedoma, J. Importance of dissolved organic carbon for phytoplankton nutrition in a eutrophic reservoir. J. Plankton Res. 2010, 32, 367–376. [Google Scholar] [CrossRef] [Green Version]
- Stadnichuk, I.N.; Tropin, I.V. Phycobiliproteins: Structure, functions and biotechnological applications. Appl. Biochem. Microbiol. 2017, 53, 1–10. [Google Scholar] [CrossRef]
- Borsari, R.R.J.; Morioka, L.R.I.; Ribeiro, M.L.L.; Buzato, J.B.; Pinotti, M.H.P. Mixotrophic growth of Nostoc sp. on glucose, sucrose and sugarcane molasses for phycobiliprotein production. Acta Sci. Biol. Sci. 2007, 29, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Elmorjani, K.; Herdman, M. Metabolic control of phycocyanin degradation in the cyanobacterium Synechocystis PCC 6803: A glucose effect. J. Gen. Microbiol. 1987, 133, 1685–1694. [Google Scholar] [CrossRef] [Green Version]
- Oesterhelt, C.; Schmälzlin, E.; Schmitt, J.M.; Lokstein, H. Regulation of photosynthesis in the unicellular acidophilic red alga Galdieria sulphuraria. Plant J. 2007, 51, 500–511. [Google Scholar] [CrossRef]
- Martínez, F.; Orús, M.I. Interactions between glucose and inorganic carbon metabolism in Chlorella vulgaris strain UAM 101. Plant Physiol. 1991, 95, 1150–1155. [Google Scholar] [CrossRef] [Green Version]
- Kanwal, S.; Rastogi, R.P.; Incharoensakdi, A. Glutamate decarboxylase activity and gamma-aminobutyric acid content in Synechocystis sp. PCC 6803 under osmotic stress and different carbon sources. J. Appl. Phycol. 2014, 26, 2327–2333. [Google Scholar] [CrossRef]
- Lee, H.; Noh, Y.J.; Hong, S.J.; Lee, H.; Kim, D.M.; Cho, B.K.; Lee, C.G.; Choi, H.K. Photosynthetic pigment production and metabolic and lipidomic alterations in the marine cyanobacteria Synechocystis sp. PCC 7338 under various salinity conditions. J. Appl. Phycol. 2020. [Google Scholar] [CrossRef]
- Rodríguez-Meizoso, I.; Jaime, L.; Santoyo, S.; Cifuentes, A.; Garcia-Blairsy Reina, G.; Senorans, F.J.; Ibáñez, E. Pressurized fluid extraction of bioactive compounds from Phormidium species. J. Agric. Food Chem. 2008, 56, 3517–3523. [Google Scholar] [CrossRef] [PubMed]
- Mustapa, A.N.; Martin, Á.; Mato, R.B.; Cocero, M.J. Extraction of phytocompounds from the medicinal plant Clinacanthus nutans Lindau by microwave-assisted extraction and supercritical carbon dioxide extraction. Ind. Crops Prod. 2015, 74, 83–94. [Google Scholar] [CrossRef]
- Raman, B.V.; Samuel, L.A.; Saradhi, M.P.; Rao, B.N.; Krishna, N.V.; Sudhakar, M.; Radhakrishnan, T.M. Antibacterial, antioxidant activity and GC-MS analysis of Eupatorium odoratum. Asian J. Pharm. Clin. Res. 2012, 5, 99–106. [Google Scholar]
- Plaza, M.; Santoyo, S.; Jaime, L.; Reina, G.G.-B.; Herrero, M.; Señoráns, F.J.; Ibáñez, E. Screening for bioactive compounds from algae. J. Pharm. Biomed. Anal. 2010, 51, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Patent Application, CN106501420A, People’s Republic of China. One Grow Tobacco Middle Neophytadiene Extraction Purification and Detection Method and Its Applicationtle. Available online: https://patents.google.com/patent/CN106501420A/en (accessed on 26 January 2021).
- Bhardwaj, M.; Sali, V.K.; Mani, S.; Vasanthi, H.R. Neophytadiene from Turbinaria ornata suppresses LPS-induced inflammatory response in RAW 264.7 macrophages and Sprague Dawley Rats. Inflammation 2020, 43, 937–950. [Google Scholar] [CrossRef]
- Cheng, K.; Zhao, X.; Zeng, J.; Zhang, J. Biotechnological production of succinic acid: Current state and perspectives. Biofuels Bioprod. Biorefining 2012, 6, 302–318. [Google Scholar] [CrossRef]
- Yan, R.; Zhu, D.; Zhang, Z.; Zeng, Q.; Chu, J. Carbon metabolism and energy conversion of Synechococcus sp. PCC 7942 under mixotrophic conditions: Comparison with photoautotrophic condition. J. Appl. Phycol. 2012, 24, 657–668. [Google Scholar] [CrossRef]
- Lea-Smith, D.J.; Bombelli, P.; Vasudevan, R.; Howe, C.J. Photosynthetic, respiratory and extracellular electron transport pathways in cyanobacteria. Biochim. Biophys. Acta Bioenerg. 2016, 1857, 247–255. [Google Scholar] [CrossRef] [Green Version]
- Peschek, G.A.; Obinger, C.; Paumann, M. The respiratory chain of blue-green algae (cyanobacteria). Physiol. Plant 2004, 120, 358–369. [Google Scholar] [CrossRef]
- Singh, D.K.; Mallick, N. Accumulation potential of lipids and analysis of fatty acid profile of few microalgal species for biodiesel feedstock. J. Microbiol. Biotechnol. Res. 2014, 4, 37–44. [Google Scholar]
- Popovich, C.A.; Damiani, C.; Constenla, D.; Martínez, A.M.; Freije, H.; Giovanardi, M.; Pancaldi, S.; Leonardi, P.I. Neochloris oleoabundans grown in enriched natural seawater for biodiesel feedstock: Evaluation of its growth and biochemical composition. Bioresour. Technol. 2012, 114, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Anne-Marie, K.; Yee, W.; Loh, S.H.; Aziz, A.; Cha, T.S. Influence of nitrogen availability on biomass, lipid production, fatty acid profile, and the expression of fatty acid desaturase genes in Messastrum gracile SE-MC4. World J. Microbiol. Biotechnol. 2020, 36, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.G.; Kim, B.H.; Ahn, C.Y.; Oh, H.M. Effect of nitrogen limitation on oleic acid biosynthesis in Botryococcus braunii. J. Appl. Phycol. 2011, 23, 1031–1037. [Google Scholar] [CrossRef]
- Mikami, K.; Murata, N. Membrane fluidity and the perception of environmental signals in cyanobacteria and plants. Prog. Lipid Res. 2003, 42, 527–543. [Google Scholar] [CrossRef]
- Sato, N.; Wada, H. Lipid Biosynthesis and its Regulation in Cyanobacteria. In Lipids in Photosynthesis: Essential and Regulatory Functions; Springer: Dordrecht, The Netherland, 2009; pp. 157–177. [Google Scholar] [CrossRef]
- Shirahashi, H.; Murakami, N.; Watanabe, M.; Nagatsu, A.; Sakakibara, J.; Tokuda, H.; Nishino, H.; Iwashima, A. Isoaltaoin and identification of anti-tumor-promoting principles from the fresh-water cyanobacterium Phormidium tenue. Chem. Pharm. Bull. 1993, 41, 1664–1666. [Google Scholar] [CrossRef] [Green Version]
- Ulivi, V.; Lenti, M.; Gentili, C.; Marcolongo, G.; Cancedda, R.; Cancedda, F.D. Anti-inflammatory activity of monogalactosyldiacylglycerol in human articular cartilage in vitro: Activation of an anti-inflammatory cyclooxygenase-2 (COX-2) pathway. Arthritis Res. Ther. 2011, 13, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Antonopoulou, S.; Nomikos, T.; Oikonomou, A.; Kyriacou, A.; Andriotis, M.; Fragopoulou, E.; Pantazidou, A. Characterization of bioactive glycolipids from Scytonema julianum (cyanobacteria). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2005, 140, 219–231. [Google Scholar] [CrossRef]
- Mykhaylenko, N.F.; Zolotareva, O.K. Cyanobacterial lipid composition with regard to the regulatory role of glucose. Adv. Res. Plant Lipids 2003, 389–392. [Google Scholar] [CrossRef]
- Siron, R.; Giusti, G.; Berland, B. Changes in the fatty acid composition of Phaeodactylum tricornutum and Dunaliella tertiolecta during growth and under phosphorus deficiency. Mar. Ecol. Prog. Ser. 1989, 55, 95–100. [Google Scholar] [CrossRef]
- Fisher, N.S.; Schwarzenbadv, R.P. Fatty acid dynamics in Thalassiosira pseudonana (Bacillariophyceae): Implications for physiological ecology. J. Phycol. 1978, 14, 143–150. [Google Scholar] [CrossRef]




| Day 1 | Day 9 | Day 18 | |
|---|---|---|---|
| Control | 0.50 ± 0.01 | 0.49 ± 0.01 | 0.48 ± 0.01 |
| 5-mM Glucose | 0.45 ± 0.01 * | 0.49 ± 0.01 | 0.49 ± 0.01 |
| % Control | 90 | 100 | 102 |
| No. | Compound | Day 9 | Day 18 | ||
|---|---|---|---|---|---|
| Control | 5-mM Glucose | Control | 5-mM Glucose | ||
| Alcohols | |||||
| 1 | Glycerol | 2.10 ± 0.15 | 0.95 ± 0.17 *,2 | 1.76 ± 1.09 | 3.06 ± 3.25 |
| 2 | Glycerol-3-phosphate | 26.80 ± 6.47 | 39.07 ± 5.84 * | 2.52 ± 2.92 | 6.65 ± 5.99 |
| Amino acids | |||||
| 3 | Alanine | 23.18 ± 6.21 | 14.43 ± 1.11 * | 11.07 ± 2.96 | 5.56 ± 2.75 * |
| 4 | Aspartic acid | 27.31 ± 5.18 | 62.70 ± 10.30 * | 48.06 ± 24.30 | 27.88 ± 18.00 |
| 5 | Glutamic acid | 44.90 ± 14.70 | 115.06 ± 8.47 * | 52.21 ± 3.92 | 94.01 ± 14.52 * |
| 6 | Glycine | 2.13 ± 0.49 | 1.74 ± 0.40 | 5.02 ± 0.37 | 3.54 ± 0.32 * |
| 7 | Pyroglutamic acid | 5.79 ± 0.67 | 6.99 ± 0.98 * | 7.19 ± 1.00 | 10.76 ± 1.49 * |
| 8 | Serine | 0.39 ± 0.12 | 0.31 ± 0.03 | 0.87 ± 0.31 | 0.51 ± 0.22 * |
| Fatty acids | |||||
| 9 | Linoleic acid | 2.68 ± 0.78 | 3.46 ± 0.52 * | 4.25 ± 0.66 | 4.96 ± 1.17 |
| 10 | Linolenic acid | 0.84 ± 0.23 | 1.58 ± 0.23 * | 1.84 ± 0.42 | 1.83 ± 0.34 |
| 11 | Oleic acid | 2.02 ± 0.41 | 9.45 ± 1.94 * | 5.01 ± 0.86 | 8.32 ± 1.89 * |
| 12 | Palmitic acid | 17.99 ± 4.15 | 25.03 ± 4.83 * | 39.84 ± 8.88 | 50.63 ± 11.27 |
| 13 | Palmitoleic acid | 0.31 ± 0.09 | 0.42 ± 0.09 * | 0.68 ± 0.11 | 0.86 ± 0.20 * |
| 14 | Stearic acid | 1.86 ± 0.31 | 2.06 ± 0.23 | 2.48 ± 0.39 | 2.49 ± 0.47 |
| Glycerolipids | |||||
| 15 | 1-Monopalmitin | 4.59 ± 0.92 | 6.09 ± 2.48 | 6.88 ± 2.78 | 7.32 ± 0.66 |
| 16 | Glycerol monostearate | 2.20 ± 0.40 | 2.94 ± 1.04 | 3.35 ± 1.33 | 3.41 ± 0.36 |
| Organic acids | |||||
| 17 | Isocitric acid | 0.79 ± 0.08 | 0.54 ± 0.14 * | 0.42 ± 0.24 | 0.64 ± 0.25 |
| 18 | Lactic acid | 0.98 ± 0.25 | 0.85 ± 0.26 | 1.01 ± 0.40 | 0.82 ± 0.14 |
| 19 | Succinic acid | 0.34 ± 0.11 | 0.79 ± 0.36 * | 0.48 ± 0.10 | 2.29 ± 0.52 * |
| Sugars | |||||
| 20 | Fructose | ND 3 | 0.43 ± 0.08 | 11.06 ± 0.60 | 6.43 ± 0.87 * |
| 21 | Glucosamine | ND | 0.42 ± 0.02 | 3.71 ± 0.97 | 3.28 ± 0.56 |
| 22 | Glucose | 0.29 ± 0.06 | 0.36 ± 0.03 * | 9.47 ± 5.72 | 20.23 ± 20.6 |
| 23 | Glucose-6-phosphate | 1.79 ± 1.18 | 0.34 ± 0.08 | ND | ND |
| 24 | Glucosylglycerol | 928.05 ± 102.33 | 947.57 ± 90.16 | 966.05 ± 97.71 | 960.70 ± 58.37 |
| 25 | Sucrose | 61.15 ± 26.60 | 117.98 ± 23.74 * | 175.79 ± 22.35 | 296.63 ± 38.42 * |
| Others | |||||
| 26 | Neophytadiene | 5.20 ± 0.83 | 10.99 ± 1.11 * | 4.45 ± 0.39 | 9.15 ± 0.96 * |
| 27 | Heptadecane | 19.50 ± 3.84 | 13.41 ± 1.40 * | 25.87 ± 6.68 | 22.97 ± 3.10 |
| No. | m/z | Lipid Species | Ion Species | Day 9 | Day 18 | ||
|---|---|---|---|---|---|---|---|
| Control | 5-mM Glucose | Control | 5-mM Glucose | ||||
| Positive ion mode | |||||||
| Digalactosyldiacylglycerol (DGDG) | |||||||
| 1 | 935 | DGDG 16:1/18:3 | [M+Na]+ | 33.75 ± 3.61 | 17.62 ± 4.36 *,2 | 30.17 ± 5.92 | 26.57 ± 2.90 |
| 2 | 937 | DGDG 16:0/18:3 | [M+Na]+ | 60.80 ± 14.09 | 35.98 ± 8.14 * | 62.28 ± 8.63 | 65.06 ± 13.61 |
| 3 | 939 | DGDG 16:0/18:2 | [M+Na]+ | 41.13 ± 5.95 | 17.62 ± 5.01 * | 39.36 ± 6.65 | 42.75 ± 10.33 |
| 4 | 941 | DGDG 16:0/18:1 | [M+Na]+ | 10.53 ± 2.72 | 10.01 ± 3.49 | 14.14 ± 2.38 | 22.72 ± 3.01 * |
| 5 | 943 | DGDG 16:0/18:0 | [M+Na]+ | 53.69 ± 37.46 | 56.22 ± 16.97 | 71.58 ± 22.68 | 57.52 ± 7.93 |
| 6 | 961 | DGDG 18:2/18:3 | [M+Na]+ | 9.48 ± 1.76 | 12.15 ± 1.89 * | 11.34 ± 3.27 | 10.09 ± 1.90 |
| Monogalactosyldiacylglycerol (MGDG) | |||||||
| 7 | 747 | MGDG 14:0/18:3 | [M+Na]+ | 29.11 ± 6.61 | 30.27 ± 2.04 | 32.02 ± 7.53 | 27.55 ± 4.85 |
| 8 | 763 | MGDG 16:0/17:2 | [M+Na]+ | 62.47 ± 25.38 | 62.50 ± 12.49 | 74.34 ± 21.78 | 50.41 ± 9.38 * |
| 9 | 773 | MGDG 16:1/18:3 | [M+Na]+ | 138.53 ± 27.91 | 144.13 ± 23.07 | 156.02 ± 16.21 | 155.10 ± 16.57 |
| 10 | 775 | MGDG 16:0/18:3 | [M+Na]+ | 398.62 ± 56.63 | 325.94 ± 64.11 | 403.47 ± 70.95 | 311.94 ± 37.64 * |
| 11 | 777 | MGDG 16:0/18:2 | [M+Na]+ | 124.28 ± 17.37 | 93.59 ± 8.26 * | 128.74 ± 18.99 | 124.63 ± 25.76 |
| 12 | 779 | MGDG 16:0/18:1 | [M+Na]+ | 2.51 ± 1.98 | 28.26 ± 9.28 * | 3.29 ± 2.87 | 17.50 ± 3.62 * |
| 13 | 799 | MGDG 18:2/18:3 | [M+Na]+ | 68.62 ± 4.81 | 84.78 ± 9.63 * | 89.77 ± 28.35 | 77.90 ± 22.35 |
| 14 | 801 | MGDG 18:2/18:2 | [M+Na]+ | 471.10 ± 45.19 | 586.25 ± 93.75 * | 593.17 ± 226.25 | 551.62 ± 177.46 |
| 15 | 803 | MGDG 18:1/18:2 | [M+Na]+ | 697.14 ± 50.39 | 885.31 ± 93.27 * | 923.49 ± 276.11 | 802.00 ± 177.73 |
| 16 | 805 | MGDG 16:0/20:2 | [M+Na]+ | 123.93 ± 9.75 | 166.84 ± 20.60 * | 154.72 ± 53.53 | 136.05 ± 30.75 |
| 17 | 807 | MGDG 16:0/20:1 | [M+Na]+ | 4.34 ± 2.39 | 6.72 ± 1.67 * | 9.17 ± 4.47 | 4.67 ± 1.07 * |
| 18 | 809 | MGDG 16:0/20:0 | [M+Na]+ | 87.78 ± 64.26 | 77.33 ± 49.96 | 171.74 ± 88.79 | 114.71 ± 27.39 |
| 19 | 823 | MGDG 16:0/21:0 | [M+Na]+ | 67.66 ± 9.15 | 68.43 ± 21.47 | 110.69 ± 50.04 | 109.86 ± 44.94 |
| Phytyl Derivatives | |||||||
| 20 | 871 | Pheophytin a | [M+H]+ | 522.39 ± 106.64 | 631.85 ± 435.75 | 75.07 ± 15.69 | 127.23 ± 29.06 * |
| 21 | 893 | Chlorophyll a | [M+H]+ | 157.53 ± 18.27 | 170.56 ± 58.57 | 111.87 ± 27.99 | 195.90 ± 35.34 * |
| Negative ion mode | |||||||
| Phosphatidylglycerol (PG) | |||||||
| 22 | 721 | PG 16:0/16:0 | [M-H]− | 227.74 ± 40.19 | 293.08 ± 19.32 * | 241.82 ± 40.15 | 286.54 ± 12.40 * |
| 23 | 731 | PG 16:0/17:2 | [M-H]− | 25.42 ± 3.40 | 46.78 ± 3.57 * | 30.48 ± 4.38 | 16.14 ± 2.14 * |
| 24 | 733 | PG 16:0/17:1 | [M-H]− | 28.81 ± 4.06 | 24.00 ± 1.76 * | 32.20 ± 5.52 | 26.12 ± 6.61 |
| 25 | 743 | PG 16:0/18:3 | [M-H]− | 184.59 ± 40.43 | 79.70 ± 18.21 * | 52.14 ± 20.42 | 40.78 ± 17.32 |
| 26 | 745 | PG 16:0/18:2 | [M-H]− | 1790.41 ± 429.45 | 1186.95 ± 268.70 * | 718.15 ± 280.60 | 560.95 ± 240.69 |
| 27 | 747 | PG 16:0/18:1 | [M-H]− | 554.97 ± 96.70 | 658.61 ± 144.52 | 242.65 ± 93.41 | 283.00 ± 117.93 |
| 28 | 749 | PG 16:0/18:0 | [M-H]− | 46.74 ± 7.80 | 16.78 ± 4.92 * | 15.53 ± 7.27 | 25.90 ± 8.04 * |
| Sulfoquinvosyldiacylglycerol (SQDG) | |||||||
| 29 | 763 | SQDG 14:1/16:0 | [M-H]− | 20.00 ± 4.76 | 6.12 ± 6.63 * | 10.46 ± 4.59 | 11.58 ± 4.62 |
| 30 | 765 | SQDG 14:0/16:0 | [M-H]− | 102.57 ± 24.01 | 21.46 ± 10.69 * | 62.01 ± 22.80 | 34.26 ± 11.14 * |
| 31 | 747 | SQDG 14:0/18:3 | [M-H]− | 37.78 ± 5.94 | 18.11 ± 3.12 * | 28.44 ± 6.26 | 20.92 ± 8.70 |
| 32 | 789 | SQDG 14:0/18:2 | [M-H]− | 88.71 ± 20.79 | 34.51 ± 5.15 * | 42.64 ± 17.24 | 28.61 ± 14.09 |
| 33 | 791 | SQDG 16:0/16:1 | [M-H]− | 1344.99 ± 318.03 | 878.52 ± 198.49 * | 738.59 ± 286.80 | 571.89 ± 227.26 |
| 34 | 793 | SQDG 16:0/16:0 | [M-H]− | 3495.78 ± 770.70 | 1810.00 ± 480.7 * | 1790.48 ± 691.01 | 1184.24 ± 469.66 * |
| 35 | 803 | SQDG 16:0/17:2 | [M-H]− | 95.83 ± 23.92 | 27.00 ± 6.81 * | 34.67 ± 13.94 | 16.27 ± 6.85 * |
| 36 | 805 | SQDG 16:0/17:1 | [M-H]− | 311.37 ± 50.70 | 246.53 ± 63.70 | 160.00 ± 66.82 | 123.67 ± 50.69 |
| 37 | 807 | SQDG 16:0/17:0 | [M-H]− | 198.33 ± 42.89 | 112.62 ± 32.15 * | 79.49 ± 31.17 | 44.82 ± 18.63 * |
| 38 | 813 | SQDG 16:0/18:4 | [M-H]− | 377.56 ± 64.81 | 265.30 ± 9.52 * | 288.50 ± 38.10 | 258.27 ± 22.99 |
| 39 | 815 | SQDG 16:0/18:3 | [M-H]− | 1020.36 ± 267.61 | 359.39 ± 56.36 * | 582.38 ± 204.28 | 501.31 ± 179.22 |
| 40 | 817 | SQDG 16:0/18:2 | [M-H]− | 6748.15 ± 1825.81 | 3287.51 ± 883.17 * | 3039.48 ± 1152.15 | 2330.66 ± 1022.07 |
| 41 | 819 | SQDG 16:0/18:1 | [M-H]− | 4661.42 ± 986.36 | 7023.10 ± 1866.88 * | 3189.82 ± 1209.63 | 3086.93 ± 1167.90 |
| 42 | 821 | SQDG 16:0/18:0 | [M-H]− | 1056.37 ± 253.67 | 1495.41 ± 398.34 * | 742.22 ± 275.01 | 655.08 ± 243.36 |
| 43 | 831 | SQDG 17:0/18:2 | [M-H]− | 38.98 ± 7.94 | 14.79 ± 4.11 * | 15.61 ± 6.32 | 11.23 ± 3.93 |
| 44 | 835 | SQDG 16:0/19:0 | [M-H]− | 64.58 ± 23.80 | 38.91 ± 11.20 * | 26.79 ± 8.83 | 31.14 ± 16.67 |
| 45 | 839 | SQDG 18:2/18:3 | [M-H]− | 15.14 ± 4.08 | 5.79 ± 0.77 * | 9.84 ± 2.04 | 8.70 ± 1.23 |
| 46 | 841 | SQDG 18:1/18:3 | [M-H]− | 24.30 ± 5.66 | 9.29 ± 3.13 * | 15.71 ± 5.42 | 11.56 ± 4.08 |
| 47 | 843 | SQDG 16:0/20:3 | [M-H]− | 76.68 ± 20.50 | 36.40 ± 8.59 * | 48.27 ± 16.34 | 46.82 ± 17.52 |
| 48 | 845 | SQDG 18:0/18:2 | [M-H]− | 83.52 ± 21.38 | 47.71 ± 12.15 * | 48.03 ± 16.39 | 48.75 ± 19.35 |
| 49 | 847 | SQDG 18:0/18:1 | [M-H]− | 49.56 ± 14.03 | 58.07 ± 15.49 * | 23.99 ± 8.64 | 33.75 ± 11.66 |
| 50 | 849 | SQDG 16:0/20:0 | [M-H]− | 28.34 ± 8.74 | 39.63 ± 9.09 | 10.23 ± 3.41 | 16.25 ± 5.92 * |
| No. | Compounds | Day 9 | No. | Compounds | Day 18 | ||
|---|---|---|---|---|---|---|---|
| Control | 5-mM Glucose | Control | 5-mM Glucose | ||||
| 1 | Aspartic acid | 11.25 ± 2.33 | 50.99 ± 8.03 *,1 | 1 | Chlorophyll a | 126.91 ± 31.78 | 146.36 ± 25.32 |
| 2 | Fructose | ND 5 | 0.35 ± 0.07 | 2 | DGDG 3 16:0/18:1 | 15.99 ± 2.25 | 16.72 ± 2.07 |
| 3 | Glucosamine | ND | 0.34 ± 0.02 | 3 | Glutamic acid | 58.27 ± 6.36 | 70.65 ± 10.14 |
| 4 | Glutamic acid | 18.48 ± 6.24 | 93.63 ± 6.38 * | 4 | MGDG 16:0/18:1 | 5.45 ± 4.81 | 12.45 ± 2.25 * |
| 5 | Glycerol-3-phosphate | 10.99 ± 2.61 | 31.78 ± 4.52 * | 5 | Neophytadiene | 5.19 ± 0.73 | 6.72 ± 0.58 * |
| 6 | Linolenic acid | 0.34 ± 0.10 | 1.29 ± 0.18 * | 6 | Oleic acid | 5.48 ± 1.42 | 6.49 ± 0.94 |
| 7 | MGDG 2 16:0/18:1 | 1.00 ± 0.76 | 22.94 ± 7.35 * | 7 | Pheophytin a | 85.38 ± 19.61 | 95.60 ± 22.08 |
| 8 | MGDG 16:0/20:2 | 50.84 ± 3.35 | 135.95 ± 18.25 * | 8 | Pyroglutamic acid | 7.84 ± 1.26 | 8.08 ± 1.00 |
| 9 | MGDG 18:1/18:2 | 286.87 ± 29.23 | 721.19 ± 82.32 * | 9 | Succinic acid | 0.68 ± 0.45 | 1.65 ± 0.36 * |
| 10 | Neophytadiene | 2.14 ± 0.33 | 8.94 ± 0.87 * | 10 | Sucrose | 200.79 ± 21.54 | 216.88 ± 24.23 |
| 11 | Oleic acid | 0.83 ± 0.18 | 7.69 ± 1.53 * | ||||
| 12 | PG 4 16:0/16:0 | 93.85 ± 19.02 | 238.55 ± 15.61 * | ||||
| 13 | PG 16:0/17:2 | 10.42 ± 1.25 | 38.06 ± 2.75 * | ||||
| 14 | Pyroglutamic acid | 2.38 ± 0.29 | 5.69 ± 0.81 * | ||||
| 15 | Sucrose | 25.31 ± 11.75 | 96.05 ± 19.30 * | ||||
| No. | Compounds | 5-mM Glucose | |
|---|---|---|---|
| Day 9 | Day 18 | ||
| 1 | Glutamic acid | 93.63 ± 6.38 | 70.65 ± 10.14 *,1 |
| 2 | MGDG 2 16:0/18:1 | 22.94 ± 7.35 | 12.45 ± 2.25 * |
| 3 | Neophytadiene | 8.94 ± 0.87 | 6.72 ± 0.58 * |
| 4 | Oleic acid | 7.69 ± 1.53 | 6.49 ± 0.94 * |
| 5 | Pyroglutamic acid | 5.69 ± 0.81 | 8.08 ± 1.00 * |
| 6 | Sucrose | 96.05 ± 19.30 | 216.88 ± 24.23 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noh, Y.; Lee, H.; Kim, M.; Hong, S.-J.; Lee, H.; Kim, D.-M.; Cho, B.-K.; Lee, C.-G.; Choi, H.-K. Enhanced Production of Photosynthetic Pigments and Various Metabolites and Lipids in the Cyanobacteria Synechocystis sp. PCC 7338 Culture in the Presence of Exogenous Glucose. Biomolecules 2021, 11, 214. https://doi.org/10.3390/biom11020214
Noh Y, Lee H, Kim M, Hong S-J, Lee H, Kim D-M, Cho B-K, Lee C-G, Choi H-K. Enhanced Production of Photosynthetic Pigments and Various Metabolites and Lipids in the Cyanobacteria Synechocystis sp. PCC 7338 Culture in the Presence of Exogenous Glucose. Biomolecules. 2021; 11(2):214. https://doi.org/10.3390/biom11020214
Chicago/Turabian StyleNoh, YuJin, Hwanhui Lee, Myeongsun Kim, Seong-Joo Hong, Hookeun Lee, Dong-Myung Kim, Byung-Kwan Cho, Choul-Gyun Lee, and Hyung-Kyoon Choi. 2021. "Enhanced Production of Photosynthetic Pigments and Various Metabolites and Lipids in the Cyanobacteria Synechocystis sp. PCC 7338 Culture in the Presence of Exogenous Glucose" Biomolecules 11, no. 2: 214. https://doi.org/10.3390/biom11020214
APA StyleNoh, Y., Lee, H., Kim, M., Hong, S.-J., Lee, H., Kim, D.-M., Cho, B.-K., Lee, C.-G., & Choi, H.-K. (2021). Enhanced Production of Photosynthetic Pigments and Various Metabolites and Lipids in the Cyanobacteria Synechocystis sp. PCC 7338 Culture in the Presence of Exogenous Glucose. Biomolecules, 11(2), 214. https://doi.org/10.3390/biom11020214